Enhanced Two Dimensional Hydrodynamic and Water Quality Model (CE-QUAL-W2) for Simulating Mercury Transport and Cycling in Water Bodies
Abstract
:1. Introduction
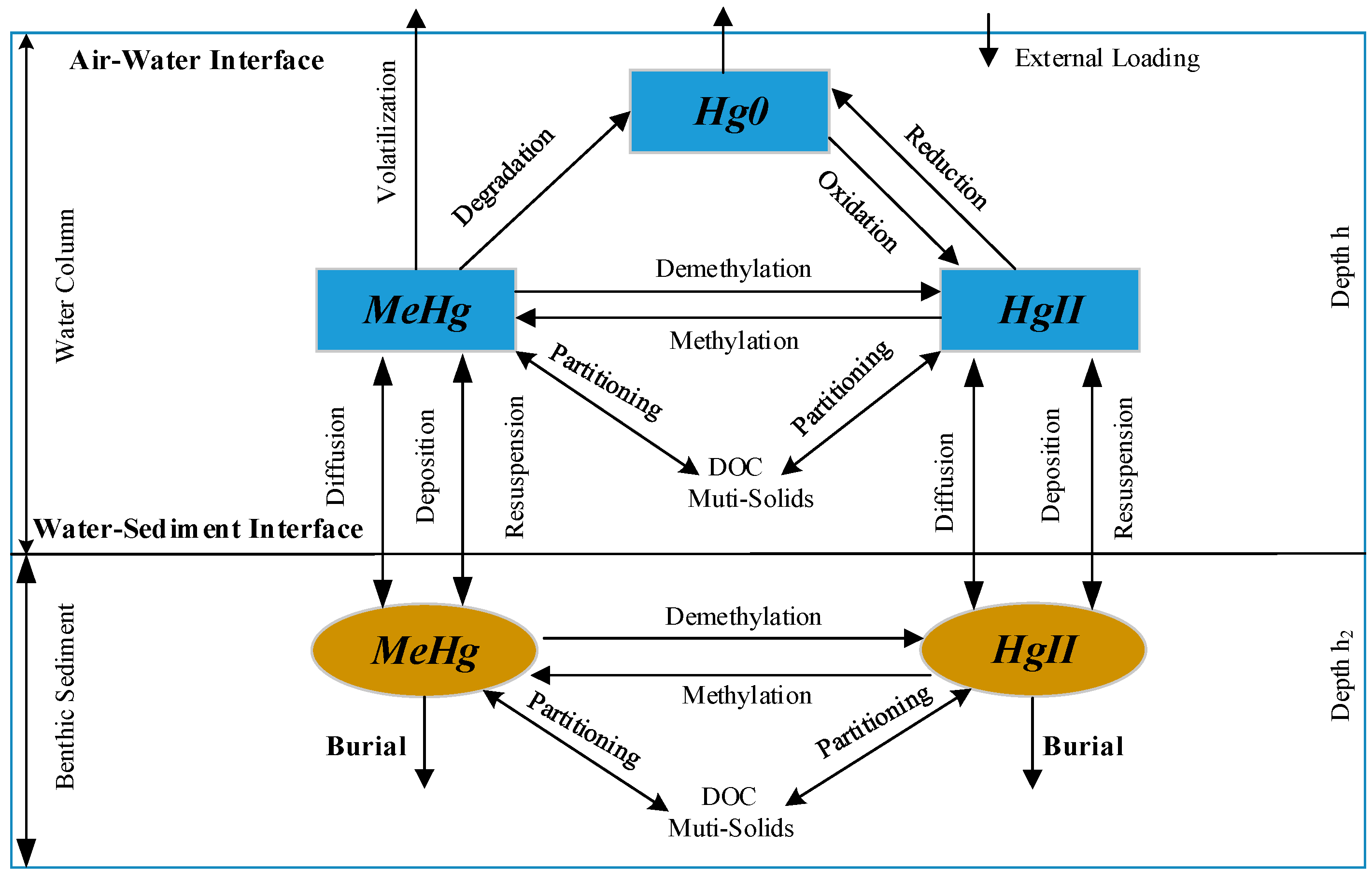
2. Hg Simulation Module within CE-QUAL-W2
2.1. Hg Partitioning
2.2. Hg Transformations and Kinetic Equations
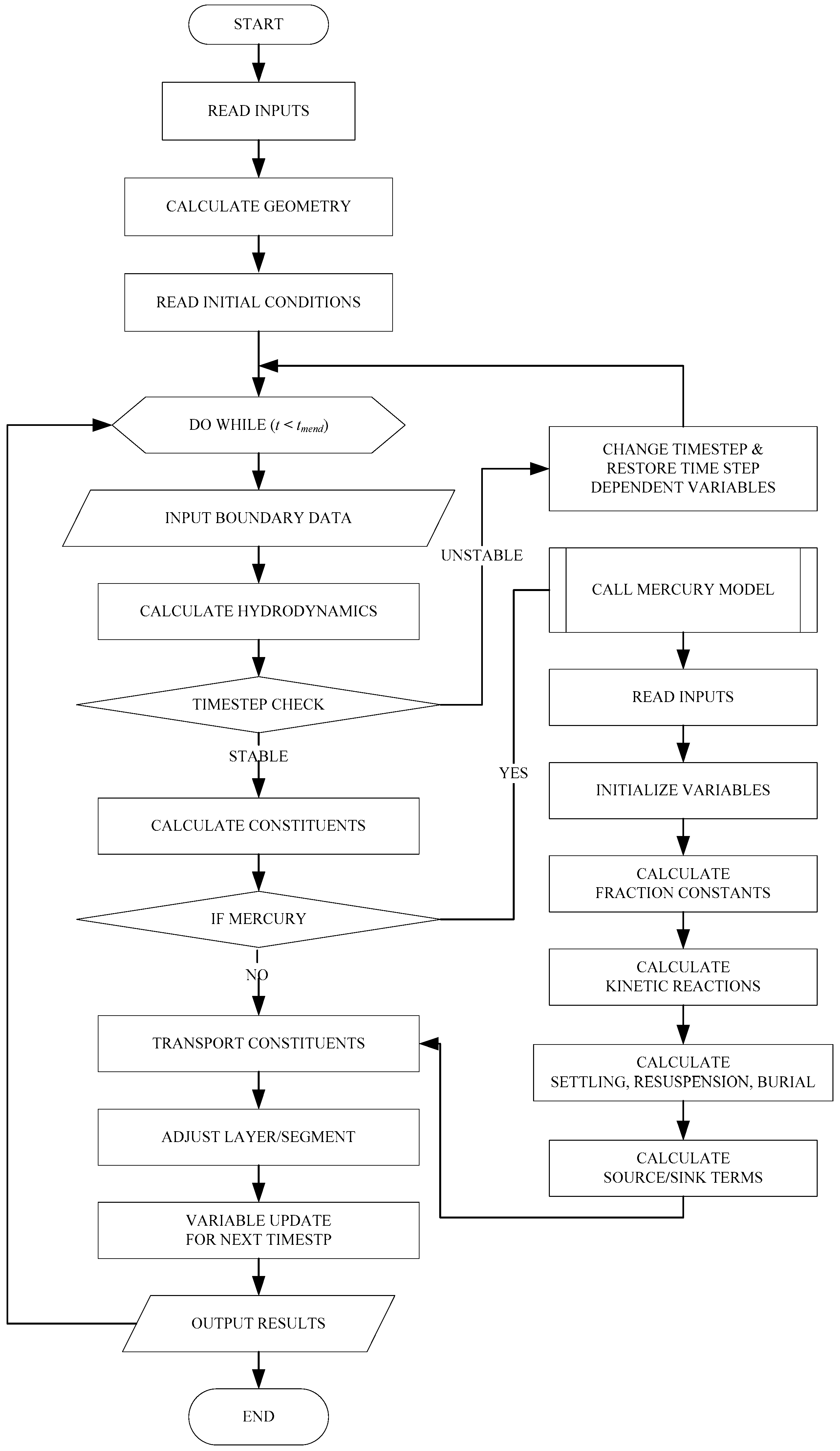
2.3. Enhanced CE-QUAL-W2 Model
3. Enhanced CE-QUAL-W2 Model Validation and Evaluation
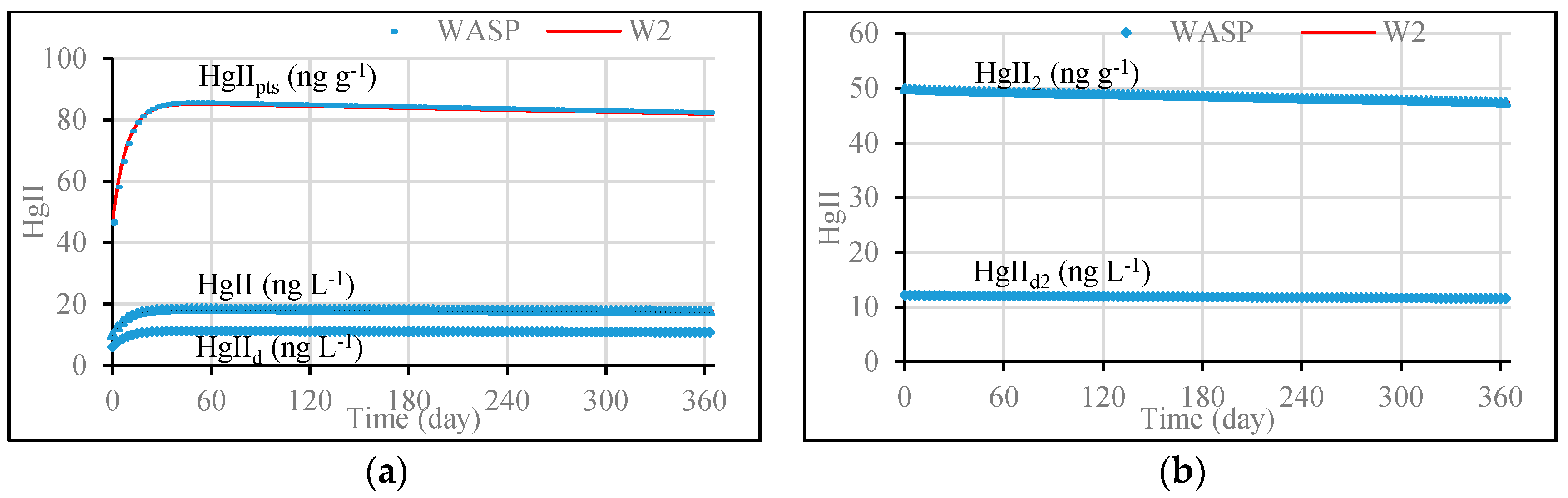
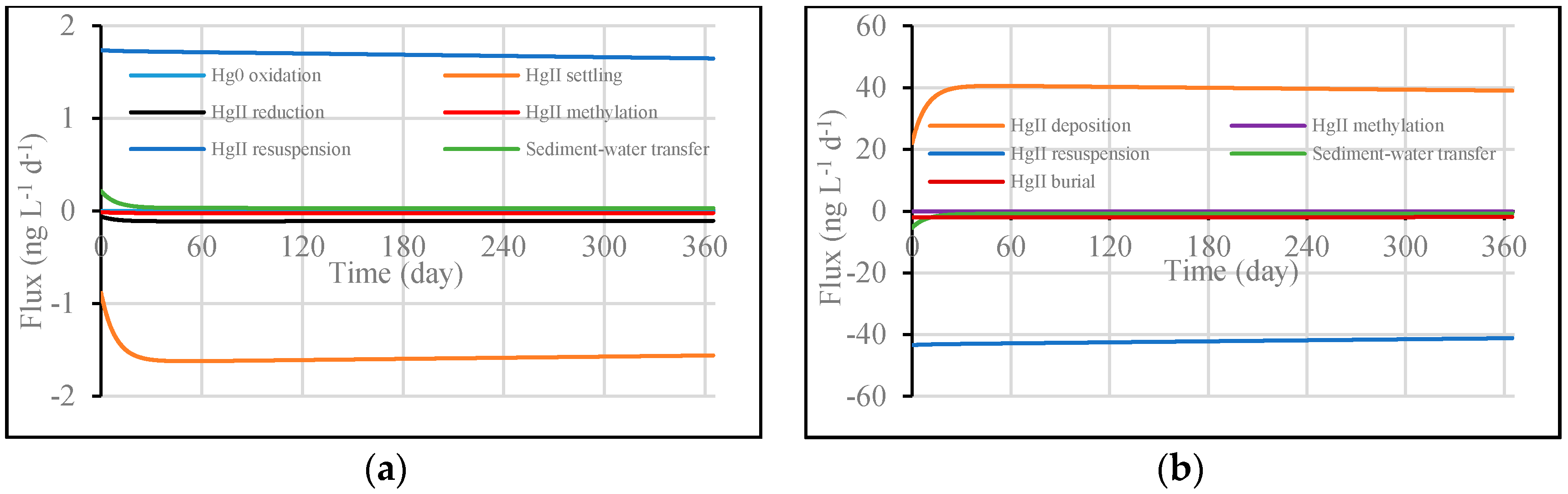
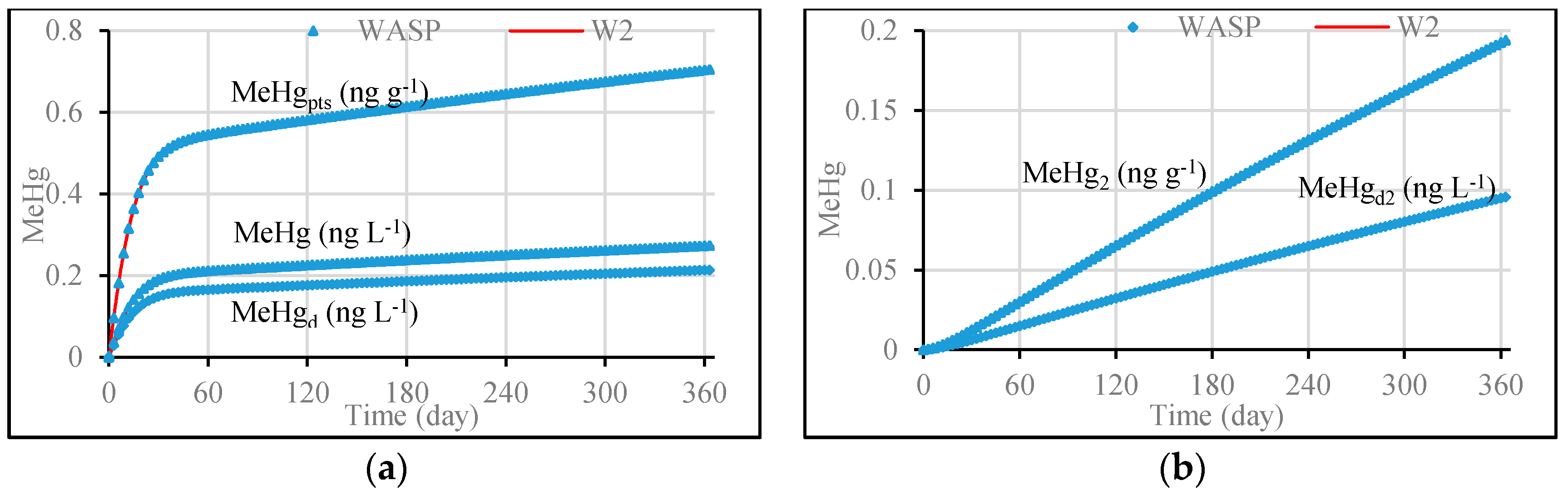
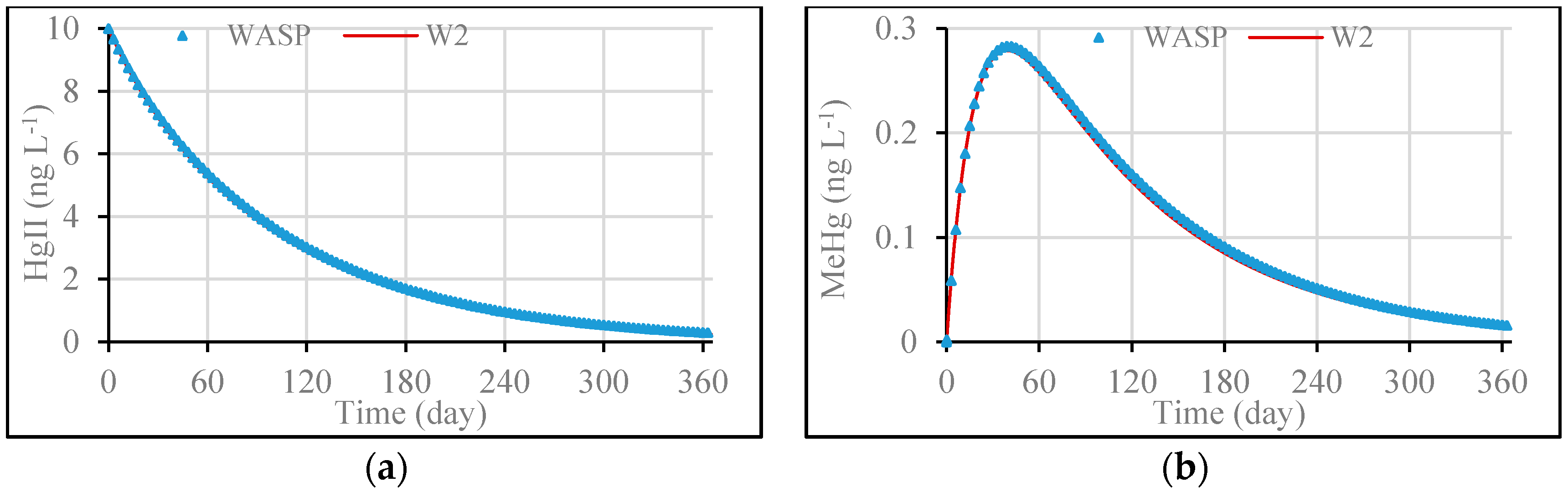
3.1. Model Validation through Comparing WASP Model Results
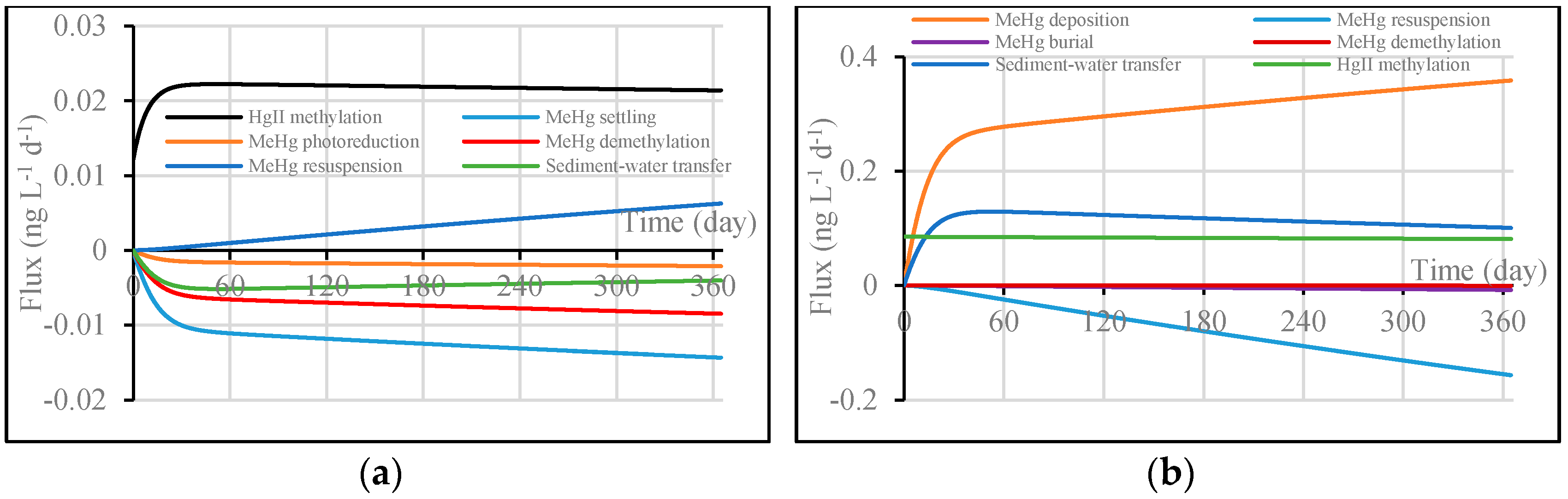
3.2. Application and Evaluation of the Enhanced W2 Model
