How Efficient Are Semi-Natural Ponds in Assimilating Wastewater Effluents? Application to Fuente de Piedra Ramsar, Mediterranean Salt Lake (South of Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Physico-Chemical and Biological Variables
3. Results
3.1. Abiotic Conditions
3.2. Biological Response
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Álvarez-Cobelas, M.; Rojo, C.; Angeler, D.G. Mediterranean limnology: Current status, gaps and future. J. Limnol. 2005, 64, 13–29. [Google Scholar] [CrossRef]
- Williams, W.D. Conservation of wetlands in drylands: A key global issue. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 517–522. [Google Scholar] [CrossRef]
- Gilbert, J.D.; de Vicente, I.; Jiménez-Melero, R.; Parra, G.; Guerrero, F. Selecting priority conservation areas based on zooplankton diversity: The case of Mediterranean wetlands. Mar. Freshw. Res. 2014, 65, 857–871. [Google Scholar] [CrossRef]
- Gilbert, J.D.; de Vicente, I.; Ortega, F.; Jiménez-Melero, R.; Parra, G.; Guerrero, F. A comprehensive evaluation of the crustacean assemblages in southern Iberian Mediterranean wetlands. J. Limnol. 2015, 74, 169–181. [Google Scholar] [CrossRef]
- Gilbert, J.D.; de Vicente, I.; Ortega, F.; García-Muñoz, E.; Jiménez-Melero, R.; Parra, G.; Guerrero, F. Linking watershed land uses and crustacean assemblages in Mediterranean wetlands. Hydrobiologia 2017, 799, 181–191. [Google Scholar] [CrossRef]
- Brinson, M.M.; Málvarez, A.I. Temperate freshwater wetlands: Types, status, and threats. Environ. Conserv. 2002, 29, 115–133. [Google Scholar] [CrossRef]
- Higueras, P.L.; Sáez-Martínez, F.J.; Reyes-Bozo, L. Characterization and remediation of contamination: Influences of mining and other human activities. Environ. Sci. Pollut. Res. 2016, 23, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramos, D.; Sánchez-Emeterio, G.; Florin, M. Changes in water quality of treated sewage effluents by their receiving environments in Tablas de Daimiel National Park, Spain. Environ. Sci. Pollut. Res. 2016, 23, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.D.; Welch, E.B.; Peterson, S.A.; Newroth, P.R. Restoration and Management of Lakes and Reservoirs, 2nd ed.; Lewis Publishers, CRC Press: Boca Raton, FL, USA, 1993; p. 548. [Google Scholar] [CrossRef]
- Harper, D. Eutrophication of Freshwaters: Principles, Problems and Restoration; Chapman and Hall: London, UK, 1992. [Google Scholar]
- Likens, G.E. Nutrients and Eutrophication. Limnol. Oceanogr. Spec. Symp. 1, American Society of Limnology and Oceanography; Allen Press: Lawrence, KS, USA, 1972. [Google Scholar]
- Smol, J.P. Eutrophication: The environmental consequences of over-fertilization. In Pollution of Lakes and Rivers: A Paleoenvironmental Perspective, 2nd ed.; Smol, J.P., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 180–228. ISBN 978-1-4051-5913-5. [Google Scholar]
- Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for the Community Action in the Field of Water Policy; The European Parliament and the Council of the European Union; Publications Office of the European Union: Luxembourg, 2000.
