Food Web Responses to Artificial Mixing in a Small Boreal Lake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lake Characteristics and Experimental Design
2.2. General Aspects of Sampling
2.3. Chlorophyll Analyses
2.4. Primary Production
2.5. Zooplankton
2.6. Benthic Macroinvertebrates
2.7. Fish
2.8. Statistical Analyses
2.9. Supplementary Materials
3. Results
3.1. Photosynthetic Bacteria
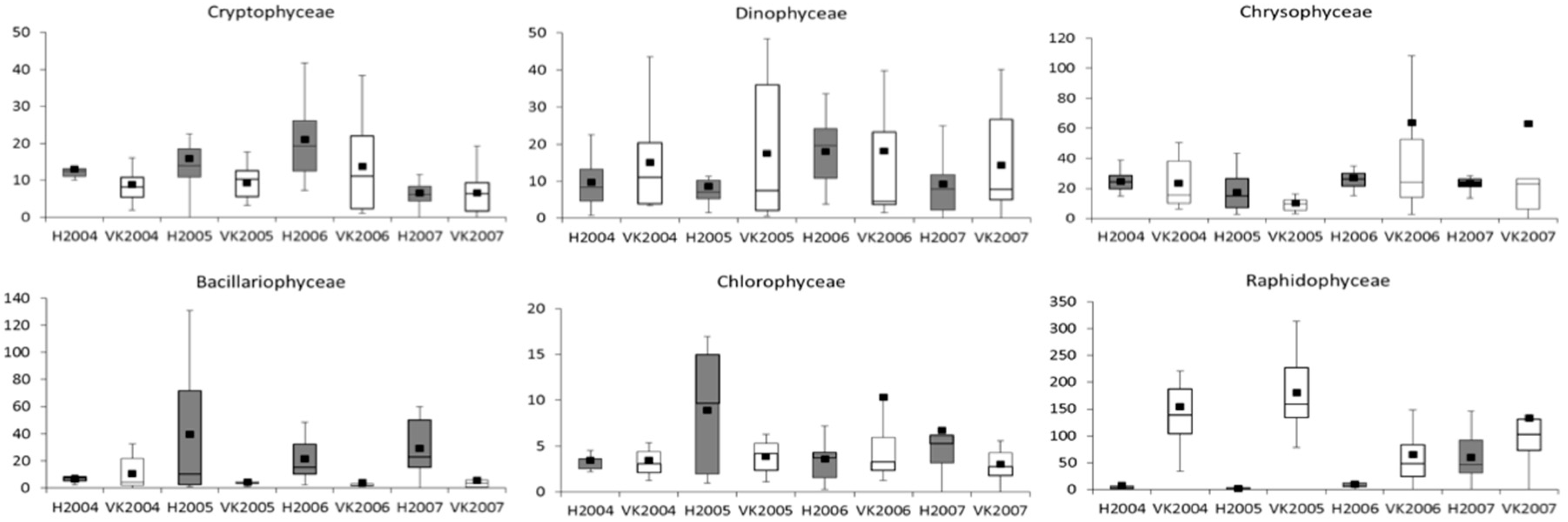
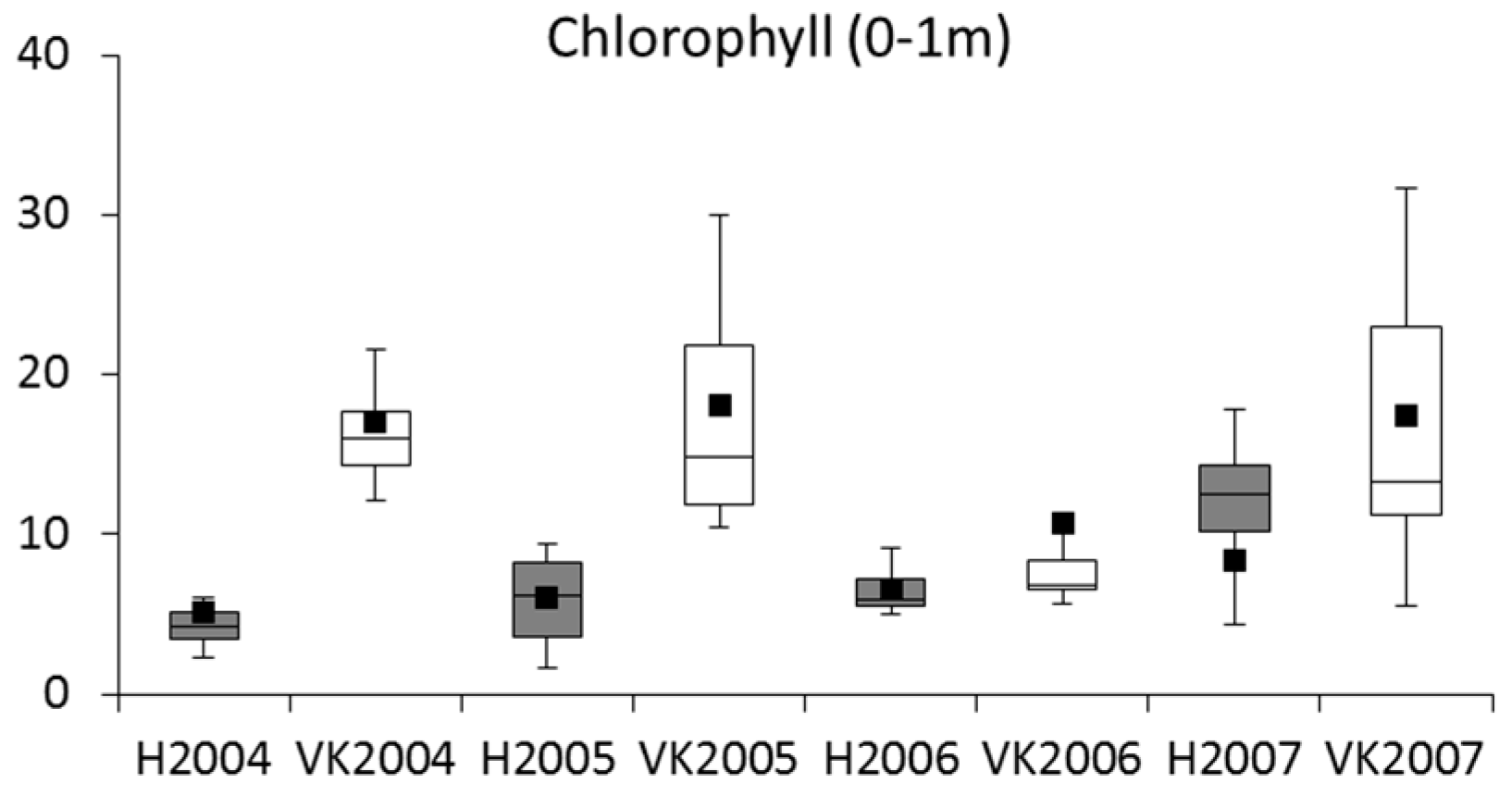
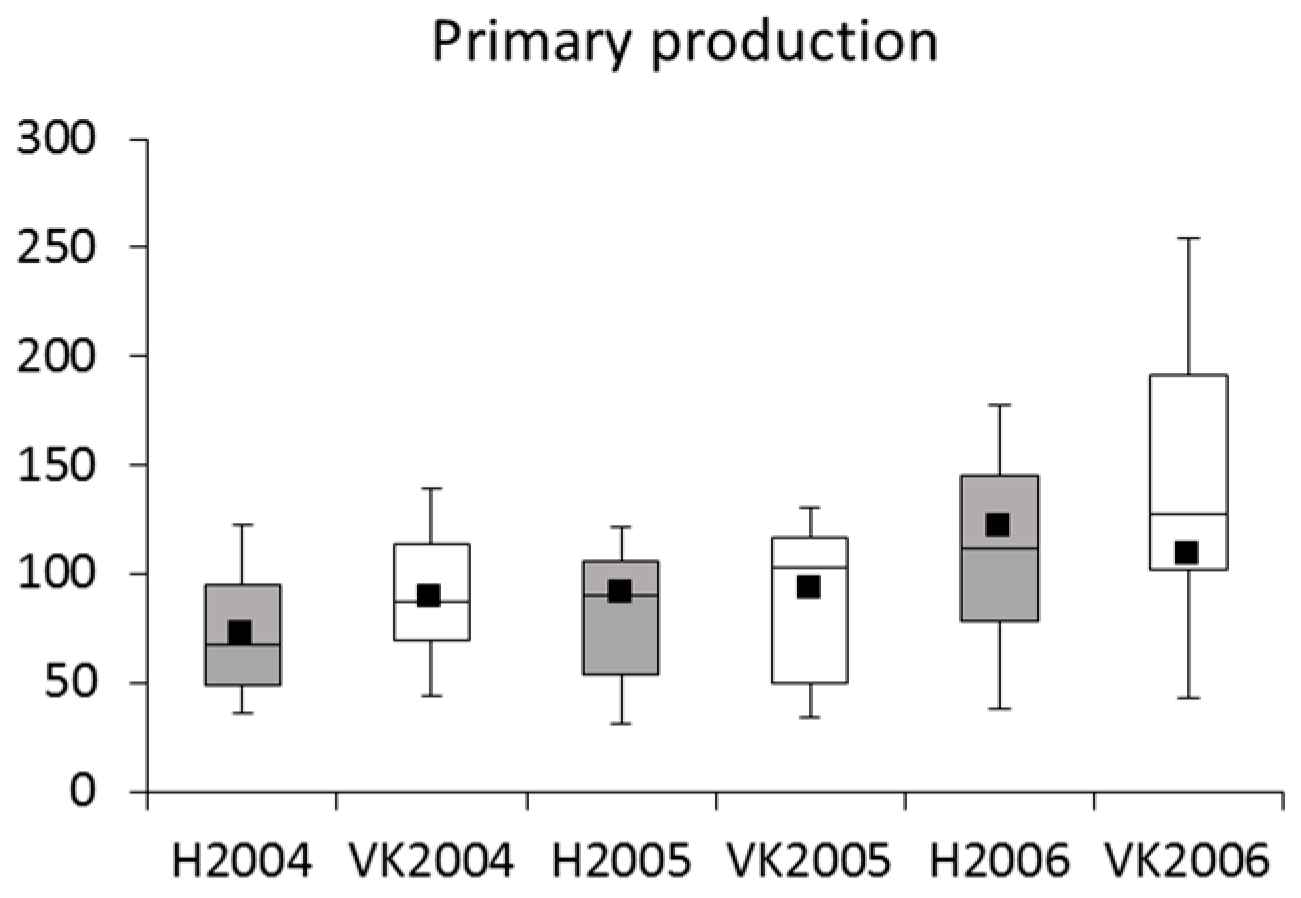
3.2. Phytoplankton and Metabolic Processes
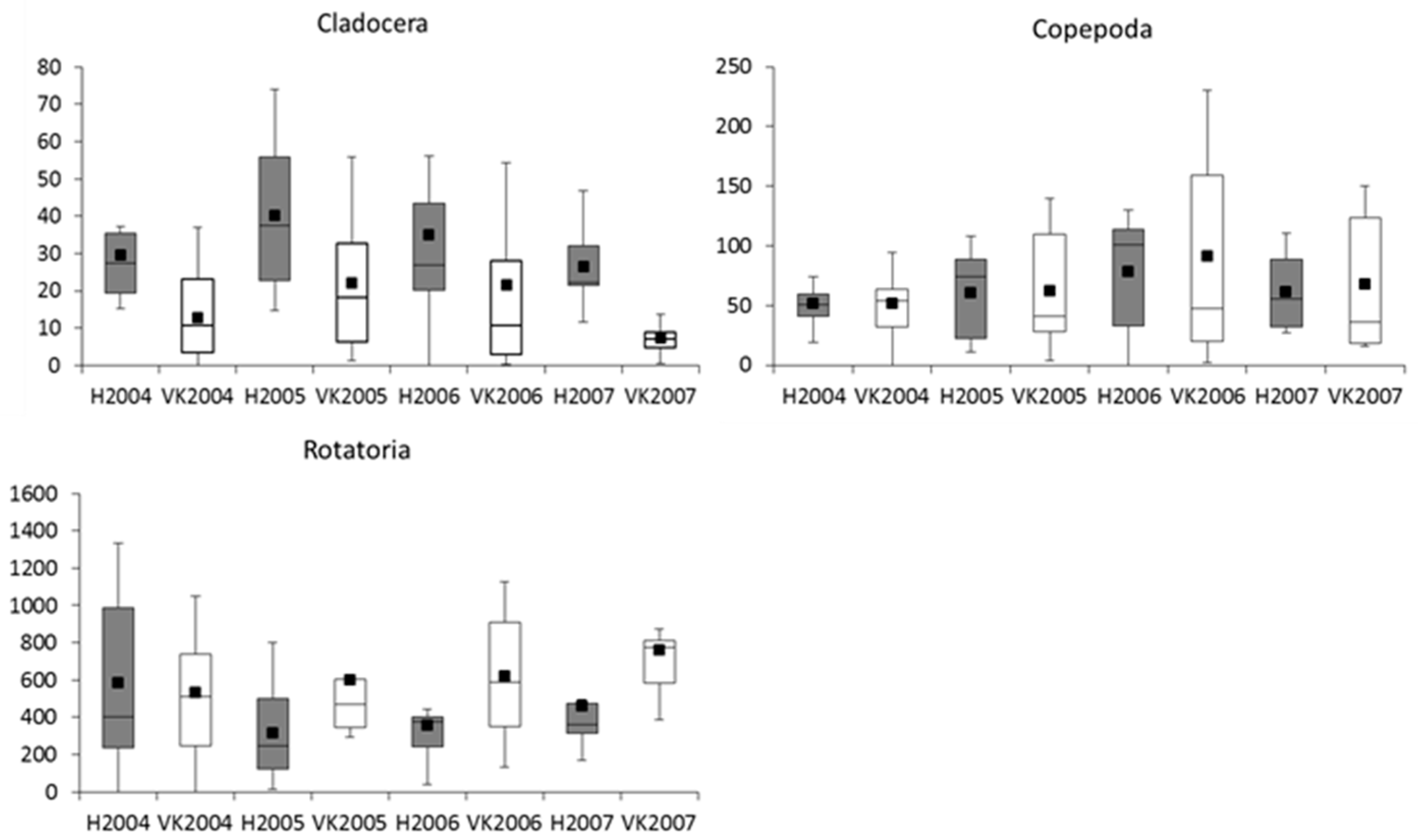
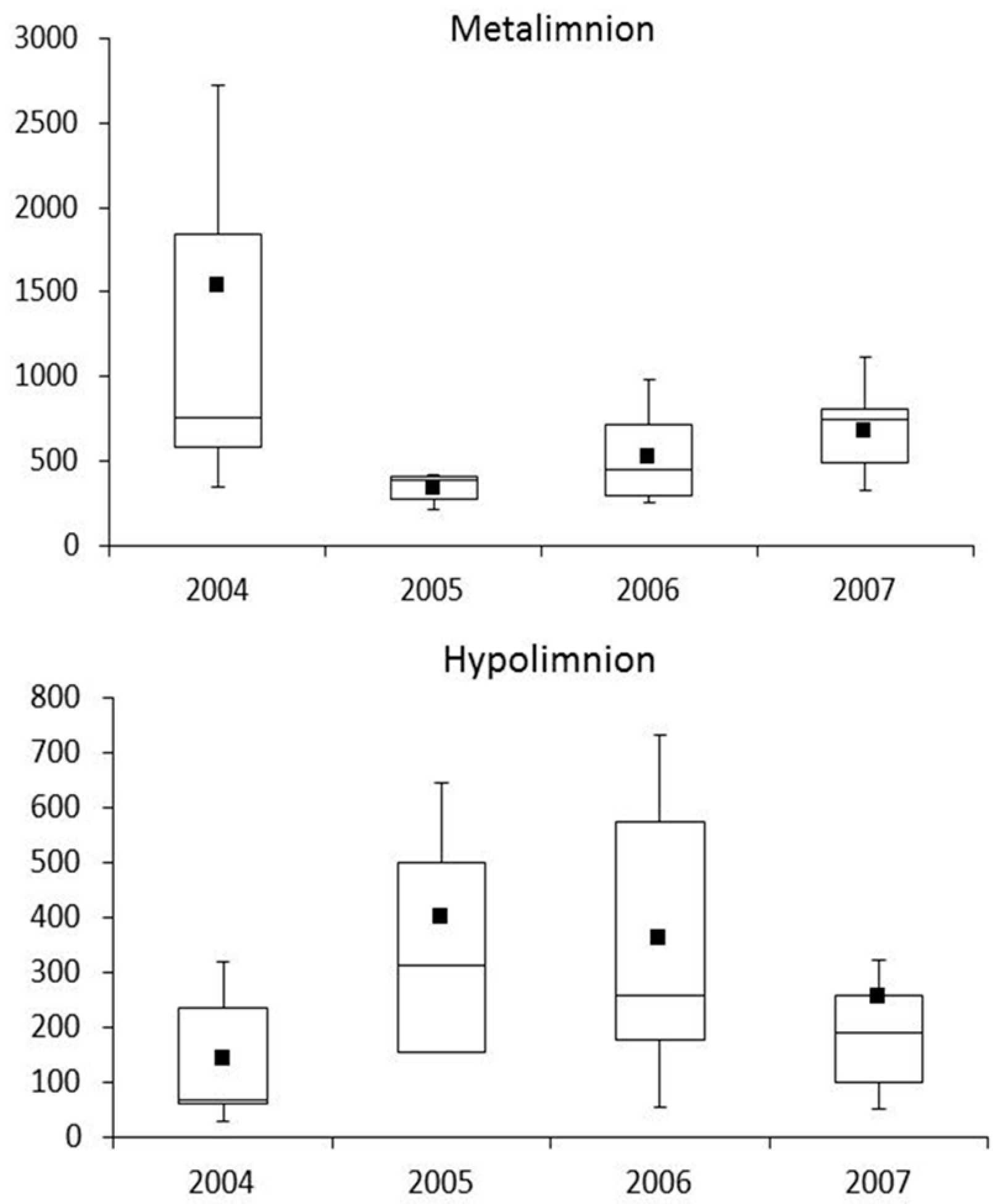
3.3. Zooplankton
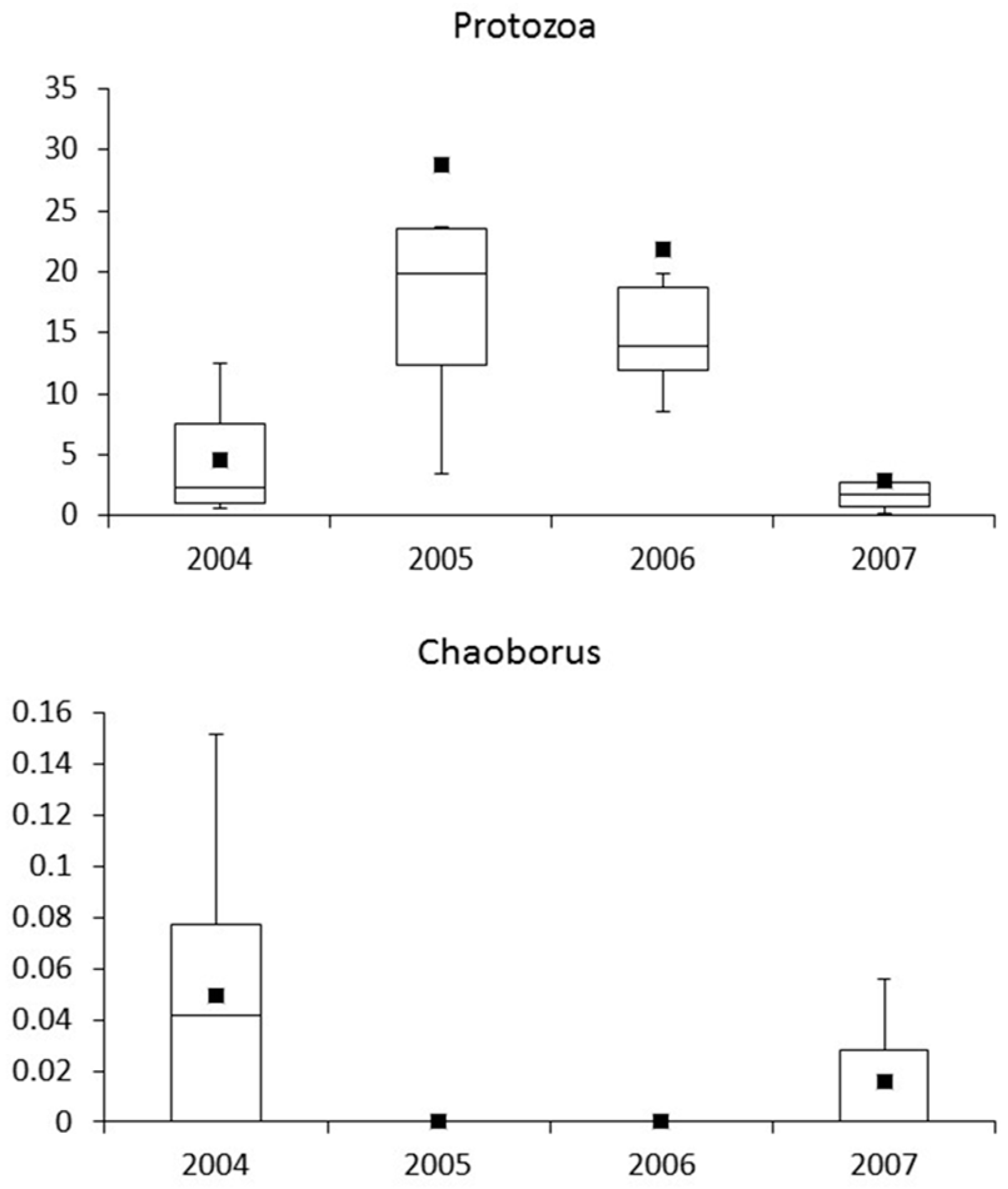
3.4. Macroinvertebrates
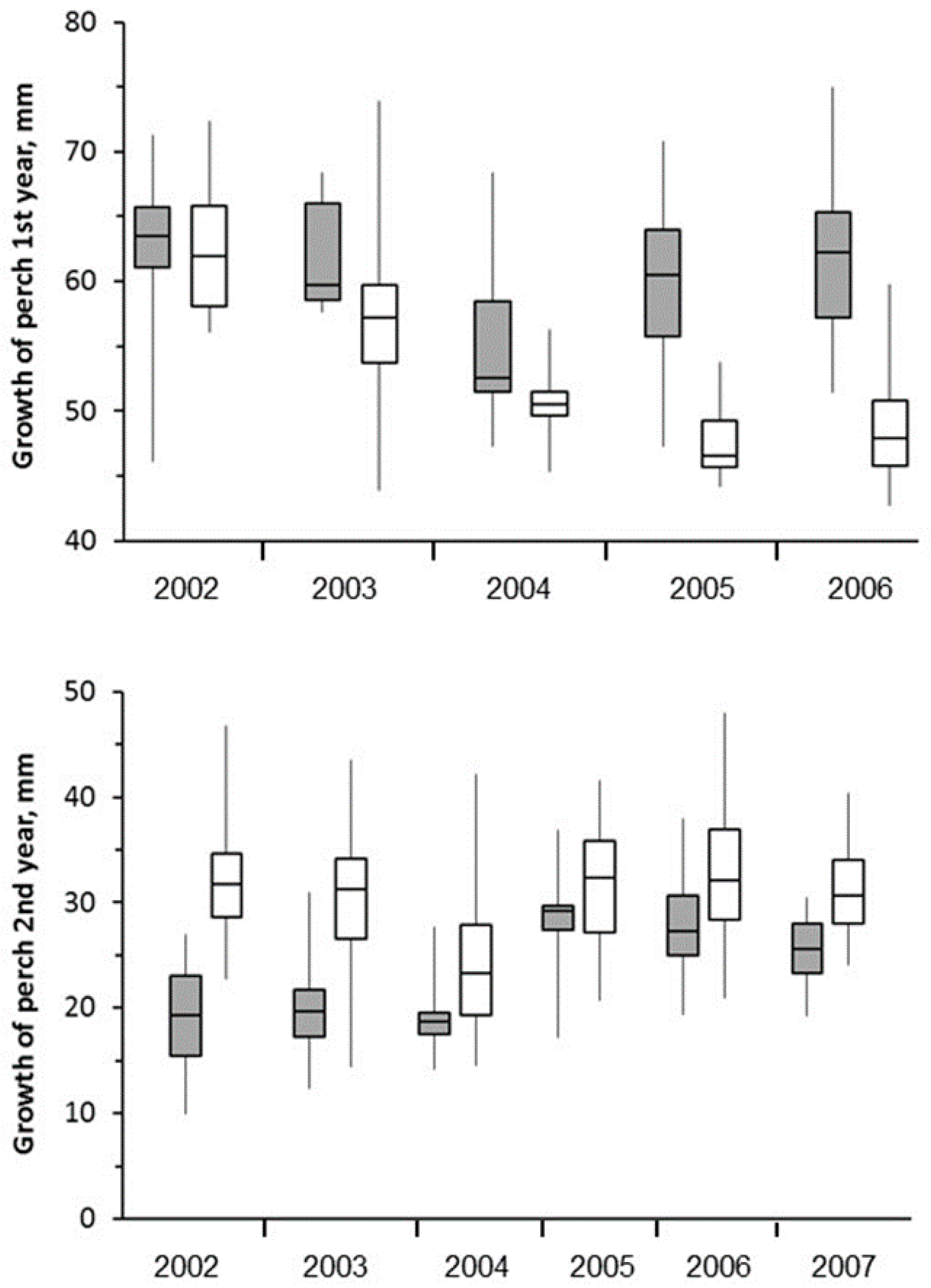
3.5. Fish
4. Discussion
4.1. Responses of GSB
4.2. Phytoplankton and Metabolic Processes
4.3. Zooplankton
4.4. Macroinvertebrates
4.5. Fish
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edinger, J.E.; Duttweiler, D.W.; Geyer, J.C. The response of water temperatures to meteorological conditions. Water Res. Res. 1968, 4, 137–1143. [Google Scholar] [CrossRef]
- Sweers, H.E. A nomogram to estimate the heat-exchange at the air-water interface as a function of wind speed and temperature; A critical survey of some literature. J. Hydrol. 1976, 30, 375–401. [Google Scholar] [CrossRef]
- Fee, E.J.; Hecky, R.E.; Kasian, S.E.M.; Cruikshank, D.R. Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnol. Oceanogr. 1996, 41, 912–920. [Google Scholar] [CrossRef]
- Arvola, L.; Rask, M.; Ruuhijärvi, J.; Tulonen, T.; Vuorenmaa, J.; Ruoho-Airola, T.; Tulonen, J. Long-term patterns in pH and colour in small acidic boreal lakes of varying hydrological and landscape settings. Biogeochemistry 2010, 101, 269–279. [Google Scholar] [CrossRef]
- Jones, R.I.; Arvola, L. Light penetration and some related characteristics in small forest lakes in southern Finland. Verh. Internat. Verein. Limnol. 1984, 22, 811–816. [Google Scholar]
- Salonen, K.; Arvola, L.; Rask, M. Autumnal and vernal circulation of small forest lakes in southern Finland. Verh. Internat. Verein. Limnol. 1984, 22, 103–107. [Google Scholar]
- Järvinen, M.; Rask, M.; Ruuhijärvi, J.; Arvola, L. Temporal coherence in water temperature and chemistry under the ice of boreal lakes (Finland). Water Res. 2002, 36, 3949–3956. [Google Scholar] [CrossRef]
