Methane Emissions in Spanish Saline Lakes: Current Rates, Temperature and Salinity Responses, and Evolution under Different Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Water Sampling and Determination of Environmental Variables
2.3. Measurements of Methane Emissions
2.4. Temperature and Salinity Dependence of Methane Emission Rates and Q10
2.5. Predicting Changes of Methane Emission Rates under Different RCP Scenarios
3. Results
3.1. Environmental Features of the Studied Lakes
3.2. Methane Emission Rates
3.3. Effect of Temperature and Salinity on Methane Emission Rates
3.4. Predicted Changes of the Methane Emission Rates Due to Temperature Effects under Different Climate Scenarios
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, B., Midgley, B., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.A.; Audet, J.; Svenning, J.C.; Lauridsen, T.L.; Søndergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication effects on greenhouse gas fluxes from shallow-lake mesocosms override those of climate warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Whiting, G.J.; Chanton, J.P. Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration. Tellus Ser. B Chem. Phys. Meteorol. 2001, 53, 521–528. [Google Scholar] [CrossRef]
- Mitra, S.; Wassmann, R.; Vlek, P.L. An appraisal of global wetland area and its organic carbon stock. Curr. Sci. 2005, 88, 25–35. [Google Scholar]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Hammer, U.T. Saline Lake Ecosystems of the World; Springer Science & Business Media: Berlin, Germany, 1986; Volume 59. [Google Scholar]
- Florin, M.; Montes, C. Functional analysis and restoration of Mediterranean lagunas in the Mancha Húmeda Biosphere Reserve. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 97–109. [Google Scholar] [CrossRef]
- Camacho, A.; Miracle, M.R.; Vicente, E. Which factors determine the abundance and distribution of picocyanobacteria in inland waters? A comparison among different types of lakes and ponds. Arch. Hydrobiol. 2003, 157, 321–338. [Google Scholar] [CrossRef]
- García-Ferrer, I.; Camacho, A.; Armengol, X.; Miracle, M.R.; Vicente, E. Seasonal and spatial heterogeneity in the water chemistry of two sewage-affected saline shallow lakes from central Spain. Hydrobiologia 2003, 506, 101–110. [Google Scholar] [CrossRef]
