Regulatory B-Cells in Transplantation
Abstract
:1. Introduction
1.1. B-Cell Subsets
1.2. Mechanisms of Tolerance by B-Cells
2. B-Cells and Transplantation

3. Regulatory B-Cells
| Subject | Phenotype | Suppressive mediator | Refs. | |
|---|---|---|---|---|
| Mice | B1-like | CD5+ | FasL | [34] |
| B10 | CD5+CD1dhigh | IL-10 | [35] | |
| B10 | CD21highCD23highCD1dhigh | IL-10 | [36] | |
| B10 | T cell Ig domain and mucin domain protein (TIM)+ | IL-10 | [37] | |
| Br3 | Unknown | TGF-β | [38] | |
| Humans | Br1 | CD24highCD38high | IL-10 | [39] |
| Br1 | CD24highCD27+ | IL-10 | [40] | |
| Br3 | Unknown | TGF-β | [41] | |
| Foxp3+ | CD19lowFoxp3+ | Unknown | [42] |
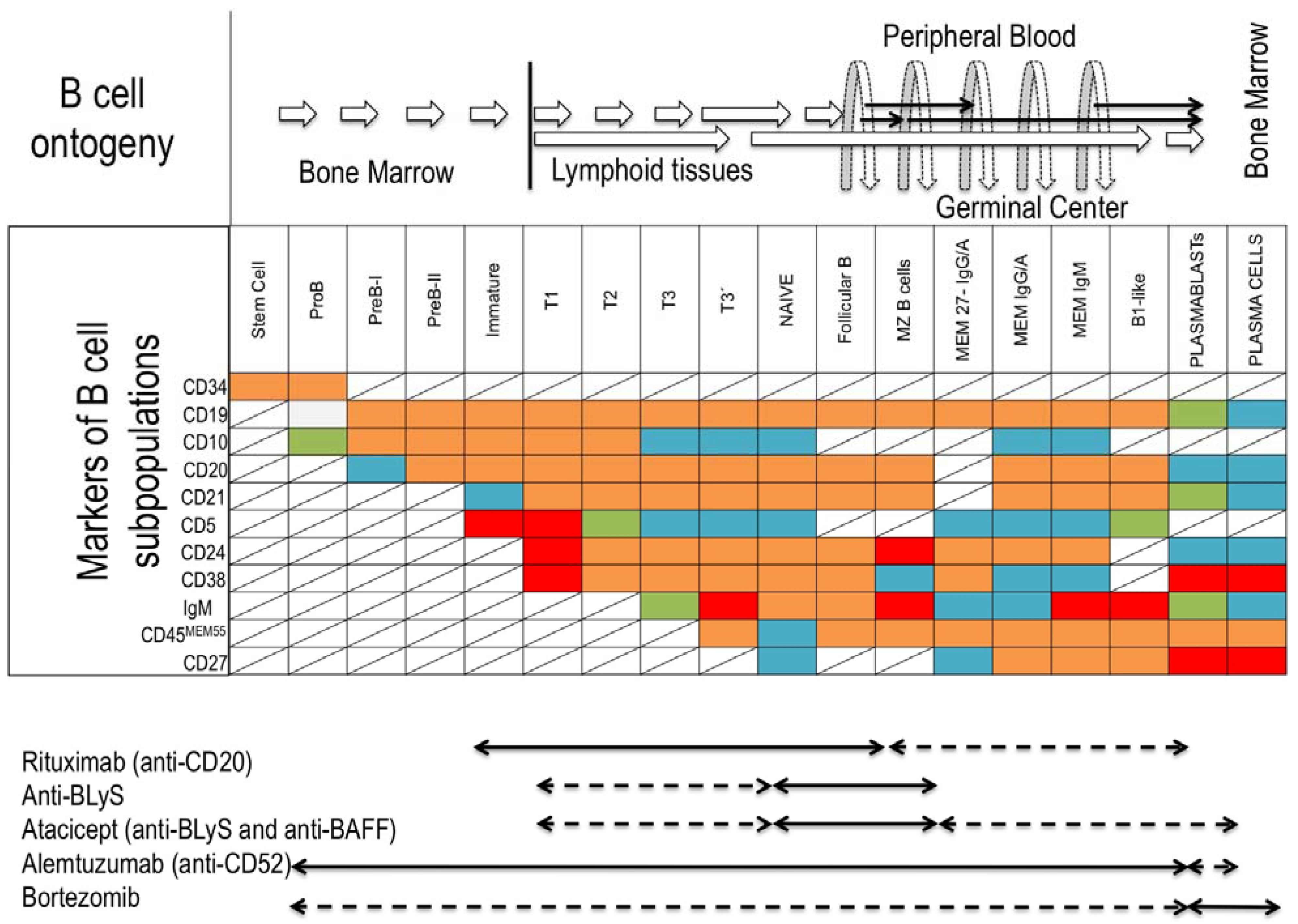
4. Bregs and Clinical Transplantation
5. Conclusion
Acknowledgments
Conflicts of Interest
References
- Griffin, D.O.; Rothstein, T.L. A small cd11b(+) human b1 cell subpopulation stimulates t cells and is expanded in lupus. J. Exp. Med. 2011, 208, 2591–2598. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.D.; Matsushita, T.; Tsubata, T.; Tedder, T.F. The development and function of regulatory b cells expressing il-10 (b10 cells) requires antigen receptor diversity and tlr signals. J. Immunol. 2009, 182, 7459–7472. [Google Scholar] [CrossRef]
- Wennhold, K.; Shimabukuro-Vornhagen, A.; Theurich, S.; von Bergwelt-Baildon, M. Cd40-activated b cells as antigen-presenting cells: The final sprint toward clinical application. Expert Rev. Vaccines 2013, 12, 631–637. [Google Scholar] [CrossRef]
- Zhang, X. Regulatory functions of innate-like b cells. Cell. Mol. Immunol. 2013, 10, 113–121. [Google Scholar] [CrossRef]
- Goodnow, C.C.; Sprent, J.; Fazekas de St Groth, B.; Vinuesa, C.G. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 2005, 435, 590–597. [Google Scholar] [CrossRef]
- Lopes-Carvalho, T.; Kearney, J.F. Development and selection of marginal zone b cells. Immunol. Rev. 2004, 197, 192–205. [Google Scholar] [CrossRef]
- Palanichamy, A.; Barnard, J.; Zheng, B.; Owen, T.; Quach, T.; Wei, C.; Looney, R.J.; Sanz, I.; Anolik, J.H. Novel human transitional b cell populations revealed by b cell depletion therapy. J. Immunol. 2009, 182, 5982–5993. [Google Scholar] [CrossRef]
- Meyer-Bahlburg, A.; Andrews, S.F.; Yu, K.O.; Porcelli, S.A.; Rawlings, D.J. Characterization of a late transitional b cell population highly sensitive to baff-mediated homeostatic proliferation. J. Exp. Med. 2008, 205, 155–168. [Google Scholar] [CrossRef]
- Parsons, R.F.; Vivek, K.; Rostami, S.Y.; Zekavat, G.; Ziaie, S.M.; Luo, Y.; Koeberlein, B.; Redfield, R.R.; Cancro, M.P.; Naji, A.; et al. Acquisition of humoral transplantation tolerance upon de novo emergence of b lymphocytes. J. Immunol. 2011, 186, 614–620. [Google Scholar] [CrossRef]