- Downing, J.A.; Prairie, Y.T.; Cole, J.J.; Duarte, C.M.; Tranvik, L.J.; Striegl, R.G.; McDowell, W.H.; Kortelainen, P.; Caraco, N.F.; Melack, J.M.; et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 2006, 51, 2388–2397. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- O'Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; McIntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 10773–10781. [Google Scholar] [CrossRef]
- Schindler, D.W.; Beatty, K.G.; Fee, E.J.; Cruikshank, D.R.; DeBruyn, E.R.; Findlay, D.L.; Linsey, G.A.; Shearer, J.A.; Stainton, M.P.; Turner, M.A. Effects of climatic warming on lakes of the central boreal forest. Science 1990, 250, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Bayley, S.E.; Parker, B.R.; Beaty, K.G.; Cruikshank, D.R.; Fee, E.J.; Schindler, E.U.; Stainton, M.P. The effects of climatic warming on the properties of boreal lakes and streams at the Experimental Lakes Area, northwestern Canada. Limnol. Oceanogr. 1996, 41, 1004–1017. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Blenckner, T.; Pettersson, K. Changes of the plankton spring outburst related to the North Atlantic Oscillation. Limnol. Oceanogr. 1999, 44, 1788–1992. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Meili, M.; Livingstone, D.M. Nonlinear temperature response of lake ice breakup. Geophys. Res. Lett. 2004, 31, L07203. [Google Scholar] [CrossRef]
- Elliott, J.A.; Jones, I.D.; Thackeray, S.J. Testing the sensitivity of phytoplankton communities to changes in water temperature and nutrient load, in a temperate lake. Hydrobiologia 2006, 559, 401–411. [Google Scholar] [CrossRef]
- Kankaala, P.; Ojala, A.; Tulonen, T.; Haapamäki, J.; Arvola, L. Changes in water chemistry and macrophyte and algal communities in experimental pond simulating climate warming in the boreal area. Verh. Internat. Verein. Limnol. 1997, 26, 496–501. [Google Scholar]
- Lydersen, E.; Aanes, K.J.; Andersen, S.; Andersen, T.; Brettum, P.; Baekken, T.; Lien, L.; Lindstrøm, E.A.; Løvik, J.L.; Mjelde, M.; et al. Ecosystem effects of thermal manipulation of a whole lake, Lake Breisjøen, southern Norway [THERMOS] project. Hydrol. Earth Syst. Sci. Discuss. 2007, 4, 3357–3394. [Google Scholar] [CrossRef]
