Multivariate Analysis of Rangeland Vegetation and Soil Organic Carbon Describes Degradation, Informs Restoration and Conservation
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
2.1.1. Environment and Soils
2.1.2. Current and Historical Land Use
2.2. Sampling
2.3. Data Analysis
2.3.1. Patterns of Degradation
2.3.2. Sources of Degradation
2.3.3. Vegetation States
3. Results
3.1. Pattern of Vegetation Degradation
| Vegetation Degradation State | Scientific Name | Common Name | Status | Mean Relative Abundance (%) ±95% CI |
|---|---|---|---|---|
| High | Schedonorus phoenix | Tall fescue | Exotic | 41 ± 5 |
| Poa pratensis | Kentucky bluegrass | Exotic | 20 ± 5 | |
| Bromus inermis | Smooth brome | Exotic | 11 ± 6 | |
| Veronia baldwinii | Baldwin’s ironweed | Native | 6 ± 4 | |
| Setaria pumila | Yellow foxtail | Exotic | 3 ± 4 | |
| Solanum carolinense | Horse nettle | Native | 2 ± 2 | |
| Daucus carota | Wild carrot | Exotic | 2± 1 | |
| Dactylis glomerata | Orchardgrass | Exotic | 2± 1 | |
| Medicago lupulina | Black medic | Exotic | 1± 1 | |
| Solidago canadensis | Tall goldenrod | Native | 1± 1 | |
| N = 3, E = 7 | Sum = 89 | |||
| Moderate | Schedonorus phoenix | Tall fescue | Exotic | 30 ± 4 |
| Medicago lupulina | Black medic | Exotic | 14 ± 4 | |
| Sporobolus clandestinus | Rough dropseed | Native | 12 ± 3 | |
| Kummerowia striata | Japanese clover | Exotic | 7 ± 4 | |
| Poa pratensis | Kentucky bluegrass | Exotic | 6 ± 2 | |
| Aristida oligantha | Prairie threeawn | Native | 5 ± 3 | |
| Lotus corniculatus | Birdsfoot trefoil | Exotic | 4 ± 4 | |
| Daucus carota | Wild carrot | Exotic | 3 ± 1 | |
| Dichanthelium oligosanthes | Scribner’s rosette grass | Native | 2 ± 1 | |
| Phleum pratense | Timothy | Exotic | 2 ± 1 | |
| N = 3, E = 7 | Sum = 83 | |||
| Low | Sorghastrum nutans | Indiangrass | Native | 11 ± 6 |
| Schedonorus phoenix | Tall fescue | Exotic | 9 ± 5 | |
| Cyperaceae | Sedges | Native | 9 ± 2 | |
| Andropogon gerardii | Big bluestem | Native | 9 ± 5 | |
| Sporobolus clandestinus | Rough dropseed | Native | 7 ± 2 | |
| Lotus corniculatus | Birdsfoot trefoil | Exotic | 6 ± 4 | |
| Poa pratensis | Kentucky bluegrass | Exotic | 6 ± 2 | |
| Solidago canadensis | Tall goldenrod | Native | 6 ± 4 | |
| Symphoricarpos orbiculatus | Coralberry | Native | 4 ± 3 | |
| Schizachyrium scoparium | Little bluestem | Native | 4 ± 3 | |
| N = 7, E = 3 | Sum = 71 |

