Comparison of Four Nitrate Removal Kinetic Models in Two Distinct Wetland Restoration Mesocosm Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Wetland Mesocosm Experiments
2.3. Sampling Plan and Analysis
2.4. Apparent NO3-N Removal Calculations
2.5. NO3-N Removal Kinetic Models
2.5.1. Zero Order (ZO) Model
2.5.2. First Order Decay (FO) Model
2.5.3. Efficiency Loss (EL) Model
2.5.4. Monod (M) Model
2.6. Temperature Adjustment of Removal Rate Coefficients
2.7. Statistical Evaluation
3. Results
3.1. NO3-N Removal and Areal Removal Rates
3.2. Model Calibrations
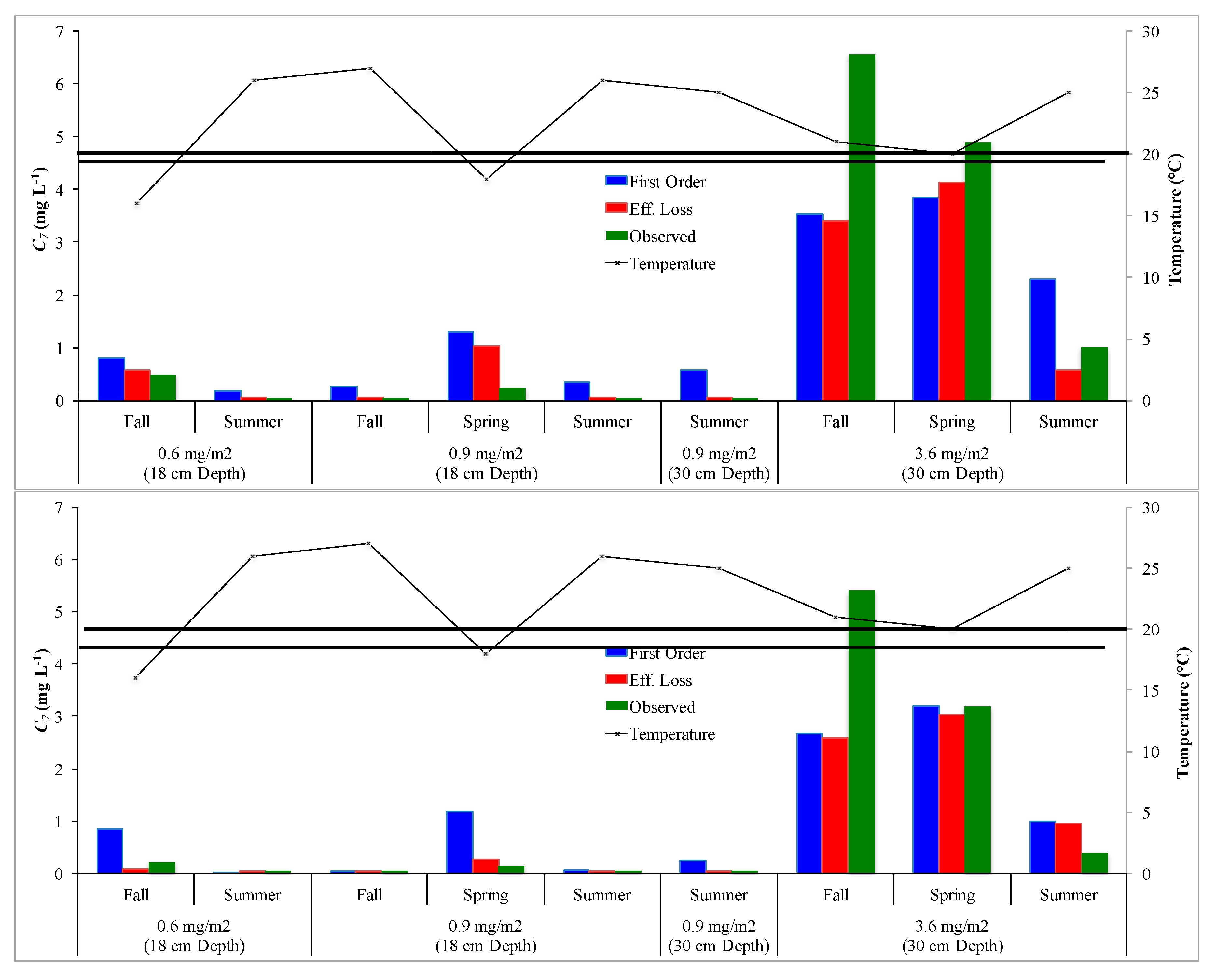
3.3. Temperature Adjustment for Removal Rate Coefficients
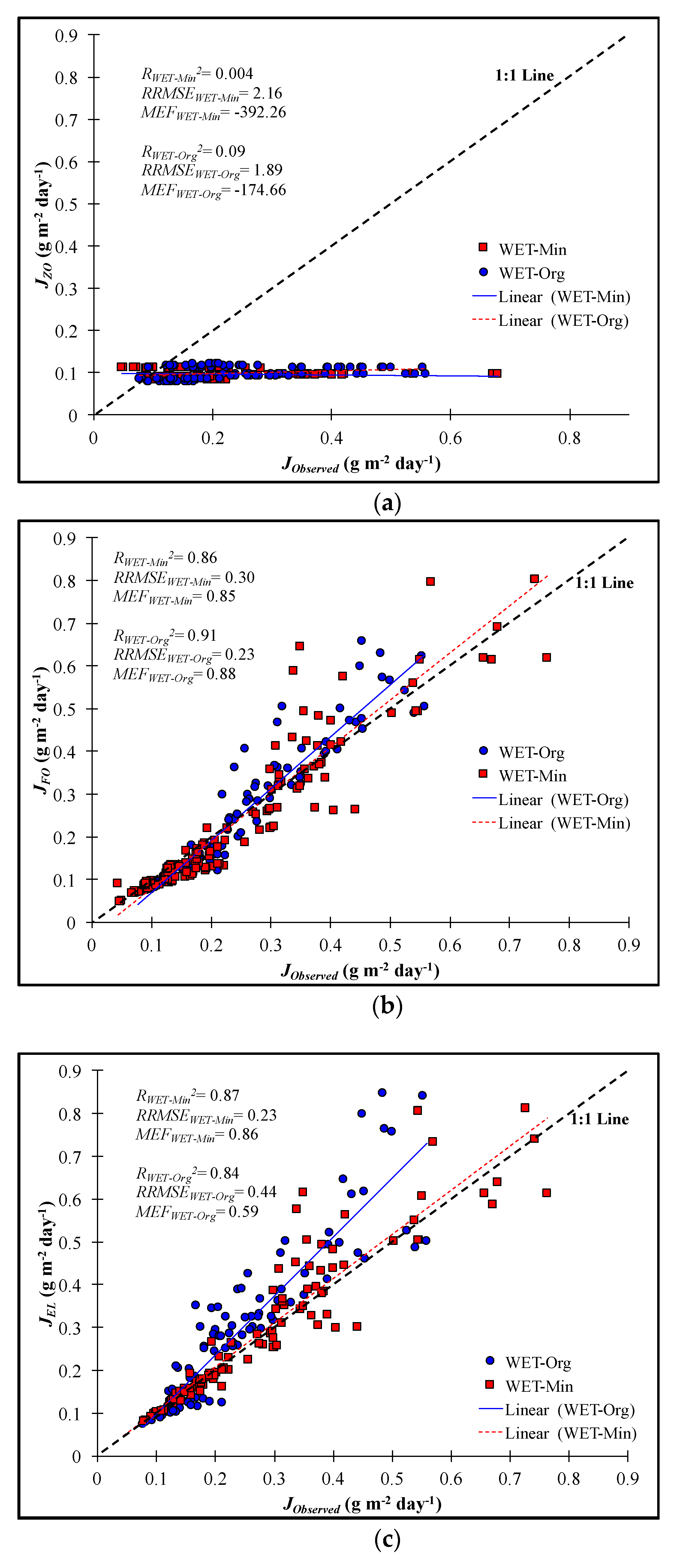
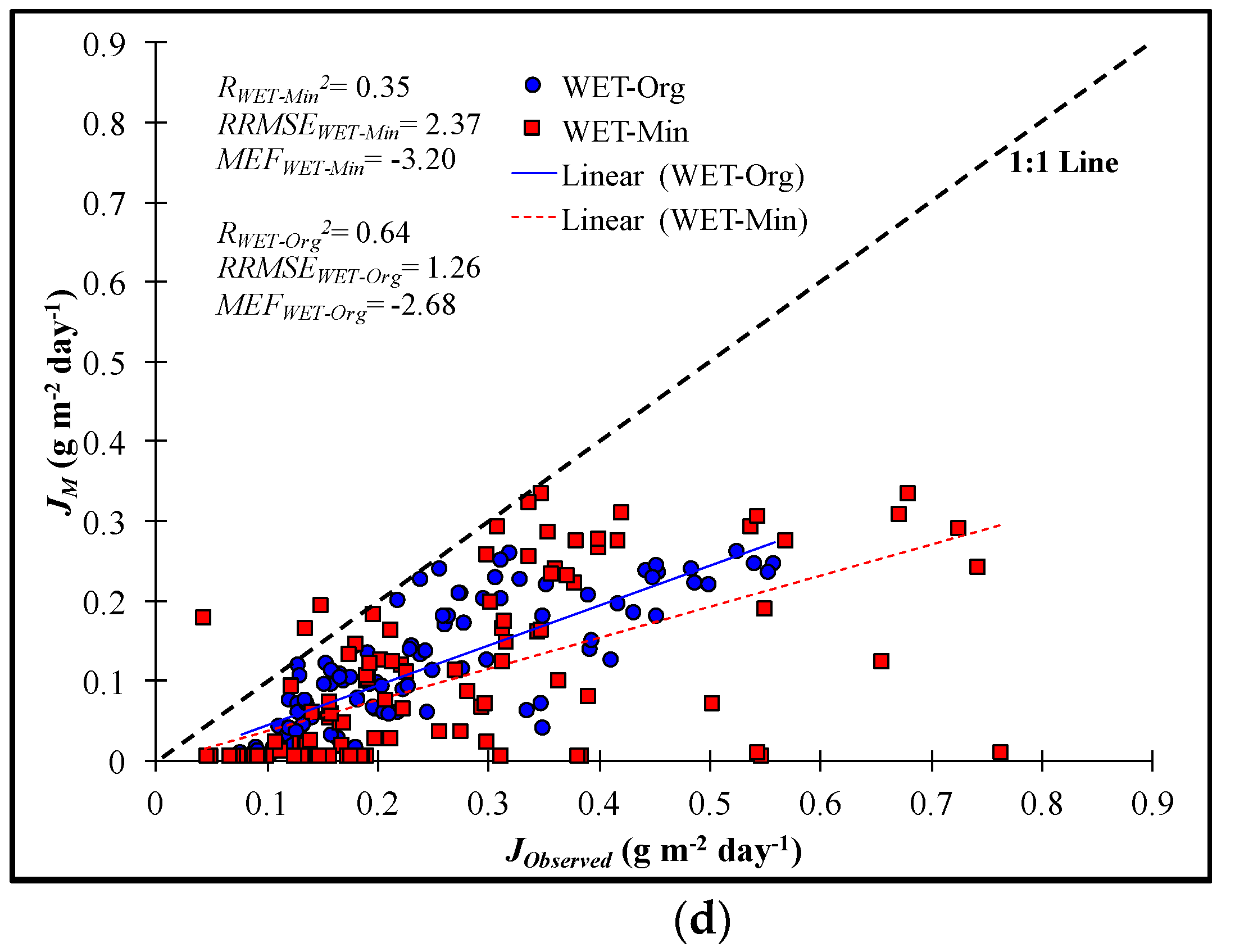
3.4. Validation of Kinetic Model Fits
4. Discussion
4.1. Prediction Power
4.2. Theoretical Wetland Predictions
5. Conclusions
- The first order decay and efficiency loss kinetic models provided stronger statistical agreement between predicted and measured NO3-N removal rates compared to other models.
