Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Samples
2.2. Bacterial Culture Conditions
2.3. Photosensitizer
2.4. Irradiation Conditions
2.5. Antimicrobial Photodynamic Therapy (PDI) Treatments
2.5.1. PDI Assays Performed in Filtered WW and in PBS
2.5.2. PDI Assays Performed in WW without the Addition of Bacteria
2.6. Photodegradation of Phenol
2.7. Photodegradation of Porphyrin
2.8. Statistical Analysis
3. Results
3.1. Antimicrobial Photodynamic Therapy (PDI) Treatments
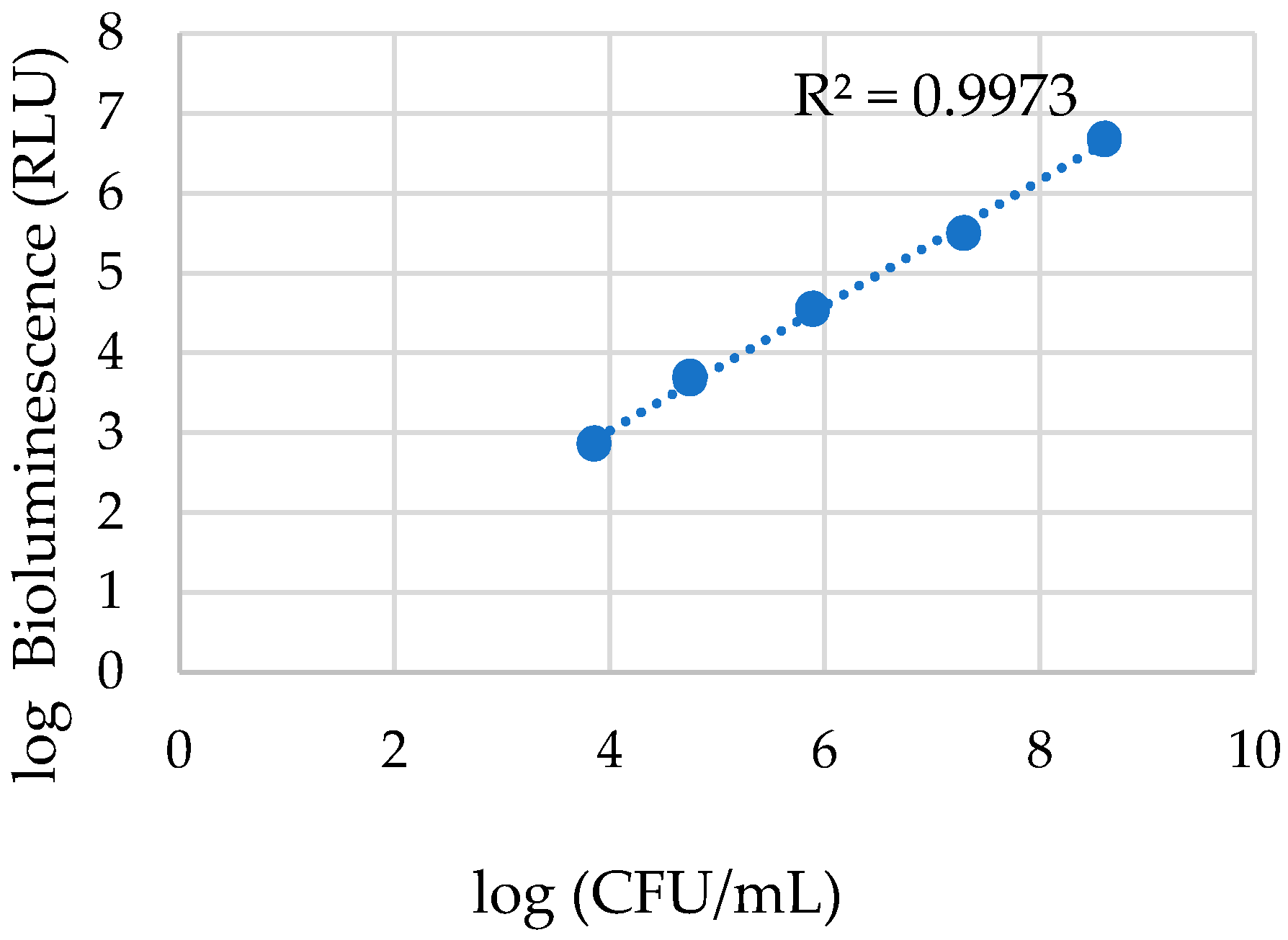
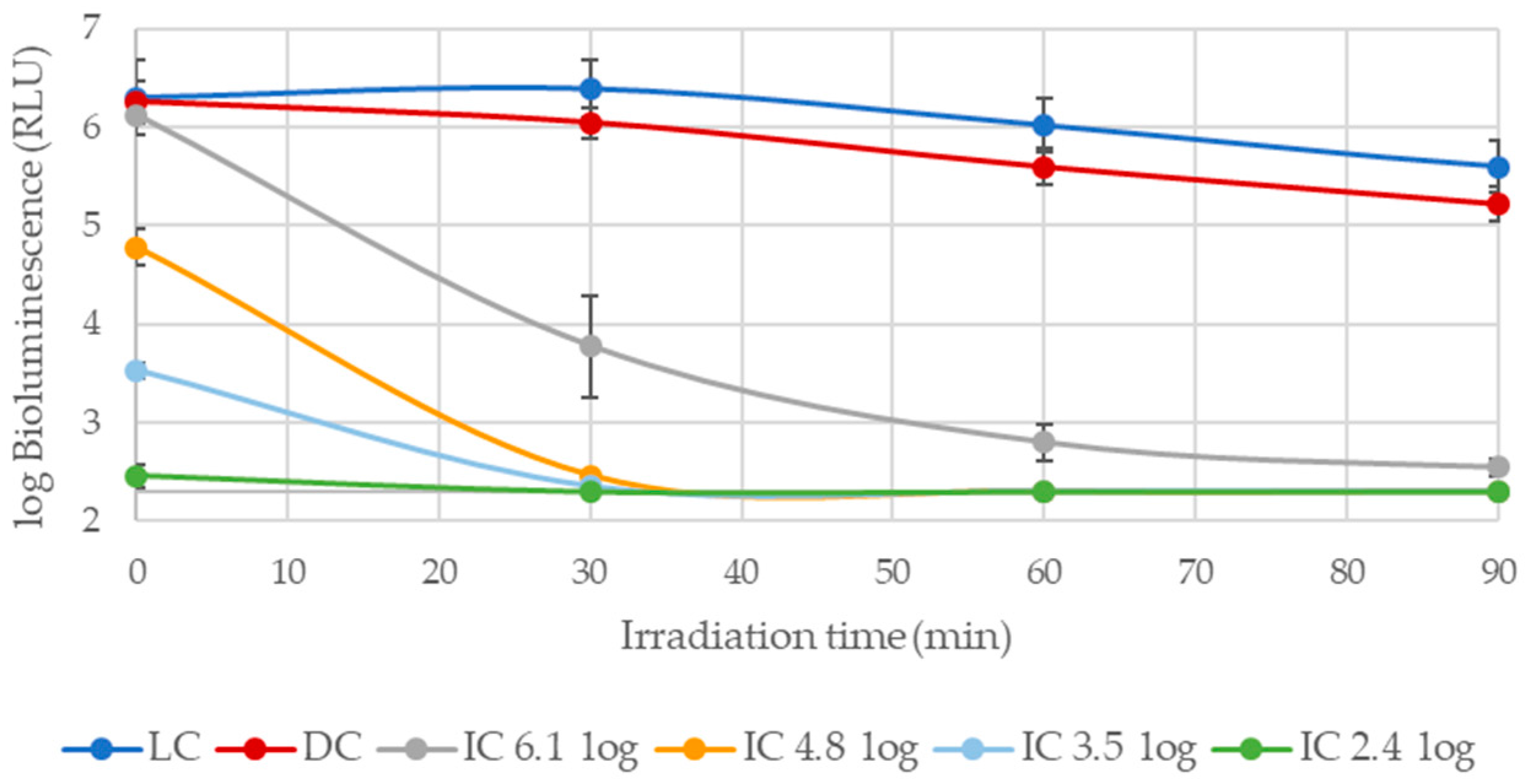
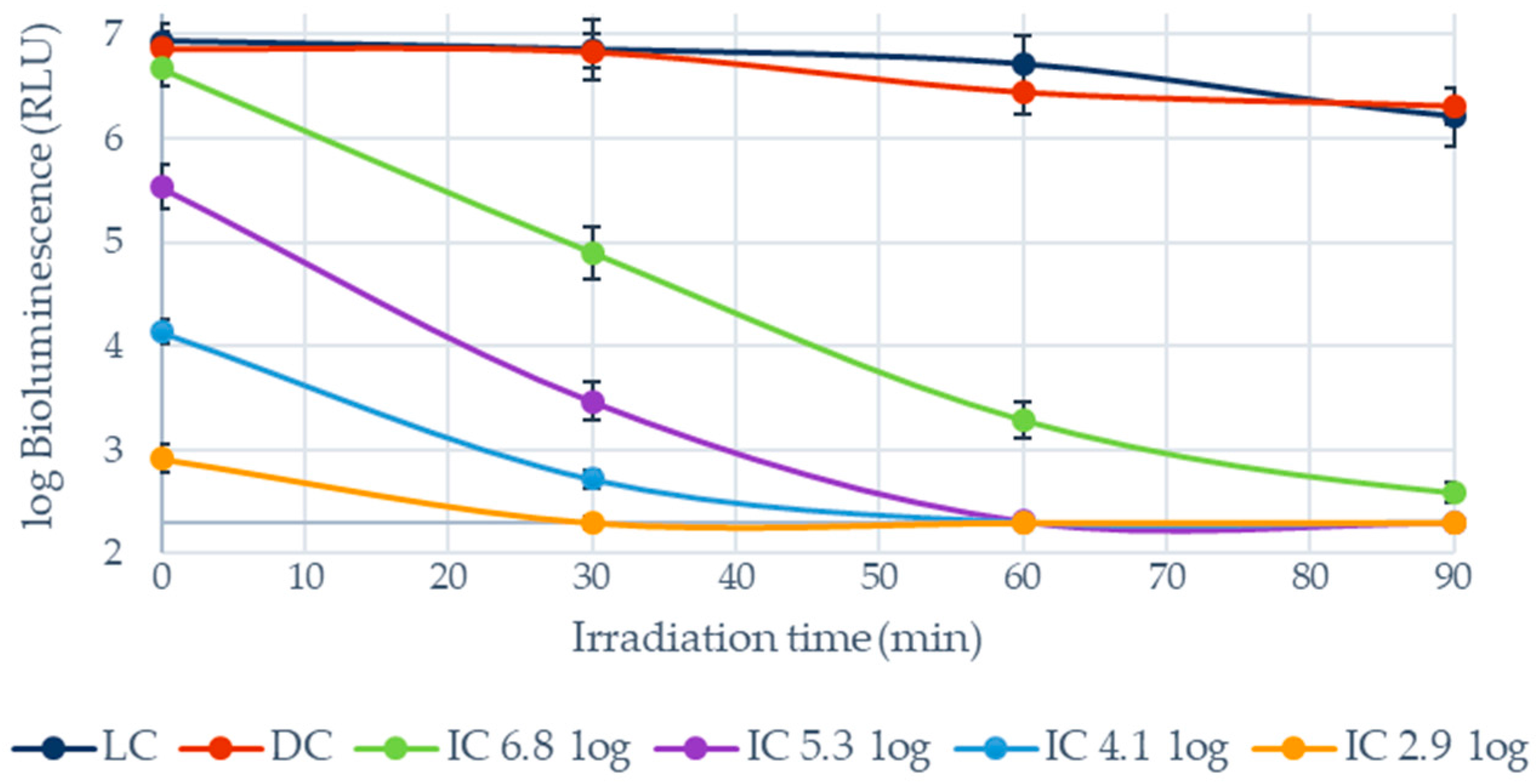
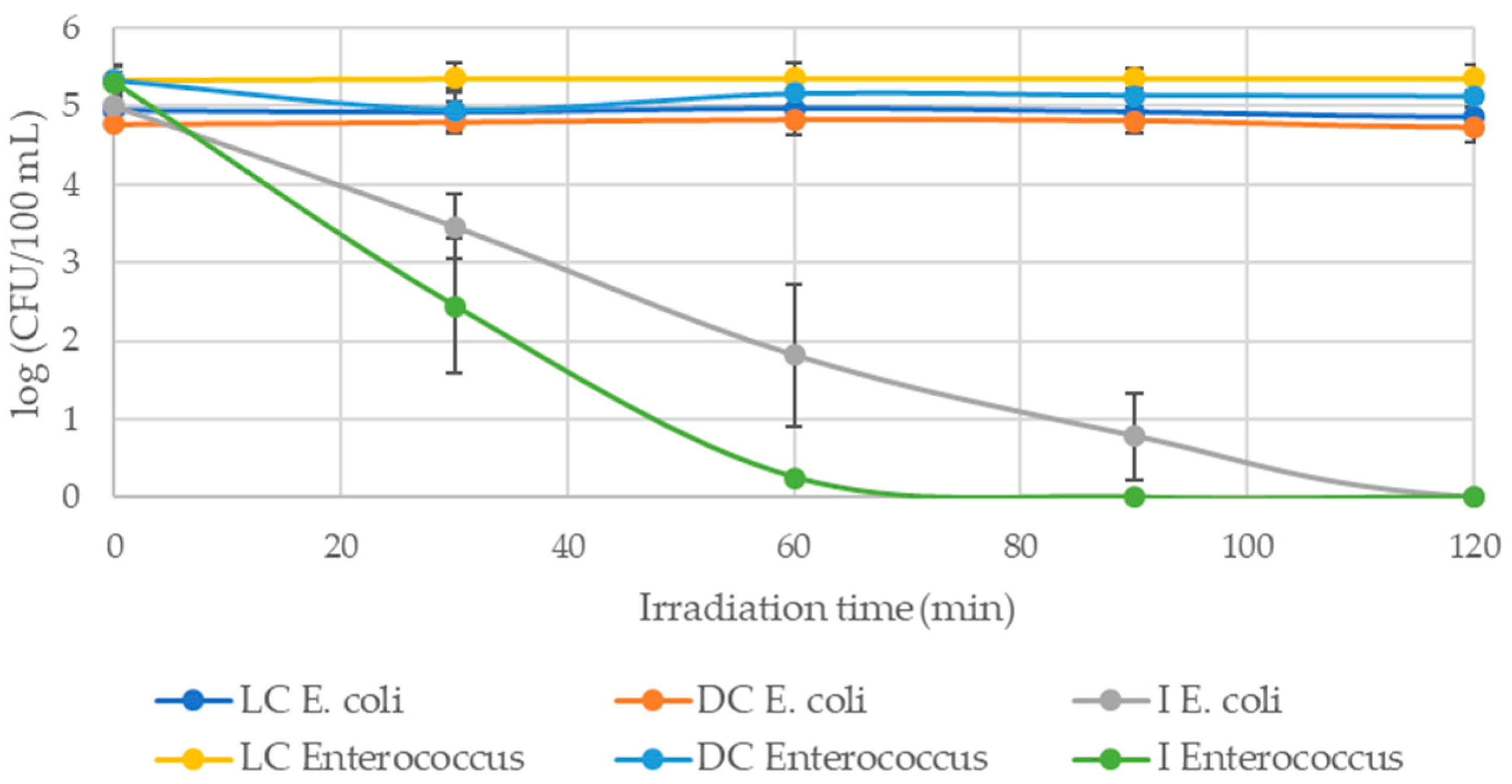
3.1.1. Photoinactivation of E. coli in Filtered WW and PBS
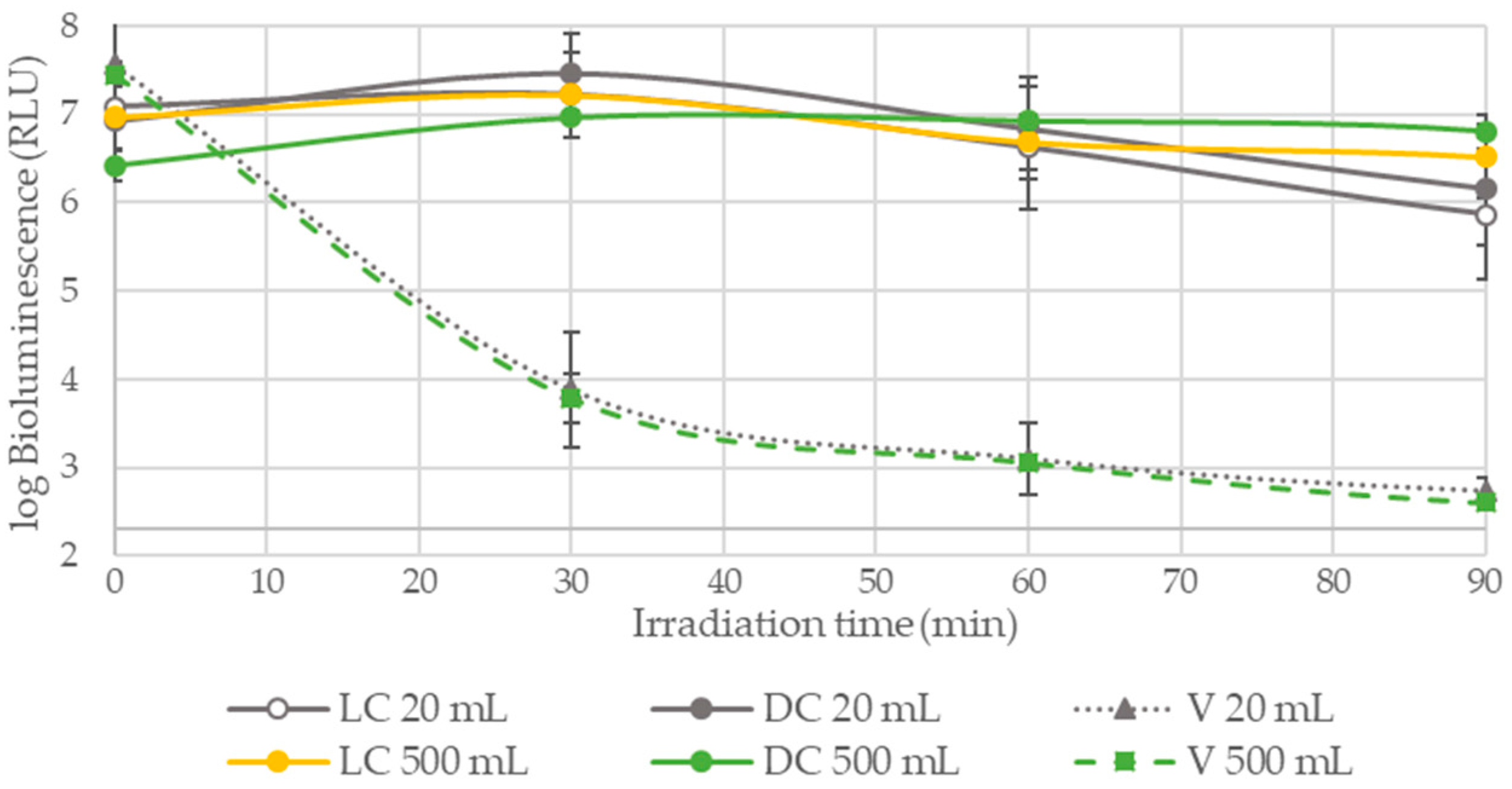
