Alpha Particle Emitter Radiolabeled Antibody for Metastatic Cancer: What Can We Learn from Heavy Ion Beam Radiobiology?
Abstract
:1. Introduction
2. Alpha-Particle Radioimmunotherapy and Heavy Ion Beam Therapy of Cancer
3. High LET Alpha-Particle Radiation, Bragg Peaks and RBE
3.1. High LET Alpha-Particle Emitters
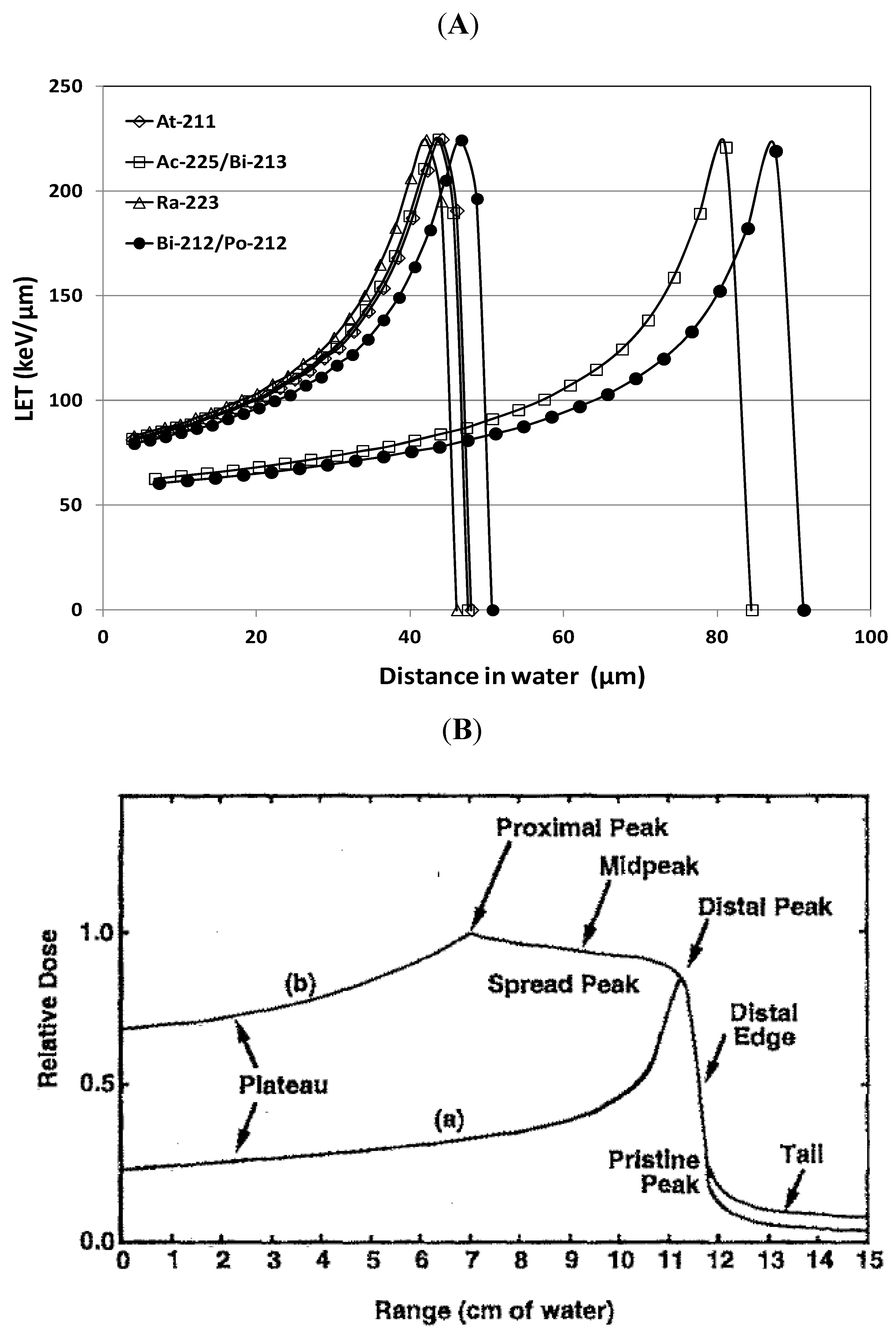
3.2. LET and RBE
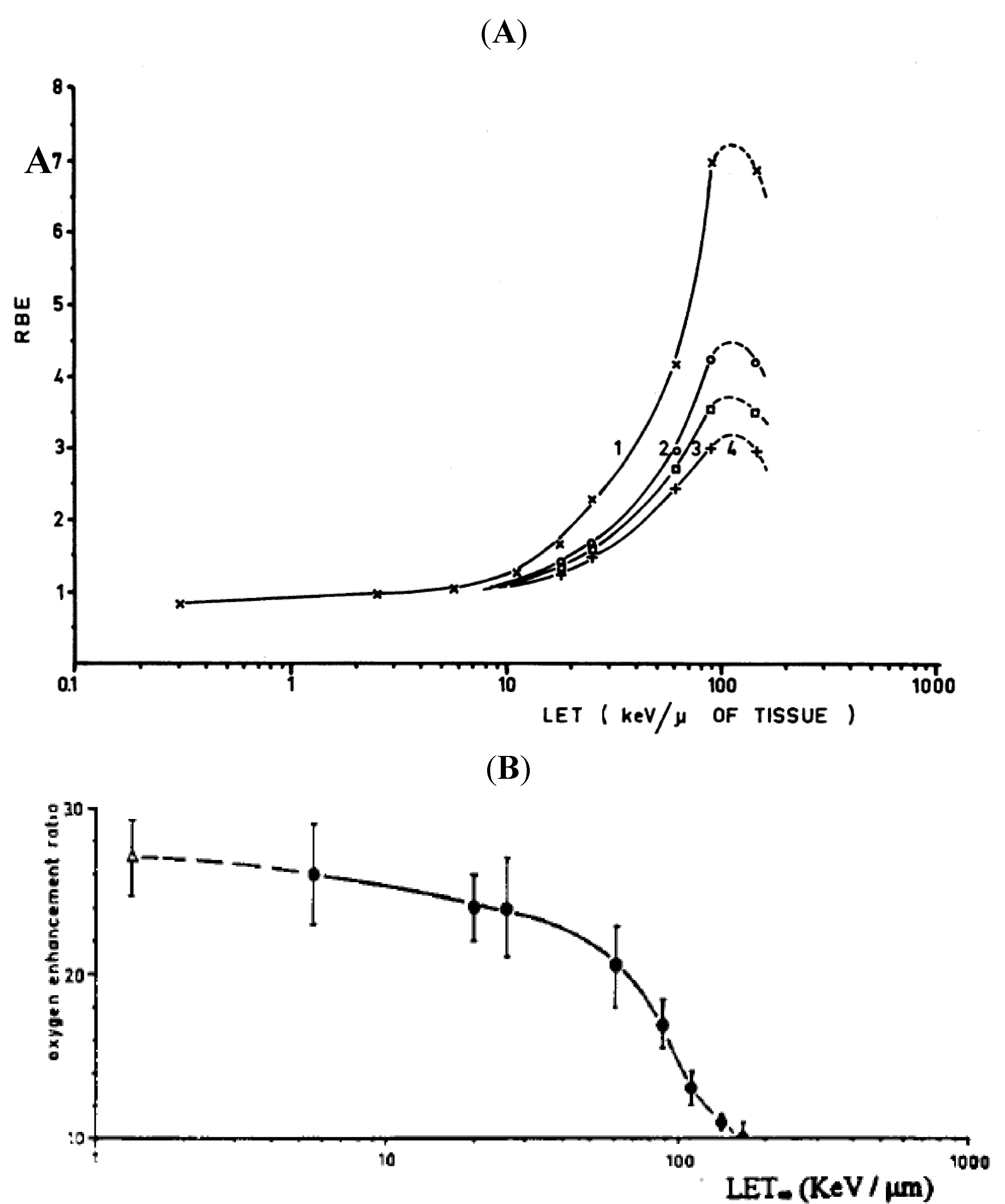
3.3. Microdosimetry
4. Biological Effects of High LET Radiation
4.1. Induction of DNA Damage by High LET Radiation
4.2. Effect of Dose-Rate and Fractionation
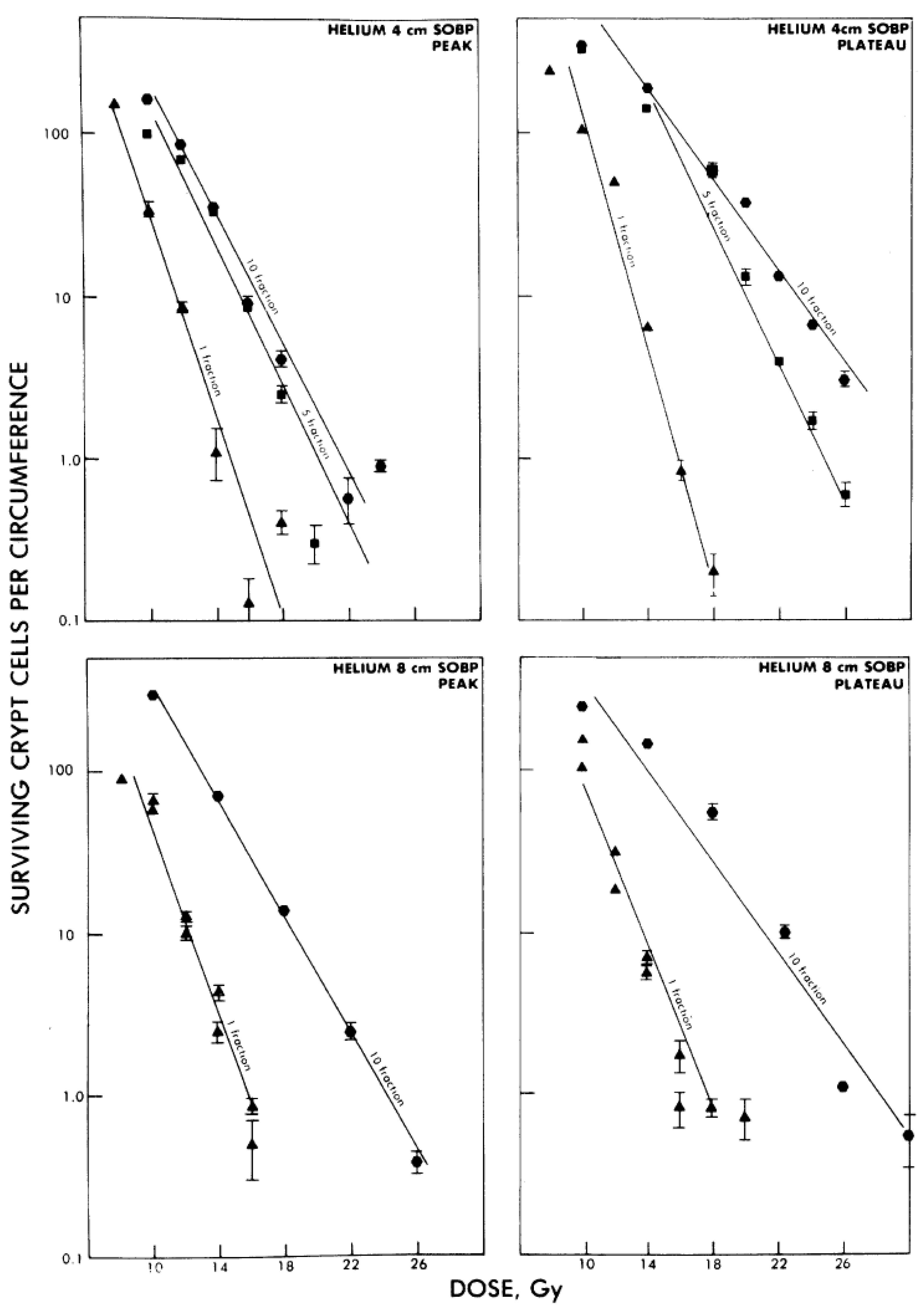
4.3. Effect of Cell Cycle and Oxygenation
5. Repair of DNA Damage by High LET Radiation
5.1. DNA DSB Repair After High LET Radiation
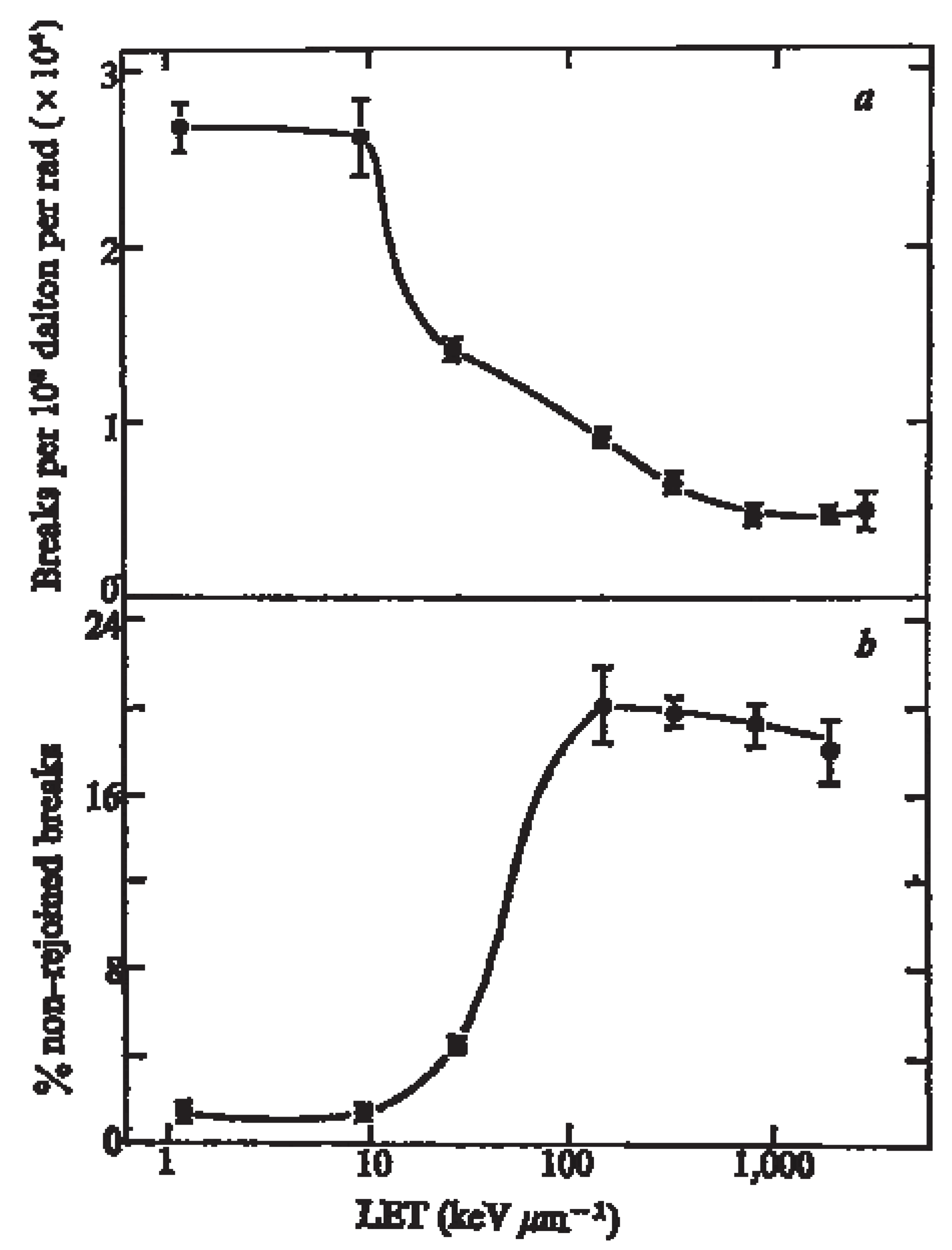
5.2. Impact of Antibodies that Dis-Regulates DNA Repair
5.3. Targeting Genetic Defects in DNA Repair by High LET Radiation
6. Conclusions
References
- Nilsson, S.; Franzen, L.; Parker, C.; Tyrrell, C.; Blom, R.; Tennvall, J.; Lennernas, B.; Petersson, U.; Johannessen, D.C.; Sokal, M.; et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007, 8, 587–594. [Google Scholar] [CrossRef]
- Skarsgard, L.D. Radiobiology with heavy charged particles: A historical review. Phys. Med. 1998, 14 Suppl. 1, 1–19. [Google Scholar]
- Blakely, E.A.; Kronenberg, A. Heavy-ion radiobiology: New approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat. Res. 1998, 150, S126–S145. [Google Scholar] [CrossRef]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef]
- Goldenberg, D.M. Targeted therapy of cancer with radiolabeled antibodies. J. Nucl. Med. 2002, 43, 693–713. [Google Scholar]
- Jain, M.; Venkatraman, G.; Batra, S.K. Optimization of radioimmunotherapy of solid tumors: Biological impediments and their modulation. Clin. Cancer Res. 2007, 13, 1374–1382. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, A.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1239. [Google Scholar]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. (212)Pb-radioimmunotherapy induces G(2) cell-cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol. Cancer Ther. 2012, 11, 639–648. [Google Scholar] [CrossRef]
- Andersson, H.; Cederkrantz, E.; Back, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)At-MX35 F(ab')2—A phase I study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef]
- Cordier, D.; Forrer, F.; Bruchertseifer, F.; Morgenstern, A.; Apostolidis, C.; Good, S.; Muller-Brand, J.; Macke, H.; Reubi, J.C.; Merlo, A. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8,Met(O2)11]-substance P: A pilot trial. Eur. J. Nucl. Med. Mol. I 2010, 37, 1335–1344. [Google Scholar] [CrossRef]
- Lawrence, J.H.; Tobiascaborn, J.L.; Gottschalk, A.; Linfoot, J.A.; Kling, R.P. Alpha Particle and Proton Beams in Therapy. JAMA 1963, 186, 236–245. [Google Scholar]
- Muramatsu, M.; Kitagawa, A. A review of ion sources for medical accelerators (invited). Rev. Sci. Instrum. 2012, 83, 02B909. [Google Scholar] [CrossRef]
- Jensen, A.D.; Munter, M.W.; Debus, J. Review of clinical experience with ion beam radiotherapy. Br. J. Radiol. 2011, 84, S35–S47. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 2007, 25, 953–964. [Google Scholar] [CrossRef]
- Chu, W.T.; Ludewigt, B.A.; Renner, T.R. Instrumentation for Treatment of Cancer Using Proton and Light-Ion Beams. Rev. Sci. Instrum. 1993, 64, 2055–2122. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Beusker, T.L. Effects of different ionizing radiations on human cells in tissue culture. I. Irradiation techniques and dosimetry. Radiat. Res. 1960, 13, 832–840. [Google Scholar] [CrossRef]