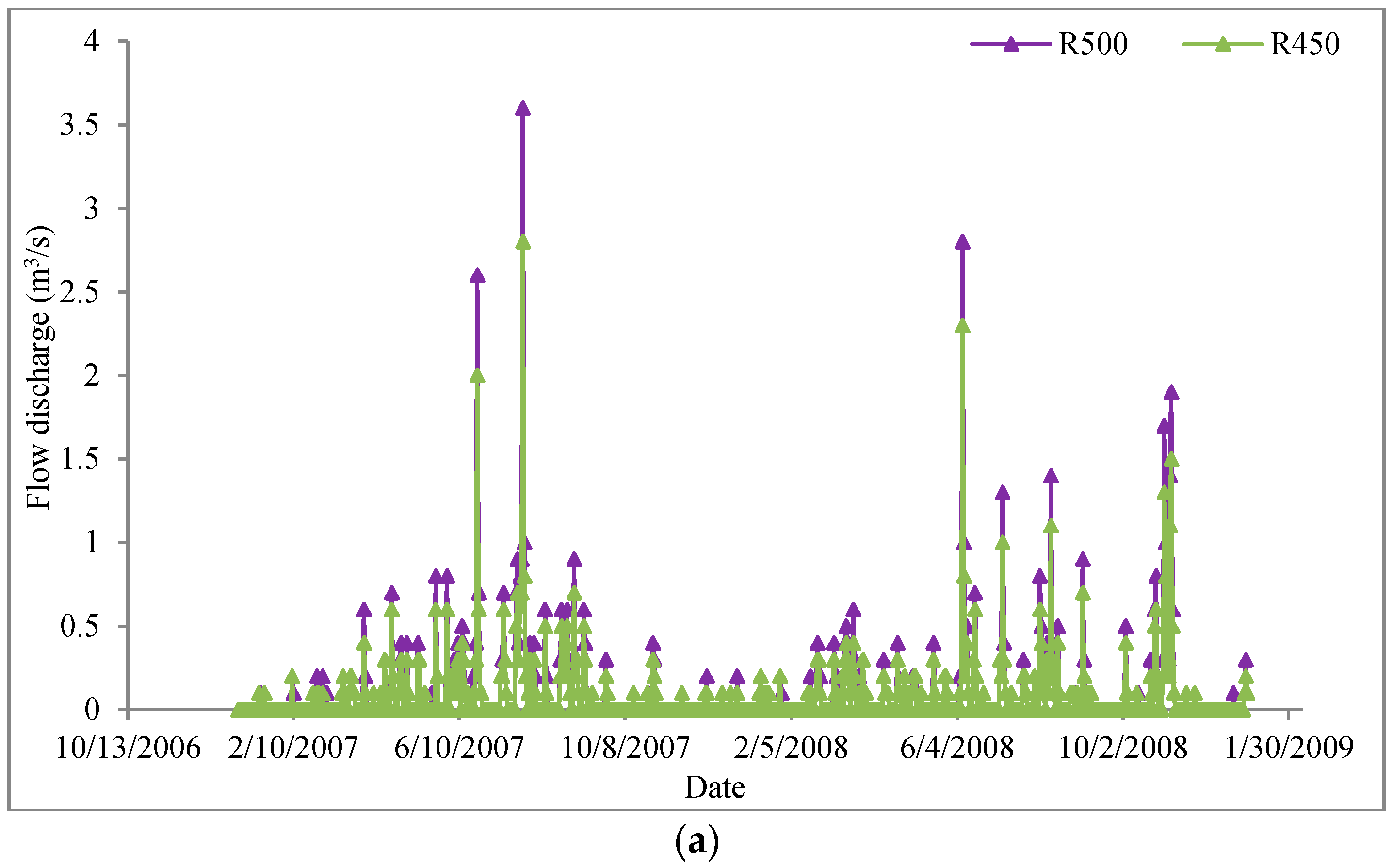
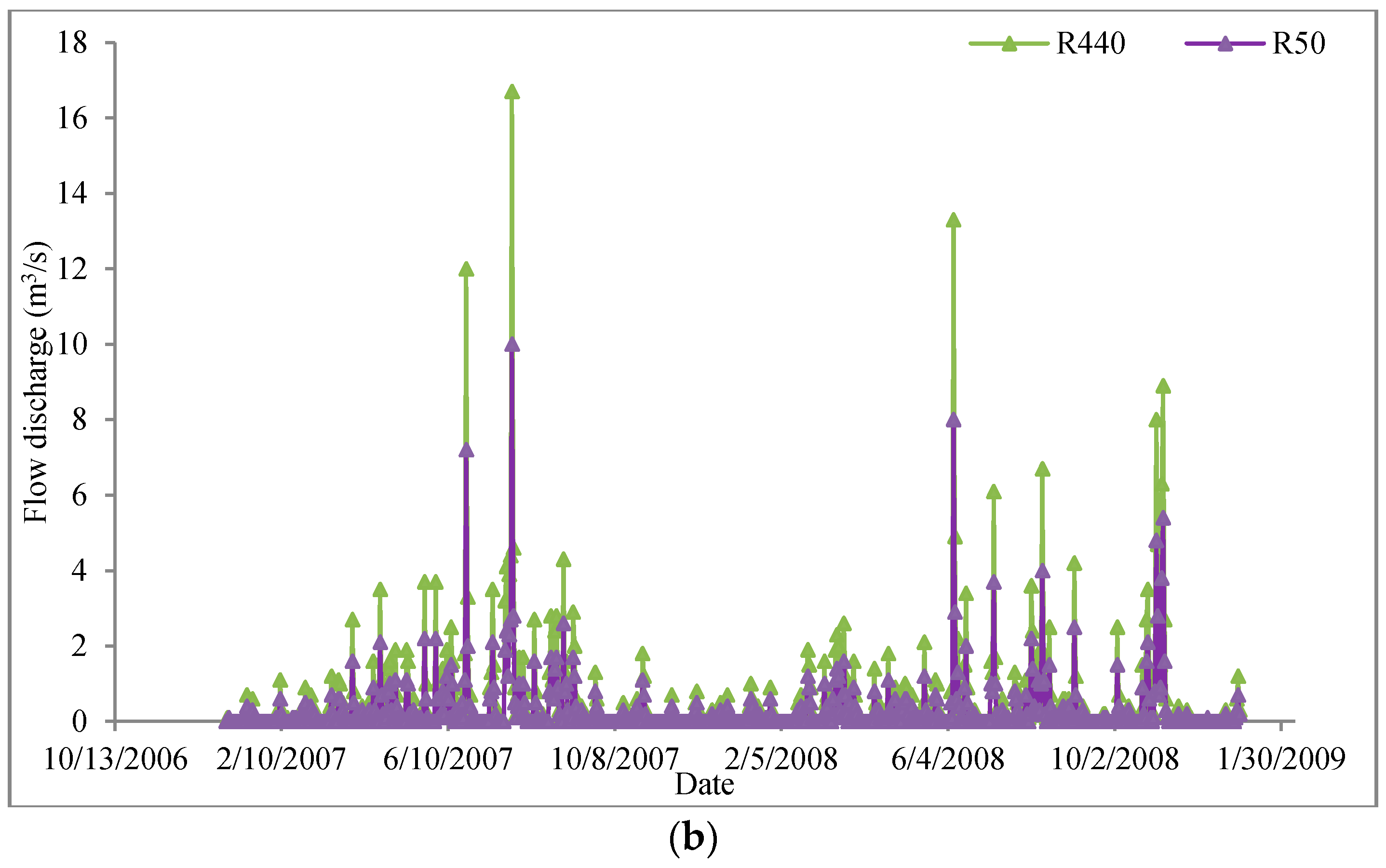
3.2.1. Study Area
3.2.2. Model Development and Calibration
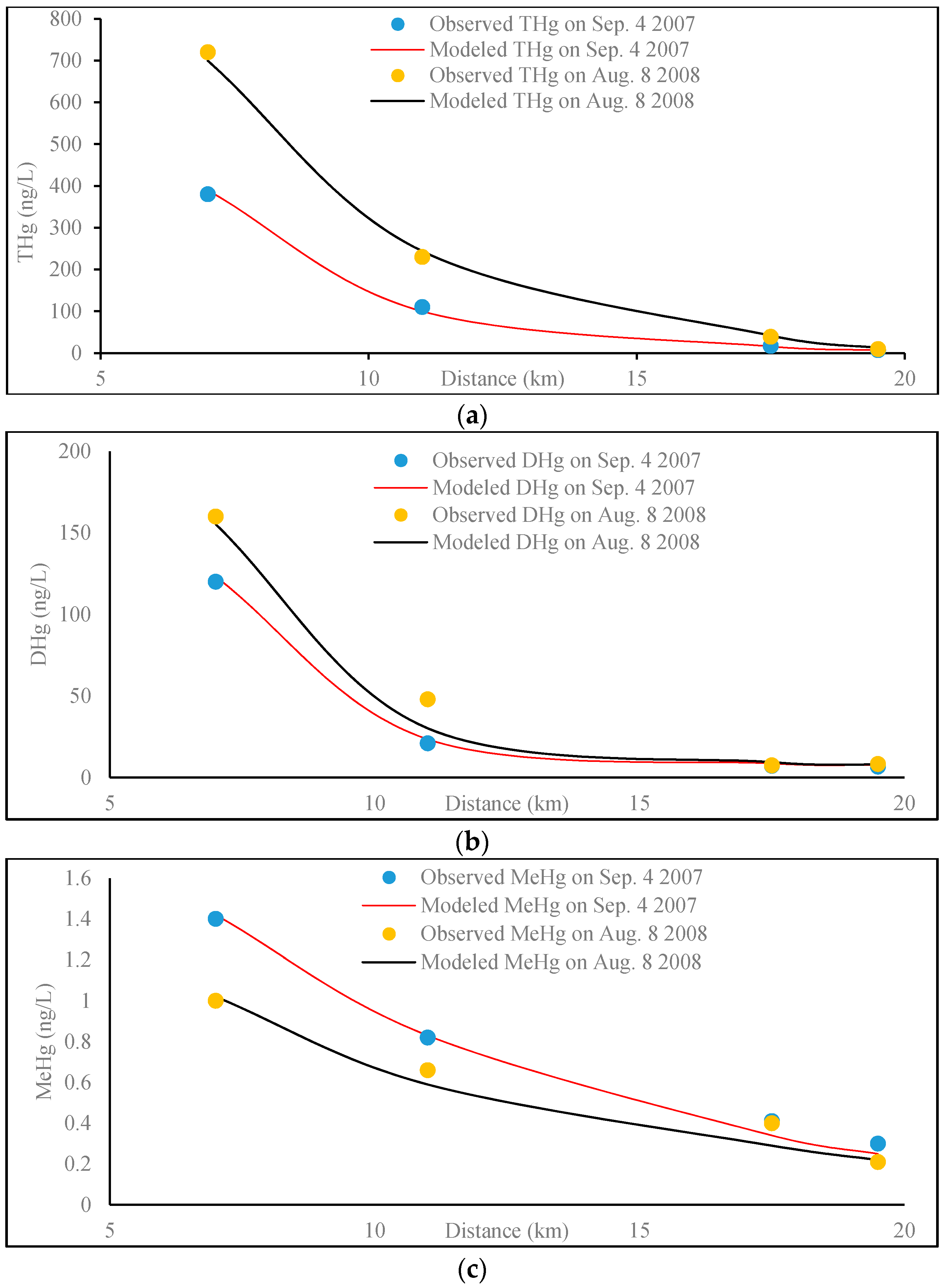
3.2.3. Results and Discussion
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, J.L. Application of two-dimensional water quality model. J. Environ. Eng. 1988, 114, 317–336. [Google Scholar] [CrossRef]
- Cole, T.M.; Wells, S.A. CE-QUAL-W2: A Two-Dimensional, Laterally Averaged, Hydrodynamic and Water Quality Model, Version 3.71; Portland State University: Portland, OR, USA, 2011. [Google Scholar]
- Kim, Y.; Kim, B. Application of a 2-dimensional water quality model (CE-QUAL-W2) to the turbidity interflow in a deep reservoir (Lake Soyang, Korea). Lake Reserv. Manag. 2006, 22, 213–222. [Google Scholar] [CrossRef]
