The Effectiveness of Conservation Reserves: Land Tenure Impacts upon Biodiversity across Extensive Natural Landscapes in the Tropical Savannahs of the Northern Territory, Australia
Abstract
:1. Introduction
2. Methods
2.1. Sampling
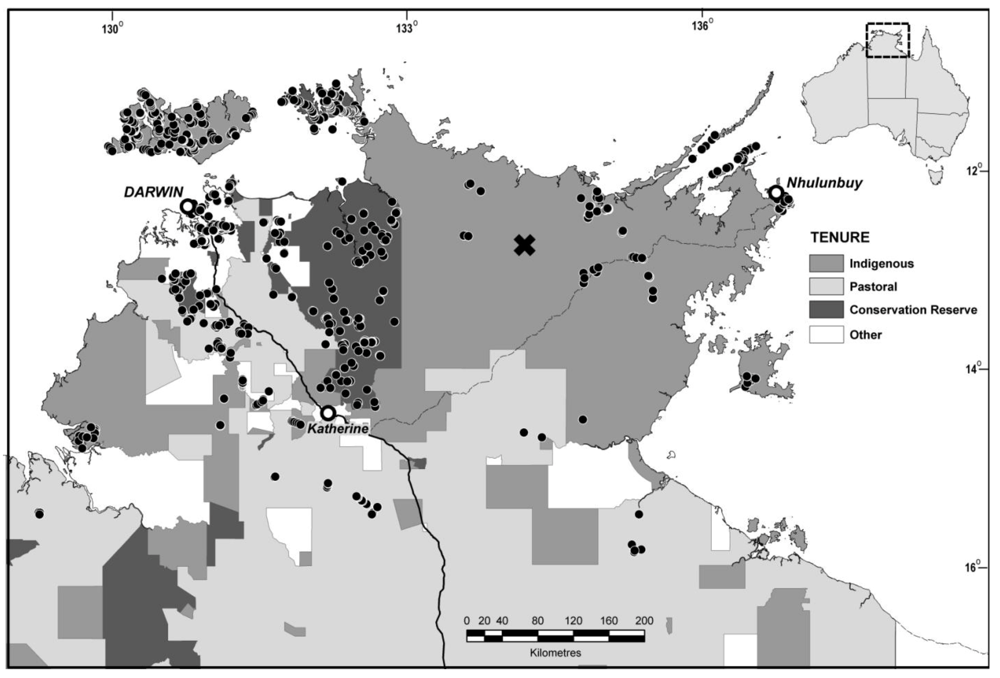
2.2. Analysis
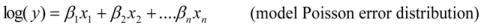
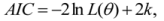
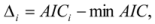
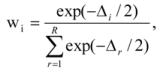
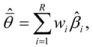
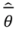 is the model averaged estimate for a set of R models [48]. We conducted the Poisson regression, model averaging and predictions (except those previously mentioned) in program R [49].
