Characterization and Treatment Proposals of Shipboard Slop Wastewater Contaminated by Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Methods
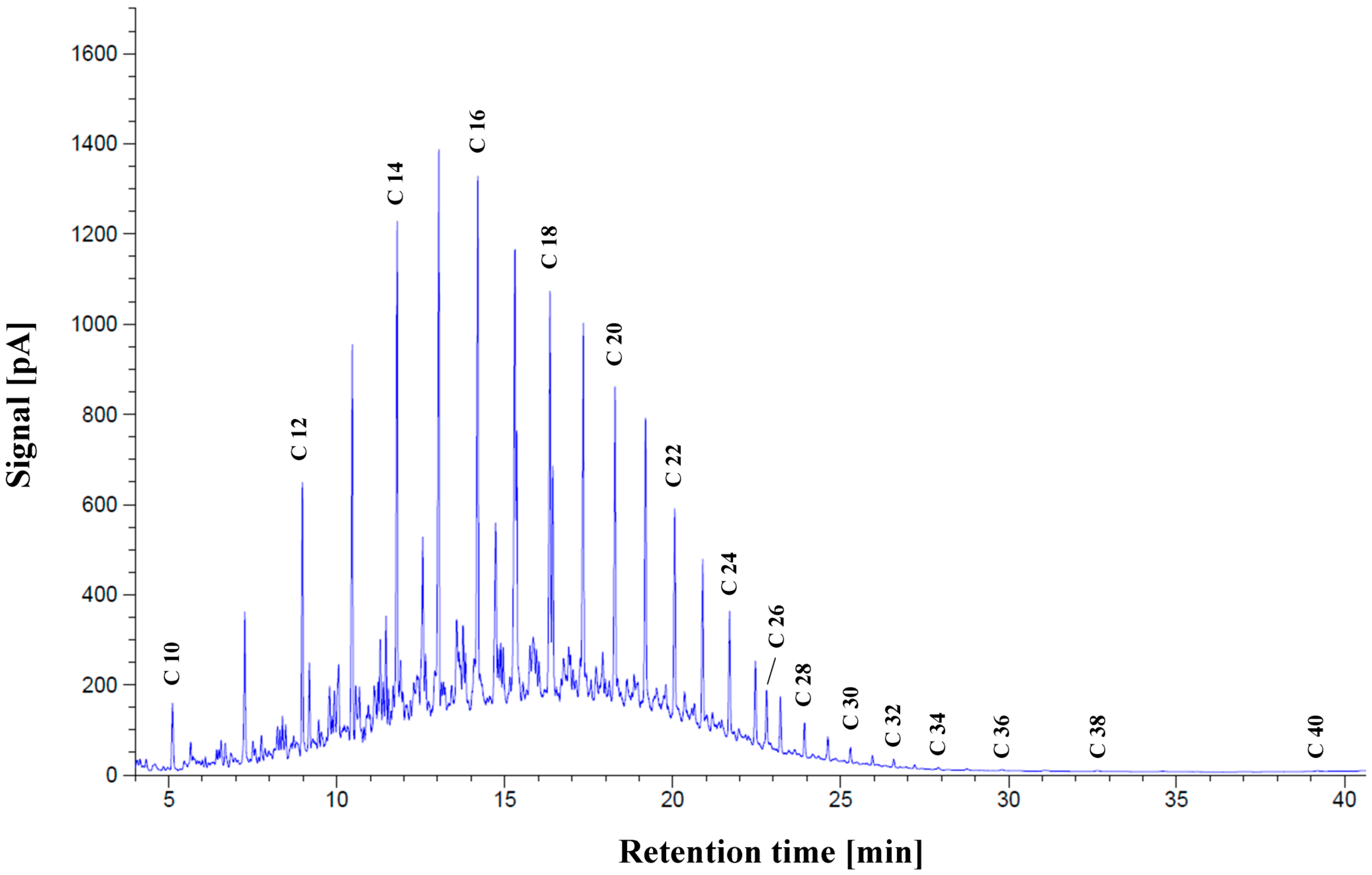
2.2. The Industrial Shipboard Wastewater: Slop
2.3. Bench Scale Processes
2.3.1. Batch Test for Analysis of Chemical Treatment
2.3.2. Batch and Dynamic Test for Physical Treatment
2.3.3. Bench Scale Application for Biological Treatment
3. Results and Discussion
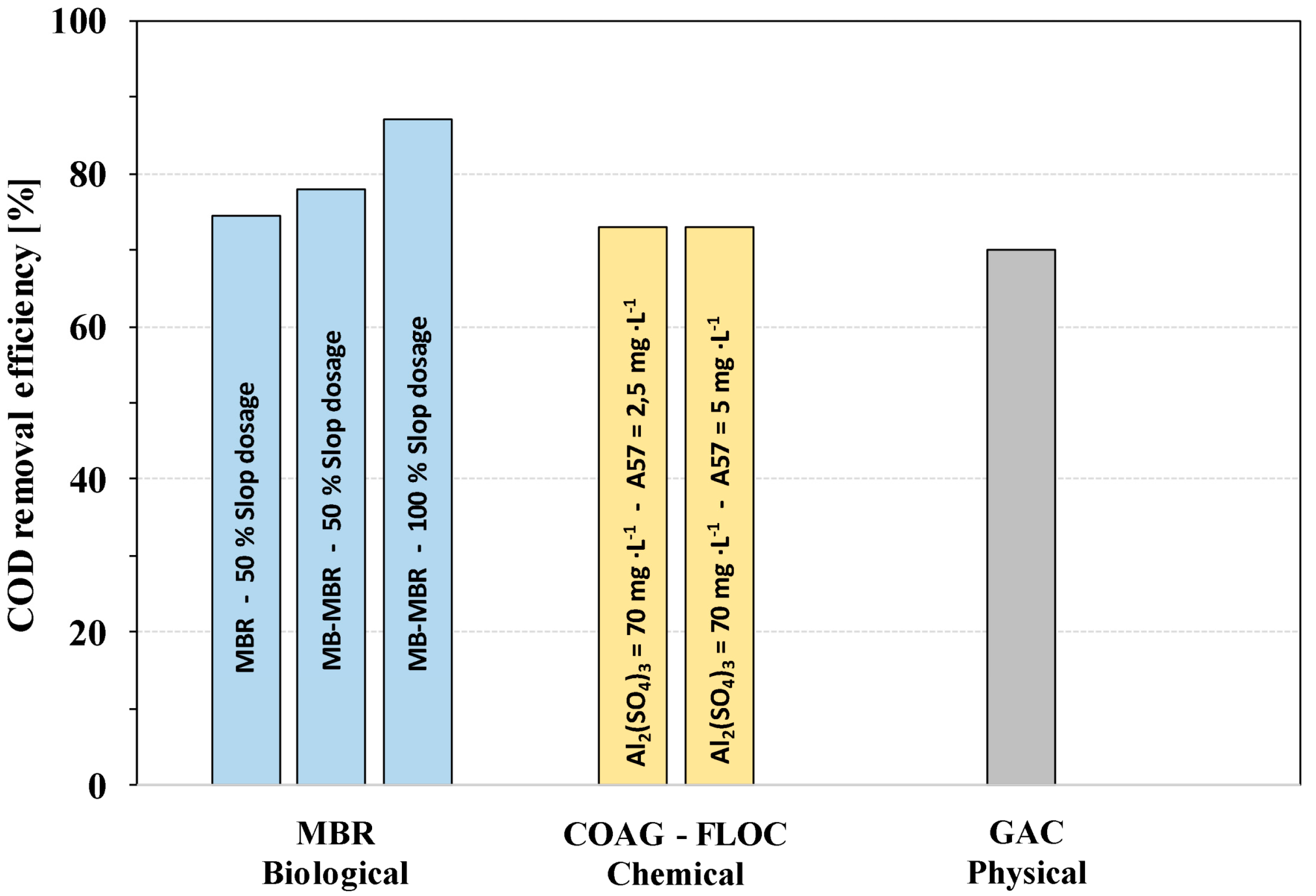
3.1. COD Removal
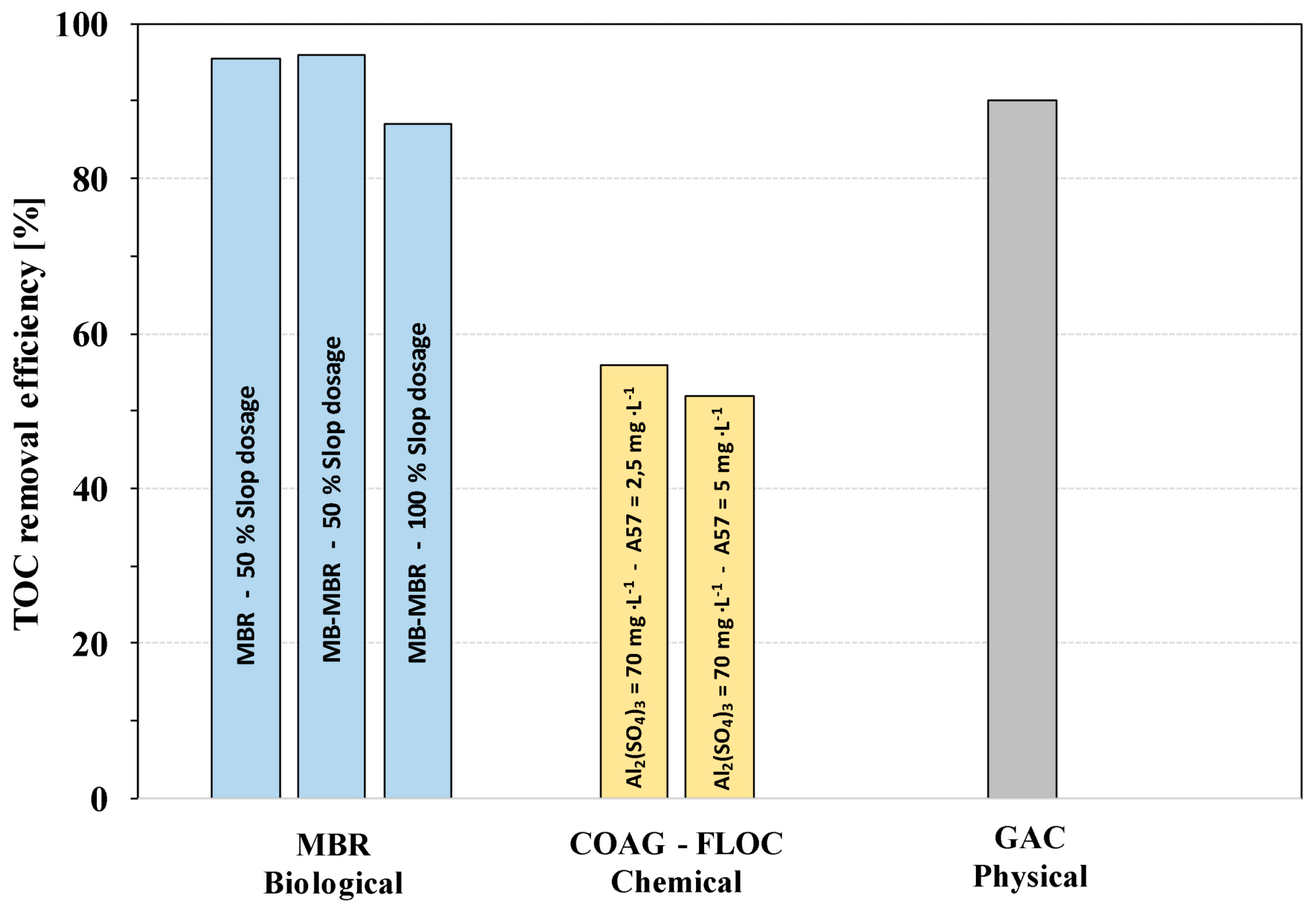
3.2. TOC Removal
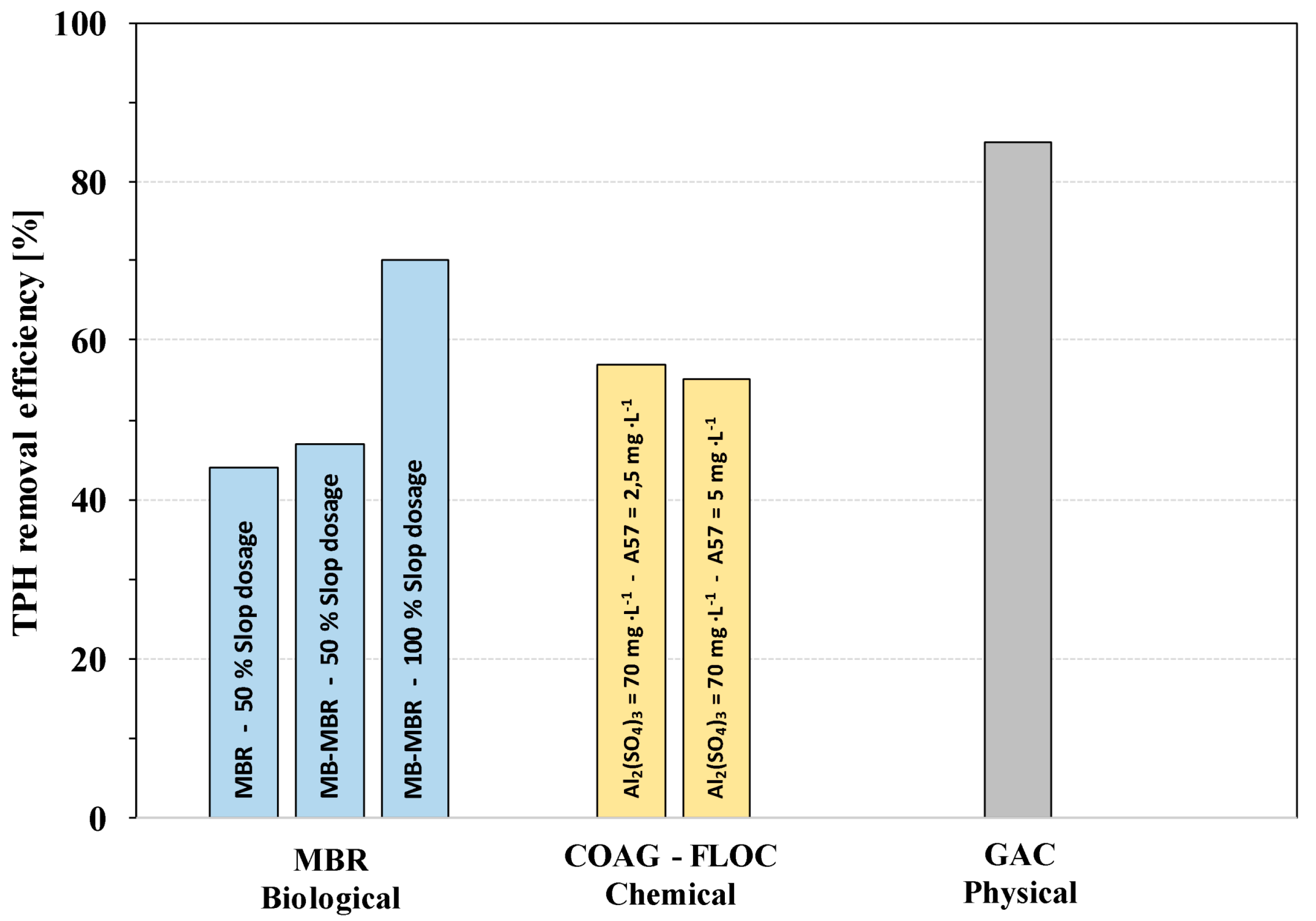
3.3. TPH Removal
3.4. Discussion about Treatment Chains Proposals
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- International Maritime Organization. MARPOL: Articles, Protocols, Annexes, Unified Interpretations of the International Convention for the Prevention of Pollution from Ships, 1973, as Modified by the Protocol of 1978 Relating Thereto; International Association of Classification Societies (IACS): London, UK, 2006. [Google Scholar]
- Di Bella, G.; Giustra, M.G.; Freni, G. Optimisation of coagulation/flocculation for pre-treatment of high strength and saline wastewater: Performance analysis with different coagulant doses. Chem. Eng. J. 2014, 254, 283–292. [Google Scholar] [CrossRef]
- Di Bella, G.; Di Prima, N.; Di Trapani, D.; Freni, G.; Giustra, M.G.; Torregrossa, M.; Viviani, G. Performance of membrane bioreactor (MBR) systems for the treatment of shipboard slops: Assessment of hydrocarbon biodegradation and biomass activity under salinity variation. J. Hazard. Mater. 