Characteristics and Biodegradability of Wastewater Organic Matter in Municipal Wastewater Treatment Plants Collecting Domestic Wastewater and Industrial Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
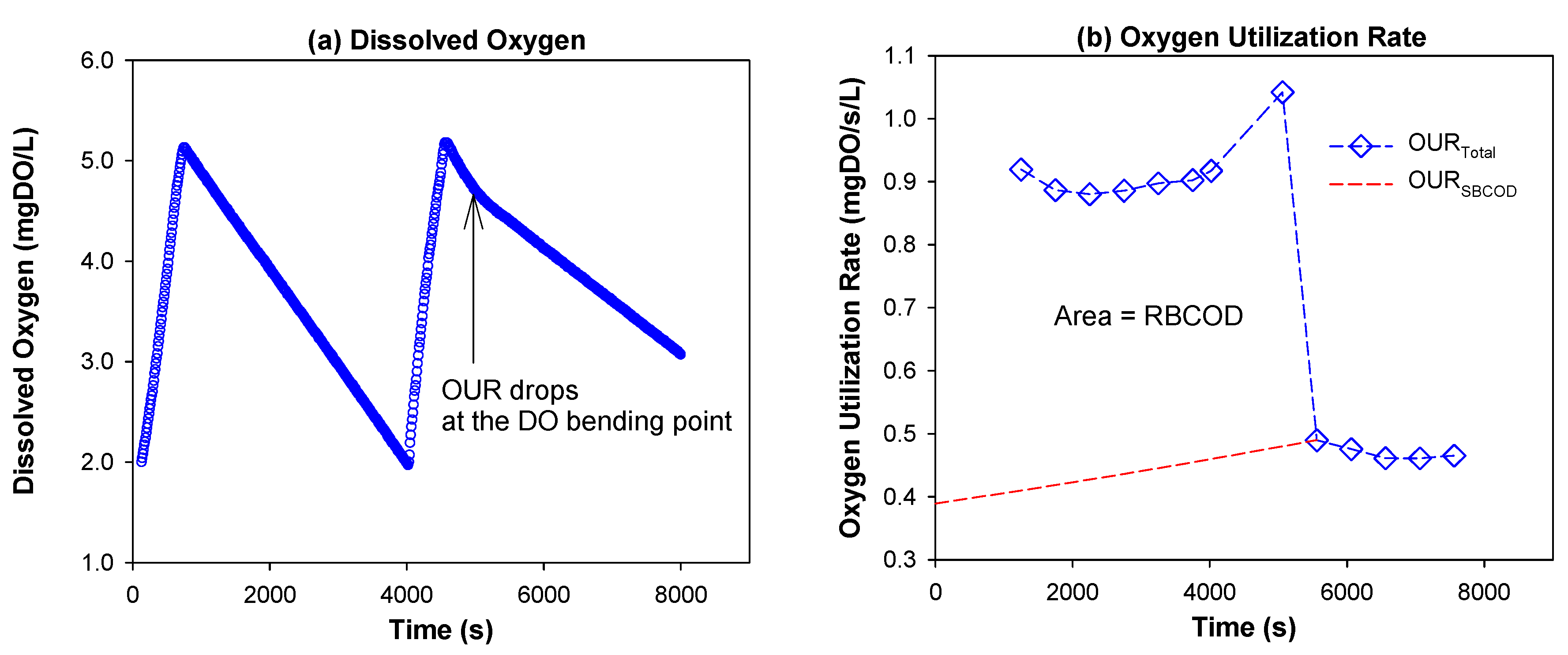
2.2. Experimental Methods
3. Results and Discussion
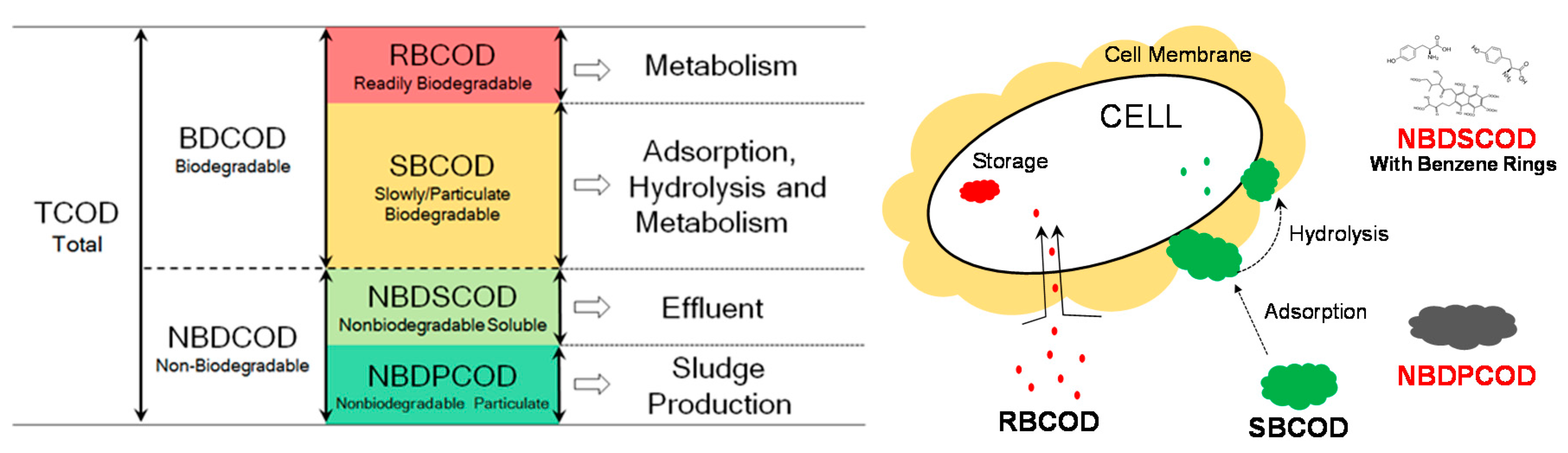
