Effect of Fe and EDTA on Freshwater Cyanobacteria Bloom Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultures
2.2. Growth Experiments
2.3. Fe Uptake Tests
2.4. Measurement of Growth
2.5. Data Analysis
3. Results
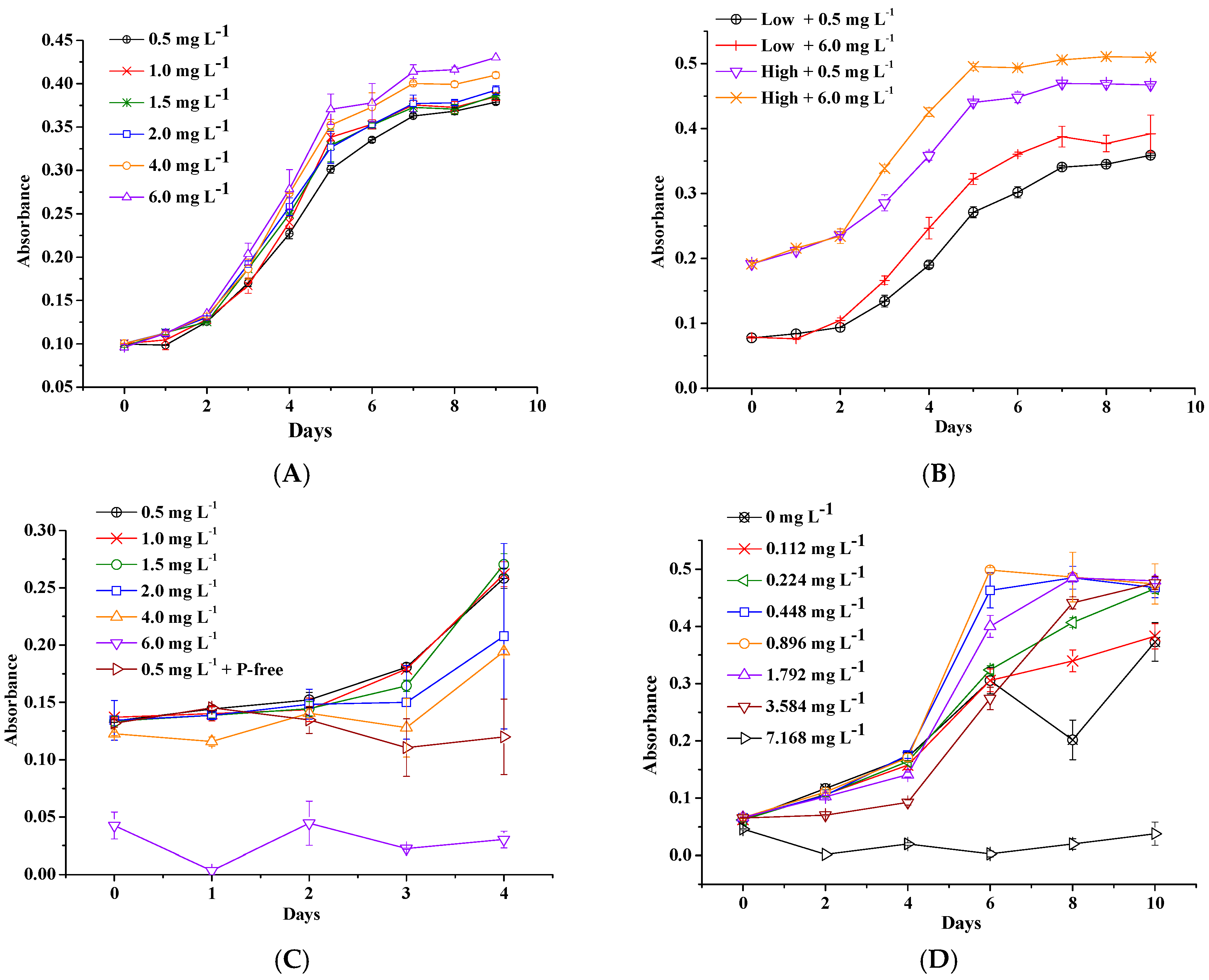
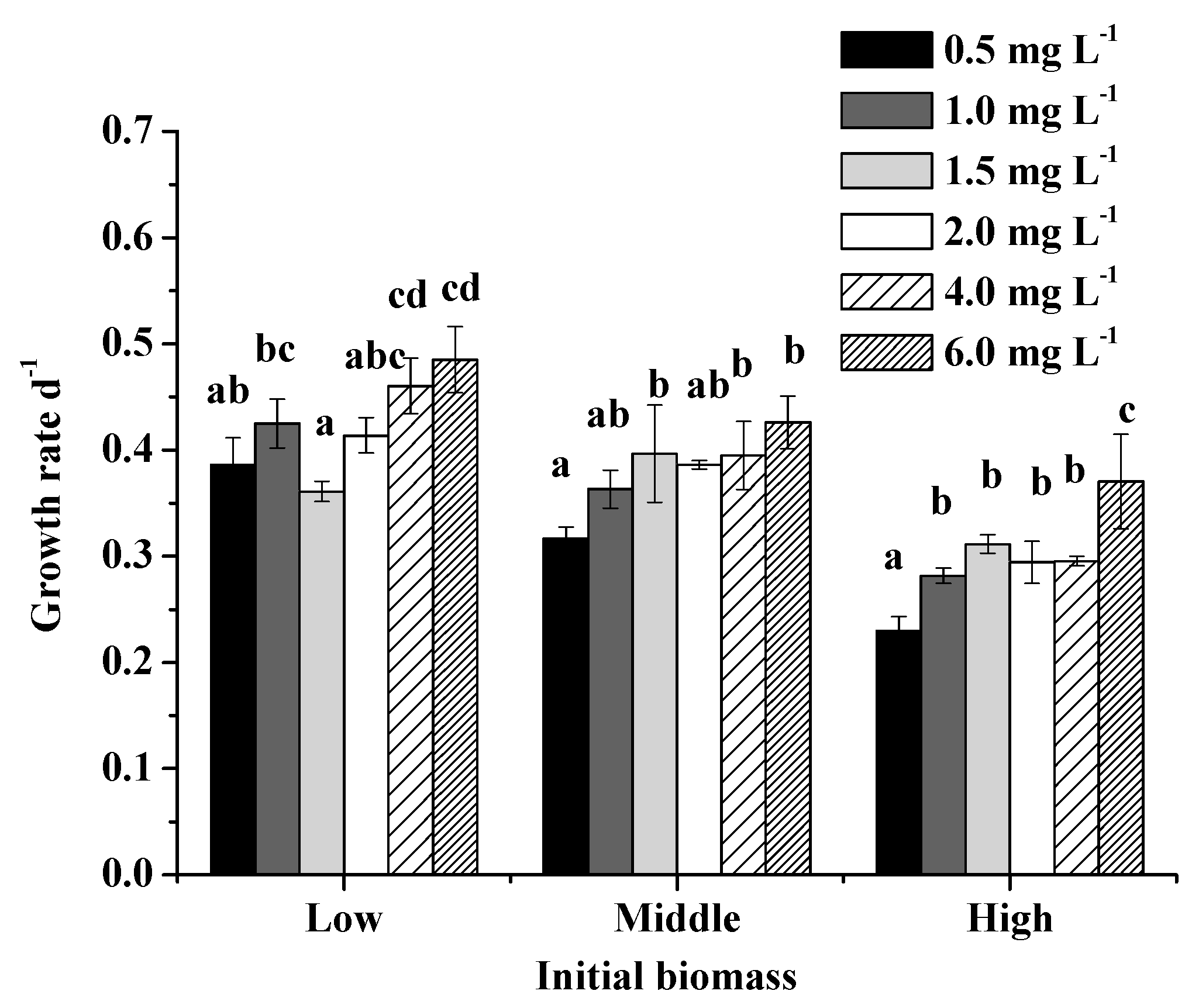
3.1. Exp 1: Effect of EDTA-Fe and Initial Biomass on M. aeruginosa Growth
3.2. Exp 2: Effect of EDTA and Fe3+ on M. Aeruginosa Growth
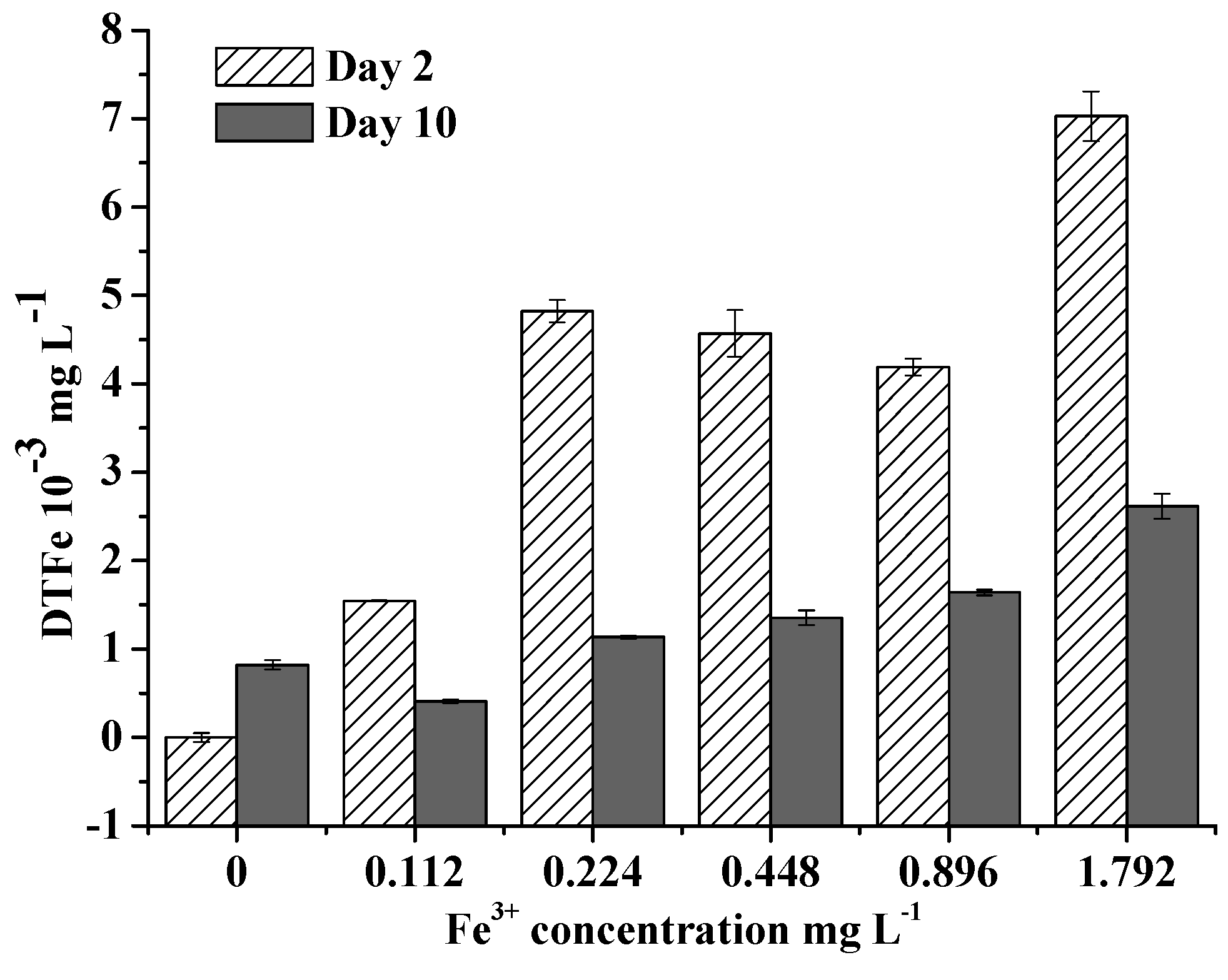
3.3. Exp 3: Effect of Fe3+ on M. Aeruginosa Growth and Fe3+ Uptake
4. Discussion
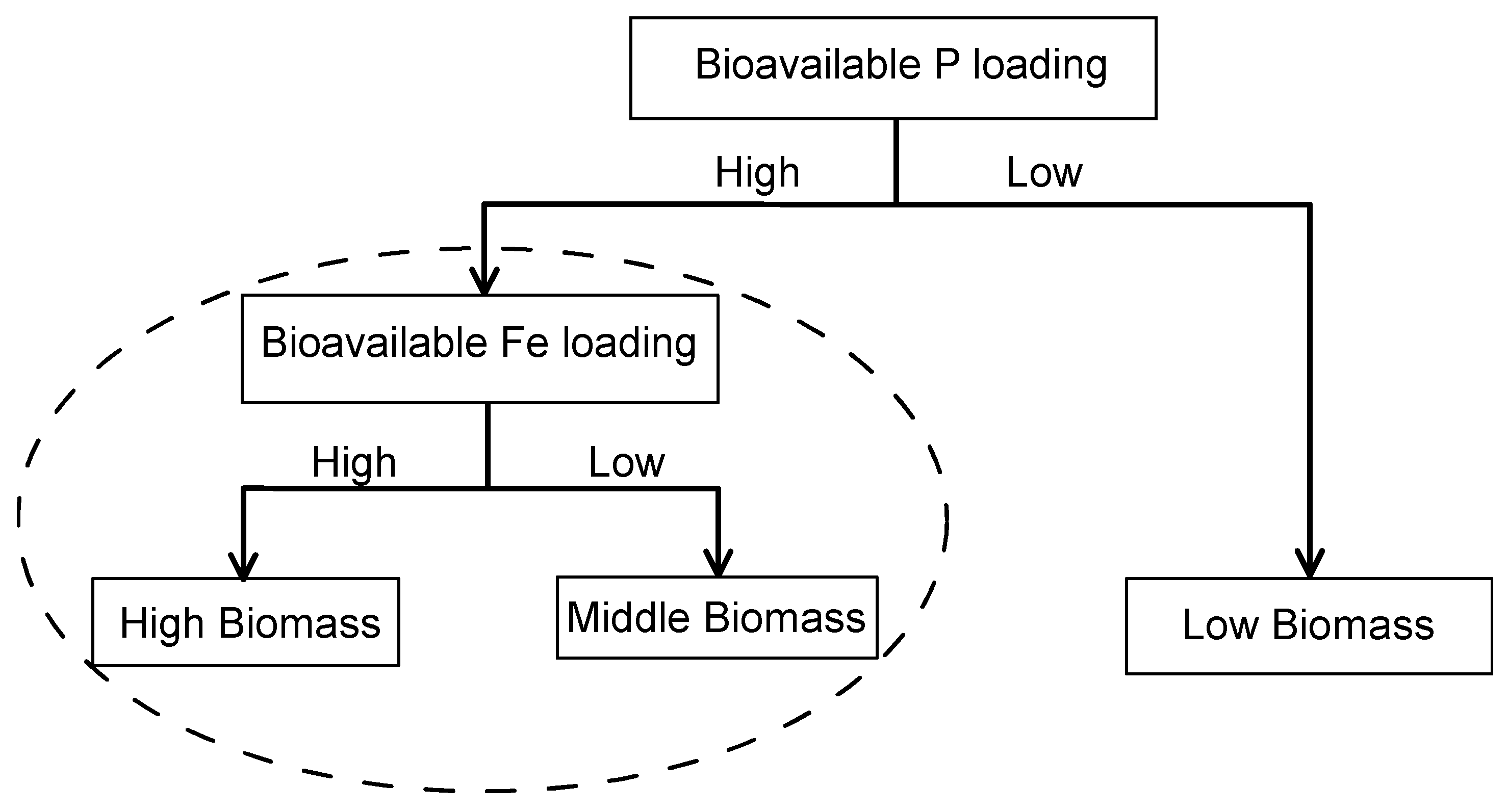
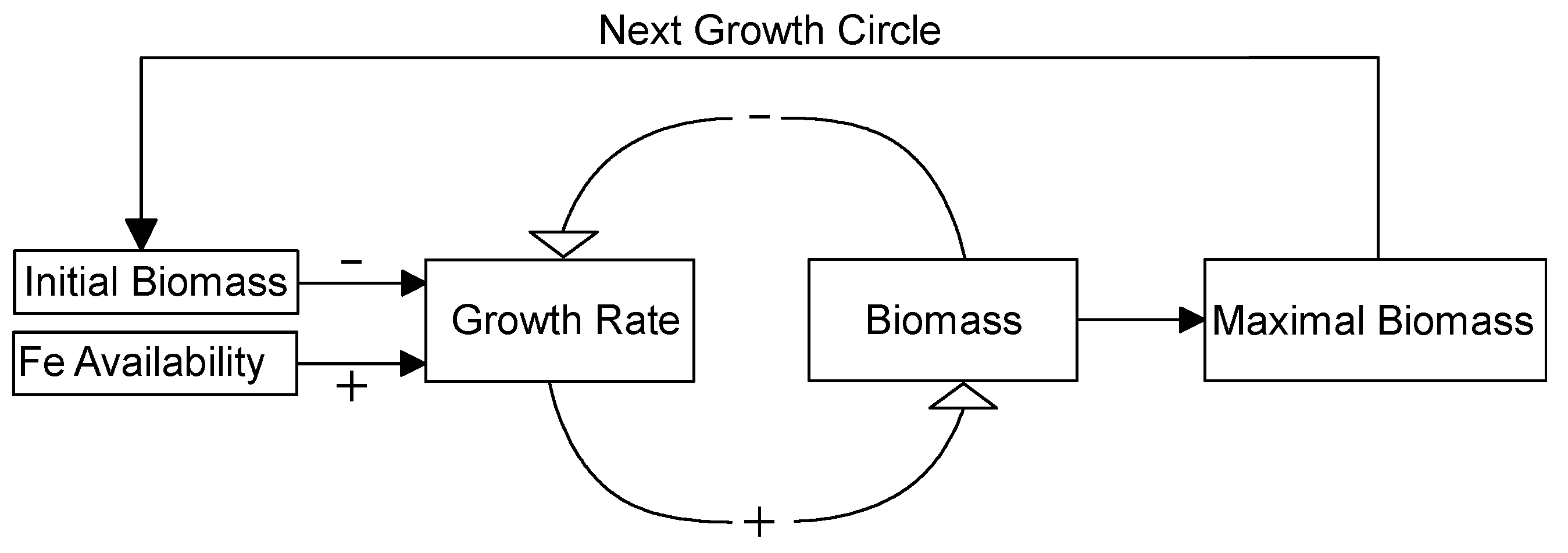
4.1. A Conceptual Model of How Fe Availability Regulates Cyanobacterial Biomass in Eutrophic Lakes
4.2. Effect of Fe Concentration and Initial Biomass
4.3. Effect of EDTA and Fe3+
4.4. Implications
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Lewitus, A. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, M.M.; Chen, H. Removal of cyanobacterial blooms in Taihu Lake using local soils. I. Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Environ. Pollut. 2006, 141, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. Lond. B Biol. Sci. 2012. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Auer, M.T.; Kieser, M.S.; Canale, R.P. Identification of critical nutrient levels through field verification of models for phosphorus and phytoplankton growth. Can. J. Fish. Aquat. Sci. 1986, 43, 379–388. [Google Scholar] [CrossRef]
