Effect of Organic Matter on Cr(VI) Removal from Groundwaters by Fe(II) Reductive Precipitation for Groundwater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examined Waters
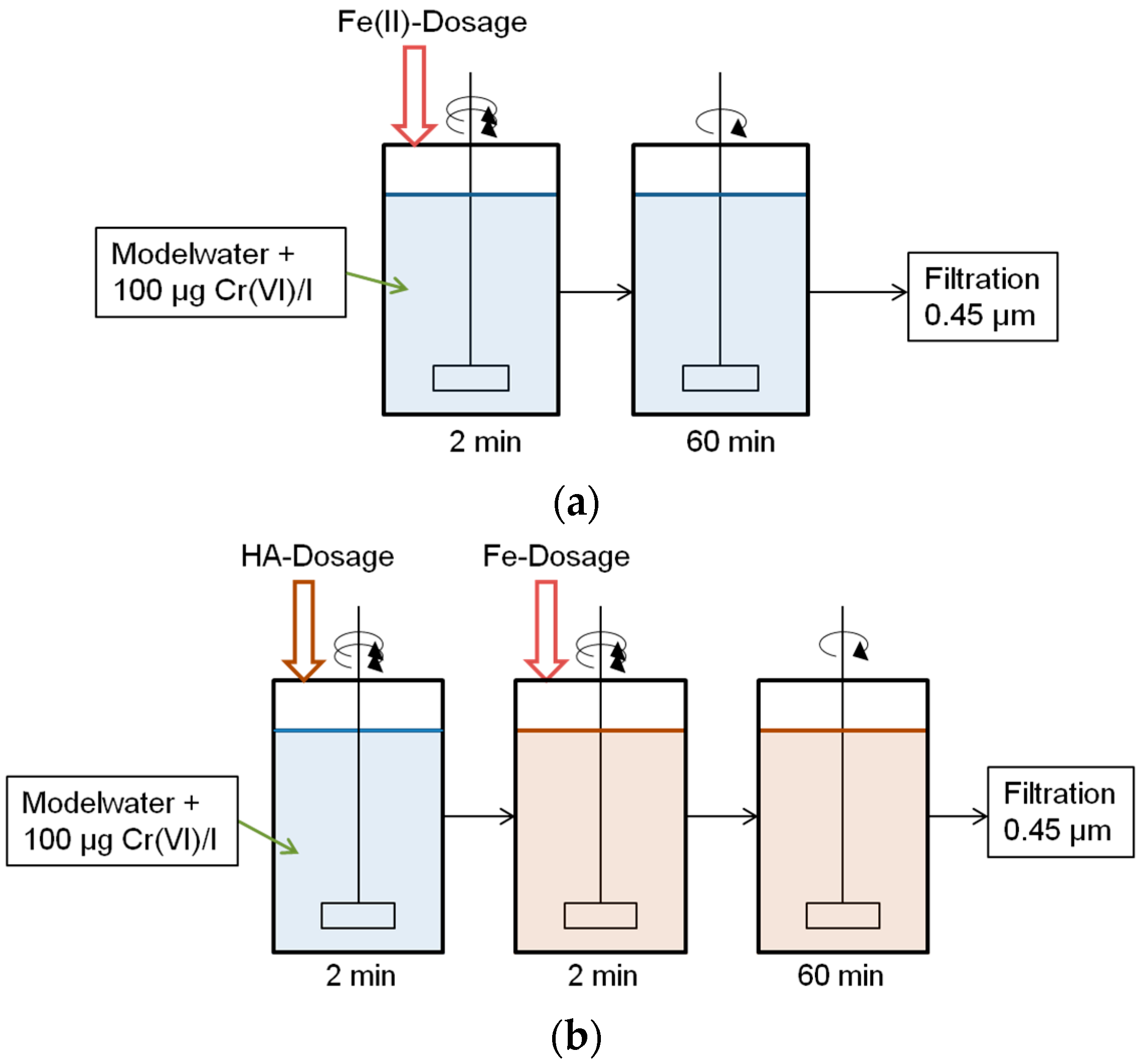
2.2. Set-Up and Procedure
2.3. Tested Reaction Conditions
2.4. Analytical Methods
3. Results
3.1. Removal of Cr(VI) in the Absence of HAs
3.1.1. Kinetics of Cr(VI) Removal
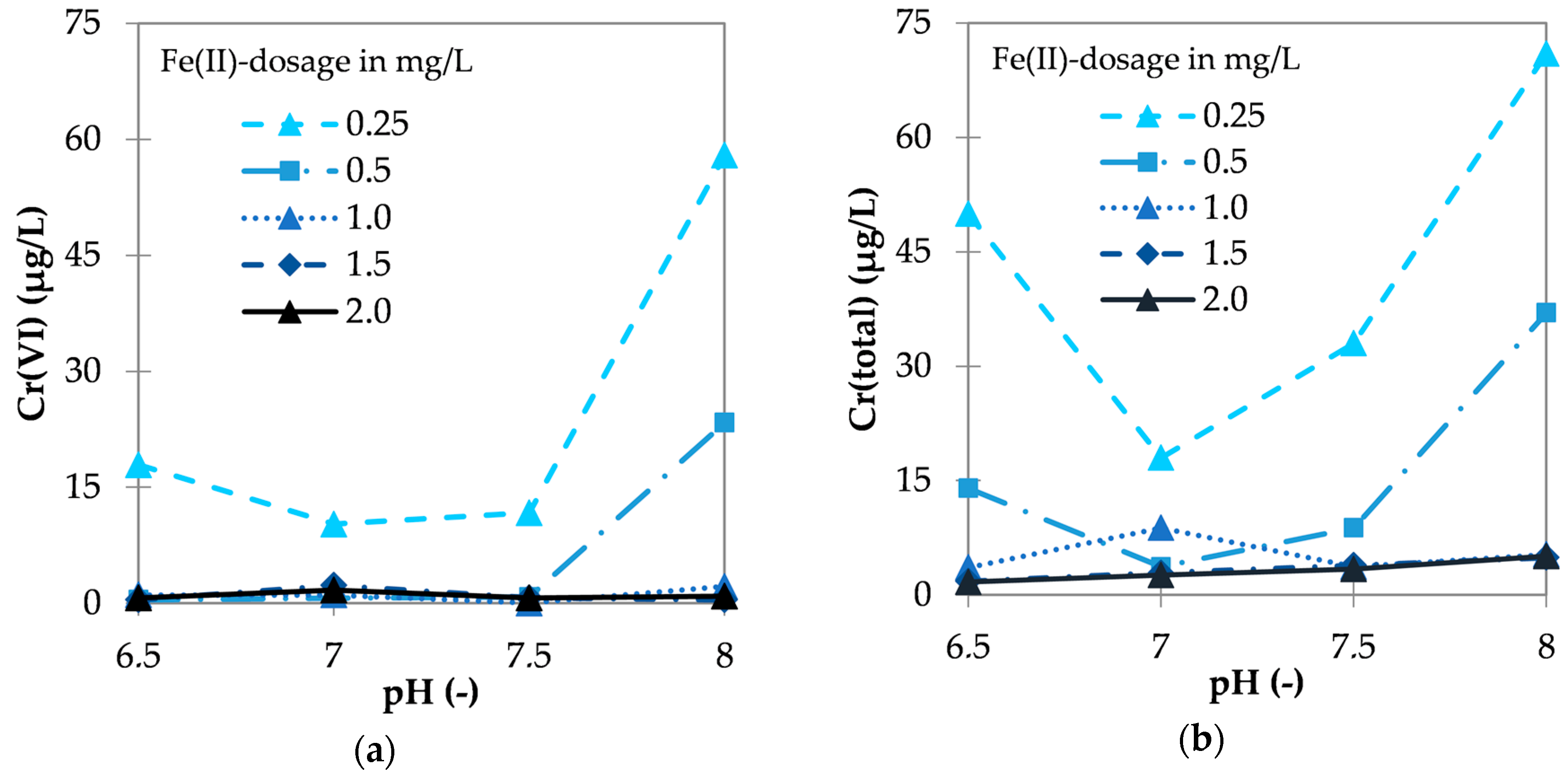
3.1.2. Influence of pH and Fe(II) Dose
3.2. Removal of Chromium in the Presence of HA
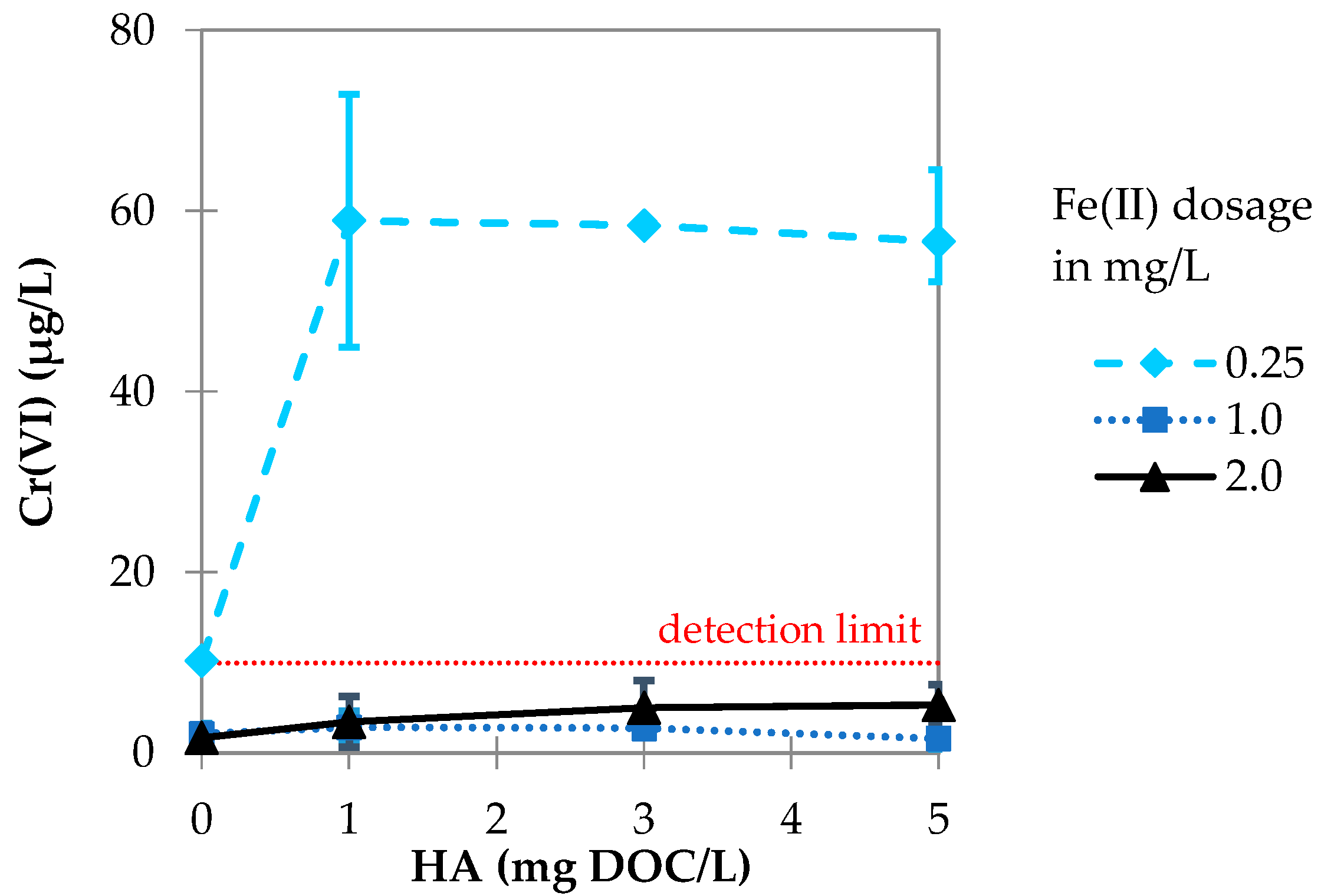
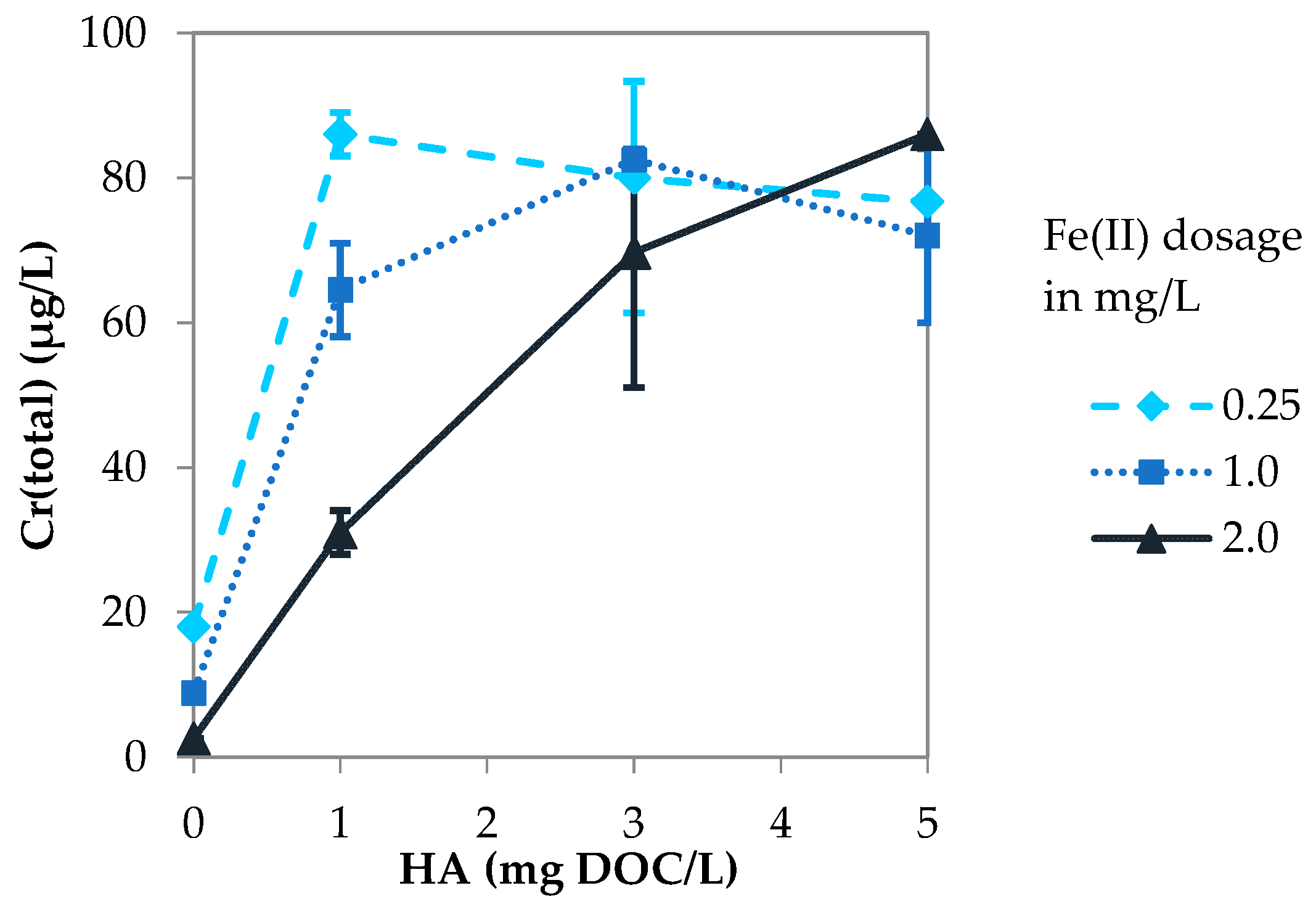
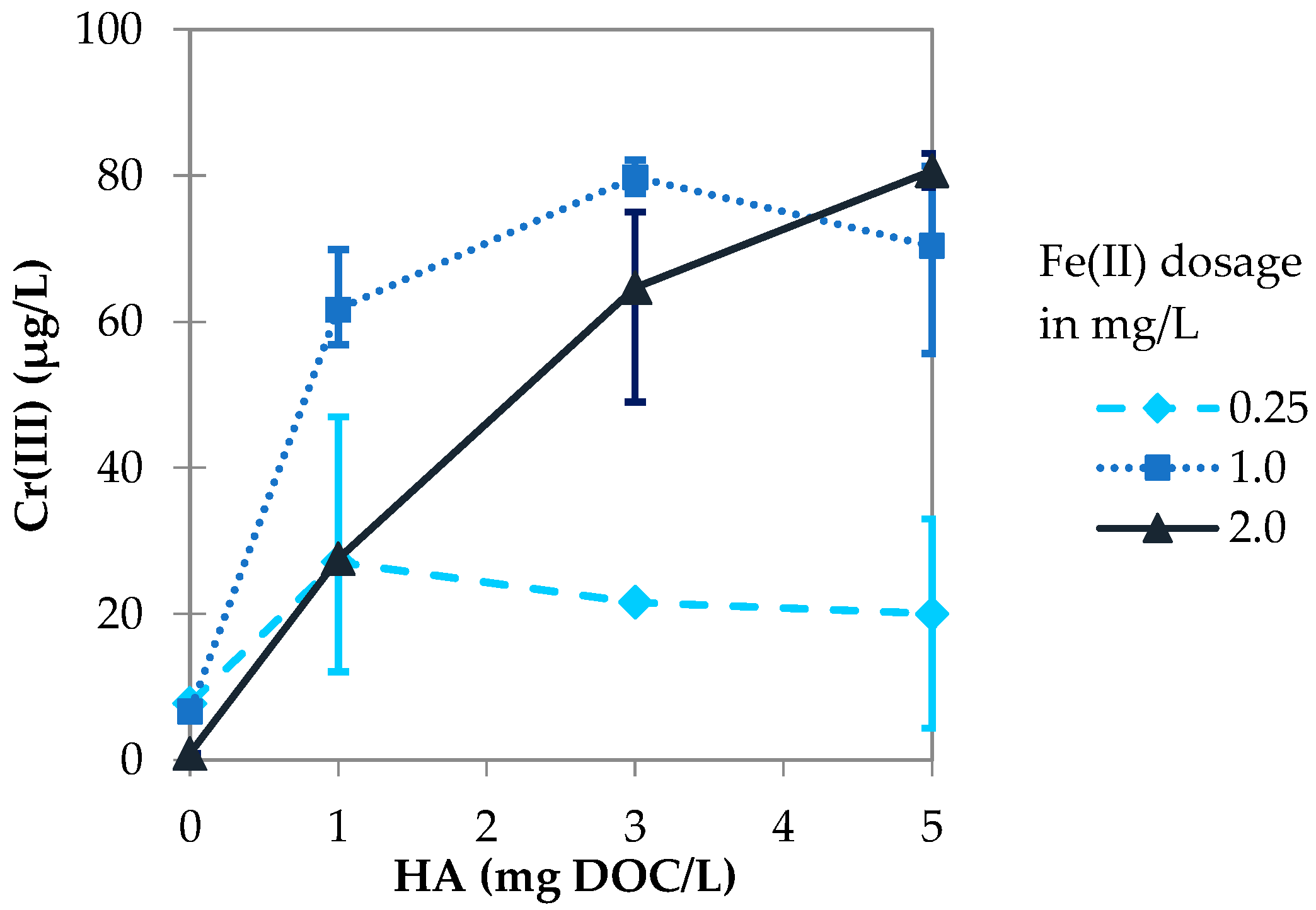
3.2.1. Influence of Fe(II) Dosage
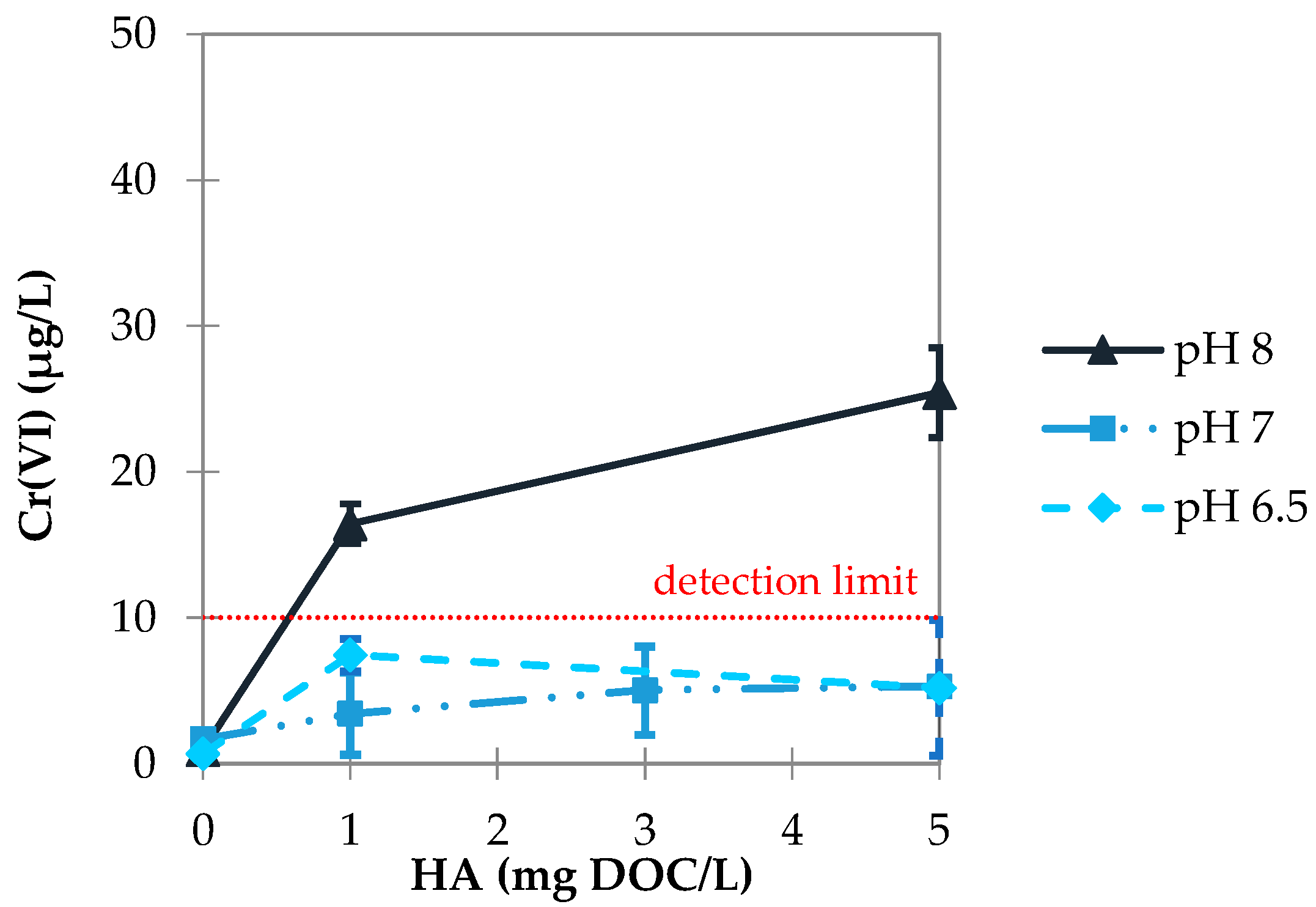
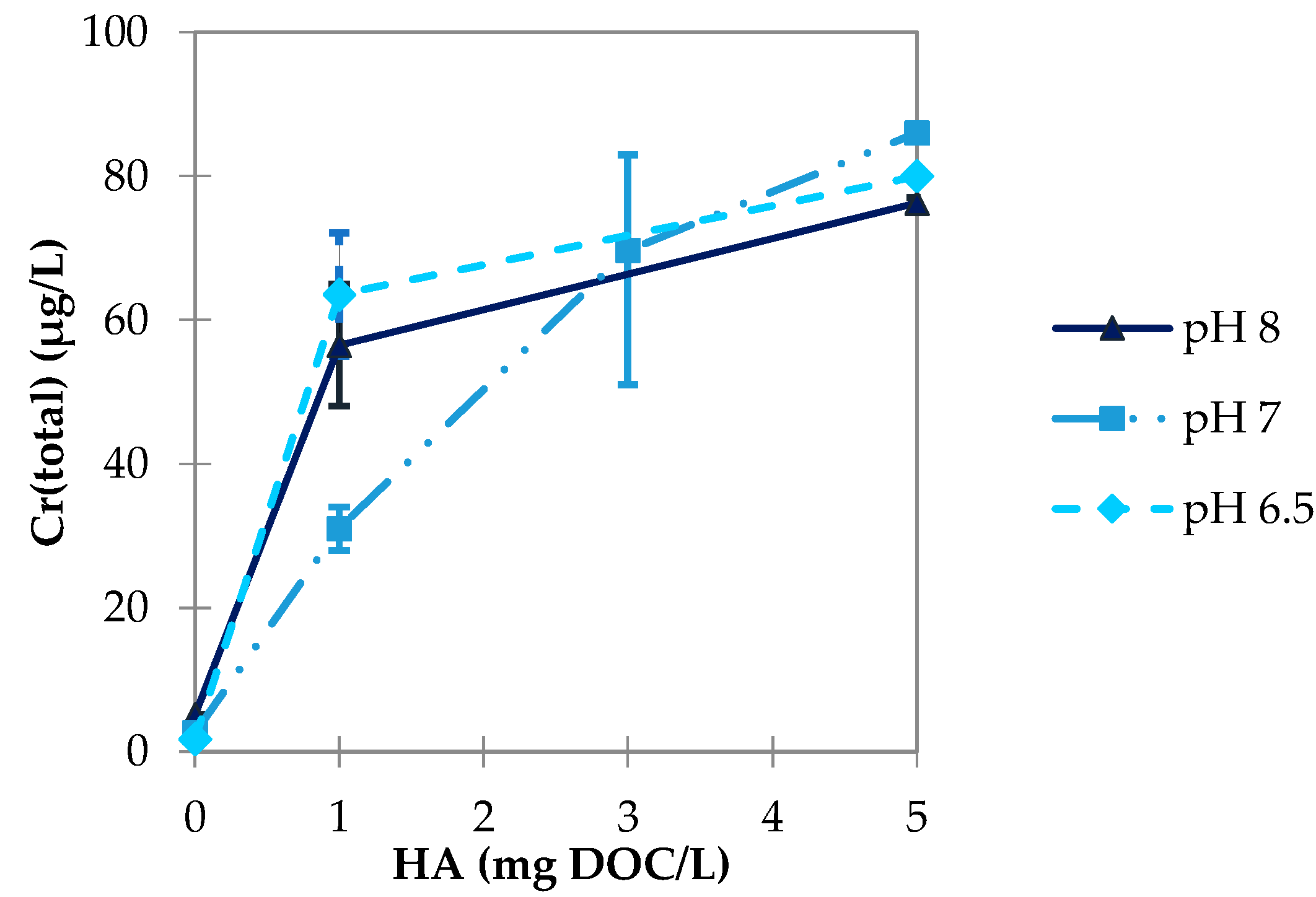
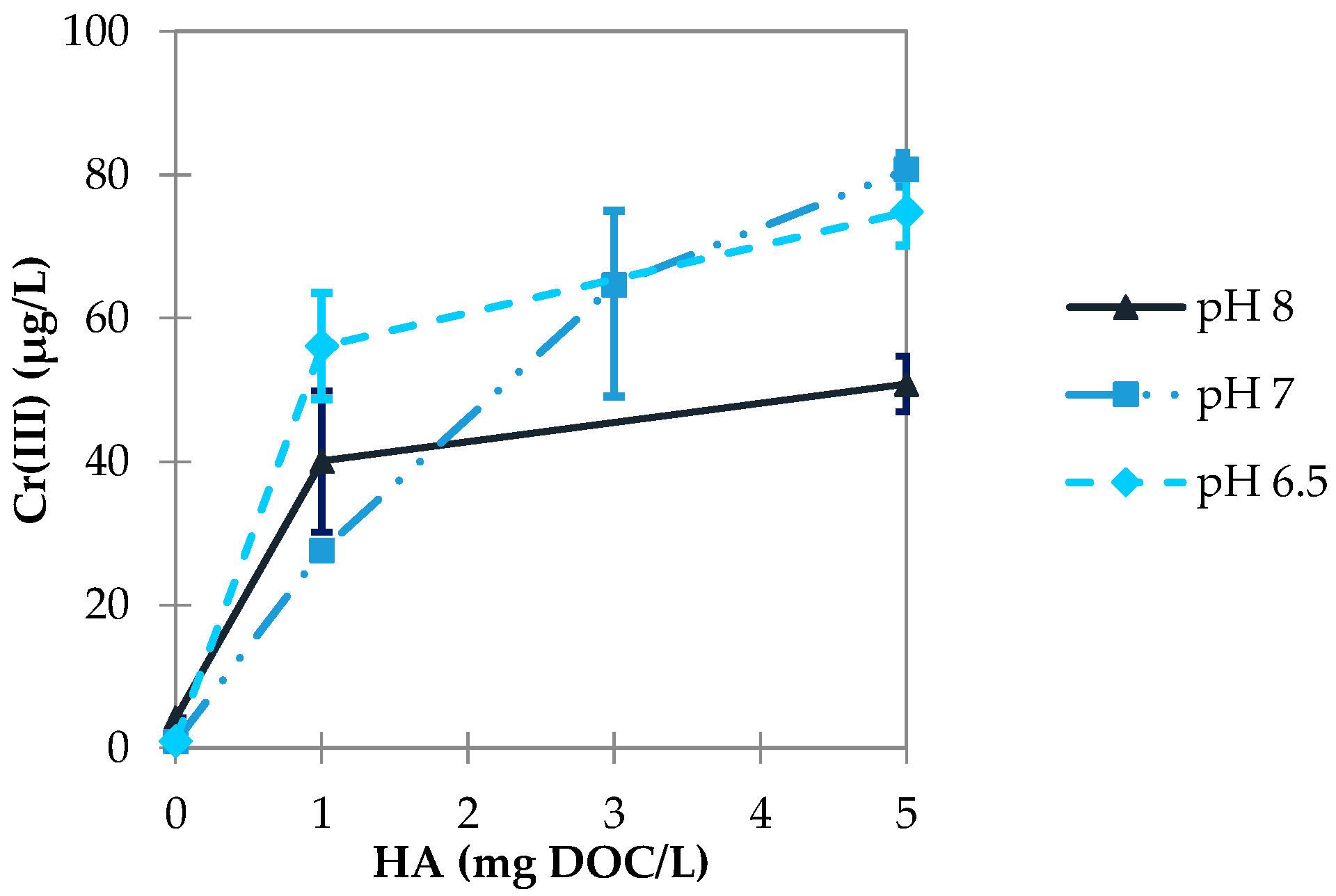
3.2.2. Influence of pH Value
3.3. Influence of HAs on the Removal of Cr(total)