- The first order decay model was determined the most practical model due to its conservative predictions, simplicity, and reasonable fit compared to the efficiency loss model, NO3-N removal rates developed at the mesocosm scale have also been reported to be conservative estimates for full-scale wetlands. Therefore, the first order decay model should provide reasonable but conservative predictions of NO3-N removal rates, particularly if maximum residence times are limited, such as to ensure tree survivability during the growing season in restored forested wetlands.
- ρ20 values determined for the first order decay model for NO3-N removal in the WET-Min and WET-Org wetland mesocosms were 4.9 cm d−1 and 4.1 cm d−1. θ values were estimated to be 1.15 and 1.09 for the WET-Min and WET-Org systems, respectively. These values can be used to develop informed water management plans for the restoration sites. This is important to ensure the wetlands are not loaded with NO3-N that exceeds their assimilation capacity; a situation that would lead to higher NO3-N export and possible unintended eutrophication of downstream water bodies.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rudd, M.A.; Fleishman, E. Policymakers’ and Scientists’ Ranks of Research Priorities for Resource-Management Policy. Bioscience 2014, 64, 219–228. [Google Scholar] [CrossRef]
- Fleishman, E.; Blockstein, D.E.; Hall, J.A.; Mascia, M.B.; Rudd, M.A.; Scott, J.M.; Sutherland, W.J.; Bartuska, A.M.; Brown, A.G.; Christen, C.A.; et al. Top 40 Priorities for Science to Inform US Conservation and Management Policy. Bioscience 2011, 61, 290–300. [Google Scholar] [CrossRef]
- US EPA. Nonpoint Source Pollution: The Nation’s Largest Water Quality Problem; U.S. Environmental Protection Agency: Washington, DC, USA, March 1996.
- Zedler, J.B.; Kercher, S. Wetland Resources: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Zedler, J.B. Wetlands at Your Service: Reducing Impacts of Agriculture at the Watershed Scale. Front. Ecol. Environ. 2012, 1, 65–72. [Google Scholar] [CrossRef]
- Dahl, T.E.; Allord, G.J. Technical Aspects of Wetlands: History of Wetlands in the Conterminous United States. Natl. Water Summ. Wetl. Resour. Tech. Asp. Wetl. 1996, 2425, 19–26. [Google Scholar]
- US EPA. Nutrient Policy and Data: Commercial Fertilizer Purchased; U.S. Environmental Protection Agency: Washington, DC, USA, 2012.