2015, 300, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Campo, R.; Di Prima, N.; Gabriella Giustra, M.; Freni, G.; Di Bella, G. Performance of a moving bed-membrane bioreactor treating saline wastewater contaminated by hydrocarbons from washing of oil tankers. Desalin. Water Treat. 2016, 57. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abidin, Z.Z.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S. Application of membrane-coupled sequencing batch reactor for oilfield produced water recycle and beneficial re-use. Bioresour. Technol. 2010, 101, 6942–6949. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Sharma, M.; Dureja, P.; Sarma, P.M.; Lal, B. Bioelectrochemical approaches for removal of sulfate, hydrocarbon and salinity from produced water. Chemosphere 2017, 166, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Poynor, T.E.; Newhart, K.B.; Regnery, J.; Coday, B.D.; Cath, T.Y. Produced water treatment using forward osmosis membranes: Evaluation of extended-time performance and fouling. J. Memb. Sci. 2017, 525, 77–88. [Google Scholar] [CrossRef]
- Bonfá, M.R.L.; Grossman, M.J.; Mellado, E.; Durrant, L.R. Biodegradation of aromatic hydrocarbons by Haloarchaea and their use for the reduction of the chemical oxygen demand of hypersaline petroleum produced water. Chemosphere 2011, 84, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Ashaghi, K.S.; Engel, L.; Willershausen, D.; Mund, P.; Bolduan, P.; Czermak, P. Characterization and application of different ceramic membranes for the oil-field produced water treatment. Desalination 2009, 245, 533–540. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Memb. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Motta, A.; Borges, C.; Esquerre, K.; Kiperstok, A. Oil Produced Water treatment for oil removal by an integration of coalescer bed and microfiltration membrane processes. J. Memb. Sci. 2014, 469, 371–378. