Performance and Microbial Diversity in a Low-Energy ANF-WDSRBC System for the Post-Treatment of Decentralized Domestic Wastewater
Abstract
:1. Introduction
2. Materials and Methods
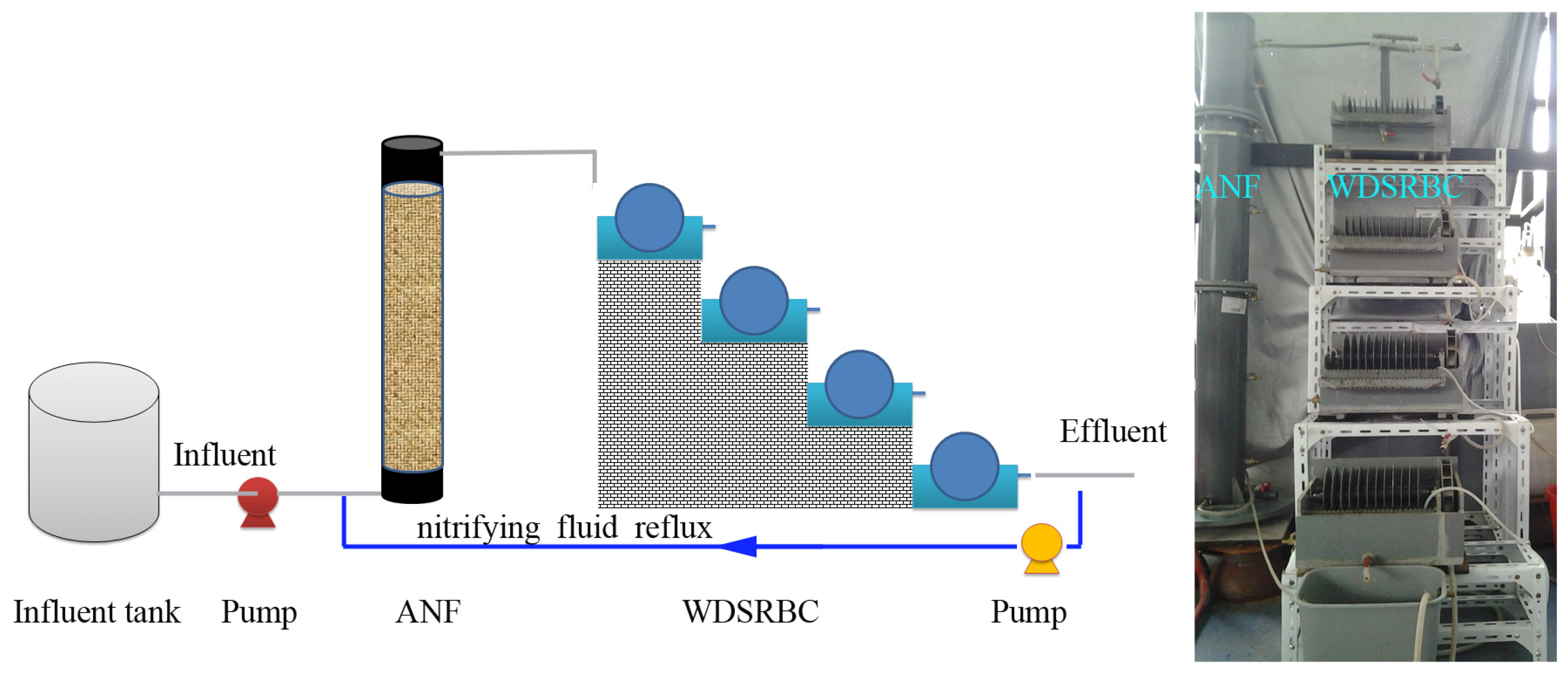
2.1. Experimental Setup
2.2. Wastewater Characteristics and Seeding Sludge
2.3. Analytical Methods
2.3.1. Chemical Analysis
2.3.2. Microbial Community Analysis by MiSeq Sequencing
3. Results and Discussion
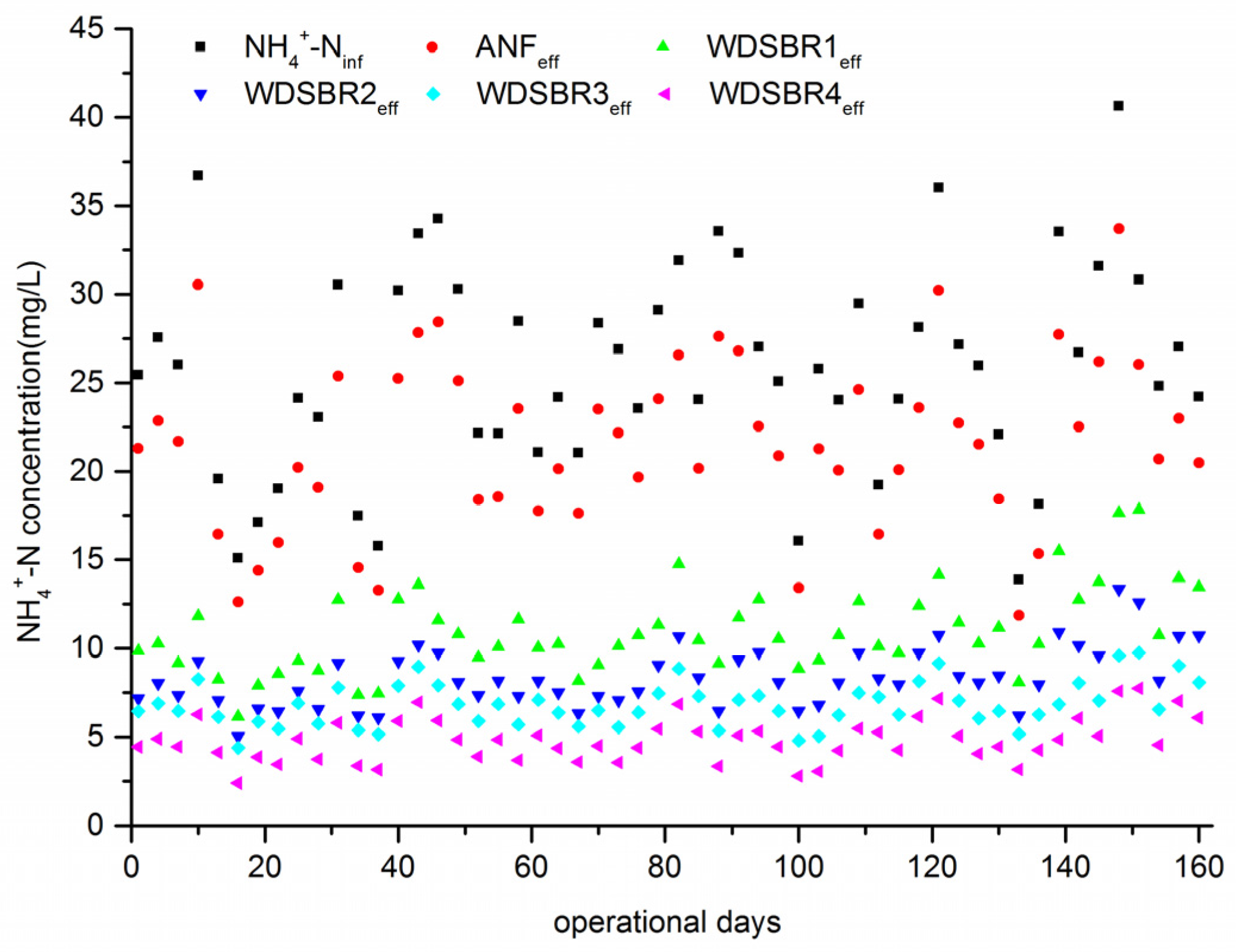
3.1. Nitrogen Removal of the ANF-WDSRBC System at Different Reflux Ratios
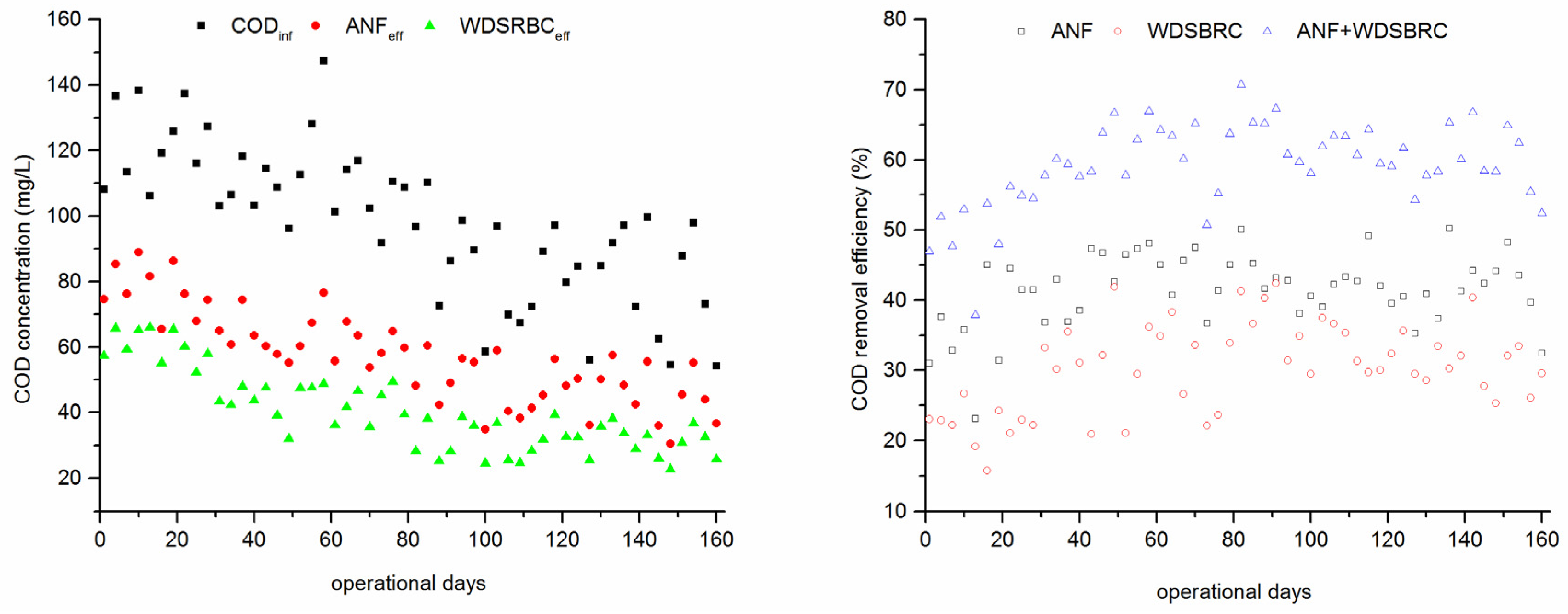
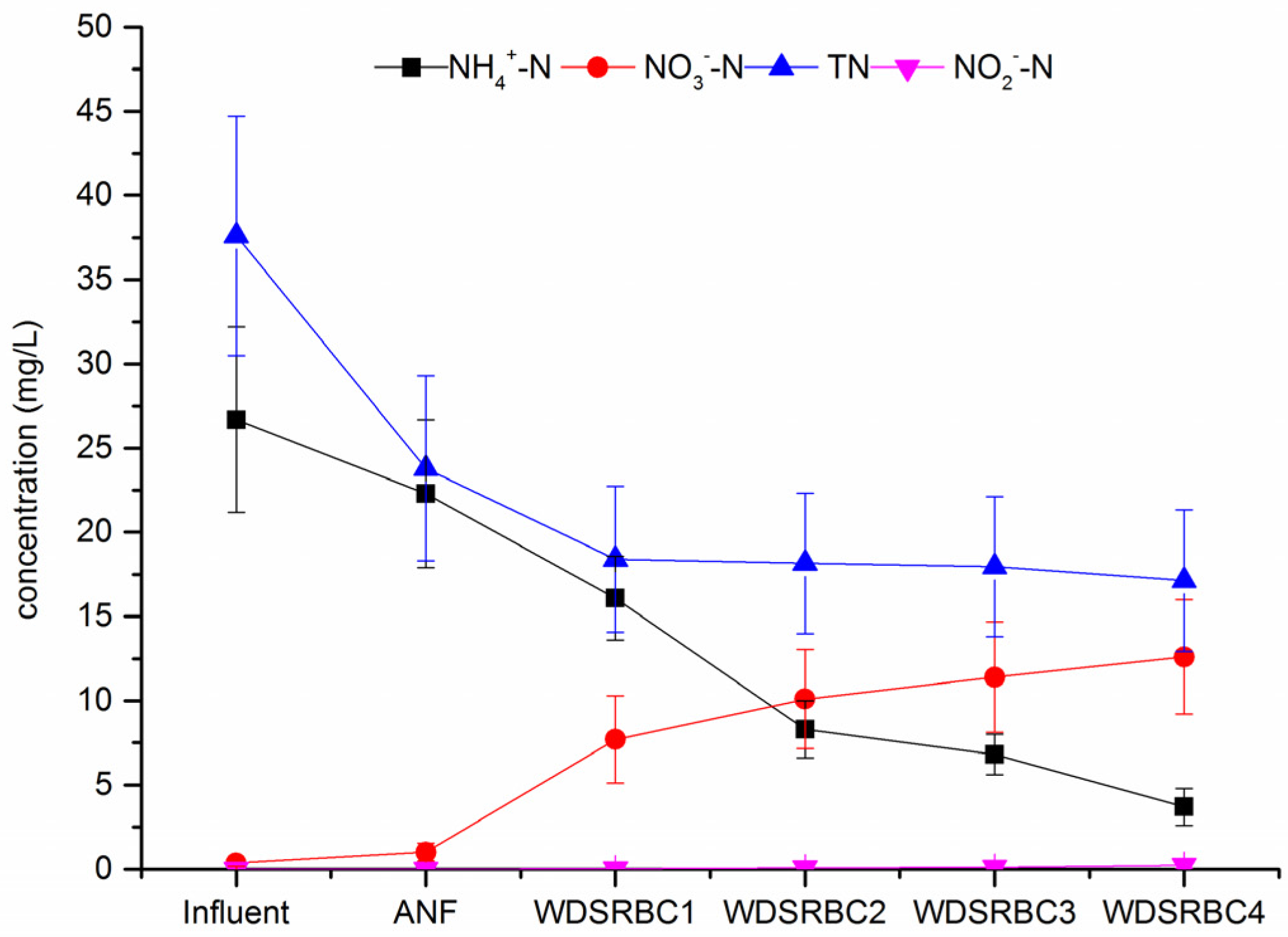
3.2. Performance of the ANF-WDSRBC System at Steady State
3.3. Diversity of Microbial Community in the ANF-WDSRBC System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Libralato, G.; Ghirardini, A.V.; Avezzù, F. To centralise or to decentralise: An overview of the most recent trends in wastewater treatment management. J. Environ. Manag. 2012, 94, 61–68. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Albarrán-Rivas, M.G.; Hernández-Mena, L.; León-Becerril, E. An assessment of an anaerobic filter packed with a low-cost material for treating domestic wastewater. Environ. Technol. 2013, 34, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Psychrophilic anaerobic membrane bioreactor treatment of domestic wastewater. Water Res. 2013, 47, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, W.; Lu, X. Identifying key parameters in a novel multistep bio-ecological wastewater treatment process for rural areas. Ecol. Eng. 2013, 61, 166–173. [Google Scholar] [CrossRef]
- Feng, H.; Hu, L.; Mahmood, Q.; Qiu, C.; Fang, C.; Shen, D. Anaerobic domestic wastewater treatment with bamboo carrier anaerobic baffled reactor. Int. Biodeterior. Biodegrad. 2008, 62, 232–238. [Google Scholar] [CrossRef]
