Seasonal Variation in Spectral Response of Submerged Aquatic Macrophytes: A Case Study at Lake Starnberg (Germany)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
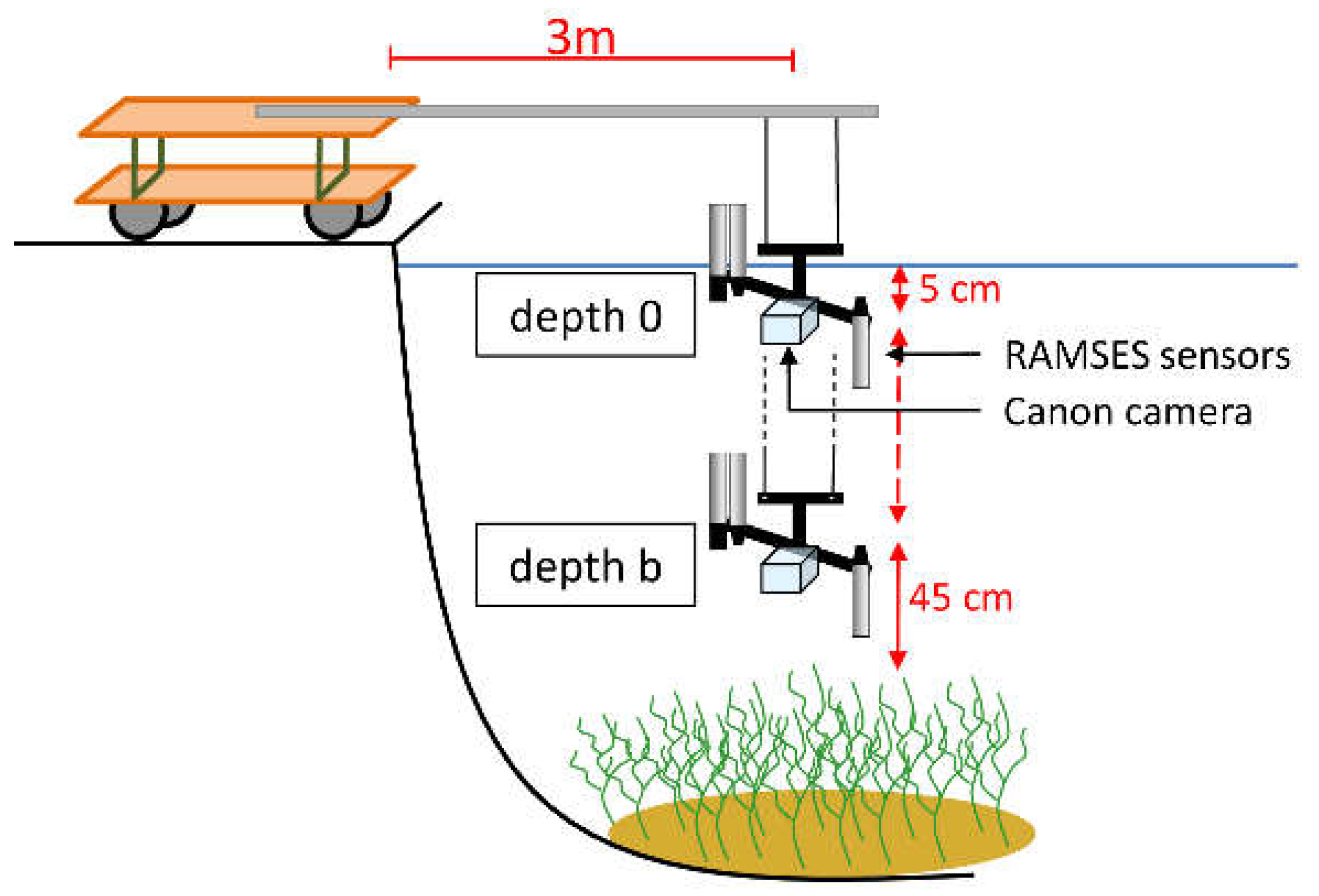
2.2. In-Situ Measurements
2.3. Data Processing
2.4. Reflectance Model
2.5. Species-Specific Spectra
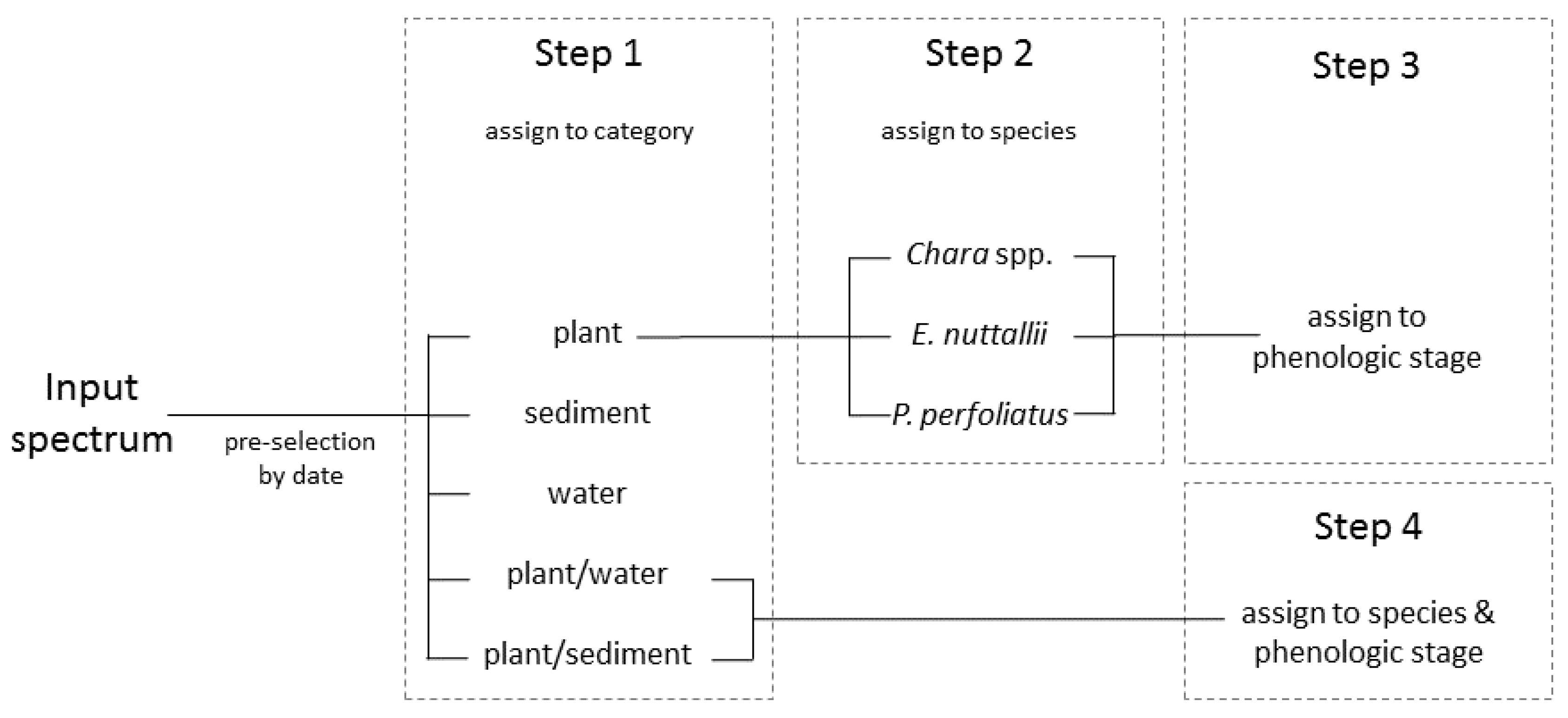
2.6. Classification Process
3. Results
3.1. Reflectance Model
3.2. Species-Specific Spectra
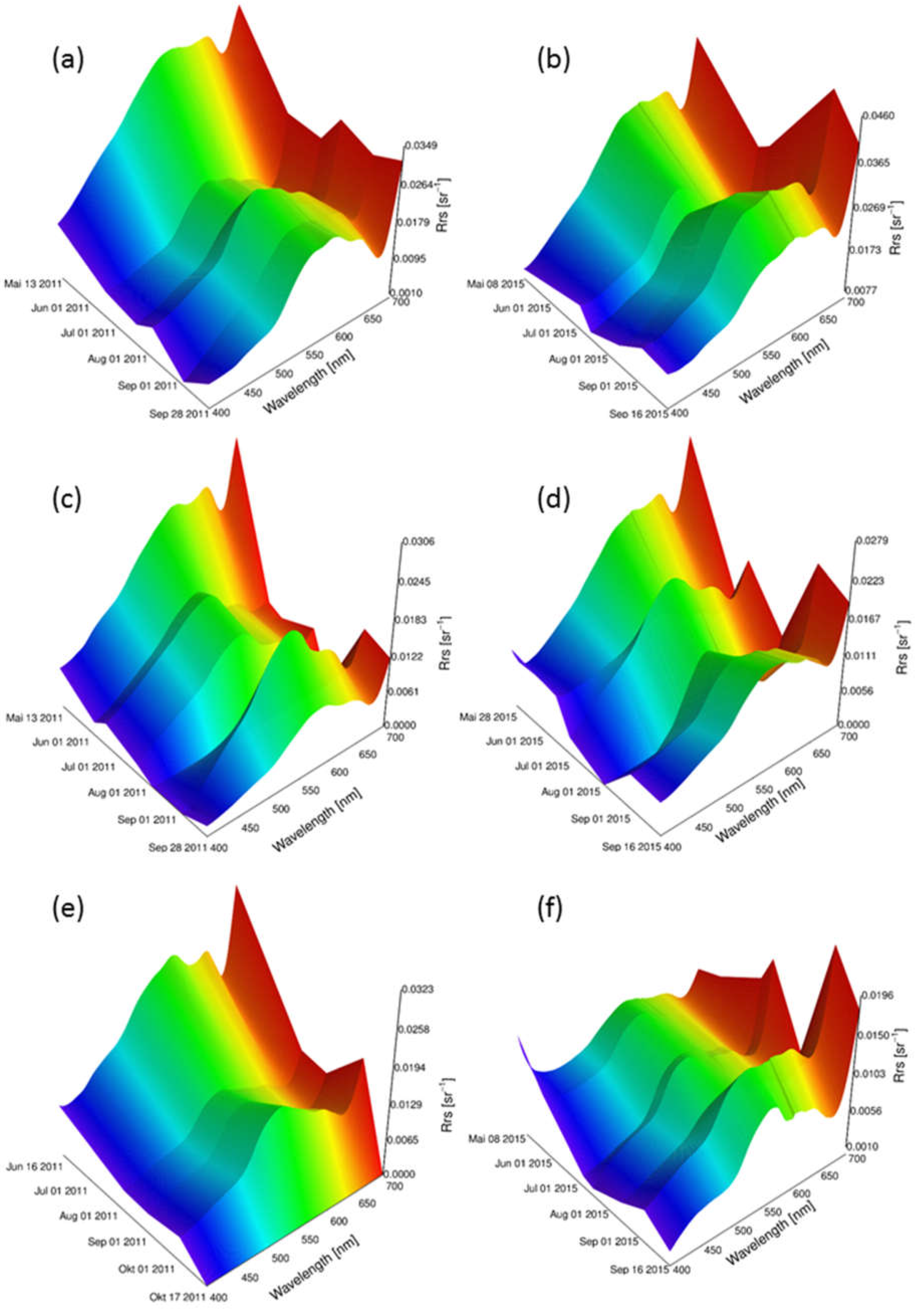
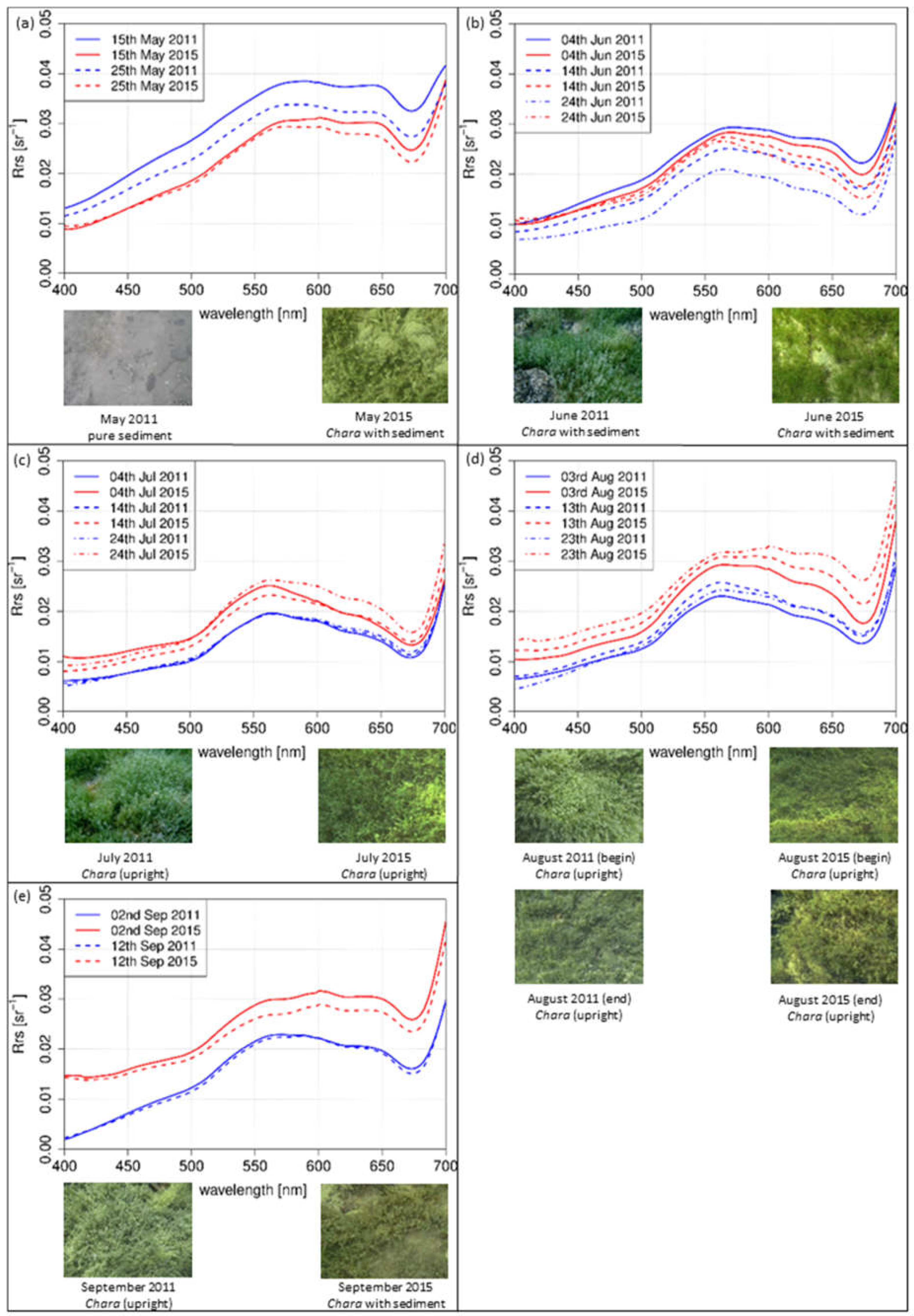
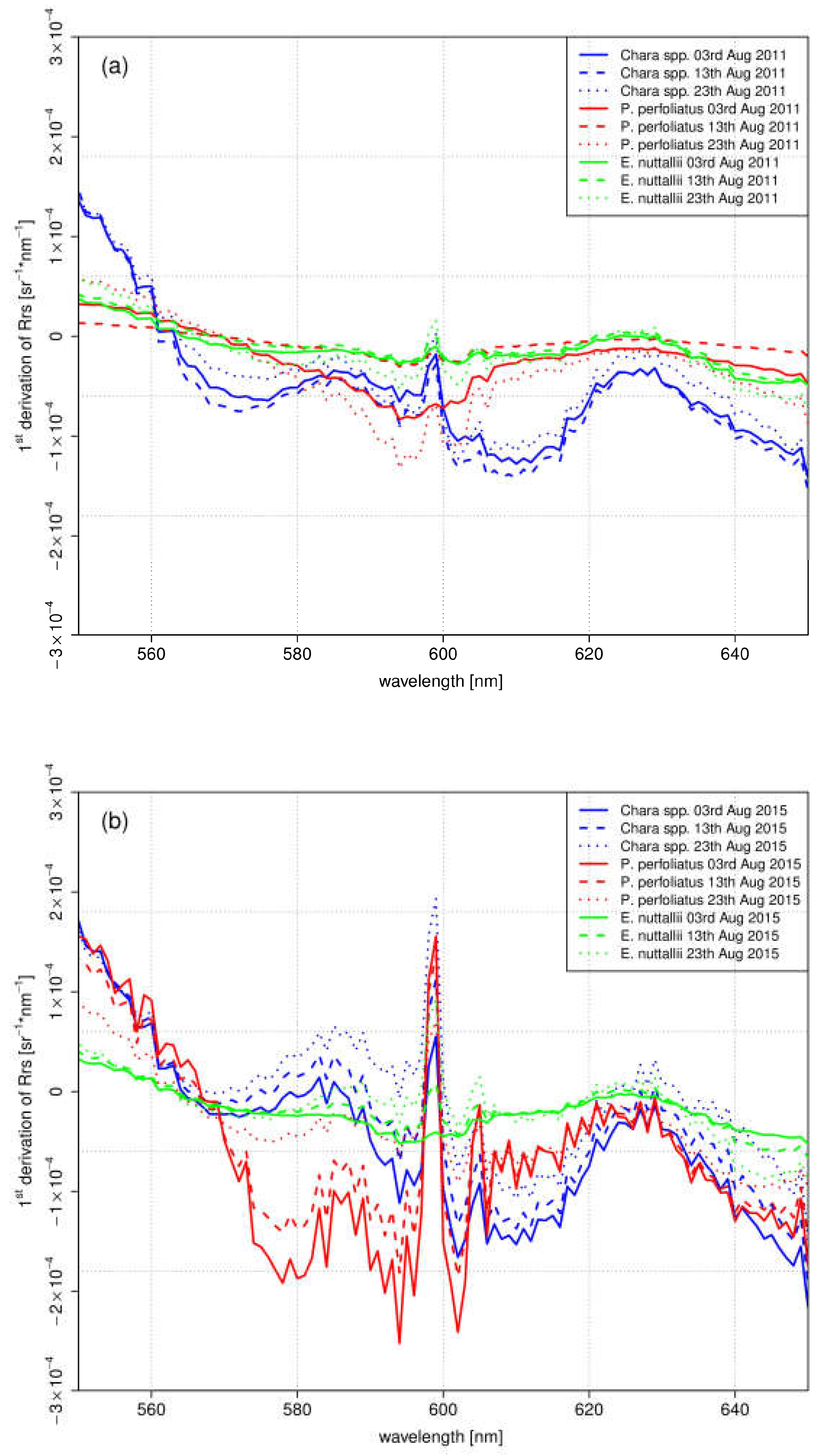
3.2.1. Test Site Chara
3.2.2. Test Site P. perfoliatus
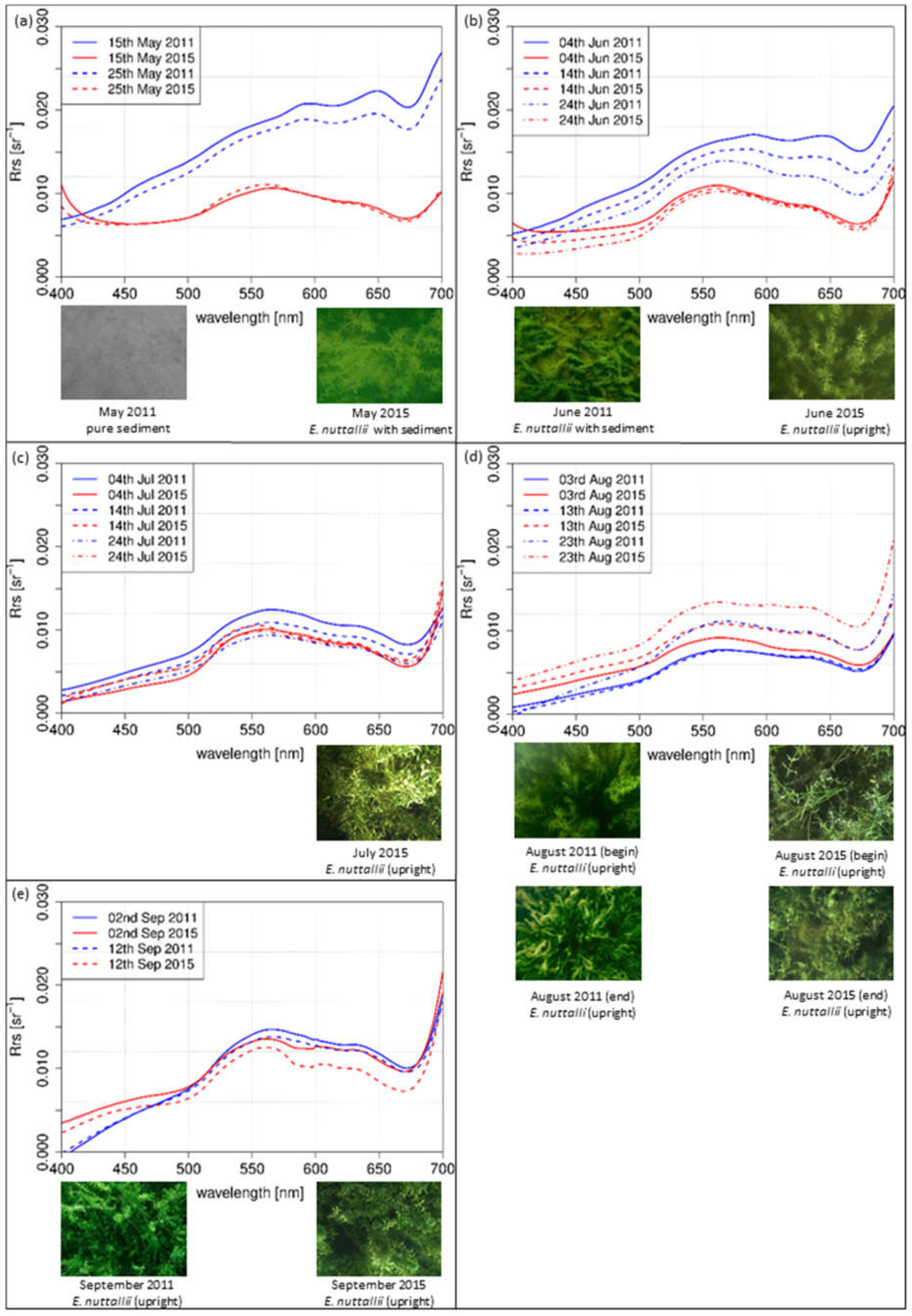
3.2.3. Test Site E. nuttallii
3.2.4. Water Temperature Effect on Species-Specific Growth
3.3. Spectral Classification on Species Level
4. Discussion
4.1. Phenologic Development in Spectra and Water Temperature
4.2. Reflectance Model
4.3. Model Inversion for Species Level Classification
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Melzer, A. Aquatic macrophytes as tools for lake management. Hydrobiologia 1999, 396, 181–190. [Google Scholar] [CrossRef]
- Søndergaard, M.; Johansson, L.S.; Lauridsen, T.L.; Jørgensen, T.B.; Liboriussen, L.; Jeppesen, E. Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 2010, 55, 893–908. [Google Scholar] [CrossRef]
- Skubinna, J.P.; Coon, T.G.; Batterson, T.R. Increased abundance and depth of submersed macrophytes in response to decreased turbidity in saginaw bay, lake huron. J. Gt. Lakes Res. 1995, 21, 476–488. [Google Scholar] [CrossRef]
