Temporal and Spatial Variations of Hydrological Processes on the Landscape Zone Scale in an Alpine Cold Region (Mafengou River Basin, China): An Update
Abstract
:1. Introduction
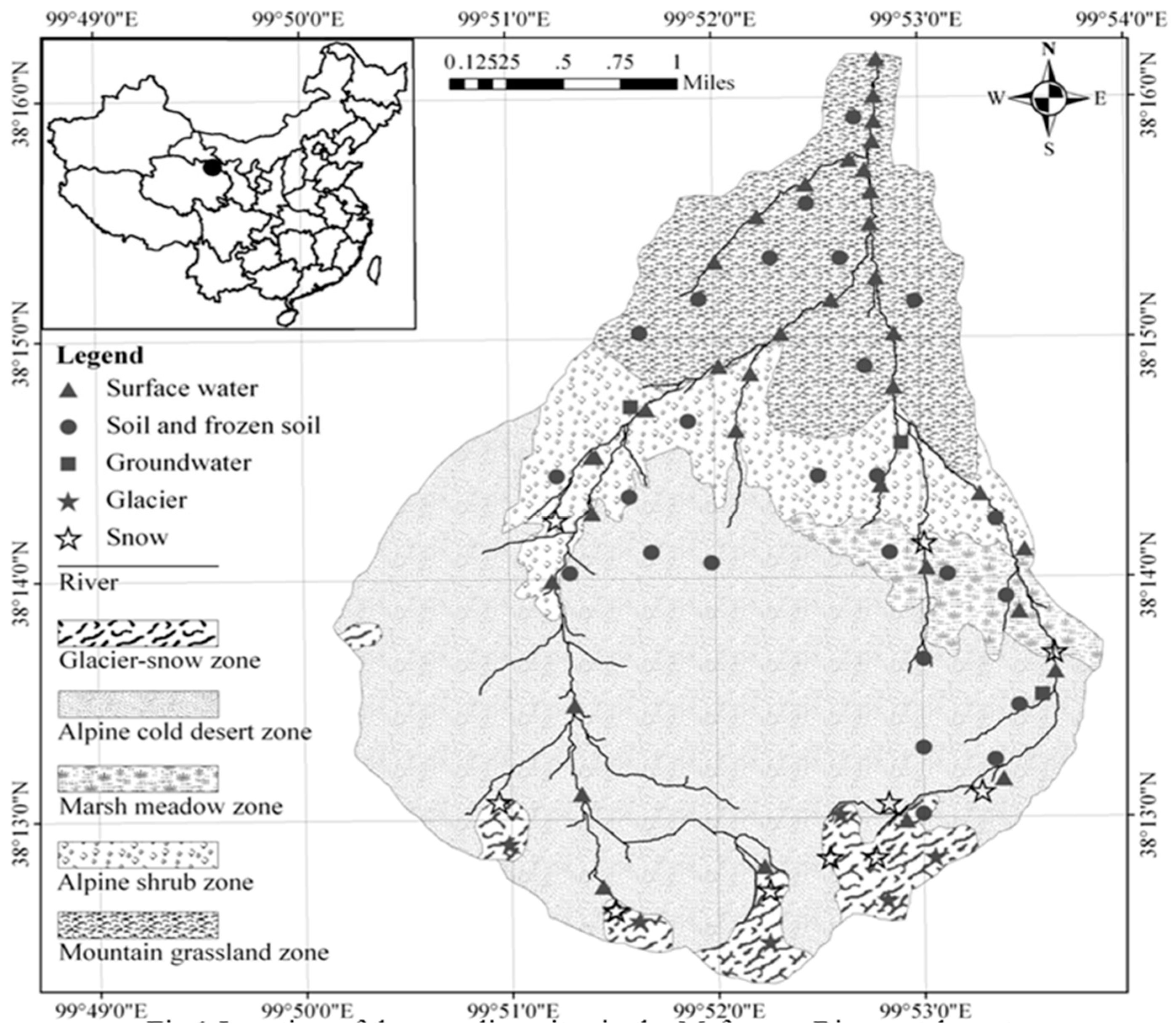
2. Area Description
2.1. Site Description
2.2. Hydrogeological Background
3. Material and Methods
3.1. Sample Collection
3.2. Laboratory Analyses
4. Results and Discussion
4.1. Temporal and Spatial Variations of Chemical Characteristics
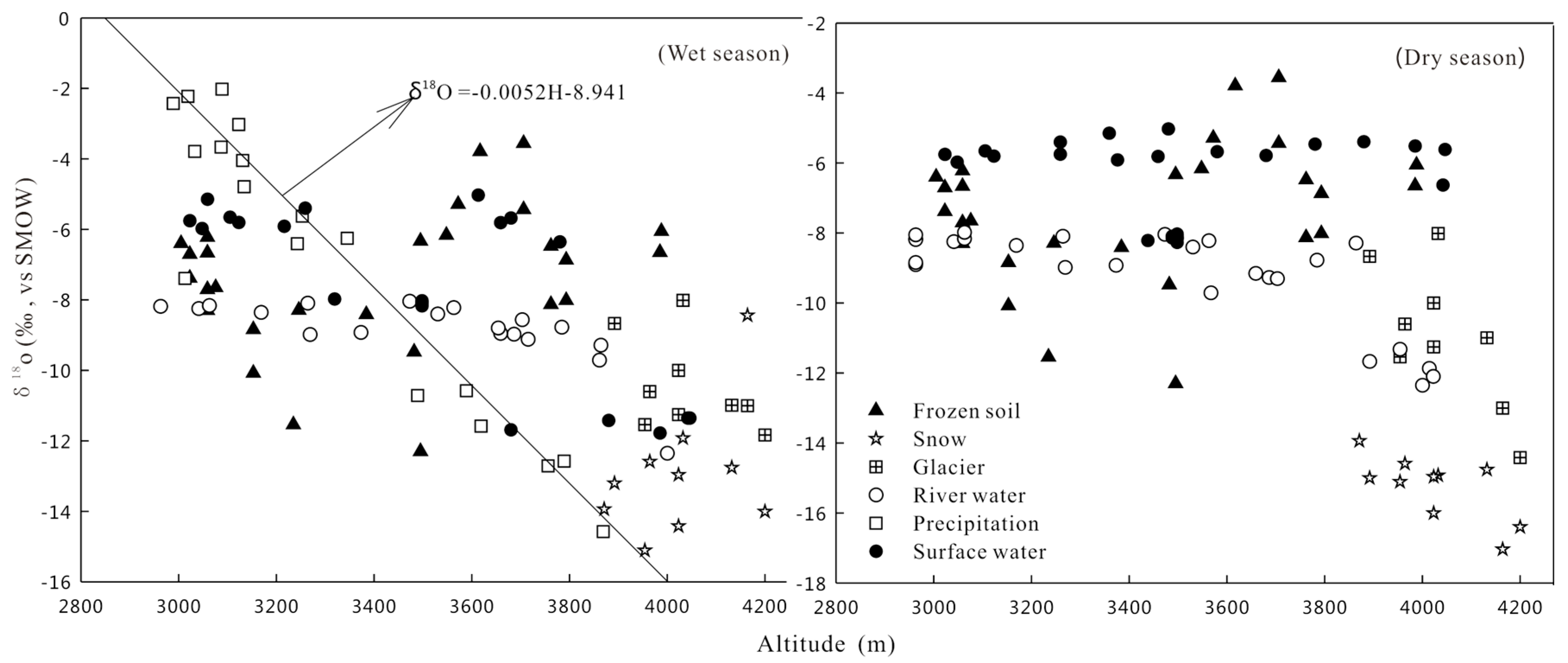