- Chappell, P.D.; Whitney, L.P.; Wallace, J.R.; Darer, A.I.; Jean-Charles, S.; Jenkins, B.D. Genetic indicators of iron limitation in wild populations of Thalassiosira oceanica from the northeast Pacific Ocean. ISME J. 2015, 9, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Legendre, L.; Jiao, N. Phytoplankton responses to nitrogen and iron limitation in the tropical and subtropical Pacific Ocean. J. Plankton Res. 2015. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Jickells, T.D. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef]
- Flynn, K.J.; Hipkin, C.R. Interactions between iron, light, ammonium, and nitrate: Insights from the construction of a dynamic model of algal physiology. J. Phycol. 1999, 35, 1171–1190. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Huntsman, S.A. High iron requirement for growth, photosynthesis, and low-light acclimation in the coastal cyanobacterium Synechococcus bacillaris. Front. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sorichetti, R.J.; Creed, I.F.; Trick, C.G. Iron and iron-binding ligands as cofactors that limit cyanobacterial biomass across a lake trophic gradient. Freshw. Biol. 2016, 61, 146–157. [Google Scholar] [CrossRef]
- Garcia, N.S.; Fu, F.; Sedwick, P.N. Iron deficiency increases growth and nitrogen-fixation rates of phosphorus-deficient marine cyanobacteria. ISME J. 2015, 9, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Abraham, E.R. Iron-mediated changes in phytoplankton photosynthetic competence during SOIREE. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 2529–2550. [Google Scholar] [CrossRef]