- Cai, J.; Terasaki, P.I. Humoral theory of transplantation: Mechanism, prevention, and treatment. Hum. Immunol. 2005, 66, 334–342. [Google Scholar] [CrossRef]
- Fidler, S.J.; Irish, A.B.; Lim, W.; Ferrari, P.; Witt, C.S.; Christiansen, F.T. Pre-transplant donor specific anti-hla antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transpl. Immunol. 2013, 28, 148–153. [Google Scholar] [CrossRef]
- Lobo, L.J.; Aris, R.M.; Schmitz, J.; Neuringer, I.P. Donor-specific antibodies are associated with antibody-mediated rejection, acute cellular rejection, bronchiolitis obliterans syndrome, and cystic fibrosis after lung transplantation. J. Heart Lung Transplant. 2013, 32, 70–77. [Google Scholar] [CrossRef]
- Kaczmarek, I.; Deutsch, M.A.; Kauke, T.; Beiras-Fernandez, A.; Schmoeckel, M.; Vicol, C.; Sodian, R.; Reichart, B.; Spannagl, M.; Ueberfuhr, P. Donor-specific hla alloantibodies: Long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp. Clin. Transplant. 2008, 6, 229–235. [Google Scholar]
- Detrait, M.; Dubois, V.; Sobh, M.; Morisset, S.; Tedone, N.; Labussiere, H.; Gillis, L.; Barraco, F.; Cannas, G.; Ducastelle, S.; et al. Impact of anti-hla antibodies on allogeneic hematopoietic stem cell transplantation outcomes after reduced-intensity conditioning regimens. Exp. Hematol. 2012, 40, 792–799. [Google Scholar] [CrossRef]
- Martinez-Llordella, M.; Puig-Pey, I.; Orlando, G.; Ramoni, M.; Tisone, G.; Rimola, A.; Lerut, J.; Latinne, D.; Margarit, C.; Bilbao, I.; et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am. J. Transplant. 2007, 7, 309–319. [Google Scholar] [CrossRef]
- Tryphonopoulos, P.; Ruiz, P.; Weppler, D.; Nishida, S.; Levi, D.M.; Moon, J.; Tekin, A.; Velez, M.; Neuman, D.R.; Island, E.; et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation 2010, 90, 1556–1561. [Google Scholar] [CrossRef]
- Sagoo, P.; Perucha, E.; Sawitzki, B.; Tomiuk, S.; Stephens, D.A.; Miqueu, P.; Chapman, S.; Craciun, L.; Sergeant, R.; Brouard, S.; et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J. Clin. Invest. 2010, 120, 1848–1861. [Google Scholar] [CrossRef]
- Newell, K.A.; Asare, A.; Kirk, A.D.; Gisler, T.D.; Bourcier, K.; Suthanthiran, M.; Burlingham, W.J.; Marks, W.H.; Sanz, I.; Lechler, R.I.; et al. Identification of a b cell signature associated with renal transplant tolerance in humans. J. Clin. Invest. 2010, 120, 1836–1847. [Google Scholar] [CrossRef]
- Patel, R.; Terasaki, P.I. Significance of the positive crossmatch test in kidney transplantation. N. Engl. J. Med. 1969, 280, 735–739. [Google Scholar] [CrossRef]
- Burns, A.M.; Chong, A.S. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive t cell priming. J. Immunol. 2011, 186, 214–221. [Google Scholar] [CrossRef]