4.2. Altitude Variations of Hydrological Processes in the Study Area
4.3. Temporal and Spatial Variations of Runoff Process
4.4. Variations of the Contributions of Runoff
5. Conclusions
- i.
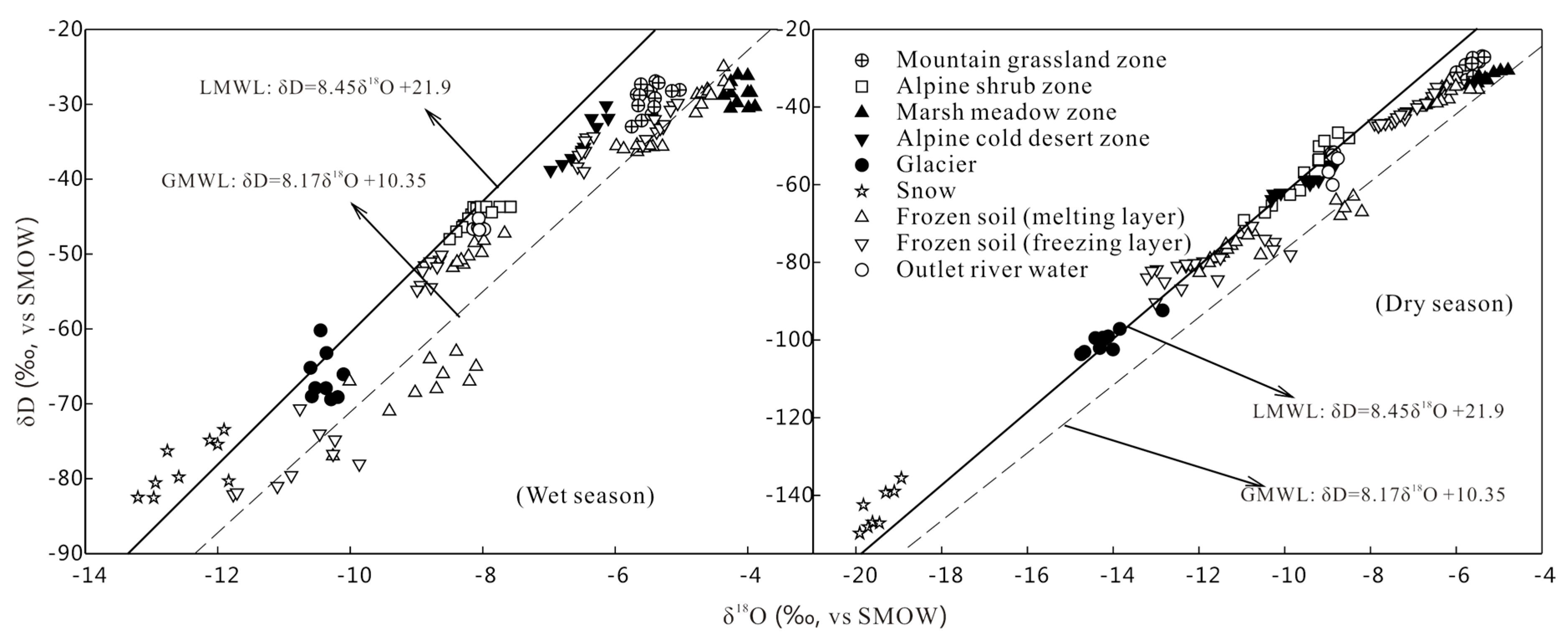
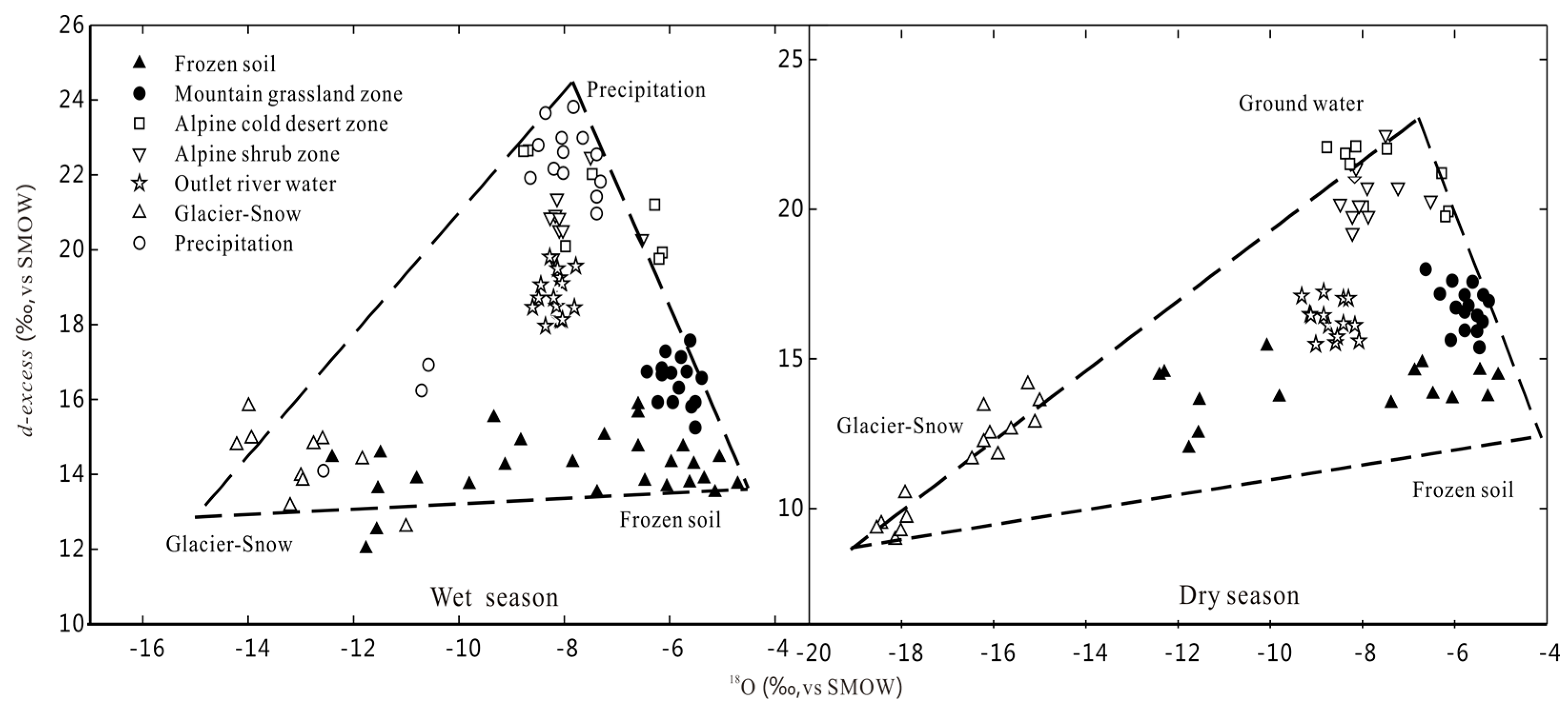
- There is not a significant difference in the water chemical characteristics of various waterbodies. There is no obvious seasonal variation, but obvious hydration credits zone existed. Various waterbodies are relatively negative in δ18O and δD in the dry season, but enriched in δ18O and δD in wet season, which exhibits seasonal variation. In the wet season, higher temperatures lead to isotope fractionation effects caused by evaporation, and secondary evaporation easily occurs during the wet season, leading to enrichment in δ18O and δD. In the dry season, the temperature is low, and water vapor comes from the evaporation of local waterbodies. Precipitation is in the solid state in the cold season and evaporation is weak. Therefore, the isotope value is low, leading to negative in δ18O and δD.
- ii.