- Barendsen, G.W. Impairment of the Proliferative Capacity of Human Cells in Culture by Alpha-Particles with Differing Linear-Energy Transfer. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1964, 8, 453–466. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Beusker, T.L.; Vergroesen, A.J.; Budke, L. Effects of different radiations on human cells in tissue culture. II. Biological experiments. Radiat. Res. 1960, 13, 841–849. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Koot, C.J.; Van Kersen, G.R.; Bewley, D.K.; Field, S.B.; Parnell, C.J. The effect of oxygen on impairment of the proliferative capacity of human cells in culture by ionizing radiations of different LET. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1966, 10, 317–327. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Walter, H.M. Effects of Different Ionizing Radiations on Human Cells in Tissue Culture. Iv. Modification of Radiation Damage. Radiat. Res. 1964, 21, 314–329. [Google Scholar] [CrossRef]
- Barendsen, G.W.; Walter, H.M.; Fowler, J.F.; Bewley, D.K. Effects of different ionizing radiations on human cells in tissue culture. III. Experiments with cyclotron-accelerated alpha-particles and deuterons. Radiat. Res. 1963, 18, 106–119. [Google Scholar] [CrossRef]
- Belli, M.; Goodhead, D.T.; Ianzini, F.; Simone, G.; Tabocchini, M.A. Direct comparison of biological effectiveness of protons and alpha-particles of the same LET. II. Mutation induction at the HPRT locus in V79 cells. Int. J. Radiat. Biol. 1992, 61, 625–629. [Google Scholar] [CrossRef]
- Goodhead, D.T.; Belli, M.; Mill, A.J.; Bance, D.A.; Allen, L.A.; Hall, S.C.; Ianzani, F.; Simone, G.; Stevens, D.L.; Stretch, A.; et al. Direct comparison between protons and alpha-particles of the same LET: I. Irradiation methods and inactivation of asynchronous V79, HeLa and C3H 10T1/2 cells. Int. J. Radiat. Biol. 1992, 61, 611–624. [Google Scholar] [CrossRef]
- Jenner, T.J.; Belli, M.; Goodhead, D.T.; Ianzini, F.; Simone, G.; Tabocchini, M.A. Direct comparison of biological effectiveness of protons and alpha-particles of the same LET. III. Initial yield of DNA double-strand breaks in V79 cells. Int. J. Radiat. Biol. 1922, 61, 631–637. [Google Scholar]
- Belli, M.; Cherubini, R.; Finotto, S.; Moschini, G.; Sapora, O.; Simone, G.; Tabocchini, M.A. RBE-LET relationship for the survival of V79 cells irradiated with low energy protons. Int. J. Radiat. Biol. 1989, 55, 93–104. [Google Scholar] [CrossRef]
- Prise, K.M.; Folkard, M.; Davies, S.; Michael, B.D. The irradiation of V79 mammalian cells by protons with energies below 2 MeV. Part II. Measurement of oxygen enhancement ratios and DNA damage. Int. J. Radiat. Biol. 1990, 58, 261–277. [Google Scholar] [CrossRef]
- Folkard, M.; Prise, K.M.; Vojnovic, B.; Newman, H.C.; Roper, M.J.; Michael, B.D. Inactivation of V79 cells by low-energy protons, deuterons and helium-3 ions. Int. J. Radiat. Biol. 1996, 69, 729–738. [Google Scholar] [CrossRef]
- Furusawa, Y.; Fukutsu, K.; Aoki, M.; Itsukaichi, H.; Eguchi-Kasai, K.; Ohara, H.; Yatagai, F.; Kanai, T.; Ando, K. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat. Res. 2000, 154, 485–496. [Google Scholar] [CrossRef]
- Folkard, M.; Prise, K.M.; Vojnovic, B.; Davies, S.; Roper, M.J.; Michael, B.D. The irradiation of V79 mammalian cells by protons with energies below 2 MeV. Part I: Experimental arrangement and measurements of cell survival. Int. J. Radiat. Biol. 1989, 56, 221–237. [Google Scholar] [CrossRef]
- Charlton, D.E.; Utteridge, T.D.; Allen, B.J. Theoretical treatment of human haemopoietic stem cell survival following irradiation by alpha particles. Int. J. Radiat. Biol. 1998, 74, 111–118. [Google Scholar] [CrossRef]