is the model averaged estimate for a set of R models [48]. We conducted the Poisson regression, model averaging and predictions (except those previously mentioned) in program R [49].3. Results: Impacts of Land Tenure and Other Variables
3.1. Fauna
| Fauna Group Attribute | Model | Deviance | AICc | Delta | Weight |
|---|---|---|---|---|---|
| Vertebrate richness | full | 2,616.0 | 6,982.4 | 0 | 0.98 |
| rock, tenure, year | 2,626.3 | 6,990.7 | 8.3 | 0.02 | |
| Vertebrate abundance | full | 2,0531 | 2,594.8 | 0 | 1 |
| rock, rainfall, tenure | 2,0619 | 2,603.5 | 86.2 | 0 | |
| Amphibian richness | full | 1,238.7 | 1,952.1 | 0 | 0.79 |
| rainfall, tenure, year | 1,243.5 | 1,954.8 | 2.7 | 0.21 | |
| Amphibian abundance | full | 4,887.5 | 5,796.7 | 0 | 1 |
| rainfall, tenure, year | 4,930.6 | 5,837.8 | 41.1 | 0 | |
| Reptile richness | rock, tenure | 1,056.5 | 4,039.0 | 0 | 0.23 |
| tenure | 1,059.7 | 4,040.1 | 1.12 | 0.13 | |
| rock, tenure, year | 1,055.8 | 4,040.2 | 1.24 | 0.12 | |
| rainfall, rock, tenure | 1,056.5 | 4,040.9 | 1.96 | 0.08 | |
| rock | 1,063.0 | 4,041.4 | 2.40 | 0.07 | |
| Reptile abundance | full | 5,785.4 | 9,525.2 | 0 | 0.74 |
| rainfall, tenure, year | 5,789.4 | 9,527.3 | 2.1 | 0.26 | |
| Bird richness | full | 2,876.7 | 6,877.5 | 0 | 1 |
| rainfall, rock, year | 2,913.4 | 6,910.1 | 32.6 | 0 | |
| Bird abundance | full | 23,250.9 | 28,366.0 | 0 | 1 |
| rainfall, rock, tenure | 23,320.5 | 28,433.3 | 67.4 | 0 | |
| Mammal richness | full | 1,248.2 | 3,091.4 | 0 | 0.99 |
| rainfall, tenure, year | 1,260.3 | 3,101.6 | 10.1 | 0.01 | |
| Mammal abundance | full | 5,387.1 | 7,677.3 | 0 | 1 |
| rainfall, tenure, year | 5,453.1 | 7,741.2 | 63.9 | 0 | |
| Threatened species richness | rainfall, tenure, year | 760.8 | 1,433.9 | 0 | 0.70 |
| full | 760.6 | 1,435.7 | 1.79 | 0.29 | |
| Threatened species abundance | full | 2,120.9 | 2,953.7 | 0 | 0.66 |
| rainfall, tenure, year | 2,124.2 | 2,955.0 | 1.35 | 0.34 |

3.2. Threats
| Variable | Conservation Reserves | Pastoral | Aboriginal Lands | H |
|---|---|---|---|---|
| Fire impact | 1.75 | 1.64 | 1.58 | 5.9 ns |
| Weed impact | 0.15 | 0.85 | 0.02 | 120.2 *** |
| Cattle impact | 0.41 | 0.70 | 0.41 | 10.6 ** |
| Pig impact | 0.42 | 0.19 | 0.14 | 41.7 *** |
4. Interpretation and Discussion
5. Conclusions
Acknowledgments
References
- Jackson, S.F.; Gaston, K.J. Land use change and the dependence of national priority species on protected areas. Glob. Change Biol. 2008, 14, 2132–2138. [Google Scholar] [CrossRef]
- Parrish, J.D.; Braun, D.P.; Unnasch, R.S. Are we conserving what we say we are? Measuring ecological integrity within protected areas. BioScience 2003, 53, 851–860. [Google Scholar] [CrossRef]
- Chape, S.; Harrison, J.; Spalding, M.; Lysenko, I. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 443–455. [Google Scholar] [CrossRef]
- Devictor, V.; Godet, L.; Julliard, R.; Couvet, D.; Jiguet, F. Can common species benefit from protected areas? Biol. Conserv. 2007, 139, 29–36. [Google Scholar] [CrossRef]
- Gaston, K.J.; Charman, K.; Jackson, S.F.; Armsworth, P.R.; Bonn, A.; Briers, R.A.; Callaghan, C.S.Q.; Catchpole, R.; Hopkins, J.; Kunin, W.E.; et al. The ecological effectiveness of protected areas: the United Kingdom. Biol. Conserv. 2006, 132, 76–87. [Google Scholar] [CrossRef]
- Rannestad, O.T.; Danielsen, T.; Moe, S.R.; Stokke, S. Adjacent pastoral areas support higher densities of wild ungulates during the wet season than the Lake Mburo National Park in Uganda. J. Trop. Ecol. 2006, 22, 675–683. [Google Scholar] [CrossRef]