- Shi, D.; Kranz, S.A.; Kim, J.M. Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions. Proc. Natl. Acad. Sci. USA 2012, 109, E3094–E3100. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R. How microgranisms transport iron. Science 1984, 225, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, W.X.; Guo, L. Phase partitioning and solubility of iron in natural seawater controlling by dissolved organic matter. Glob. Biogeochem. Cycles 2004. [Google Scholar] [CrossRef]
- Orihel, D.M.; Schindler, D.W.; Ballard, N.C.; Wilson, L.R.; Vinebrooke, R.D. Experimental iron amendment suppresses toxic cyanobacteria in a hypereutrophic lake. Ecol. Appl. 2016. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.M.; Geider, R.J.; Kolber, Z.; Falkowski, P.G. Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae. Plant Physiol. 1992, 100, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Huntsman, S.A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 1997, 390, 389–392. [Google Scholar] [CrossRef]
- Hyenstrand, P.; Rydin, E.; Gunnerhed, M.; Linder, J.; Blomqvist, P. Response of the cyanobacterium Gloeotrichia echinulata to iron and boron additions—An experiment from Lake Erken. Freshw. Biol. 2001, 46, 735–741. [Google Scholar] [CrossRef]
- Molot, L.A.; Li, G.; Findlay, D. L; Watson, S.B. Iron-mediated suppression of bloom-forming cyanobacteria by oxine in a eutrophic lake. Freshw. Biol. 2010, 55, 1102–1117. [Google Scholar] [CrossRef]