- Gao, D.-W.; An, R.; Tao, Y.; Li, J.; Li, X.-X.; Ren, N.-Q. Simultaneous methane production and wastewater reuse by a membrane-based process: Evaluation with raw domestic wastewater. J. Hazard. Mater. 2011, 186, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.-W.; Tao, Y.; An, R. Digested sewage treatment using membrane-based process at different hydraulic retention times. Desalination 2012, 286, 187–192. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Fossing, H.; Hansen, J.W.; Manscher, O.H.; Murray, C.; Petersen, D.L. Nitrogen inputs from agriculture: Towards better assessments of eutrophication status in marine waters. Ambio 2014, 43, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, S.I.; Fawzy, M.E.; Elgendy, A.S. Potential of using biological aerated filter as a post treatment for municipal wastewater. Ecol. Eng. 2015, 84, 53–57. [Google Scholar] [CrossRef]
- Bastos, R.; Calijuri, M.; Bevilacqua, P.; Rios, E.; Dias, E.; Capelete, B.; Magalhães, T. Post-treatment of UASB reactor effluent in waste stabilization ponds and in horizontal flow constructed wetlands: A comparative study in pilot scale in southeast brazil. Water Sci. Technol. 2010, 61, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, M.A.; Alherrawy, A.Z.; Kamel, M.M.; Elgohary, F.A. Use of wetlands as post-treatment of anaerobically treated effluent. Desalination 2009, 245, 50–59. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Q.; Lu, X. Constructed wetland in a compact rural domestic wastewater treatment system for nutrient removal. Environ. Eng. Sci. 2012, 29, 751–757. [Google Scholar] [CrossRef]