- Saloranta, T.M.; Forsius, M.; Järvinen, M.; Arvola, L. Impacts of projected climate change on thermodynamics of a shallow and deep lake in Finland: Model simulations and Bayesian uncertainty analysis. Hydrol. Res. 2009, 40, 234–248. [Google Scholar] [CrossRef]
- Gauthier, J.; Prairie, Y.T.; Beisner, B.E. Thermocline deepening and mixing alter zooplankton phenology, biomass and body size in a whole-lake experiment. Freshw. Biol. 2014, 59, 998–1011. [Google Scholar] [CrossRef]
- Räisänen, J.; Hansson, U.; Ullerstig, A.; Döscher, R.; Graham, L.P.; Jones, C.; Meier, H.E.M.; Samuelsson, P.; Willén, U. European climate in the late twenty-first century: Regional simulations with two driving global models and two forcing scenarios. Clim. Dyn. 2004, 22, 13–31. [Google Scholar] [CrossRef]
- Forsius, M.; Saloranta, T.; Arvola, L.; Salo, S.; Verta, M.; Ala-Opas, P.; Rask, M.; Vuorenmaa, J. Physical and chemical consequences of artificially deepened thermocline in a small humic lake—A paired whole-lake climate change experiment. Hydrol. Earth Syst. Sci. 2010, 14, 629–2642. [Google Scholar] [CrossRef]
- Rask, M.; Verta, M.; Korhonen, M.; Salo, S.; Forsius, M.; Arvola, L.; Jones, R.I.; Kiljunen, M. Does lake thermocline depth affect methyl mercury concentrations in fish? Biogeochemistry 2010, 101, 311–322. [Google Scholar] [CrossRef]
- Verta, M.; Salo, S.; Korhonen, M.; Porvari, P.; Paloheimo, A.; Munthe, J. Climate induced thermocline change has an effect on the methyl mercury cycle in small boreal lakes. Sci. Total Environ. 2010, 408, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, J.; Arvola, L.; Peura, S.; Tiirola, M. Green sulphur bacteria as a component of the photosynthetic plankton community in oxygen-stratified boreal humic lakes. Aquat. Microbial. Ecol. 2013, 68, 267–272. [Google Scholar] [CrossRef]
- Perron, T.J.; Chételat, J.; Gunn, J.; Beisner, B.E.; Amyot, M. Effect of experimental deepening of the thermocline and oxycline on methylmercury accumulation in a Canadian Shield Lake. Environ. Sci. Technol. 2014, 48, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
- Ouellet Jobin, V.; Beisner, B.E. Deep chlorophyll maxima, spatial overlap and diversity in phytoplankton exposed to experimentally altered thermal stratification. J. Plankton Res. 2014, 36, 933–942. [Google Scholar] [CrossRef]