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530. [Google Scholar] [CrossRef]
- Olsen, C.R.; Cutshall, N.H.; Larsen, I. Pollutant particle associations and dynamics in coastal marine environments. Mar. Chem. 1982, 11, 501–533. [Google Scholar] [CrossRef]
- Ufnar, D.; Ufnar, J.A.; Ellender, R.D.; Rebarchik, D.; Stone, G. Influence of coastal processes on high fecal coliform counts in the Mississippi Sound. J. Coast. Res. 2006, 22, 1515–1526. [Google Scholar] [CrossRef]
- FDEP (Florida Department of Environmental Protection). Ground Water Protection Section. In Resource Management Groundwater Standards and Guidance Concentration Used in Watershed Assessments; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2004; p. 17. [Google Scholar]
- Kadlec, H.R.; Knight, R.L. Treatment Wetlands; Lewis Publishers: Boca Raton, FL, USA, 1996. [Google Scholar]
- Pinney, M.L.; Westerhoff, P.K.; Baker, L. Transformations in dissolved organic carbon through constructed wetlands. Water Res. 2000, 34, 1897–1911. [Google Scholar] [CrossRef]
- Hammer, D.A.; Bastian, R.K. Wetland Ecosystem: Natural Water Purifiers? In Constructed Wetlands for Wastewater Treatment: Municipal, Industrial and Agriculture; Hammer, D.A., Ed.; Lewis Publishers, CRC Press Company: Boca Raton, FL, USA, 1989; pp. 5–19. [Google Scholar]
- Angelakis, A.N.; Snyder, S.A. Wastewater Treatment and Reuse: Past, Present, and Future. Water 2015, 7, 4887–4895. [Google Scholar] [CrossRef]
- Reinoso, R.; Torres, L.A.; Bécares, E. Efficiency of natural systems for removal of bacteria and pathogenic parasites from wastewater. Sci. Total Environ. 2008, 395, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.A.; Meuleman, A.F.M. Wetlands for wastewater treatment: Opportunities and limitations. Ecol. Eng. 1999, 12, 5–12. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M. Hydrogeology of ponds, pools and playa-lakes of southern Spain. Wetlands 2007, 27, 819–830. [Google Scholar] [CrossRef]
- Ley 1/1984, de 9 de Enero, de la Declaración de la Laguna de Fuente de Piedra como Reserva Integral; BOJA n° 4; Consejería de la Presidencia de la Junta de Andalucía: Sevilla, Spain, 1984.
- Kadlec, H.R. Large Constructed Wetlands for Phosphorus Control: A Review. Water 2016, 8, 243. [Google Scholar] [CrossRef]
- Montes, C.; Rendón-Martos, M.; Varela, L.; Cappa, M.J. Manual de Restauración de Humedales Mediterráneos; Consejería de Medio Ambiente de la Junta de Andalucía: Sevilla, Spain, 2007; p. 239. [Google Scholar]
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüring, M.; Meyerhöfer, U.-P.; Hansen, H.D. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Leboulanger, C.; Bouvy, M.; Carré, C.; Cecchi, P.; Amalric, L.; Bouchez, A.; Pagano, M.; Sarazin, G. Comparison of the effects of two herbicides and an insecticide on tropical freshwater plankton in microcosms. Arch. Environ. Contam. Toxicol. 2011, 61, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Reul, A.; Muñoz, M.; Bautista, B.; Neale, P.J.; Sobrino, C.; Mercado, J.M.; Segovia, M.; Salles, S.; Kulk, G.; León, P.; et al. Effect of CO2, nutrients and light on coastal plankton. III. Trophic cascade, size structure and composition. Aquat. Biol. 2014, 22, 59–76. [Google Scholar] [CrossRef]
- Dordio, A.; Carvalho, A.J.P.; Pinto, A.P.H. Wetlands: Water “living filetrs”? In Wetlands: Ecology, Conservation and Restoration; Russo, R.E., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 15–71. [Google Scholar]
- Lazarova, V.; Levine, B.; Sack, J.; Cirelli, G.; Jeffrey, P.; Muntau, H.; Salgot, M.; Brissaud, F. Role of water reuse for enhancing integrated water managementin Europe and Mediterranean countries. Water Sci. Technol. 2001, 43, 25–33. [Google Scholar] [PubMed]
- Angelakis, A.N.; Spyridakis, S.V. The status of water resources in Minoan times: A preliminary study. In Diachronic Climatic Impacts on Water Resources with Emphasis on Mediterranean Region; Angelakis, A.N., Issar, A., Eds.; Springer-Verlag: Heidelberg, Germany, 1996; pp. 161–192. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Marecos so Monte, M.H.F.; Bontoux, L.; Asano, T. The status of wastewater reuse practice in the Mediterranean basin: Need for guidelines. Water Res. 1999, 33, 2201–2217. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Reddy, K.R. Temperature effects in treatment wetlands. Water Environ. Res. 2001, 73, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M. Ecology of Shallow Lakes; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Moreno-Ostos, E.; Paracuellos, M.; de Vicente, I.; Nevado, J.C.; Cruz-Pizarro, L. Response of waterbirds to alternating clear and turbid water phases in two shallow Mediterranean lakes. Aquat. Ecol. 2008, 42, 701–706. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Liu, L.; Hall, G.; Champagne, P. Effects of Environmental Factors on the Disinfection Performance of a Wastewater Stabilization Pond Operated in a Temperate Climate. Water 2016, 8, 5. [Google Scholar] [CrossRef]
- Alderisio, K.A.; DeLuca, N. Seasonal Enumeration of Fecal Coliform Bacteria from the Feces of Ring-Billed Gulls (Larusdelawarensis) and Canada Geese (Brantacanadensis). Appl. Environ. Microbiol. 1999, 65, 5628–5630. [Google Scholar] [PubMed]
- Edge, T.A.; Hill, S. Multiple lines of evidence to identify the sources of fecal pollution at a freshwater beach in Hamilton Harbour, Lake Ontario. Water Res. 2007, 41, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto 140/2003, de 7 de Febrero, Por el Que se Establecen los Criterios Sanitarios de la Calidad del Agua de Consumo Humano; BOE n° 45; Agencia Estatal Boletín Oficial del Estado: Madrid, Spain, 2003.