- Hiras, D.N.; Manariotis, I.D.; Grigoropoulos, S.G. Organic and nitrogen removal in a two-stage rotating biological contactor treating municipal wastewater. Bioresour. Technol. 2004, 93, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Guo, F.; Ju, F.; Zhang, T. Shifts in the microbial community, nitrifiers and denitrifiers in the biofilm in a full-scale rotating biological contactor. Environ. Sci. Technol. 2014, 48, 8044–8052. [Google Scholar] [CrossRef] [PubMed]
- Courtens, E.; Boon, N.; De Clippeleir, H.; Berckmoes, K.; Mosquera, M.; Seuntjens, D.; Vlaeminck, S.E. Control of nitratation in an oxygen-limited autotrophic nitrification/denitrification rotating biological contactor through disc immersion level variation. Bioresour. Technol. 2014, 155, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Klapwijk, A.; Elgohary, F.A.; Lettinga, G. Potentials of using a rotating biological contactor (RBC) for post-treatment of anaerobically pre-treated domestic wastewater. Biochem. Eng. J. 2005, 25, 89–98. [Google Scholar] [CrossRef]
- Malachova, K.; Rybkova, Z.; Sezimova, H.; Cerven, J.; Novotny, C. Biodegradation and detoxification potential of rotating biological contactor (RBC) with irpex lacteus for remediation of dye-containing wastewater. Water Res. 2013, 47, 7143–7148. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.; Vergara, M.; Moreno, Y. Landfill leachate treatment using a rotating biological contactor and an upward-flow anaerobic sludge bed reactor. Waste Manag. 2007, 27, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Hassard, F.; Biddle, J.; Cartmell, E.; Jefferson, B.; Tyrrel, S.F.; Stephenson, T. Rotating biological contactors for wastewater treatment—A review. Process Saf. Environ. 2015, 94, 285–306. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Terzakis, S.; Chatzinotas, A.; Brix, H.; Kalogerakis, N.; Manios, T. Pilot-scale comparison of constructed wetlands operated under high hydraulic loading rates and attached biofilm reactors for domestic wastewater treatment. Sci. Total Environ. 