- Poikane, S.; Birk, S.; Böhmer, J.; Carvalho, L.; de Hoyos, C.; Gassner, H.; Hellsten, S.; Kelly, M.; Lyche Solheim, A.; Olin, M.; et al. A hitchhiker’s guide to european lake ecological assessment and intercalibration. Ecol. Indic. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Penning, W.E.; Dudley, B.; Mjelde, M.; Hellsten, S.; Hanganu, J.; Kolada, A.; van den Berg, M.; Poikane, S.; Phillips, G.; Willby, N.; et al. Using aquatic macrophyte community indices to define the ecological status of european lakes. Aquat. Ecol. 2008, 42, 253–264. [Google Scholar] [CrossRef]
- Silva, T.S.F.; Costa, M.P.F.; Melack, J.M.; Novo, E.M.L.M. Remote sensing of aquatic vegetation: Theory and applications. Environ. Monit. Assess. 2008, 140, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Short, F.T.; Neckles, H.A. The effects of global climate change on seagrasses. Aquat. Bot. 1999, 63, 169–196. [Google Scholar] [CrossRef]
- Rooney, N.; Kalff, J. Inter-annual variation in submerged macrophyte community biomass and distribution: The influence of temperature and lake morphometry. Aquat. Bot. 2000, 68, 321–335. [Google Scholar] [CrossRef]
- European Commission. The Water Framework Directive (Directive 2000/60/ec of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy); Official Journal of the European Communities: Brussels, Belgium, 2000; pp. 1–72. [Google Scholar]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote sensing of inland waters: Challenges, progress and future directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Dörnhöfer, K.; Oppelt, N. Remote sensing for lake research and monitoring—Recent advances. Ecol. Indic. 2016, 64, 105–122. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, L.-Q. Mapping large-scale distribution of submerged aquatic vegetation coverage using remote sensing. Ecol. Inform. 2008, 3, 245–251. [Google Scholar] [CrossRef]
- Wolf, P.; Rößler, S.; Schneider, T.; Melzer, A. Collecting in situ remote sensing reflectances of submersed macrophytes to build up a spectral library for lake monitoring. Eur. J. Remote Sens. 2013, 46, 401–416. [Google Scholar] [CrossRef]