3.3.1. Influence of Fe(II) Dosage
3.3.2. Influence of pH Value
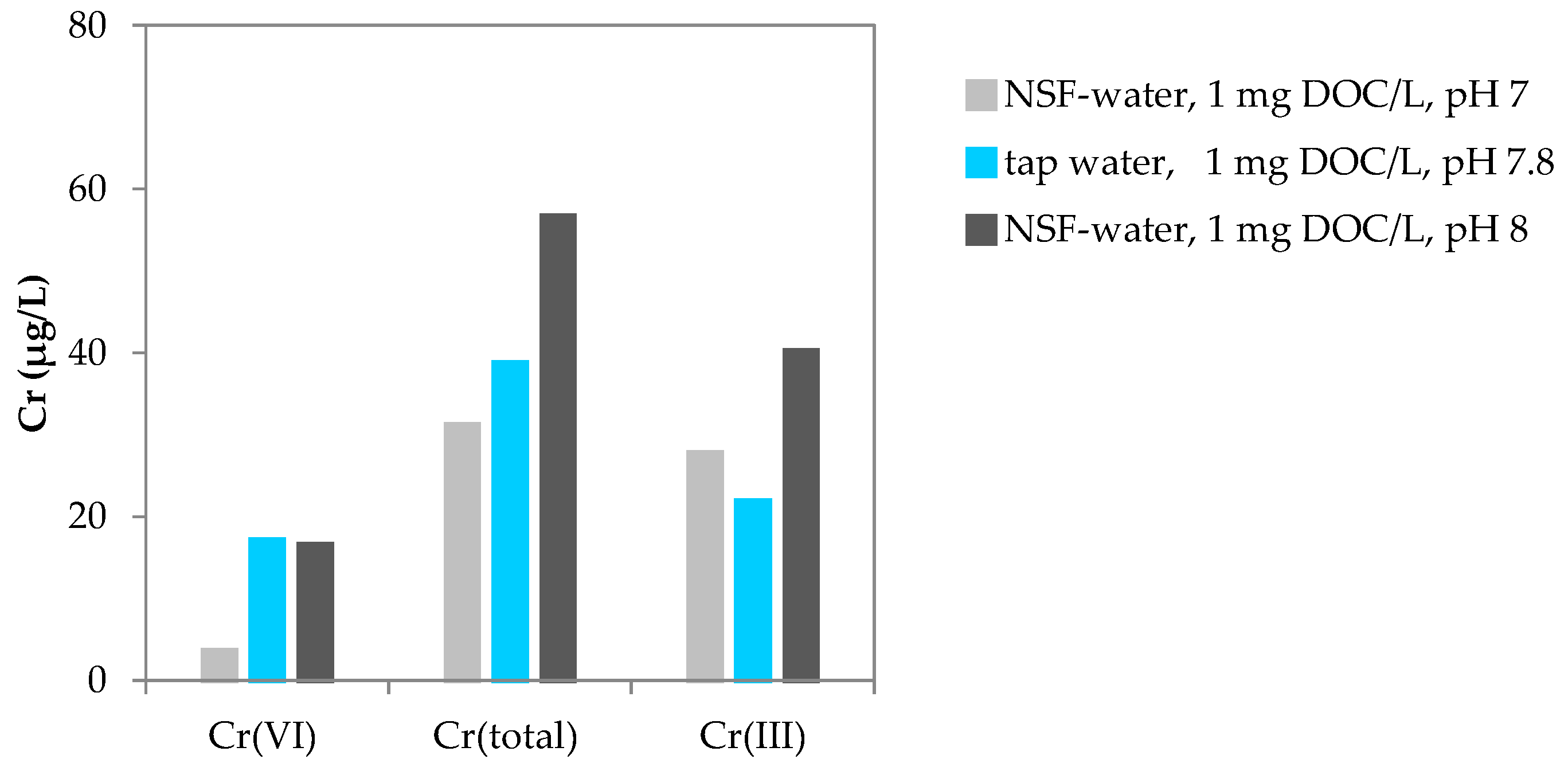
3.4. Cr(VI) Removal in Tap Water Containing NOM, Spiked with Cr(VI)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, J.G.; Dixon, J.B. Oxidation and fate of chromium in soils. Soil Sci. Plant Nutr. 2002, 48, 483–490. [Google Scholar] [CrossRef]
- Ludwig, A. Chrom(III) und Chrom(VI) in Einer mit Gerbereischlamm Belasteten Ackerfläche bei Weinheim. Master’s Thesis, Universität Heidelberg, Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Kaprara, E.; Kazakis, N.; Simeonidis, K.; Coles, S.; Zouboulis, A.I.; Samaras, P.; Mitrakas, M. Occurrence of Cr(VI) in drinking water of Greece and relation to the geological background. J. Hazard. Mater. 2015, 281, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Kazakis, N.; Kantiranis, N.; Voudouris, K.S.; Mitrakas, M.; Kaprara, E.; Pavlou, A. Geogenic