2009, 407, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Burgara-Montero, O.; El-Baz, A.A.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Optimal design of a distributed treatment system for increasing dissolved oxygen in watersheds through self-rotating discs. Sustain. Chem. Eng. 2013, 1, 1267–1279. [Google Scholar] [CrossRef]
- EI Monayerie, D.S.; Atta, N.N.; EI Din, D.S.; Daif, S. Modeling of oxygen transfer in self-rotating biological contactors (SRBC). Proceeding of the Sixteenth International Water Technology Conference (16th IWTC), Istanbul, Turkey, 7–10 May 2012. [Google Scholar]
- Chen, Z.; Wen, Q.; Wang, J.; Li, F. Simultaneous removal of carbon and nitrogen from municipal-type synthetic wastewater using net-like rotating biological contactor (NRBC). Process Biochem. 2006, 41, 2468–2472. [Google Scholar] [CrossRef]
- Sayess, R.R.; Saikaly, P.E.; El-Fadel, M.; Li, D.; Semerjian, L. Reactor performance in terms of COD and nitrogen removal and bacterial community structure of a three-stage rotating bioelectrochemical contactor. Water Res. 2013, 47, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shao, M.; Ye, L. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.; Raskin, L. Diversity and dynamics of microbial communities in engineered environments and their implications for process stability. Curr. Opin. Biotech. 2003, 14, 270–276. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Chiou, R.J.; Ouyang, C.F. The effect of recycle ratio on nitrogen removal in the combined pre-denitrification/nitrification biofilter system. J. Chem. Technol. Biotechnol. 2001, 76, 559–564. [Google Scholar] [CrossRef]