- Roessler, S.; Wolf, P.; Schneider, T.; Melzer, A. Multispectral remote sensing of invasive aquatic plants using rapideye. In Earth Observation of Global Changes (EOGC); Krisp, M.J., Meng, L., Pail, R., Stilla, U., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2013; pp. 109–123. [Google Scholar]
- Pinnel, N.; Heege, T.; Zimmermann, S. Spectral discrimination of submerged macrophytes in lakes using hyperspectral remote sensing data. SPIE Proc. Ocean Opt. XVII 2004, 1, 1–16. [Google Scholar]
- Malthus, T.J.; George, D.G. Airborne remote sensing of macrophytes in cefni reservoir, anglesey, UK. Aquat. Bot. 1997, 58, 317–332. [Google Scholar] [CrossRef]
- Giardino, C.; Bartoli, M.; Candiani, G.; Bresciani, M.; Pellegrini, L. Recent changes in macrophyte colonisation patterns: An imaging spectrometry-based evaluation of southern Lake Garda (Northern Italy). APPRES 2007, 1, 011509–011517. [Google Scholar]
- George, D.G. The airborne remote sensing of phytoplankton chlorophyll in the lakes and tarns of the english lake district. Int. J. Remote Sens. 1997, 18, 1961–1975. [Google Scholar] [CrossRef]
- Dekker, A.G.; Vos, R.J.; Peters, S.W.M. Analytical algorithms for lake water TSM estimation for retrospective analyses of TM and SPOT sensor data. Int. J. Remote Sens. 2002, 23, 15–35. [Google Scholar] [CrossRef]
- Malthus, T.J.; Karpouzli, E. Integrating field and high spatial resolution satellite-based methods for monitoring shallow submersed aquatic habitats in the Sound of Eriskay, Scotland, UK. Int. J. Remote Sens. 2003, 24, 2585–2593. [Google Scholar] [CrossRef]
- Barko, J.W.; Gunnison, D.; Carpenter, S.R. Sediment interactions with submersed macrophyte growth and community dynamics. Aquat. Bot. 1991, 41, 41–65. [Google Scholar] [CrossRef]