Cr oxidation on the surface of mafic minerals and the hydrogeological conditions influencing hexavalent chromium concentrations in groundwater. Sci. Total Environ. 2015, 514, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- McLean, J.E.; McNeill, L.S.; Edwards, M.; Parks, J.L. Hexavalent chromium review: Part 1, Health effects, regulations, and analysis. J. Am. Water Works Assoc. 2012, 104, E348–E357. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Stumm, W. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996; ISBN 0471511846. [Google Scholar]
- McNeill, L.S.; McLean, J.E.; Parks, J.L.; Edwards, M. Hexavalent chromium review: Part 2: Chemistry, occurrence, and treatment. J. Am. Water Works Assoc. 2012, 104, E395–E405. [Google Scholar] [CrossRef]
- Palmer, C.D.; Puls, R. EPA Ground Water Issue: Natural Attenuation of Hexavalent Chromium in Groundwater and Soils; Technology Innovation Office, Office of Solid Waste and Emergency Response, US EPA: Washington, DC, USA, 1994.
- Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption. 3 November 1998. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:01998L0083-20151027 (accessed on 5 January 2017).
- California Environmental Protection Agency. Chromium-6 Drinking Water MCL. 2016. Available online: http://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/Chromium6.shtml (accessed on 5 January 2017).
- Mitrakas, M.G.; Pantazatou, A.S.; Tzimou-Tsitouridou, R.; Sikalidis, C.A. Influence of pH and temperature on Cr(VI) removal from a natural water using Fe(II): A pilot and full scale case study. Desalination Water Treat. 2011, 33, 77–85. [Google Scholar] [CrossRef]
- Buerge, I.J.; Hug, S.J. Kinetics and pH dependence of chromium(VI) reduction by iron(II). Environ. Sci. Technol. 1997, 31, 1426–1432. [Google Scholar] [CrossRef]
