Fish but Not Macroinvertebrates Promote Trophic Cascading Effects in High Density Submersed Plant Experimental Lake Food Webs in Two Contrasting Climate Regions
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sampling and Sample Analysis
2.4. Data Analysis
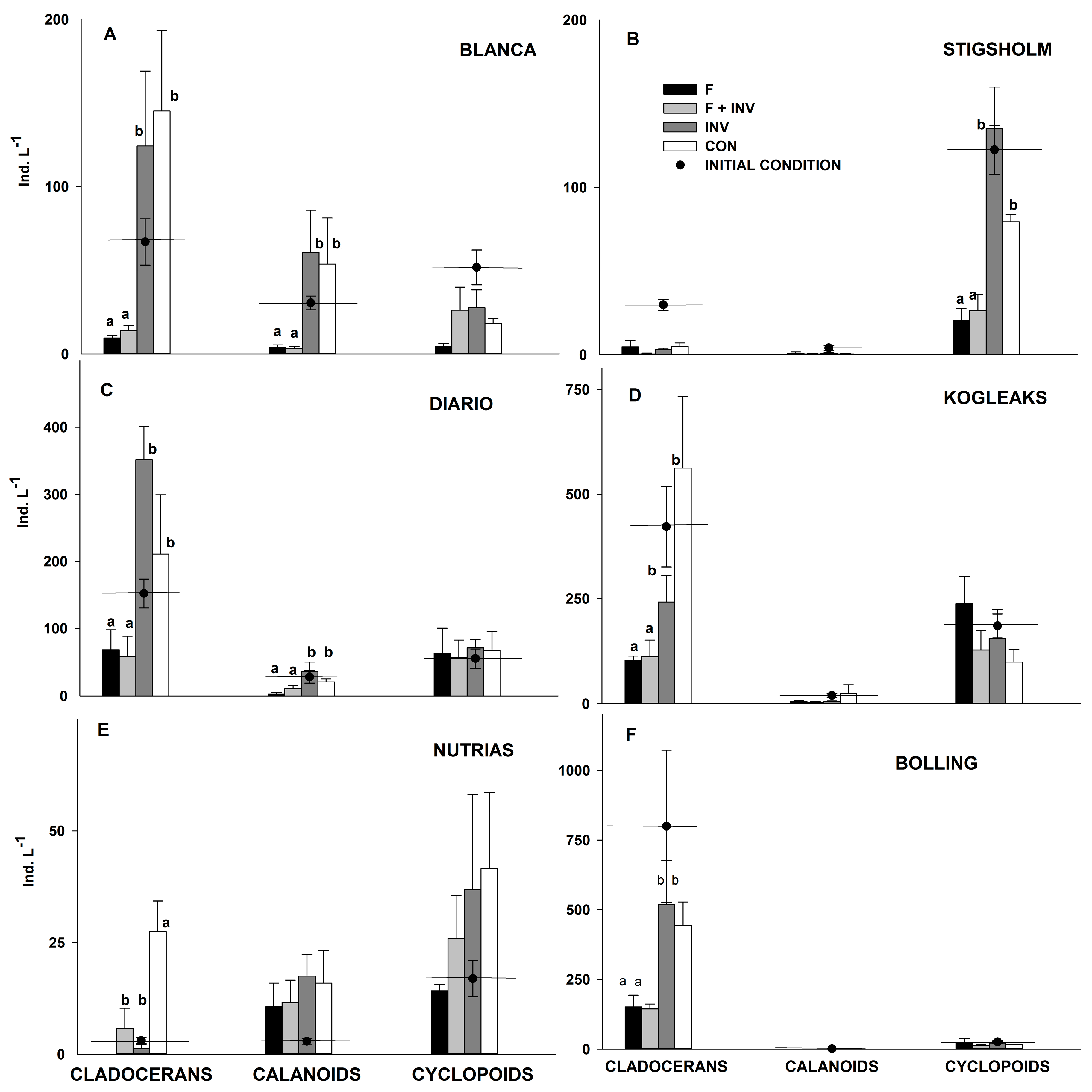
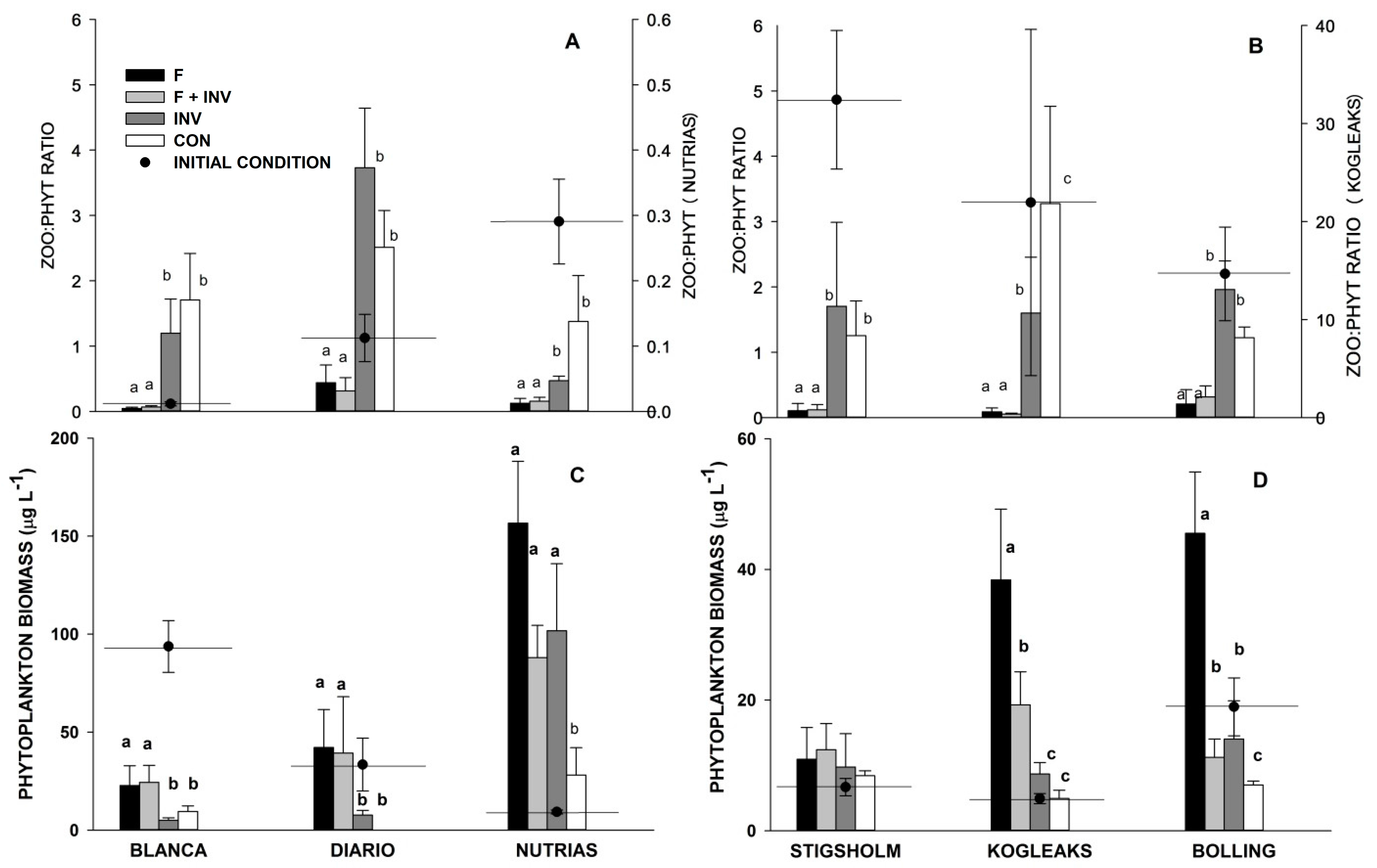
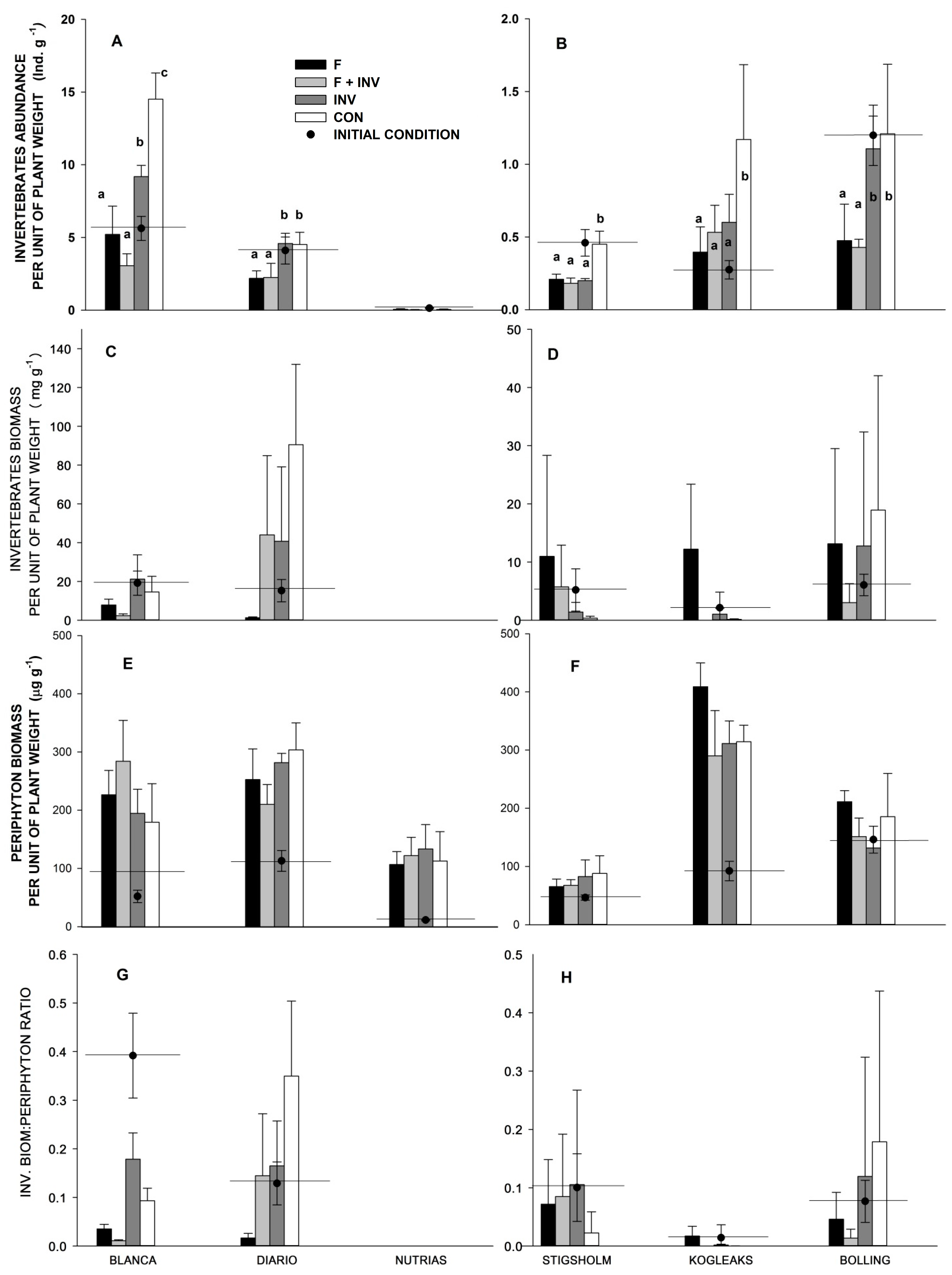
3. Results
3.1. Pelagic Trophic Interactions
3.2. Littoral Trophic Interactions
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R.; Cochran, P.A.; Elser, J.J.; Elser, M.M.; Lodge, D.M.; Kretchmer, D.; He, X.; von Ende, C.N. Regulation of lake primary productivity by food web structure. Ecology 1987, 68, 1863–1876. [Google Scholar] [CrossRef]
- McQueen, D.J.; Post, J.R.; Mills, E.L. Trophic relationships in freshwater pelagic ecosystems. Can. J. Fish. Aquat. Sci. 1986, 43, 1571–1581. [Google Scholar] [CrossRef]
- Burks, R.L.; Lodge, D.M.; Jeppesen, E.; Lauridsen, T.L. Diel horizontal migration of zooplankton: Costs and benefits of inhabiting littoral zones. Freshw. Biol. 2002, 47, 343–365. [Google Scholar] [CrossRef]
- Schindler, D.E.; Scheuerell, M.D. Habitat coupling in lake ecosystems. Oikos 2002, 98, 177–189. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Vadeboncoeur, Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 2002, 83, 2152–2161. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, body size and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Brucet, S.; Boix, D.; Nathansen, L.W.; Quintana, X.D.; Jensen, E.; Balayla, D.; Meerhoff, M.; Jeppesen, E. Effects of temperature, salinity and fish in structuring the macroinvertebrate community in shallow lakes: Implications for effects of climate change. PLoS ONE 2012, 7, e30877. [Google Scholar] [CrossRef] [PubMed]
- Hrbácek, J.; Dvorakova, M.; Korinek, V.; Procházkóva, L. Demonstration of the effect of the fish stock on the species composition of zooplankton and the intensity of metabolism of the whole plankton association. Verh. Int. Ver. Limnol. 1961, 14, 192–195. [Google Scholar]
- Burks, R.L.; Lodge, D.M. Cued in: Advances and opportunities in freshwater chemical ecology. J. Chem. Ecol. 2002, 28, 1901–1917. [Google Scholar] [CrossRef] [PubMed]
- Kairesalo, T.; Tátrai, I.; Luokkanen, E. Impacts of waterweed (elodea canadiensis michx) on fish-plankton interactions in the lake littoral. Verh. Int. Ver. Limnol. 1998, 26, 1846–1851. [Google Scholar]
- Lauridsen, T.L.; Lodge, D.M. Avoidance of daphnia magna by fish and macrophytes: Chemical cues and predator-mediated use of macrophyte habitat. Limnol. Oceanogr. 1996, 41, 794–798. [Google Scholar] [CrossRef]
- Romare, P.; Hansson, L.A. A behavioural cascade: Top-predator induced behavioural shifts in planktivorous fish and zooplankton. Limnol. Oceanogr. 2003, 48, 1956–1964. [Google Scholar] [CrossRef]
- Timms, R.M.; Moss, B. Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing, in the presence of zooplanktivorous fish, in a shallow wetland ecosystem. Limnol. Oceanogr. 1984, 29, 472–486. [Google Scholar] [CrossRef]
- Brönmark, C.; Weisner, S.E.B. Indirect effects of fish community structure on submerged vegetation in shallow, eutrophic lakes: An alternativa mechanism. Hydrobiologia 1992, 243, 293–301. [Google Scholar] [CrossRef]