- Charlton, D.E.; Turner, M.S. Use of chord lengths through the nucleus to simulate the survival of mammalian cells exposed to high LET alpha-radiation. Int. J. Radiat. Biol. 1996, 69, 213–217. [Google Scholar] [CrossRef]
- Claesson, K.; Magnander, K.; Kahu, H.; Lindegren, S.; Hultborn, R.; Elmroth, K. RBE of alpha-particles from (211)At for complex DNA damage and cell survival in relation to cell cycle position. Int. J. Radiat. Biol. 2010, 87, 372–384. [Google Scholar]
- Claesson, A.K.; Stenerlow, B.; Jacobsson, L.; Elmroth, K. Relative biological effectiveness of the alpha-particle emitter (211)At for double-strand break induction in human fibroblasts. Radiat. Res. 2007, 167, 312–318. [Google Scholar] [CrossRef]
- Aurlien, E.; Larsen, R.H.; Akabani, G.; Olsen, D.R.; Zalutsky, M.R.; Bruland, O.S. Exposure of human osteosarcoma and bone marrow cells to tumour-targeted alpha-particles and gamma-irradiation: Analysis of cell survival and microdosimetry. Int. J. Radiat. Biol. 2000, 76, 1129–1141. [Google Scholar] [CrossRef]
- Back, T.; Andersson, H.; Divgi, C.R.; Hultborn, R.; Jensen, H.; Lindegren, S.; Palm, S.; Jacobsson, L. 211At radioimmunotherapy of subcutaneous human ovarian cancer xenografts: evaluation of relative biologic effectiveness of an alpha-emitter in vivo. J. Nucl. Med. 2005, 46, 2061–2067. [Google Scholar]
- Howell, R.W.; Azure, M.T.; Narra, V.R.; Rao, D.V. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat. Res. 1994, 137, 352–360. [Google Scholar] [CrossRef]
- Howell, R.W.; Goddu, S.M.; Narra, V.R.; Fisher, D.R.; Schenter, R.E.; Rao, D.V. Radiotoxicity of gadolinium-148 and radium-223 in mouse testes: Relative biological effectiveness of alpha-particle emitters in vivo. Radiat. Res. 1997, 147, 342–348. [Google Scholar] [CrossRef]
- Behr, T.M.; Behe, M.; Stabin, M.G.; Wehrmann, E.; Apostolidis, C.; Molinet, R.; Strutz, F.; Fayyazi, A.; Wieland, E.; Gratz, S.; Koch, L.; Goldenberg, D.M.; Becker, W. High-linear energy transfer (LET) alpha versus low-LET beta emitters in radioimmunotherapy of solid tumors: Therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17–1A Fab' fragments in a human colonic cancer model. Cancer Res. 1999, 59, 2635–2643. [Google Scholar]
- Behr, T.M.; Behe, M.; Sgouros, G. Correlation of red marrow radiation dosimetry with myelotoxicity: Empirical factors influencing the radiation-induced myelotoxicity of radiolabeled antibodies, fragments and peptides in pre-clinical and clinical settings. Cancer Biother. Radiopharm. 2002, 17, 445–464. [Google Scholar] [CrossRef]
- Nayak, T.K.; Norenberg, J.P.; Anderson, T.L.; Prossnitz, E.R.; Stabin, M.G.; Atcher, R.W. Somatostatin-receptor-targeted alpha-emitting Bi-213 is therapeutically more effective than beta-emitting Lu-177 in human pancreatic adenocarcinoma cells. Nucl. Med. Biol. 2007, 34, 185–193. [Google Scholar] [CrossRef]
- Feinendegen, L.E.; McClure, J.J. Meeting report—Alpha-emitters for medical therapy—Workshop of the United States Department of Energy—Denver, Colorado, May 30–31, 1996. Radiat. Res. 1997, 148, 195–201. [Google Scholar] [CrossRef]
- Rossi, H.H. Radiation physics and radiobiology. Health Phys. 1996, 70, 828–831. [Google Scholar] [CrossRef]
- Kellerer, A.M.; Chmelevsky, D. Criteria for the applicability of LET. Radiat. Res. 1975, 63, 226–234. [Google Scholar] [CrossRef]
- Chouin, N.; Bardies, M. Alpha-particle microdosimetry. Curr. Radiopharm. 2011, 4, 266–280. [Google Scholar]