- Burns, A.M.; Ma, L.; Li, Y.; Yin, D.; Shen, J.; Xu, J.; Chong, A.S. Memory alloreactive b cells and alloantibodies prevent anti-cd154-mediated allograft acceptance. J. Immunol. 2009, 182, 1314–1324. [Google Scholar]
- Deng, S.; Moore, D.J.; Huang, X.; Lian, M.M.; Mohiuddin, M.; Velededeoglu, E.; Lee, M.K.t.; Sonawane, S.; Kim, J.; Wang, J.; et al. Cutting edge: Transplant tolerance induced by anti-cd45rb requires b lymphocytes. J. Immunol. 2007, 178, 6028–6032. [Google Scholar]
- Battaglia, M.; Roncarolo, M.G. The role of cytokines (and not only) in inducing and expanding t regulatory type 1 cells. Transplantation 2004, 77, S16–S18. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse t helper cell. Iv. Th2 clones secrete a factor that inhibits cytokine production by th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Gregori, S.; Goudy, K.S.; Roncarolo, M.G. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory t cells. Front. Immunol. 2012, 3, 30. [Google Scholar]
- Spits, H.; de Waal Malefyt, R. Functional characterization of human il-10. Int Arch. Allergy Immunol. 1992, 99, 8–15. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(il-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of il-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Brooks, D.G.; Walsh, K.B.; Elsaesser, H.; Oldstone, M.B. Il-10 directly suppresses cd4 but not cd8 t cell effector and memory responses following acute viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 3018–3023. [Google Scholar]
- Levings, M.K.; Sangregorio, R.; Galbiati, F.; Squadrone, S.; de Waal Malefyt, R.; Roncarolo, M.G. Ifn-alpha and il-10 induce the differentiation of human type 1 t regulatory cells. J. Immunol. 2001, 166, 5530–5539. [Google Scholar]
- Tilg, H.; van Montfrans, C.; van den Ende, A.; Kaser, A.; van Deventer, S.J.; Schreiber, S.; Gregor, M.; Ludwiczek, O.; Rutgeerts, P.; Gasche, C.; et al. Treatment of crohn's disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 2002, 50, 191–195. [Google Scholar] [CrossRef]
- Lauw, F.N.; Pajkrt, D.; Hack, C.E.; Kurimoto, M.; van Deventer, S.J.; van der Poll, T. Proinflammatory effects of il-10 during human endotoxemia. J. Immunol. 2000, 165, 2783–2789. [Google Scholar]
- Furukawa, Y.; Becker, G.; Stinn, J.L.; Shimizu, K.; Libby, P.; Mitchell, R.N. Interleukin-10 (il-10) augments allograft arterial disease: Paradoxical effects of il-10 in vivo. Am. J. Pathol. 1999, 155, 1929–1939. [Google Scholar] [CrossRef]
- Zhao, G.; Moore, D.J.; Lee, K.M.; Kim, J.I.; Duff, P.E.; O’Connor, M.R.; Hirohashi, T.; Lei, J.; Yang, M.; Markmann, J.F.; et al. An unexpected counter-regulatory role of il-10 in b-lymphocyte-mediated transplantation tolerance. Am. J. Transplant. 2010, 10, 796–801. [Google Scholar] [CrossRef]