- Debele, B.; Srinivasan, R.; Parlange, J.Y. Coupling upland watershed and downstream waterbody hydrodynamic and water quality models (SWAT and CE-QUAL-W2) for better water resources management in complex river basins. Environ. Model. Assess. 2008, 13, 135–153. [Google Scholar] [CrossRef]
- Afshar, A.; Kazemi, H.; Saadatpour, M. Particle swarm optimization for automatic calibration of large scale water quality model (CE-QUAL-W2): Application to Karkheh reservoir, Iran. Water Resour. Manag. 2011, 25, 2613–2632. [Google Scholar] [CrossRef]
- Ma, J.; Liu, D.; Wells, S.A.; Tang, H.; Ji, D.; Yang, Z. Modeling density currents in a typical tributary of the Three Gorges Reservoir, China. Ecol. Model. 2015, 296, 113–125. [Google Scholar] [CrossRef]
- Noori, R.; Yeh, H.-D.; Ashrafi, K.; Rezazadeh, N.; Bateni, S.M.; Karbassi, A.; Kachoosangi, F.T.; Moazami, S. A reduced-order based CE-QUAL-W2 model for simulation of nitrate concentration in dam reservoirs. J. Hydrol. 2015, 530, 645–656. [Google Scholar] [CrossRef]
- Brown, C.A.; Sharp, D.; Collura, T.C. Effect of climate change on water temperature and attainment of water temperature criteria in the Yaquina Estuary, Oregon (USA). Estuar. Coast. Shelf Sci. 2016, 169, 136–146. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, B.; Johnson, B.E. Integration of a benthic sediment diagenesis module into the two dimensional hydrodynamic and water quality model—CE-QUAL-W2. Ecol. Model. 2015, 297, 213–231. [Google Scholar] [CrossRef]
- Keating, M.H.; Mahaffey, K.R.; Schoeny, R.; Rice, G.E.; Bullock, O.R. Mercury Study Report to Congress; Volume I. Executive Summary. 1997. Available online: https://www3.epa.gov/airtoxics/112nmerc/volume1.pdf (accessed on 1 July 2017).
- US EPA (U.S. Environmental Protection Agency). Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories Volume 2 Risk Assessment and Fish Consumption Limits. 2000. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/volume2.pdf (accessed on 1 July 2017).
- EFSA (European Food Safety Authority). EFSA Provides Risk Assessment on Mercury in Fish: Precautionary Advice Given to Vulnerable Groups, Press Release. 18 March 2004. Available online: https://www.efsa.europa.eu/fr/press/news/contam040318 (accessed on 1 July 2017).