- Sastri, A.; Gauthier, J.; Juneau, P.; Beisner, B.E. Biomass and productivity responses of zooplankton communities to experimental thermocline deepening. Limn. Oceanogr. 2014, 59, 1–16. [Google Scholar] [CrossRef]
- Arvola, L.; Salonen, K.; Keskitalo, J.; Tulonen, T.; Järvinen, M. Long-term trends in chlorophyll and metabolic processes of plankton and organic matter sedimentation in a small, pristine e boreal lake. Boreal Environ. Res. 2014, 19, 83–96. [Google Scholar]
- Lehtovaara, A.; Arvola, L.; Keskitalo, J.; Olin, M.; Rask, M.; Salonen, K.; Sarvala, J.; Tulonen, T.; Vuorenmaa, J. Responses of zooplankton to long-term environmental changes in a small boreal lake. Boreal Environ. Res. 2014, 19, 97–111. [Google Scholar]
- Rask, M.; Sairanen, S.; Vesala, S.; Arvola, L.; Estlander, S.; Olin, M. Population dynamics and growth of perch in a small, humic lake over a 20-year period—Importance of abiotic and biotic factors. Boreal Environ. Res. 2014, 112–123. [Google Scholar]
- Vuorenmaa, J.; Keskitalo, J.; Tulonen, T.; Salonen, K.; Arvola, L. Long-term trends in water chemistry of a small pristine boreal lake in the course of a dramatic decrease in sulphur deposition. Boreal Environ. Res. 2014, 19, 47–65. [Google Scholar]
- Arvola, L.; Metsälä, T.R.; Similä, A.; Rask, M. Phyto- and zooplankton in relation to water pH and humic content in small lakes in southern Finland. Verh. Internat. Verein. Limnol. 1990, 24, 688–692. [Google Scholar]
- Jylhä, K.; Laapas, M.; Ruosteenoja, K.; Arvola, L.; Drebs, A.; Kersalo, J.; Saku, S.; Gregow, H.; Hannula, H.-R.; Pirinen, P. Climate variability and trends in the Valkea-Kotinen region, southern Finland: Comparisons between the past, current and projected climate. Boreal Environ. Res. 2014, 19, 4–30. [Google Scholar]
- Ruoho-Airola, T.; Hatakka, T.; Kyllönen, K.; Makkonen, U.; Porvari, P. Temporal trends in the bulk deposition and atmospheric concentration of acidifying compounds and trace elements in the Finnish Integrated Monitoring catchment Valkea-Kotinen during 1988–2011. Boreal Environ. Res. 2014, 19, 31–46. [Google Scholar]
- Finnish Environment Institute. HERTTA Data Base. Available online: https://wwwp2.ymparisto.fi/scripts/hearts/welcome.asp (accessed on 10 July 2017).