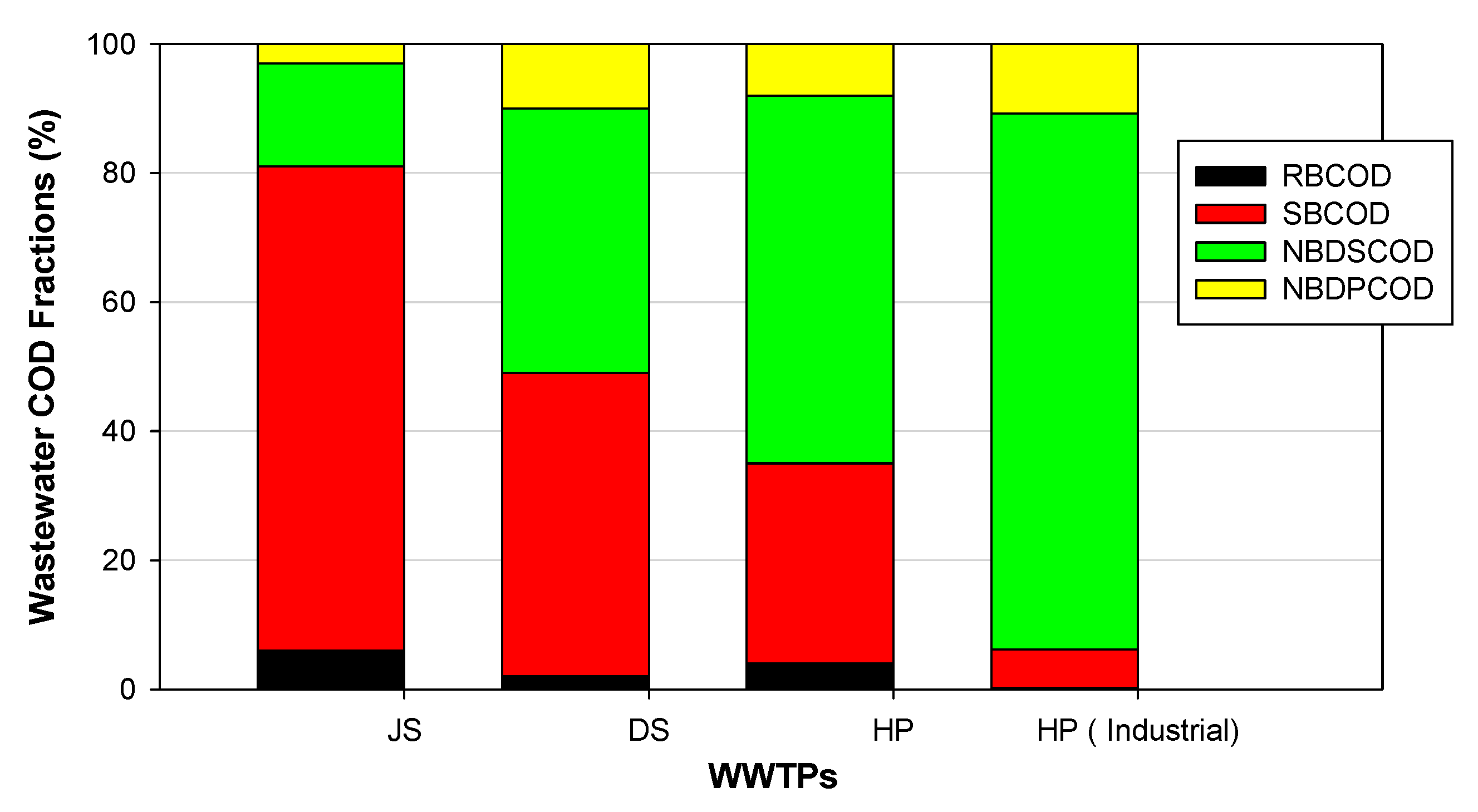
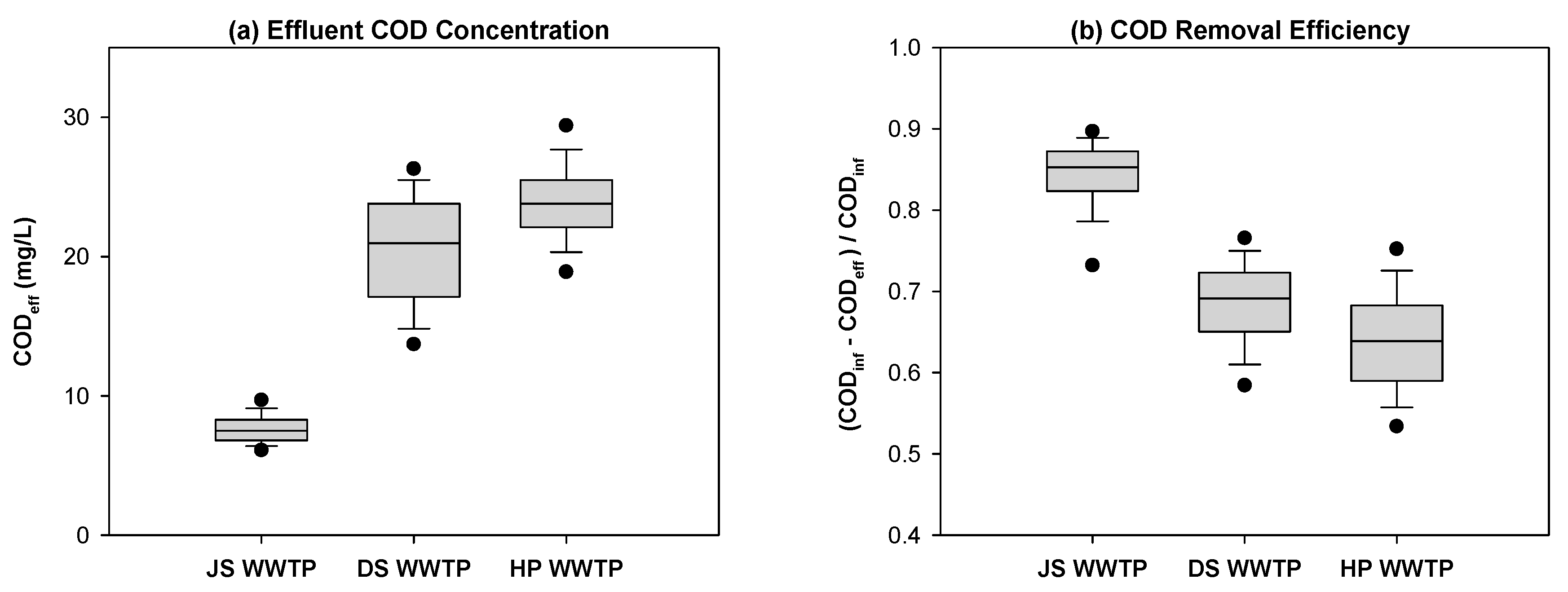
3.1. COD Fractionation
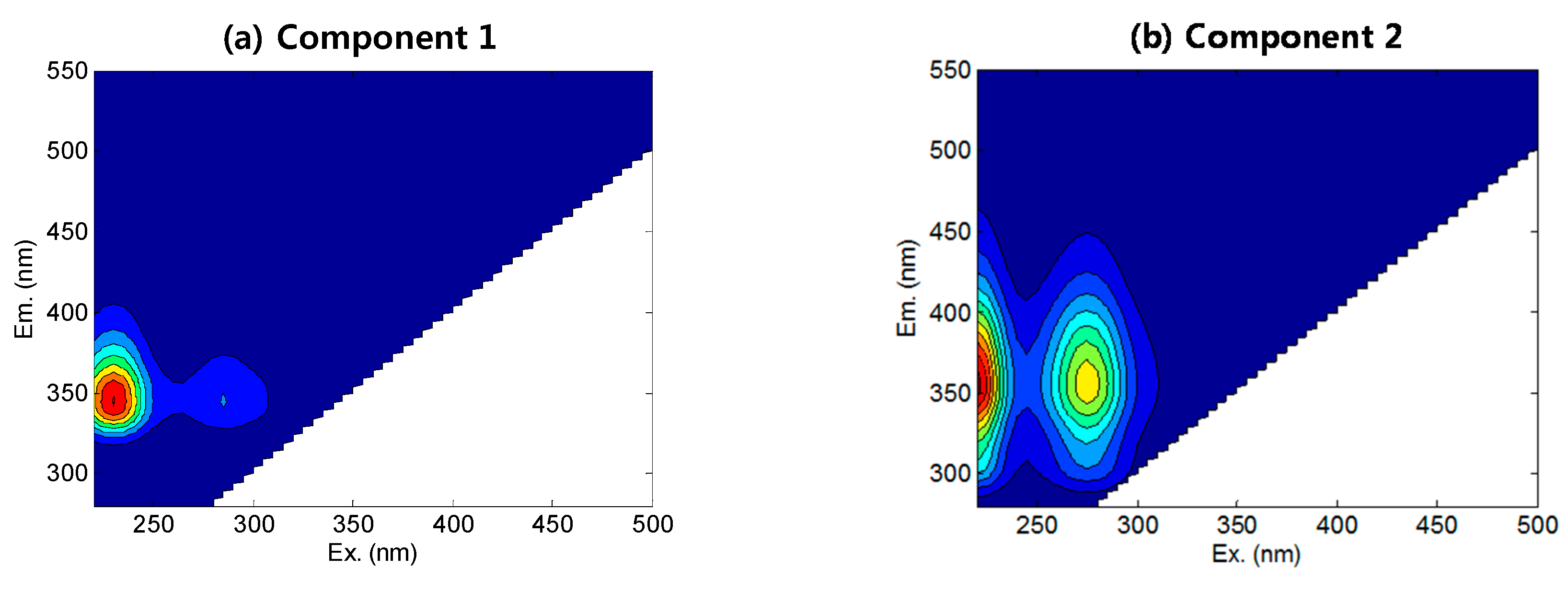
