IgE and Drug Allergy: Antibody Recognition of ‘Small’ Molecules of Widely Varying Structures and Activities
Abstract
:1. Introduction: IgE Antibody Recognition of Drugs
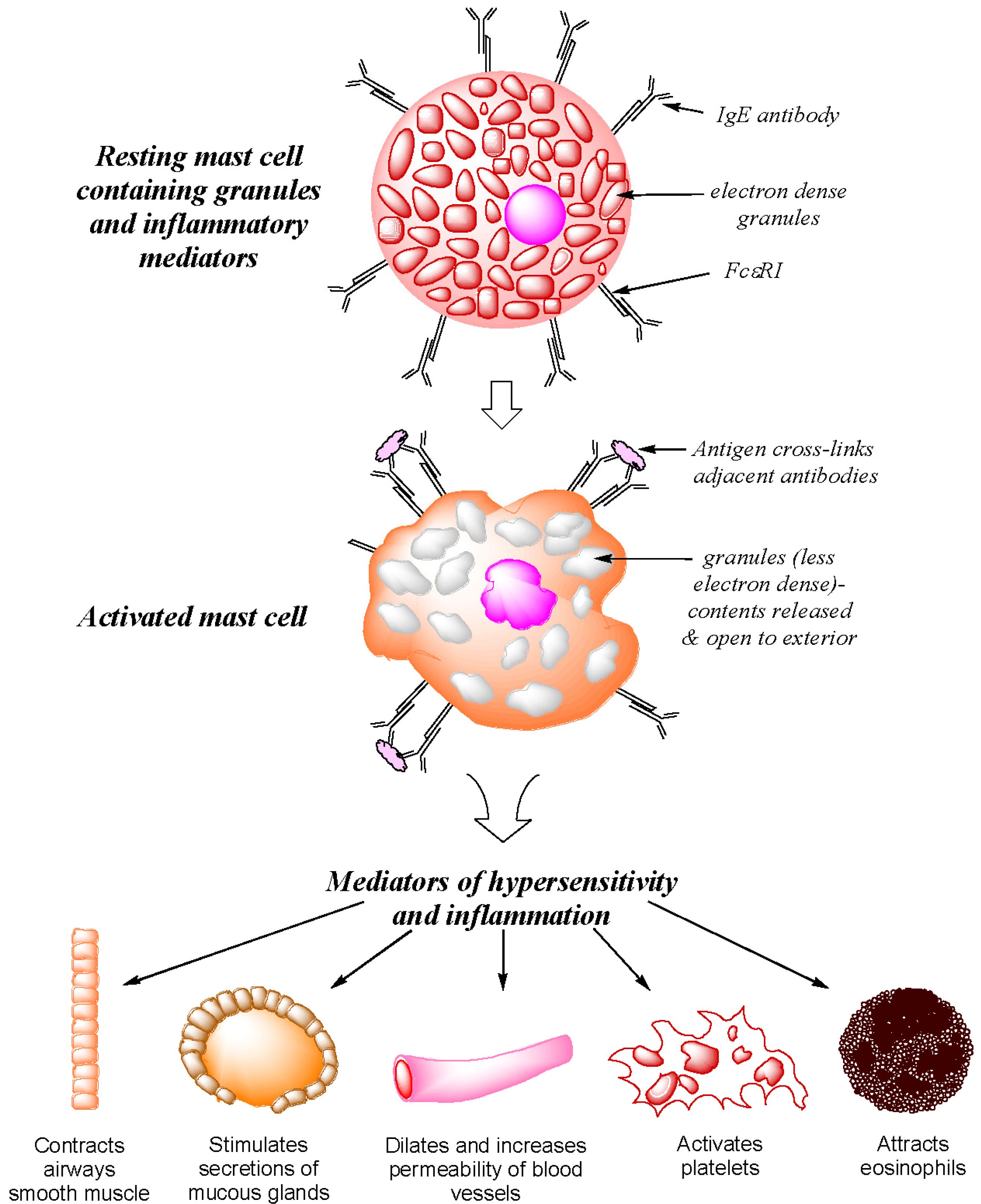