| MANOVA Model Terms | Wilks’ Approx. F | P |
|---|---|---|
| Tall fescue canopy cover (TF) | 50.16 | <0.001 |
| Severe grazing history (Gr) | 31.56 | <0.001 |
| Soil Organic carbon (SOC) | 12.90 | <0.001 |
| TF × SOC | 2.24 | 0.08 |
| SOC × Gr | 2.08 | 0.25 |
| TF × SOC × Gr | 1.43 | 0.24 |
| TF × Gr | 1.41 | 0.25 |

3.2. Sources of Degradation

3.3. Degradation States
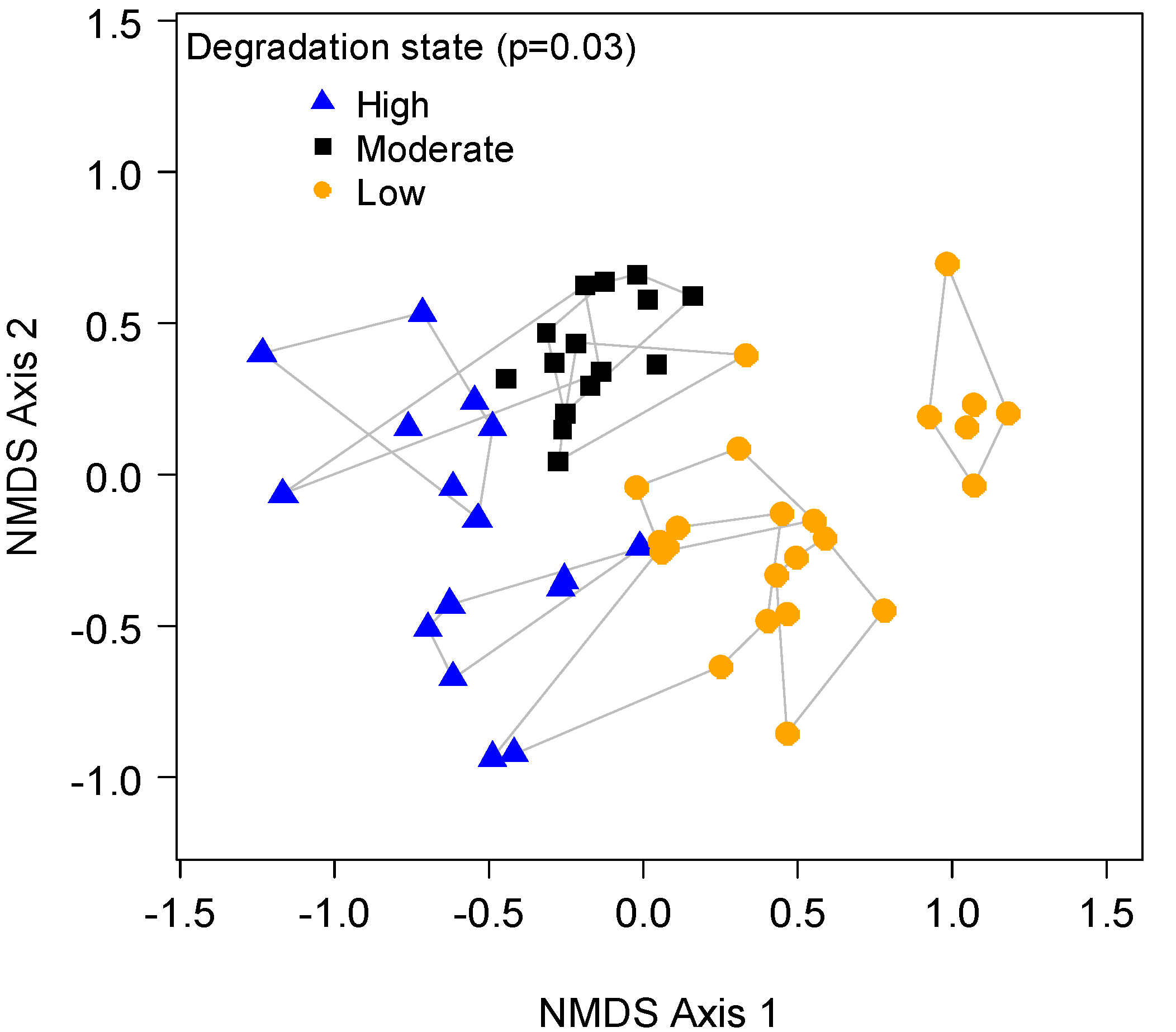

4. Discussion
4.1. Degradation Pattern and Pathways

4.2. Managing Pathways between States for Restoration
4.3. Biodiversity Conservation in Working Grassland Landscapes
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Ellis, E.C.; Ramankutty, N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ. 2008, 6, 439–447. [Google Scholar] [CrossRef]
- Foley, J.A.; Defries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Reidsma, P.; Tekelenburg, T.; Vandenberg, M.; Alkemade, R. Impacts of land-use change on biodiversity: An assessment of agricultural biodiversity in the European Union. Agr. Ecosyst. Environ. 2006, 114, 86–102. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B.; Manning, A.D. Biodiversity, ecosystem function, and resilience: Ten guiding principles for commodity production landscapes. Front. Ecol. Environ. 2006, 4, 80–86. [Google Scholar] [CrossRef]
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of “ecoagriculture” landscapes. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008, 363, 477–494. [Google Scholar] [CrossRef]
- Jordan, N.; Warner, K.D. Enhancing the multifunctionality of US agriculture. BioScience 2010, 60, 60–66. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Whisenant, S.G. Repairing Damaged Wildlands: A Process-Oriented, Landscape-Scale Approach; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Sluis, W.J. Patterns of species richness and composition in re-created grassland. Restor. Ecol. 2002, 10, 677–684. [Google Scholar] [CrossRef]
- Polley, H.W.; Derner, J.D.; Wilsey, B.J. Patterns of plant species diversity in remnant and restored tallgrass prairies. Restor. Ecol. 2005, 13, 480–487. [Google Scholar] [CrossRef]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Tugel, A.J.; Peacock, G.L.; Robinett, D.G.; Shaver, P.L.; Brown, J.R.; Herrick, J.E.; Sanchez, H.; Havstad, K.M. State-and-transition models for heterogeneous landscapes: A strategy for development and application. Rangel. Ecol. Manag. 2009, 62, 1–15. [Google Scholar] [CrossRef]