- Jones, J.I.; Waldron, S. Combined stable isotope and gut contents analysis of food webs in plant dominated, shallow lakes. Freshw. Biol. 2003, 48, 1396–1407. [Google Scholar] [CrossRef]
- Liboriussen, L.; Jeppesen, E. Temporal dynamics in epipelic, pelagic and epiphytic algal production in a clear and a turbid shallow lake. Freshw. Biol. 2003, 48, 418–431. [Google Scholar] [CrossRef]
- Jones, J.I.; Sayer, C.D. Does the fish-invertebrate-periphyton cascade precipitate plant loss in shallow lakes? Ecology 2003, 84, 2155–2167. [Google Scholar] [CrossRef]
- Bertolo, A.; Lacroix, G.; Lescher-Moutoué, F.; Cardinal-Legrand, C. Plankton dynamics in planktivore and piscivore dominated mesocosm. Arch. Hydrobiol. 2000, 147, 327–349. [Google Scholar] [CrossRef]
- Havens, K.E. Size structure and energetics in a plankton food web. Oikos 1998, 81, 346–358. [Google Scholar] [CrossRef]
- Meerhoff, M.; Clemente, J.M.; Teixeira de Mello, F.; Iglesias, C.; Pedersen, A.R.; Jeppesen, E. Can warm climate-related structure of littoral predator assemblies weaken the clear water state in shallow lakes? Glob. Chang. Biol. 2007, 13, 1888–1897. [Google Scholar] [CrossRef]
- Havens, K.E.; Pinto-Coelho, R.M.; Beklioğlu, M.; Christoffersen, K.S.; Jeppesen, E.; Lauridsen, T.L.; Mazumder, A.; Méthot, G.; Alloul, B.P.; Tavşanoğlu, U.N.; et al. Temperature effects on body size of freshwater crustacean zooplankton from greenland to the tropics. Hydrobiologia 2015, 743, 27–35. [Google Scholar] [CrossRef]
- Lazzaro, X. Do the trophic cascade hypothesis and classical biomanipulation approaches apply to tropical lakes and reservoirs? Verh. Int. Ver. Limnol. 1997, 26, 719–730. [Google Scholar]
- Teixeira-de Mello, F.; Meerhoff, M.; Pekcan-Hekim, Z.; Jeppesen, E. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshw. Biol. 2009, 54, 1202–1215. [Google Scholar] [CrossRef]
- Boveri, M.; Quirós, R. Cascading trophic effects in pampean shallow lakes: Results of a mesocosm experiment using two coexisting fish species with different feeding strategies. Hydrobiologia 2007, 584, 215–222. [Google Scholar] [CrossRef]
- Iglesias, C.; Goyenola, G.; Mazzeo, N.; Meerhoff, M.; Rodó, E.; Jeppesen, E. Horizontal dynamics of zooplankton in subtropical lake blanca (uruguay) hosting multiple zooplankton predators and aquatic plant refuges. Hydrobiologia 2007, 584, 179–189. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ü.N.; Cakiroğlu, A.I.; Erdogan, Ş.; Meerhoff, M.; Jeppesen, E.; Beklioğlu, M. Sediments, not plants, offer the preferred refuge for daphnia against fish predation in mediterranean shallow lakes: An experimental demonstration. Freshw. Biol. 2012, 57, 795–802. [Google Scholar]
- Sinistro, R. Top-down and bottom-up regulation of planktonic communities in a warm temperate wetland. J. Plankton Res. 2009, 32, 209–220. [Google Scholar] [CrossRef]
- Castro, B.B.; Marques, S.M.; Gonçalves, F. Habitat selection and diel distribution of the crustacean zooplankton from a shallow mediterranean lake during the turbid and clear water phases. Freshw. Biol. 2007, 52, 421–433. [Google Scholar] [CrossRef]
- Meerhoff, M.; Iglesias, C.; Teixeira-de Mello, F.; Clemente, J.M.; Jensen, E.; Lauridsen, T.L.; Jeppesen, E. Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshw. Biol. 2007, 52, 1009–1021. [Google Scholar] [CrossRef]