- Squires, M.M.; Lesack, L.F. Spatial and temporal patterns of light attenuation among lakes of the mackenzie delta. Freshw. Biol. 2003, 48, 1–20. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Sediment-related mechanisms of growth limitation in submersed macrophytes. Ecology 1986, 67, 1328–1340. [Google Scholar] [CrossRef]
- Shuchman, R.A.; Sayers, M.J.; Brooks, C.N. Mapping and monitoring the extent of submerged aquatic vegetation in the laurentian great lakes with multi-scale satellite remote sensing. J. Gt. Lakes Res. 2013, 39, 78–89. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Zhu, B.; Mayer, C.M.; Rudstam, L.G.; Mills, E.L.; Ritchie, M.E. A comparison of irradiance and phosphorus effects on the growth of three submerged macrophytes. Aquat. Bot. 2008, 88, 358–362. [Google Scholar] [CrossRef]
- Madsen, T.V.; Brix, H. Growth, photosynthesis and acclimation by two submerged macrophytes in relation to temperature. Oecologia 1997, 110, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Raeder, U.; Melzer, A. Influence of environmental conditions on the regenerative capacity and the survivability of elodea nuttallii fragments. J. Limnol. 2014, 74. [Google Scholar] [CrossRef]
- Hoffmann, M.; Raeder, U. Predicting the potential distribution of neophytes in southern Germany using native Najas marina as invasion risk indicator. Environ. Earth Sci. 2016, 75, 1217. [Google Scholar] [CrossRef]
- Hoffmann, M.; Sacher, M.; Lehner, S.; Raeder, U.; Melzer, A. Influence of sediment on the growth of the invasive macrophyte Najas marina ssp intermedia in lakes. Limnologica 2013, 43, 265–271. [Google Scholar] [CrossRef]
- Morel, A.; Belanger, S. Improved detection of turbid waters from ocean color sensors information. Remote Sens. Environ. 2006, 102, 237–249. [Google Scholar] [CrossRef]