- Sorichetti, R.J.; Creed, I.F.; Trick, C.G. The influence of iron, siderophores and refractory DOM on cyanobacterial biomass in oligotrophic lakes. Freshw. Biol. 2014, 59, 1423–1436. [Google Scholar] [CrossRef]
- Lis, H.; Kranzler, C.; Keren, N. A comparative study of iron uptake rates and mechanisms amongst marine and fresh water cyanobacteria: Prevalence of reductive iron uptake. Life 2015, 5, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Jinhua, S. The Assessment Methodology for Eutrophication Level of Lakes in China. Environ. Pollut. Control 1990, 12, 2–7. [Google Scholar]
- Xing, W.; Huang, W.M.; Liu, Y.D.; Li, D.H.; Shen, Y.W.; Li, G.B. Environmental mechanism of change in cyanobacterial species composition in the Northeastern part of Lake Dianchi (China). Fresenius Environ. Bull. 2007, 16, 82–90. [Google Scholar]
- Sun, B.K.; Tanji, Y.; Unno, H. Influences of iron and humic acid on the growth of the cyanobacterium Anabaena circinalis. Biochem. Eng. J. 2005, 24, 195–201. [Google Scholar] [CrossRef]
- Clark, F.; Brook, B.W.; Delean, S.; Reşit Akçakaya, H.; Bradshaw, C.J. The theta-logistic is unreliable for modelling most census data. Methods Ecol. Evol. 2010, 1, 253–262. [Google Scholar] [CrossRef]
- Reynolds, S.A.; Brassil, C.E. When can a single-species, density-dependent model capture the dynamics of a consumer-resource system? J. Theor. Biol. 2013, 339, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Sæther, B.E.; Engen, S. Pattern of variation in avian population growth rates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1185–1195. [Google Scholar]