- Pettine, M.; D’Ottone, L.; Campanella, L.; Millero, F.J.; Passino, R. The reduction of chromium(VI) by iron(II) in aqueous solutions. Geochim. Cosmochim. Acta 1998, 62, 1509–1519. [Google Scholar] [CrossRef]
- Fendorf, S.E.; Li, G. Kinetics of Chromate Reduction by Ferrous Iron. Environ. Sci. Technol. 1996, 30, 1614–1617. [Google Scholar] [CrossRef]
- Schlautman, M.A.; Han, I. Effects of pH and dissolved oxygen on the reduction of hexavalent chromium by dissolved ferrous iron in poorly buffered aqueous systems. Water Res. 2001, 35, 1534–1546. [Google Scholar] [CrossRef]
- El-Shoubary, Y.; Speizer, N.; Seth, S.; Savoia, H. A pilot plant to treat chromium-contaminated groundwater. Environ. Prog. 1998, 17, 209–213. [Google Scholar] [CrossRef]
- Hering, J.G.; Lee, G. Removal of chromium(VI) from drinking water by redox-assisted coagulation with iron(II). J. Water Supply Res. Technol.-Aqua 2003, 52, 319–332. [Google Scholar]
- Buerge, I.J.; Hug, S.J. Influence of organic ligands on chromium(VI) reduction by iron(II). Environ. Sci. Technol. 1998, 32, 2092–2099. [Google Scholar] [CrossRef]
- Hori, M.; Shozugawa, K.; Matsuo, M. Reduction process of Cr(VI) by Fe(II) and humic acid analyzed using high time resolution XAFS analysis. J. Hazard. Mater. 2015, 285, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.G.; Fimmen, R.L.; Chin, Y.-P. Reduction of Cr(VI) to Cr(III) by Fe(II) in the presence of fulvic acids and in lacustrine pore water. Chem. Geol. 2009, 262, 328–335. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) Adsorption and Reduction by Humic Acid Coated on Magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, P.R.; Palmer, C.D. Effect of temperature, ionic strength, background electrolytes, and Fe(III) on the reduction of hexavalent chromium by soil humic substances. Environ. Sci. Technol. 1996, 30, 2470–2477. [Google Scholar] [CrossRef]
- Amy, G.; Chen, H.; Drizo, A.; von Gunten, U.; Brandhuber, P.; Hund, R.; Chowdhury, Z.; Kommineni, S.; Sinha, S.; Jekel, M.; et al. Adsorbent Treatment Technologies for Arsenic Removal; Awwa Research Foundation, American Water Works Association: Denver, CO, USA, 2005; ISBN 1-58321-399-6. [Google Scholar]
- DVGW. Arbeitsblatt W 218 Flockung in der Wasseraufbereitung-Flockungstestverfahren: DVGW W 218; DVGW Deutscher Verein des Gas- und Wasserfaches e.V.: Bonn, Germany, 1998; ISSN 0176-3504. [Google Scholar]
- Rüdel, H.; Kösters, J.; Schörmann, J. Bestimmung von Elementgehalten in Umweltproben Durch ICP-MS. 2011. Available online: http://www.ime.fraunhofer.de/content/dam/ime/de/documents/AE/UPB_SOP_ICP-MS_de.pdf (accessed on 5 January 2017).