- Hong, B.; Swaney, D.P.; Howarth, R.W. Estimating net anthropogenic nitrogen inputs to U.S. watersheds: Comparison of methodologies. Environ. Sci. Technol. 2013, 47, 5199–5207. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, J.; Sardans, J.; Stefanescu, C.; Ubach, A.; Bartrons, M.; Asensio, D.; Peñuelas, J. Global biodiversity, stoichiometry and ecosystem function responses to human-induced C-N-P imbalances. J. Plant Physiol. 2015, 172, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, M.; Delgado, J.; Hansen, L.; Livingston, M.; Mosheim, R.; Williamson, J. Nitrogen in Agricultural Systems: Implications For Conservation Policy; ERR-127; U.S. Department of Agriculture: Quilcene, WA, USA, 2011.
- Matson, P.A.; Parton, W.; Power, A.; Swift, M. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.K.; Tilman, D.G. Human Alteration of the Global Nitrogen Cycle: Sources and Consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Ardón, M.; Morse, J.L.; Doyle, M.W.; Bernhardt, E.S. The Water Quality Consequences of Restoring Wetland Hydrology to a Large Agricultural Watershed in the Southeastern Coastal Plain. Ecosystems 2010, 13, 1060–1078. [Google Scholar] [CrossRef]
- Chescheir, G.M.; Gilliam, J.W.; Skaggs, R.W.; Broadhead, R.G. Nutrient and sediment removal in forested wetlands receiving pumped agricultural drainage water. Wetlands 1991, 11, 87–103. [Google Scholar] [CrossRef]
- Bruland, G.L.; Richardson, C.J. Comparison of soil organic matter in created, restored and paired natural wetlands in North Carolina. Wetl. Ecol. Manag. 2006, 14, 245–251. [Google Scholar] [CrossRef]
- Arheimer, B.; Wittgren, H.B. Modelling nitrogen removal in potential wetlands at the catchment scale. Ecol. Eng. 2002, 19, 63–80. [Google Scholar] [CrossRef]
- Woltemade, C.J. Ability of Restored Wetlands to Reduce Nitrogen and Phosphorus Concentrations in Agricultural Drainage Water. J. Soil Water Conserv. 2000, 55, 303–309. [Google Scholar]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Sun, G. Kinetic modelling of nitrogen and organics removal in vertical and horizontal flow wetlands. Water Res. 2011, 45, 3137–3152. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R.H. Nitrate dynamics in event-driven wetlands. Ecol. Eng. 2010, 36, 503–516. [Google Scholar] [CrossRef]
- Lee, C.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Comín, F.A.; Sorando, R.; Darwiche-Criado, N.; García, M.; Masip, A. A protocol to prioritize wetland restoration and creation for water quality improvement in agricultural watersheds. Ecol. Eng. 2014, 66, 10–18. [Google Scholar] [CrossRef]
- Kumar, J.L.G.; Zhao, Y.Q. A review on numerous modeling approaches for effective, economical and ecological treatment wetlands. J. Environ. Manag. 2011, 92, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Burchell, M.R.; Skaggs, R.W.; Lee, C.R.; Broome, S.; Chescheir, G.M.; Osborne, J. Substrate organic matter to improve nitrate removal in surface-flow constructed wetlands. J. Environ. Qual. 2007, 36, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Karpuzcu, M.E.; Stringfellow, W.T. Kinetics of nitrate removal in wetlands receiving agricultural drainage. Ecol. Eng. 2012, 42, 295–303. [Google Scholar] [CrossRef]
- Kadlec, R.H. The inadequacy of first-order treatment wetland models. Ecol. Eng. 2000, 15, 105–119. [Google Scholar] [CrossRef]
- Chapman, M.G.; Underwood, Z.J. The need for practical scientific protocol to measure successful restoration. Wetl. Aust. J. 2000, 19, 28–49. [Google Scholar]
- Ahn, C.; Mitsch, W.J. Scaling considerations of mesocosm wetlands in simulating large created freshwater marshes. Ecol. Eng. 2002, 18, 327–342. [Google Scholar] [CrossRef]