- Bull, J.C.; Bonsall, M.B. Overcompensatory population dynamic responses to environmental stochasticity. J. Anim. Ecol. 2008, 77, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.L.; Anderson, M.G.; Steury, T.D. Temporal shift in density dependence among North American breeding duck populations. Ecology 2010, 91, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Hixon, M.A.; Johnson, D.W. Density dependence and independence. Encycl. Life Sci. 2009. [Google Scholar] [CrossRef]
- Borlestean, A.; Frost, P.C.; Murray, D.L. A mechanistic analysis of density dependence in algal population dynamics. Front. Ecol. Evol. 2015, 3, 37. [Google Scholar] [CrossRef]
- Hassler, C.S.; Twiss, M.R. Bioavailability of iron sensed by a phytoplanktonic Fe-bioreporter. Environ. Sci. Technol. 2006, 40, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liu, G. Iron biogeochemistry and its environmental impacts in freshwater lakes. Fresenius Environ. Bull. 2011, 20, 1339–1345. [Google Scholar]
- Wilhelm, S.W. Ecology of iron-limited cyanobacteria: A review of physiological responses and implications for aquatic systems. Aquat. Microb. Ecol. 1995, 9, 295–303. [Google Scholar] [CrossRef]
- Ferreira, F.; Straus, N.A. Iron deprivation in cyanobacteria. J. Appl. Phycol. 1994, 6, 199–210. [Google Scholar] [CrossRef]
- Gress, C.D.; Treble, R.G.; Matz, C.J.; Weger, H.G. Biologicalavailability of iron to the freshwater cyanobacterium Anabaena flos-aquae1. J. Phycol. 2004, 40, 879–886. [Google Scholar] [CrossRef]