2. Type I Immediate Hypersensitivity: IgE Antibodies, Mast Cells, Mediators and Clinical Reactions
3. Recognition of β-Lactam Antibiotics by IgE Antibodies
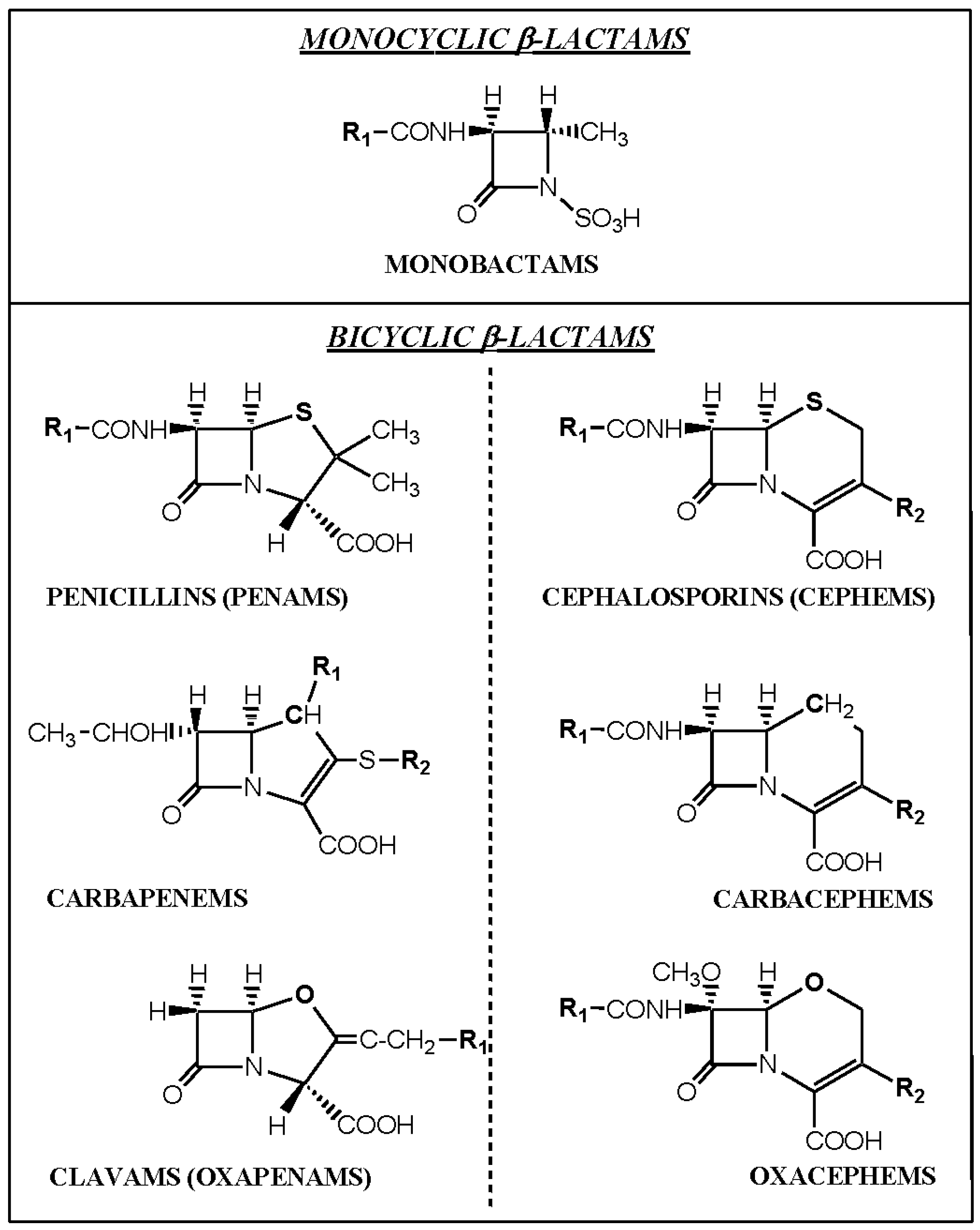
