Fate of Trace Organic Compounds in Granular Activated Carbon (GAC) Adsorbers for Drinking Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Quality
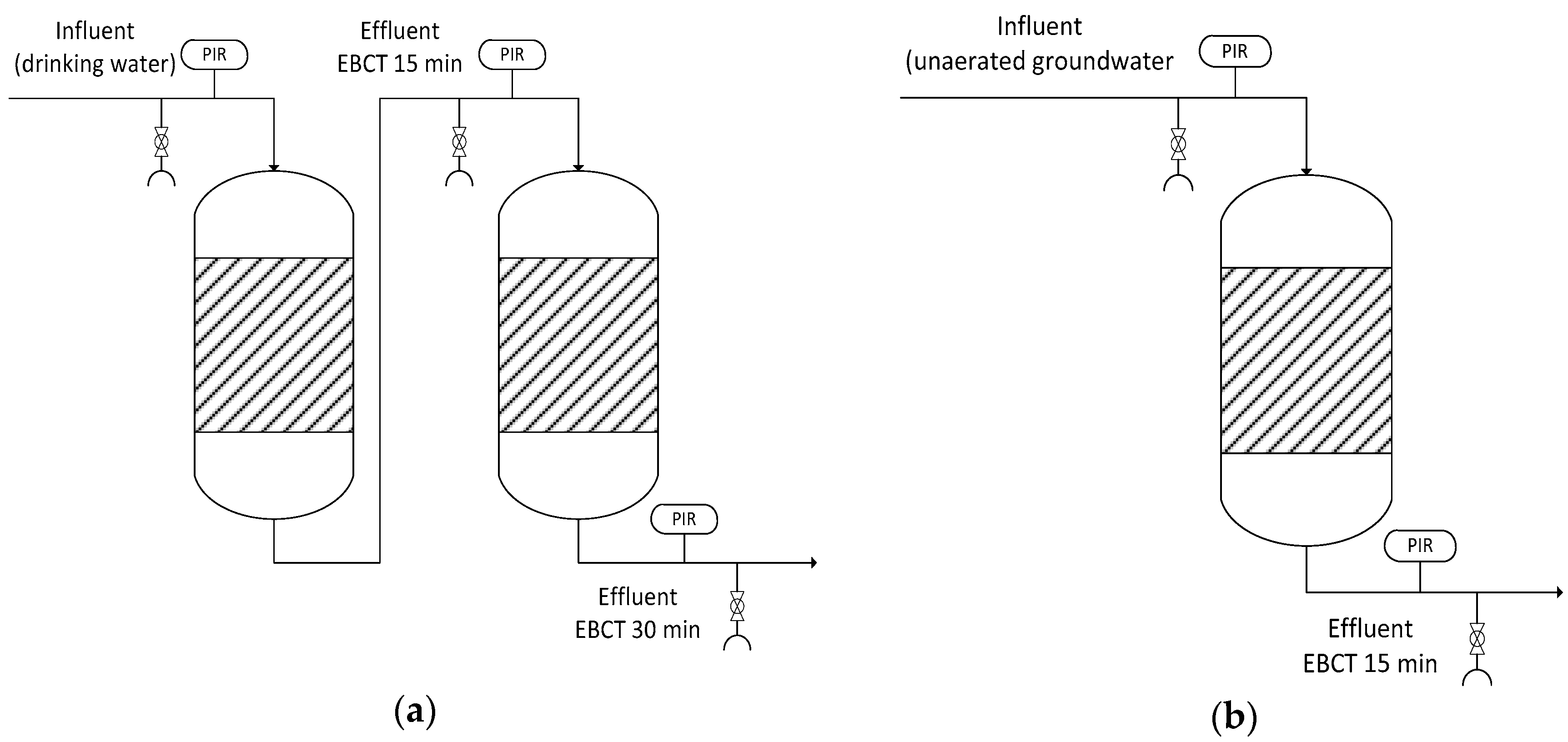
2.2. GAC Pilot Plant
2.3. Analytical Methods
3. Results and Discussion
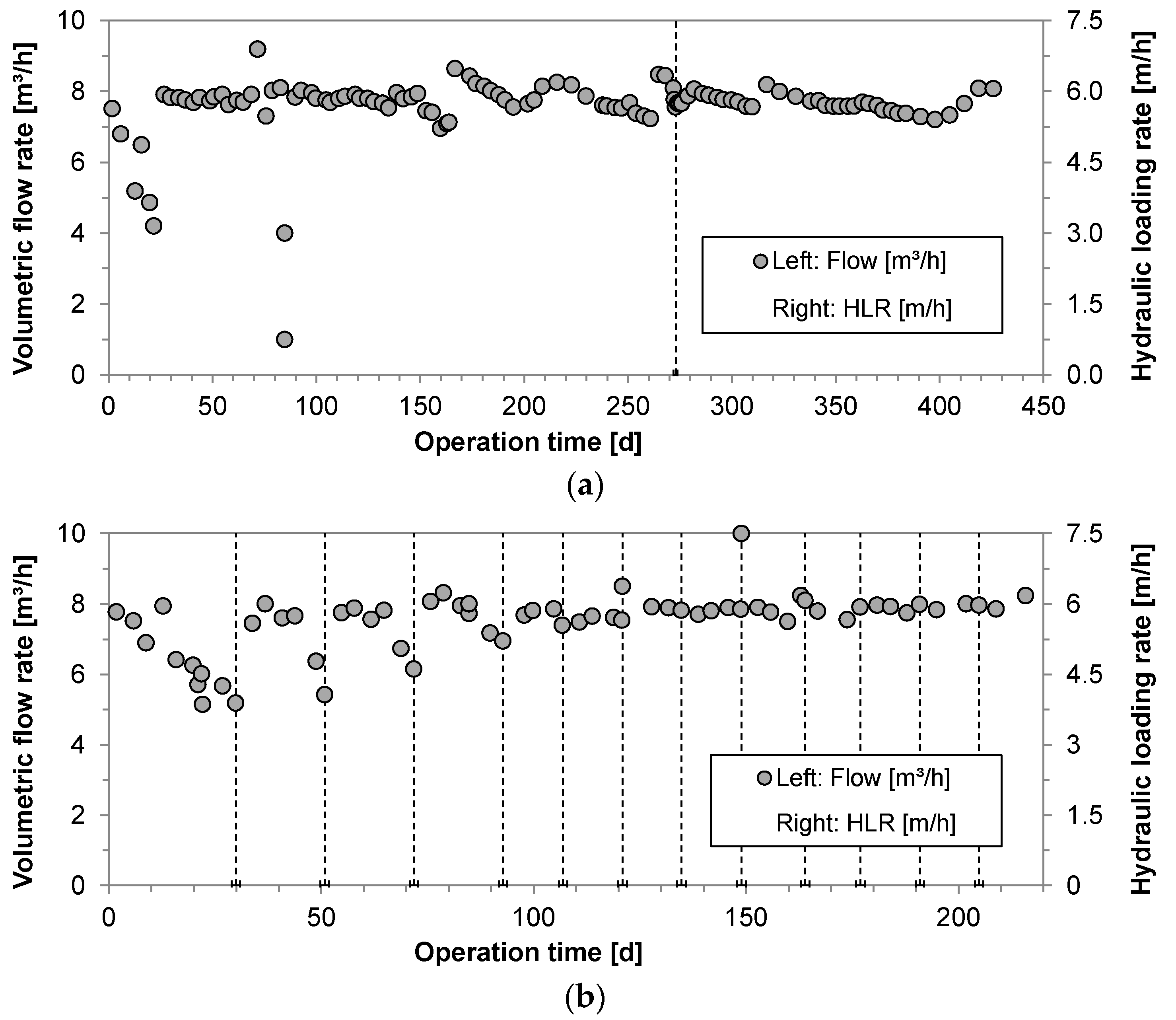
3.1. Pilot Operation
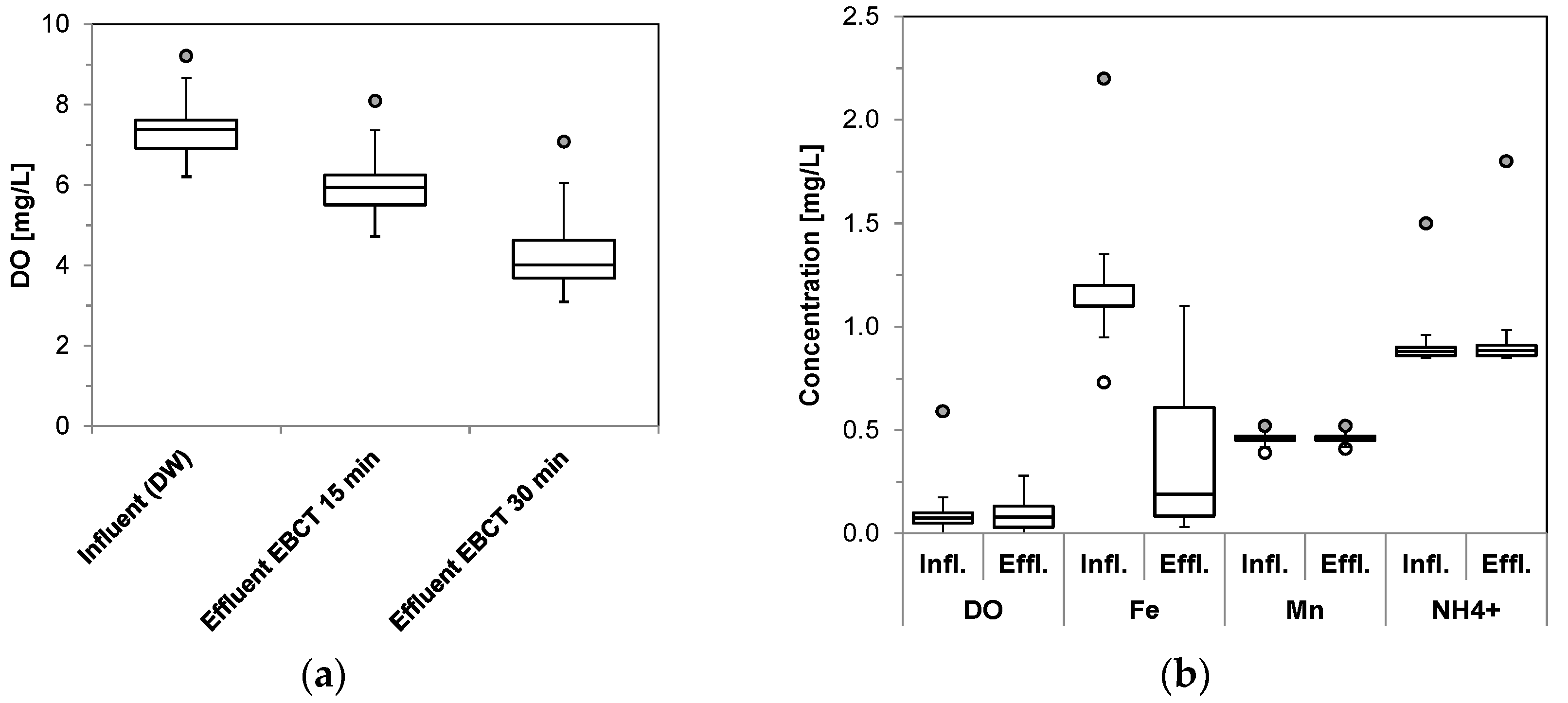
3.2. Redox Parameters
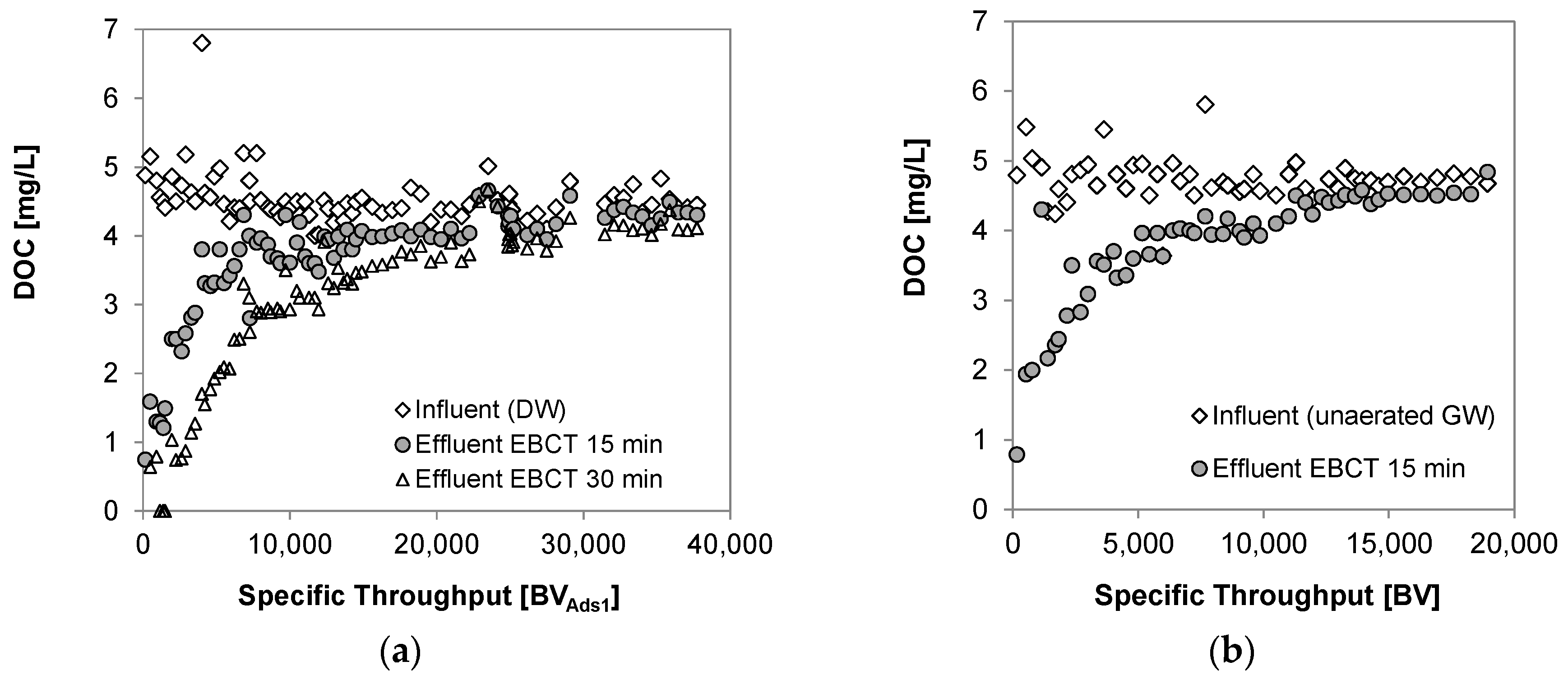