- Montoya, L.; Vizioli, C.; Rodríguez, N.; Rastoll, M.J.; Amils, R.; Marin, I. Microbial community composition of Tirez lagoon (Spain), a highly sulfated athalassohaline environment. Aquat. Biosyst. 2013, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Escrivà, A.; Valls, L.; Rochera, C.; Camacho, A.; Mesquita-Joanes, F. Metacommunity dynamics of Ostracoda in temporary lakes: Overall strong niche effects except at the onset of the flooding period. Limnol. Ecol. Manag. Inland Waters 2017, 62, 104–110. [Google Scholar] [CrossRef]
- Ortiz-Llorente, M.; Alvarez-Cobelas, M. Comparison of biogenic methane emissions from unmanaged estuaries, lakes, oceans, rivers and wetlands. Atmos. Environ. 2012, 59, 328–337. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Contribution of working groups I, II and III to the fourth assessment report of the Intergovernmental Panel on Climate Change. In Climate Change: Synthesis Report; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Altor, A.E.; Mitsch, W.J. Methane and carbon dioxide dynamics in wetland mesocosms: Effects of hydrology and soils. Ecol. Appl. 2008, 18, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M.; Bartha, R. Microbial Ecology: Fundamentals and Applications, 4th ed.; Addison-Wesley Publishing Company: Boston, MA, USA, 1997. [Google Scholar]
- Doña, C.; Chang, N.B.; Caselles, V.; Sánchez, J.M.; Pérez-Planells, L.; Bisquert, M.D.M.; García-Santos, V.; Imen, S.; Camacho, A. Monitoring hydrological patterns of temporary lakes using remote sensing and machine learning models: Case study of la Mancha Húmeda Biosphere Reserve in central Spain. Remote Sens. 2016, 8, 618. [Google Scholar] [CrossRef]
- Pacheco, F.S.; Roland, F.; Downing, J.A. Eutrophication reverses whole-lake carbon budgets. Inland Waters 2014, 4, 41–48. [Google Scholar] [CrossRef]
- Bastviken, D. Methane. In Encyclopedia of Inland Waters; Likens, E., Ed.; Elsevier: Oxford, UK, 2009; Volume 2. [Google Scholar]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; Del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lai, D.Y.; Tong, C.; Wang, W.; Huang, J.; Zeng, C. Variations in temperature sensitivity (Q10) of ch4 emission from a subtropical estuarine marsh in southeast china. PLoS ONE 2015, 10, e0125227. [Google Scholar] [CrossRef] [PubMed]