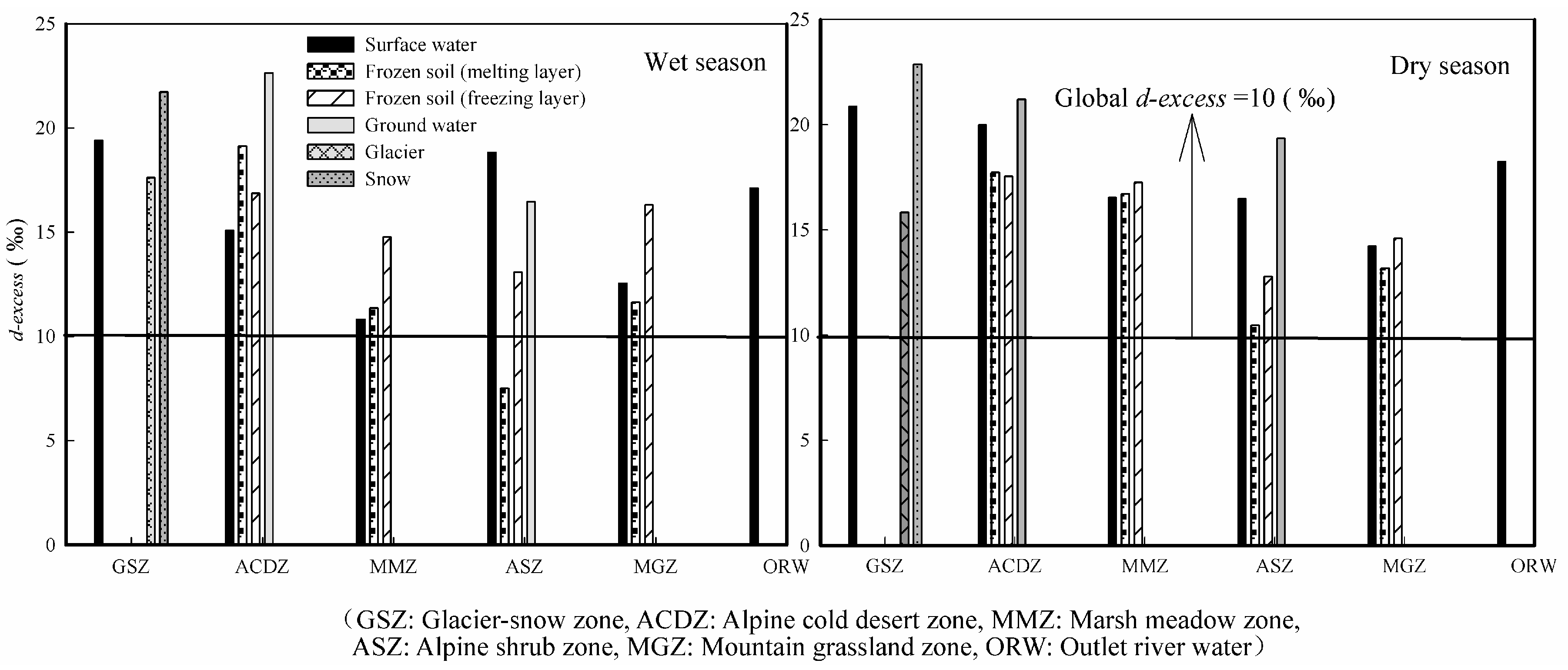
- The difference of d-excess value in the alpine cold mountainous region reflects the complexity of moisture sources and the extreme climate. The d-excess values of various waterbodies in the each of the landscape zones are higher than the global average value (10‰) due to the high altitude, strong solar radiation and strong evaporation in the Mafengou River basin. It is strongly affected by the local climate and environment, and leads to high d-excess.
- iii.
- The isotopic components of precipitation exhibits altitude effects. The δ18O and δD values are decreased with increased altitude. However, there are no obvious altitude effects among surface water during the wet and dry season. This is because it is not only recharged by precipitation, but also by meltwater of glacier, snow and frozen soil. The transformations of groundwater and surface water lead to the change of isotopic composition. Therefore, homogenization effects are the controlling factor of the change of isotopic components of various waterbodies. Therefore elevation effects cannot be applied to determine the recharged level of groundwater or river water in the Mafengou River Basin.
- iv.