3.1.2. PDI Assays Performed in WW without the Addition of Bacteria
3.2. Photodegradation Tests of Phenol
3.2.1. Photodegradation Test of Phenol with Artificial Light
3.2.2. Photodegradation Test of Phenol with Solar Light
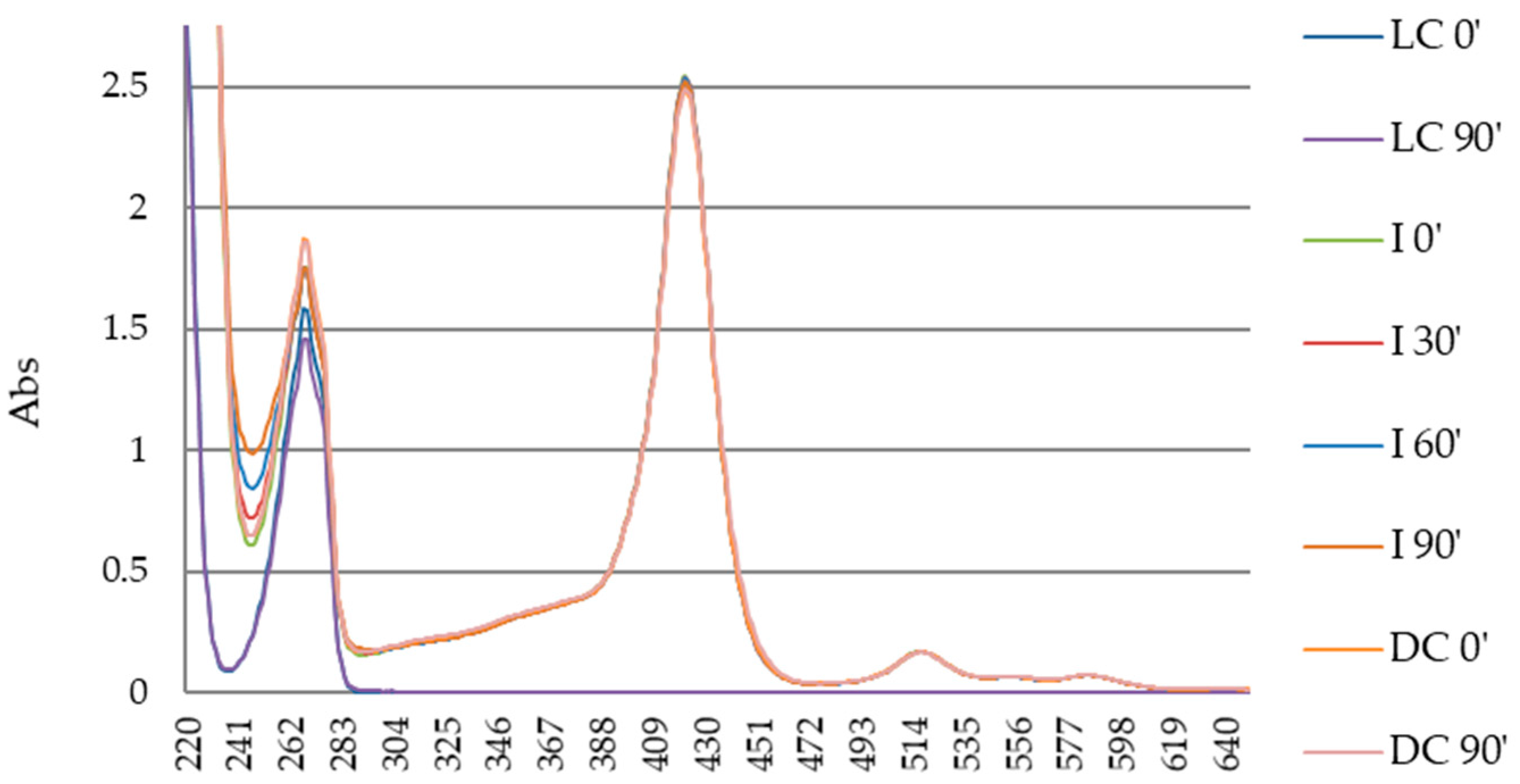
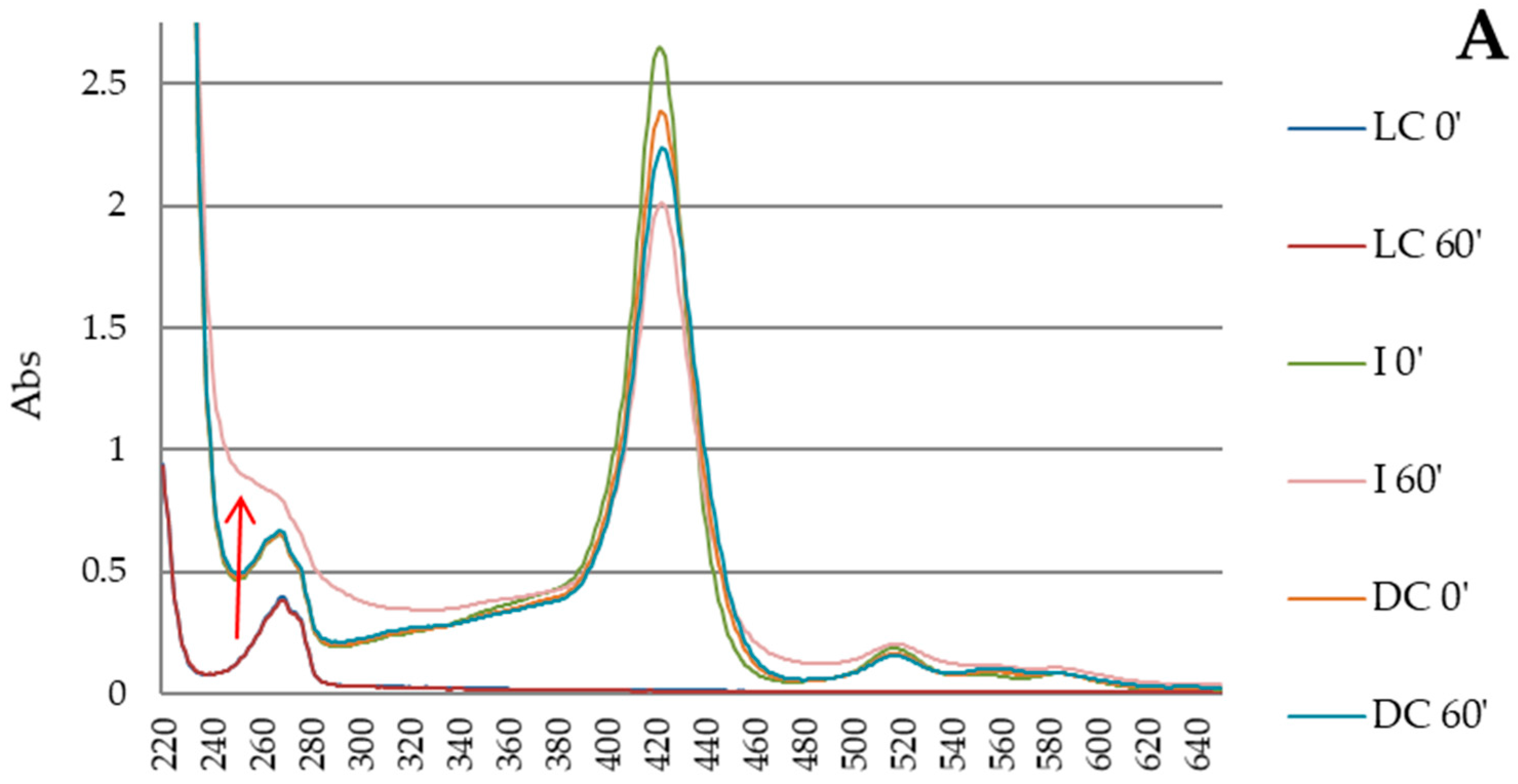
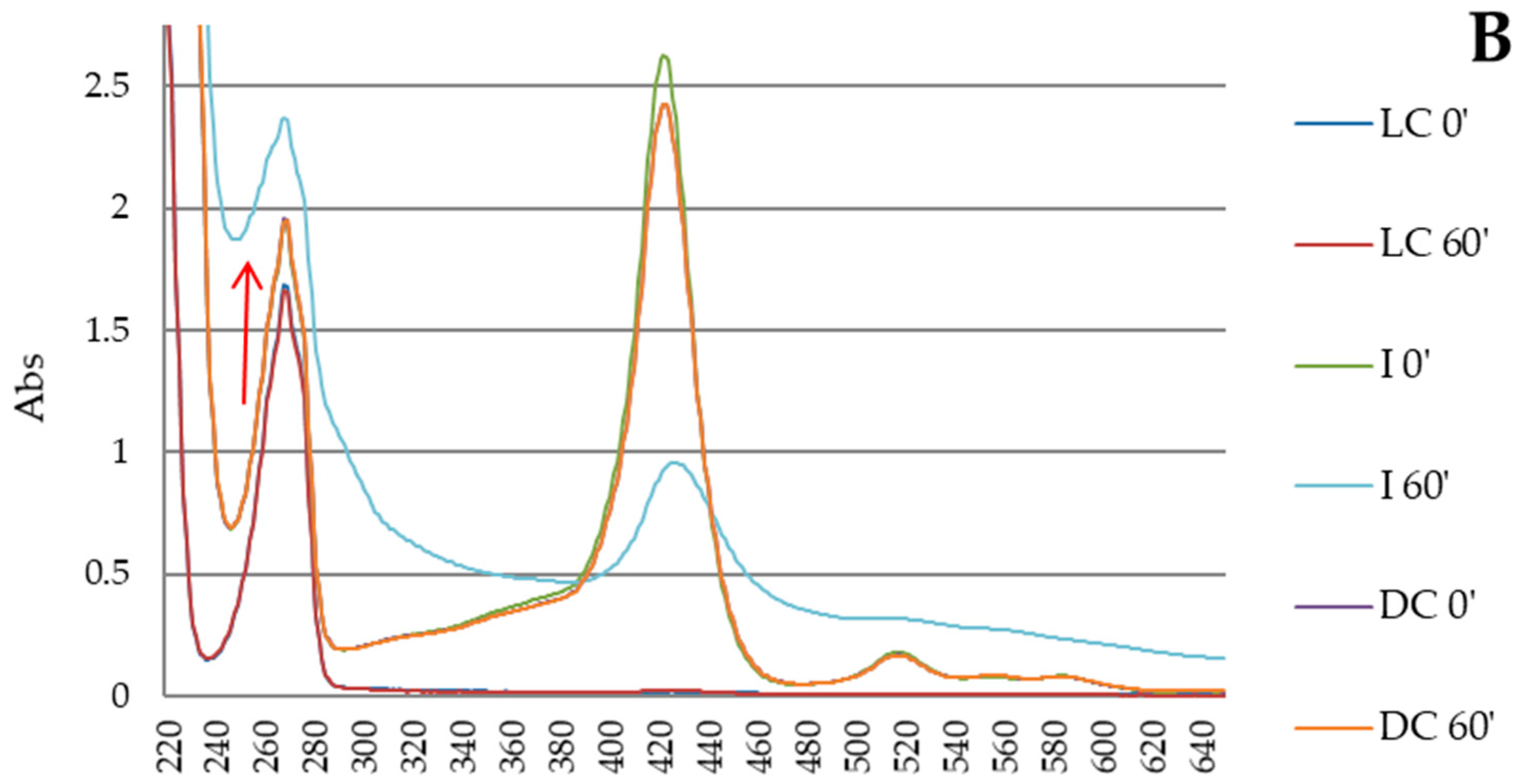
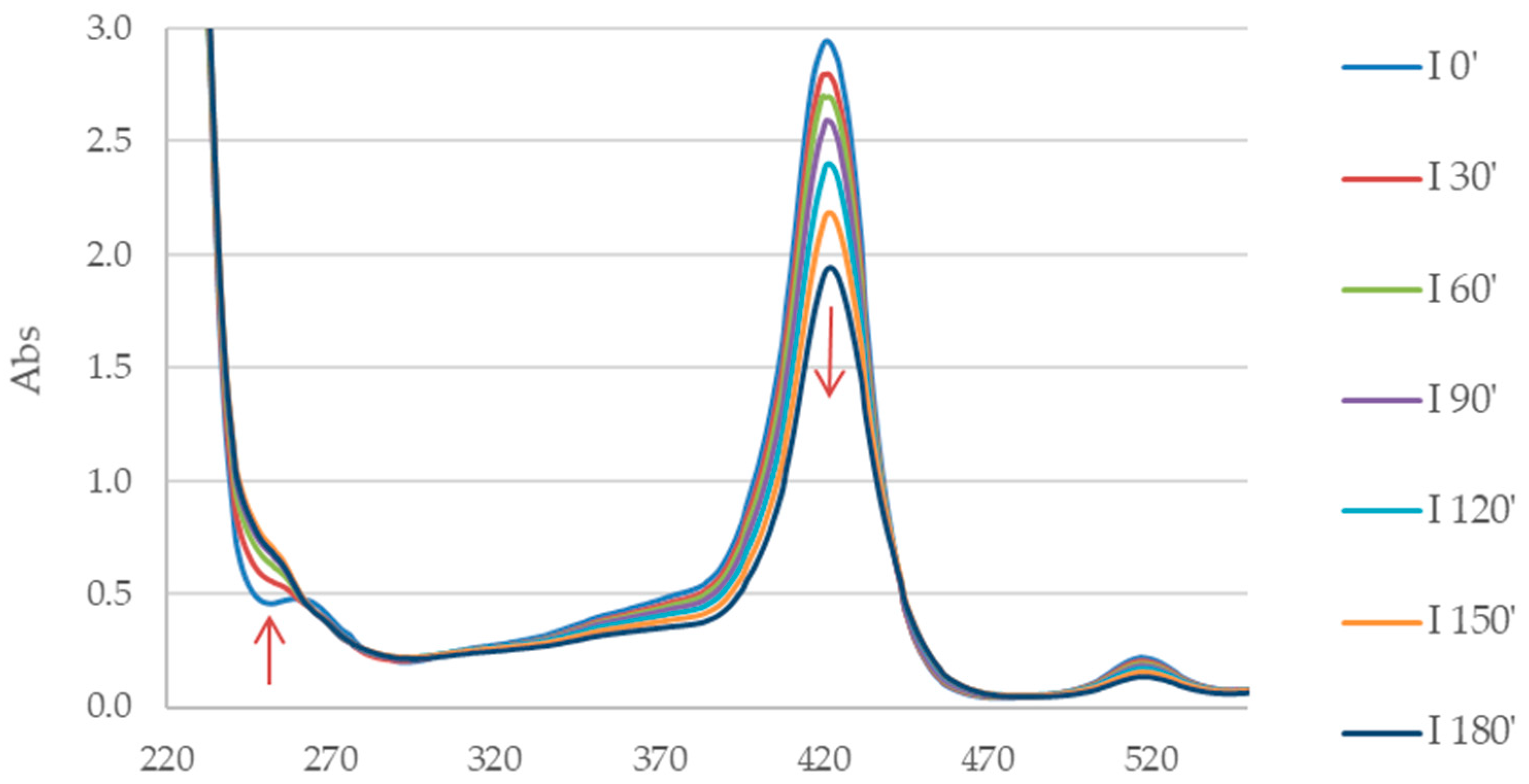
3.3. Photodegradation of Porphyrin
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.H. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Sewage bacteriophage inactivation by cationic porphyrins: Influence of light parameters. Photochem. Photobiol. Sci. 2010, 9, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Frigon, D.; Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R. Biological