- Kellerer, A.M. Radiobiological challenges posed by microdosimetry. Health Phys. 1996, 70, 832–836. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Ovid Technologies Inc. Radiobiology for the Radiologist, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; p. 456. [Google Scholar]
- Franken, N.A.; ten Cate, R.; Krawczyk, P.M.; Stap, J.; Haveman, J.; Aten, J.; Barendsen, G.W. Comparison of RBE values of high-LET alpha-particles for the induction of DNA-DSBs, chromosome aberrations and cell reproductive death. Radiat. Oncol. 2011, 6, 64. [Google Scholar] [CrossRef]
- Belli, M.; Campa, A.; Dini, V.; Esposito, G.; Furusawa, Y.; Simone, G.; Sorrentino, E.; Tabocchini, M.A. DNA fragmentation induced in human fibroblasts by accelerated (56)fe ions of differing energies. Radiat. Res. 2006, 165, 713–720. [Google Scholar] [CrossRef]
- Newman, H.C.; Prise, K.M.; Folkard, M.; Michael, B.D. DNA double-strand break distributions in X-ray and alpha-particle irradiated V79 cells: Evidence for non-random breakage. Int. J. Radiat. Biol. 1997, 71, 347–363. [Google Scholar] [CrossRef]
- Terato, H.; Tanaka, R.; Nakaarai, Y.; Nohara, T.; Doi, Y.; Iwai, S.; Hirayama, R.; Furusawa, Y.; Ide, H. Quantitative analysis of isolated and clustered DNA damage induced by gamma-rays, carbon ion beams, and iron ion beams. J. Radiat. Res. 2008, 49, 133–146. [Google Scholar] [CrossRef]
- Hada, M.; Sutherland, B.M. Spectrum of complex DNA damages depends on the incident radiation. Radiat. Res. 2006, 165, 223–230. [Google Scholar] [CrossRef]
- Karlsson, K.H.; Stenerlow, B. Focus formation of DNA repair proteins in normal and repair-deficient cells irradiated with high-LET ions. Radiat. Res. 2004, 161, 517–527. [Google Scholar] [CrossRef]
- Friedland, W.; Dingfelder, M.; Jacob, P.; Paretzke, H.G. Calculated DNA double-strand break and fragmentation yields after irradiation with He ions. Radiat. Phys. Chem. 2005, 72, 279–286. [Google Scholar]
- Ward, J.F. The complexity of DNA damage: Relevance to biological consequences. Int. J. Radiat. Biol. 1994, 66, 427–432. [Google Scholar] [CrossRef]
- Hada, M.; Georgakilas, A.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef]
- Gollapalle, E.; Wang, R.; Adetolu, R.; Tsao, D.; Francisco, D.; Sigounas, G.; Georgakilas, A.G. Detection of oxidative clustered DNA lesions in X-irradiated mouse skin tissues and human MCF-7 breast cancer cells. Radiat. Res. 2007, 167, 207–216. [Google Scholar] [CrossRef]
- Sutherland, B.M.; Bennett, P.V.; Sidorkina, O.; Laval, J. Clustered DNA damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 2000, 97, 103–108. [Google Scholar]
- Tsao, D.; Kalogerinis, P.; Tabrizi, I.; Dingfelder, M.; Stewart, R.D.; Georgakilas, A.G. Induction and processing of oxidative clustered DNA lesions in Fe-56-Ion-irradiated human monocytes. Radiat. Res. 2007, 168, 87–97. [Google Scholar] [CrossRef]
- Milligan, J.R.; Aguilera, J.A.; Paglinawan, R.A.; Ward, J.F.; Limoli, C.L. DNA strand break yields after post-high LET irradiation incubation with endonuclease-III and evidence for hydroxyl radical clustering. Int. J. Radiat. Biol. 2001, 77, 155–164. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. Fast Monte Carlo simulation of DNA damage formed by electrons and light ions. Phys. Med. Biol. 2006, 51, 1693–1706. [Google Scholar] [CrossRef]
- Nikjoo, H.; O'Neill, P.; Wilson, W.E.; Goodhead, D.T. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat. Res. 2001, 156, 577–583. [Google Scholar] [CrossRef]
- Semenenko, V.A.; Stewart, R.D. Monte carlo simulation of base and nucleotide excision repair of clustered DNA damage sites. II. Comparisons of model predictions to measured data. Radiat. Res. 2005, 164, 194–201. [Google Scholar] [CrossRef]