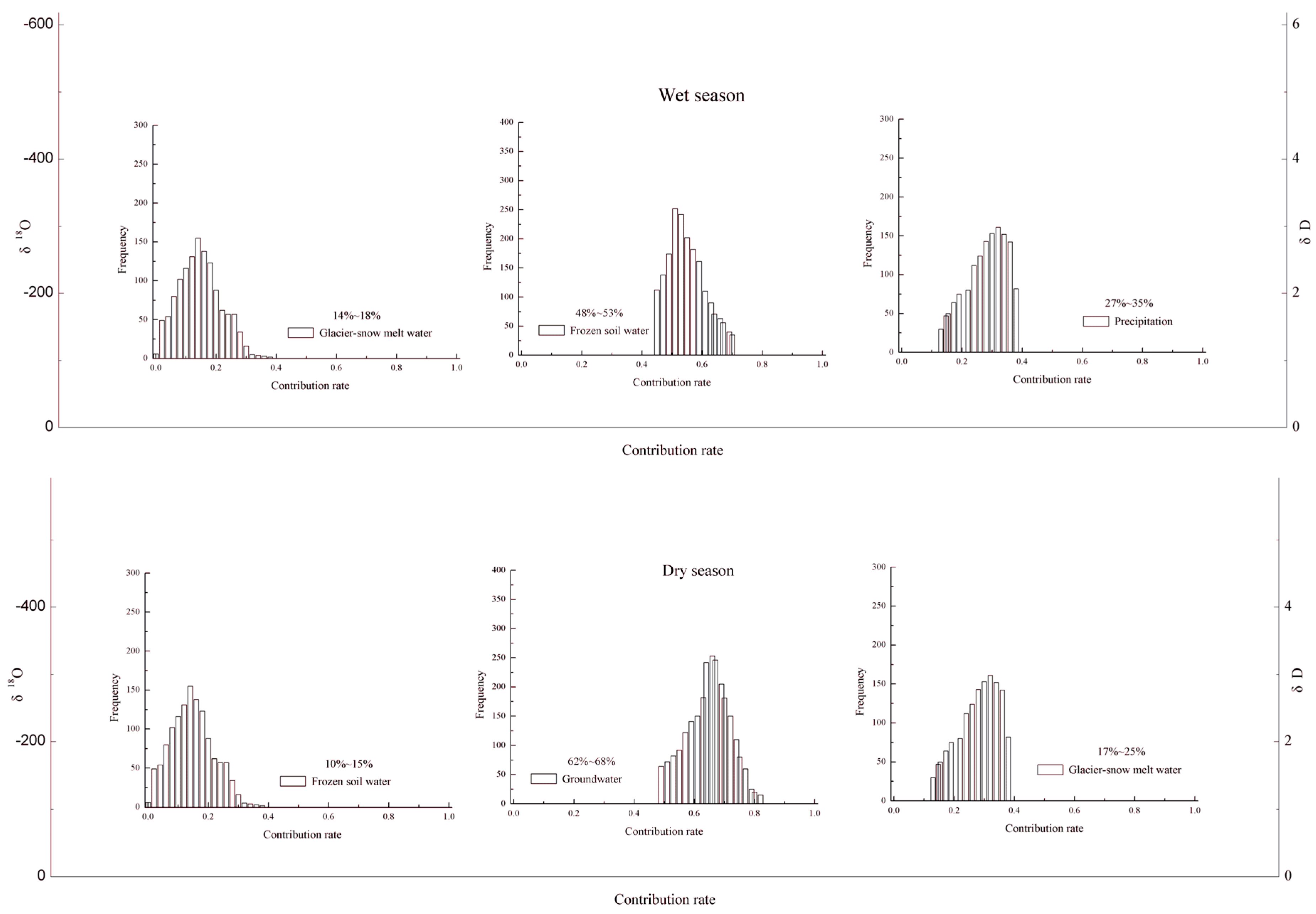
- The river water is mainly recharged by thawed frozen soil water and precipitation in the wet season, but glacier snow meltwater with negative in δ18O and δD is relatively less (14~18%). In the dry season, glacier snow meltwater and groundwater are the dominant source of the outlet river water, but thawed frozen soil water is relatively less (10~15%).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Geldern, R.; Hayashi, T.; Böttcher, M.E.; Mottl, M.J.; Barth, J.A.C.; Stadler, S. Stable isotope geochemistry of pore waters and marine sediments from the New Jersey shelf: Methane formation and fluid origin. Geosphere 2013, 9, 96–112. [Google Scholar] [CrossRef]
- Steen-Larsen, H.C.; Sveinbjörnsdottir, A.E.; Jonsson, T.H.; Johnsen, S.J. Monitoring the water vapor isotopic composition in the temperate North Atlantic. Geophys. Res. Abstr. 2012, 14, 1562. [Google Scholar]
- Yang, Y.G.; Xiao, H.L.; Zou, S.B.; Zhao, L.J.; Hou, L.G.; Wang, F. Hydrochemical and hydrological processes in the different landscape zones of alpine cold region in China. Environ. Earth Sci. 2012, 65, 609–620. [Google Scholar] [CrossRef]
- Chagué-Goff, C.; Mark, A.F.; Dickinson, K.J.M. Hydrological processes and chemical characteristics of low-alpine patterned wetlands, south-central New Zealand. J. Hydrol. 2010, 385, 105–119. [Google Scholar] [CrossRef]
- Stumpp, C.; Maloszewski, P.; Stichler, W.; Fank, J. Environmental isotope (δ18O) and hydrological data to assess water flow in unsaturated soils planted with different crops: Case study lysimeter station “Wagna” (Austria). J. Hydrol. 2009, 369, 198–208. [Google Scholar] [CrossRef]
- Mul, M.L.; Mutiibwa, R.K.; Uhlenbrook, S.; Savenije, H.H.G. Hydrograph separation using hydrochemical tracers in the Makanya catchment, Tanzania. Phys. Chem. Earth 2008, 33, 151–156. [Google Scholar] [CrossRef]
- Malinovsky, D.; Hammarlund, D.; Ilyashuk, B.; Martinsson, O.; Gelting, J. Variations in the isotopic composition of molybdenum in freshwater lake systems. Chem. Geol. 2007, 236, 181–198. [Google Scholar] [CrossRef]
- Zeng, H.A.; Wu, J.L. Water isotopic and hydrochemical characteristics and causality in Tajikistan. Adv. Water Sci. 2013, 24, 272–279. [Google Scholar]
- Yang, Y.G.; Xiao, H.L.; Wei, Y.P.; Zou, S.B.; Yang, Q.; Yin, Z.L. Hydrological processes in the different landscape zones of alpine cold regions in the wet season, combining isotopic and hydrochemical tracers. Hydrol. Process. 2012, 26, 1457–1466. [Google Scholar] [CrossRef]
- McInerney, F.A.; Helliker, B.R.; Freeman, K.H. Hydrogen isotope ratios of leaf wax n-alkanes in grasses are insensitive to transpiration. Geochim. Cosmochim. Acta 2011, 75, 541–554. [Google Scholar] [CrossRef]
- Han, D.M.; Liang, X.; Jin, M.G.; Currell, M.J.; Song, X.F.; Liu, C.M. Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou Basin. J. Volcanol. Geotherm. Res. 2010, 189, 92–104. [Google Scholar] [CrossRef]
- Wu, P.; Tang, C.Y.; Zhu, L.J.; Liu, C.Q.; Cha, X.F.; Tao, X.Z. Hydrogeochemical characteristics of surface water and groundwater in the karst basin, southwest China. Hydrol. Process. 2009, 23, 2012–2022. [Google Scholar] [CrossRef]
- Liu, F.; Parmenter, R.; Brooks, P.D.; Conklin, M.H.; Bales, R.C. Seasonal and interannual variation of streamflow pathways and biogeochemical implications in semi-arid, forested catchments in Valles Caldera, New Mexico. Ecohydrology 2008, 1, 239–252. [Google Scholar] [CrossRef]