- Meerhoff, M.; Fosalba, C.; Bruzzone, C.; Mazzeo, N.; Noordoven, W.; Jeppesen, E. An experimental study of habitat choice by Daphnia: Plants signal danger more than refuge in subtropical lakes. Freshw. Biol. 2006, 51, 1320–1330. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ü.N.; Brucet, S.; Levi, E.E.; Bucak, T.; Bezirci, G.; Özen, A.; Johansson, L.S.; Jeppesen, E.; Beklioğlu, M. Size-based diel migration of zooplankton in mediterranean shallow lakes assessed from in situ experiments with artificial plants. Hydrobiologia 2015, 753, 47–59. [Google Scholar] [CrossRef]
- González-Sagrario, M.; Balseiro, E.; Ituarte, R.; Spivak, E. Macrophytes as refuge or risky area for zooplankton: A balance set by littoral predacious macroinvertebrates. Freshw. Biol. 2009, 54, 1042–1053. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Meerhoff, M.; Lacerot, G.; Clemente, J.; Scasso, F.; Kruk, C.; Goyenola, G.; García-Alonso, J.; Amsinck, S.; et al. High predation is of key importance for dominance of small-bodied zooplankton in warm shallow lakes: Evidence from lakes, fish exclosures and surface sediments. Hydrobiologia 2011, 667, 133–147. [Google Scholar] [CrossRef]
- Branco, C.W.C.; Rocha, M.I.A.; Pinto, G.F.S.; Gomara, G.A.; Filippo, R.D. Limnological features of funil reservoir (R.J., Brazil) and indicator properties of rotifers and cladocerans of the zooplankton community. Lakes Reser. Res. Manag. 2002, 7, 87–92. [Google Scholar] [CrossRef]
- Crisman, T.L.; Beaver, J.R. Applicability of planktonic biomanipulation for managing eutrophication in the subtropics. Hydrobiologia 1990, 200/201, 177–185. [Google Scholar] [CrossRef]
- Dumont, H.J. On the diversity of the cladocera in the tropics. Hydrobiologia 1994, 272, 27–38. [Google Scholar] [CrossRef]
- Fernando, C.H. Zooplankton, fish and fisheries in tropical freshwaters. Hydrobiologia 1994, 272, 105–123. [Google Scholar] [CrossRef]
- García, P.R.; Nandini, S.; Sarma, S.S.S.; Valderrama, E.R.; Cuesta, I.; Hurtado, M.D. Seasonal variations of zooplankton abundance in the freshwater reservoir valle bravo (Mexico). Hydrobiolgia 2002, 467, 99–108. [Google Scholar] [CrossRef]
- Kruk, C.; Rodríguez-Gallego, L.; Meerhoff, M.; Quintans, F.; Lacerot, G.; Mazzeo, N.; Scasso, F.; Paggi, J.C.; Peeters, E.T.H.M.; Scheffer, M. Determinants of biodiversity in subtropical shallow lakes (Atlantic coast, Uruguay). Freshw. Biol. 2009, 54, 2628–2641. [Google Scholar] [CrossRef]
- Meerhoff, M.; Teixeira-de Mello, F.; Kruk, C.; Alonso, C.; Gonzalez-Bergonzoni, I.; Pacheco, J.P.; Arim, M.; Beklioglu, M.; Brucet, S.; Goyenola, G.; et al. Environmental warming in shallow lakes: A review of effects on community structure as evidenced from space-for-time substitution approach. Adv. Ecol. Res. 2012, 46. [Google Scholar] [CrossRef]
- Iglesias, C.; Meerhoff, M.; Johansson, L.S.; González-Bergonzoni, I.; Mazzeo, N.; Pacheco, J.P.; Teixeira-de Mello, F.; Goyenola, G.; Lauridsen, T.L.; Søndergaard, M.; et al. Stable isotope analysis confirms substantial differences between subtropical and temperate shallow lake food webs. Hydrobiologia 2017, 784, 111–123. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Lodge, D.M. Effects of submersed macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Pedersen, L.J.; Jensen, L. Top-down control in freshwater lakes: The role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 1997, 342, 151–164. [Google Scholar] [CrossRef]