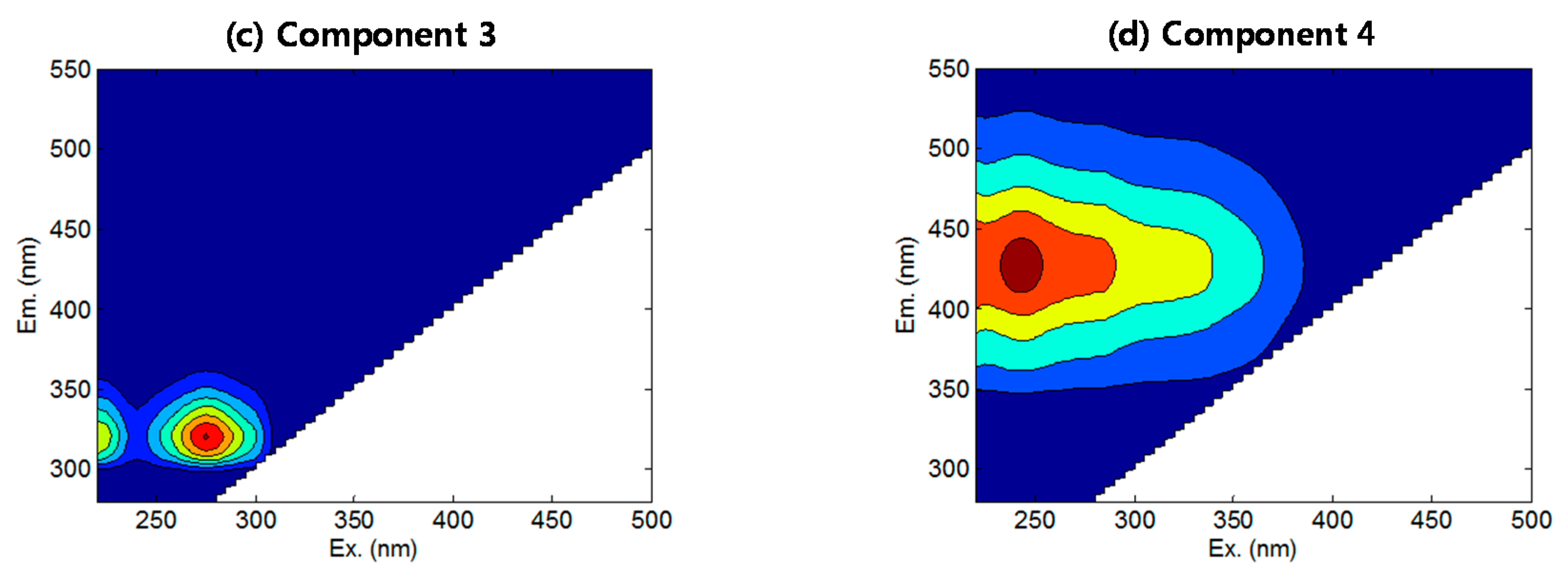
3.2. FEEM-PARAFAC
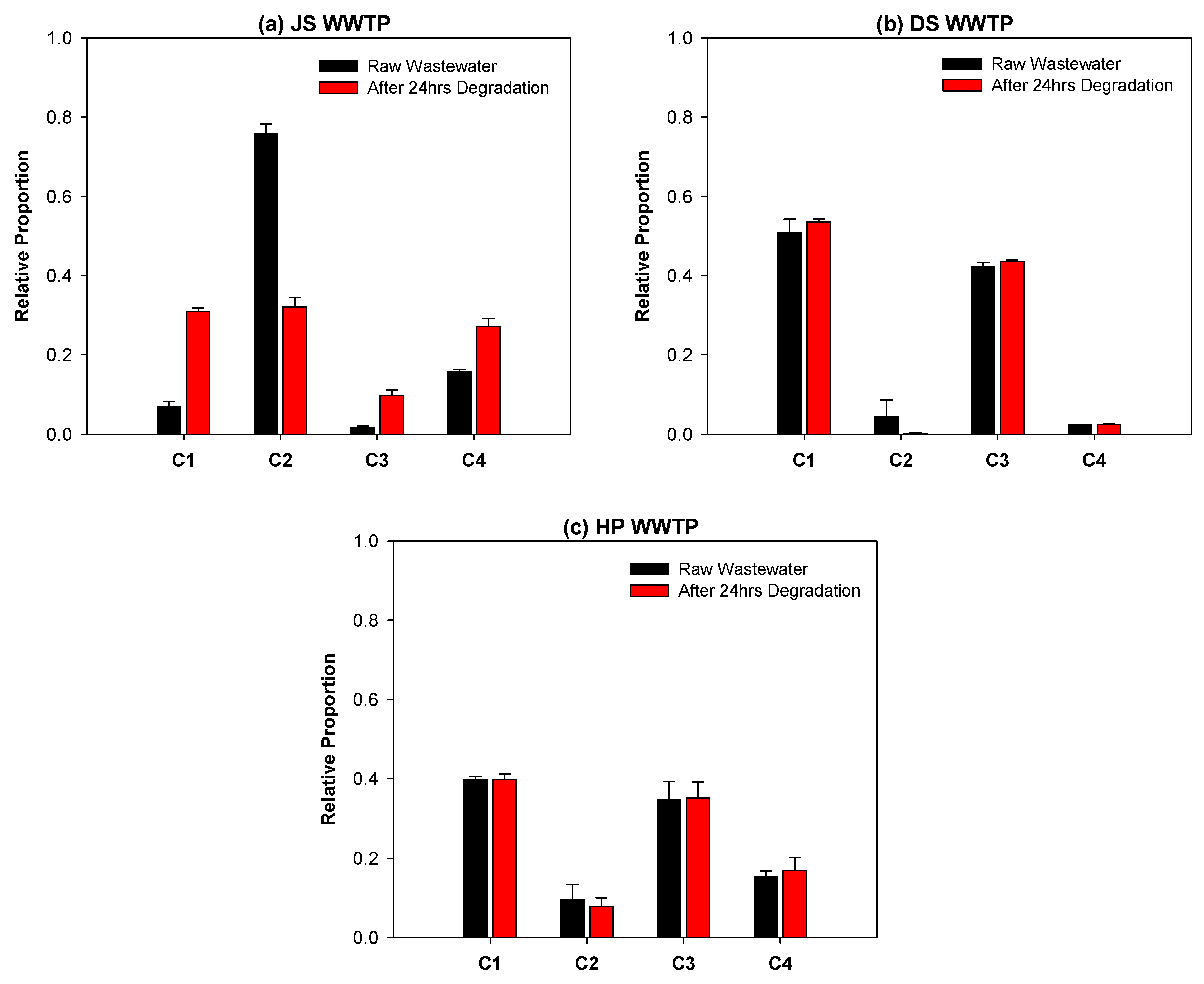
3.3. Fate of Wastewater Organic Matter in a Biological WWTP
4. Conclusions
- (1)
- The COD fractionation tests could quantify an integrated index of the non-biodegradable soluble organic matter (i.e., NBDSCOD), which is abundant in industrial discharges.