- Asaithamby, A.; Hu, B.; Chen, D.J. Unrepaired clustered DNA lesions induce chromosome breakage in human cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8293–8298. [Google Scholar] [CrossRef]
- Levy, R.P.; Blakely, E.A.; Chu, W.T.; Coutrakon, G.B.; Hug, E.B.; Kraft, G.; Tsujii, H. The Current Status and Future Directions of Heavy Charged Particle Therapy in Medicine. Appl. Acc. Res. Ind. 2009, 1099, 410–425. [Google Scholar]
- Barendsen, G.W. Modification of Radiation Damage by Fractionation of the Dose, Anoxia, and Chemical Protectors in Relation to Let. Ann. NY Acad. Sci. 1964, 114, 96–114. [Google Scholar] [CrossRef]
- Hill, C.K.; Buonaguro, F.M.; Myers, C.P.; Han, A.; Elkind, M.M. Fission-Spectrum Neutrons at Reduced Dose-Rates Enhance Neoplastic Transformation. Nature 1982, 298, 67–69. [Google Scholar]
- Brenner, D.J.; Hall, E.J. The inverse dose-rate effect for oncogenic transformation by neutrons and charged particles: A plausible interpretation consistent with published data. Int. J. Radiat. Biol. 1990, 58, 745–758. [Google Scholar]
- Brenner, D.J.; Hall, E.J.; Randerspehrson, G.; Miller, R.C. Mechanistic Considerations on the Dose-Rate Let Dependence of Oncogenic Transformation by Ionizing-Radiations. Radiat. Res. 1993, 133, 365–369. [Google Scholar] [CrossRef]
- Tauchi, H.; Waku, H.; Matsumoto, E.; Yara, S.; Okumura, S.; Iwata, Y.; Komatsu, K.; Furusawa, Y.; Eguchi-Kasai, K.; Tachibana, A. Two major factors involved in the reverse dose-rate effect for somatic mutation induction are the cell cycle position and LET value. J. Radiat. Res. 2009, 50, 441–448. [Google Scholar] [CrossRef]
- Rossi, H.H.; Kellerer, A.M. The Dose-Rate Dependence of Oncogenic Transformation by Neutrons May Be Due to Variation of Response during the Cell-Cycle. Int. J. Radiat. Biol. 1986, 50, 353–361. [Google Scholar]
- Miller, R.C.; Randers-Pehrson, G.; Hieber, L.; Marino, S.A.; Richards, M.; Hall, E.J. The inverse dose-rate effect for oncogenic transformation by charged particles is dependent on linear energy transfer. Radiat. Res. 1993, 133, 360–364. [Google Scholar] [CrossRef]
- Bettega, D.; Calzolari, P.; Chiorda, G.N.; Tallonelombardi, L. Transformation of C3h 10t1/2 Cells with 4.3 Mev Alpha-Particles at Low-Doses—Effects of Single and Fractionated Doses. Radiat. Res. 1992, 131, 66–71. [Google Scholar]
- Hieber, L.; Ponsel, G.; Roos, H.; Fenn, S.; Fromke, E.; Kellerer, A.M. Absence of a dose-rate effect in the transformation of C3H 10T1/2 cells by alpha-particles. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 52, 859–869. [Google Scholar]
- Goldstein, L.S.; Philips, T.L.; Ross, G.Y. Enhancement by fractionation of biological peak-to-plateau relative biological effectiveness ratios for heavy ions. Int. J. Radiat. Oncol. Biol. Phys. 1978, 4, 1033–1037. [Google Scholar] [CrossRef]
- Fukutsu, K.; Kanai, T.; Furusawa, Y.; Ando, K. Response of mouse intestine after single and fractionated irradiation with accelerated carbon ions with a spread-out Bragg peak. Radiat. Res. 1997, 148, 168–174. [Google Scholar] [CrossRef]
- Goldstein, L.S.; Phillips, T.L.; Ross, G.Y. Biological effects of accelerated heavy ions. II. Fractionated irradiation of intestinal crypt cells. Radiat. Res. 1981, 86, 542–558. [Google Scholar] [CrossRef]
- Chang, P.Y.; Bakke, J.; Puey, A. Fractionated exposure of high energy iron ions has a sparing effect in vivo. Adv. Space Res. 2007, 40, 568–575. [Google Scholar] [CrossRef]
- Suzuki, M.; Kase, Y.; Kanai, T.; Ando, K. Change in radiosensitivity with fractionated-dose irradiation of carbon-ion beams in five different human cell lines. Int. J. Radiat. Oncol. 2000, 48, 251–258. [Google Scholar]