- Mertes, L.A.K.; Smith, M.O.; Adams, J.B. Estimating suspended sediment concentrations in surface waters of the Amazon River wetlands from Landsat images. Remote Sens. Environ. 1993, 43, 281–301. [Google Scholar] [CrossRef]
- Bostater, J.C.R.; Ghir, T.; Bassetti, L.; Hall, C.; Reyeier, E.; Lowers, R.; Holloway-Adkins, K.; Virnstein, R. Hyperspectral Remote Sensing Protocol Development for Submerged Aquatic Vegetation in Shallow Waters; SPIE: Bellingham, WA, USA, 2004; pp. 199–215. [Google Scholar]
- Mobley, C.D. Estimation of the remote-sensing reflectance from above-surface measurements. Appl. Opt. 1999, 38, 7442–7455. [Google Scholar] [CrossRef] [PubMed]
- Mumby, P.J.; Clark, C.D.; Green, E.P.; Edwards, A.J. Benefits of water column correction and contextual editing for mapping coral reefs. Int. J. Remote Sens. 1998, 19, 203–210. [Google Scholar] [CrossRef]
- Heblinski, J.; Schmieder, K.; Heege, T.; Agyemang, T.K.; Sayadyan, H.; Vardanyan, L. High-resolution satellite remote sensing of littoral vegetation of lake sevan (armenia) as a basis for monitoring and assessment. Hydrobiologia 2011, 661, 97–111. [Google Scholar] [CrossRef]
- Fritz, C.; Doernhoefer, K.; Schneider, T.; Geist, J.; Oppelt, N. Mapping submerged aquatic vegetation using rapideye satellite data: The example of Lake Kummerow (Germany). Water 2017, 9, 510. [Google Scholar] [CrossRef]
- Pinnel, N. A Method for Mapping Submerged Macrophytes in Lakes Using Hyperspectral Remote Sensing. Ph.D. Thesis, Technische Universität München, München, Germany, 2007. [Google Scholar]
- Williams, D.J.; Rybicki, N.B.; Lombana, A.V.; O’Brien, T.M.; Gomez, R.B. Preliminary investigation of submerged aquatic vegetation mapping using hyperspectral remote sensing. Environ. Monit. Assess. 2003, 81, 383–392. [Google Scholar] [CrossRef]