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Elters, C.; Simon, A.; Tatsuya, T.; Price, W. Coal seam gas produced water treatment by ultrafiltration, reverse osmosis and multi-effect distillation: A pilot study. Sep. Purif. Technol. 2015, 146, 94–100. [Google Scholar] [CrossRef]
- Wandera, D.; Himstedt, H.H.; Marroquin, M.; Wickramasinghe, S.R.; Husson, S.M. Modification of ultrafiltration membranes with block copolymer nanolayers for produced water treatment: The roles of polymer chain density and polymerization time on performance. J. Memb. Sci. 2012, 403–404, 250–260. [Google Scholar] [CrossRef]
- Zhou, F.S.; Zhao, M.F.; Ni, W.X.; Dang, Y.S.; Pu, C.S.; Lu, F.J. Inorganic polymeric flocculent FMA for purifying oilfield produced water: Preparation and uses. Oilfield Chem. 2000, 17, 256–259. [Google Scholar]
- Houcine, M. Solution for Heavy Metals Decontamination in Produced Water/Case Study in Southern Tunisia. In Proceedings of the SPE International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Kuala Lumpur, Malaysia, 20–22 March 2002; Society of Petroleum Engineers: Richardson, TX, USA, 2002. [Google Scholar]
- Hansen, B.R. Review of potential, technologies for the removal of dissolved components from produced water. Chem. Eng. Res. Des. 1994, 72, 176–188. [Google Scholar]
- Roccaro, P.; Lombardo, G.; Vagliasindi, F.G.A. Offline bioregeneration of spent activated carbon loaded with real Produced Water and its adsorption capacity for benzene and toluene. Desalin. Water Treat. 2015, 55, 756–766. [Google Scholar] [CrossRef]