- Von Utermöhl, H. Neue Wege in der quantitativen Erfassung des Planktons. [Mit besondere Beriicksichtigung des Ultraplanktons]. Verh. Int. Verein. Theor. Angew. Limnol. 1931, 5, 567–595. (In Germany) [Google Scholar]
- Salonen, K. Rapid and precise determination of total inorganic carbon and some gases in aqueous solutions. Water Res. 1981, 15, 403–406. [Google Scholar] [CrossRef]
- Keskitalo, J.; Salonen, K. Manual for Integrated Monitoring; Subprogramme Hydrobiology of Lakes; Publications of the Water and Environment Administration series B 16; National Board of Waters and the Environment: Helsinki, Finland, 1994; p. 41. [Google Scholar]
- CEN. Water Quality—Sampling of Fish with Multi-Mesh Gillnets; EN 14757; European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- Olin, M.; Rask, M.; Ruuhijärvi, J.; Tammi, J. Development and evaluation of the Finnish fish-based lake classification method. Hydrobiologia 2013, 713, 149–166. [Google Scholar] [CrossRef]
- Bagenal, T.B.; Tesch, F.W. Age and growth. In Fish Production of Fresh Waters; IBP-Handbook; Bagenal, T.B., Ed.; Blackwell Science Publishers: Oxford, UK, 1978; Volume 3, pp. 101–136. [Google Scholar]
- Raitaniemi, J.; Rask, M.; Vuorinen, P.J. The growth of perch, Perca fluviatilis L., in small Finnish lakes at different stages of acidification. Ann. Zool. Fennici 1988, 25, 209–219. [Google Scholar]
- Carpenter, S.R.; Frost, T.M.; Heisley, D.; Kratz, T.K. Random intervention analysis and the interpretation of whole-ecosystem experiments. Ecology 1989, 70, 1142–1152. [Google Scholar] [CrossRef]
- Bried, J.T.; Ervin, G.N. Randomized intervention analysis for detecting non-random change and management impact: Dragonfly examples. Ecol. Indic. 2011, 11, 535–539. [Google Scholar] [CrossRef]
- Salonen, K.; Kononen, K.; Arvola, L. Respiration of plankton in two small, polyhumic lakes. Hydrobiologia 1983, 101, 65–70. [Google Scholar] [CrossRef]
- Einola, E.; Rantakari, M.; Kankaala, P.; Kortelainen, P.; Ojala, A.; Pajunen, P.; Mäkelä, S.; Arvola, L. Carbon pools and fluxes in a chain of five boreal lakes: A dry and wet year comparison. J. Geophys. Res. 2011, 116, G03009. [Google Scholar] [CrossRef]
- Salonen, K.; Jokinen, S. Flagellate grazing on bacteria in a small dystrophic lake. Hydrobiologia 1988, 161, 203–209. [Google Scholar] [CrossRef]
- Salonen, K.; Lehtovaara, A. Migrations of haemoglobin-rich Daphnia longispina in a small, steeply stratified, humic lake with an anoxic hypolimnion. Hydrobiologia 1992, 161, 271–288. [Google Scholar] [CrossRef]
- Arvola, L.; Salonen, K.; Kankaala, P.; Lehtovaara, A. Vertical distributions of bacteria and algae in a steeply stratified humic lake under high grazing pressure from Daphnia longispina. Hydrobiologia 1992, 229, 253–269. [Google Scholar] [CrossRef]
- Arvola, L.; Eloranta, P.; Järvinen, M.; Keskitalo, J.; Holopainen, A.-L. Phytoplankton. In Limnology of Humic Waters; Eloranta, P., Keskitalo, J., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 137–171. [Google Scholar]
- Arvola, L.; Salonen, K.; Rask, M. Trophic interactions. In Limnology of Humic Waters; Eloranta, P., Keskitalo, J., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 265–279. [Google Scholar]