and physicochemical wastewater treatment processes reduce the prevalence of virulent Escherichia coli. Appl. Environ. Microbiol. 2013, 79, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Almeida, A.; Carvalho, C.M.B.; Tomé, J.P.C.; Fautino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â. Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores. J. Appl. Microbiol. 2009, 106, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014, 38, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Darcy, C.; Lescure, I.; Payot, V.; Rouland, G. Effluents des Établissements Hospitaliers: Teneur en Microorganismes Pathogènes, Risques Sanitaires, Procédures Particulières D’épuration et de Gestion des Boues; Office International de l’eau: Limoges, France, 2002. [Google Scholar]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Costa, L.; Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Sewage bacteriophage photoinactivation by cationic porphyrins: A study of charge effect. Photochem. Photobiol. Sci. 2008, 7, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [PubMed]
- Jemli, M.; Alouini, Z.; Sabbahi, S.; Gueddari, M. Destruction of fecal bacteria in wastewater by three photosensitizers. J. Environ. Monit. 2002, 4, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Gomes, A.T.P.C.; Fernandes, S.C.D.; Prata, A.C.B.; Almeida, A.; Cunha, A.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; et al. Photoinactivation of bacteria in wastewater by porphyrins: Bacterial β-galactosidase activity and leucine-uptake as methods to monitor the process. J. Photochem. Photobiol. 2007, 88, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Cunha, Â.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Porphyrins as antimibrobial photosensitizing agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications, 11th ed.; Hamblin, M.R., Jori, G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 83–160. [Google Scholar]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Caveleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Costa, L.; Alves, E.; Oliveira, A.; Cunha, Â.; et al. Antimicrobial photodynamic activity of