- Boyd, C.S.; Svejcar, T.J. Managing complex problems in rangeland ecosystems. Rangel. Ecol. Manag. 2009, 62, 491–499. [Google Scholar] [CrossRef]
- Cramer, V.A.; Hobbs, R.J.; Standish, R.J. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef]
- Cousins, S.A.O. Landscape history and soil properties affect grassland decline and plant species richness in rural landscapes. Biol. Conserv. 2009, 142, 2752–2758. [Google Scholar] [CrossRef]
- Briske, D.D.; Fuhlendorf, S.D.; Smeins, F.E. State-and-transition models, thresholds, and rangeland health: A synthesis of ecological concepts and perspectives. Rangel. Ecol. Manag. 2005, 58, 1–10. [Google Scholar] [CrossRef]
- Debinski, D.M.; Moranz, R.A.; Delaney, J.T.; Miller, J.R.; Engle, D.M.; Winkler, L.B.; McGranahan, D.A.; Barney, R.J.; Trager, J.C.; Stephenson, A.L.; et al. A cross-taxonomic comparison of insect responses to grassland management and land-use legacies. Ecosphere 2011, 2, art131. [Google Scholar] [CrossRef]
- Hovick, T.J.; Miller, J.R.; Dinsmore, S.J.; Engle, D.M.; Debinski, D.M.; Fuhlendorf, S.D. Effects of fire and grazing on grasshopper sparrow nest survival. J. Wildl. Manag. 2012, 76, 19–27. [Google Scholar] [CrossRef]
- Moranz, R.A.; Debinski, D.M.; McGranahan, D.A.; Engle, D.M.; Miller, J.R. Untangling the effects of fire, grazing, and land-use legacies on grassland butterfly communities. Biodivers. Conserv. 2012, 21, 2719–2746. [Google Scholar] [CrossRef]
- Friedel, M.H. Range condition assessment and the concept of thresholds: A viewpoint. J. Range Manag. 1991, 44, 422–426. [Google Scholar] [CrossRef]
- Van der Westhuizen, H.; Snyman, H.; Fouché, H. A degradation gradient for the assessment of rangeland condition of a semi-arid sourveld in southern Africa. Afr. J. Range Forage Sci. 2005, 22, 47–58. [Google Scholar] [CrossRef]
- Sadler, R.J.; Hazelton, M.; Boer, M.M.; Grierson, P.F. Deriving state-and-transition models from an image series of grassland pattern dynamics. Ecol. Model. 2010, 221, 433–444. [Google Scholar] [CrossRef]
- Papini, R.; Valboa, G.; Favilli, F.; L’Abate, G. Influence of land use on organic carbon pool and chemical properties of Vertic Cambisols in central and southern Italy. Agr. Ecosyst. Environ. 2011, 140, 68–79. [Google Scholar] [CrossRef]
- Bacon, C.W. Toxic endophyte-infected tall fescue and range grasses: Historic perspectives. J. Anim. Sci. 1995, 73, 861–870. [Google Scholar]