- EN 1233:1996: Water Quality—Determination of Chromium—Atomic Absorption Spectrometric Methods; European Committee for Standardization: Brussels, Belgium, 1996.
- Eaton, A.D.; American Public Health Association; American Water Works Association; Water Pollution Control Federation (Eds.) Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, WA, USA, 2005; ISBN 0875530478. [Google Scholar]
- Sedlak, D.L.; Chan, P.G. Reduction of hexavalent chromium by ferrous iron. Geochim. Cosmochim. Acta 1997, 61, 2185–2192. [Google Scholar] [CrossRef]
- Mertineit, S.; Raue, B.; Thoma, A.; Sacher, F. Studie zur Belastung von Trinkwasser in Deutschland mit Chromat; DVGW Deutscher Verein des Gas- und Wasserfaches e.V.: Bonn, Germany, 2013. [Google Scholar]
- Lindsay, D.R.; Farley, K.J.; Carbonaro, R.F. Oxidation of Cr(III) to Cr(VI) during chlorination of drinking water. J. Environ. Monit. 2012, 14, 1789–1797. [Google Scholar] [CrossRef] [PubMed]

| Substance | NSF-Water | Tap Water |
|---|---|---|
| Na+ (mg/L) | 88 | 26 |
| Mg2+ (mg/L) | 12.5 | 4 |
| Ca2+ (mg/L) | 40 | 67 |
| HCO3− (mg/L) | 183 | - |
| SO42− (mg/L) | 50 | 15 |
| Cl− (mg/L) | 71 | 35 |
| NO3− (mg/L) | 2 | <0.4 |
| F− (mg/L) | 1 | 0.15 |
| SiO2 (mg/L) | 20 | Not specified |
| SO42− (mg/L) | - | 15 |
| Carbonate alkalinity (mmol/L) | Not specified | 1.7 |
| Parameter | Set-Up A | Set-Up B |
|---|---|---|
| Stirring device | Hei-Torque Precision 100 | Aqualytic |
| Volume | 1.8 L | 1.0 L |
| Rapid mixing | 2 min at 250 rpm (500 s−1) | 2 min at 150 rpm (115 s−1) |
| Slow stirring | 60 min at 50 rpm (initially), stirring speed was adjusted keeping G-value at 50 s−1 during extensive sampling | 60 min at 30 rpm (10 s−1) |
| HA (mg DOC/L) | 0.25 mg Fe(II)/L | 1 mg Fe(II)/L | 2 mg Fe(II)/L |
|---|---|---|---|
| 0 | A | A | A |
| 1 | A *, B | A * | A, B |
| 3 | B | A * | A, B* |
| 5 | A, B * | A * | A, B |
| HA (mg DOC/L) | pH 6.5 | pH 7 | pH 8 |
|---|---|---|---|
| 0 | A | A | A |
| 1 | B * | A, B | A, B * |
| 3 | - | A, B | - |
| 5 | B * | A, B | B * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gröhlich, A.; Langer, M.; Mitrakas, M.; Zouboulis, A.; Katsoyiannis, I.; Ernst, M. Effect of Organic Matter on Cr(VI) Removal from Groundwaters by Fe(II) Reductive Precipitation for Groundwater Treatment. Water 2017, 9, 389. https://doi.org/10.3390/w9060389
Gröhlich A, Langer M, Mitrakas M, Zouboulis A, Katsoyiannis I, Ernst M. Effect of Organic Matter on Cr(VI) Removal from Groundwaters by Fe(II) Reductive Precipitation for Groundwater Treatment. Water. 2017; 9(6):389. https://doi.org/10.3390/w9060389
Chicago/Turabian StyleGröhlich, Anna, Margarethe Langer, Manassis Mitrakas, Anastasios Zouboulis, Ioannis Katsoyiannis, and Mathias Ernst. 2017. "Effect of Organic Matter on Cr(VI) Removal from Groundwaters by Fe(II) Reductive Precipitation for Groundwater Treatment" Water 9, no. 6: 389. https://doi.org/10.3390/w9060389