porphyrin derivatives: Potential application on medical and water disinfection. J. Porphyr. Phthalocyanines 2009, 13, 574–577. [Google Scholar] [CrossRef]
- Carvalho, C.M.B.; Alves, E.; Costa, L.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A. Functional cationic nanomagnet-porphyrin hybrids for the photoinactivation of microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Rocha, S.; Cunha, Â.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Effect of photodynamic therapy on the virulence factors of Staphylococcus aureus. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R. Chemical Aspects of Photodynamic Therapy; Gordon and Breach Science Publishers: London, UK; Newark, NJ, USA, 2000. [Google Scholar]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2016, 3099, 1–7. [Google Scholar] [CrossRef]
- St. Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.A.; Parasca, S.V. Light sources for photodynamic inactivation of bacteria. Lasers Med. Sci. 2009, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, K.T.; de Souza, J.M.; Gobo, N.R.D.S.; de Assis, F.F.; Brocksom, T.J. Basic concepts and applications of porphyrins, chlorins and phthalocyanines as photosensitizers in photonic therapies. Rev. Virtual Quím. 2015, 7, 310–335. [Google Scholar] [CrossRef]
- European Environment Agency. Bathing Water Quality. 2015. Available online: http://www.eea.europa.eu/data-and-maps/indicators/bathing-water-quality (accessed on 27 April 2017).