- Doyle, D.; Brown, A. Produced water treatment and hydrocarbon removal with organoclay. Soc. Pet. Eng. 2000, 1–11. [Google Scholar] [CrossRef]
- Means, C.M.; Braden, M.L. Process for Removing Water Soluble Organic Compounds from Produced Water. U.S. Patent 5,104,545, 14 April 1992. [Google Scholar]
- Campo, R.; Di Prima, N.; Freni, G.; Giustra, M.G.; Di Bella, G. Start-up of two moving bed membrane bioreactors treating saline wastewater contaminated by hydrocarbons. Water Sci. Technol. 2016, 73. [Google Scholar] [CrossRef]
- Kargi, F.; Uygur, A. Improved nutrient removal from saline wastewater in an SBR by Halobacter supplemented activated sludge. Environ. Eng. Sci. 2005, 22, 170–176. [Google Scholar] [CrossRef]
- Kargi, F.; Konya, I. COD, para-chlorophenol and toxicity removal from para-chlorophenol containing synthetic wastewater in an activated sludge unit. J. Hazard. Mater. 2006, 132, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Freire, D.D.C.; Cammarota, M.C.; Sant’Anna, G.L. Biological treatment of oil field wastewater in a sequencing batch reactor. Environ. Technol. 2001, 22, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.T.; Nirmalakhandan, N. Bioreclamation of oilfield produced wastewaters: Characterization and feasibility study. In Produced Water; Ray, J.P., Engelhardt, F.R., Eds.; Springer: New York, NY, USA, 1992; pp. 523–533. [Google Scholar]
- Cheryan, M.; Rajagopalan, N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Memb. Sci. 1998, 151, 13–28. [Google Scholar] [CrossRef]
- Judd, S.; Judd, C. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Soltani, S.; Mowla, D.; Vossoughi, M.; Hesampour, M. Experimental investigation of oily water treatment by membrane bioreactor. Desalination 2010, 250, 598–600. [Google Scholar] [CrossRef]