- Woinarski, J.; Mackey, B.; Nix, H.; Traill, B. The Nature of Northern Australia: Natural Values, Ecological Processes and Future Prospects; ANU E-Press: Canberra, ACT, Australia, 2007. [Google Scholar]
- Garnett, S.T.; Woinarski, J.C.Z.; Crowley, G.M.; Kutt, A.S. Biodiversity conservation in Australian tropical rangelands. In Wild Rangelands: Conserving Wildlife While Maintaining Livestock in Semi-Arid Ecosystems; du Toit, J., Kock, R., Deutsch, J., Eds.; Wiley-Blackwell: Chichester, UK, 2010; pp. 191–234. [Google Scholar]
- Yibarbuk, D.; Whitehead, P.J.; Russell-Smith, J.; Jackson, D.; Godjuwa, C.; Fisher, A.; Cooke, P.; Choquenot, D.; Bowman, D.M.J.S. Fire ecology and Aboriginal land management in central Arnhem Land, northern Australia: a tradition of ecosystem management. J. Biogeogr. 2001, 28, 325–343. [Google Scholar]
- Altman, J.C.; Whitehead, P.J. Caring for Country and Sustainable Indigenous Development: Opportunities, Constraints and Innovation; Australian National University Centre for Aboriginal Economic Policy Research Working Paper No. 20/2003; ANU: Canberra, ACT, Australia, 2003. [Google Scholar]
- Luckert, M.K.; Campbell, B.M.; Gorman, J.T.; Garnett, S.T. Investing in Indigenous Natural Resource Management; Charles Darwin University: Darwin, NT, Australia, 2007. [Google Scholar]
- Whitehead, P.J.; Bowman, D.M.J.S.; Preece, N.; Fraser, F.; Cooke, P. Customary use of fire by Indigenous peoples in northern Australia: its contemporary role in savannah management. Int. J. Wildland Fire 2003, 12, 415–425. [Google Scholar] [CrossRef]
- Altman, J.C.; Buchanan, G.J.; Larsen, L. The Environmental Significance of the Indigenous Estate: Natural Resource Management as Economic Development in Remote Australia; CAEPR Discussion Paper 286/2007; ANU College of Arts & Social Sciences: Canberra, ACT, Australia, 2007. [Google Scholar]
- Gilligan, B. The Indigenous Protected Area Programme; Department of the Environment and Heritage: Canberra, ACT, Australia, 2006. [Google Scholar]
- Director of National Parks. In Kakadu National Park. Management Plan 2007–2014; Australian Government Director of National Parks: Canberra, ACT, Australia, 2007.
- Franklin, D.C.; Petty, A.M.; Williamson, G.J.; Brook, B.W.; Bowman, D.M.J.S. Monitoring contrasting land management in the savannah landscapes of northern Australia. Environ. Manage. 2008, 41, 501–515. [Google Scholar] [CrossRef]
- Caro, T.M. Species richness and abundance of small mammals inside and outside and African national park. Biol. Conserv. 2001, 98, 251–257. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Ash, A.J. Responses of vertebrates to pastoralism, military land use and landscape position in an Australian tropical savannah. Austral. Ecol. 2002, 27, 311–323. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Andersen, A.N.; Churchill, T.; Ash, A.J. Response of ant and terrestrial spider assemblages to pastoral and military land use and to landscape position, in a tropical savannah woodland in northern Australia. Austral. Ecol. 2002, 27, 324–333. [Google Scholar] [CrossRef]
- Kutt, A.S.; Gordon, I.J. Variation in terrestrial mammal abundance on pastoral and conservation land tenures in north-eastern Australian tropical savannas. Anim. Conserv. 2012, 15, 416–425. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Risler, J.; Kean, L. The response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral. Ecol. 2004, 29, 156–176. [Google Scholar] [CrossRef]