- Jones, R.I. Phytoplankton, primary production and nutrient cycling. In Aquatic Humic Substances, Ecology and Biogeochemistry; Ecological Studies 133; Hessen, D.O., Tranvik, L.J., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; pp. 145–194. [Google Scholar]
- Kankaala, P.; Taipale, S.; Grey, J.; Sonninen, E.; Arvola, L.; Jones, R. Experimental δ13C evidence for a contribution of methane to pelagic food webs in lakes. Limnol. Oceanogr. 2006, 51, 2821–2827. [Google Scholar] [CrossRef]
- Zingel, P.; Huitu, E.; Mäkelä, S.; Arvola, L. The abundance and diversity of planktonic ciliates in 12 boreal lakes of varying trophic state. Arch. Hydrobiol. 2002, 155, 315–332. [Google Scholar] [CrossRef]
- Devlin, S.P.; Saarenheimo, J.; Syväranta, J.; Jones, R.I. Top consumer abundance influences lake methane efflux. Nat. Commun. 2015, 6, 8787. [Google Scholar] [CrossRef] [PubMed]
- Arvola, L. Spring phytoplankton of 54 small lakes in southern Finland. Hydrobiologia 1986, 137, 125–134. [Google Scholar] [CrossRef]
- Ilmavirta, V.; Kotimaa, A.-L. Spatial and seasonal variations in phytoplanktonic primary production and biomass in the oligotrophic lake Pääjärvi, southern Finland. Ann. Bot. Fennici 1974, 11, 112–120. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; p. 545. [Google Scholar]
- Ramberg, L. Relations between phytoplankton and light climate in two Swedish forest lakes. Int. Rev. Ges Hydrobiol. 1979, 64, 749–782. [Google Scholar] [CrossRef]
- Salonen, K.; Arvola, L.; Rosenberg, M. Diel vertical migrations of phyto- and zooplankton in a small steeply stratified humic lake with low nutrient concentration. Verh. Internat. Verein. Limnol. 1993, 25, 539–543. [Google Scholar]
- Arvola, L. Primary production and phytoplankton production in two small, polyhumic forest lakes in southern Finland. Hydrobiologia 1983, 101, 105–110. [Google Scholar] [CrossRef]
- Taipale, S.J.; Vuorio, K.; Brett, M.T.; Peltomaa, E.; Hiltunen, M.; Kankaala, P. Lake zooplankton delta C-13 values are strongly correlated with the delta C-13 values of distinct phytoplankton taxa. Ecosphere 2016, 7, 01392. [Google Scholar] [CrossRef]
- Arvola, L.; Salonen, K. Plankton community of a polyhumic lake with and without Daphnia longispina [Cladocera]. Hydrobiologia 2001, 445, 141–150. [Google Scholar] [CrossRef]
- Jones, R.I.; Carter, C.E.; Kelly, A.; Ward, S.; Kelly, D.J.; Grey, J. Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 2008, 89, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Rask, M. The diet and diel feeding activity of perch, Perca fluviatilis L., in a small lake in southern Finland. Ann. Zool. Fennici 1986, 23, 49–56. [Google Scholar]
- Estlander, S.; Nurminen, L.; Olin, M.; Vinni, M.; Immonen, S.; Rask, M.; Ruuhijärvi, J.; Horppila, J.; Lehtonen, H. Diet shift and food selection of (Perca fluviatilis) and roach [Rutilus rutilus] in humic lakes of varying water colour. J. Fish Biol. 2010, 77, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Tonn, W.M.; Magnuson, J.J.; Rask, M.; Toivonen, J. Intercontinental comparison of small-lake fish assemblages: The balance between local and regional processes. Am. Nat. 1990, 136, 345–375. [Google Scholar] [CrossRef]