- (2)
- FEEM-PARAFAC revealed that domestic wastewater contained biodegradable tryptophan-like components, whereas the industrial discharge contained larger amounts of the non-biodegradable protein-, tyrosine-, and fulvic-like components.
- (3)
- NBDSCOD contained in industrial discharge cannot be treated effectively in a conventional biological treatment process, and hence, escaped treatment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, S.J.; Park, S.M.; Kwon, O.S.; Park, S.J.; Yeom, I.T. Improvement on Sewerage Effluent Standard of Public Sewerage Treatment Plants. J. Korean Soc. Water Environ. 2013, 29, 276–287. (In Korean) [Google Scholar]
- NIER. Study on Appropriate Treatment & Management of the Public Sewage Treatment Works Entering the Industrial Wastewater; Ministry of Environment: Seoul, Korea, 2011. (In Korean)
- NARS. Improvement Plans for the Current Industrial Effluent Guidlines; National Assembly Research Service: Seoul, Korea, 2015. (In Korean)
- Carmen, Z.; Daniela, S. Textile organic dyes—Characteristics, polluting effects and separation/elimination procedures from industrial effluents—A critical overview. In Organic Pollutants Ten Years after the Stockholm Convention—Environmental and Analytical Update; InTech Euro: Rijeka, Croatia, 2012; pp. 55–85. [Google Scholar]
- Hubbe, M.A.; Metts, J.R.; Hermosilla, D.; Blanco, A.; Yerushalmi, L.; Haghighat, F.; Lindholm-Lehto, P.; Khodaparast, Z.; Kamali, M.; Elliott, A. Pulp and paper effluent. Bioresouces 2016, 11, 1–139. [Google Scholar]
- Wentzel, M.C.; Mbewe, A.; Lakay, M.T.; Ekama, G.A. Batch test for characterisation of the carbonaceous materials in municipal wastewaters. Water SA 1999, 25, 327–336. [Google Scholar]
- Henze, M.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjandnovic, D. Biological Wastewater Treatment—Principles, Modeling and Design; IWA Publishing: London, UK, 2008. [Google Scholar]
- Takahashi, N.; Kumagai, T. Removal of dissolved organic carbon and color from dyeing wastewater by pre-ozonation and subsequent biological treatment. Ozone Sci. Eng. 2006, 28, 199–205. [Google Scholar] [CrossRef]
- Bae, W.; Won, H.; Hwang, B.; Toledo, R.A.; Chung, J.W.; Kwon, K.; Shim, H. Characterization of refractory matters in dyeing wastewater during a full-scale Fenton process following pure-oxygen activated sludge treatment. J. Hazard. Mater. 2015, 287, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lindholm-Lehto, P.C.; Knuutinen, J.S.; Ahkola, H.S.J.; Herve, S.H. Refractory organic pollutants and toxicity in pulp and paper mill wastewaters. Environ. Sci. Pollut. Res. 2015, 22, 6473–6499. [Google Scholar] [CrossRef] [PubMed]
- Carstea, F.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence spectroscopy for wastewater monitoring: A review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Plosz, B.G.; Vogelsang, C.; Macrae, K.; Heiaas, H.; Lopez, A.; Liltved, H.; Langford, K. The BIOZO process—A biofilm system combined with ozonation: Occurrence of xenobiotic organic micro-pollutants in and removal of polycyclic aromatic hydrocarbons and nitrogen from landfill leachate. Water Sci. Technol. 2010, 61, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Ince, B.K.; Cetecioglu, Z.; Ince, O. Pollution prevention in the pulp and paper industries. In Environmental Management in Practice; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Wentzel, M.C.; Mbewe, A.; Ekama, G.A. Batch test for measurement of readily biodegradable COD and active organism concentrations in municipal waste waters. Water SA 1995, 21, 118–124. [Google Scholar]