- Bachand, P.A.M.; Horne, A.J. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol. Eng. 1999, 14, 17–32. [Google Scholar] [CrossRef]
- Kangas, P.; Adey, W. Mesocosms and ecological engineering. Ecol. Eng. 1996, 6, 1–5. [Google Scholar] [CrossRef]
- US EPA. National Estuary Program Costal Condition Report—NEP CCR Factsheet: Chapter 4; U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Adame, M.F.; Hermoso, V.; Perhans, K.; Lovelock, C.E.; Herrera-Silveira, J.A. Selecting cost-effective areas for restoration of ecosystem services. Conserv. Biol. 2015, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R.H.; Tanner, C.C.; Hally, V.M.; Gibbs, M.M. Nitrogen spiraling in subsurface-flow constructed wetlands: Implications for treatment response. Ecol. Eng. 2005, 25, 365–381. [Google Scholar] [CrossRef]
- Messer, T.L.; Burchell, M.R.; Birgand, F.; Broome, S.W.; Chescheir, G.M. Nitrate removal potential of restored wetlands loaded with agricultural drainage water: A mesocosm scale experimental approach. Ecol. Eng. 2017, 106, 541–554. [Google Scholar] [CrossRef]
- Shih, S.S.; Zeng, Y.Q.; Lee, H.Y.; Otte, M.L.; Fang, W.T. Tracer experiments and hydraulic performance improvements in a treatment pond. Water 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Bachand, P.A.M. Effects of Managing Vegetative Species, Hydraulic Retention Time, Wetland Age and Water Depth on Removing Nitrate from Nitrified Wastewater in Constructed Wetland Macrocosms in the Prado Basin, Riverside County, California. Ph.D. Dissertation, University of California, Berkeley, CA, USA, 1996. [Google Scholar]
- Turlan, T.; Birgand, F.; Marmonier, P. Comparative use of field and laboratory mesocosms for in-stream nitrate uptake measurement. Ann. Limnol.-Int. J. Limnol. 2007, 43, 41–51. [Google Scholar] [CrossRef]
- Birgand, F.; Aveni-Deforge, K.; Smith, B.; Horstman, M.; Gerling, A.B.; Carey, C.C. First report of a novel multiplexer pumping system coupled to a water quality probe to collect high temporal frequency in situ water chemistry measurements at multiple sites. Limnol. Oceanogr. Methods 2016, 767–783. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Hamilton, S.K.; Podzikowski, L.; Ostrom, N. The fate of assimilated nitrogen in streams: An in situ benthic chamber study. Freshw. Biol. 2012, 57, 1113–1125. [Google Scholar] [CrossRef]
- Bowie, G.L.; Mills, W.B.; Porcella, D.B.; Campbell, C.L.; Pagenkopf, J.R.; Rupp, G.L.; Johnson, K.M.; Chan, P.W.H.; Gherini, S.A. Rates, Constants, and Kinetics Formulations in Surface Water Quality Modeling; US EPA: Athens, GA, USA, 1985.
- Anderson, C.J.; Mitsch, W.J. Effect of Pulsing on Macrophyte Productivity and Nutrient Uptake: A Wetland Mesocosm Experiment. Am. Midl. Nat. 2005, 154, 305–319. [Google Scholar] [CrossRef]
- Horne, A.J. Nitrogen removal from waste treatment pond or activated sludge plant effluents with free-surface wetlands. Water Sci. Technol. 1995, 31, 341–351. [Google Scholar] [CrossRef]
- Bekins, B.A.; Warren, E.; Godsy, E.M. A comparison of zero-order, first order, and Monod biotransformation models. Groundwater 1998, 36, 261–268. [Google Scholar] [CrossRef]
- Bollag, J.M.; Stotzky, G. Soil Biochemisiry; Marcel Dekker, Inc.: New York, NY, USA, 2000; Volume 10. [Google Scholar]
- O’Brien, J.M.; Dodds, W.K.; Wilson, K.C.; Murdock, J.N.; Eichmiller, J. The saturation of N cycling in Central Plains streams: 15N experiments across a broad gradient of nitrate concentrations. Biogeochemistry 2007, 84, 31–49. [Google Scholar] [CrossRef]
- Aumen, N. Concepts and Methods for Assessing Solute Dynamics in Stream Ecosystems A. J. N. Am. Benthol. Soc. 1990, 9, 95–119. [Google Scholar]