- Canfield, D.E., Jr.; Shireman, J.V.; Colle, D.E.; Haller, W.T.; Watkins, C.E.I.; Maceina, M.J. Prediction of chlorophyll a concentrations in florida lakes: Importance of aquatic macrophytes. Can. J. Fish. Aquat. Sci. 1984, 41, 497–501. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.-L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Jakobsen, B.A.; Hansen, R.S.; Søndengaard, M.; Jensen, J.P.; Lauridsen, T.; Mazzeo, N.; Branco, C.C. Restoration of shallow lakes by nutrient control and biomanipulation—The successful strategy varies with lake size and climate. Hydrobiologia 2007, 581, 269–285. [Google Scholar] [CrossRef]
- Kosten, S.; Lacerot, G.; Jeppesen, E.; da Motta Marques, D.; van Nes, E.; Mazzeo, N.; Scheffer, M. Effects of submerged vegetation on water clarity across climates. Ecosystems 2009, 12, 1117–1129. [Google Scholar] [CrossRef]
- Conrow, R.; Zale, A.V.; Gregory, R.W. Distributions and abundances of early stages of fishes in a florida lake dominated by aquatic macrophytes. Trans. Am. Fish. Soc. 1990, 119, 521–528. [Google Scholar] [CrossRef]
- Meerhoff, M.; Mazzeo, N.; Moss, B.; Rodríguez-Gallego, L. The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat. Ecol. 2003, 37, 377–391. [Google Scholar] [CrossRef]
- Liboriussen, L.; Jeppesen, E.; Bramm, M.E.; Lassen, M.F. Periphyton-macroinvertebrate interactions in light and fish manipulated enclosures in a clear and a turbid shallow lake. Aquat. Ecol. 2005, 39, 23–39. [Google Scholar] [CrossRef]
- Wilhelm, F.M.; Schindler, D.W. Effects of gammarus lacustris (crustacea: Amphipoda) on plankton community structure in an alpine lake. Can. J. Fish Aquat. Sci. 1999, 56, 1401–1408. [Google Scholar] [CrossRef]
- Paggi, J.C.; de Paggi, S.J. Primeros estudios sobre el zooplancton de las aguas lóticas del paraná medio. Physis 1974, 33, 94–114. [Google Scholar]
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The dry weight estimate of biomass in a selection of cladocera, copepoda and rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 1975, 19, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Søndergaard, M.; Kanstrup, E.; Petersen, B.; Henriksen, R.B.; Hammershøj, M.; Mortensen, E.; Jensen, J.P.; Have, A. Does the impact of nutrients on the biological structure and function of brackish and freshwater lakes differ? Hydrobiologia 1994, 275–276, 15–30. [Google Scholar] [CrossRef]
- Jespersen, A.M.; Christoffersen, K. Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Arch. Hydrobiol. 1987, 109, 445–454. [Google Scholar]
- Merrit, W.; Cummings, K. An Introduction to the Aquatic Insects of North America; Kendal/Hunt: Dubuque, Iowa, 1984. [Google Scholar]