- Segers, R. Methane production and methane consumption: A review of processes underlying wetland methane fluxes. Biogeochemistry 1998, 41, 23–51. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Noges, T.; Noges, P.; Attayde, J.L.; Zohary, T.; Coppens, J. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Den Elzen, M.G.; Lucas, P.L.; Eickhout, B.; Strengers, B.J.; Van Ruijven, B.; Wonink, S.; van Houdt, R. Stabilizing greenhouse gas concentrations at low levels: An assessment of reduction strategies and costs. Clim. Chang. 2007, 81, 119–159. [Google Scholar] [CrossRef]
- Wise, M.; Calvin, K.; Thomson, A.; Clarke, L.; Bond-Lamberty, B.; Sands, R.; Smith, S.J.; Janetos, A.; Edmonds, J. Implications of limiting CO2 concentrations for land use and energy. Science 2009, 324, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nature 2009, 458, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.; Stehfest, E.; Den Elzen, M.; Deetman, S.; Beltran, A.; Oostenrijk, R. Exploring the possibility to keep global mean temperature change below 2 °C. Clim. Chang. 2011, 109, 95–106. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Masui, T.; Matsumoto, K.; Hijioka, Y.; Kinoshita, T.; Nozawa, T.; Ishiwatari, S.; Kato, E.; Shukla, P.; Yamagata, Y.; Kainuma, M. An emission pathway for stabilization at 6 w m−2 radiative forcing. Clim. Chang. 2011, 109, 59–76. [Google Scholar] [CrossRef]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E. Rcp 4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Chang. 2011, 109, 77–94. [Google Scholar] [CrossRef]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.; Lamarque, J.; Matsumoto, K.; Montzka, S.; Raper, S.; Riahi, K. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213–248. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Riahi, K.; Krey, V.; Rao, S.; Chirkov, V.; Fischer, G.; Kolp, P.; Kindermann, G.; Nakicenovic, N.; Rafai, P. Rcp-8.5: Exploring the consequence of high emission trajectories. Clim. Chang. 2011, 109, 33–57. [Google Scholar] [CrossRef]
- Rogelj, J.; Meinshausen, M.; Knutti, R. Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nat. Clim. Chang. 2012, 2, 8–253. [Google Scholar] [CrossRef]
- Castillo-Escrivà, A.; Valls, L.; Rochera, C.; Camacho, A.; Mesquita-Joanes, F. Spatial and environmental analysis of an ostracod metacommunity from endorheic lakes. Aquat. Sci. 2016, 78, 707–716. [Google Scholar] [CrossRef]
