Phosphorus Dynamics in Long-Term Flooded, Drained, and Reflooded Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling and Preparation
2.3. Experimental Set-up
2.4. Water and Soil Analysis
2.5. Statistical Analysis
3. Results and Discussion
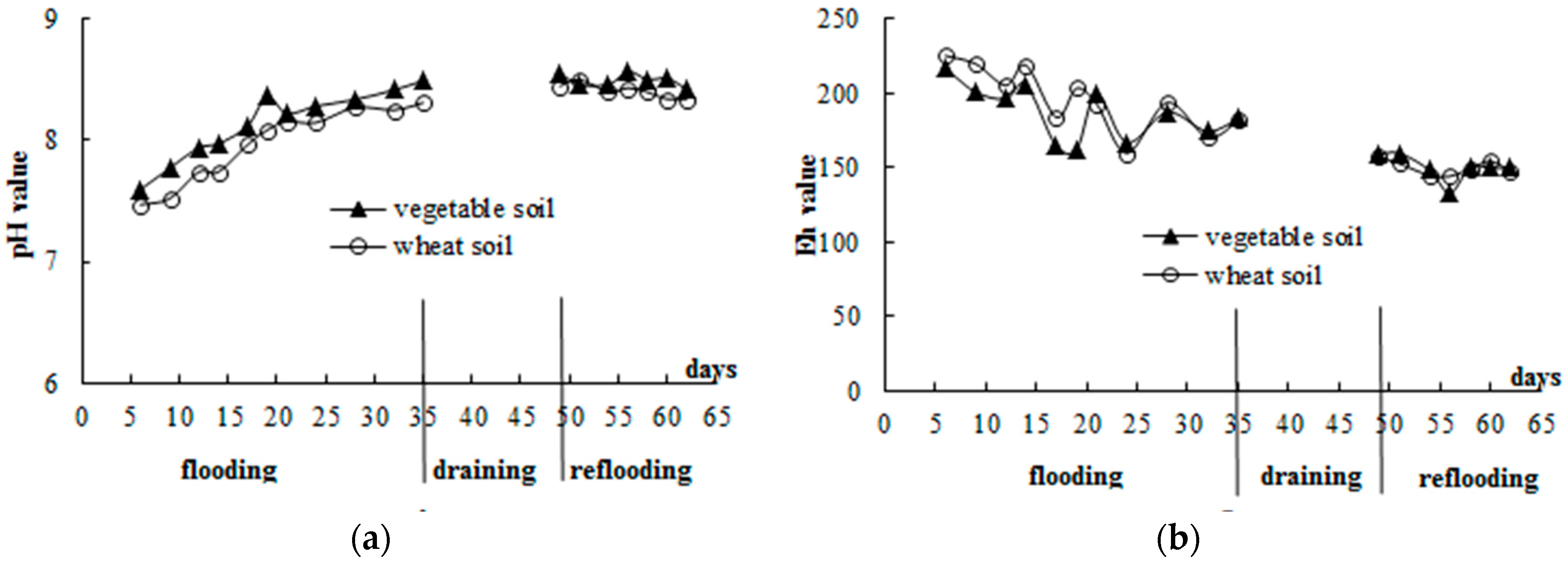
3.1. General Soil Properties
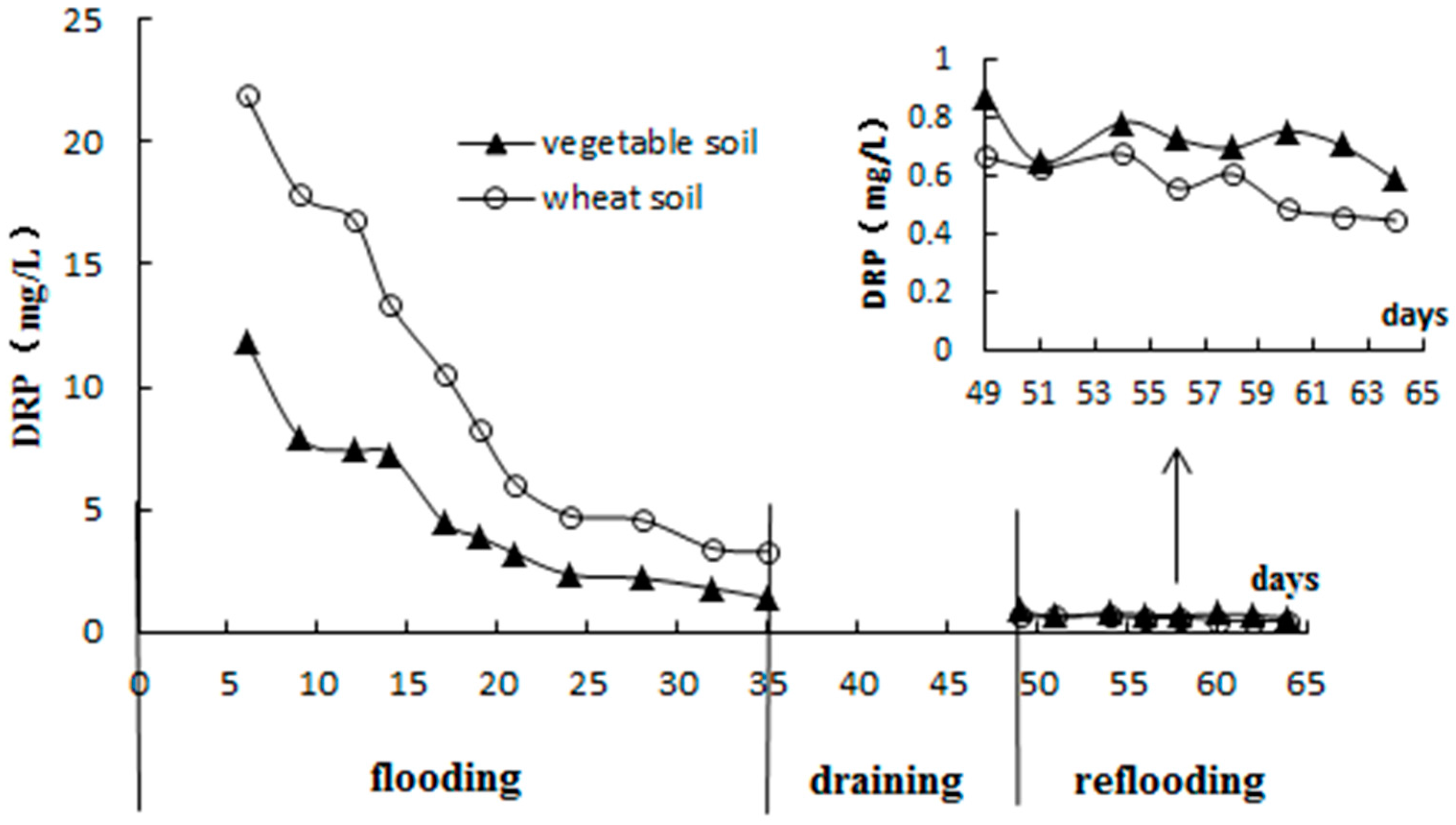
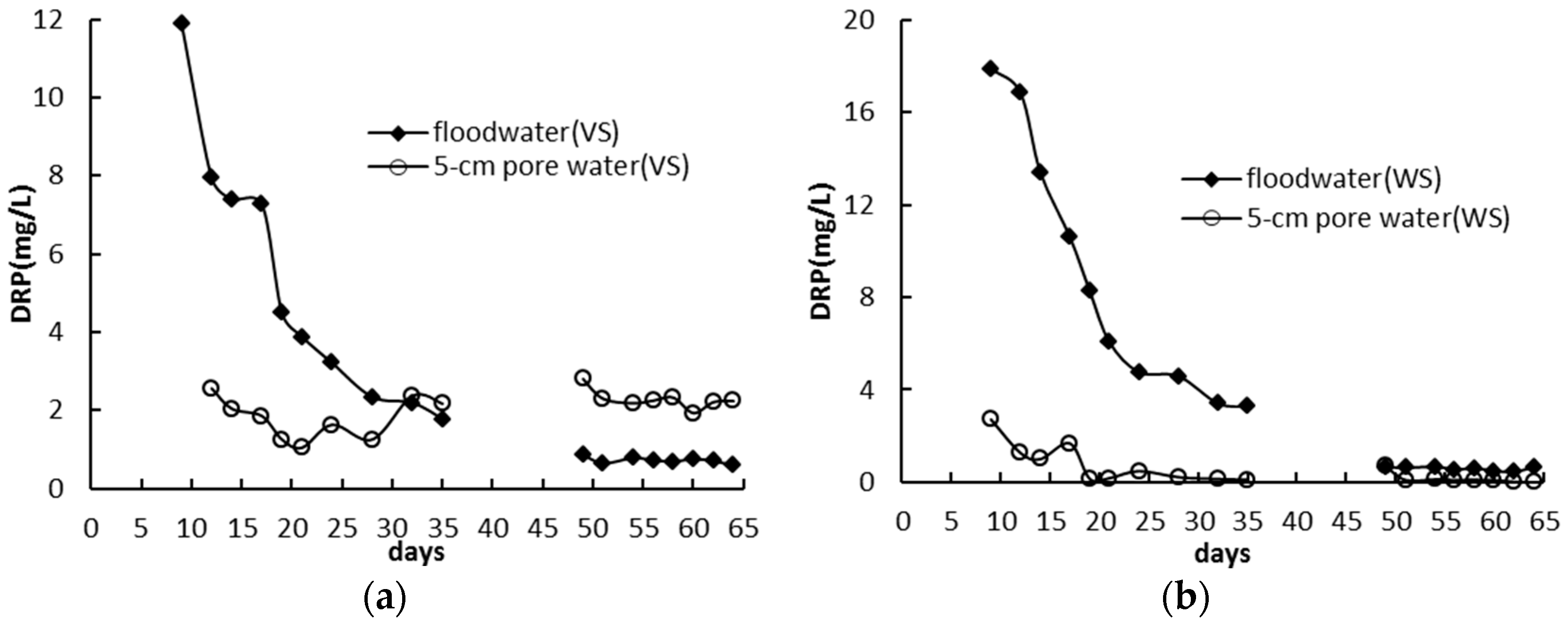
3.2. Standing Floodwater P Following Long-Term Flooding, Draining, and Reflooding
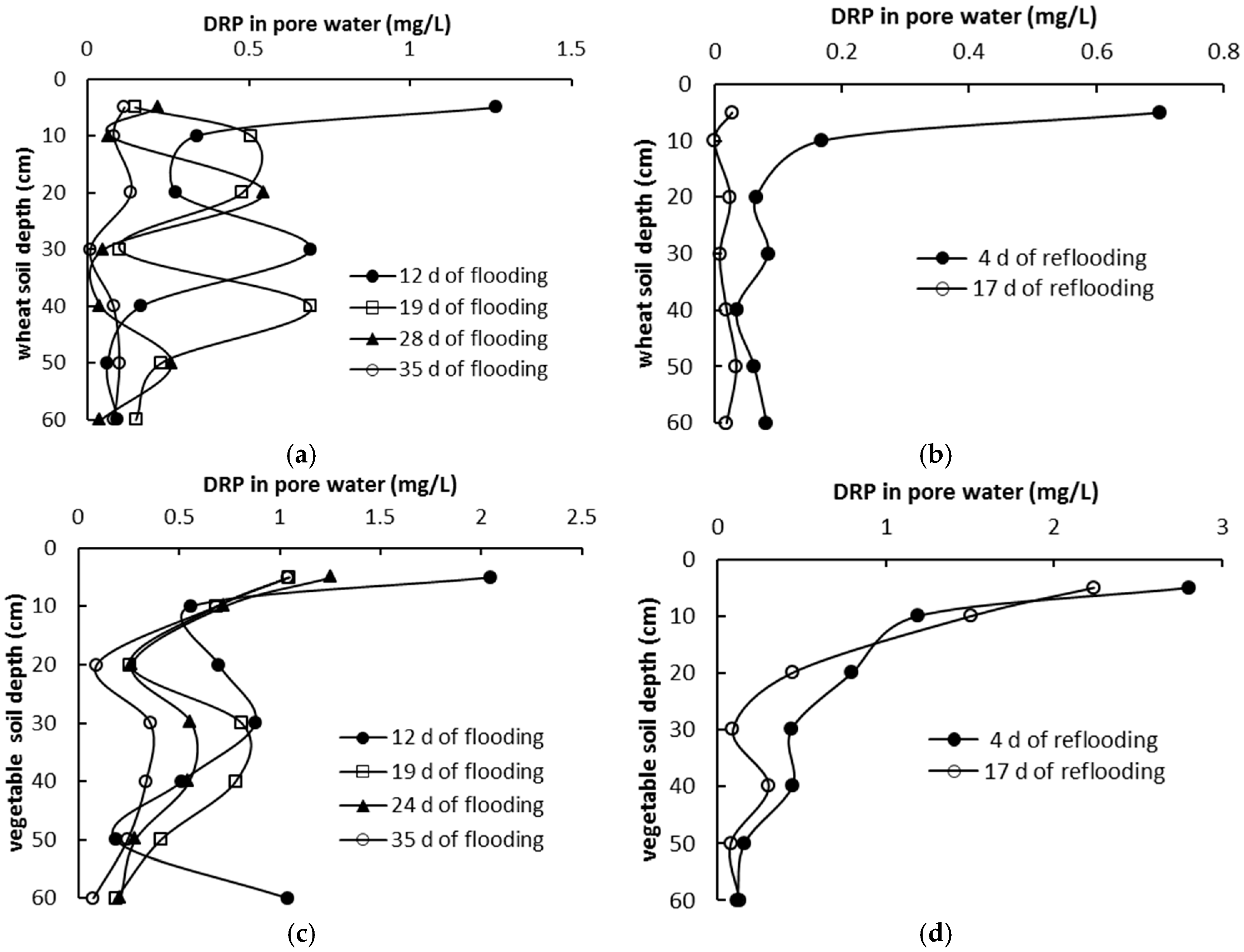
3.3. Phosphorus Distribution in Pore Water
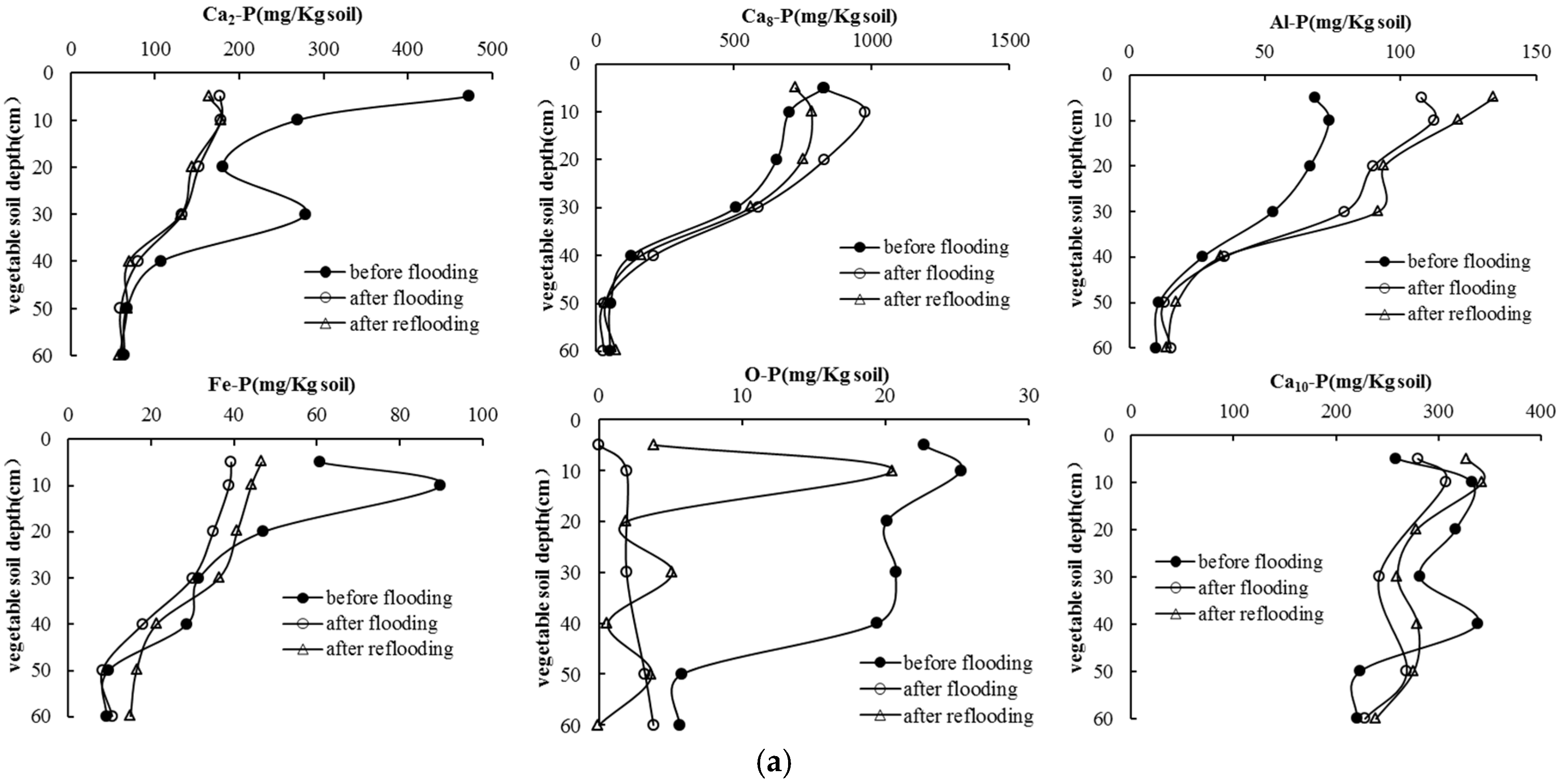
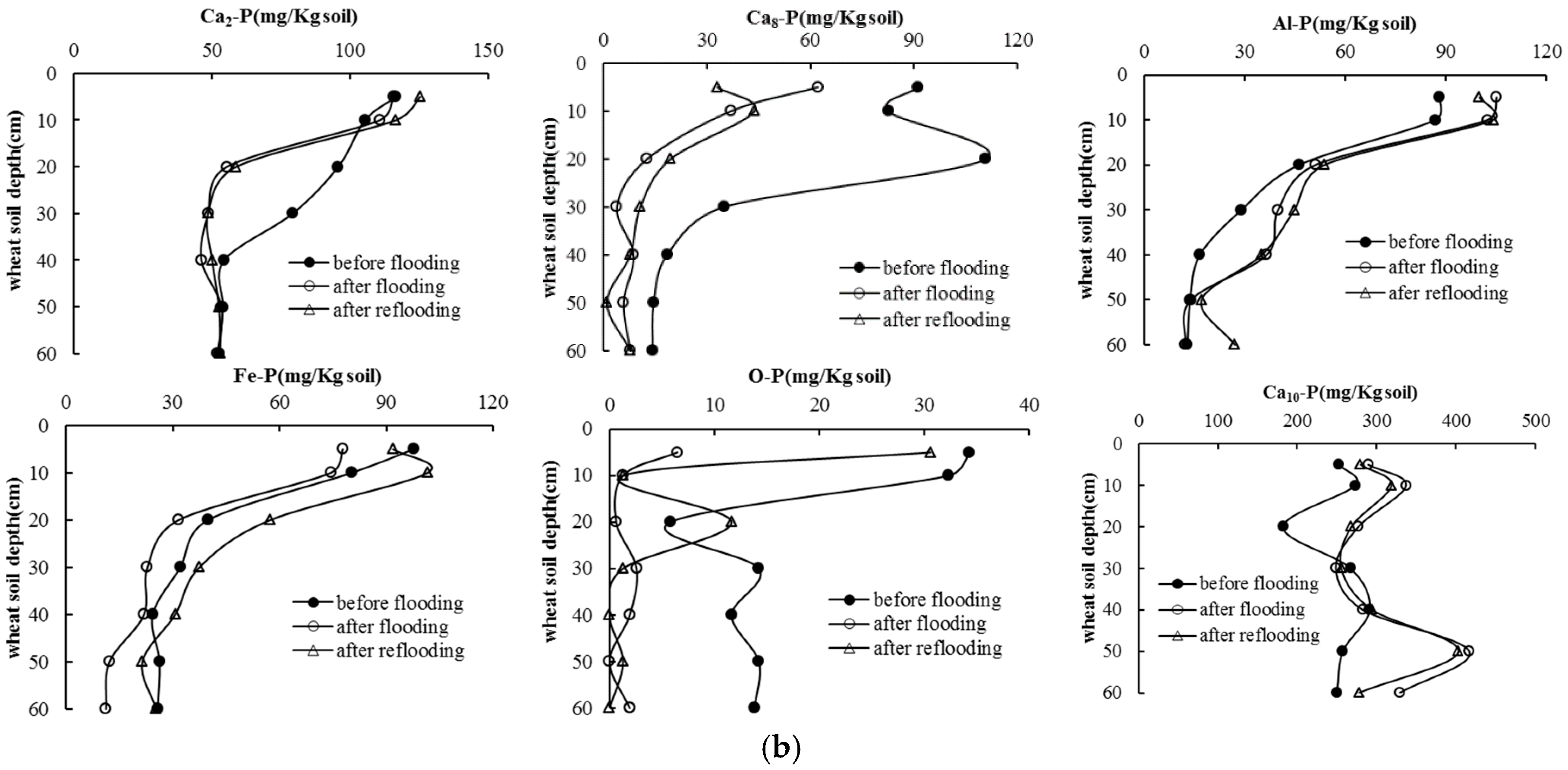
3.4. Soil P Following Long-Term Flooding, Draining, and Reflooding
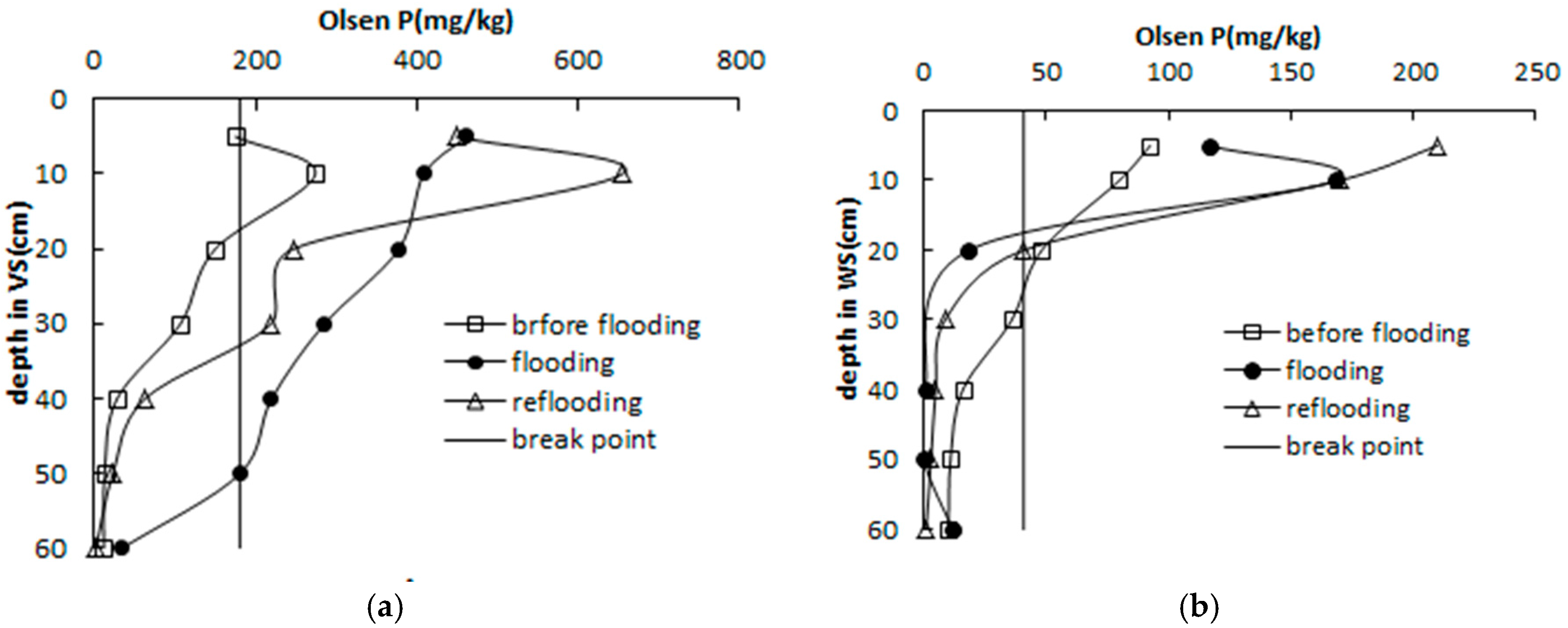
3.5. Relationships between Olsen P and Dissolved P in Pore Water
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| P | phosphorus |