- Ohlanders, N.; Rodriguez, M.; McPhee, J. Stable water isotope variation in a Central Andean watershed dominated by glacier and snowmelt. Hydrol. Earth Syst. Sci. 2013, 17, 1035–1050. [Google Scholar] [CrossRef]
- Turner, K.W.; Wolfe, B.B.; Edwards, T.W.D. Characterizing the role of hydrological processes on lake water balances in the old Crow Flats, Yukon Territory, Canada, using water isotope tracers. J. Hydrol. 2010, 386, 103–117. [Google Scholar] [CrossRef]
- Long, A.J. Hydrograph separation for karst watersheds using a two-domain rainfall-discharge model. J. Hydrol. 2009, 364, 249–256. [Google Scholar] [CrossRef]
- Longinelli, A.; Stenni, B.; Genoni, L.; Flora, O.; Defrancesco, C.; Pellegrini, G. A stable isotope study of the Garda Lake, northern Italy: Its hydrological balance. J. Hydrol. 2008, 360, 103–116. [Google Scholar] [CrossRef]
- Dutton, A.; Wilkinson, B.H.; Welker, J.M.; Bowen, G.J.; Lohmann, K.C. Spatial distribution and seasonal variation in 18O/16O of modern precipitation and river water across the conterminous USA. Hydrol. Process. 2005, 19, 4121–4146. [Google Scholar] [CrossRef]
- Caley, T.; Roche, D.M. δ18O water isotope in the iLOVECLIM model (version 1.0)—Part 3: A paleo-perspective based on present-day data-model comparison for oxygen stable isotopes in carbonates. Geosci. Model Dev. 2013, 6, 1505–1516. [Google Scholar] [CrossRef]
- Barbieri, M.; Battistel, M.; Boschetti, T. Chemical and isotope monitoring at Lake Albano (central Italy): Water-rock interaction and climate change effects. Procedia Earth Planet. Sci. 2013, 7, 53–56. [Google Scholar] [CrossRef]
- MacKinnon, G.; MacKenzie, A.B.; Cook, G.T.; Pulford, I.D.; Duncan, H.J.; Scott, E.M. Spatial and temporal variations in Pb concentrations and isotopic composition in road dust, farmland soil and vegetation in proximity to roads since cessation of use of leaded petrol in the UK. Sci. Total Environ. 2011, 409, 5010–5019. [Google Scholar] [CrossRef] [PubMed]
- Mul, M.L.; Savenije, H.H.G.; Uhlenbrook, S. Spatial rainfall variability and runoff response during an extreme event in a semi-arid catchment in the South Pare Mountains, Tanzania. Hydrol. Earth Syst. Sci. Discuss. 2009, 13, 1659–1670. [Google Scholar] [CrossRef]
- Liu, Y.H.; Fan, N.J.; Yang, H.B.; An, S.Q.; Wang, Z.S.; Wu, C.; Zhan, J.H.; Liu, S.R.; Cheng, W.L. Spatial and temporal variation of runoff and contributions of different water sources in the alpine valley. J. Nanjing For. Univ. 2007, 31, 23–26. [Google Scholar]
- Harvey, F.E.; Welker, J.M. Stable isotopic composition of precipitation in the semi-arid north-central portion of the US Great Plains. J. Hydrol. 2000, 238, 90–109. [Google Scholar] [CrossRef]
- Singh, B.P. Isotopic composition of water in precipitation in a region or place. Appl. Radiat. Isot. 2013, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Carucci, V.; Petitta, M.; Aravena, R. Interaction between shallow and deep aquifers in the Tivoli Plain (Central Italy) enhanced by groundwater extraction: A multi-isotope approach and geochemical modeling. Appl. Geochem. 2012, 27, 266–280. [Google Scholar] [CrossRef]
- Engström, E.; Rodushkin, I.; Ingri, J.; Baxter, D.C.; Ecke, F.; Österlund, H.; Öhlander, B. Temporal isotopic variations of dissolved silicon in a pristine boreal river. Chem. Geol. 2010, 271, 142–152. [Google Scholar] [CrossRef]
- Iqbal, M.Z. Short-term variability in isotopic composition of precipitation: A case study from the Midwestern United States. Hydrol. Process. 2008, 22, 4609–4619. [Google Scholar] [CrossRef]