- Yang, L.; Han, D.H.; Lee, B.M.; Hur, J. Characterizing treated wastewaters of different industries using clustered fluorescence EEM–PARAFAC and FT-IR spectroscopy: Implications for downstream impact and source identification. Chemosphere 2015, 127, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ekama, G.A.; Dold, P.A.; Marais, G.v.R. Procedures for determining influent COD fractions and the maximum specific growth rate of heterotrophs in activated sludge. Water Sci. Technol. 1986, 18, 91–114. [Google Scholar]
- Grady, C.P.L.; Daigger, G.T.; Love, N.G.; Filipe, C.D.M. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Orhon, D.; Cokgor, U.E. COD Fractionation in Wastewater Characterization—The State of the Art. J. Chem. Technol. Biotechnol. 1997, 68, 283–293. [Google Scholar] [CrossRef]
- Hu, Z.; Chanadran, K.; Smets, B.F.; Grasso, D. Evaluation of a rapid physical-chemical method for the determination of extant soluble COD. Water Res. 2002, 36, 617–624. [Google Scholar] [CrossRef]
- Mamais, D.; Jenkins, D.; Pitt, P. A rapid physical-chemical method for the determination of readily biodegradable soluble COD in municipal wastewater. Water Res. 1993, 27, 195–197. [Google Scholar] [CrossRef]
- Hur, J.; Cho, J. Prediction of BOD, COD, and total nitrogen concentrations in a typical urban river using a fluorescence excitation–emission matrix with PARAFAC and UV absorption indices. Sensors 2012, 12, 972–986. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Choi, E.; Rhu, D.; Yun, Z.; Lee, E. Temperature effects on biological nutrient removal system with weak municipal wastewater. Water Sci. Technol. 1998, 37, 219–226. [Google Scholar] [CrossRef]
- Mikola, A.M.K.; Vahala, R.; Rautiainen, J.A. Factors affecting the quality of the plant influent and its suitability for prefermentation and the biological nutrient removal process. J. Environ. Eng. 2011, 137, 1185–1192. [Google Scholar] [CrossRef]
- EnviroSim Associates. General Activated Dludge-Digestion Model (General ASDM); EnviroSim Associates: Hamilton, ON, Canada, 2007. [Google Scholar]
- Ni, B.J.; Fang, F.; Xie, W.M.; Sun, M.; Sheng, G.P.; Li, W.H.; Yu, H.Q. Characterization of extracellular polymeric substances produced by mixed microorganisms in activated sludge with gel-permeating chromatography, excitation–emission matrix fluorescence spectroscopy measurement and kinetic modeling. Water Res. 2009, 43, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.X.; Xiao, K.; Liang, P.; Sun, J.Y.; Sai, S.J.; Huang, X. Characterization of soluble microbial products in 10 large-scale membrane bioreactors for municipal wastewater treatment in China. J. Membr. Sci. 2012, 415, 336–345. [Google Scholar] [CrossRef]
- Fan, J.; Li, H.; Shuang, C.; Li, W.; Li, A. Dissolved organic matter removal using magnetic anion exchange resin treatment on biological effluent of textile dyeing wastewater. J. Environ. Sci. 2014, 26, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Song, Y.H.; Tu, X.; Du, E.D.; Liu, R.X.; Peng, J.F. Assessing removal efficiency of dissolved organic matter in wastewater treatment using fluorescence excitation emission matrices with parallel factor analysis and second derivative synchronous fluorescence. Bioresour. Technol. 