| TP | total phosphorus |
| DRP | dissolved reactive phosphorus |
| Al-P | aluminum-P, extracted with 0.5 M NH4F |
| Fe-P | iron-P, extracted with 0.1 M NaOH-0.1 M Na2CO3 |
| Ca2-P | CaHPO4·2H2O, extracted with 0.25 M NaHCO3 |
| Ca8-P | Ca8H2(PO4)6·5H2O, extracted with 0.5 M H3COONH4 |
| Ca10-P | Ca10(PO4)6(OH)2, extracted with 0.5 M H2SO4 |
| O-P | occluded P |
| Olsen-P | extracted with 0.5 M NaHCO3 |
| VS | vegetable-growing soil |
| WS | wheat-growing soil |
| OM | organic matter |
References
- Schindler, D.W. Evolution of phosphorus limitation in lakes. Science 1977, 195, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Foy, R.H.; Withers, P.J.A. The Contribution of Agricultural Phosphorus to Eutrophication; Proceedings-Fertilizer Society No. 365; Greenhill House: Thorpe Wood, Petersborough, UK, 1995. [Google Scholar]
- Sims, J.T.; Simard, R.R.; Joern, B.C. Phosphorus loss in agricultural drainage: Historical perspective and current research. J. Environ. Qual. 1998, 27, 277–293. [Google Scholar] [CrossRef]
- Van der Molen, D.T.; Breeuwsma, A.; Boers, P.C.M. Agricultural nutrient losses to surface water in the Netherlands: Impact, strategies, and perspectives. J. Environ. Qual. 1998, 27, 4–11. [Google Scholar] [CrossRef]
- Yuan, Y.; Locke, M.A.; Bingner, R.L.; Rebich, R.A. Phosphorus losses from agricultural watersheds in the Mississippi Delta. J. Environ. Manag. 2013, 115, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5878. [Google Scholar] [CrossRef] [Green Version]
- Logan, T.J. Soils and environmental quality. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 155–169. [Google Scholar]