- Clay, K.; Holah, J. Fungal endophyte symbiosis and plant diversity in successional fields. Science 1999, 285, 1742–1744. [Google Scholar] [CrossRef]
- Madison, L.A.; Barnes, T.G.; Sole, J.D. Effectiveness of fire, disking, and herbicide to renovate tall fescue fields to Northern Bobwhite habitat. Wildl. Soc. Bull. 2001, 29, 706–712. [Google Scholar]
- Rudgers, J.A.; Koslow, J.M.; Clay, K. Endophytic fungi alter relationships between diversity and ecosystem properties. Ecol. Lett. 2004, 7, 42–51. [Google Scholar] [CrossRef]
- McGranahan, D.A.; Engle, D.M.; Fuhlendorf, S.D.; Miller, J.R.; Debinski, D.M. An invasive cool-season grass complicates prescribed fire management in a native warm-season grassland. Nat. Areas J. 2012, 32, 208–214. [Google Scholar] [CrossRef]
- McGranahan, D.A.; Engle, D.M.; Miller, J.R.; Debinski, D.M. An invasive grass increases live fuel proportion and reduces fire spread in a simulated grassland. Ecosystems 2013, 16, 158–169. [Google Scholar] [CrossRef]
- Westoby, M.; Walker, B.; Noy-Meir, I. Opportunistic management for rangelands not at equilibrium. J. Range Manag. 1989, 42, 266–274. [Google Scholar] [CrossRef]
- Stringham, T.K.; Krueger, W.C.; Shaver, P.L. State and transition approach modeling: An ecological process. J. Range Manag. 2003, 56, 106–113. [Google Scholar] [CrossRef]
- IEM “Climodat” Reports; IEM Iowa Environmental Mesonet, Iowa State University Department of Agronomy: Lamoni, IA, USA. Available online: http://mesonet.agron.iastate.edu/climodat/index.phtml?station=IA4585&report=17 (accessed on 31 May 2011).
- USDA-SCS, Soil Survey of Ringgold County, Iowa; United States Department of Agriculture: Washington, DC, USA, 1992.
- USDA-NRCS. Web Soil Survey Data for Ringgold County, Iowa; Natural Resource Conservation Service, United States Department of Agriculture. Available online: http://websoilsurvey.nrcs.usda.gov (accessed on 25 February 2011).
- Miller, J.R.; Morton, L.W.; Engle, D.M.; Debinski, D.M.; Harr, R.N. Nature reserves as catalysts for landscape change. Front. Ecol. Environ. 2012, 10, 144–152. [Google Scholar] [CrossRef]
- Rosburg, T.R.; Glenn-Lewin, D.C. Effects of Fire and Atrazine on Pasture and Remnant Prairie Plant Species in Southern Iowa. In Proceedings of the Twelfth North American Prairie Conference, Cedar Falls, IA, USA, 5–9 August 1990; pp. 107–112.