- Elgqvist, J.; Andersson, H.; Back, T.; Claesson, I.; Hultborn, R.; Jensen, H.; Lindegren, S.; Olsson, M.; Palm, S.; Warnhammar, E.; Jacobsson, L. Fractionated radioimmunotherapy of intraperitoneally growing ovarian cancer in nude mice with 211At-MX35 F(ab')2: Therapeutic efficacy and myelotoxicity. Nucl. Med. Biol. 2006, 33, 1065–1072. [Google Scholar] [CrossRef]
- Lucke-Huhle, C.; Blakely, E.A.; Chang, P.Y.; Tobias, C.A. Drastic G2 arrest in mammalian cells after irradiation with heavy-ion beams. Radiat. Res. 1979, 79, 97–112. [Google Scholar] [CrossRef]
- Luckehuhle, C. Alpha-Irradiation-Induced G2 Delay—A Period of Cell Recovery. Radiat. Res. 1982, 89, 298–308. [Google Scholar] [CrossRef]
- Bird, R.P.; Burki, H.J. Survival of synchronized Chinese hamster cells exposed to radiation of different linear-energy transfer. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1975, 27, 105–120. [Google Scholar] [CrossRef]
- Wenzl, T.; Wilkens, J.J. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys. Med. Biol. 2011, 56, 3251–3268. [Google Scholar]
- Michalik, V. Model of DNA Damage Induced by Radiations of Various Qualities. Int. J. Radiat. Biol. 1992, 62, 9–20. [Google Scholar] [CrossRef]
- Fyles, A.W.; Milosevic, M.; Wong, R.; Kavanagh, M.C.; Pintilie, M.; Sun, A.; Chapman, W.; Levin, W.; Manchul, L.; Keane, T.J.; Hill, R.P. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother. Oncol. 1998, 48, 149–156. [Google Scholar] [CrossRef]
- Fyles, A.; Milosevic, M.; Hedley, D.; Pintilie, M.; Levin, W.; Manchul, L.; Hill, R.P. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J. Clin. Oncol. 2002, 20, 680–687. [Google Scholar] [CrossRef]
- Hockel, M.; Schlenger, K.; Aral, B.; Mitze, M.; Schaffer, U.; Vaupel, P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996, 56, 4509–4515. [Google Scholar]
- Nakano, T.; Suzuki, Y.; Ohno, T.; Kato, S.; Suzuki, M.; Morita, S.; Sato, S.; Oka, K.; Tsujii, H. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin. Cancer Res. 2006, 12, 2185–2190. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- O'Hara, J.A.; Blumenthal, R.D.; Grinberg, O.Y.; Demidenko, E.; Grinberg, S.; Wilmot, C.M.; Taylor, A.M.; Goldenberg, D.M.; Swartz, H.M. Response to radioimmunotherapy correlates with tumor pO2 measured by EPR oximetry in human tumor xenografts. Radiat. Res. 2001, 155, 466–473. [Google Scholar] [CrossRef]
- Adams, G.P.; Shaller, C.C.; Chappell, L.L.; Wu, C.; Horak, E.M.; Simmons, H.H.; Litwin, S.; Marks, J.D.; Weiner, L.M.; Brechbiel, M.W. Delivery of the alpha-emitting radioisotope bismuth-213 to solid tumors via single-chain Fv and diabody molecules. Nucl. Med. Biol. 2000, 27, 339–346. [Google Scholar] [CrossRef]
- Ritter, M.A.; Cleaver, J.E.; Tobias, C.A. High-Let Radiations Induce a Large Proportion of Non-Rejoining DNA Breaks. Nature 1977, 266, 653–655. [Google Scholar]
- Roots, R.; Yang, T.C.; Craise, L.; Blakely, E.A.; Tobias, C.A. Impaired repair capacity of DNA breaks induced in mammalian cellular DNA by accelerated heavy ions. Radiat. Res. 1979, 78, 38–49. [Google Scholar] [CrossRef]
- Fillingham, J.; Keogh, M.C.; Krogan, N.J. GammaH2AX and its role in DNA double-strand break repair. Biochem. Cell Biol. 2006, 84, 568–577. [Google Scholar] [CrossRef]
- Schmid, T.E.; Dollinger, G.; Beisker, W.; Hable, V.; Greubel, C.; Auer, S.; Mittag, A.; Tarnok, A.; Friedl, A.A.; Molls, M.; Roper, B. Differences in the kinetics of gamma-H2AX fluorescence decay after exposure to low and high LET radiation. Int. J. Radiat. Biol. 2010, 86, 682–691. [Google Scholar]