- Tammi, J.; Appelberg, M.; Hesthagen, T.; Beier, U.; Lappalainen, A.; Rask, M. Fish status survey of Nordic lakes: Effects of acidification, eutrophication and stocking activity on present fish species composition. Ambio 2003, 32, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E. Foraging abilities and niche breadths of two percids, Perca fluviatilis and Gymnocephalus cernua, under different environmental conditions. J. Anim. Ecol. 1988, 57, 443–453. [Google Scholar] [CrossRef]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P. Impacts of climate warming on lake fish assemblages: Evidence from 24 European long-term data series. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef]
- Bergman, E. Effects of roach Rutilus rutilus on two percids, Perca fluviatilis and Gymnocephalus cernua: Importance of species interactions for diet shifts. Oikos 1990, 57, 241–249. [Google Scholar] [CrossRef]
- Olin, M.; Rask, M.; Estlander, S.; Horppila, J.; Nurminen, L.; Tiainen, J.; Vinni, M.; Lehtonen, H. Roach (Rutilus rutilus) populations respond to varying environment by altering size structure and growth rate. Boreal Environ. Res. 2017, 22, 119–136. [Google Scholar]
- Rask, M.; Raitaniemi, J. The growth of perch, Perca fluviatilis L., in recently acidified lakes of southern Finland—A comparison with unaffected waters. Arch. Hydrobiol. 1988, 112, 387–397. [Google Scholar]
- Nyberg, K.; Vuorenmaa, J.; Tammi, J.; Nummi, P.; Väänänen, V.-M.; Mannio, J.; Rask, M. Re-establishment of perch in three lakes recovering from acidification: Rapid growth associated with abundant food resources. Boreal Environ. Res. 2010, 15, 480–490. [Google Scholar]
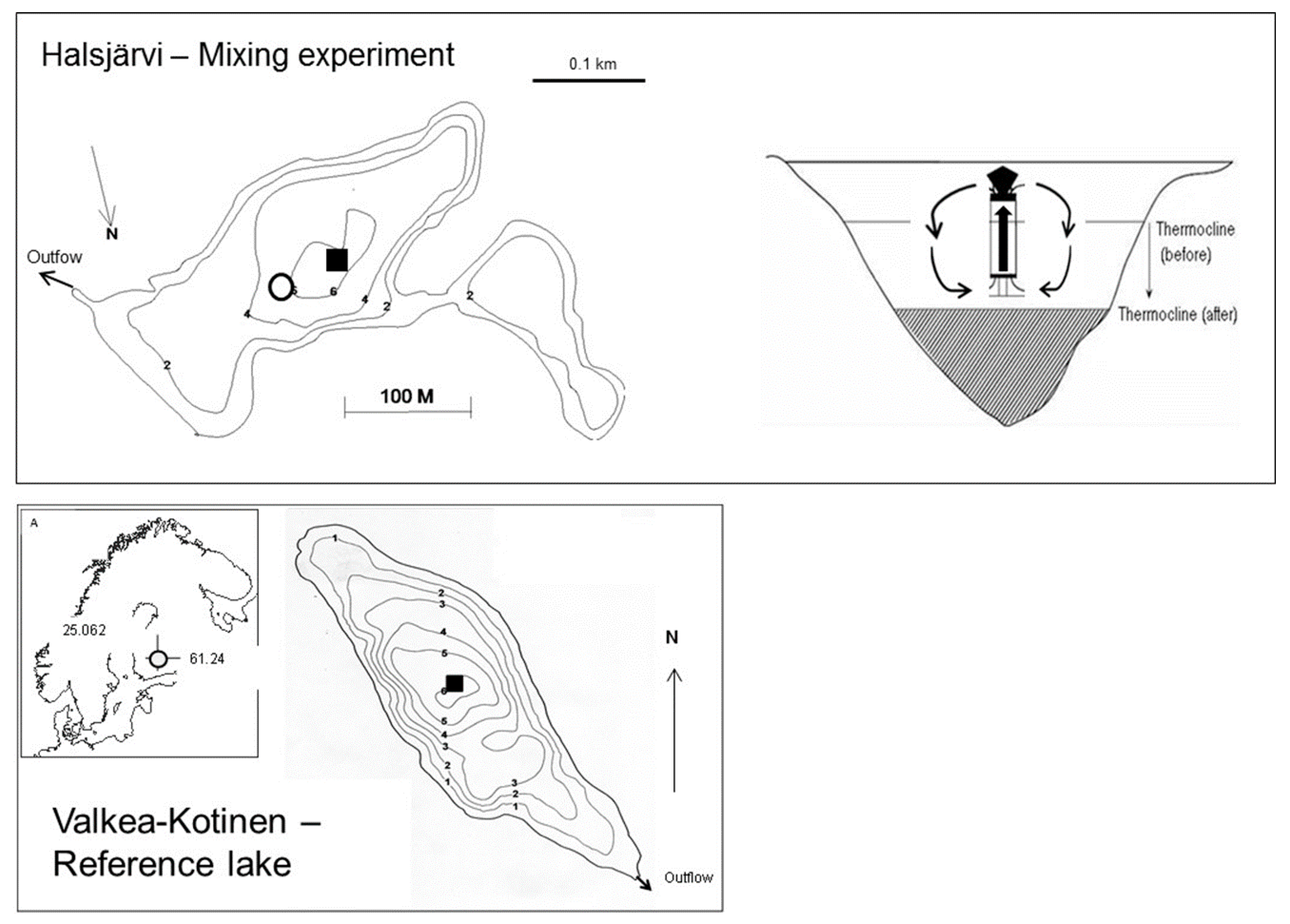
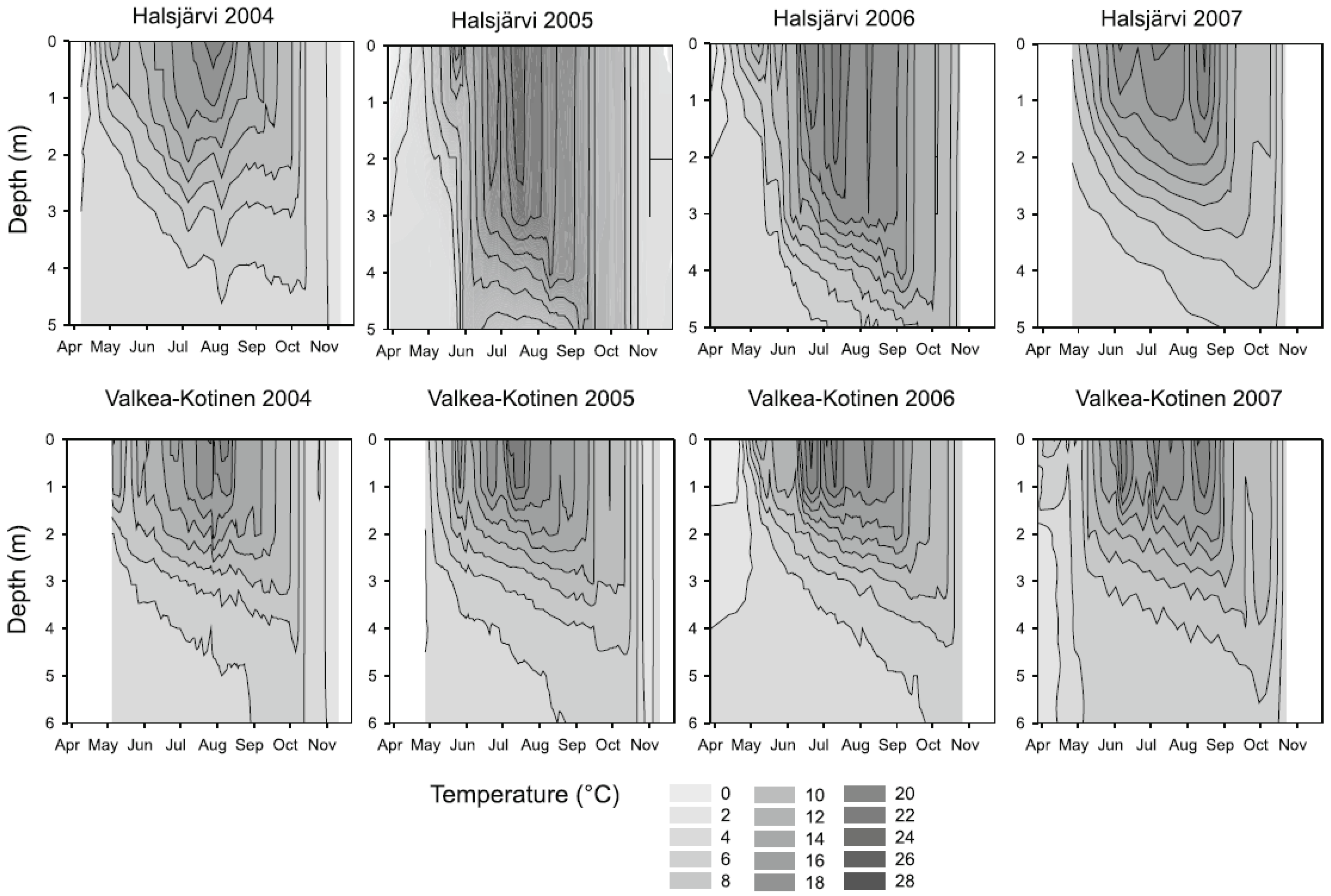
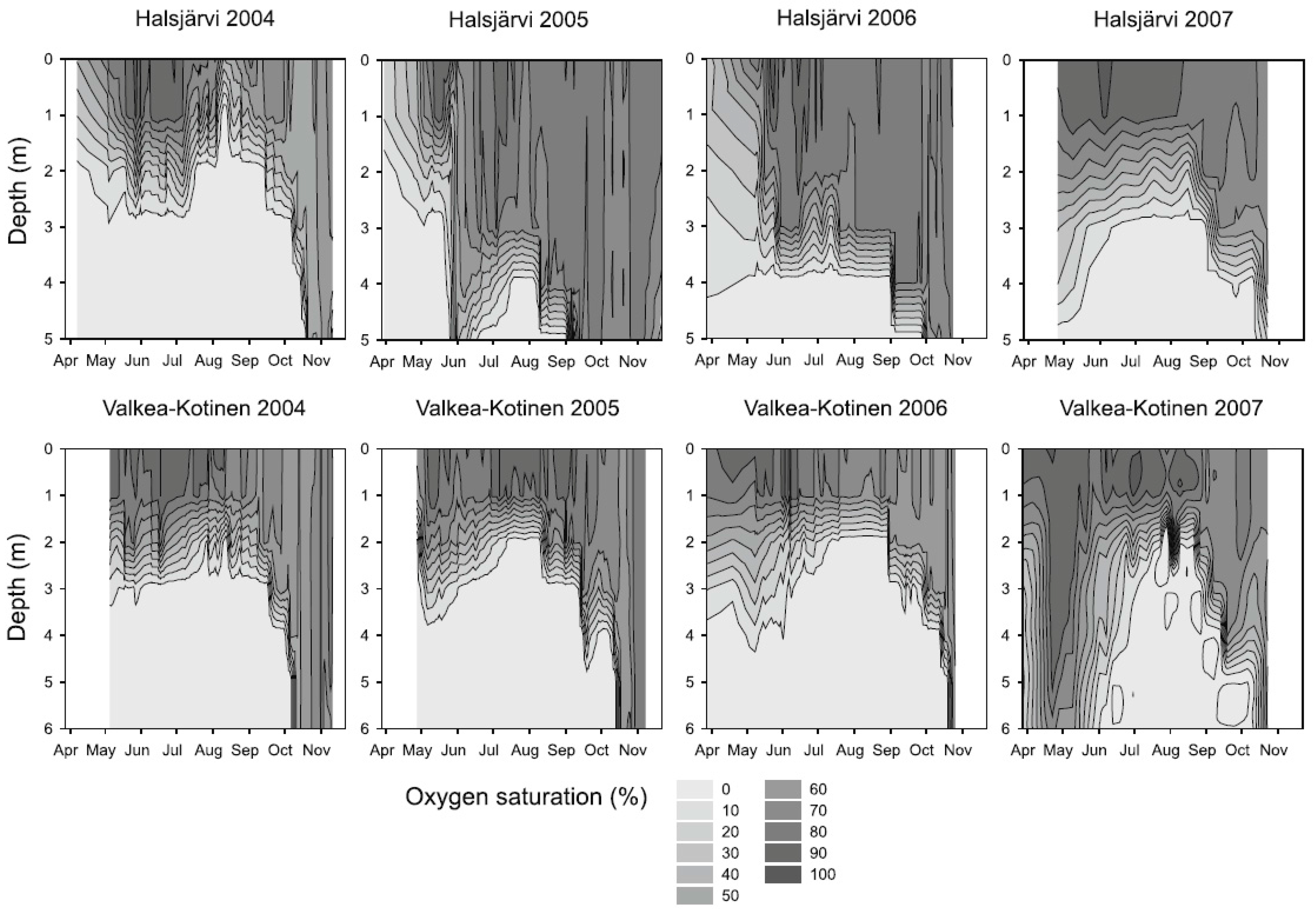
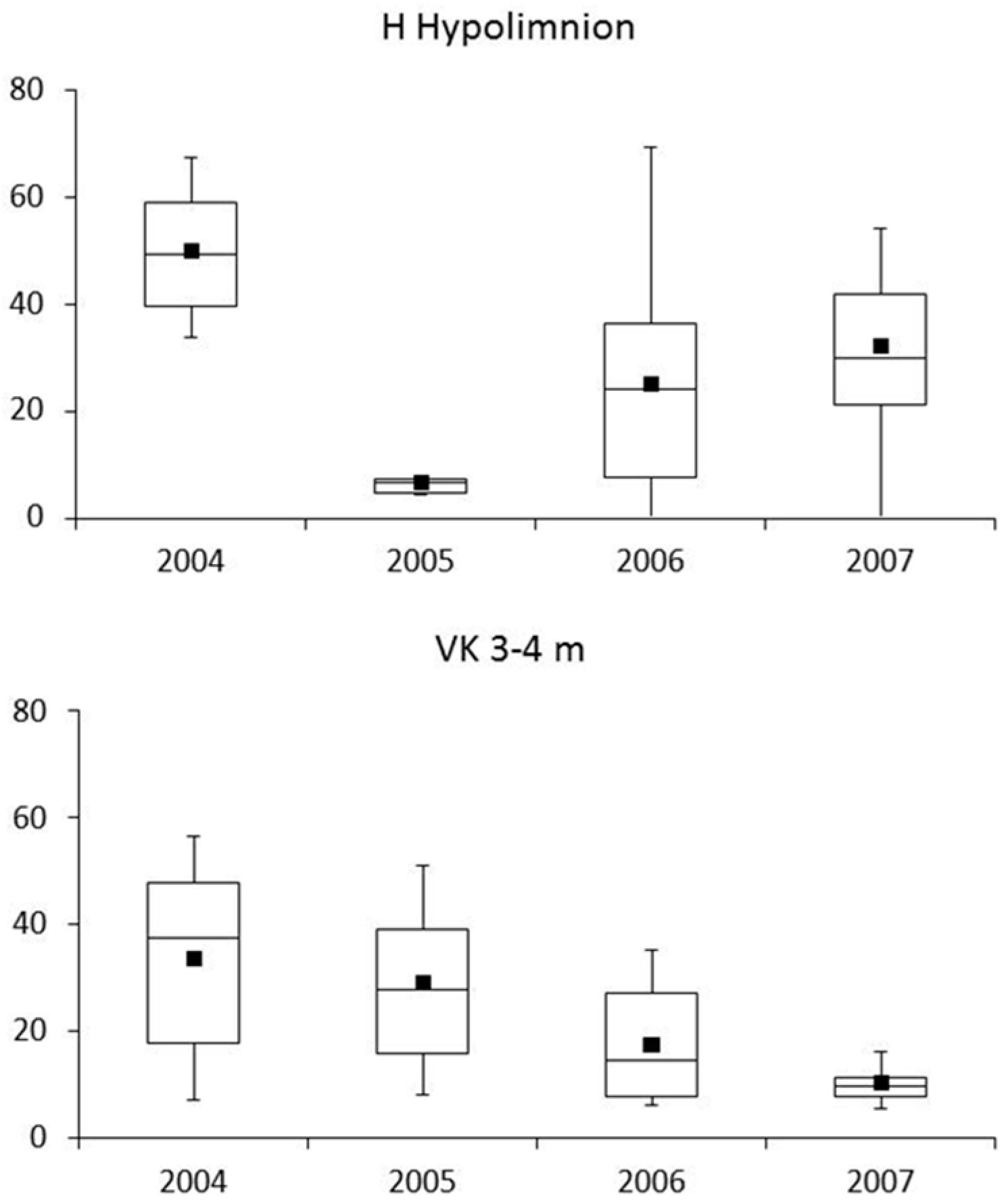


| Variable | H | VK |
|---|---|---|
| Latitude | 6,792,190 | 6,794,162 |
| Longitude | 3,400,181 | 3,396,164 |
| Elevation [m] | 131 | 156 |
| Lake area [ha] | 4.7 | 4.1 |
| Lake maximum depth [m] | 5.9 | 6.4 |
| Lake volume [m3] | 130,000 | 103,000 |
| Catchment area [ha] | 35 | 30 |
| pH | 6.4 (6.6) | 5.4 (5.2) |
| Ca [mg·L−1] | 6.2 (5.4) | 2.4 (2.2) |
| Fe [mg·L−1] | 3.1 (1.5) | 0.44 (0.42) |
| SO4 [mg·L−1] | 9.3 (8.8) | 5.2 (4.5) |
| Alkalinity [mmol·L−1] | 0.26 (0.18) | 0.024 (0.023) |
| TOC [mg·L−1] | 10.9 (8.0) | n.a. (13.7) |
| Colour [mg·Pt·L−1] | 237 (147) | 207 (198) |
| Chlorophyll a [μg·L−1] | 15 (6) | 14 (16) |
| Tot Hg [ng·L−1]1 | 3.7 (1.5) | 3.8 (2.6) |
| Variable | Variable | Treat-Pre | Treat-Post | Hals-Valk | Treat-Pre | Treat-Post | RIA Sign | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hals | Valk | Hals | Valk | Pre | Treat | Post | Treat/Pre | Treat/Post | ||||
| Chlorophyll | HJ epilimnion vs. VK depth 0–1 m | 0.56 | 0.33 | −1.89 | −1.14 | −8.15 | −7.92 | −7.18 | 0.23 | −0.75 | NS | NS |
| HJ depth 0–5 m vs. VK depth 0–1 m | −22.30 | 0.33 | −11.61 | −1.14 | 16.71 | −5.92 | 4.55 | −22.63 | −10.47 | <0.01 | <0.01 | |
| Zooplankton 1 | Cladocera | 5.69 | 6.39 | 9.50 | 11.24 | 17.45 | 16.75 | 18.49 | −0.70 | −1.74 | NS | NS |