- Fontanarrosa, M.; Chaparro, G.; de Tezanos Pinto, P.; Rodriguez, P.; O’Farrell, I. Zooplankton response to shading effects of free-floating plants in shallow warm temperate lakes: A field mesocosm experiment. Hydrobiologia 2010, 646, 231–242. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Goyenola, G.; Fosalba, C.; Teixeira-de Mello, F.; Garcia, S.; Jeppesen, E. Field and experimental evidence of the effect of jenynsia multidentata, a small omnivorous-planktivorous fish, on the size distribution of zooplankton in subtropical lakes. Freshw. Biol. 2008, 53, 1797–1807. [Google Scholar] [CrossRef]
- Mazzeo, N.; Iglesias, C.; Teixeira-de Mello, F.; Borthagaray, A.; Fosalba, C.; Ballabio, R.; Larrea, D.; Vilches, J.; García, S.; Pacheco, J.; et al. Trophic cascade effects of Hoplias malabaricus (Characiformes, Erythrinidae) in subtropical lakes food webs: A mesocosm approach. Hydrobiologia 2010, 644, 325–335. [Google Scholar] [CrossRef]
- Nagdali, S.S.; Gupta, P.K. Impact of mass mortality of a mosquito fish, gambussia affinis, on the ecology of a freshwater eutrophic lake (Lake Naini Tal, India). Hydrobiologia 2002, 468, 45–52. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F. The Trophic Cascade in Lakes; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Gyllström, M.; Hansson, L.A.; Jeppesen, E.; García-Criado, F.; Gross, E.; Irvine, K.; Kairesalo, T.; Kornijow, R.; Miracle, M.R.; Nykänen, M.; et al. The role of climate in shaping zooplankton communities of shallow lakes. Limnol. Oceanogr. 2005, 50, 2008–2021. [Google Scholar] [CrossRef]
- Moss, B.; Stephen, D.; Balayla, D.M.; Bécares, E.; Collings, S.E.; Fernández-Aláez, C.; Fernández-Aláez, M.; Ferriol, C.; García, P.; Gomá, J.; et al. Continental-scale patterns of nutrient and fish effects on shallow lakes: Synthesis of a pan-european mesocosm experiment. Freshw. Biol. 2004, 49, 1633–1649. [Google Scholar] [CrossRef]
- Vakkilainen, K.; Kairesalo, T.; Hietala, J.; Balayla, D.M.; Becares, E.; Van de Bund, W.J.; Van Donk, E.; Fernández-Aláez, M.; Gyllström, M.; Hansson, L.A. Response of zooplankton to nutrient enrichment and fish in shallow lakes: A pan-european mesocosm experiment. Freshw. Biol. 2004, 49, 1619–1632. [Google Scholar] [CrossRef]
- Hayden, B.; Myllykangas, J.P.; Rolls, R.J.; Kahilainen, K.K. Climate and productivity shape fish and invertebrate community structure in subarctic lakes. Freshw. Biol. 2017, 62, 990–1003. [Google Scholar] [CrossRef]
- Skoglund, S.; Knudsen, R.; Amundsen, P. Selective predation on zooplankton by pelagic arctic charr, salvelinus alpinus, in six subarctic lakes. J. Ichthyol. 2013, 53, 849–855. [Google Scholar] [CrossRef]
- Schriver, P.; Bøgestrand, J.; Jeppesen, E.; Søndergaard, M. Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: Large-scale enclosure experiments in a shallow eutrophic lake. Freshw. Biol. 1995, 33, 255–270. [Google Scholar]
- Ekvall, M.K.; Urrutia-Cordero, P.; Hansson, L.-A. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS ONE 2014, 9, e112956. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Lauridsen, T.L.; Kairesalo, T.; Perrow, M. Impact of submerged macrophytes on fish-zooplankton interactions in lakes. In The Structuring Role of Submerged Macrophytes in Lakes; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Springer: New York, NY, USA, 1997; Volume 131, pp. 91–114. [Google Scholar]