- Barros, P.; Ruiz, I.; Soto, M. Performance of an anaerobic digester-constructed wetland system for a small community. Ecol. Eng. 2008, 33, 142–149. [Google Scholar] [CrossRef]
- Sumino, H.; Takahashi, M.; Yamaguchi, T.; Abe, K.; Araki, N.; Yamazaki, S.; Shimozaki, S.; Nagano, A.; Nishio, N. Feasibility study of a pilot-scale sewage treatment system combining an up-flow anaerobic sludge blanket (UASB) and an aerated fixed bed (AFB) reactor at ambient temperature. Bioresour. Technol. 2007, 98, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mittal, A.K. Characterization of biofilm of a rotating biological contactor treating synthetic wastewater. Water Sci. Technol. 2012, 66, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.C.; Khardenavis, A.A.; Kapley, A. Shifts in microbial community in response to dissolved oxygen levels in activated sludge. Bioresour. Technol. 2014, 165, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, S.K. Simultaneous carbon and nitrogen removal from high strength domestic wastewater in an aerobic RBC biofilm. Water Res. 2001, 35, 1714–1722. [Google Scholar] [CrossRef]
- Blackburne, R.; Yuan, Z.; Keller, J. Demonstration of nitrogen removal via nitrite in a sequencing batch reactor treating domestic wastewater. Water Res. 2008, 42, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, C.; Munz, G.; Petroni, G.; Lubello, C.; Mori, G.; Verni, F.; Vannini, C. Characterization and comparison of bacterial communities selected in conventional activated sludge and membrane bioreactor pilot plants: A focus on nitrospira and planctomycetes bacterial phyla. Curr. Microbiol. 2013, 67, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Pizzetti, I.; Gobet, A.; Fuchs, B.M.; Amann, R.; Fazi, S. Abundance and diversity of planctomycetes in a tyrrhenian coastal system of central italy. Aquat. Microb. Ecol. 2011, 65, 129–141. [Google Scholar] [CrossRef]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [PubMed]