- Hudson, R.J.M.; Gherini, S.A.; Watras, C.J.; Porcella, D.S. Modeling the biogeochemical cycle of mercury in lakes: The mercury cycling model (MCM) and its application to the MTL study lakes. In Mercury Pollution—Integration and Synthesis; Lewis Publishers: Ann Arbor, MI, USA, 1994; pp. 473–523. [Google Scholar]
- Martin, J.L. MERC4: A Mercury Transport and Kinetics Model (beta 1.0); U.S. Environmental Protection Agency, Center for Exposure Assessment Modeling: Athens, GA, USA, 1992.
- Knightes, C.D. Development and test application of a screening-level mercury fate model and tool for evaluating wildlife exposure risk for surface waters with mercury-contaminated sediments (SERAFM). Environ. Model. Softw. 2008, 23, 495–510. [Google Scholar] [CrossRef]
- Wool, T.A.; Ambrose, R.B.; Martin, J.L.; Comer, E.A. Water Quality Analysis Simulation Program (WASP), Version 6, User’s Manual; U.S. Environmental Protection Agency: Athens, GA, USA, 2006.
- Knightes, C.D.; Sunderland, E.M.; Barber, M.C.; Johnston, J.M.; Ambrose, R.B. Application of ecosystem-scale fate and bioaccumulation models to predict fish mercury response times to changes in atmospheric deposition. Environ. Toxicol. Chem. 2009, 28, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Larssen, T.; Vogt, R.D.; Feng, X.B.; Zhang, H. Modelling transport and transformation of mercury fractions in heavily contaminated mountain streams by coupling a GIS-based hydrological model with a mercury chemistry model. Sci. Total Environ. 2011, 409, 4596–4605. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.R. Speciation cycling of arsenic, cadmium, lead and mercury in natural waters. In Lead, Mercury, Cadmium and Arsenic in the Environment; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1987; pp. 175–186. [Google Scholar]
- Stein, E.D.; Cohen, Y.; Winer, A.M. Environmental distribution and transformation of mercury compounds. Crit. Rev. Environ. Sci. Technol. 1996, 26, 1–43. [Google Scholar] [CrossRef]
- Jackson, T.A. Long-range atmospheric transport of mercury to ecosystems, and the importance of anthropogenic emissions: A critical review and evaluation of the published evidence. Environ. Rev. 1997, 5, 99–120. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Zhang, Z.; Johnson, B.E. Aquatic Contaminant and Mercury Simulation Modules Developed for Hydrological and Hydraulic Models; ERDC/EL TR-16-8; US Army Engineer Research and Development Center: Vicksburg, MS, USA, 2016.
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Thibodeaux, L.J.; Valsaraj, K.T.; Reible, D.D. Bioturbation-driven transport of hydrophobic organic contaminants from bed sediment. Environ. Eng. Sci. 2001, 18, 215–223. [Google Scholar] [CrossRef]
- Ditoro, D.M. Sediment Flux Modeling; Wiley-Interscience: New York, NY, USA, 2001. [Google Scholar]
- Boyer, J.M.; Chapra, S.C.; Ruiz, C.E.; Dortch, M.S. RECOVERY: A Mathematical Model to Predict the Temporal Response of Surface Water to Contaminated Sediments; U.S. Army Corps of Engineers Waterways Experiment Station: Vicksburg, MS, USA, 1994.
- Schink, D.R.; Guinasso, N.L. Modelling the influence of bioturbation and other processes on calcium carbonate dissolution at the sea floor. In The Fate of Fossil Fuel CO2 in the Oceans; Plenum Press: New York, NY, USA, 1977; pp. 375–399. [Google Scholar]
- Feng, X.; Qiu, G. Mercury pollution in Guizhou, Southwestern China—An overview. Sci. Total Environ. 2008, 400, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.L.; Feng, X.B.; Wang, S.F.; Shang, L.H. Mercury and methylmercury in riparian soil, sediments, mine-waste calcines, and moss from abandoned Hg mines in east Guizhou province, southwestern China. Appl. Geochem. 2005, 20, 627–638. [Google Scholar] [CrossRef]
- King, J.K.; Michael, S.F.; Lee, R.F.; Jahnke, R.A. Coupling mercury methylation rates to sulfate reduction rates in marine sediments. Environ. Toxicol. Chem. 1999, 18, 1362–1369. [Google Scholar] [CrossRef]
- Gilmour, C.C.; Elias, D.A.; Kucken, A.M.; Brown, S.D.; Palumbo, A.V.; Wall, J.D. Sulfate-reducing bacterium Desulfovibrio desulfuricans ND132 as a model for understanding bacterial mercury methylation. Appl. Environ. Microbiol. 2011, 77, 3938–3951. [Google Scholar] [CrossRef] [PubMed]
- Achá, D.; Hintelmann, J.; Yee, J. Importance of sulfate reducing bacteria in mercury methylation and demethylation in periphyton from Bolivian Amazon region. Chemosphere 2011, 82, 911–916. [Google Scholar] [CrossRef]
- Shao, D.; Kang, Y.; Wu, S.; Wong, M.H. Effects of sulfate reducing bacteria and sulfate concentrations on mercury methylation in freshwater sediments. Sci. Total Environ. 2012, 424, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hsukim, H. Photolytic degradation of methylmercury enhanced by binding to natural organic ligands. Nat. Geosci. 2010, 3, 473–476. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zheng, W.; Liang, L.; Gu, B. Mercury photolytic transformation affected by low-molecular-weight natural organics in water. Sci. Total Environ. 2012, 416, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Fleck, J.A.; Gill, G.; Bergamaschi, B.A.; Kraus, T.E.; Downing, B.D.; Alpers, C.N. Concurrent photolytic degradation of aqueous methylmercury and dissolved organic matter. Sci. Total Environ. 2014, 484, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Kim, M.; Ahn, J.H. Prediction of Chlorophyll-a Changes due to Weir Constructions in the Nakdong River Using EFDC-WASP Modelling. Environ. Eng. Res. 2012, 17, 95–102. [Google Scholar] [CrossRef]
- Xiao, D.; Jia, H.; Wang, Z. Modeling Megacity Drinking Water Security under a DSS Framework in a Tidal River at the North Pearl River Delta, China. J. Am. Water Resour. Assoc. 2015, 51, 637–654. [Google Scholar] [CrossRef]
- Chen, C.X.; Zheng, B.H.; Jiang, X.; Zhao, Z.; Zhan, Y.Z.; Yi, F.J.; Ren, J.Y. Spatial distribution and pollution assessment of mercury in sediments of Lake Taihu, China. J. Environ. Sci. 2013, 25, 316–325. [Google Scholar] [CrossRef]
- Allison, J.D.; Allison, T.L. Partition Coefficients for Metals in Surface Water, Soil, and Waste; EPA-600/R-05/074; Rep. U.S. Environmental Protection Agency, Office of Research and Development: Washington DC, USA, 2005.