- McGranahan, D.A.; Engle, D.M.; Wilsey, B.J.; Fuhlendorf, S.D.; Miller, J.R.; Debinski, D.M. Grazing and an invasive grass confound spatial pattern of exotic and native grassland plant species richness. Basic Appl. Ecol. 2012, 13, 654–662. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Bull, A.; Otsuki, Y. Comparison of rangeland vegetation sampling techniques in the Central Grasslands. J. Range Manag. 1998, 51, 164–172. [Google Scholar] [CrossRef]
- Daubenmire, R. A canopy-coverage method of vegetational analysis. Northwest Sci. 1959, 33, 43–64. [Google Scholar]
- Mann, L.K. Changes in soil carbon storage after cultivation. Soil Sci. 1986, 142, 279–288. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Zhang, H.; Tunnell, T.R.; Engle, D.M.; Cross, A.F. Effects of grazing on restoration of southern mixed prairie soils. Restor. Ecol. 2002, 10, 401–407. [Google Scholar] [CrossRef]
- He, N.P.; Zhang, Y.H.; Yu, Q.; Chen, Q.S.; Pan, Q.M.; Zhang, G.M.; Han, X.G. Grazing intensity impacts soil carbon and nitrogen storage of continental steppe. Ecosphere 2011, 2, art8. [Google Scholar] [CrossRef]
- United States Department of Agriculture-National Cooperative Soil Survey (USDA-NCSS). National Cooperative Soil Characterization Data. Available online: http://ssldata.nrcs.usda.gov/default.htm (accessed on 25 February 2011).
- Altesor, A.; Pineiro, G.; Lezama, F.; Jackson, R.B.; Sarasola, M.; Paruelo, J.M. Ecosystem changes associated with grazing in subhumid South American grasslands. J. Veg. Sci. 2006, 17, 323–332. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.G.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. Available online: http://cran.r-project.org/package=vegan (accessed on 25 January 2011).
- R Development Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2011.
- Beals, M.L. Understanding community structure: A data-driven multivariate approach. Oecologia 2006, 150, 484–495. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. Available online: http://cran.r-project.org/web/packages/nlme/index.html (accessed on 22 May 2011).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Milchunas, D.G.; Sala, O.E.; Lauenroth, W.K. A generalized model of the effects of grazing by large herbivores on grassland community structure. Am. Nat. 1988, 132, 87–106. [Google Scholar]
- Burns, C.E.; Collins, S.L.; Smith, M.D. Plant community response to loss of large herbivores: Comparing consequences in a South African and a North American grassland. Biodivers. Conserv. 2009, 18, 2327–2342. [Google Scholar] [CrossRef]
- Pieper, R.D. Ecological Implications of Livestock Grazing. In Ecological Implications of Livestock Herbivory in the West; Vavra, M., Laycock, W.A., Pieper, R.D., Eds.; USA Society for Range Management: Denver, CO, USA, 1994; pp. 177–211. [Google Scholar]
- Laycock, W.A. Stable states and thresholds of range condition on North American rangelands: A viewpoint. J. Range Manag. 1991, 44, 427–433. [Google Scholar] [CrossRef]
- Gardner, J.L. Effects of thirty years of protection from grazing in desert grassland. Ecology 1950, 31, 44–50. [Google Scholar] [CrossRef]
- Fleischner, T.L. Ecological costs of livestock grazing in western North America. Conserv. Biol. 1994, 8, 629–644. [Google Scholar]
- Harrison, Y.; Shackleton, C. Resilience of South African communal grazing lands after the removal of high grazing pressure. Land Degrad. Dev. 1999, 10, 225–239. [Google Scholar] [CrossRef]
- DiTomaso, J.M.; Brooks, M.L.; Allen, E.B.; Minnich, R.; Rice, P.M.; Kyser, G.B. Control of invasive weeds with prescribed burning. Weed Technol. 2006, 20, 535–548. [Google Scholar] [CrossRef]
- Cummings, D.C.; Fuhlendorf, S.D.; Engle, D.M. Is altering grazing selectivity of invasive forage species with patch burning more effective than herbicide treatments? Rangel. Ecol. Manag. 2007, 60, 253–260. [Google Scholar] [CrossRef]