- Castillo-Escrivà, A.; Valls, L.; Rochera, C.; Camacho, A.; Mesquita-Joanes, F. Disentangling environmental, spatial, and historical effects on ostracod communities in shallow lakes. Hydrobiologia 2017, 787, 61–72. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Ferree, M.A.; Shannon, R.D. Evaluation of a second derivative UV/Visible spectroscopy technique for nitrate and total nitrogen analysis of wastewater samples. Water Res. 2001, 35, 327–332. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.; Dekkers, E. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Picazo, A.; Rochera, C.; Vicente, E.; Miracle, M.R.; Camacho, A. Spectrophotometric methods for the determination of photosynthetic pigments in stratified lakes: A critical analysis based on comparisons with HPLC determinations in a model lake. Limnetica 2013, 32, 139–158. [Google Scholar]
- Bastviken, D.; Ejlertsson, J.; Tranvik, L. Measurement of methane oxidation in lakes: A comparison of methods. Environ. Sci. Technol. 2002, 36, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Sander, B.O.; Wassmann, R. Common practices for manual greenhouse gas sampling in rice production: A literature study on sampling modalities of the closed chamber method. Greenh. Gas Meas. Manag. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Yuesi, W.; Yinghong, W. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv. Atmos. Sci. 2003, 20, 842–844. [Google Scholar] [CrossRef]
- Lambert, M.; Fréchette, J.L. Analytical techniques for measuring fluxes of CO2 and CH4 from hydroelectric reservoirs and natural water bodies. In Greenhouse Gas Emissions—Fluxes and Processes: Hydroelectric Reservoirs and Natural Environments; Tremblay, A., Varfalvy, L., Roehm, C., Garneau, M., Eds.; Springer: Berlin, Germany, 2005; pp. 37–60. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Ferriol, C.; Miracle, M.R.; Vicente, E. Effects of nutrient addition, recovery thereafter and the role of macrophytes in nutrient dynamics of a Mediterranean shallow lake: A mesocosm experiment. Mar. Freshw. Res. 2017, 68, 506–518. [Google Scholar] [CrossRef]
- Bartlett, K.B.; Harriss, R.C. Review and assessment of methane emissions from wetlands. Chemosphere 1993, 26, 261–320. [Google Scholar] [CrossRef]
- Cao, M.; Marshall, S.; Gregson, K. Global carbon exchange and methane emissions from natural wetlands: Application of a process-based model. J. Geophys. Res. Atmos. 1996, 101, 14399–14414. [Google Scholar] [CrossRef]
- Kang, H.; Jang, I.; Kim, S. Key processes in CH4 dynamics in wetlands and possible shifts with climate change. In Global Change and the Function and Distribution of Wetlands; Springer: Berlin, Germany, 2012; pp. 99–114. [Google Scholar]