- Tanner, J.E.; Alfieri, C. Epstein-barr virus induces fas (cd95) in t cells and fas ligand in b cells leading to t-cell apoptosis. Blood 1999, 94, 3439–3447. [Google Scholar]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory b cell subset with a unique cd1dhicd5+ phenotype controls t cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Wang, S.; Ko, K.H.; Xu, H.; Zheng, B.J.; Cao, X.; Lu, L. Novel function of b cell-activating factor in the induction of il-10-producing regulatory b cells. J. Immunol. 2010, 184, 3321–3325. [Google Scholar] [CrossRef]
- Ding, Q.; Yeung, M.; Camirand, G.; Zeng, Q.; Akiba, H.; Yagita, H.; Chalasani, G.; Sayegh, M.H.; Najafian, N.; Rothstein, D.M. Regulatory b cells are identified by expression of tim-1 and can be induced through tim-1 ligation to promote tolerance in mice. J. Clin. Invest. 2011, 121, 3645–3656. [Google Scholar] [CrossRef]
- Lotz, M.; Ranheim, E.; Kipps, T.J. Transforming growth factor beta as endogenous growth inhibitor of chronic lymphocytic leukemia b cells. J. Exp. Med. 1994, 179, 999–1004. [Google Scholar] [CrossRef]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. Cd19(+)cd24(hi)cd38(hi) b cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Zha, B.; Wang, L.; Liu, X.; Liu, J.; Chen, Z.; Xu, J.; Sheng, L.; Li, Y.; Chu, Y. Decrease in proportion of cd19+ cd24(hi) cd27+ b cells and impairment of their suppressive function in graves' disease. PLoS One 2012, 7, e49835. [Google Scholar]
- Lee, J.H.; Noh, J.; Noh, G.; Choi, W.S.; Cho, S.; Lee, S.S. Allergen-specific transforming growth factor-beta-producing cd19+cd5+ regulatory b-cell (br3) responses in human late eczematous allergic reactions to cow's milk. J. Interferon Cytokine Res. 2011, 31, 441–449. [Google Scholar] [CrossRef]
- Noh, J.; Choi, W.S.; Noh, G.; Lee, J.H. Presence of foxp3-expressing cd19(+)cd5(+) b cells in human peripheral blood mononuclear cells: Human cd19(+)cd5(+)foxp3(+) regulatory b cell (breg). Immune Netw. 2010, 10, 247–249. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates il-10-producing regulatory b cell subset characterized by cd1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef]
- Matsushita, T.; Yanaba, K.; Bouaziz, J.D.; Fujimoto, M.; Tedder, T.F. Regulatory b cells inhibit eae initiation in mice while other b cells promote disease progression. J. Clin. Invest. 2008, 118, 3420–3430. [Google Scholar]
- Kitabayashi, A.; Hirokawa, M.; Miura, A.B. The role of interleukin-10 (il-10) in chronic b-lymphocytic leukemia: Il-10 prevents leukemic cells from apoptotic cell death. Int. J. Hematol. 1995, 62, 99–106. [Google Scholar] [CrossRef]