- Fyfe, S. Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct? Limnol. Oceanogr. 2003, 48, 464–479. [Google Scholar] [CrossRef]
- Armstrong, R.A. Remote sensing of submerged vegetation canopies for biomass estimation. Int. J. Remote Sens. 1993, 14, 621–627. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gausman, H.W. Evaluation of factors causing reflectance differences between sun and shade leaves. Remote Sens. Environ. 1984, 15, 177–181. [Google Scholar] [CrossRef]
- Hestir, E.L.; Khanna, S.; Andrew, M.E.; Santos, M.J.; Viers, J.H.; Greenberg, J.A.; Rajapakse, S.S.; Ustin, S.L. Identification of invasive vegetation using hyperspectral remote sensing in the California delta ecosystem. Remote Sens. Environ. 2008, 112, 4034–4047. [Google Scholar] [CrossRef]
- Elatawneh, A.; Kalaitzidis, C.; Petropoulos, G.P.; Schneider, T. Evaluation of diverse classification approaches for land use/cover mapping in a mediterranean region utilizing hyperion data. Int. J. Digit. Earth 2014, 7, 194–216. [Google Scholar] [CrossRef]
- Stoffels, J.; Hill, J.; Sachtleber, T.; Mader, S.; Buddenbaum, H.; Stern, O.; Langshausen, J.; Dietz, J.; Ontrup, G. Satellite-based derivation of high-resolution forest information layers for operational forest management. Forests 2015, 6, 1982–2013. [Google Scholar] [CrossRef]
- Arle, J.; Blondzik, K.; Claussen, U.; Duffek, A.; Grimm, S.; Hilliges, F.; Hoffmann, A.; Leujak, W.; Mohaupt, V.; Naumann, S.; et al. Wasserwirtschaft in Deutschland. Teil 2. Gewässergüte; Umweltbundesamt (UBA): Bonn, Germany, 2013. (In Germany) [Google Scholar]
- Wöbbecke, K.; Klett, G.; Rechenberg, B. Wasserbeschaffenheit der Wichtigsten Seen in der Bundesrepublik Deutschland: Datensammlung 1981–2000; Umweltbundesamt: Dessau-Roßlau, Germany, 2003. (In Germany) [Google Scholar]