- Böhlke, J.K.; Antweiler, R.C.; Harvey, J.W.; Laursen, A.E.; Smith, L.K.; Smith, R.L.; Voytek, M.A. Multi-scale measurements and modeling of denitrification in streams with varying flow and nitrate concentration in the upper Mississippi River basin, USA. Biogeochemistry 2009, 93, 117–141. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H.; Phillips, R.E. The Role of Nitrate Diffusion in Determining the Order and Rate of Denitrification in Flooded Soil: I. Experimental Results. Soil Sci. Soc. Am. J. 1978, 42, 268–272. [Google Scholar] [CrossRef]
- Christensen, S.; Simkins, S.; Tiedje, J.M. Spatial Variation in Denitrification: Dependency of Activity Centers on the Soil Environment. Soil Sci. Soc. Am. J. 1990, 54, 1608. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Messer, J.J.; Brezonik, P.L. Laboratory evaluation of kinetic parameters for lake sediment denitrification models. Ecol. Model. 1984, 21, 277–286. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Karpuzcu, M.E.; Spier, C.; Hanlon, J.S.; Graham, J. Sizing mitigation wetlands in agricultural watersheds. Water Sci. Technol. 2013, 67, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Dzakpasu, M.; Hofmann, O.; Scholz, M.; Harrington, R.; Jordan, S.N.; McCarthy, V. Nitrogen removal in an integrated constructed wetland treating domestic wastewater. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2011, 46, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Uyanik, S.; Yesilnacar, M.I.; Sahinkaya, E. Side-by-side comparison of horizontal subsurface flow and free water surface flow constructed wetlands and artificial neural network (ANN) modelling approach. Ecol. Eng. 2009, 35, 1255–1263. [Google Scholar] [CrossRef]
- Youssef, M.A.; Skaggs, R.W.; Chescheir, G.M.; Gilliam, J.W. Field evaluation of a model for predicting nitrogen losses from drained lands. J. Environ. Qual. 2006, 35, 2026–2042. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H.M.; Heuberger, P.S.C. Calibration of process-oriented models. Ecol. Model. 1995, 83, 55–66. [Google Scholar] [CrossRef]
- Smith, L.K.; Voytek, M.A.; Böhlke, J.K.; Harvey, J.W. Denitrification in nitrate-rich streams: Application of N2:Ar and 15N-tracer methods in intact cores. Ecol. Appl. 2006, 16, 2191–2207. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Valett, H.M.; Webster, J.R.; Thomas, S.A.; Cooper, L.W.; Hamilton, S.K.; Kellogg, W.K.; Peterson, B.J. Stream denitrification and total nitrate uptake rates measured using a field 15 N tracer addition approach. Limnol. Ocean. 2004, 49, 809–820. [Google Scholar] [CrossRef]
- Puckett, L.J. Hydrogeologic controls on the transport and fate of nitrate in ground water beneath riparian buffer zones: Results from thirteen studies across the United States. Water Sci. Technol. 2004, 49, 47–53. [Google Scholar] [PubMed]
- Gebremariam, S.Y.; Beutel, M.W. Nitrate removal and DO levels in batch wetland mesocosms: Cattail (Typha spp.) versus bulrush (Scirpus spp.). Ecol. Eng. 2008, 34, 1–6. [Google Scholar] [CrossRef]
- Horne, A.J.; Bachand, P.A. Denitrification in constructed free-water surface wetlands: I. Very high nitrate removal rates in a macrocosm study. Ecol. Eng. 2000, 14, 9–15. [Google Scholar]
- Wollheim, W.M.; Harms, T.K.; Peterson, B.J.; Morkeski, K.; Hopkinson, C.S.; Stewart, R.J.; Gooseff, M.N.; Briggs, M.A. Nitrate uptake dynamics of surface transient storage in stream channels and fluvial wetlands. Biogeochemistry 2014, 120, 239–257. [Google Scholar] [CrossRef]
- Rossi, F.; Motta, O.; Matrella, S.; Proto, A.; Vigliotta, G. Nitrate removal from wastewater through biological denitrification with OGA 24 in a batch reactor. Water 2015, 7, 51–62. [Google Scholar] [CrossRef]
- Kadlec, R.H. Constructed Marshes for Nitrate Removal. Crit. Rev. Environ. Sci. Technol. 2012, 42, 934–1005. [Google Scholar] [CrossRef]