- Jang, D.; Hwang, Y.; Shin, H.; Lee, W. Effects of salinity on the characteristics of biomass and membrane fouling in membrane bioreactors. Bioresour. Technol. 2013, 141, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johir, M.A.H.; Vigneswaran, S.; Kandasamy, J.; BenAim, R.; Grasmick, A. Effect of salt concentration on membrane bioreactor (MBR) performances: Detailed organic characterization. Desalination 2013, 322, 13–20. [Google Scholar] [CrossRef]
- Pendashteh, A.R.; Abdullah, L.C.; Fakhru’L-Razi, A.; Madaeni, S.S.; Zainal Abidin, Z.; Awang Biak, D.R. Evaluation of membrane bioreactor for hypersaline oily wastewater treatment. Process. Saf. Environ. Prot. 2012, 90, 45–55. [Google Scholar] [CrossRef]
- Ødegaard, H. Innovations in wastewater treatment: The moving bed biofilm process. Water Sci. Technol. 2006, 53, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, D.; Di Bella, G.; Mannina, G.; Torregrossa, M.; Viviani, G. Comparison between moving bed-membrane bioreactor (MB-MBR) and membrane bioreactor (MBR) systems: Influence of wastewater salinity variation. Bioresour. Technol. 2014, 162, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Díaz, J.C.; Calderón, K.; Rodríguez, F.A.; González-López, J.; Hontoria, E.; Poyatos, J.M. Comparative kinetic study between moving bed biofilm reactor-membrane bioreactor and membrane bioreactor systems and their influence on organic matter and nutrients removal. Biochem. Eng. J. 2013, 77, 28–40. [Google Scholar] [CrossRef]