- Bavarian Environmental Agency. Bavarian Hyrological Service. Available online: http://www.Gkd.Bayern.De (accessed on 13 July 2017).
- TriOS. Ramses Radiometer. Available online: http://www.Trios.De/en/products/sensors/ramses.html (accessed on 13 July 2017).
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. 1995, 100, 13321. [Google Scholar] [CrossRef]
- Brando, V.E.; Dekker, A.G. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1376–1387. [Google Scholar] [CrossRef]
- Giardino, C.; Candiani, G.; Bresciani, M.; Lee, Z.; Gagliano, S.; Pepe, M. Bomber: A tool for estimating water quality and bottom properties from remote sensing images. Comput. Geosci. 2012, 45, 313–318. [Google Scholar] [CrossRef]
- Albert, A.; Mobley, C.D. An analytical model for subsurface irradiance and remote sensing reflectance in deep and shallow case-2 waters. Opt. Express 2003, 11, 2873–2890. [Google Scholar] [CrossRef] [PubMed]
- Gege, P. Wasi-2d: A software tool for regionally optimized analysis of imaging spectrometer data from deep and shallow waters. Comput. Geosci. 2013, 62, 208–215. [Google Scholar] [CrossRef]
- Gege, P. The water color simulator wasi: An integrating software tool for analysis and simulation of optical in situ spectra. Comput. Geosci. 2004, 30, 523–532. [Google Scholar] [CrossRef]
- Maritorena, S. Remote sensing of the water attenuation in coral reefs: A case study in french polynesia. Int. J. Remote Sens. 1996, 17, 155–166. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 13 July 2017).
- Akima, H.; Gebhardt, A.; Petzoldt, T.; Maechler, M. Akima: Interpolation of Irregularly Spaced Data, R package version 0.5-11; Available online: https://cran.r-project.org/web/packages/akima/index.html (accessed on 13 July 2017).