- Petru, B.J.; Chescheir, G.M.; Ahn, C. Assessment of water budgets and the hydrologic performance of a created mitigation wetland-A modeling approach. Ecol. Eng. 2014, 71, 667–676. [Google Scholar] [CrossRef]
- Teskey, R.O.; Hinckley, T.M. Impact of Water Level Changes on Woody Riparian and Wetland Communities. Volume 2: Southern Forest Region; U.S. Fish and Wildlife Service: Washington, DC, USA, 1977.
- Reed, S.C.; Brown, D.S. Subsurface Flow Wetlands: A Performance Evaluation. Water Environ. Res. 1995, 67, 244–248. [Google Scholar] [CrossRef]


| Season | Date | Experiment Period | Average Daily Water Temperature | Water Depth Prior to Loading | Water Depth after Loading | Target NO3-N | Target NO3-N Load |
|---|---|---|---|---|---|---|---|
| Day/Month/Year | Days | °C | cm | mg L−1 | g N m−2 | ||
| Fall a | 25/9–4/10/12 | 10 | 22 | 4 | 30 | 2.5 | 0.9 |
| Fall a | 16/10–26/10/12 | 10 | 17 | 4 | 18 | 5 | 0.9 |
| Fall a | 5/11–15/11/12 | 10 | 11 | 18 | 30 | 10 | 2.2 |
| Fall b | 24/9–4/10/13 | 10 | 21 | 4 | 30 | 10 | 3.6 |
| Fall b | 15/10–25/10/13 | 10 | 16 | 4 | 18 | 2.5 | 0.6 |
| Fall b | 2/9–9/9/14 | 7 | 27 | −5 † | 20 | 2.5 | 0.9 |
| Winter a | 22/1–1/2/13 | 10 | 9 | 4 | 15 | 2.5 | 0.6 |
| Winter a | 11/2–21/2/13 | 10 | 11 | 4 | 18 | 5 | 0.9 |
| Spring a | 28/5–7/6/13 | 10 | 25 | 4 | 18 | 2.5 | 0.6 |
| Spring b | 8/4–18/4/14 | 10 | 18 | 4 | 18 | 5 | 0.9 |
| Spring b | 21/4–1/5/14 | 10 | 20 | 4 | 30 | 10 | 3.6 |
| Spring b | 27/5–6/6/14 | 10 | 25 | 4 | 30 | 2.5 | 0.9 |
| Summer a | 2/7–12/7/13 | 10 | 27 | 4 | 30 | 2.5 | 0.9 |
| Summer a | 6/8–16/8/13 | 10 | 27 | 4 | 30 | 5 | 2.0 |
| Summer a | 20/8–27/8/13 | 7 | 25 | 4 | 30 | 2.5 | 0.9 |
| Summer b | 13/6–20/6/14 | 7 | 26 | 4 | 18 | 2.5 | 0.6 |
| Summer b | 22/7–1/8/14 | 10 | 26 | 4 | 30 | 10 | 3.6 |
| Summer b | 12/8–19/8/14 | 7 | 25 | 4 | 18 | 5 | 0.9 |
| Acronym | Parameter | Definition | Range | Increased Strength as Approaches: | Equation |
|---|---|---|---|---|---|
| R2 | Coefficient of determination: | Measures the extent of linear correlation between two datasets. | 0–1 | 1 | |
| RRMSE | Relative root mean square error: | Measures the differences between the predicted and the measured values. | 0–∞ | 0 | |
| MEF | Model efficiency: | Measures the variations accounted for by the model. | −∞ to 1 | 1 |
| Season | Mean Water Temperature | Water Depth | Load | Mean NO3-N (Initial–Final) | Time Required to Achieve Reported % Reduction | Mean NO3-N % Reduction | JNN ± SD (Min to Max Between Daily Sampling) | JNN ± SD (Average for Day 0 to Final Sampling Day) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WET-Min | WET-Org | WET-Min | WET-Org | WET-Min | WET-Org | WET-Min | WET-Org | WET-Min | WET-Org | ||||
| °C | cm | g m−2 d−1 | ---------------mg L−1------------ | --------------d--------------- | ----------------%-------------- | -----------mg m−2 d−1---------- | -----------mg m−2 d−1---------- | ||||||
| Fall 2013 | 18 | 18 | 0.6 | 3.56–0.22 | 3.56–0.14 | 7 † | 10 | 94 | 96 | 49 to 162 | 33 to 214 | 134 ± 4 | 103 ± 18 |
| Winter 2013 | 9 | 15 | 0.6 | 2.28–1.02 | 2.28–1.00 | 10 | 10 | 55 | 56 | 4 to 88 | 4 to 17 | 14 ± 3 | 13 ± 1 |
| Spring 2013 | 25 | 18 | 0.6 | 3.45–0.89 | 3.45–0.13 | 3 † | 7 † | 74 | 96 | 37 to 194 | 17 to 153 | 116 ± 21 | 72 ± 3 |
| Summer 2014 | 26 | 18 | 0.6 | 3.38–0.13 | 3.38–0.53 | 3.3 † | 3.3 † | 96 | 84 | 57 to 153 | 85 to 197 | 183 ± 38 | 127 ± 6 |
| Fall 2012 | 17 | 18 | 0.9 | 4.7–0.91 | 5.00–0.99 | 10 | 10 | 81 | 80 | 36 to 117 | 15 to 127 | 69 ± 13 | 59 ± 16 |
| Winter 2013 | 11 | 18 | 0.9 | 6.13–2.63 | 6.13–2.94 | 10 | 10 | 57 | 52 | 23 to 99 | 18 to 212 | 56 ± 15 | 42 ± 14 |
| Spring 2014 | 20 | 18 | 0.9 | 6.02–0.14 | 6.02–0.23 | 6.9 † | 6.9 † | 98 | 96 | 46 to 148 | 12 to 346 | 127 ± 28 | 88 ± 16 |