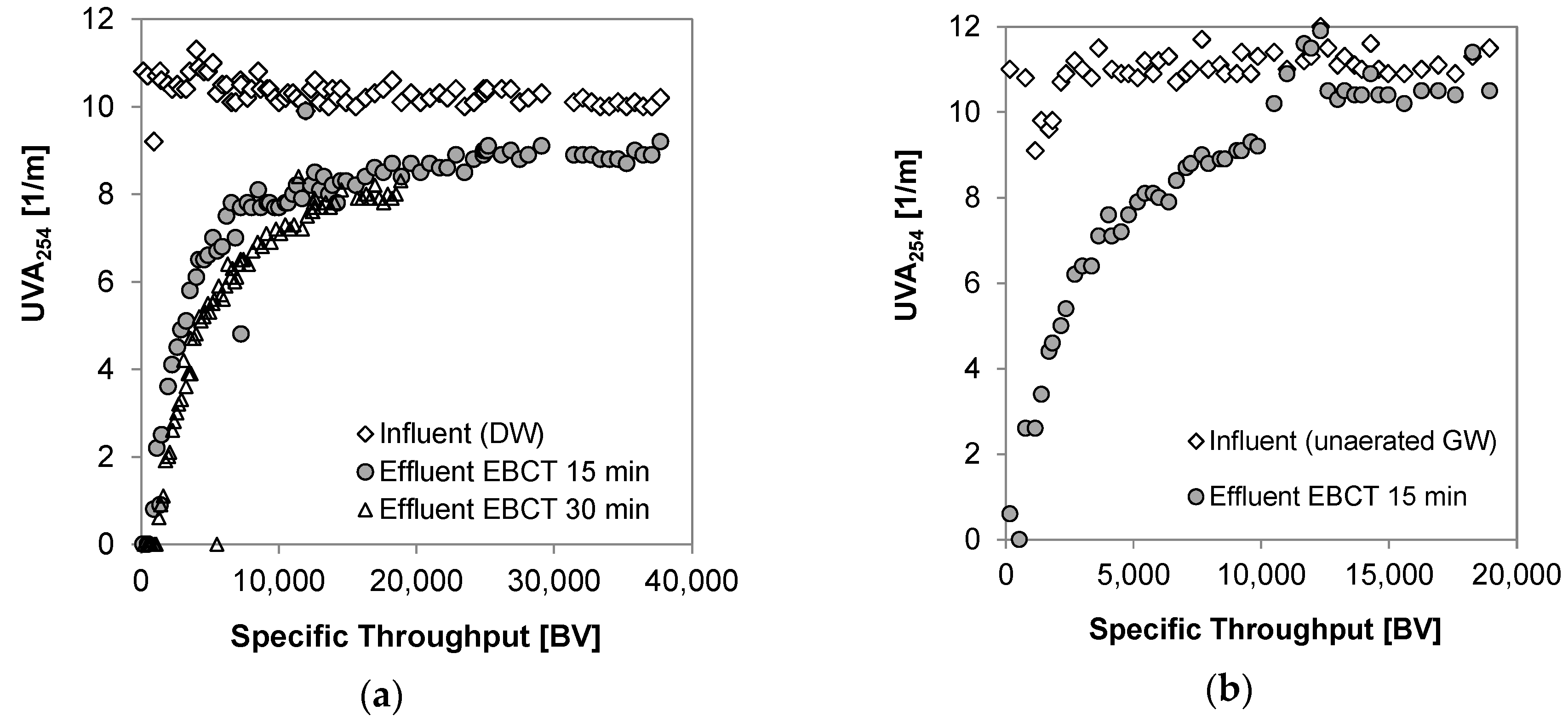
3.3. Breakthrough of Dissolved Organic Carbon
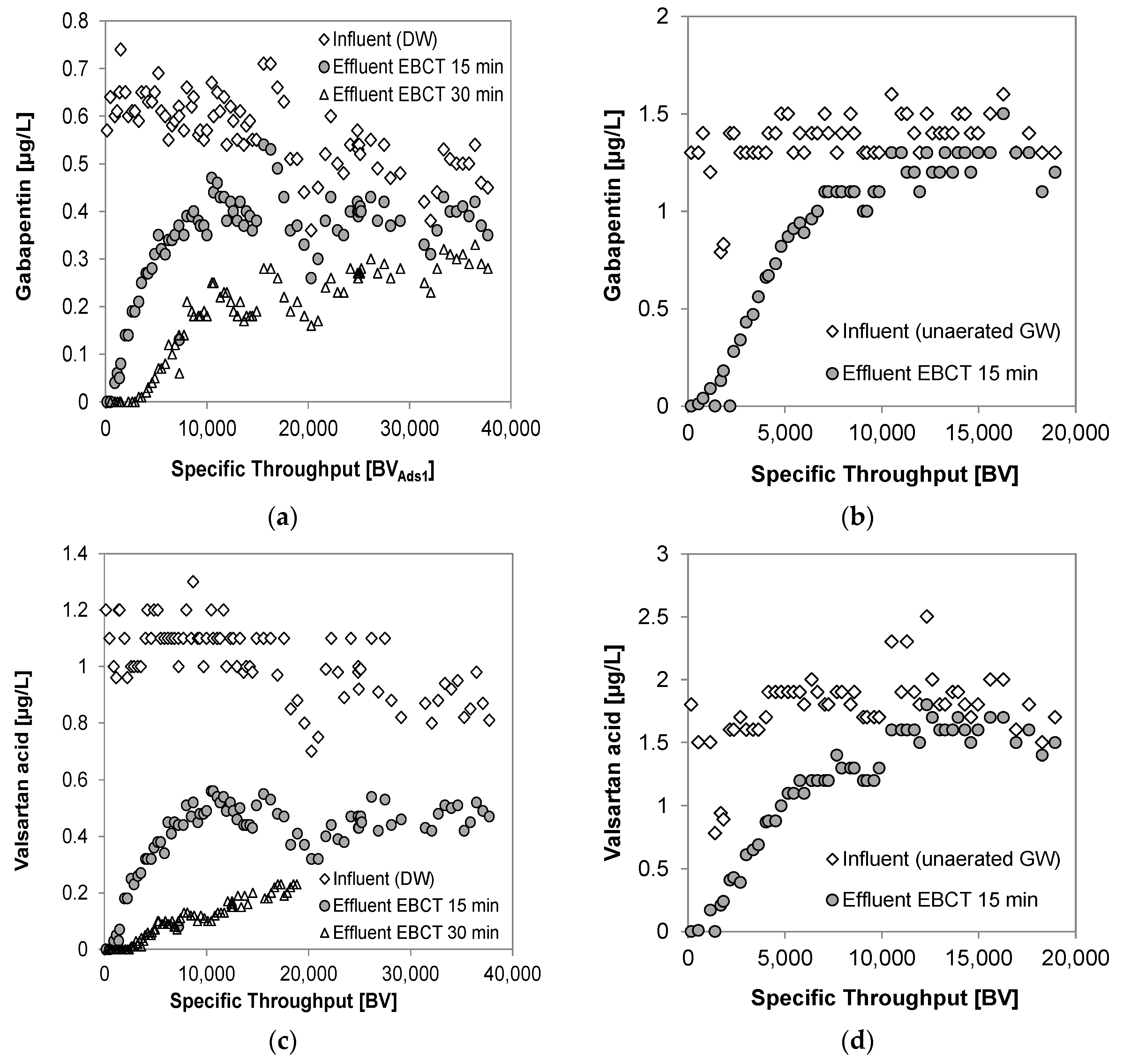
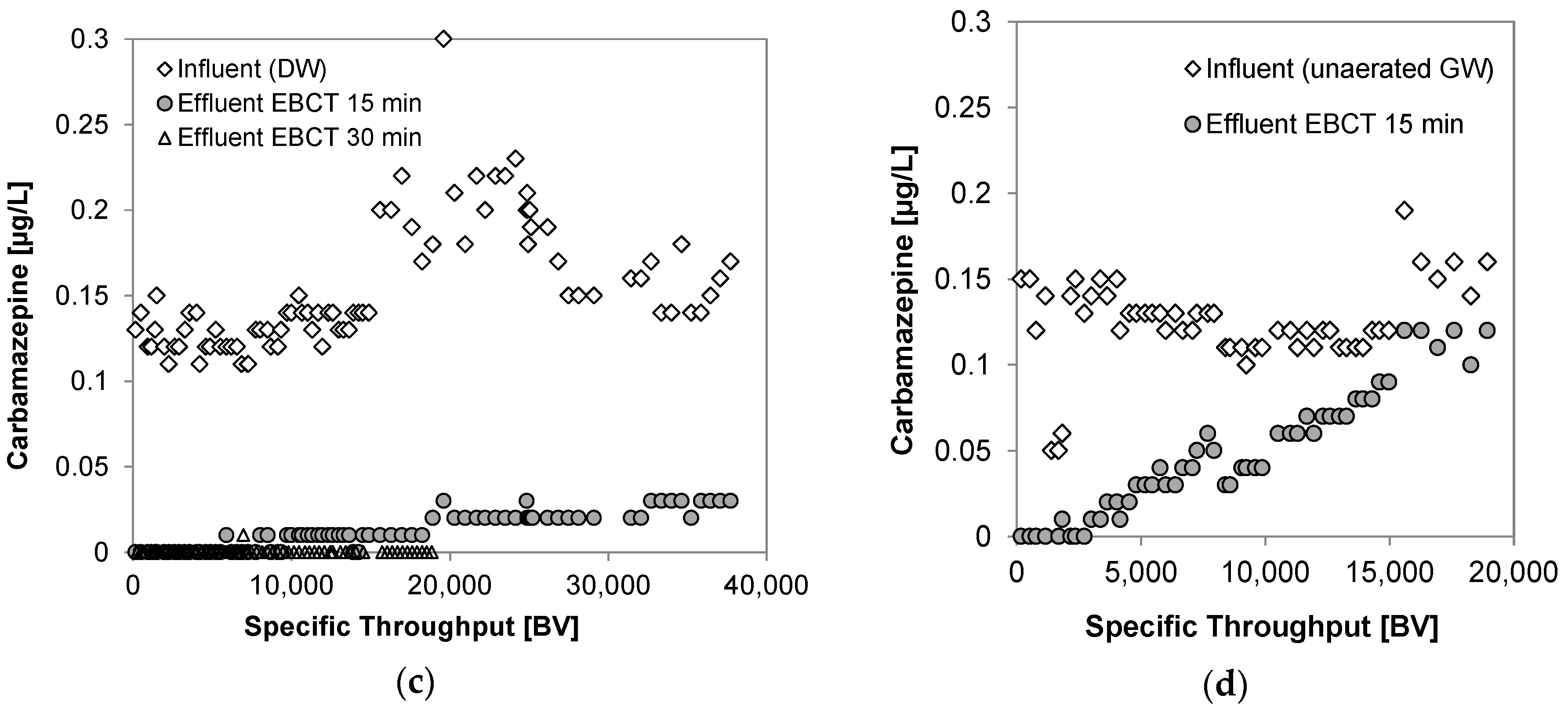
3.4. Breakthrough of Trace Organic Chemicals
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Grunheid, S.; Amy, G.; Jekel, M. Removal of bulk dissolved organic carbon (DOC) and trace organic compounds by bank filtration and artificial