- Vasilas, B.L.; Rabenhorst, M.; Fuhrmann, J.; Chirnside, A.; Inamdar, S. Wetland biogeochemistry techniques. In Wetland Techniques; Springer: Berlin, Germany, 2013; pp. 355–442. [Google Scholar]
- Jeppesen, E.; Meerhoff, M.; Jacobsen, B.A.; Hansen, R.S.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Mazzeo, N.; Branco, C.W.C. Restoration of shallow lakes by nutrient control and biomanipulation—The successful strategy varies with lake size and climate. Hydrobiologia 2007, 581, 269–285. [Google Scholar] [CrossRef]
- Bohn, T.; Lettenmaier, D.; Sathulur, K.; Bowling, L.; Podest, E.; McDonald, K.; Friborg, T. Methane emissions from western Siberian wetlands: Heterogeneity and sensitivity to climate change. Environ. Res. Lett. 2007, 2, 045015. [Google Scholar] [CrossRef]

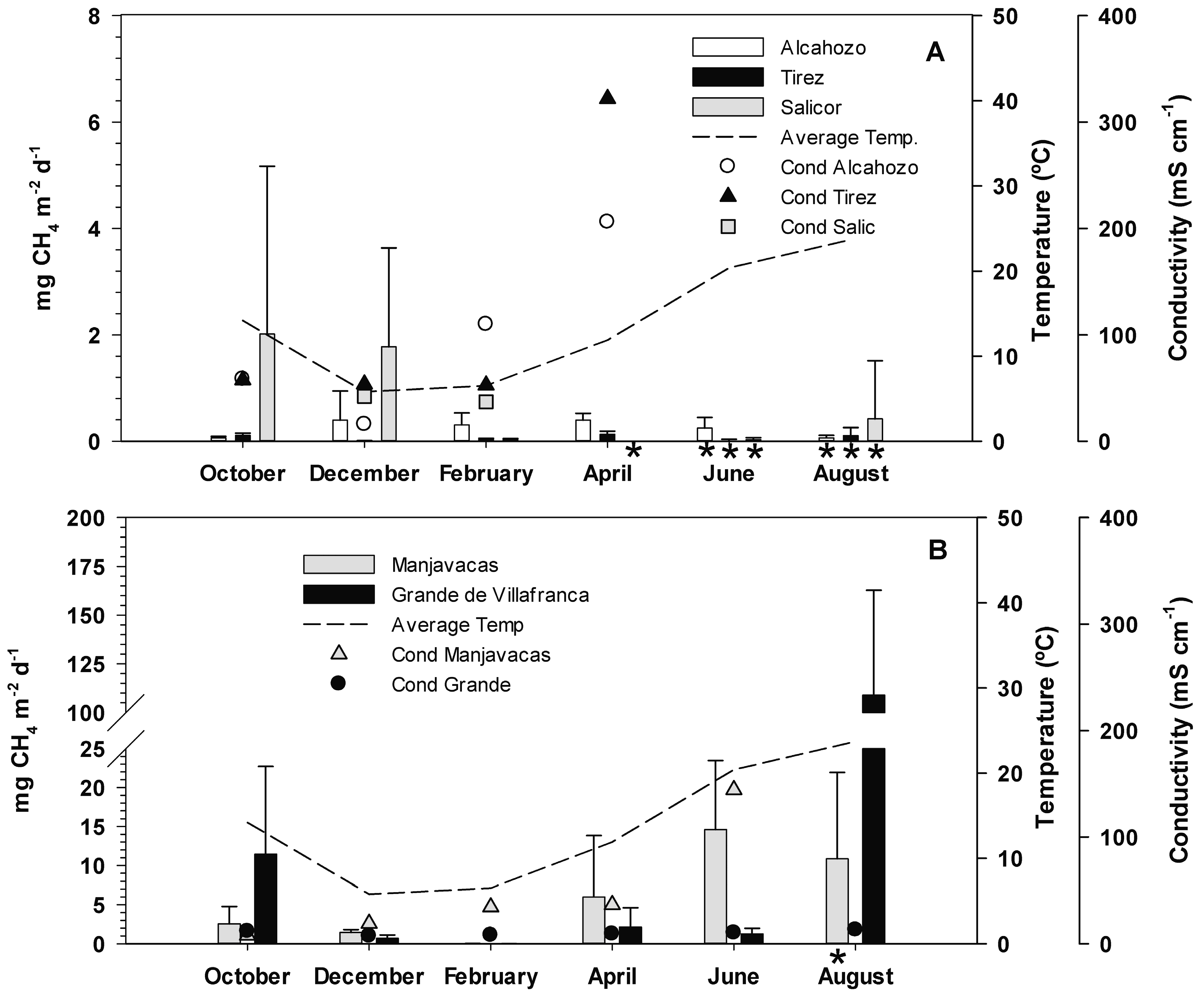

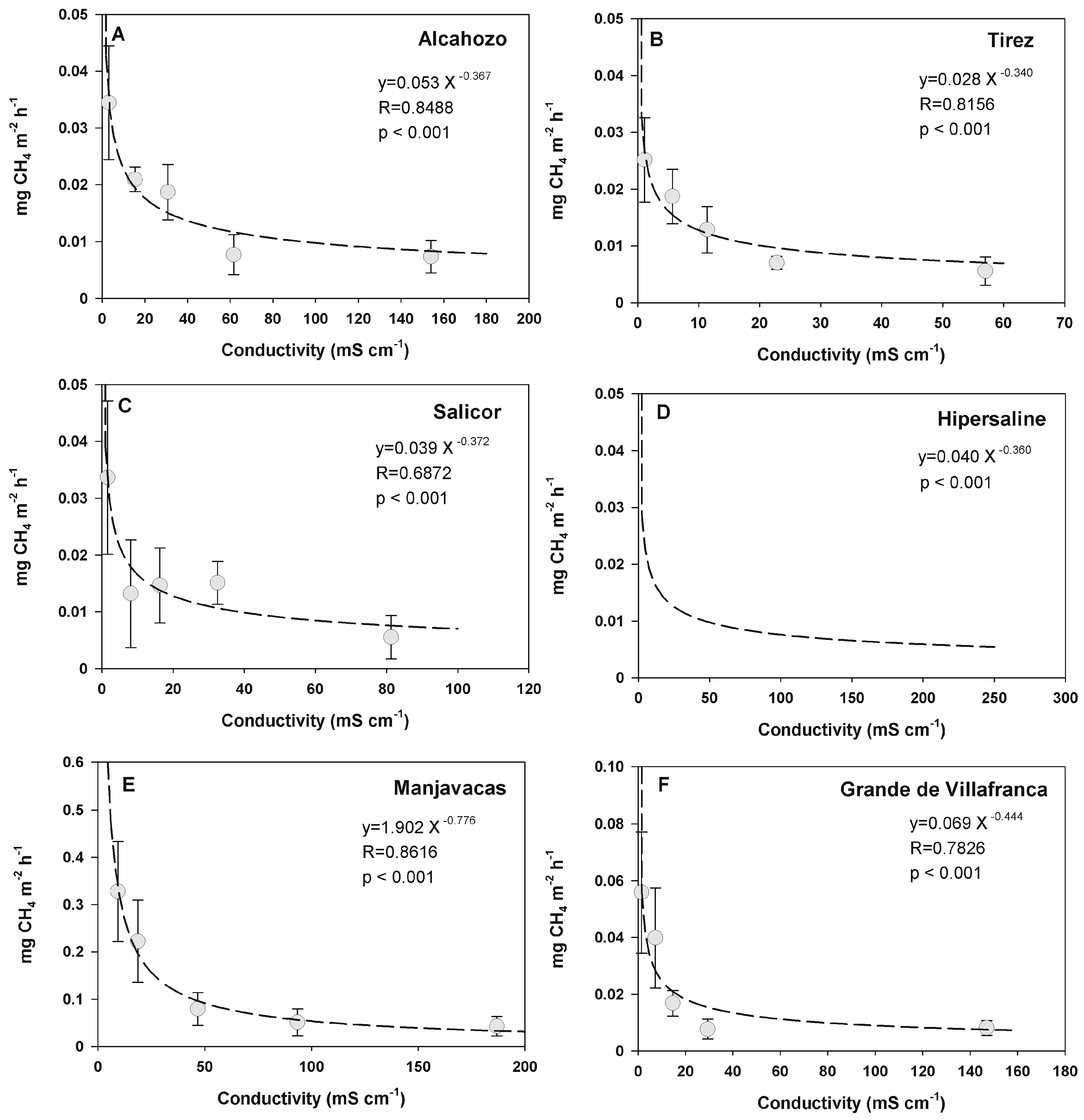
| Lake | Tírez | Salicor | Alcahozo | Manjavacas | Grande de Villafranca |
|---|---|---|---|---|---|
| UTM coordinates (Datum WGS 84; zone 30S) | 469.172X | 484.695X | 510.761X | 511.712X | 470.949X |
| 4.376.321Y | 4.368.329Y | 4.360.263Y | 4.362.992Y | 4.366.816Y | |
| Geographic coordinates | 39°32′21.62″ N | 39°27′57.49″ N | 39°23′24.72″ N | 39°24′59.47″ N | 39°27′4.63″ N |
| 3°21′24.69″ W | 3°10′25.81″ W | 2°52′38.61″ W | 2°51′49.83″ W | 3°20′13.37″ W | |
| Length of the flooding period (months) | 6 (October to April) | 5.5 (October to March) | 6.5 (October to May) | 10 (September to July) | 12 (Permanent) |
| Maximum Area (ha) | 92.5 | 58.2 | 70.0 | 230.4 | 135.6 |
| Helophytes cover (ha) | 0 | 0 | 0 | 21.2 | 41.0 |
| Maximum depth (cm) | 30 | 20 | 31 | 25 | 106 |
| Water temperature (°C) | 8.1–19.1 | 9.4–12.0 | 4.9–15.8 | 5.3–27.1 | 6.0–27.9 |
| Cond (mS cm−1) | 52.5–322.1 | 37.0–298.2 | 15.9–206.0 | 7.8–144.4 | 7.2–12.9 |
| pH | 7.6–8.4 | 8.3–8.6 | 8.0–8.6 | 8.4–9.0 | 7.6–9.1 |
| O2 (mg L−1) | 8.3–9.7 | 7.7–9 | 6.7–11.0 | 6.6–12.9 | 4.7–10.8 |
| Alkalinity (meq·L−1) | 3.5–29.7 | 4.9–5.6 | 2.5–19.2 | 5.6–33.7 | 2.7–3.9 |
| Soluble reactive Phosphorus (µmol·L−1) | 0.95–18.90 | 0.24–0.87 | 0.15–0.46 | 0.22–15.91 | 0.30–1.72 |
| NO3 (µmol·L−1) | 8.4–112.1 | 65.8–73.2 | 25.9–294.3 | 87.0–115.1 | 24.9–111.6 |
| Ammonium (µmol·L−1) | 12.7–61.2 | 8.0–13.0 | 2.2–60.6 | 2.3–83.9 | 6.2–41.6 |
| Chl-a (µg·L−1) | 7.1–14.8 | 8.1–13.6 | 0.3–8.6 | 0.8–73.3 | 0.2–7.3 |
| (A) | |||||||||||||||