- Legge, S.; Kennedy, M.S.; Lloyd, R.; Murphy, S.A.; Fisher, A. Rapid recovery of mammal fauna in the central Kimberley, northern Australia, following the removal of introduced herbivores. Austral. Ecol. 2011, 36, 791–799. [Google Scholar] [CrossRef]
- Kutt, A.S.; Vanderduys, E.P.; Perry, J.J.; Perkins, G.C.; Kemp, J.E.; Bateman, B.L.; Kanowski, J.; Jensen, R. Signals of change in tropical savannah woodland vertebrate fauna 5 years after cessation of livestock grazing. Wildlife Res. 2012, 39, 386–396. [Google Scholar]
- Edwards, A.; Kennett, R.; Price, O.; Russell-Smith, J.; Spiers, G.; Woinarski, J. Monitoring the impacts of fire regimes on biodiversity in northern Australia: an example from Kakadu National Park. Int. J. Wildland Fire 2003, 12, 427–440. [Google Scholar]
- Russell-Smith, J.; Edwards, A.C.; Woinarski, J.C.Z.; McCartney, J.; Kerin, S.; Winderlich, S.; Murphy, B.P.; Watt, F. The First Ten Years of the ‘Three Parks’ (Kakadu, Litchfield, Nitmiluk) Fire Regime and Biodiversity Monitoring Program. In Culture, Ecology and Economy of Fire Management in Northern Australia: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P., Eds.; CSIRO Publications: Melbourne, VIC, Australia, 2009; pp. 257–286. [Google Scholar]
- Woinarski, J.C.Z.; Russell-Smith, J.; Andersen, A.; Brennan, K. Fire management and biodiversity of the western Arnhem Land plateau. In Culture, Ecology and Economy of Fire Management in Northern Australia: Rekindling the Wurrk Tradition; Russell-Smith, J., Whitehead, P.J., Cooke, P., Eds.; CSIRO Publications: Melbourne, VIC, Australia, 2009; pp. 201–228. [Google Scholar]
- Woinarski, J.C.Z.; Armstrong, M.; Brennan, K.; Fisher, A.; Griffiths, A.D.; Hill, B.; Milne, D.J.; Palmer, C.; Ward, S.; Watson, M.; et al. Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Res. 2010, 37, 116–126. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Panton, W.J. Decline of Callitris intratropica R.T. Baker & H.G. Smith in the Northern Territory: implications for pre- and post-European colonization ire regimes. J. Biogeogr. 1993, 20, 373–381. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Prior, L.D. Impact of Aboriginal landscape burning on woody vegetation in Eucalyptus tetrodonta savannah in Arnhem Land, northern Australia. J. Biogeogr. 2004, 31, 807–817. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Price, O.; Whitehead, P.J.; Walsh, A. The ‘wilderness effect’ and the decline of Callitris intratropica on the Arnhem Land Plateau, northern Australia. Aust. J. Bot. 2001, 49, 665–672. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Walsh, A.; Prior, L.D. Landscape analysis of Aboriginal fire management in central Arnhem Land, north Australia. J. Biogeogr. 2004, 31, 207–223. [Google Scholar] [CrossRef]
- Price, O.; Bowman, D.M.J.S. Fire-stick forestry: a matrix model in support of skilful fire management of Callitris intratropica R.T. Baker by north Australian Aborigines. J. Biogeogr. 1994, 21, 573–580. [Google Scholar] [CrossRef]
- Prior, L.D.; Bowman, D.M.J.S.; Brook, B.W. Growth and survival of two north Australian relictual tree species, Allosyncarpia ternata (Myrtaceae) and Callitris intratropica (Cupressaceae). Ecol. Res. 2007, 22, 228–236. [Google Scholar] [CrossRef]