- Chen, Y.; Barak, P. Iron nutrition of plants in calcareous soils. Adv. Agron. 1982, 35, 217–240. [Google Scholar]
- Wilhelm, S.W.; Trick, C.G. Iron-limited growth of cyanobacteria: Multiple siderophore production is a common response. Limnol. Oceanogr. 1994, 39, 1979–1984. [Google Scholar] [CrossRef]
- Church, M.J.; Hutchins, D.A.; Ducklow, H.W. Limitation of bacterial growth by dissolved organic matter and iron in the Southern Ocean. Appl. Environ. Microbiol. 2000, 66, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Koukal, B.; Gueguen, C.; Pardos, M.; Dominik, J. Influence of humic substances on the toxic effects of cadmium and zinc to the green alga Pseudokirchneriella subcapitata. Chemosphere 2003, 53, 953–961. [Google Scholar] [CrossRef]
- Imai, A.; Fukushima, T.; Matsushige, K. Effects of iron limitation and aquatic humic substances on the growth of Microcystis aeruginosa. Can. J. Fish. Aquat. Sci. 1999, 56, 1929–1937. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Dupuy, C.; Cruzado, A.; Velasquez, Z.; Achterberg, E.P.; Braungardt, C.B. Influence of sorption processes by iron oxides and algae fixation on arsenic and phosphate cycle in an acidic estuary (Tinto river, Spain). Water Res. 2000, 34, 3222–3230. [Google Scholar] [CrossRef]
- Wang, W.X.; Dei, R.C. Bioavailability of iron complexed with organic colloids to the cyanobacteria Synechococcus and Trichodesmium. Aquat. Microb. Ecol. 2003, 33, 247–259. [Google Scholar] [CrossRef]