- Natarajan, P.; Singh, A.; McNamara, J.T.; Secor, E.R., Jr.; Guernsey, L.A.; Thrall, R.S.; Schramm, C.M. Regulatory b cells from hilar lymph nodes of tolerant mice in a murine model of allergic airway disease are cd5+, express tgf-beta, and co-localize with cd4+foxp3+ t cells. Mucosal Immunol. 2012, 5, 691–701. [Google Scholar] [CrossRef]
- Vitale, G.; Mion, F.; Pucillo, C. Regulatory b cells: Evidence, developmental origin and population diversity. Mol. Immunol. 2010, 48, 1–8. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral cd4+cd25- naive t cells to cd4+cd25+ regulatory t cells by tgf-beta induction of transcription factor foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Marie, J.C.; Letterio, J.J.; Gavin, M.; Rudensky, A.Y. Tgf-beta1 maintains suppressor function and foxp3 expression in cd4+cd25+ regulatory t cells. J. Exp. Med. 2005, 201, 1061–1067. [Google Scholar] [CrossRef]
- Zheng, S.G. Regulatory t cells vs th17: Differentiation of th17 versus treg, are the mutually exclusive? Am. J. Clin. Exp. Immunol. 2013, 2, 94–106. [Google Scholar]
- Parsons, R.F.; Vivek, K.; Redfield, R.R., 3rd; Migone, T.S.; Cancro, M.P.; Naji, A.; Noorchashm, H. B-lymphocyte homeostasis and blys-directed immunotherapy in transplantation. Transplant. Rev. (Orlando) 2010, 24, 207–221. [Google Scholar] [CrossRef]
- Heidt, S.; Hester, J.; Shankar, S.; Friend, P.J.; Wood, K.J. B cell repopulation after alemtuzumab induction-transient increase in transitional b cells and long-term dominance of naive b cells. Am. J. Transplant. 2012, 12, 1784–1792. [Google Scholar] [CrossRef]
- Todeschini, M.; Cortinovis, M.; Perico, N.; Poli, F.; Innocente, A.; Cavinato, R.A.; Gotti, E.; Ruggenenti, P.; Gaspari, F.; Noris, M.; et al. In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces b cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-hla antibody development. J. Immunol. 2013, 191, 2818–2828. [Google Scholar] [CrossRef]
- Parsons, R.F.; Vivek, K.; Redfield, R.R.; Migone, T.S.; Cancro, M.P.; Naji, A.; Noorchashm, H. B-cell tolerance in transplantation: Is repertoire remodeling the answer? Expert Rev. Clin. Immunol. 2009, 5, 703–723. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
San Segundo, D.; López-Hoyos, M.; Arias, M. Regulatory B-Cells in Transplantation. Antibodies 2013, 2, 587-597. https://doi.org/10.3390/antib2040587
San Segundo D, López-Hoyos M, Arias M. Regulatory B-Cells in Transplantation. Antibodies. 2013; 2(4):587-597. https://doi.org/10.3390/antib2040587
Chicago/Turabian StyleSan Segundo, David, Marcos López-Hoyos, and Manuel Arias. 2013. "Regulatory B-Cells in Transplantation" Antibodies 2, no. 4: 587-597. https://doi.org/10.3390/antib2040587