- Jin, G.; Jeng, H.-W.; Bradford, H.; Englande, A.J. Comparison of E. coli, enterococci, and fecal coliform as indicators for brackish water quality assessment. Water Environ. Res. 2005, 76, 245–255. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawskp, A.; Ratajczak, W.; Porebski, T.; Capata, W.; Ostrowska, I. Removal of phenol from wastewater by different separation techniques. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Maleki, A.; Alimohamadi, M.; Ghasri, A. Photo-oxidation of phenol in aqueous solution: Toxicity of intermediates. Korean J. Chem. Eng. 2007, 24, 79–82. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Phenol. PubChem Compound Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/996 (accessed on 27 April 2017).
- Santo, C.M. A Indústria de Refinação de Petróleo: Características e Tratamento das Águas Residuais. e-LP Eng. Technol. J. 2010, 1, 21–46. Available online: http://recil.grupolusofona.pt/handle/10437/2853 (accessed on 27 April 2017).
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.-Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Mendo, S.; Almeida, A. Photodynamic inactivation of recombinant bioluminescent Escherichia coli by cationic porphyrins under artificial and solar irradiation. J. Ind. Microbiol. Biotechnol. 2008, 35, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Krysteva, M.A.; Lalov, I.G.; Artarsky, S.V. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006, 40, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Arrojado, C.; Pereira, C.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; et al. Applicability of photodynamic antimicrobial chemotherapy as an alternative to inactivate fish pathogenic bacteria in aquaculture systems. Photochem. Photobiol. Sci. 2011, 10, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Photodynamic antimicrobial chemotherapy in aquaculture: Photoinactivation studies of Vibrio fischeri. PLoS ONE 2011, 6, e20970. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent Organic Matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef] [Green Version]
- Ogilby, P.R.; Foote, C.S. Chemistry of singlet oxygen. 42. Effect of solvent, solvent isotopic substitution, and temperature on the lifetime of singlet molecular oxygen (1.DELTA.g). J. Am. Chem. Soc. 1983, 105, 3423–3430. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J. Phys. Chem. 1995, 24, 663–677. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Photosensitising agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeterior. Biodegrad. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Filipe, O.M.S.; Mota, N.; Santos, S.A.O.; Domingues, M.R.M.; Silvestre, A.J.D.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Santos, E.B.H. Identification and characterization of photodegradation products of metoprolol in the presence of natural fulvic acid by HPLC-UV-MSn. J. Hazard. Mater. 2017, 323, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Decreto-Lei nº 113/2012. Gestão da Qualidade das Águas Balneares. Ministério da Agricultura, do Mar, do Ambiente e do Ordenamento do Território: Portugal. Available online: https://dre.pt/web/guest/pesquisa/-/search/177865/details/maximized (accessed on 22 August 2017).
- Sistema Nacional de Informação de Recursos Hídricos, Qualidade das Águas Balneares. 2017. Available online: http://snirh.apambiente.pt/index.php?idMain=1&idItem=2.1 (accessed on 28 April 2017).
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Commission Communication on the Results of the Risk Evaluation and the Risk Reduction Strategies for the Substances: Piperazine; Cyclohexane; Methylenediphenyl Diisocyanate; But-2yne-1,4-diol; Methyloxirane; Aniline; 2-Ethylhexylacrylate; 1,4-Dichlorobenz. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1492559033778&uri=CELEX:52008XC0207(02) (accessed on 19 April 2017).
- Drozd, D.; Szczubiałka, K.; Skiba, M.; Kepczynski, M.; Nowakowska, M. Porphyrin−nanoclay photosensitizers for visible light induced oxidation of phenol in aqueous media. J. Phys. Chem. 2014, 118, 9196–9202. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Gerdes, R.; Wöhrle, D.; Spiller, W.; Schneider, G.; Schnurpfeil, G.; Schulz-Ekloff, G. Photo-oxidation of phenol and monochlorophenols in oxygen-saturated aqueous solutions by different photosensitizers. J. Photochem. Photobiol. A Chem. 1997, 111, 65–74. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram-negative bacteria. Dyes Pigment. 2014, 110, 123–133. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomeu, M.; Reis, S.; Fontes, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact. Water 2017, 9, 630. https://doi.org/10.3390/w9090630
Bartolomeu M, Reis S, Fontes M, Neves MGPMS, Faustino MAF, Almeida A. Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact. Water. 2017; 9(9):630. https://doi.org/10.3390/w9090630
Chicago/Turabian StyleBartolomeu, Maria, Sílvia Reis, Milton Fontes, Maria Graça P. M. S. Neves, Maria Amparo F. Faustino, and Adelaide Almeida. 2017. "Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact" Water 9, no. 9: 630. https://doi.org/10.3390/w9090630