recharge. Water Res. 2005, 39, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Van Baar, P. Entwicklung und Anwendung von UHPLC-MS Verfahren für Organische Spurenstoffe zur Bewertung der Sicherheit der Rohwasserressourcen der Wasserwerke der Stadt Berlin; Technische Universität Berlin: Berlin, Germany, 2015. [Google Scholar]
- Yu, J.T.; Bouwer, E.J.; Coelhan, M. Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agric. Water Manag. 2006, 86, 72–80. [Google Scholar] [CrossRef]
- Altmann, J.; Rehfeld, D.; Trader, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.; Sperlich, A.; Jekel, M. Pilot-scale study of powdered activated carbon recirculation for micropollutant removal. Water Sci. Technol. 2016, 74, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Nodler, K.; Hillebrand, O.; Idzik, K.; Strathmann, M.; Schiperski, F.; Zirlewagen, J.; Licha, T. Occurrence and fate of the angiotensin II receptor antagonist transformation product valsartan acid in the water cycle—A comparative study with selected beta-blockers and the persistent anthropogenic wastewater indicators carbamazepine and acesulfame. Water Res. 2013, 47, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Funke, J.; Prasse, C.; Lutke Eversloh, C.; Ternes, T.A. Oxypurinol—A novel marker for wastewater contamination of the aquatic environment. Water Res. 2015, 74, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Konig, A.; Weidauer, C.; Seiwert, B.; Reemtsma, T.; Unger, T.; Jekel, M. Reductive transformation of carbamazepine by abiotic and biotic processes. Water Res. 2016, 101, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wiese, B.; Massmann, G.; Jekel, M.; Heberer, T.; Dünnbier, U.; Orlikowski, D.; Grützmacher, G. Removal kinetics of organic compounds and sum parameters under field conditions for managed aquifer recharge. Water Res. 2011, 45, 4939–4950. [Google Scholar] [CrossRef] [PubMed]
- Berliner Wasserbetriebe. Analysedaten des Wasserwerks Tegel. Available online: http://www.bwb.de/content/language1/downloads/WW_Analysedaten_2016_tegel_.pdf (accessed on 28 April 2017).