- Russell-Smith, J. Recruitment dynamics of the long-lived obligate seeders Callitris intratropica (Cupressaceae) and Petraeomyrtus punicea (Myrtaceae). Aust. J. Bot. 2006, 54, 479–485. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Lucas, D.; Gapindi, M.; Gunbunuka, B.; Kapirigi, N.; Namingum, G.; Lucas, K.; Giuliani, P.; Chaloupka, G. Aboriginal resource utilization and fire management practice in western Arnhem Land, monsoonal northern Australia: Notes for prehistory, lessons for the future. Hum. Ecol. 1997, 25, 159–195. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Ryan, P.G.; Klessa, D.; Waight, G.; Harwood, R. Fire regimes, fire-sensitive vegetation and fire management of the sandstone Arnhem Plateau, monsoonal northern Australia. J. Appl. Ecol. 1998, 35, 829–846. [Google Scholar]
- Russell-Smith, J.; Ryan, P.G.; Cheal, D.C. Fire regimes and the conservation of sandstone heath in monsoonal northern Australia: frequency, interval, patchiness. Biol. Conserv. 2002, 104, 91–106. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Armstrong, M.; Price, O.; McCartney, J.; Griffiths, T.; Fisher, A. The terrestrial vertebrate fauna of Litchfield National Park, Northern Territory: monitoring over a 6-year period and response to fire history. Wildlife Res. 2004, 31, 1–10. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Rankmore, B.; Hill, B.; Griffiths, A.D.; Stewart, A.; Grace, B. Fauna assemblages in regrowth vegetation in tropical open forests of the Northern Territory, Australia. Wildlife Res. 2010, 36, 675–690. [Google Scholar]
- Houlder, D.J. ANUCLIM (Version 5.1); Centre for Resource and Environmental Studies, Australian National University: Canberra, ACT, Australia, 2000. [Google Scholar]
- Firth, R.S.C.; Woinarski, J.C.Z.; Brennan, K.G.; Hempel, C. Environmental relationships of the brush-tailed rabbit-rat Conilurus penicillatus and other small mammals on the Tiwi Islands, northern Australia. J. Biogeogr. 2006, 33, 1820–1837. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Gambold, N. Gradient analysis of a tropical herpetofauna: distribution patterns of terrestrial reptiles and amphibians in Stage III of Kakadu National Park, Australia. Wildlife Res. 1992, 19, 105–127. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Braithwaite, R.W.; Menkhorst, K.A.; Griffin, S.; Fisher, A.; Preece, N. Gradient analysis of the distribution of mammals in Stage III of Kakadu National Park, with a review of the distribution patterns of mammals across north-western Australia. Wildlife Res. 1992, 19, 233–262. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Fisher, A.; Milne, D. Distribution patterns of vertebrates in relation to an extensive rainfall gradient and soil variation in the tropical savannas of the Northern Territory, Australia. J. Trop. Ecol. 1999, 15, 381–398. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Legge, S.; Fitzsimons, J.A.; Traill, B.J.; Burbidge, A.A.; Fisher, A.; Firth, R.S.C.; Gordon, I.J.; Griffiths, A.D.; Johnson, C.N.; et al. The disappearing mammal fauna of northern Australia: context, cause and response. Conserv. Lett. 2011, 4, 192–201. [Google Scholar] [CrossRef]
- Woinarski, J.; Pavey, C.; Kerrigan, R.; Cowie, I.; Ward, S. Lost from Our Landscape: Threatened Species of the Northern Territory; NT Government Printer: Darwin, NT, Australia, 2007. [Google Scholar]
- Dobson, A.J. An Introduction to Generalized Linear Models; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- R Development Core Team, R: A Language and Environment for Statistical Computing, Reference Index Version 2.9.2; R Foundation for Statistical Computing: Vienna, Austria, 2009.