3.1. IgE-Mediated Clinical Responses to Penicillins
3.2. Reaction of Penicillins with IgE Antibodies and Identification of Allergenic Determinants
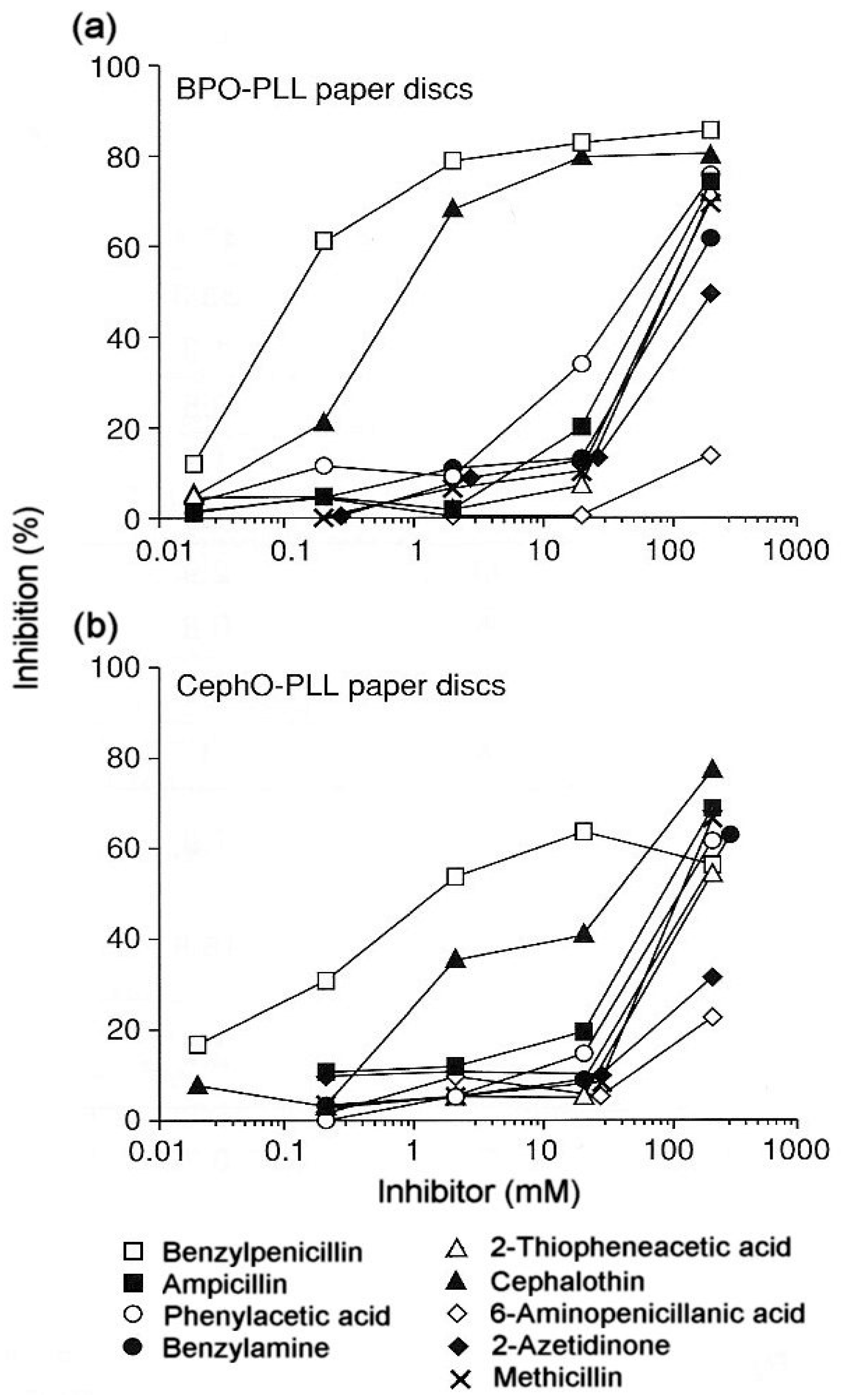