- Dieter, H.H. Health related guide values for drinking-water since 1993 as guidance to assess presence of new analytes in drinking-water. Int. J. Hyg. Environ. Health 2014, 217, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Wode, F.; Reilich, C.; van Baar, P.; Dünnbier, U.; Jekel, M.; Reemtsma, T. Multiresidue analytical method for the simultaneous determination of 72 micropollutants in aqueous samples with ultra high performance liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2012, 1270, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wode, F.; van Baar, P.; Dünnbier, U.; Hecht, F.; Taute, T.; Jekel, M.; Reemtsma, T. Search for over 2000 current and legacy micropollutants on a wastewater infiltration site with a UPLC-high resolution MS target screening method. Water Res. 2015, 69, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hellauer, K.; Mergel, D.; Ruhl, A.; Filter, J.; Hübner, U.; Jekel, M.; Drewes, J. Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes. Water 2017, 9, 221. [Google Scholar] [CrossRef]
- Altmann, J.; Sperlich, A.; Jekel, M. Integrating organic micropollutant removal into tertiary filtration: Combining PAC adsorption with advanced phosphorus removal. Water Res. 2015, 84, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Onesios, K.M.; Bouwer, E.J. Biological removal of pharmaceuticals and personal care products during laboratory soil aquifer treatment simulation with different primary substrate concentrations. Water Res. 2012, 46, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Velten, S.; Hammes, F.; Boller, M.; Egli, T. Rapid and direct estimation of active biomass on granular activated carbon through adenosine tri-phosphate (ATP) determination. Water Res. 2007, 41, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Jekel, M.; Dott, W.; Bergmann, A.; Dunnbier, U.; Gnirss, R.; Haist-Gulde, B.; Hamscher, G.; Letzel, M.; Licha, T.; Lyko, S.; et al. Selection of organic process and source indicator substances for the anthropogenically influenced water cycle. Chemosphere 2015, 125, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography–organic carbon detection–organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Snoeyink, V.L.; Mariñas, B.J.; Campos, C. Pore blockage effect of NOM on atrazine adsorption kinetics of PAC: The roles of PAC pore size distribution and NOM molecular weight. Water Res. 2003, 37, 4863–4872. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Knappe, D.R.U.; Iwaki, K.; Ohira, H. Pesticide Adsorption by Granular Activated Carbon Adsorbers. 2. Effects of Pesticide and Natural Organic Matter Characteristics on Pesticide Breakthrough Curves. Environ. Sci. Technol. 2002, 36, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Zietzschmann, F.; Worch, E.; Altmann, J.; Ruhl, A.S.; Sperlich, A.; Meinel, F.; Jekel, M. Impact of EfOM size on competition in activated carbon adsorption of organic micro-pollutants from treated wastewater. Water Res. 2014, 65, 297–306. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Median Value | Limit Value 1 | HRIV 2 |
|---|---|---|---|
| Iron (mg/L) | <0.03 | 0.2 | - |
| Manganese (mg/L) | <0.005 | 0.05 | - |
| Conductivity (µS/cm) | 750 | 2790 | - |
| Turbidity (NTU) | <0.2 | 1.0 | - |
| pH | 7.5 | 6.5–9.5 | - |
| Temperature (°C) | 12.9 | - | - |
| Dissolved oxygen (mg/L) | 8.0 | - | - |
| TOC (mg/L) | 4.5 | - | - |
| UVA254 (L/m) | 10.4 | - | - |
| Colour (L/m) | 0.3 | 0.5 | - |
| Carbamazepine (µg/L) | 0.13 | - | 0.3 |
| Gabapentin (µg/L) | 0.55 | - | 1.0 |
| Oxypurinol (µg/L) | 0.43 | - | 0.3 |
| Valsartan acid (µg/L) | 1.0 | - | 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperlich, A.; Harder, M.; Zietzschmann, F.; Gnirss, R. Fate of Trace Organic Compounds in Granular Activated Carbon (GAC) Adsorbers for Drinking Water Treatment. Water 2017, 9, 479. https://doi.org/10.3390/w9070479
Sperlich A, Harder M, Zietzschmann F, Gnirss R. Fate of Trace Organic Compounds in Granular Activated Carbon (GAC) Adsorbers for Drinking Water Treatment. Water. 2017; 9(7):479. https://doi.org/10.3390/w9070479
Chicago/Turabian StyleSperlich, Alexander, Mareike Harder, Frederik Zietzschmann, and Regina Gnirss. 2017. "Fate of Trace Organic Compounds in Granular Activated Carbon (GAC) Adsorbers for Drinking Water Treatment" Water 9, no. 7: 479. https://doi.org/10.3390/w9070479