2013, 144, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Levy, G.J.; Borisover, M. Fluorescent components of organic matter in wastewater: Efficacy and selectivity of the water treatment. Water Res. 2014, 55, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stueyz, R.; Khan, S.J. Organic matter fluorescence in municipal water recycling schemes: Toward a unified PARAFAC model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Chen, S.Y.; Xu, Z.X.; Li, Y.; Shuang, C.D.; Li, A.M. Characterization of dissolved organic matter in municipal wastewater using fluorescence PARAFAC analysis and chromatography multi-excitation/emission scan: A comparative study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.S.; Wei, C.H.; Mo, C.H.; Wu, H.Z.; Ren, Y.; Feng, C.H. Novel insights into anoxic/aerobic1/aerobic2 biological fluidized-bed system for coke wastewater treatment by fluorescence excitation–emission matrix spectra coupled with parallel factor analysis. Chemosphere 2014, 113, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kreetachat, T.; Damrongsri, M.; Punsuwon, V.; Vaithanomsat, P.; Chiemchaisri, C. Effects of ozonation process on lignin-derived compounds in pulp and paper mill effluents. J. Hazard. Mater. 2007, 142, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, M.Y.; Jamil, T.S.; EL-Seesy, I.E.; Souaya, E.R.; Nasr, R.A. Treatment of highly polluted paper mill wastewater by lolar photocatalytic oxidation with synthesized nano TiO2. Chem. Eng. J. 2011, 168, 446–454. [Google Scholar] [CrossRef]
| ITEM | First Sampling Campaign | Second Sampling Campaign | ||||
|---|---|---|---|---|---|---|
| JS WWTP | DS WWTP | HP WWTP | JS WWTP | DS WWTP | HP WWTP | |
| Total COD | 155.5 ± 0.5 | 110.7 ± 3.3 | 112 ± 3.2 | 150 ± 3.7 | 105 ± 3.7 | 129 ± 4.0 |
| CODZn24 (a) | 28.5 ± 0.5 | 43.7 ± 2.1 | 67 ± 1.2 | 21 ± 1.0 | 45.5 ± 1.5 | 71 ± 1.7 |
| BODu (b) | 124 | 52.4 | 38.8 | 123.2 | 52.6 | 43.8 |
| RBCOD | 9.2 | 4.7 | 0.7 | 7.7 | 0.3 | 8 |
| SBCOD | 114.8 | 47.7 | 38.1 | 115.5 | 52.3 | 35.8 |
| NBDSCOD | 28.5 | 43.7 | 67.0 | 21.0 | 45.5 | 71.0 |
| NBDPCOD | 3.0 | 14.6 | 6.2 | 5.8 | 6.9 | 14.2 |
| This Study | Previous Study | |||
|---|---|---|---|---|
| Comp. | λex/λem | λex/λem | Substance | Reference |
| C1 | 230/345 | 230/350 | Protein-like | Shen et al. (2012) [27] |
| 280/340 | Protein-like | Ni et al. (2009) [26] | ||
| 250–280/<380 | Protein-like | Fan et al. (2014) [28] | ||
| C2 | 220(275)/355 | 220(280)/350 | Tryptophan-like, SMP | Yu et al. (2013) [29] |
| 275(240)/346 | Tryptophan-like | Cohen et al. (2014) [30] | ||
| 220(275)/343 | Tryptophan-like | Shen et al. (2012) [27] | ||
| C3 | 275(220)/320 | 270/300 | Tyrosine-like | Murphy et al. (2011) [31] |
| 279/315 | Protein-like | Shen et al. (2012) [27] | ||
| 280(230)/310 | Tyrosine-like | Li et al. (2014) [32] | ||
| 280/320 | Phenol-like, protein-like | Ou et al. (2014) [33] | ||
| C4 | 243/430 | 230–245/425–430 | Fulvic-like | Yu et al. (2013) [29] |
| 270(350)/432 | Humic-like | Cohen et al. (2014) [30] | ||
| 230–275/400–520 | Fulvic-like, Lignins | Carstea et al. (2016) [11] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-Y.; Baek, S.-R.; Kim, J.-I.; Choi, J.-W.; Hur, J.; Lee, T.-U.; Park, C.-J.; Lee, B.J. Characteristics and Biodegradability of Wastewater Organic Matter in Municipal Wastewater Treatment Plants Collecting Domestic Wastewater and Industrial Discharge. Water 2017, 9, 409. https://doi.org/10.3390/w9060409
Choi Y-Y, Baek S-R, Kim J-I, Choi J-W, Hur J, Lee T-U, Park C-J, Lee BJ. Characteristics and Biodegradability of Wastewater Organic Matter in Municipal Wastewater Treatment Plants Collecting Domestic Wastewater and Industrial Discharge. Water. 2017; 9(6):409. https://doi.org/10.3390/w9060409
Chicago/Turabian StyleChoi, Yun-Young, Seung-Ryong Baek, Jae-In Kim, Jeong-Woo Choi, Jin Hur, Tae-U Lee, Cheol-Joon Park, and Byung Joon Lee. 2017. "Characteristics and Biodegradability of Wastewater Organic Matter in Municipal Wastewater Treatment Plants Collecting Domestic Wastewater and Industrial Discharge" Water 9, no. 6: 409. https://doi.org/10.3390/w9060409