- Mulqueen, J.; Rodgers, M.; Scally, P. Phosphorus transfer from soil to surface waters. J. Agric. Water Manag. 2004, 68, 91–105. [Google Scholar] [CrossRef]
- Sah, R.N.; Mikkelsen, D.S. Sorption and bioavailability of phosphorus during the drainage period of flooded-drained soils. Plant Soil 1985, 92, 265–278. [Google Scholar] [CrossRef]
- Young, E.O.; Ross, D.S. Phosphate release from seasonally flooded soils: A laboratory microcosm study. J. Environ. Qual. 2001, 30, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Elie, O.H.; Kirk, G.J.D.; Frossard, E. Phosphorus uptake by rice from soil that is flooded, drained or flooded then drained. Eur. J. Soil Sci. 2003, 54, 77–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Werner, W. Effect of aerobic conditions in the rhizosphere of rice on the dynamics and availability of phosphorus in a flooded soil—A model experiment. J. Plant Nutr. Soil Sci. 2004, 167, 66–71. [Google Scholar] [CrossRef]
- Yang, C.M.; Yang, L.Z.; Lee, J.H. Organic phosphorus fraction in organically amended paddy soils in continuously and intermittently flooded conditions. J. Environ. Qual. 2006, 35, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Quintero, C.E.; Gutiérrez-Boem, F.H.; Befani, M.R.; Boschetti, N.G. Effect of soil flooding on P transformation in soils of the Mesopotamia region, Argentina. J. Plant Nutr. Soil Sci. 2007, 170, 500–505. [Google Scholar] [CrossRef]
- Tian, J.; Liu, L.; Ding, H.S.; Chen, T. Mobilization and transformation of phosphorus from water-soil interface of flooded soils. Environ. Sci. 2008, 29, 1818–1823. [Google Scholar]
- Wang, G.; Zhai, Z.; Liu, J.; Wang, J. Forms and profile distribution of soil phosphorus in four wetlands across gradients of sand desertification in Northeast China. Geoderma 2008, 145, 50–59. [Google Scholar] [CrossRef]
- Ma, L.; Rena, D.; Zhang, M.; Zhao, J. Phosphorus fractions and soil release in alternately waterlogged and drained environments at water-fluctuation-zone of the Three Gorges Reservoir. J. Food Agric. Environ. 2010, 8, 1329–1335. [Google Scholar]
- Amarawansha, E.A.G.; Kumaragamage, D.; Flater, D.; Zvomuya, F.; Tenuta, M. Phosphorus mobilization from manure-amended and unamended alkaline soils to overlying water during simulated flooding. J. Environ. Qual. 2015, 44, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, S.; Simard, R.R.; Cluis, D. Forms and concentration of phosphorus in drainage water of twenty-seven tile-drained soils. J. Environ. Qual. 1998, 27, 721–728. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Water Quality in Ireland (1995–1997); Environmental Protection Agency: Wexford, Ireland, 1999.