- Barnes, T.G. Using herbicides to rehabilitate native grasslands. Nat. Areas J. 2007, 27, 56–65. [Google Scholar] [CrossRef]
- Bouressa, E.L.; Tugel, A.J.; Peacock, G.L.; Jackson, R.D. Burning and grazing to promote persistence of warm-season grasses sown into a cool-season pasture. Ecol. Restor. 2010, 28, 40–45. [Google Scholar] [CrossRef]
- McGranahan, D.A.; Engle, D.M.; Fuhlendorf, S.D.; Winter, S.J.; Miller, J.R.; Debinski, D.M. Spatial heterogeneity across five rangelands managed with pyric-herbivory. J. Appl. Ecol. 2012, 49, 903–910. [Google Scholar] [CrossRef]
- McGranahan, D.A.; Engle, D.M.; Fuhlendorf, S.D.; Winter, S.L.; Miller, J.R.; Debinski, D.M. Inconsistent outcomes of heterogeneity-based management underscore importance of matching evaluation to conservation objectives. Environ. Sci. Policy 2013, 31, 53–60. [Google Scholar] [CrossRef]
- Rietkerk, M.; Van de Koppel, J. Alternate stable states and threshold effects in semi-arid grazing systems. Oikos 1997, 79, 69–76. [Google Scholar] [CrossRef]
- Baer, S.G.; Kitchen, D.J.; Blair, J.M.; Rice, C.W. Changes in ecosystem structure and function along a chronosequence of restored grasslands. Ecol. Appl. 2002, 12, 1688–1701. [Google Scholar] [CrossRef]
- Young, T.P. Restoration ecology and conservation biology. Biol. Conserv. 2000, 92, 73–83. [Google Scholar] [CrossRef]
- Poschlod, P.; Bakker, J.P.; Kahmen, S. Changing land use and its impact on biodiversity. Basic Appl. Ecol. 2005, 6, 93–98. [Google Scholar] [CrossRef]
- Öckinger, E.; Smith, H.G. Landscape composition and habitat area affects butterfly species richness in semi-natural grasslands. Oecologia 2006, 149, 526–534. [Google Scholar] [CrossRef]
- Lindborg, R.; Bengtsson, J.; Berg, Å.; Cousins, S.A.O.; Eriksson, O.; Gustafsson, T.; Hasund, K.P.; Lenoir, L.; Pihlgren, A.; Sjödin, E.; et al. A landscape perspective on conservation of semi-natural grasslands. Agr. Ecosyst. Environ. 2008, 125, 213–222. [Google Scholar] [CrossRef]
- Efetha, Å.A.; Eriksson, O.; Berglund, H. Species abundance patterns of plants in Swedish semi-natural pastures. Ecography 2006, 18, 310–317. [Google Scholar]
- Kleijn, D.; Baquero, R.A.; Clough, Y.; Díaz, M.; Esteban, J.; Fernández, F.; Gabriel, D.; Herzog, F.; Holzschuh, A.; Jöhl, R.; et al. Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol. Lett. 2006, 9, 243–254. [Google Scholar] [CrossRef]
- Ricketts, T.H.; Daily, G.C.; Ehrlich, P.R.; Fay, J.P. Countryside biogeography of moths in a fragmented landscape: Biodiversity in native and agricultural habitat. Conserv. Biol. 2001, 15, 378–388. [Google Scholar] [CrossRef]
- Lindborg, R.; Eriksson, O. Effects of restoration on plant species richness and composition in scandinavian semi-natural grasslands. Restor. Ecol. 2004, 12, 318–326. [Google Scholar] [CrossRef]
- Pykälä, J. Plant species responses to cattle grazing in mesic semi-natural grassland. Agr. Ecosyst. Environ. 2005, 108, 109–117. [Google Scholar] [CrossRef]
- Pykälä, J. Effects of restoration with cattle grazing on plant species composition and richness of semi-natural grasslands. Biodivers. Conserv. 2003, 12, 2211–2226. [Google Scholar] [CrossRef]
- Pillsbury, F.C.; Miller, J.R.; Debinski, D.M.; Engle, D.M. Another tool in the toolbox? Using fire and grazing to promote bird diversity in highly fragmented landscapes. Ecosphere 2011, 2, art28. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Hobbs, R.J.; Suding, K.N. Management of novel ecosystems: Are novel approaches required? Front. Ecol. Environ. 2008, 6, 547–553. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Engle, D.M.; Elmore, R.D.; Limb, R.F.; Bidwell, T.G. Conservation of pattern and process: Developing an alternative paradigm of rangeland management. Rangel. Ecol. Manag. 2012, 65, 579–589. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. Available online: http://cc.oulu.fi/~jarioksa/opetus/metodi/vegantutor.pdf (accessed on 12 March 2013).