- Muresan, B.; Cossa, D.; Richard, S.; Burban, B. Mercury speciation and exchanges at the air-water interface of a tropical artificial reservoir, French Guiana. Sci. Total Environ. 2007, 385, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, E.G.; Pacyna, J.M. Global emission of mercury from anthropogenic sources in 1995. Water Air Soil Pollut. 2002, 137, 149–165. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Breivik, K.; Münch, J.; Fudala, J. European atmospheric emissions of selected persistent organic pollutants, 1970–1995. Atmos. Environ. 2003, 37, 119–131. [Google Scholar] [CrossRef]
- Dastoor, A.P.; Larocque, Y. Global circulation of atmospheric mercury: A modelling study. Atmos. Environ. 2004, 38, 147–161. [Google Scholar] [CrossRef]
- Feng, X. Mercury pollution in China—An Overview. In Dynamics of Mercury Pollution on Regional and Global Scales; Springer: New York, NY, USA, 2005; pp. 657–678. [Google Scholar]
- Zhang, H.; Feng, X.; Larssen, T.; Shang, L.; Vogt, R.D.; Rothenberg, S.E.; Li, P.; Zhang, H.; Lin, Y. Fractionation, distribution and transport of mercury in rivers and tributaries around Wanshan Hg mining district, Guizhou province, southwestern China: Part 1—Total mercury. Appl. Geochem. 2010, 25, 633–641. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Larssen, T.; Shang, L.; Vogt, R.D.; Lin, Y.; Li, P.; Zhang, H. Fractionation, distribution and transport of mercury in rivers and tributaries around Wanshan Hg mining district, Guizhou province, southwestern China: Part 2—Methyl mercury. Appl. Geochem. 2010, 25, 642–649. [Google Scholar] [CrossRef]
- Lin, Y.; Larssen, T.; Vogt, R.D.; Feng, X.B. Identification of fractions of mercury in water, soil and sediment from a typical Hg mining area in Wanshan, Guizhou province, China. Appl. Geochem. 2010, 25, 60–68. [Google Scholar] [CrossRef]
- Rice, G.E.; Ambrose, R.B.; Bullock, O.R.; Swartout, J. Mercury Study Report to Congress. Volume 3. Fate and Transport of Mercury in the Environment. 1997. Available online: https://www3.epa.gov/airtoxics/112nmerc/volume3.pdf (accessed on 1 July 2017).
- Eckley, C.S.; Watras, C.J.; Hintelmann, H.; Morrison, K.; Kent, A.D.; Regnell, O. Mercury methylation in the hypolimnetic waters of lakes with and without connection to wetlands in northern Wisconsin. Can. J. Fish. Aquat. Sci. 2005, 62, 400–411. [Google Scholar] [CrossRef]
- Carroll, R.W.H.; Warwick, J.J.; Heim, K.J.; Bonzongo, J.C.; Miller, J.R.; Lyons, W.B. Simulation of mercury transport and fate in the Carson River, Nevada. Ecol. Model. 2000, 125, 255–278. [Google Scholar] [CrossRef]
- Oremland, R.S.; Miller, L.G.; Dowdle, P.; Connell, T.; Barkay, T. Methyl-mercury oxidative degradation potentials in contaminated and pristine sediments of the Carson River, Nevada. Appl. Environ. Microbiol. 1995, 61, 2745–2753. [Google Scholar] [PubMed]




| Symbol | Definition | Units |
|---|---|---|
| Hg0 | Hg0 concentration in water | ng·L−1 |
| HgII | Concentration of total HgII in water | ng·L−1 |
| HgIId | Concentration of dissolved HgII in water | ng·L−1 |
| HgIIdoc | Concentration of DOC adsorbed HgII in water | ng·L−1 |
| HgIIpom | Concentration of POM adsorbed HgII in water | ng·L−1 |
| HgIIpt | Total concentration of solids adsorbed HgII in water | ng·L−1 |
| HgIIpts | Total concentration of solids adsorbed HgII in water | ng·g−1 |
| HgII2 | Concentration of total HgII in sediment | ng·L−1 |
| HgIIdp2 | Concentration of dissolved HgII in pore water | ng·L−1 |
| HgIIdocp2 | Concentration of DOC adsorbed HgII in pore water | ng·L−1 |
| HgIIpom2 | Concentration of POM adsorbed HgII in sediment | ng·L−1 |
| HgIIpt2 | Total concentration of solids adsorbed HgII in sediment | ng·L−1 |
| HgIIpts2 | Total concentration of solids adsorbed HgII in sediment | ng·g−1 |
| MeHg | Concentration of total MeHg in water | ng·L−1 |
| MeHgd | Concentration of dissolved MeHg in water | ng·L−1 |
| MeHgdoc | Concentration of DOC adsorbed MeHg in water | ng·L−1 |
| MeHgpom | Concentration of POM adsorbed MeHg in water | ng·L−1 |
| MeHgpt | Total concentration of solids adsorbed MeHg in water | ng·L−1 |
| MeHgpts | Total concentration of solids adsorbed MeHg in water | ng·g−1 |
| MeHg2 | Concentration of total MeHg in sediment | ng·L−1 |
| MeHgdp2 | Concentration of dissolved MeHg in pore water | ng·L−1 |
| MeHgdocp2 | Concentration of DOC adsorbed MeHg in pore water | ng·L−1 |
| MeHgpom2 | Concentration of POM adsorbed MeHg in sediment | ng·L−1 |
| MeHgpt2 | Concentration of solids adsorbed MeHg in sediment | ng·L−1 |
| MeHgpts2 | Concentration of solids adsorbed MeHg in sediment | ng·g−1 |
| Symbol | Definition | Units |
|---|---|---|
| CL | cloud cover | / |
| DOC | dissolved organic carbon in water | mg·L−1 |
| DOC2 | dissolved organic carbon in pore water | mg·L−1 |
| fd | fraction of dissolved phase in water | / |
| fdoc | fraction of DOC adsorbed phase in water | / |
| fd2 | fraction of dissolved phase in sediment | / |
| fdoc2 | fraction of DOC adsorbed phase in sediment | / |
| fpom | fraction of POM adsorbed phase in water | / |
| Fpom2 | fraction of POM adsorbed phase in sediment | / |
| fpn | fraction of inorganic solid “n” adsorbed phase in water | / |
| fpn2 | fraction of inorganic solid “n” adsorbed phase in sediment | / |
| h | water depth | m |
| h2 | sediment layer thickness | m |
| Hg00 | air concentration of Hg0 | ng·L−1 |
| i | either HgII or MeHg | / |
| I0 | solar radiation at the water surface | W·m−2 |