- Heinonen-Tanski, H.; Matikka, V. Chemical and Microbiological Quality of Effluents from Different On-Site Wastewater Treatment Systems across Finland and Sweden. Water 2017, 9, 47. [Google Scholar] [CrossRef]
- Rozema, E.R.; VanderZaag, A.C.; Wood, J.D.; Drizo, A.; Zheng, Y.; Madani, A.; Gordon, R.J. Constructed Wetlands for Agricultural Wastewater Treatment in Northeastern North America: A Review. Water 2016, 8, 173. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Knight, R.L.; Vymazal, J.; Brix, H.; Cooper, P.; Harberl, R. Constructed Wetland for Polution Control; IWA Publishing: London, UK, 2000; p. 156. ISBN 9781900222051. [Google Scholar]
- Valipour, A.; Kalyan Raman, V.; Ahn, Y.-H. Effectiveness of Domestic Wastewater Treatment Using a Bio-Hedge Water Hyacinth Wetland System. Water 2015, 7, 329–347. [Google Scholar] [CrossRef]
- Phuong Tram, V.O.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Nguyen, P.D.; Listowski, A.; Wang, X.C. A mini-review on the impacts of climate change on wastewater reclamation and reuse. Sci. Total Environ. 2014, 494–495, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I.; et al. Towards the review of the European Union Water Framework Directive: Recommendations for more efficient assessment and management of chemical contamination in European surface water resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, A.; Balch, G.C.; Wootton, B.C.; Jørgensen, S.E.; Anderson, B.C. SubWet 2.0. Modeling the Performance of Treatment Wetlands. In Developments in Environmental Modelling; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
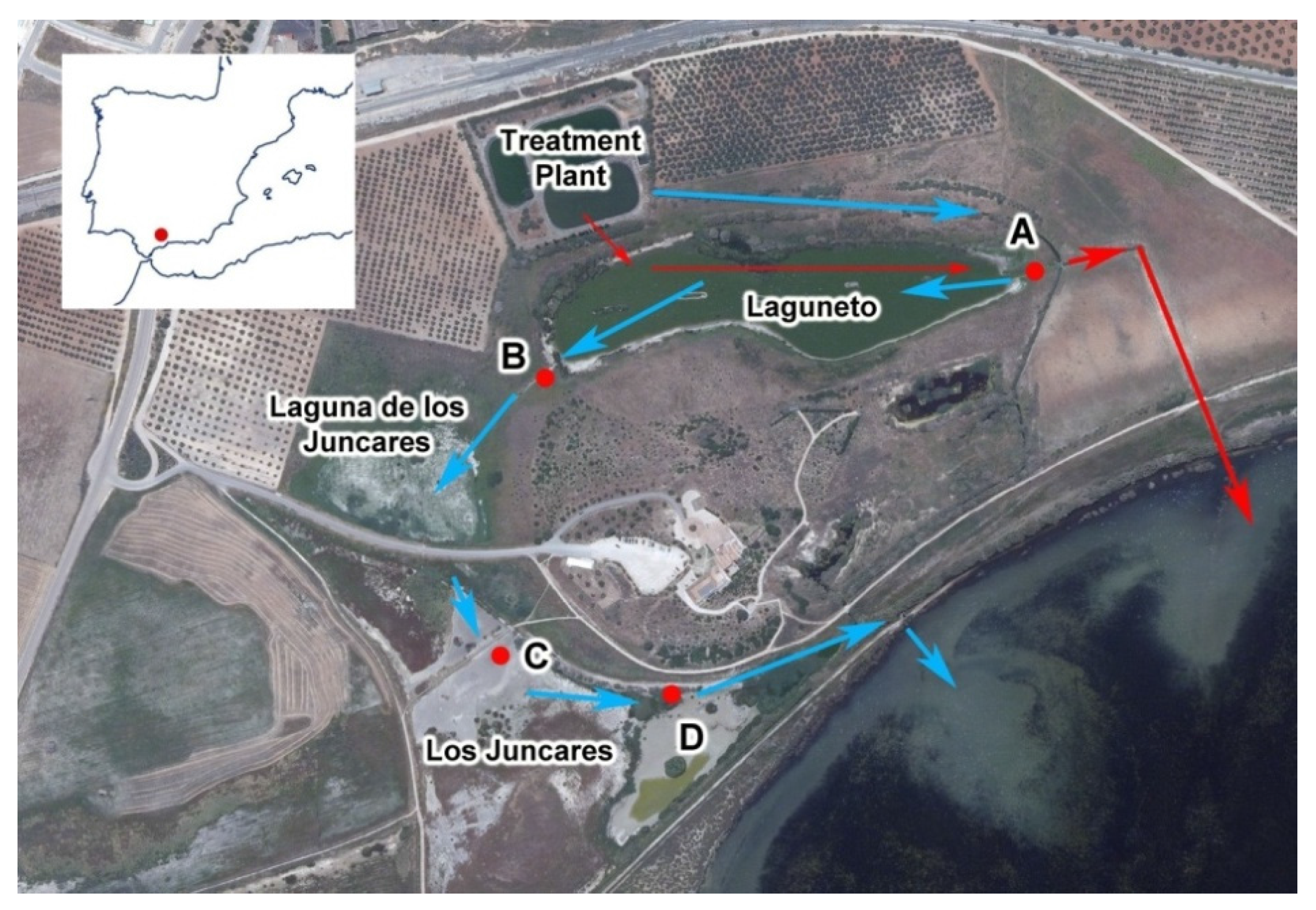
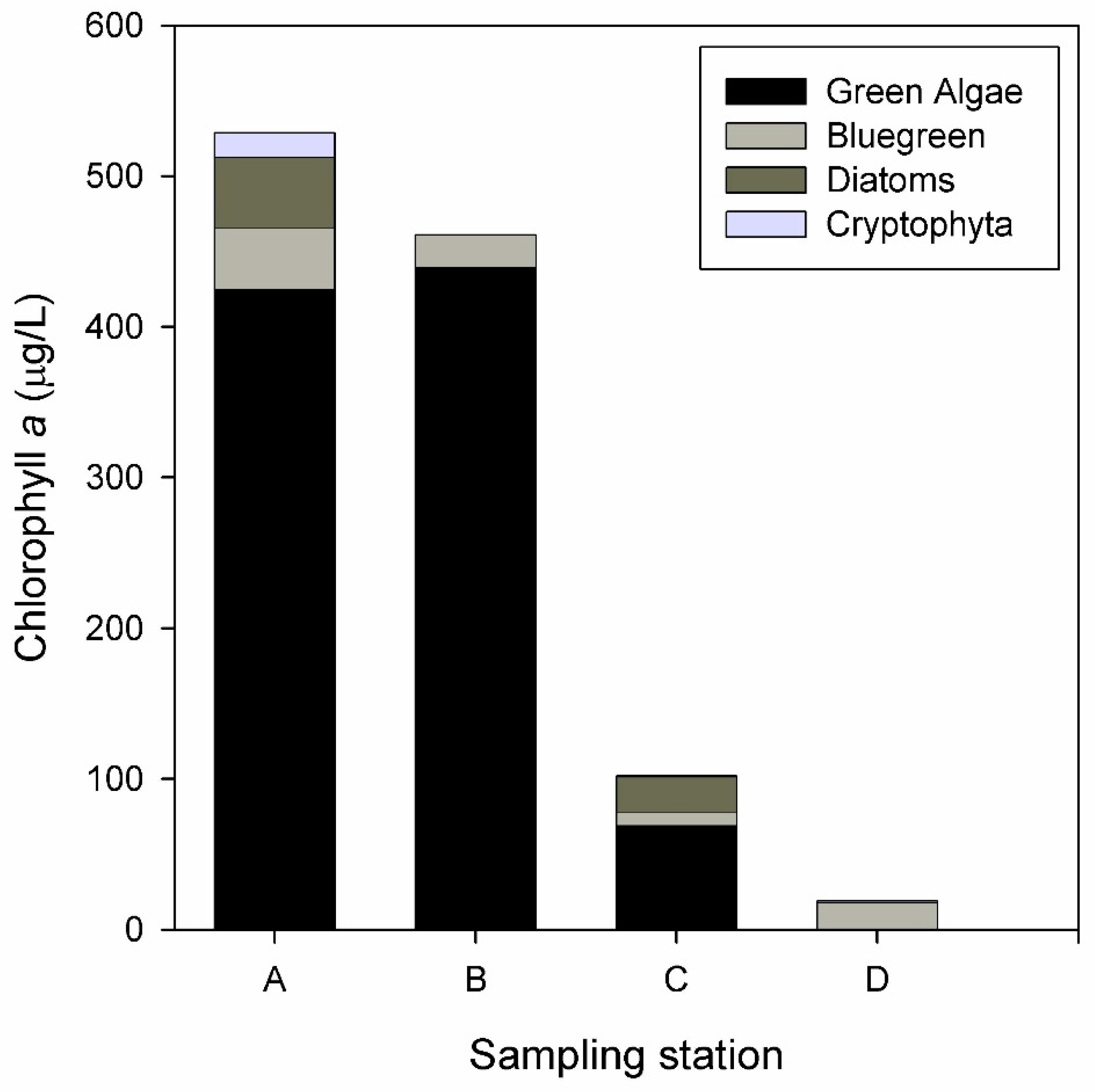
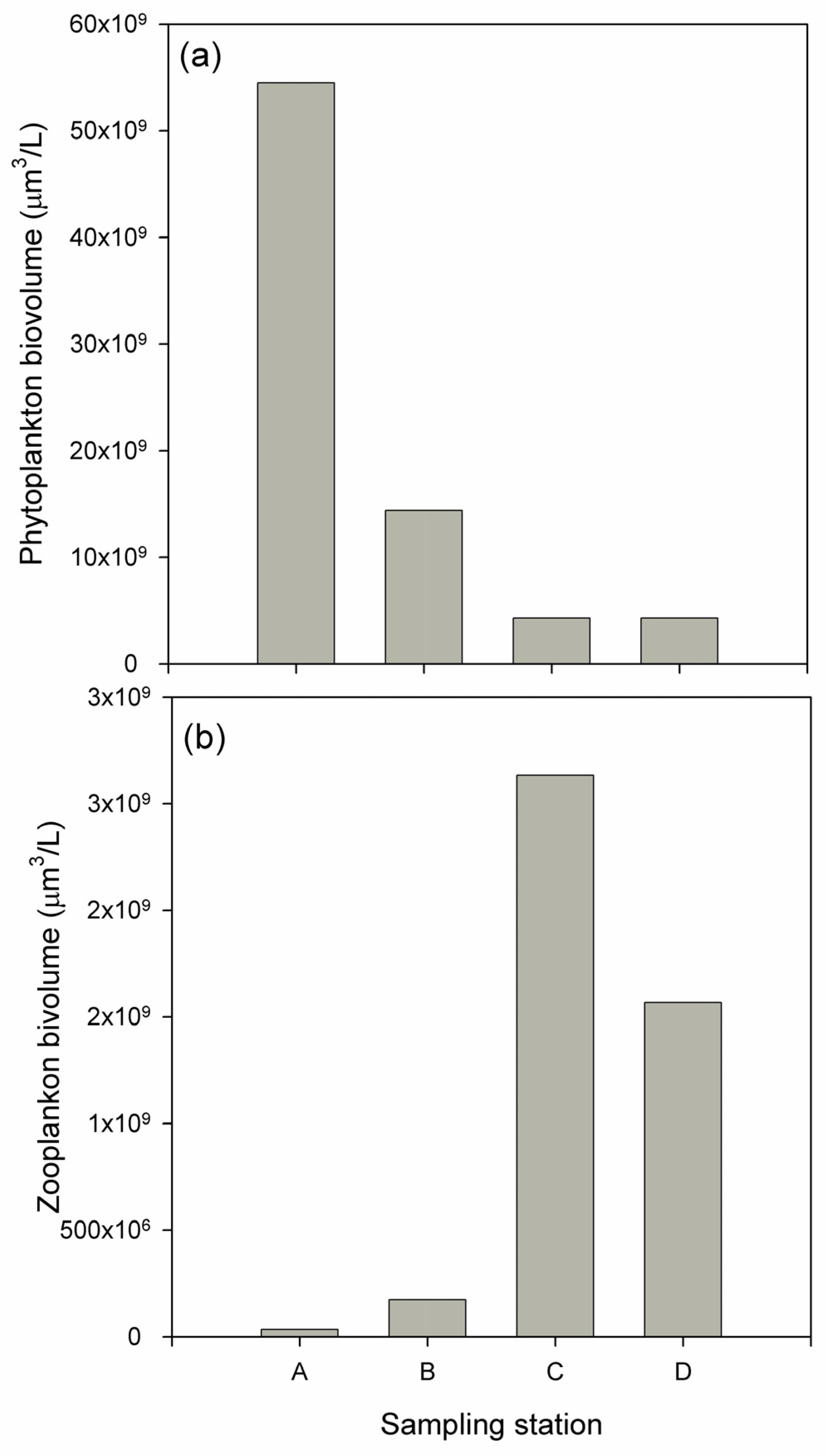
| Water Quality Parameters | Decrease (%) | Inlet Mean (mg/L) | Outlet Mean (mg/L) | Flow Mean (m3/h) | Hydraulic Retention Time (d) |
|---|---|---|---|---|---|
| Biochemical oxygen demand (BOD) | 84 ± 15 | 181 ± 95 | 25 ± 17 | ||
| Chemical oxygen demand (COD) | 74 ± 12 | 451 ± 220 | 104 ± 41 | ||