- Carey, E.; Taillefert, M. The role of soluble Fe (III) in the cycling of iron and sulfur in coastal marine sediments. Limnol. Oceanogr. 2005, 50, 1129. [Google Scholar] [CrossRef]
- Martynova, M.V. Iron compound occurrence forms in freshwater deposits: Analytical review. Water Res. 2010, 37, 488–496. [Google Scholar] [CrossRef]
- Nürnberg, G.K.; Dillon, P.J. Iron budgets in temperate lakes. Can. J. Fish. Aquat. Sci. 1993, 50, 1728–1737. [Google Scholar]
- Schindler, D.W.; Donahue, W.F. An impending water crisis in Canada's western prairie provinces. Proc. Natl. Acad. Sci. USA 2006, 103, 7210–7216. [Google Scholar] [CrossRef] [PubMed]

| Parameters | µmax | Km | µmax/Km | Adj. R-Square |
|---|---|---|---|---|
| Fe3+ | 0.5628 | 1.7040 | 0.330 | 0.8668 |
| EDTA-Fe | - | - | - | - |
| Low initial biomass | 0.4609 | 2.9981 | 0.154 | 0.5278 |
| Middle initial biomass | 0.4203 | 3.0543 | 0.138 | 0.8666 |
| High initial biomass | 0.3528 | 4.9090 | 0.072 | 0.6830 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; He, J.; Luo, X. Effect of Fe and EDTA on Freshwater Cyanobacteria Bloom Formation. Water 2017, 9, 326. https://doi.org/10.3390/w9050326
Zhang T, He J, Luo X. Effect of Fe and EDTA on Freshwater Cyanobacteria Bloom Formation. Water. 2017; 9(5):326. https://doi.org/10.3390/w9050326
Chicago/Turabian StyleZhang, Ting, Jian He, and Xingzhang Luo. 2017. "Effect of Fe and EDTA on Freshwater Cyanobacteria Bloom Formation" Water 9, no. 5: 326. https://doi.org/10.3390/w9050326