- Kose-Mutlu, B.; Ersahin, M.E.; Ozgun, H.; Kaya, R.; Kinaci, C.; Koyuncu, I. Influence of powdered and granular activated carbon system as a pre-treatment alternative for membrane filtration of produced water. J. Chem. Technol. Biotechnol. 2017, 92, 283–291. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Roddick, F.A. Pre-treatments for removing colour from secondary effluent: Effectiveness and influence on membrane fouling in subsequent microfiltration. Sep. Purif. Technol. 2013, 103, 313–320. [Google Scholar] [CrossRef]
- Dialynas, E.; Diamadopoulos, E. Integration of immersed membrane ultrafiltration with coagulation and activated carbon adsorption for advanced treatment of municipal wastewater. Desalination 2008, 230, 113–127. [Google Scholar] [CrossRef]
- Gur-Reznik, S.; Katz, I.; Dosoretz, C.G. Removal of dissolved organic matter by granular-activated carbon adsorption as a pretreatment to reverse osmosis of membrane bioreactor effluents. Water Res. 2008, 42, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); World Economic Forum (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- US EPA. Epa Method 3510C: Separatory Funnel Liquid-Liquid Extraction; Test Methods For Evaluating Solid Waste Physicalchemical Methods; United States Environmental Protection Agency: Washington, DC, USA, 1996; pp. 1–8. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; Metcalf & Eddy, Inc.: Wakefield, MA, USA, 2003; Volume 4. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]

| Parameter | Symbol | Units | Value |
|---|---|---|---|
| Chemical Oxygen demand | COD | mg·L−1 | 1.327 ± 328 |
| Total Organic Carbon | TOC | mg·L−1 | 428 ± 62 |
| Total Petroleum Hydrocarbons | TPH | mg·L−1 | 135 ± 38 |
| Sodium Chloride | NaCl | g·L−1 | 38.67 ± 0.19 |
| Conductivity | - | mS·cm−1 | 48 ± 2 |
| pH | - | - | 7.78 ± 0.62 |
| Total Suspended Solids | mg·L−1 | 352 ± 84 | |
| Metals | |||