Appendix
| Plot Code | Tract | Grazing History | Floristic Degradation State | Native Species Richness | Total Species Richness | Proportion Native Abundance | Simpson’s Diversity (1/D) |
|---|---|---|---|---|---|---|---|
| Gil.C.N | Gilleland | Severely grazed | High | 14 | 31 | 0.3 | 3.04 |
| Gil.C.S | Gilleland | Severely grazed | High | 18 | 39 | 0.14 | 3.88 |
| Gil.N.N | Gilleland | Severely grazed | High | 18 | 38 | 0.06 | 2.4 |
| Gil.N.S | Gilleland | Severely grazed | High | 15 | 31 | 0.03 | 5.92 |
| Gil.S.N | Gilleland | Severely grazed | High | 8 | 28 | 0.01 | 3.23 |
| Gil.S.S | Gilleland | Severely grazed | High | 28 | 51 | 0.38 | 4.06 |
| LT. C.S | Lee Trail | Ungrazed | Medium | 28 | 34 | 0.80 | 6.02 |
| LT.C.N | Lee Trail | Ungrazed | Medium | 35 | 46 | 0.47 | 7.67 |
| LT.E.N | Lee Trail | Ungrazed | High | 13 | 20 | 0.28 | 2.81 |
| LT.E.S | Lee Trail | Ungrazed | High | 16 | 26 | 0.05 | 2.77 |
| LT.W.N | Lee Trail | Ungrazed | Medium | 26 | 32 | 0.85 | 3.58 |
| LT.W.S | Lee Trail | Ungrazed | Medium | 26 | 32 | 0.45 | 3.23 |
| Paw.E.N | Pawnee | Ungrazed | Medium | 41 | 52 | 0.97 | 5.5 |
| Paw.E.S | Pawnee | Ungrazed | Medium | 39 | 45 | 0.94 | 5.28 |
| Paw.S.N | Pawnee | Ungrazed | Medium | 49 | 53 | 0.99 | 4.22 |
| Paw.S.S | Pawnee | Ungrazed | Medium | 46 | 53 | 0.83 | 5.74 |
| Paw.W.N | Pawnee | Ungrazed | Medium | 31 | 39 | 0.90 | 4.98 |
| Paw.W.S | Pawnee | Ungrazed | Medium | 40 | 51 | 0.98 | 2.93 |
| PyN.N.N | Pyland North | Severely grazed | High | 12 | 29 | 0.02 | 3.44 |
| PyN.N.S | Pyland North | Severely grazed | High | 26 | 43 | 0.31 | 4.8 |
| PyN.S.N | Pyland North | Severely grazed | Low | 21 | 39 | 0.22 | 9.69 |
| PyN.S.S | Pyland North | Severely grazed | Low | 36 | 54 | 0.28 | 4.52 |
| PyN.W.N | Pyland North | Severely grazed | Low | 28 | 47 | 0.12 | 7.18 |
| PyN.W.S | Pyland North | Severely grazed | Low | 26 | 46 | 0.37 | 6.35 |
| PyS.N.N | Pyland South | Severely grazed | Low | 35 | 49 | 0.41 | 4.98 |
| PyS.N.S | Pyland South | Severely grazed | Low | 33 | 53 | 0.45 | 7.99 |
| PyS.S.N | Pyland South | Severely grazed | Low | 33 | 50 | 0.31 | 5.88 |
| PyS.S.S | Pyland South | Severely grazed | Low | 29 | 48 | 0.13 | 4.62 |
| PyS.W.N | Pyland South | Severely grazed | Low | 32 | 48 | 0.52 | 7.06 |
| PyS.W.S | Pyland South | Severely grazed | Low | 25 | 42 | 0.22 | 3.71 |
| PyW.C.N | Pyland West | Severely grazed | Low | 23 | 37 | 0.14 | 4.48 |
| PyW.C.S | Pyland West | Severely grazed | Medium | 30 | 47 | 0.29 | 3.42 |