- Seng, V.; Bell, R.W.; Willett, I.R.; Nesbitt, H.J. Phosphorus nutrition of rice in relation to flooding and temporary loss of soil-water saturation in two lowland soils of Cambodia. Plant Soil 1999, 207, 121–132. [Google Scholar] [CrossRef]
- Vadas, P.A.; Sims, J.T. Phosphorus sorption in matured Atlantic coastal plain soils under flooded and drained conditions. J. Environ. Qual. 1999, 28, 1870–1877. [Google Scholar] [CrossRef]
- Chacon, N.; Flores, S.; Gonzalez, A. Implications of iron solubilization on soil phosphorus release in seasonally flooded forests of the lower Orinoco River, Venezuela. Soil Biol. Biochem. 2006, 38, 1494–1499. [Google Scholar] [CrossRef]
- Szilas, C.P.; Borggard, O.K.; Hansedn, H.C.B. Potential iron and phosphate mobilization during flooding of soil material. Water Air Soil Pollut. 1998, 106, 97–109. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar]
- Olila, O.G.; Reddy, K.R.; Stites, D.L. Influence of draining on soil phosphorus forms and distribution in a constructed wetland. Ecol. Engn. 1997, 9, 157–169. [Google Scholar] [CrossRef]
- Twinch, A.J. Phosphate exchange characteristics of wet and dried sediment samples from a hypereutrophic reservoir: Implications for the measurements of sediment phosphorus status. J. Water Res. 1987, 21, 1225–1230. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Sun, F. Agricultural phosphorus flow and its environmental impacts in China. Sci. Total Environ. 2008, 405, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kolahchi, Z.; Jalali, M. Phosphorus movement and retention by two calcareous soils. Soil Sediment Contam. 2013, 22, 21–38. [Google Scholar] [CrossRef]
- Ding, X.; Wei, C.; Wang, R. Phosphorus leaching risk assessment with manure fertilizer application in South China. Bull. Environ. Contam. Toxicol. 2014, 93, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). World Reference Base for Soil Resource; World Soil Resources Report 89; FAO: Rome, Italy, 2014. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.; Watanabe, F.C.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture (USDA): Washington, DC, USA, 1954.
- Lu, R.K. Soil Agrochemistry Analysis; China Agriculture Scientech Press: Beijing, China, 2000. [Google Scholar]
- Pizzerghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- Tian, J. Study on Phosphorus Chemical Behavior and Release Mechanics in Submerged Soils. Ph.D. Thesis, Hohai University, Nanjing, China, 2009. [Google Scholar]
- Karjalainen, S.M.; Ronkanen, A.; Heikkinen, K.; Kløve, B. Long-term accumulation and retention of Al, Fe and P in peat soils of northern treatment wetlands. Ecol. Eng. 2016, 93, 91–103. [Google Scholar] [CrossRef]
- Moore, P.A.; Reddy, K.R. Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida. J. Environ. Qual. 1994, 23, 955–964. [Google Scholar] [CrossRef]
- Sallade, Y.E.; Sims, J.T. Phosphorus transformation in the sediments of Delaware’s agricultural drainage ways: II Effect of reducing conditions on phosphorus release. J. Environ. Qual. 1997, 26, 1579–1588. [Google Scholar] [CrossRef]
- Bartlett, R.J.; James, B.R. System for categorizing redox status by chemical field testing. Geoderma 1995, 68, 211–218. [Google Scholar] [CrossRef]
- Hogan, D.M.; Jordan, T.E.; Walbridge, M.R. Phosphorus retention and soil organic carbon in restored and natural freshwater wetlands. Wetlands 2004, 24, 573–585. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Liu, S.; Liu, Y.J.; Zhang, L.Q.; Cao, Q.; Sun, D.Z. Seasonal variations and bioavailability of inorganic phosphorus in soils of Yeyahu Wetland in Beijing, China. Intl. J. Sediment Res. 2011, 26, 181–192. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, B. Distribution and availability of various forms of inorganic-P in calcareous soils. Soil Sci. 1992, 29, 80–86. [Google Scholar]
- Leher, J.R.; Brown, W.E. Calcium phosphate fertilizers. II. A petrographie study of their alteration in soils. Soil Sci. Soc. Am. Proc. 1958, 22, 29–32. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Frazier, A.W.; Stephenson, H.F. Identification of reaction products from phosphate fertilizers in soils. Soil Sci. Soc. Am. Proc. 1962, 26, 446–452. [Google Scholar] [CrossRef]
- Brandon, D.M. Phosphorus Transformation in Alternately Flooded Soils and Their Effects on Rice-Rotation Crops. Ph.D. Thesis, University of California, Davis, CA, USA, 1977. [Google Scholar]
- Dieter, D.; Herzog, C.; Hupfer, M. Effects of drying on phosphorus uptake in re-flooded lake sediments. Environ. Sci. Pollut. Res. 2015, 22, 17065–17081. [Google Scholar] [CrossRef] [PubMed]
- Heckrath, G.; Brookes, P.C.; Poulton, P.R.; Goulding, K.W.T. Phosphorus leaching from soils containing different phosphorus concentrations in the Broadbalk experiment. J. Environ. Qual. 1995, 24, 904–910. [Google Scholar] [CrossRef]
- Hesketh, N.; Brookes, P.C. Development of an indicator for risk of phosphorus leaching. J. Environ. Qual. 2000, 29, 105–110. [Google Scholar] [CrossRef]
- UK Ministry of Agriculture Fisheries and Food (MAFF). Fertilizer Recommendations for Agricultural and Horticultural Crops; Reference Book 209; His Majesty’s Stationery Office (HMSO): London, UK, 1991.