| Total Suspended Solids (TSS) | 52 ± 30 | 197 ± 63 | 164 ± 31 | ||
| Winter, Spring and Autumn | 13.54 | 80 | |||
| Summer | 16.67 | 65 |
| Abiotic Parameters | Point A | Point B | Point C | Point D |
|---|---|---|---|---|
| Temperature (°C) | 19.62 ± 0.10 | 20.83 ± 0.04 | 21.95 ± 0.05 | 21.17 ± 0.09 |
| pH | 7.99 ± 0.03 | 8.63 ± 0.01 | 7.45 ± 0.02 | 7.94 ± 0.02 |
| Electric conductivity (µS/cm) | 3262.67 ± 4.37 | 2561.00 ± 1.87 | 2688.75 ± 25.86 | 4481.00 ± 4.24 |
| Total phosphorus (mg/L) | 5.00 ± 3.46 | 2.30 ± 0.58 | 2.30 ± 0.58 | 3.00 ± 0.00 |
| Total nitrogen (mg/L) | 14.70 ± 0.58 | 7.30 ± 0.58 | 11.30 ± 2.89 | 11.30 ± 2.08 |
| Bacteria | A | B | D |
|---|---|---|---|
| Heterotrophic bacteria at 22 °C (cfu/mL) | (1.29 ± 0.60) × 105 | (2.10 ± 1.39) × 104 | 388 ± 151 |
| Heterotrophic bacteria at 37 °C (cfu/mL) | (2.39 ± 2.23) × 105 | (3.18 ± 1.17) × 104 | 247 ± 135 |
| Faecal coliforms (cfu/100 mL) | 65 ± 40 | 655 ± 18 | 17 ± 21 |