| PyW.N.N | Pyland West | Severely grazed | Medium | 27 | 47 | 0.2 | 5.61 |
| PyW.N.S | Pyland West | Severely grazed | Medium | 31 | 48 | 0.16 | 3.92 |
| PyW.S.N | Pyland West | Severely grazed | Medium | 24 | 39 | 0.33 | 6.8 |
| PyW.S.S | Pyland West | Severely grazed | Medium | 30 | 47 | 0.52 | 4.96 |
| Rch.N.N | Richardson | Ungrazed | High | 9 | 18 | 0.07 | 2.81 |
| Rch.N.S | Richardson | Ungrazed | High | 21 | 32 | 0.07 | 2.02 |
| Rch.S.N | Richardson | Ungrazed | High | 13 | 27 | 0.1 | 2.81 |
| Rch.S.S | Richardson | Ungrazed | High | 21 | 33 | 0.15 | 3.35 |
| Rch.W.N | Richardson | Ungrazed | High | 13 | 20 | 0.11 | 3.21 |
| Rch.W.S | Richardson | Ungrazed | High | 17 | 30 | 0.3 | 3.7 |
| RN.E.N | Ringgold North | Ungrazed | Low | 33 | 36 | 0.99 | 6.31 |
| RN.E.S | Ringgold North | Ungrazed | Low | 22 | 23 | 1 | 4.71 |
| RN.S.N | Ringgold North | Ungrazed | Low | 29 | 30 | 0.98 | 3.94 |
| RN.S.S | Ringgold North | Ungrazed | Low | 27 | 28 | 0.99 | 3.81 |
| RN.W.N | Ringgold North | Ungrazed | Low | 35 | 39 | 0.99 | 9.42 |
| RN.W.S | Ringgold North | Ungrazed | Low | 39 | 43 | 1 | 7.27 |
| RS.C.N | Ringgold South | Ungrazed | Low | 31 | 43 | 0.55 | 7.24 |
| RS.C.S | Ringgold South | Ungrazed | Low | 45 | 52 | 0.67 | 8.85 |
| RS.N.N | Ringgold South | Ungrazed | Low | 21 | 31 | 0.39 | 4.16 |
| RS.N.S | Ringgold South | Ungrazed | Low | 30 | 42 | 0.35 | 4.87 |
| RS.S.N | Ringgold South | Ungrazed | Low | 30 | 36 | 0.38 | 4.94 |
| RS.S.S | Ringgold South | Ungrazed | Low | 33 | 42 | 0.28 | 5.27 |

© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McGranahan, D.A.; Engle, D.M.; Fuhlendorf, S.D.; Miller, J.R.; Debinski, D.M. Multivariate Analysis of Rangeland Vegetation and Soil Organic Carbon Describes Degradation, Informs Restoration and Conservation. Land 2013, 2, 328-350. https://doi.org/10.3390/land2030328
McGranahan DA, Engle DM, Fuhlendorf SD, Miller JR, Debinski DM. Multivariate Analysis of Rangeland Vegetation and Soil Organic Carbon Describes Degradation, Informs Restoration and Conservation. Land. 2013; 2(3):328-350. https://doi.org/10.3390/land2030328
Chicago/Turabian StyleMcGranahan, Devan Allen, David M. Engle, Samuel D. Fuhlendorf, James R. Miller, and Diane M. Debinski. 2013. "Multivariate Analysis of Rangeland Vegetation and Soil Organic Carbon Describes Degradation, Informs Restoration and Conservation" Land 2, no. 3: 328-350. https://doi.org/10.3390/land2030328