- Edwards, A.C.; Withers, P.J.A. Soil phosphorus management and water quality: A UK Perspective. Soil Use Manag. 1998, 14, 124–130. [Google Scholar] [CrossRef]
- Zhang, M.K.; Zhou, C.; Fang, L.P. Study on break point of environment in paddy soils. J. Agro-Environ. Sci. 2006, 2, 170–174. [Google Scholar]
- Wright, R.B.; Lockaby, B.G.; Walbridge, M.R. Phosphorus availability in an artificially flooded southeastern floodplain forest soil. Soil Sci. Soc. Am. J. 2001, 65, 1293–1302. [Google Scholar] [CrossRef]
- Sah, R.N.; Mikkelsen, D.S.; Hafez, A.A. Phosphorus behavior in flooded-drained soils: III. Phosphorus description and availability. Soil Sci. Soc. Am. J. 1989, 53, 1729–1732. [Google Scholar] [CrossRef]

| P Forms in Soil | Extractant | Reference | |
|---|---|---|---|
| TP | HNO3, HClO4, and H2SO4 at a 3:1:1 ratio | [32] | |
| Olsen P (soil available phosphorus) | 0.5 M NaHCO3 | [33] | |
| inorganic P fractions | Ca2-P (CaHPO4·2H2O) | 0.25 M NaHCO3 (pH 7.5) | [34] |
| Ca8-P (Ca8H2(PO4)6·5H2O) | 0.5 M H3COONH4 (pH 4.2) | [34] | |
| Al-P | 0.5 M NH4F (pH 8.2) | [34] | |
| Fe-P | 0.1 M NaOH-0.1 M Na2CO3 | [34] | |
| O-P (occluded P) | 0.3 M Na2S2O4-0.5 M NaOH | [34] | |
| Ca10-P (Ca10(PO4)6(OH)2) | 0.5 M H2SO4 | [34] | |
| Soil Profile cm | pH | Total P g/kg | OM mg/kg | Olsen P mg/kg | Sand % | Silt % | Clay % |
|---|---|---|---|---|---|---|---|
| Vegetable-growing soil | |||||||
| 0–5 | 7.31 | 1.91 | 203.86 | 175.29 | 5.23 | 55.41 | 39.36 |
| 5–15 | 7.31 | 2.01 | 210.32 | 274.21 | 0.38 | 57.87 | 41.75 |
| 15–25 | 7.41 | 1.71 | 147.63 | 150.02 | 5.48 | 53.18 | 41.34 |
| 25–35 | 7.48 | 1.44 | 109.40 | 106.88 | 0.54 | 64.87 | 34.59 |
| 35–45 | 7.57 | 0.75 | 104.33 | 28.37 | 5.32 | 43.83 | 50.85 |
| 45–60 | 7.52 | 0.64 | 96.61 | 13.02 | 3.99 | 36.77 | 59.24 |
| Wheat-growing soil | |||||||
| 0–5 | 7.62 | 1.13 | 188.11 | 92.50 | 9.77 | 73.00 | 17.23 |
| 5–15 | 7.64 | 1.06 | 82.12 | 79.87 | 9.60 | 72.85 | 17.55 |
| 15–25 | 7.83 | 0.62 | 34.60 | 48.19 | 12.00 | 75.05 | 12.95 |
| 25–35 | 7.90 | 0.55 | 26.17 | 35.95 | 6.82 | 73.62 | 19.56 |
| 35–45 | 7.96 | 0.54 | 58.78 | 15.93 | 4.65 | 54.46 | 40.89 |
| 45–60 | 7.96 | 0.58 | 24.48 | 10.88 | 5.35 | 33.98 | 60.67 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Dong, G.; Karthikeyan, R.; Li, L.; Harmel, R.D. Phosphorus Dynamics in Long-Term Flooded, Drained, and Reflooded Soils. Water 2017, 9, 531. https://doi.org/10.3390/w9070531
Tian J, Dong G, Karthikeyan R, Li L, Harmel RD. Phosphorus Dynamics in Long-Term Flooded, Drained, and Reflooded Soils. Water. 2017; 9(7):531. https://doi.org/10.3390/w9070531
Chicago/Turabian StyleTian, Juan, Guiming Dong, Raghupathy Karthikeyan, Lin Li, and R. Daren Harmel. 2017. "Phosphorus Dynamics in Long-Term Flooded, Drained, and Reflooded Soils" Water 9, no. 7: 531. https://doi.org/10.3390/w9070531