- Leatherbarrow, E.L.; Harper, J.V.; Cucinotta, F.A.; O'Neill, P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int. J. Radiat. Biol. 2006, 82, 111–118. [Google Scholar] [CrossRef]
- Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef]
- Anderson, J.A.; Harper, J.V.; Cucinotta, F.A.; O'Neill, P. Participation of DNA-PKcs in DSB Repair after Exposure to High- and Low-LET Radiation. Radiat. Res. 2010, 174, 195–205. [Google Scholar] [CrossRef]
- Kinashi, Y.; Takahashi, S.; Kashino, G.; Okayasu, R.; Masunaga, S.; Suzuki, M.; Ono, K. DNA double-strand break induction in Ku80-deficient CHO cells following Boron Neutron Capture Reaction. Radiat. Oncol. 2011, 6, 106. [Google Scholar] [CrossRef]
- Okayasu, R.; Okada, M.; Okabe, A.; Noguchi, M.; Takakura, K.; Takahashi, S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat. Res. 2006, 165, 59–67. [Google Scholar] [CrossRef]
- Zafar, F.; Seidler, S.B.; Kronenberg, A.; Schild, D.; Wiese, C. Homologous recombination contributes to the repair of DNA double-strand breaks induced by high-energy iron ions. Radiat. Res. 2010, 173, 27–39. [Google Scholar] [CrossRef]
- Xue, L.; Yu, D.; Furusawa, Y.; Okayasu, R.; Tong, J.; Cao, J.; Fan, S. Regulation of ATM in DNA double strand break repair accounts for the radiosensitivity in human cells exposed to high linear energy transfer ionizing radiation. Mutat. Res. 2009, 670, 15–23. [Google Scholar] [CrossRef]
- Friesen, C.; Glatting, G.; Koop, B.; Schwarz, K.; Morgenstern, A.; Apostolidis, C.; Debatin, K.M.; Reske, S.N. Breaking chemoresistance and radioresistance with [Bi-213]anti-CD45 antibodies in leukemia cells. Cancer Res. 2007, 67, 1950–1958. [Google Scholar]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Kriegs, M.; Kasten-Pisula, U.; Rieckmann, T.; Holst, K.; Saker, J.; Dahm-Daphi, J.; Dikomey, E. The epidermal growth factor receptor modulates DNA double-strand break repair by regulating non-homologous end-joining. DNA Repair 2010, 9, 889–897. [Google Scholar] [CrossRef]
- Myllynen, L.; Rieckmann, T.; Dahm-Daphi, J.; Kasten-Pisula, U.; Petersen, C.; Dikomey, E.; Kriegs, M. In tumor cells regulation of DNA double strand break repair through EGF receptor involves both NHEJ and HR and is independent of p53 and K-Ras status. Radiother. Oncol. 2011, 101, 147–151. [Google Scholar] [CrossRef]
- Dittmann, K.; Mayer, C.; Rodemann, H.P. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother. Oncol. 2005, 76, 157–161. [Google Scholar] [CrossRef]
- Jensen, A.D.; Nikoghosyan, A.; Hinke, A.; Debus, J.; Munter, M.W. Combined treatment of adenoid cystic carcinoma with cetuximab and IMRT plus C12 heavy ion boost: ACCEPT [ACC, Erbitux (R) and particle therapy]. BMC Cancer 2011, 11, 70. [Google Scholar] [CrossRef]
- Allen, G.W.; Saba, C.; Armstrong, E.A.; Huang, S.M.; Benavente, S.; Ludwig, D.L.; Hicklin, D.J.; Harari, P.M. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007, 67, 1155–1162. [Google Scholar]
- Macaulay, V.M.; Salisbury, A.J.; Bohula, E.A.; Playford, M.P.; Smorodinsky, N.I.; Shiloh, Y. Downregulation of the type 1 insulin-like growth factor receptor in mouse melanoma cells is associated with enhanced radiosensitivity and impaired activation of Atm kinase. Oncogene 2001, 20, 4029–4040. [Google Scholar]
- Szabo, C.I.; King, M.C. Inherited breast and ovarian cancer. Hum. Mol. Genet. 1995, 4, 1811–1817. [Google Scholar]
- Fackenthal, J.D.; Olopade, O.I. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat. Rev. Cancer 2007, 7, 937–948. [Google Scholar] [CrossRef]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Kwan, E.; Jack, E.; Vesprini, D.J.; Kuperstein, G.; Abrahamson, J.L.; et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 2001, 68, 700–710. [Google Scholar] [CrossRef]