- Yoshimura, K.; Oki, T.; Ohte, N.; Kanae, S. A quantitative analysis of short-term 18O variability with a Rayleigh-type isotope circulation model. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef]
- Takao, O.; Kunihiko, S.; Kazuki, U. Oxygen and hydrogen isotopic preference in hydration spheres of Magnesium and Calcium Ions. Z. Naturforsch. 2013, 68, 362–370. [Google Scholar]
- Kurita, N.; Noone, D.; Risi, C.; Schmidt, G.A.; Yamada, H.; Yoneyama, K. Intraseasonal isotopic variation associated with the Madden-Julian Oscillation. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Rashid, U.; Ahmed, I.; Alam, F.; Mohammad, M.K. Hydrochemical characteristics and seasonal variations in groundwater quality of an alluvial aquifer in parts of Central Ganga Plain, Western Uttar Pradesh, India. Environ. Geol. 2009, 58, 1295–1300. [Google Scholar]
- Yao, Z.J.; Liu, J.; Huang, H.Q.; Song, X.F.; Dong, X.H.; Liu, X. Characteristics of isotope in precipitation, river water and lake water in the Manasarovar basin of Qinghai–Tibet Plateau. Environ. Geol. 2009, 57, 551–556. [Google Scholar] [CrossRef]
- Ogrinc, N.; Kanduc, T.; Stichler, W.; Vreca, P. Spatial and seasonal variations in δ18O and δD values in the River Sava in Slovenia. J. Hydrol. 2008, 359, 303–312. [Google Scholar] [CrossRef]
- Yamanaka, T.; Tsujimura, M.; Oyunbaatar, D.; Davaa, G. Isotopic variation of precipitation over eastern Mongolia and its implication for the atmospheric water cycle. J. Hydrol. 2007, 333, 21–34. [Google Scholar] [CrossRef]
- Weyhenmeyer, C.E.; Burns, S.J.; Waber, H.N. Isotope study of moisture sources, recharge areas, and groundwater flow paths within the eastern Batinah coastal plain, Sultanate of Oman. Water Resour. Res. 2002, 38, 1184–1206. [Google Scholar] [CrossRef]
- Lee, K.S.; Wenner, D.B.; Lee, I. Using H-and O-isotopic data for estimating the relative contributions of rainy and dry season precipitation to groundwater: Example from Cheju Island, Korea. J. Hydrol. 1999, 222, 65–74. [Google Scholar] [CrossRef]
- Yang, Y.G.; Xiao, H.L.; Wei, Y.P.; Zhao, L.J.; Zou, S.B.; Yin, Z.L.; Yang, Q. Hydrologic processes in the different landscape zones of Mafengou River basin in the alpine cold region during the melting period. J. Hydrol. 2011, 409, 149–156. [Google Scholar] [CrossRef]
- Nie, Z.L. Ental isotopes as tracers of hydrologic cycle in the recharge of the Heihe River. Geogr. Inf. Sci. 2005, 21, 104–108. [Google Scholar]
- Zhu, G.F.; Su, Y.H.; Feng, Q. The hydrochemical characteristics and evolution of groundwater and surface water in the Heihe River Basin, northwest China. Hydrol. J. 2008, 16, 167–182. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, W.; Su, Y.H.; Zhang, Y.W.; Si, J.H. Distribution and evolution of water chemistry in Heihe River basin. Environ. Geol. 2004, 45, 947–956. [Google Scholar] [CrossRef]
- Appelo, C.A.J. Geochemical experimentation and modeling are tools for understanding the origin of as in groundwater in Bangladesh and elsewhere. In Arsenic in Groundwater—A World Problem; NNC-IAH Pub.: Utrecht, The Netherlands, 2008; Volume 5, pp. 33–50. [Google Scholar]
- Appelo, C.A.J.; Van Loon, L.R.; Wersin, P. Multicomponent diffusion of a suite of tracers (HTO, Cl, Br, I, Na, Sr, Cs) in a single sample of Opalinus Clay. Geochim. Cosmochim. Acta 2010, 74, 1201–1219. [Google Scholar] [CrossRef]
- Etzel, T.M.; Bowman, J.R.; McCulloch, J.M.; Moore, J.N.; Spicuzza, M.J.; Valley, J.W. Oxygen isotopic evidence of water-rock interactions in the Coso geothermal system. In Proceedings of the 38th Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 11–13 February 2013. [Google Scholar]