| Aluminum | Al | mg·L−1 | <0.005 |
| Arsenic | As | mg·L−1 | <0.001 |
| Boron | Bo | mg·L−1 | 5.02 |
| Cadmium | Cd | mg·L−1 | <0.001 |
| Chrome | Cr | mg·L−1 | <0.005 |
| Iron | Fe | mg·L−1 | 1.18 |
| Manganese | Mn | mg·L−1 | <0.005 |
| Nickel | Ni | mg·L−1 | <0.005 |
| Copper | Cu | mg·L−1 | <0.005 |
| Selenium | Se | mg·L−1 | <0.005 |
| Lead | Pb | mg·L−1 | <0.005 |
| Zinc | Zn | mg·L−1 | <0.005 |
| Influent Characteristics | Treatment Chain | Law Limit (MARPOL 73/78) [4] | ||||
|---|---|---|---|---|---|---|
| Physical | Chemical | Biological | ||||
| Low load influent <30 mgTPH·L−1 → | - | (Coagulation-Flocculation) ηTPH ≈ 57% | + | MBR or MB-MBR ηTPH ≈ 70% | 5 mgTPH·L−1 | |
| Medium load influent 30 < mgTPH·L−1 < 150 → | GAC ηTPH ≈ 85% | + | (Coagulation-Flocculation) ηTPH ≈ 57% | + | MBR or MB-MBR ηTPH ≈ 70% | |
| High load influent >150 mgTPH·L−1 → | GAC ηTPH ≈ 85% | + | Coagulation-Flocculation ηTPH ≈ 57% | + | MBR or MB-MBR ηTPH ≈ 70% | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campo, R.; Giustra, M.G.; De Marchis, M.; Freni, G.; Di Bella, G. Characterization and Treatment Proposals of Shipboard Slop Wastewater Contaminated by Hydrocarbons. Water 2017, 9, 581. https://doi.org/10.3390/w9080581
Campo R, Giustra MG, De Marchis M, Freni G, Di Bella G. Characterization and Treatment Proposals of Shipboard Slop Wastewater Contaminated by Hydrocarbons. Water. 2017; 9(8):581. https://doi.org/10.3390/w9080581
Chicago/Turabian StyleCampo, Riccardo, Maria Gabriella Giustra, Mauro De Marchis, Gabriele Freni, and Gaetano Di Bella. 2017. "Characterization and Treatment Proposals of Shipboard Slop Wastewater Contaminated by Hydrocarbons" Water 9, no. 8: 581. https://doi.org/10.3390/w9080581