- Wolf, P.K.-H. In Situ-Messungen als Basis Für Wachstums-/Reflexionsmodelle Submerser Makrophyten. Ph.D. Thesis, Technische Universität München, München, Germany, 2014. (In Germany). [Google Scholar]
- Blindow, I. Long- and short-term dynamics of submerged macrophytes in two shallow eutrophk lakes. Freshw. Biol. 1992, 28, 15–27. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Comparative influences of light and temperature on the growth and metabolism of selected submersed freshwater macrophytes. Ecol. Monogr. 1981, 51, 219–235. [Google Scholar] [CrossRef]
- McKee, D.; Hatton, K.; Eaton, J.W.; Atkinson, D.; Atherton, A.; Harvey, I.; Moss, B. Effects of simulated climate warming on macrophytes in freshwater microcosm communities. Aquat. Bot. 2002, 74, 71–83. [Google Scholar] [CrossRef]
- Wolter, P.T.; Johnston, C.A.; Niemi, G.J. Mapping submergent aquatic vegetation in the US great lakes using quickbird satellite data. Int. J. Remote Sens. 2005, 26, 5255–5274. [Google Scholar] [CrossRef]
- Sawaya, K.E.; Olmanson, L.G.; Heinert, N.J.; Brezonik, P.L.; Bauer, M.E. Extending satellite remote sensing to local scales: Land and water resource monitoring using high-resolution imagery. Remote Sens. Environ. 2003, 88, 144–156. [Google Scholar] [CrossRef]



| Date | Test site Chara 2011 | Test Site Chara 2015 | Test Site P. perfoliatus 2011 | Test Site P. perfoliatus 2015 | Test Site E. nuttallii 2011 | Test Site E. nuttallii 2015 | |
|---|---|---|---|---|---|---|---|
| 15 May | 100% sediment | 100% plant/sediment 100% Chara spp. 1.1 | - | - | 100% sediment | 99.99% plant 100% E. nuttallii 100% E. nuttallii 2.1 | |
| 25 May | 100% sediment | 100% plant/sediment 100% Chara spp. 1.2 | 30 May 100% sediment | 30 May 100% sediment | 100% sediment | 100% plant 100% E. nuttallii 100% E. nuttallii 2.1 | |
| 4 June | 100% sediment | 100% plant/sediment 100% Chara spp. 1.2 | 100% plant/water 100% P. perfoliatus 1 | 100% sediment | 100% plant/sediment 100% Chara spp. 1.2 | 100% plant/water 100% P. perfoliatus 1 | |
| 14 June | 100% plant/sediment 100% Chara spp. 1.2 | 100% plant/sediment 100% Chara spp. 1.2 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/sediment no stage classifiable | 100% plant/sediment no stage classifiable | |
| 24 June | 100% plant/sediment 100% Chara spp. 1.2 | 100% plant 100% Chara spp. 100% Chara spp. 2 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 2.2 | |
| 4 July | 100% plant/sediment 100% Chara spp. 1.2 | 100% plant 100% Chara spp. 100% Chara spp. 2 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 2.2 | |
| 14 July | 100% plant/sediment 100% Chara spp. 1.1 | 100% plant 100% Chara spp. 100% Chara spp. 2 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 2 | 100% plant/water 100% P. perfoliatus 1 | 100% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 2.2 | |
| 24 July | 100% plant/sediment 100% Chara spp. 1.1 | 100% plant 100% Chara spp. 100% Chara spp. 2 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 2 | 100% plant/water 100% P. perfoliatus 1 | 99.99% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 3 | |
| 3 August | 99.91% plant/sediment 100% Chara spp. 1.1 | 100% plant 100% Chara spp. 100% Chara spp. 2 | 100% plant99.48% E. nuttallii 100% E. nuttallii 2.3 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 2 | 99.82% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 2.3 | |
| 13 August | 89.58% plant/sediment no stage classifiable | 99.99% plant 100% Chara spp. 100% Chara spp. 2 | 99.99% plant 100% E. nuttallii 100% E. nuttallii 2.3 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 2 | 99.99% plant/sediment no stage classifiable | 99.99% plant 100% E. nuttallii 100% E. nuttallii 2.3 | |
| 23 August | 99.90% plant/sediment no stage classifiable | 100% plant 100% Chara spp. 100% Chara spp. 3.1 | 99.99% plant 99.98% P. perfoliatus 100% P. perfoliatus 3.1 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 3.1 | 100% plant/sediment no stage classifiable | 99.99% plant 100% E. nuttallii 100% E. nuttallii 2.3 | |
| 2 September | 100% plant/sediment no stage classifiable | 99.99% plant 100% Chara 100% Chara 3.1 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 3.1 | 100% plant 100% P. perfoliatus 100% P. perfoliatus 3.1 | 100% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 2.3 | |
| 12 September | 100% plant/sediment no stage classifiable | 100% plant/sediment no stage classifiable | 100% plant 100% P. perfoliatus 100% P. perfoliatus 3.1 | 100% plant/water no stage classifiable | 100% plant/sediment no stage classifiable | 100% plant 100% E. nuttallii 100% E. nuttallii 2.3 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, C.; Schneider, T.; Geist, J. Seasonal Variation in Spectral Response of Submerged Aquatic Macrophytes: A Case Study at Lake Starnberg (Germany). Water 2017, 9, 527. https://doi.org/10.3390/w9070527
Fritz C, Schneider T, Geist J. Seasonal Variation in Spectral Response of Submerged Aquatic Macrophytes: A Case Study at Lake Starnberg (Germany). Water. 2017; 9(7):527. https://doi.org/10.3390/w9070527
Chicago/Turabian StyleFritz, Christine, Thomas Schneider, and Juergen Geist. 2017. "Seasonal Variation in Spectral Response of Submerged Aquatic Macrophytes: A Case Study at Lake Starnberg (Germany)" Water 9, no. 7: 527. https://doi.org/10.3390/w9070527