- Wang, X.; Hu, M.; Xia, Y.; Wen, X.; Ding, K. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl. Environ. Microb. 2012, 78, 7042–7047. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Shao, M.; Zhang, T.; Tong, A.H.Y.; Lok, S. Analysis of the bacterial community in a laboratory-scale nitrification reactor and a wastewater treatment plant by 454-pyrosequencing. Water Res. 2011, 45, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.-N.; Park, S.-J.; Kim, S.-J.; Jeon, C.O.; Chae, J.-C.; Rhee, S.-K. Isolation, characterization, and abundance of filamentous members of caldilineae in activated sludge. J. Microbiol. 2010, 48, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Windey, K.; De Bo, I.; Verstraete, W. Oxygen-limited autotrophic nitrification–denitrification (OLAND) in a rotating biological contactor treating high-salinity wastewater. Water Res. 2005, 39, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Ducey, T.F.; Vanotti, M.B.; Shriner, A.D.; Szogi, A.A.; Ellison, A.Q. Characterization of a microbial community capable of nitrification at cold temperature. Bioresour. Technol. 2010, 101, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Li, A.; Gopalakrishnan, S.; Shin, P.K.S.; Wu, R.S.S.; Pointing, S.B.; Chiu, J.M.Y. Interactive effects of hypoxia and polybrominated diphenyl ethers (PBDES) on microbial community assembly in surface marine sediments. Mar. Pollut. Bull. 2014, 85, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y. Cultivation of uncultured Chloroflexi subphyla: Significance and ecophysiology of formary uncultured Chloroflexi ‘subphylum I’ with natural and biotechnological relevance. Microbes Environ. 