| Copepoda | 14.28 | 17.32 | 3.89 | −0.08 | 0.66 | −2.38 | −6.34 | −3.04 | 3.96 | NS | NS | |
| Rotatoria | −240.22 | 70.83 | −122.82 | −152.54 | 37.98 | −273.08 | −302.79 | −311.06 | 29.71 | <0.05 | NS | |
| Phytoplankton | Cyano | −0.69 | 0.07 | −1.47 | −1.08 | 1.19 | 0.43 | 0.82 | −0.76 | −0.39 | NS | NS |
| Crypto | 4.94 | 2.62 | 11.41 | 4.92 | 4.08 | 6.41 | −0.09 | 2.33 | 6.50 | NS | <0.05 | |
| Dino | 3.56 | 2.69 | 4.11 | 3.51 | −5.39 | −4.52 | −5.13 | 0.87 | 0.61 | NS | NS | |
| Chryso | −2.36 | 13.49 | −0.89 | −26.19 | 1.08 | −14.76 | −40.05 | −15.84 | 25.30 | NS | NS | |
| Diatomo | 23.97 | −6.26 | 1.27 | −1.32 | −3.72 | 26.51 | 23.92 | 30.23 | 2.59 | <0.01 | NS | |
| Raphido | 2.77 | 3.63 | −0.43 | 4.08 | 0.03 | −0.83 | 3.68 | −0.86 | −4.51 | NS | NS | |
| Chloro | −1.74 | −31.51 | −53.59 | −9.86 | −147.14 | −117.37 | −73.63 | 29.77 | −43.73 | NS | NS | |
| Phytopl_biom | 31.75 | −17.86 | −36.72 | −15.65 | −163.74 | −114.13 | −93.05 | 49.61 | −21.07 | NS | NS | |
| -Taxon | 2004 | 2005 | 2006 | 2007 | K-W Statistic | p |
|---|---|---|---|---|---|---|
| H | ||||||
| Asellus aquaticus | 63 | 901 | 671 | 985 | 6.47 | 0.09 |
| Ephemeroptera | 31 | 807 | 818 | 482 | 8.14 | 0.04 |
| Odonata | 31 | 94 | 63 | 105 | 3.1 | 0.38 |
| Trichoptera | 21 | 105 | 388 | 262 | 8.81 | 0.04 |
| Chironomidae | 315 | 650 | 1405 | 613 | 6.33 | 0.1 |
| Total | 503 | 2673 | 3566 | 2640 | 7.64 | 0.05 |
| VK | ||||||
| Asellus aquaticus | 84 | 189 | 629 | 346 | 3.33 | 0.34 |
| Ephemeroptera | 3826 | 1604 | 1269 | 1384 | 1.04 | 0.79 |
| Odonata | 115 | 115 | 115 | 178 | 1.04 | 0.99 |
| Trichoptera | 335 | 209 | 262 | 220 | 3.48 | 0.32 |
| Chironomidae | 4402 | 4749 | 8815 | 2757 | 9.46 | 0.02 |
| Total | 9151 | 7086 | 11342 | 5084 | 0.73 | 0.06 |
| Variable | Response | Response |
|---|---|---|
| Bacteriochlorophyll d | R | Collapse of GSB due to complete mixing |
| Chlorophyll a | R | Minor increase in the course of the study |
| Phytoplankton | R | Diatoms increased and Gonyostomum semen decreased |
| Primary production | R | Minor increase in the course of the study |
| Respiration | No | |
| Zooplankton | R | A clear increase in the hypolimnion |
| Macroinvertebrates | R | Chironomidae increased and Chaoborus decreased |
| Fish | R | 2nd year growth of perch increased |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvola, L.; Rask, M.; Forsius, M.; Ala-Opas, P.; Keskitalo, J.; Kulo, K.; Kurkilahti, M.; Lehtovaara, A.; Sairanen, S.; Salo, S.; et al. Food Web Responses to Artificial Mixing in a Small Boreal Lake. Water 2017, 9, 515. https://doi.org/10.3390/w9070515
Arvola L, Rask M, Forsius M, Ala-Opas P, Keskitalo J, Kulo K, Kurkilahti M, Lehtovaara A, Sairanen S, Salo S, et al. Food Web Responses to Artificial Mixing in a Small Boreal Lake. Water. 2017; 9(7):515. https://doi.org/10.3390/w9070515
Chicago/Turabian StyleArvola, Lauri, Martti Rask, Martin Forsius, Pasi Ala-Opas, Jorma Keskitalo, Katja Kulo, Mika Kurkilahti, Anja Lehtovaara, Samuli Sairanen, Simo Salo, and et al. 2017. "Food Web Responses to Artificial Mixing in a Small Boreal Lake" Water 9, no. 7: 515. https://doi.org/10.3390/w9070515