- Gelós, M.; Teixeira-de Mello, F.; Goyenola, G.; Iglesias, C.; Fosalba, C.; García-Rodríguez, F.; Pacheco, J.; García, S.; Meerhoff, M. Seasonal and diel changes in fish activity and potential cascading effects in subtropical shallow lakes with different water transparency. Hydrobiologia 2010, 646, 173–185. [Google Scholar] [CrossRef]
- Pacheco, J.; Iglesias, C.; Meerhoff, M.; Fosalba, C.; Goyenola, G.; Teixeira-de Mello, F.; García, S.; Gelós, M.; García-Rodríguez, F. Phytoplankton community structure in five subtropical shallow lakes with different trophic status (Uruguay): A morphology-based approach. Hydrobiologia 2010, 646, 187–197. [Google Scholar] [CrossRef]
- Persson, L.; Anderson, G.; Hamrin, S.F.; Johansson, L. Predation regulation and primary production a long the productivity gradient of temperate lake ecosystem. In Complex Interactions in Lake Communities; Carpenter, S.E., Ed.; Springer: New York, NY, USA, 1988; pp. 45–65. [Google Scholar]
- Winemiller, K.O. Spatial and temporal variation in tropical fish trophic networks. Ecol. Monogr. 1990, 60, 331–367. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Meerhoff, M.; Davidson, T.; Teixeira-de Mello, F.; Baattrup-Pedersen, A.; Jeppesen, E. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems 2012, 15, 492–503. [Google Scholar] [CrossRef]
- Weetman, D.; Atkinson, D. Evaluation of alternative hypotheses to explain temperature-induced life history shifts in daphnia. J. Plankton Res. 2004, 26, 107–116. [Google Scholar] [CrossRef]
- Collins, P.A.; Paggi, J.C. Feeding ecology of Macrobrachium borellii (Nobili) (Decapoda: Palaemonidae) in the flood valley of the river paraná, argentina. Hydrobiologia 1998, 362, 21–30. [Google Scholar] [CrossRef]
- Felten, V.; Tixier, G.; Guerold, F.; De Billy, V.D.C.; Dangles, O. Quantification of diet variability in a stream amphipod: Implications for ecosystem functioning. Fundam. Appl. Limnol. 2008, 170, 303–313. [Google Scholar] [CrossRef]
- Hellmann, C.; Worischka, S.; Mehler, E.; Becker, J.; Gergs, R.; Winkelmann, C. The trophic function of Dikerogammarus villosus (Sowinsky, 1894) in invaded rivers: A case study in the Elbe and Rhine. Aquat. Invasions 2015, 10, 385–397. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Vander Zanden, M.J.; Lodge, D.M. Putting the lake back together: Reintegrating benthic pathways into lake food web models. Bioscience 2002, 52, 44–54. [Google Scholar] [CrossRef]
- Diehl, S.; Kornijów, R. Influence of submerged macrophytes on trophic interactions among fish and macroinvertebrates. In The Structuring Role of Submerged Macrophytes in Lakes; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Springer: New York, NY, USA, 1997; Volume 131, pp. 24–46. [Google Scholar]
- Arcifa, M.S. Feeding habits of chaoboridae larvae in a tropical brazilian reservoir. Rev. Bras. Biol. 2000, 60, 591–597. [Google Scholar] [CrossRef]
- Castilho-Noll, M.S.M.; Arcifa, M.S. Mesocosm experiment on the impact of invertebrate predation on zooplankton of a tropical lake. Aquat. Ecol. 2007, 41, 587–598. [Google Scholar] [CrossRef]
- Bécares, E.; Gomá, J.; Fernández-Aláez, M.; Fernández-Aláez, C.; Romo, S.; Miracle, M.R.; Ståhl-Delbanco, A.; Hansson, L.-A.; Gyllström, M.; Van de Bund, W.J. Effects of nutrients and fish on periphyton and plant biomass across a european latitudinal gradient. Aquat. Ecol. 2008, 42, 561–574. [Google Scholar] [CrossRef]