- Ramus, S.J.; Gayther, S.A. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef]
- Goggins, M.; Schutte, M.; Lu, J.; Moskaluk, C.A.; Weinstein, C.L.; Petersen, G.M.; Yeo, C.J.; Jackson, C.E.; Lynch, H.T.; Hruban, R.H.; et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996, 56, 5360–5364. [Google Scholar]
- Lynch, H.T.; de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Van Assen-Bolt, A.J.; Van Waarde-Verhagen, M.A.; Sijmons, R.H.; Van der Hout, A.H.; Bauch, T.; Streffer, C.; Kampinga, H.H. BRCA1 and BRCA2 heterozygosity and repair of X-ray-induced DNA damage. Int. J. Radiat. Biol. 2002, 78, 285–295. [Google Scholar]
- Trenz, K.; Schutz, P.; Speit, G. Radiosensitivity of lymphoblastoid cell lines with a heterozygous BRCA1 mutation is not detected by the comet assay and pulsed field gel electrophoresis. Mutagenesis 2005, 20, 131–137. [Google Scholar] [CrossRef]
- Baeyens, A.; Thierens, H.; Claes, K.; Poppe, B.; de Ridder, L.; Vral, A. Chromosomal radiosensitivity in BRCA1 and BRCA2 mutation carriers. Int. J. Radiat. Biol. 2004, 80, 745–756. [Google Scholar] [CrossRef]
- Beucher, A.; Deckbar, D.; Schumann, E.; Krempler, A.; Frankenberg-Schwager, M.; Lobrich, M. Elevated radiation-induced gammaH2AX foci in G2 phase heterozygous BRCA2 fibroblasts. Radiother. Oncol. 2011, 101, 46–50. [Google Scholar]
- Leong, T.; Whitty, J.; Keilar, M.; Mifsud, S.; Ramsay, J.; Birrell, G.; Venter, D.; Southey, M.; McKay, M. Mutation analysis of BRCA1 and BRCA2 cancer predisposition genes in radiation hypersensitive cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 959–965. [Google Scholar] [CrossRef]
- Clarke, R.A.; Goozee, G.R.; Birrell, G.; Fang, Z.M.; Hasnain, H.; Lavin, M.; Kearsley, J.H. Absence of ATM truncations in patients with severe acute radiation reactions. Int. J. Radiat. Oncol. 1998, 41, 1021–1027. [Google Scholar] [CrossRef]
- Shayeghl, M.; Seal, S.; Regan, J.; Collins, N.; Barfoot, R.; Rahman, N.; Ashton, A.; Moohan, M.; Wooster, R.; Owen, R.; et al. Heterozygosity for mutations in the ataxia telangiectasia gene is not a major cause of radiotherapy complications in breast cancer patients. Br. J. Cancer 1998, 78, 922–927. [Google Scholar]
- Zhou, G.; Smilenov, L.B.; Lieberman, H.B.; Ludwig, T.; Hall, E.J. Radiosensitivity to high energy iron ions is influenced by heterozygosity for Atm, Rad9 and Brca1. Adv. Space Res. 2010, 46, 681–686. [Google Scholar] [CrossRef]
- Worgul, B.V.; Smilenov, L.; Brenner, D.J.; Vazquez, M.; Hall, E.J. Mice heterozygous for the ATM gene are more sensitive to both X-ray and heavy ion exposure than are wildtypes. Adv. Space Res. 2005, 35, 254–259. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, H.; Senthamizhchelvan, S.; Hobbs, R.F.; Sgouros, G. Alpha Particle Emitter Radiolabeled Antibody for Metastatic Cancer: What Can We Learn from Heavy Ion Beam Radiobiology? Antibodies 2012, 1, 124-148. https://doi.org/10.3390/antib1020124
Song H, Senthamizhchelvan S, Hobbs RF, Sgouros G. Alpha Particle Emitter Radiolabeled Antibody for Metastatic Cancer: What Can We Learn from Heavy Ion Beam Radiobiology? Antibodies. 2012; 1(2):124-148. https://doi.org/10.3390/antib1020124
Chicago/Turabian StyleSong, Hong, Srinivasan Senthamizhchelvan, Robert F. Hobbs, and George Sgouros. 2012. "Alpha Particle Emitter Radiolabeled Antibody for Metastatic Cancer: What Can We Learn from Heavy Ion Beam Radiobiology?" Antibodies 1, no. 2: 124-148. https://doi.org/10.3390/antib1020124