| I0pht | light intensity when kpht is measured | W·m−2 |
| k12 | Hg0 oxidation rate in water | d−1 |
| kd21 | photoreduction rate of dissolved HgII in water | d−1 |
| kdoc21 | photoreduction rate of DOC adsorbed HgII in water | d−1 |
| kd23 | methylation rate of dissolved HgII in water | d−1 |
| kdoc23 | methylation rate of DOC adsorbed HgII in water | d−1 |
| kd31 | photolytic degradation rate of dissolved MeHg in water | d−1 |
| kdoc31 | photolytic degradation rate of DOC adsorbed MeHg in water | d−1 |
| kd32 | demethylation rate of dissolved MeHg in water | d−1 |
| kdoc32 | demethylation rate of DOC adsorbed MeHg in water | d−1 |
| kd32-2 | dissolved MeHg demethylation rate in sediment | d−1 |
| kso42 | sediment sulfate reduction rate | d−1 |
| KH | Henry’s law constant | Pa·m3·mol−1 |
| Kdoc | equilibrium partition coefficient for DOC in water | L·kg−1 |
| Kdoc2 | equilibrium partition coefficient for sediment DOC | L·kg−1 |
| Kpom | equilibrium partition coefficient for POM in water | L·kg−1 |
| Kpom2 | equilibrium partition coefficient for sediment POM | L·kg−1 |
| Kpn | equilibrium partition coefficient for inorganic solids in water | L·kg−1 |
| Kpn2 | equilibrium partition coefficient for sediment solids “n” | L·kg−1 |
| KSO4 | half-saturation constant for the effect of sulfate on methylation | mg-O2·L−1 |
| LHgII | atmospheric deposition rates of HgII | μg·m−2·d−1 |
| LMeHg | atmospheric deposition rates of MeHg | μg·m−2·d−1 |
| mn | inorganic solid “n” concentration in water | mg·L−1 |
| mn2 | inorganic solid “n” concentration in sediment | mg·L−1 |
| MeHg0 | air concentration of MeHg | ng·L−1 |
| POM | particulate organic matter in water | mg L−1 |
| POM2 | particulate organic matter in sediment | mg·L−1 |
| rmso4 | ratio of sediment methylation rate and sulfate reduction rate | L·mg−1 |
| R | universal gas constant | Pa·m3·mol−1·K−1 |
| SO42 | sediment pore water sulfate concentration | mg-O2·L−1 |
| SSHg0 | source/sink terms of Hg0 in water | μg·L−1·d−1 |
| SSHgII | source/sink terms of HgII in water | μg·L−1·d−1 |
| SSMeHg | source/sink terms of MeHg in water | μg·L−1·d−1 |
| SSHgII2 | source/sink terms of HgII in sediment | μg·L−1·d−1 |
| SSMeHg2 | source/sink terms of MeHg in sediment | μg·L−1·d−1 |
| Twk | water temperature | K |
| Y12 | Hg0 oxidation yield coefficient | g·g−1 |
| Y21 | HgII photoreduction yield coefficient | g·g−1 |
| Y23 | HgII methylation yield coefficient | g·g−1 |
| Y31 | MeHg photolytic degradation yield coefficient | g·g−1 |
| Y32 | MeHg demethylation yield coefficient | g·g−1 |
| ϕ | porosity | / |
| λmax | maximum light extinction coefficient | m−1 |
| vv-Hg0 | volatilization velocity of Hg0 | m·d−1 |
| vv-MeHg | volatilization velocity of MeHg | m·d−1 |
| vb | sediment burial velocity | m·d−1 |
| vpn | settling velocity of inorganic solid “n” | m·d−1 |
| vrn | sediment resuspension velocity of solid “n” | m·d−1 |
| vsom | settling velocity of POM | m·d−1 |
| Variable | Units | Value |
|---|---|---|
| Kp-HgII Kpom-HgII Kp-MeHg Kpom-MeHg | L·kg−1 L·kg−1 L·kg−1 L·kg−1 | 2.0 × 103 (Silt and fine), 1.0 × 103 (Sand) 1.0 × 104 |
| 1.0 × 103 (Silt and fine), 0.5 × 103 (Sand) 0.5 × 104 | ||
| KH-Hg0 | atm·m3·mol−1 | 0.0071 |
| vv-Hg0 | m·d−1 | 0.8 |
| Hg00 | ng·L−1 | 0.0 |
| k12 | d−1 | 0.001 |
| Y12 | g·g−1 | 1.0 |
| kd21 | d−1 | 0.01 |
| Y21 | g·g−1 | 1.0 |
| I0pht | W·m−2 | 100.0 |
| kd23 | d−1 | 0.002 |
| Y23 | g·g−1 | 1.07 |
| kd32 | d−1 | 0.04 |
| kd23_2 | d−1 | 0.01 |
| kd31 | d−1 | 0.01 |
| Y31 | g·g−1 | 0.93 |
| kd32_2 | d−1 | 0.005 |
| Y32 | g·g−1 | 0.93 |
| Reach Number | Reach Name | Length (m) | Slope | Remark |
|---|---|---|---|---|
| 1 | R450 | 646 | 0.17301 | Hg tailing |
| 2 | R500 | 2675 | 0.04434 | Hg tailing |
| 3 | R320 | 4687 | 0.02238 | Mainstem |
| 4 | R410 | 3388 | 0.02766 | Mainstem |
| 5 | R180 | 6813 | 0.01000 | Mainstem |
| 6 | R20 | 2017 | 0.01000 | Mainstem |
| 7 | R440 | 5688 | 0.03769 | Tributary |
| 8 | R50 | 7353 | 0.02745 | Tributary |
| W2 model Segment No. | TSS (mg·L−1) | TSS (mg·L−1) | ||
|---|---|---|---|---|
| 4 September 2007 | 8 August 2008 | |||
| Modelled | Observed | Modelled | Observed | |
| 5 | 1.75 | 1.7 | 4.26 | 4.4 |
| 22 | 0.7 | 0.76 | 1.95 | 2 |
| 36 | 0.55 | 0.44 | 2.15 | 2.2 |
| 51 | 0.55 | 0.7 | 2.14 | 2.1 |
| Kd for Fine Solids/Kd for Coarse Solids | 102 | 103 | 104 | 105 | 106 |
|---|---|---|---|---|---|
| 104 | 33.47 | 30.95 | 29.54 | 29.26 | 32.79 |
| 105 | 29.16 | 26.30 | 24.68 | 24.37 | 27.97 |
| 106 | 18.53 | 14.23 | 11.75 | 12.29 | 15.42 |
| 107 | 39.06 | 35.03 | 32.67 | 32.20 | 38.84 |
| M/D | 10−2 | 10−1 | 1 |
|---|---|---|---|
| 10−1 | 15.98 | 28.45 | 25.06 |
| 10−2 | 11.81 | 9.93 | 13.37 |
| 10−3 | 12.20 | 14.14 | 19.53 |
| 10−4 | 12.69 | 14.65 | 20.18 |
| 10−5 | 14.27 | 16.10 | 20.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Zhang, Z.; Liu, X. Enhanced Two Dimensional Hydrodynamic and Water Quality Model (CE-QUAL-W2) for Simulating Mercury Transport and Cycling in Water Bodies. Water 2017, 9, 643. https://doi.org/10.3390/w9090643
Zhu S, Zhang Z, Liu X. Enhanced Two Dimensional Hydrodynamic and Water Quality Model (CE-QUAL-W2) for Simulating Mercury Transport and Cycling in Water Bodies. Water. 2017; 9(9):643. https://doi.org/10.3390/w9090643
Chicago/Turabian StyleZhu, Senlin, Zhonglong Zhang, and Xiaobo Liu. 2017. "Enhanced Two Dimensional Hydrodynamic and Water Quality Model (CE-QUAL-W2) for Simulating Mercury Transport and Cycling in Water Bodies" Water 9, no. 9: 643. https://doi.org/10.3390/w9090643