- Merlivat, L.; Jouzel, J. Global climatic interpretation of the deuterium-oxygen 18 relationship for precipitation. Geophys. Res. 1979, 84, 5029–5033. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
| Sampling Site | Location Area | SI(anhydrite) | SI(aragonite) | SI(calcite) | CO2(g) | SI(dolomite) | SI(fluorite) | SI(gypsum) | SI(salt) |
|---|---|---|---|---|---|---|---|---|---|
| G-09-06 | Glacier snow | −3.57 | −0.81 | −0.66 | −1.82 | −1.89 | −3.84 | −3.35 | −11.00 |
| C-09-1 | Alpine cold desert | −2.96 | −1.11 | −0.97 | −1.92 | −1.95 | −3.90 | −2.74 | −10.47 |
| S-09-1 | Alpine shrub | −2.96 | 0.31 | 0.47 | −3.93 | 0.55 | −3.56 | −2.70 | −10.48 |
| SP-09-6 | Spring lower | −1.94 | 0.70 | 0.86 | −3.42 | 1.47 | −3.08 | −1.69 | −9.07 |
| Flow Path | Anhydrite | Aragonite | Calcite | Dolomite | Fluorite | Gypsum | Salt |
|---|---|---|---|---|---|---|---|
| G-09-06→S-09-1 | 2.281 × 10−5 | 2.165 × 10−4 | 3.646 × 10−5 | 1.375 × 10−5 | |||
| G-09-06→C-09-1 | 8.116 × 10−5 | −3.574 × 10−4 | 3.506 × 10−4 | 9.243 × 10−5 | 7.936 × 10−6 | ||
| C-09-1→S-09-1 | 3.297 × 10−4 | 6.359 × 10−7 | 9.089 × 10−6 |
| Landscape Zones | Samples Type | NO (420) | Wet Season | Dry Season | ||
|---|---|---|---|---|---|---|
| δ18O (‰) | δD (‰) | δ18O (‰) | δD (‰) | |||
| Glacier snow | Surface water | 36 | −9.69 ± 0.73 | −67.03 ± 4.6 | −16.03 ± 0.8 | −78.14 ± 1.11 |
| Glacier | 20 | −12.82 ± 0.51 | −82.34 ± 3.1 | −17.30 ± 1.02 | −99.34 ± 3.3 | |
| Snow | 20 | −17.13 ± 1.21 | −107.3 ± 2.86 | −19.60 ± 1.62 | −113.42 ± 8.5 | |
| Alpine cold desert | Surface water | 36 | −7.20 ± 1.2 | −39.86 ± 5.2 | −9.47 ± 1.8 | −57.56 ± 3.7 |
| Groundwater | 24 | −11.95 ± 0.18 | −80.23 ± 2.8 | −12.15 ± 0.23 | −81.64 ± 1.9 | |
| Frozen soil (surface) | 24 | −10.45 ± 2.2 | −72.82 ± 2.1 | −11.36 ± 1.74 | −76.12 ± 1.2 | |
| Frozen soil (deep) | 24 | −11.64 ± 0.42 | −80.71 ± 2.3 | −11.43 ± 0.74 | −80.19 ± 2.4 | |
| Marsh meadow | Surface water | 36 | −4.75 ± 0.21 | −30.37 ± 1.4 | −6.82 ± 0.21 | −32.44 ± 2.16 |
| Frozen soil (surface) | 24 | −4.96 ± 0.41 | −31.56 ± 1.3 | −6.71 ± 0.34 | −36.71 ± 0.21 | |
| Frozen soil (deep) | 24 | −5.38 ± 1.2 | −33.68 ± 0.4 | −7.49 ± 0.67 | −43.07 ± 1.3 | |
| Alpine shrub | Surface water | 32 | −6.84 ± 0.32 | −35.62 ± 0.8 | −7.83 ± 0.24 | −37.89 ± 2.46 |
| Groundwater | 36 | −8.19 ± 0.56 | −44.23 ± 1.3 | −9.47 ± 0.47 | 60.58 ± 1.12 | |
| Alpine grassland | Surface water | 36 | −5.54 ± 0.11 | −27.33 ± 0.7 | −6.39 ± 0.21 | −30.17 ± 1.82 |
| Frozen soil (surface) | 24 | −6.02 ± 0.81 | −34.53 ± 2.2 | −6.47 ± 0.23 | −37.67 ± 1.11 | |
| Frozen soil (deep) | 24 | −7.80 ± 0.84 | −44.43 ± 0.9 | −7.91 ± 0.11 | −39.25 ± 1.73 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, B. Temporal and Spatial Variations of Hydrological Processes on the Landscape Zone Scale in an Alpine Cold Region (Mafengou River Basin, China): An Update. Water 2017, 9, 574. https://doi.org/10.3390/w9080574
Yang Y, Li B. Temporal and Spatial Variations of Hydrological Processes on the Landscape Zone Scale in an Alpine Cold Region (Mafengou River Basin, China): An Update. Water. 2017; 9(8):574. https://doi.org/10.3390/w9080574
Chicago/Turabian StyleYang, Yonggang, and Bin Li. 2017. "Temporal and Spatial Variations of Hydrological Processes on the Landscape Zone Scale in an Alpine Cold Region (Mafengou River Basin, China): An Update" Water 9, no. 8: 574. https://doi.org/10.3390/w9080574