- Hansson, L. Factors regulating periphytic algal biomass. Limnol. Oceanogr. 1992, 37, 322–328. [Google Scholar] [CrossRef]
- Brönmark, C.; Vermaat, J.E. Complex fish-snail-epiphyton interactions and their effects on submerged freshwater macrophytes. In The Structuring Role of Submerged Macrophytes in Lakes; Jeppesen, E., Søndergaard, M., Søndergaard, M., Christoffersen, K., Eds.; Springer: New York, NY, USA, 1997; pp. 47–68. [Google Scholar]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.; De Meester, L.; Søndergaard, M.; Lauridsen, T.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Jeppesen, E. The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: A microcosm approach. Hydrobiologia 2014, 738, 49–59. [Google Scholar] [CrossRef]
- Mahdy, A.; Hilt, S.; Filiz, N.; Beklioğlu, M.; Hejzlar, J.; Özkundakci, D.; Papastergiadou, E.; Scharfenberger, U.; Šorf, M.; Stefanidis, K.; et al. Effects of water temperature on summer periphyton biomass in shallow lakes: A pan-european mesocosm experiment. Aquat. Sci. 2015, 77, 499–510. [Google Scholar] [CrossRef]
- Hart, D. Intraguild predation, invertebrate predators, and trophic cascades in lake food webs. J. Theor. Biol. 2002, 218, 111–128. [Google Scholar] [CrossRef] [PubMed]

| Diario | Blanca | Nutrias | Kogleaks | Stigsholm | Bølling | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| start | end | start | end | Start | end | start | end | start | end | start | End | |
| Temperature (oC) | 28.0 | 20.5 | 22.5 | 21.4 | 27.0 | 20.2 | 16.6 | 8.3 | 15.1 | 10.4 | 14.7 | 13.9 |
| DO2 (mg L−1) | 9.7 | 7.0 | 6.7 | 10.4 | 7.4 | 8.4 | 5.7 | 9.1 | 10.5 | 9.3 | 8.7 | 7.6 |
| pH | 8.6 | 7.5 | 8.4 | 7.61 | 6.14 | 5.8 | 6.9 | 7.5 | 8.4 | 7.5 | 7.1 | 7.2 |
| Conductivity (mS cm−1) | 566 | 617 | 318 | 316 | 75 | 82 | 595 | 542 | 210 | 185 | 116 | 116 |
| Turbidity (NTU) | 6.3 | 20 | 14.2 | 18.2 | 26.3 | 39.7 | 12.4 | 2.1 | 3.6 | 3.9 | 14.7 | 17.3 |
| Phyto. Chl-a (µg L−1) | 15.7 | 9.6 | 46.5 | 56.1 | 15.7 | 4.7 | 8.9 | 4.7 | 11.4 | 8.2 | 8.4 | 10.7 |
| Distinctive traits | Slightly brackish | Cyanobacteria blooms | Humic | Slightly brackish | Cyanobacteria blooms | Humic | ||||||
| PVI (%) | >75% | <25% | 0% | ca. 50% | ca. 50% | 0% | ||||||
| TN (µg L−1) | 970 | 1391.5 | 670 | 2330 | 2275 | 1600 | ||||||
| TP (µg L−1) | 89.2 | 65.9 | 122.5 | 214.2 | 55 | 182.8 | ||||||
| Fish Species 1 | Fish Species 2 | Macroinvertebrates | ||
|---|---|---|---|---|
| Subtropical | Name | Cnesterodon decemmaculatus | Jenynsia multidentata | Palaemonetes argentinus |
| Density | 50 (42) * | 40 (33) * | 120 (100) * | |
| Biomass | 26.5 | 80.8 | 56 | |
| Temperate | Name | Gasterosteus aculeatus | Perca fluviatilis | Gammarus lacustris |
| Density | 12 (10) + | 6 (5) * | 240 (200) ¤ | |
| Biomass | 29.2 | 81.8 | 12 |
| Diario | Blanca | Nutrias | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To | CON | F | INV | F + INV | To | CON | F | INV | F + INV | To | CON | F | INV | F + INV | |
| Predator | 0,01 (0,0) | 0,0 (-) | 0,0 (-) | 0,04 (0,1) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) |
| Gatherer | 14,8 (5,8) | 90,5 (41,4) | 1,3 (0,4) | 2,9 (0,7) | 3,2 (0,1) | 17,2 (6,3) | 19,3 (9,2) | 10,1 (2,0) | 21,2 (12,5) | 2,3 (1,0) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,6 (0,2) | 0,0 (-) |