| Summer 2014 | 26 | 18 | 0.9 | 6.49–0.35 | 6.49–0.10 | 3 † | 5 † | 95 | 98 | 170 to 451 | 107 to 374 | 322 ± 61 | 215 ± 34 |
| Fall 2012 | 22 | 30 | 0.9 | 2.35–0.15 | 2.35–0.72 | 9 | 9 | 94 | 69 | 46 to 109 | 21 to 86 | 62 ± 9 | 63 ± 16 |
| Fall 2014 † | 27 | 20 | 0.9 | 3.25–0.33 | 3.25–0.09 | 3 † | 4.9 † | 90 | 97 | 80 to 349 | 125 to 262 | 275 ± 23 | 173 ± 1 |
| Spring 2014 | 26 | 30 | 0.9 | 3.29–0.11 | 3.29–0.38 | 5 † | 5 † | 97 | 89 | 103 to 276 | 123 to 205 | 216 ± 44 | 164 ± 5 |
| Summer 2013 | 27 | 30 | 0.9 | 3.09–0.36 | 3.09–0.09 | 5 † | 10 | 88 | 97 | 105 to 178 | 57 to 202 | 137 ± 18 | 98 ± 9 |
| Summer 2013 | 25 | 30 | 0.9 | 3.66–0.27 | 3.66–0.27 | 6.8 † | 6.8 † | 93 | 93 | 4 to 274 | 76 to 242 | 144 ± 4 | 144 ± 5 |
| Fall 2012 | 11 | 30 | 2.2 | 6.44–3.41 | 6.52–3.17 | 10 | 10 | 47 | 51 | 32 to 88 | 6 to 281 | 45 ± 2 | 176 ± 12 |
| Summer 2013 | 27 | 30 | 2.0 | 3.66–0.27 | 6.64–1.01 | 6.9 † | 6.9 † | 95 | 85 | 138 to 452 | 119 to 373 | 283 ± 17 | 212 ± 31 |
| Fall 2013 | 16 | 30 | 3.6 | 14.32–2.84 | 14.32–4.47 | 10.2 | 10.2 | 80 | 69 | 186 to 388 | 115 to 298 | 316 ± 2 | 260 ± 40 |
| Spring 2014 | 25 | 30 | 3.6 | 11.88–1.19 | 11.88–3.19 | 10.1 | 10.1 | 90 | 73 | 175 to 453 | 146 to 400 | 288 ± 14 | 234 ± 5 |
| Summer 2014 | 25 | 30 | 3.6 | 12.97–0.38 | 12.97–1.01 | 7 † | 9.9 | 97 | 92 | 171 to 868 | 198 to 452 | 603 ± 140 | 344 ± 8 |
| Wetland System | Initial Target Concentration (mg L−1) | NO3-N Removal Rate Coefficients for T20 | EL Constant (α) (Unitless) | Reference | |||
|---|---|---|---|---|---|---|---|
| JZO (mg m−2 d−1) * [θ: R2] | ρFO (cm d−1) * [θ: R2] | ρEL (cm d−1) * [θ: R2] | Jmax (g m−2 d−1) * [θ: R2] | ||||
| WET-Min | 2.5–15 | 94 ± 11 [1.03 ± 0.10: 0.58 to 0.66] | 4.9 ± 0.8 [1.15 ± 0.02: 0.89 to 0.98] | 10.2 [1.10: 0.72] | 0.50 ± 0.08 [No Correlation] | 0.6 (15 cm depth) 0.7 (30 cm depth) | This study |
| WET-Org | 2.5–15 | 92 ± 8 [1.04 ± 0.01: 0.06 to 0.15] | 4.1 ± 1.0 [1.09 ± 0.06: 0.82 to 0.93] | 8.0 [1.18: 0.67] | 0.38 ± 0.1 [No Correlation] | 0.7 (15 cm depth) 0.7 (30 cm depth) | This study |
| Constructed Treatment Mesocosm | 19 | 2.1–2.9 | Gebremariam and Beutel (2008) | ||||
| Lab Scale Wetland | 0.0–12.1 | 0.25 | Saeed et al., 2011 | ||||
| Surface-Flow Constructed Mesocosm | 32–117 | 5.7–16.5 | Burchell et al., 2007 | ||||
| Fluvial Wetlands | 0.1–3.7 | 3–9.3 | Not Reported | Wollheim et al., 2014 | |||
| Free Surface Constructed Wetlands | 10–26 | 200–5000 | Horne, 1995 | ||||
| NO3-N % Reduction | 96% | 80% | 60% | 30% | ||||
|---|---|---|---|---|---|---|---|---|
| Target Ceff (mg L−1) | 0.1 | 0.5 | 1.0 | 1.75 | ||||
| Avg. Water Temperature (°C) | WET-Min (cm Day−1) | WET-Org (cm Day−1) | WET-Min (cm Day−1) | WET-Org (cm Day−1) | WET-Min (cm Day−1) | WET-Org (cm Day−1) | WET-Min (cm Day−1) | WET-Org (cm Day−1) |
| 10 | 0.4 | 0.5 | 0.7 | 1.0 | 1.3 | 1.8 | 3.2 | 4.6 |
| 15 | 0.7 | 0.8 | 1.4 | 1.6 | 2.5 | 2.7 | 6.5 | 7.0 |
| 20 | 1.5 | 1.2 | 2.9 | 2.4 | 5.1 | 4.2 | 13.1 | 10.8 |
| 25 | 2.9 | 1.8 | 5.8 | 3.7 | 10.3 | 6.5 | 26.4 | 16.6 |
| 30 | 5.9 | 2.8 | 11.7 | 5.7 | 20.6 | 10.0 | 53.0 | 25.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messer, T.L.; Burchell, M.R.; Bírgand, F. Comparison of Four Nitrate Removal Kinetic Models in Two Distinct Wetland Restoration Mesocosm Systems. Water 2017, 9, 517. https://doi.org/10.3390/w9070517
Messer TL, Burchell MR, Bírgand F. Comparison of Four Nitrate Removal Kinetic Models in Two Distinct Wetland Restoration Mesocosm Systems. Water. 2017; 9(7):517. https://doi.org/10.3390/w9070517
Chicago/Turabian StyleMesser, Tiffany L., Michael R. Burchell, and François Bírgand. 2017. "Comparison of Four Nitrate Removal Kinetic Models in Two Distinct Wetland Restoration Mesocosm Systems" Water 9, no. 7: 517. https://doi.org/10.3390/w9070517