- Parr, C.L.; Woinarski, J.C.Z.; Pienaar, D.J. Cornerstones of biodiversity conservation? Comparing the management effectiveness of Kruger and Kakadu National Parks, two key savannah reserves. Biodivers. Conserv. 2009, 18, 3643–3662. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z. A Difficult and Destructive Metamorphosis: Conservation and Land Management in the Northern Territory in the 1950s. In Modern Frontier: Aspects of the 1950s in Australia’s Northern Territory; Wells, J.T., Dewar, M., Parry, S., Eds.; Charles Darwin University Press: Darwin, NT, Australia, 2005; pp. 33–55. [Google Scholar]
- Russell-Smith, J.; Bowman, D.M.J.S. Conservation of monsoon rainforest isolates in the Northern Territory, Australia. Biol. Conserv. 1992, 59, 51–63. [Google Scholar] [CrossRef]
- Preece, N.; Harvey, K.; Hempel, C.; Woinarski, J.C.Z. Uneven distribution of weeds along extensive transects in Australia's Northern Territory points to management solutions. Ecol. Manag. Restor. 2010, 11, 127–134. [Google Scholar] [CrossRef]
- Garnett, S.T.; Sithole, B.; Whitehead, P.J.; Burgess, C.P.; Johnston, F.H.; Lea, T. Healthy Country, Healthy People: policy implications of links between Indigenous human heath and environmental condition in tropical Australia. Aust. J. Publ. Admin. 2009, 68, 53–66. [Google Scholar] [CrossRef]
- Johnston, F.H.; Burgess, P.; Bowman, D.M.J.S. A Case for Indigenous Natural Resource Management and Health. In Investing in Indigenous Natural Resource Management; Luckert, M.K., Campbell, B.M., Gorman, J.T., Garnett, S.T., Eds.; Charles Darwin University Press: Darwin, NT, Australia, 2007; pp. 91–95. [Google Scholar]
- Hill, R.; Harding, E.K.; Edwards, D.; O’Dempsey, J.; Hill, D.; Martin, A.; McIntyre-Tamwoy, S. A Cultural and Conservation Economy for Northern Australia; Australian Conservation Foundation: Melbourne, ACT, Australia, 2007. [Google Scholar]
- Fitzsimons, J.; Russell-Smith, J.; James, G.; Vigilante, T.; Lipsett-Moore, G.; Morrison, J.; Looker, M. Insights into the biodiversity and social benchmarking components of the Northern Australian fire management and carbon abatement programmes. Ecol. Manag. Restor. 2012, 13, 51–57. [Google Scholar] [CrossRef]
- Preuss, K.; Dixon, M. ‘Looking after country two-ways’: insights into Indigenous community-based conservation form the Southern Tanami. Ecol. Manag. Restor. 2012, 13, 2–15. [Google Scholar] [CrossRef]
- Wiens, J. The dangers of black-and-white conservation. Conserv. Biol. 2007, 21, 1371–1372. [Google Scholar] [CrossRef]
- Hansen, A.J.; Rotella, J.J. Biophysical factors, land use and species viability in and around Nature Reserves. Conserv. Biol. 2002, 16, 1112–1122. [Google Scholar] [CrossRef]
- Berger, J. Is it acceptable to let a species go extinct in a National Park? Conserv. Biol. 2003, 17, 1451–1454. [Google Scholar] [CrossRef]
- Struhsaker, T.T.; Struhsaker, P.J.; Siex, K.S. Conserving Africa’s rain forests: Problems in protected areas and possible solutions. Biol. Conserv. 2005, 123, 45–54. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Woinarski, J.C.Z.; Green, J.; Fisher, A.; Ensbey, M.; Mackey, B. The Effectiveness of Conservation Reserves: Land Tenure Impacts upon Biodiversity across Extensive Natural Landscapes in the Tropical Savannahs of the Northern Territory, Australia. Land 2013, 2, 20-36. https://doi.org/10.3390/land2010020
Woinarski JCZ, Green J, Fisher A, Ensbey M, Mackey B. The Effectiveness of Conservation Reserves: Land Tenure Impacts upon Biodiversity across Extensive Natural Landscapes in the Tropical Savannahs of the Northern Territory, Australia. Land. 2013; 2(1):20-36. https://doi.org/10.3390/land2010020
Chicago/Turabian StyleWoinarski, John C.Z., Jon Green, Alaric Fisher, Michelle Ensbey, and Brendan Mackey. 2013. "The Effectiveness of Conservation Reserves: Land Tenure Impacts upon Biodiversity across Extensive Natural Landscapes in the Tropical Savannahs of the Northern Territory, Australia" Land 2, no. 1: 20-36. https://doi.org/10.3390/land2010020