| Shredder | 1,9 (1) | 0,0 (-) | 0,0 (-) | 0,23 (0,3) | 0,0 (-) | 0,8 (0,5) | 0,0 (-) | 3,8 (0,6) | 0,0 (-) | 0,0 (-) | 0,1 (0,0) | 0,0 (-) | 0,0 (-) | 0,4 (0,1) | 0,2 (0,1) |
| Filterer | 0,09 (0,09) | 0,03 (0,01) | 0,04 (0,02) | 0,0 (-) | 0,0 (-) | 1,9 (1,9) | 0,06 (0,06) | 0,0 (-) | 0,05 (0,03) | 0,0 (-) | 0,0 (-) | 0,01 (0,0) | 0,0 (-) | 0,0 (-) | 0,0 (-) |
| Scrapers | 0,14 (0,14) | 0,0 (-) | 0,0 (-) | 37,8 (37,8) | 40,9 (40,9) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) |
| Piercer | 0,02 (0,02) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) |
| Total Biomass | 15,3 (5,8) | 90,5(41,4) | 1,3 (0,4) | 40,7 (37,8) | 44,0 (40,9) | 19,1 (6,2) | 19,4 (9,1) | 10,5 (2,1) | 21,2 (12,5) | 22,3 (0,9) | 0,01 (0,0) | 0,01 (0,0) | 0,0 (-) | 0,7 (0,2) | 0,02 (0,01) |
| Stigsholm | Kogleaks | Bøllingso | |||||||||||||
| To | CON | F | INV | F + INV | To | CON | F | INV | F + INV | To | CON | F | INV | F + INV | |
| Predator | 0,3 (0,2) | 0,6 (0,6) | 0,1 (0,02) | 0,5 (0,2) | 0,09 (0,09) | 0,01 (0,0) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,02 (0,01) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,02 (0,02) |
| Gatherer | 1,5 (0,9) | 3,4 (0,3) | 0,02 (0,0) | 0,8 (0,3) | 0,1 (0,08) | 6,4 (2,3) | 36,7 (19,4) | 0,9 (1,7) | 0,01 (0,01) | 0,4 (0,2) | 4,5 (1,4) | 12,8 (8,2) | 4,2 (1,4) | 6,2 (2,5) | 2,9 (1,6) |
| Shredder | 3,7 (2,7) | 7,6 (6,0) | 1,4 (0,8) | 4,9 (3,95) | 0,01 (0,01) | 0,02 (0,01) | 0,05 (0,0) | 0,01 (0,01) | 0,0 (-) | 0,01 (0,01) | 1,0 (0,9) | 0,3 (0,3) | 8,4 (8,4) | 12,5 (12,5) | 0,02 (0,01) |
| Filterer | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 2,2 (2,2) | 0,0 (-) | 0,0 (-) | 0,0(-) | 0,0 (-) | 0,0 (-) | 0,5 (0,2) | 0,0 (-) | 2,1 (2,1) | 2,2 (2,2) | 0,8 (0,8) |
| Scrapers | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) |
| Piercer | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) | 0,0 (-) |
| Total Biomass | 5,5 (3,7) | 11,6 (9,3) | 1,6 (0,8) | 6,2 (3,3) | 0,4 (0,1) | 6,4 (2,3) | 36,7 (1,9) | 0,9(1,7) | 0,01 (0,0) | 0,4 (0,2) | 6,1 (1,2) | 13,1 (8,2) | 12,8 (9,8) | 18,9 (1,3) | 3,0 (1,6) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, C.; Jeppesen, E.; Mazzeo, N.; Pacheco, J.P.; Mello, F.T.-d.; Landkildehus, F.; Fosalba, C.; Clemente, J.M.; Meerhoff, M. Fish but Not Macroinvertebrates Promote Trophic Cascading Effects in High Density Submersed Plant Experimental Lake Food Webs in Two Contrasting Climate Regions. Water 2017, 9, 514. https://doi.org/10.3390/w9070514
Iglesias C, Jeppesen E, Mazzeo N, Pacheco JP, Mello FT-d, Landkildehus F, Fosalba C, Clemente JM, Meerhoff M. Fish but Not Macroinvertebrates Promote Trophic Cascading Effects in High Density Submersed Plant Experimental Lake Food Webs in Two Contrasting Climate Regions. Water. 2017; 9(7):514. https://doi.org/10.3390/w9070514
Chicago/Turabian StyleIglesias, Carlos, Erik Jeppesen, Néstor Mazzeo, Juan Pablo Pacheco, Franco Teixeira-de Mello, Frank Landkildehus, Claudia Fosalba, Juan M. Clemente, and Mariana Meerhoff. 2017. "Fish but Not Macroinvertebrates Promote Trophic Cascading Effects in High Density Submersed Plant Experimental Lake Food Webs in Two Contrasting Climate Regions" Water 9, no. 7: 514. https://doi.org/10.3390/w9070514