| IPCC (2014) | Scenario | Lake | Yearly Change | January | February | March | April | May | June | July | August | September | October | November | December |
| RCP2.6 | 2050 | Alcahozo | 58 | 63 | 31 | 21 | 63 | 49 | 81 | 71 | 61 | ||||
| RCP4.5 | 2050 | Alcahozo | 83 | 68 | 47 | 33 | 87 | 78 | 107 | 87 | 71 | ||||
| RCP6 | 2050 | Alcahozo | 72 | 58 | 41 | 39 | 78 | 63 | 95 | 84 | 66 | ||||
| RCP8.5 | 2050 | Alcahozo | 120 | 89 | 71 | 61 | 113 | 120 | 151 | 116 | 98 | ||||
| RCP2.6 | 2050 | Tirez | 43 | 42 | 20 | 19 | 53 | 39 | 56 | 44 | 35 | ||||
| RCP4.5 | 2050 | Tirez | 58 | 47 | 31 | 29 | 69 | 58 | 73 | 53 | 39 | ||||
| RCP6 | 2050 | Tirez | 51 | 39 | 26 | 31 | 63 | 47 | 63 | 53 | 38 | ||||
| RCP8.5 | 2050 | Tirez | 82 | 60 | 47 | 47 | 89 | 85 | 101 | 71 | 58 | ||||
| RCP2.6 | 2050 | Salicor | 56 | 60 | 28 | 23 | 71 | 55 | 49 | ||||||
| RCP4.5 | 2050 | Salicor | 75 | 64 | 43 | 35 | 96 | 69 | 55 | ||||||
| RCP6 | 2050 | Salicor | 67 | 55 | 35 | 39 | 83 | 66 | 53 | ||||||
| RCP8.5 | 2050 | Salicor | 107 | 83 | 64 | 60 | 134 | 93 | 81 | ||||||
| RCP2.6 | 2050 | Manjavacas | 48 | 38 | 19 | 14 | 38 | 30 | 44 | 54 | 56 | 48 | 43 | 37 | |
| RCP4.5 | 2050 | Manjavacas | 66 | 41 | 29 | 21 | 51 | 47 | 65 | 75 | 70 | 62 | 51 | 43 | |
| RCP6 | 2050 | Manjavacas | 53 | 36 | 25 | 24 | 47 | 38 | 53 | 56 | 60 | 56 | 50 | 40 | |
| RCP8.5 | 2050 | Manjavacas | 96 | 53 | 43 | 37 | 65 | 69 | 101 | 109 | 94 | 84 | 67 | 57 | |
| RCP2.6 | 2050 | Grande de Villafranca | 73 | 45 | 26 | 18 | 58 | 42 | 62 | 83 | 74 | 79 | 66 | 49 | 37 |
| RCP4.5 | 2050 | Grande de Villafranca | 101 | 51 | 39 | 29 | 76 | 62 | 90 | 114 | 104 | 104 | 85 | 60 | 42 |
| RCP6 | 2050 | Grande de Villafranca | 79 | 42 | 32 | 32 | 70 | 51 | 70 | 87 | 79 | 87 | 74 | 58 | 40 |
| RCP8.5 | 2050 | Grande de Villafranca | 142 | 64 | 56 | 49 | 99 | 92 | 141 | 163 | 139 | 136 | 117 | 79 | 62 |
| (B) | |||||||||||||||
| IPCC (2014) | Scenario | Lake | Yearly Change | January | February | March | April | May | June | July | August | September | October | November | December |
| RCP2.6 | 2070 | Alcahozo | 62 | 61 | 35 | 27 | 56 | 52 | 92 | 78 | 61 | ||||
| RCP4.5 | 2070 | Alcahozo | 110 | 92 | 61 | 58 | 110 | 98 | 151 | 120 | 92 | ||||
| RCP6 | 2070 | Alcahozo | 100 | 89 | 63 | 56 | 95 | 89 | 137 | 104 | 87 | ||||
| RCP8.5 | 2070 | Alcahozo | 228 | 155 | 116 | 123 | 200 | 233 | 286 | 223 | 166 | ||||