2009, 24, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, X.; Wang, X.; Ngo, H.H.; Jin, X. A new step aeration approach towards the improvement of nitrogen removal in a full scale carrousel oxidation ditch. Bioresour. Technol. 2015, 198, 23–30. [Google Scholar] [CrossRef] [PubMed]

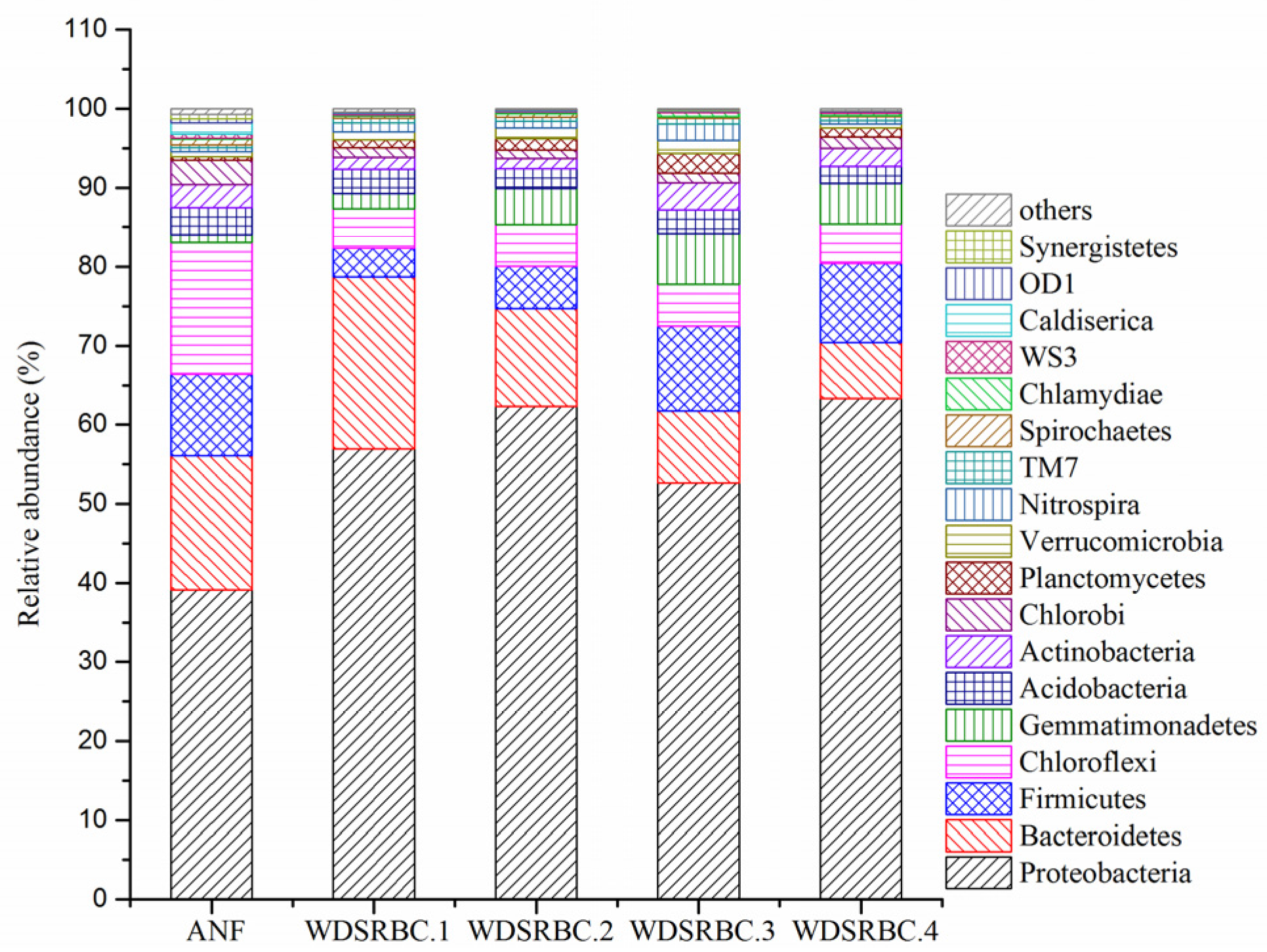
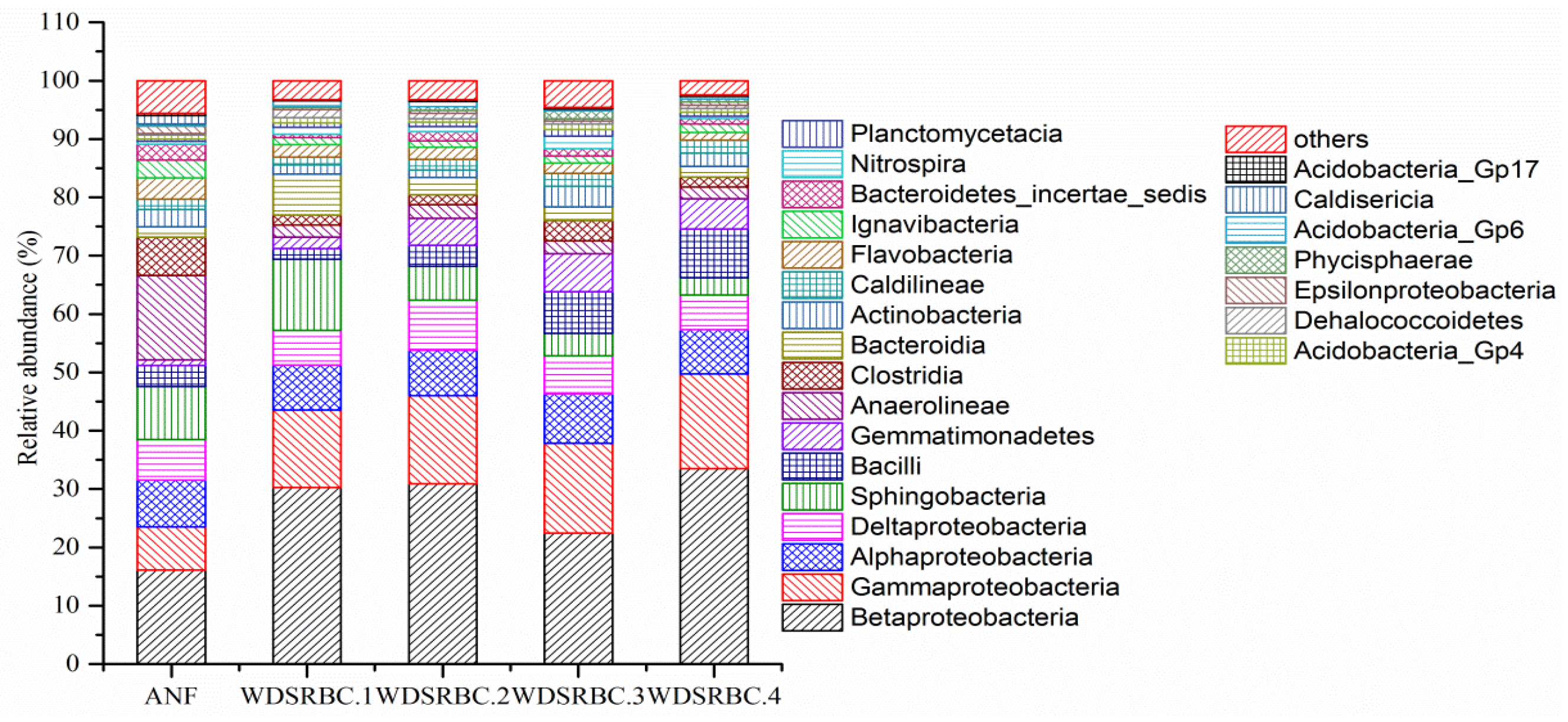
| pH | COD (mg/L) | NH4+–N (mg/L) | NO3−–N (mg/L) | NO2−–N (mg/L) | TN (mg/L) | DO (mg/L) |
|---|---|---|---|---|---|---|
| 7.1 ± 0.2 | 91.4 ± 21.0 | 26.7 ± 5.5 | 0.37 ± 0.2 | ND * | 37.6 ± 7.1 | 0.20 ± 0.03 |
| Unit (mg/L) | COD | NH4+–N | NO3−–N | NO2−–N | TN | DO |
|---|---|---|---|---|---|---|
| Influent | 91.4 ± 21.0 | 26.7 ± 5.5 | 0.37 ± 0.2 | ND # | 37.6 ± 7.1 | 0.20 ± 0.03 |
| ANF | 51.6 ± 10.4 | 22.3 ± 4.4 | 1.01 ± 0.5 | ND # | 23.8 ± 5.5 | 0.35 ± 0.02 |
| Four-stages WDSRBC | 35.0 ± 7.7 | 3.7 ± 1.1 | 12.6 ± 3.4 | 0.23 ± 0.18 | 17.1 ± 4.2 | 5.15 ± 0.05 |
| Re * (%) | 61.4 ± 4.3 | 86.1 ± 3.7 | - | - | 54.5 ± 3.9 | - |
| Post-Treatment | CODinf (mg/L) | Removal (%) | NH4+–Ninf (mg/L) | Removal (%) | TNinf (mg/L) | Removal (%) | Energy Consumption | Reference |
|---|---|---|---|---|---|---|---|---|
| AT-MBR | 186.8 ± 10.4 | 87 | 44.1 ± 4.8 | 91 | 58.8 | 58 | Higher | [7] |
| AFB | 103 ± 25 | 47.6 | - | - | 38 ± 6 | 21 | High | [31] |
| ABR-WDASB | 167 | 53.3 | 22.3 | 50 | 22.3 | 33 | Low | [4] |
| CW | 175 ± 89 | 72 | 27.9 ± 14.0 | 38 | 55 ± 9.5 | 32 | Lower | [30] |
| ANF-WDSRBC | 91.4 ± 21.0 | 61.4 ± 4.3 | 26.7 ± 5.5 | 86.1 ± 3.7 | 37.6 ± 7.1 | 54.5 ± 3.9 | Low | This study |
| Phylum | Class | Genus | ANF | WDSRBC1 | WDSRBC2 | WDSRBC3 | WDSRBC4 |
|---|---|---|---|---|---|---|---|
| Proteobacteria | Alphaproteobacteria | Nitrobacter | 0 | 0.05 | 0.10 | 0.03 | 0.03 |