| Faecal enterococci (cfu/100 mL) | 1033 ± 351 | 388 ± 68 | 1 ± 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De-los-Ríos-Mérida, J.; Reul, A.; Muñoz, M.; Arijo, S.; Tapia-Paniagua, S.; Rendón-Martos, M.; Guerrero, F. How Efficient Are Semi-Natural Ponds in Assimilating Wastewater Effluents? Application to Fuente de Piedra Ramsar, Mediterranean Salt Lake (South of Spain). Water 2017, 9, 600. https://doi.org/10.3390/w9080600
De-los-Ríos-Mérida J, Reul A, Muñoz M, Arijo S, Tapia-Paniagua S, Rendón-Martos M, Guerrero F. How Efficient Are Semi-Natural Ponds in Assimilating Wastewater Effluents? Application to Fuente de Piedra Ramsar, Mediterranean Salt Lake (South of Spain). Water. 2017; 9(8):600. https://doi.org/10.3390/w9080600
Chicago/Turabian StyleDe-los-Ríos-Mérida, Jesús, Andreas Reul, María Muñoz, Salvador Arijo, Silvana Tapia-Paniagua, Manuel Rendón-Martos, and Francisco Guerrero. 2017. "How Efficient Are Semi-Natural Ponds in Assimilating Wastewater Effluents? Application to Fuente de Piedra Ramsar, Mediterranean Salt Lake (South of Spain)" Water 9, no. 8: 600. https://doi.org/10.3390/w9080600