| RCP2.6 | 2070 | Tirez | 44 | 39 | 23 | 23 | 47 | 39 | 63 | 49 | 35 | ||||
| RCP4.5 | 2070 | Tirez | 76 | 60 | 41 | 44 | 85 | 71 | 101 | 75 | 53 | ||||
| RCP6 | 2070 | Tirez | 70 | 60 | 42 | 44 | 75 | 67 | 92 | 65 | 51 | ||||
| RCP8.5 | 2070 | Tirez | 146 | 101 | 75 | 89 | 143 | 158 | 179 | 138 | 98 | ||||
| RCP2.6 | 2070 | Salicor | 65 | 55 | 32 | 28 | 83 | 62 | 49 | ||||||
| RCP4.5 | 2070 | Salicor | 106 | 86 | 55 | 55 | 134 | 96 | 73 | ||||||
| RCP6 | 2070 | Salicor | 95 | 83 | 57 | 55 | 119 | 83 | 71 | ||||||
| RCP8.5 | 2070 | Salicor | 198 | 141 | 101 | 116 | 249 | 184 | 138 | ||||||
| RCP2.6 | 2070 | Manjavacas | 47 | 37 | 22 | 17 | 34 | 32 | 44 | 51 | 54 | 54 | 47 | 37 | |
| RCP4.5 | 2070 | Manjavacas | 90 | 54 | 37 | 36 | 64 | 57 | 90 | 105 | 92 | 84 | 69 | 54 | |
| RCP6 | 2070 | Manjavacas | 82 | 53 | 38 | 34 | 56 | 53 | 82 | 94 | 86 | 77 | 60 | 51 | |
| RCP8.5 | 2070 | Manjavacas | 169 | 86 | 67 | 70 | 107 | 122 | 168 | 196 | 176 | 145 | 118 | 92 | |
| RCP2.6 | 2070 | Grande de Villafranca | 70 | 42 | 29 | 23 | 53 | 42 | 60 | 79 | 70 | 76 | 74 | 54 | 37 |
| RCP4.5 | 2070 | Grande de Villafranca | 137 | 66 | 49 | 45 | 94 | 76 | 127 | 166 | 127 | 136 | 117 | 83 | 56 |
| RCP6 | 2070 | Grande de Villafranca | 126 | 64 | 51 | 45 | 83 | 72 | 111 | 144 | 127 | 127 | 106 | 72 | 54 |
| RCP8.5 | 2070 | Grande de Villafranca | 264 | 109 | 90 | 94 | 160 | 176 | 243 | 306 | 260 | 264 | 207 | 153 | 106 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho, A.; Picazo, A.; Rochera, C.; Santamans, A.C.; Morant, D.; Miralles-Lorenzo, J.; Castillo-Escrivà, A. Methane Emissions in Spanish Saline Lakes: Current Rates, Temperature and Salinity Responses, and Evolution under Different Climate Change Scenarios. Water 2017, 9, 659. https://doi.org/10.3390/w9090659
Camacho A, Picazo A, Rochera C, Santamans AC, Morant D, Miralles-Lorenzo J, Castillo-Escrivà A. Methane Emissions in Spanish Saline Lakes: Current Rates, Temperature and Salinity Responses, and Evolution under Different Climate Change Scenarios. Water. 2017; 9(9):659. https://doi.org/10.3390/w9090659
Chicago/Turabian StyleCamacho, Antonio, Antonio Picazo, Carlos Rochera, Anna C. Santamans, Daniel Morant, Javier Miralles-Lorenzo, and Andreu Castillo-Escrivà. 2017. "Methane Emissions in Spanish Saline Lakes: Current Rates, Temperature and Salinity Responses, and Evolution under Different Climate Change Scenarios" Water 9, no. 9: 659. https://doi.org/10.3390/w9090659