| Betaproteobacteria | Nitrosomonas | 0.12 | 0.26 | 1.59 | 0.47 | 0.23 | |
| Nitrosospira | 1.17 | 2.59 | 3.25 | 3.95 | 4.01 | ||
| Thiobacillus | 2.01 | 3.70 | 4.12 | 3.78 | 2.96 | ||
| Thauera | 1.02 | 0.04 | 0.04 | 0.07 | 0.03 | ||
| Epsilonproteobacteria | Nitratifractor | 0.02 | 0 | 0 | 0 | 0 | |
| Gammaproteobacteria | Luteimonas | 0.03 | 0.14 | 0.06 | 0.08 | 0.03 | |
| Pseudomonas | 0.12 | 0.05 | 0.20 | 0.12 | 0.16 | ||
| Bacteroidetes | Flavobacteria | Flavobacterium | 0.25 | 0.18 | 0.30 | 0.58 | 0.32 |
| Sphingobacteria | Meniscus | 4.69 | 0.04 | 0.07 | 0.10 | 0.04 | |
| Firmicutes | Bacilli | Bacillus | 2.43 | 0.73 | 4.29 | 1.12 | 2.65 |
| Falsibacillus | 0.52 | 1.07 | 2.41 | 2.39 | 3.81 | ||
| Clostridia | Anaerovorax | 1.07 | 0.01 | 0 | 0 | 0.01 | |
| Chloroflexi | Anaerolineae | Bellilinea | 4.55 | 0.43 | 0.36 | 0.35 | 0.31 |
| Longilinea | 7.50 | 1.82 | 2.02 | 2.11 | 1.80 | ||
| Caldilineae | Caldilinea | 1.91 | 1.57 | 2.10 | 2.47 | 3.12 | |
| Dehalococcoidetes | Dehalogenimonas | 0.29 | 1.69 | 0.53 | 1.11 | 0.60 | |
| Nitrospira | Nitrospira | Nitrospira | 0.53 | 1.37 | 0.96 | 2.39 | 0.47 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, X. Performance and Microbial Diversity in a Low-Energy ANF-WDSRBC System for the Post-Treatment of Decentralized Domestic Wastewater. Water 2017, 9, 330. https://doi.org/10.3390/w9050330
Li J, Lu X. Performance and Microbial Diversity in a Low-Energy ANF-WDSRBC System for the Post-Treatment of Decentralized Domestic Wastewater. Water. 2017; 9(5):330. https://doi.org/10.3390/w9050330
Chicago/Turabian StyleLi, Juanhong, and Xiwu Lu. 2017. "Performance and Microbial Diversity in a Low-Energy ANF-WDSRBC System for the Post-Treatment of Decentralized Domestic Wastewater" Water 9, no. 5: 330. https://doi.org/10.3390/w9050330