3.3. Recognition of Cephalosporin R1 and R2 Side Chains by IgE Antibodies
3.4. Summary of IgE Antibody Recognition of the Bicyclic β-Lactams, Penicillins and Cephalosporins
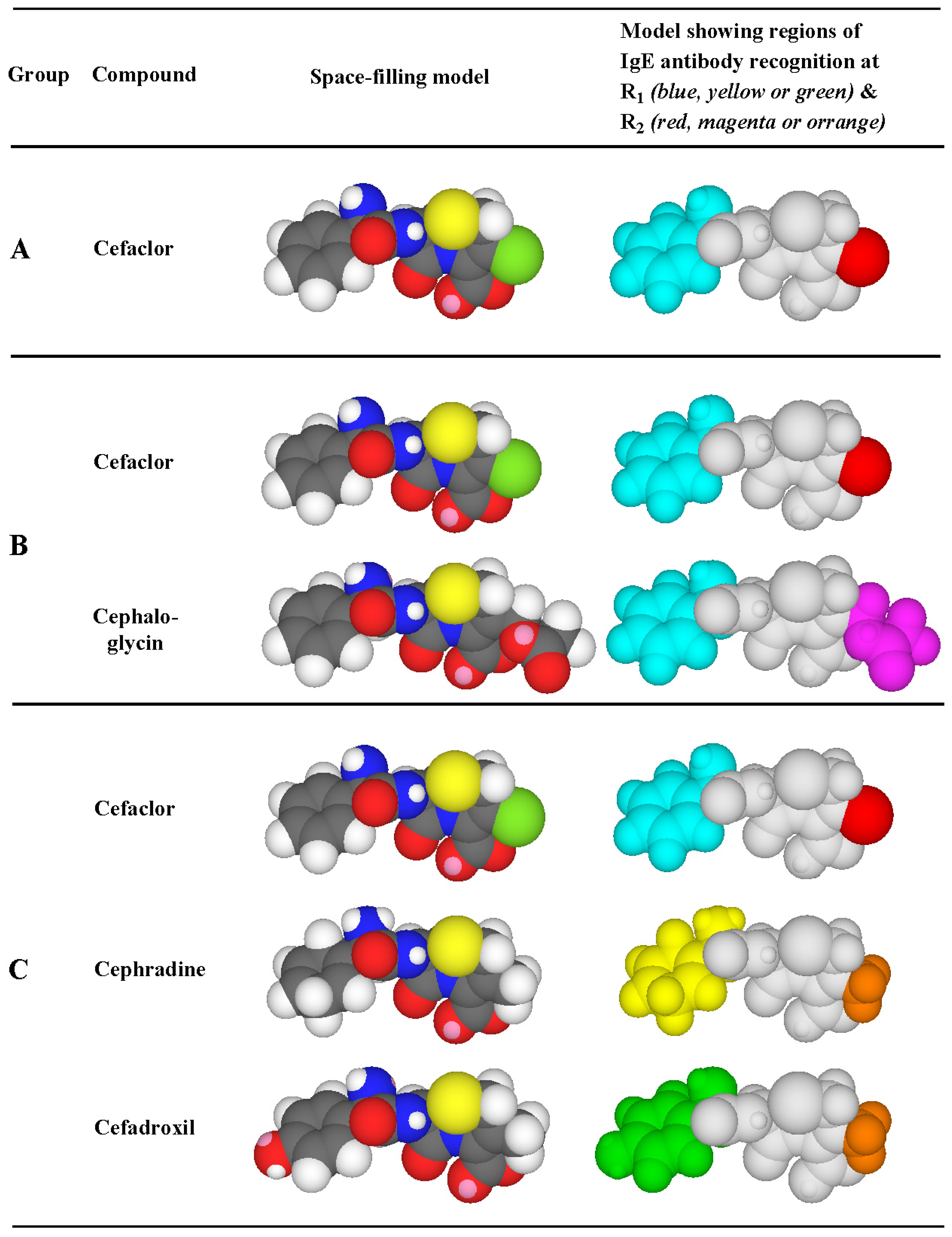
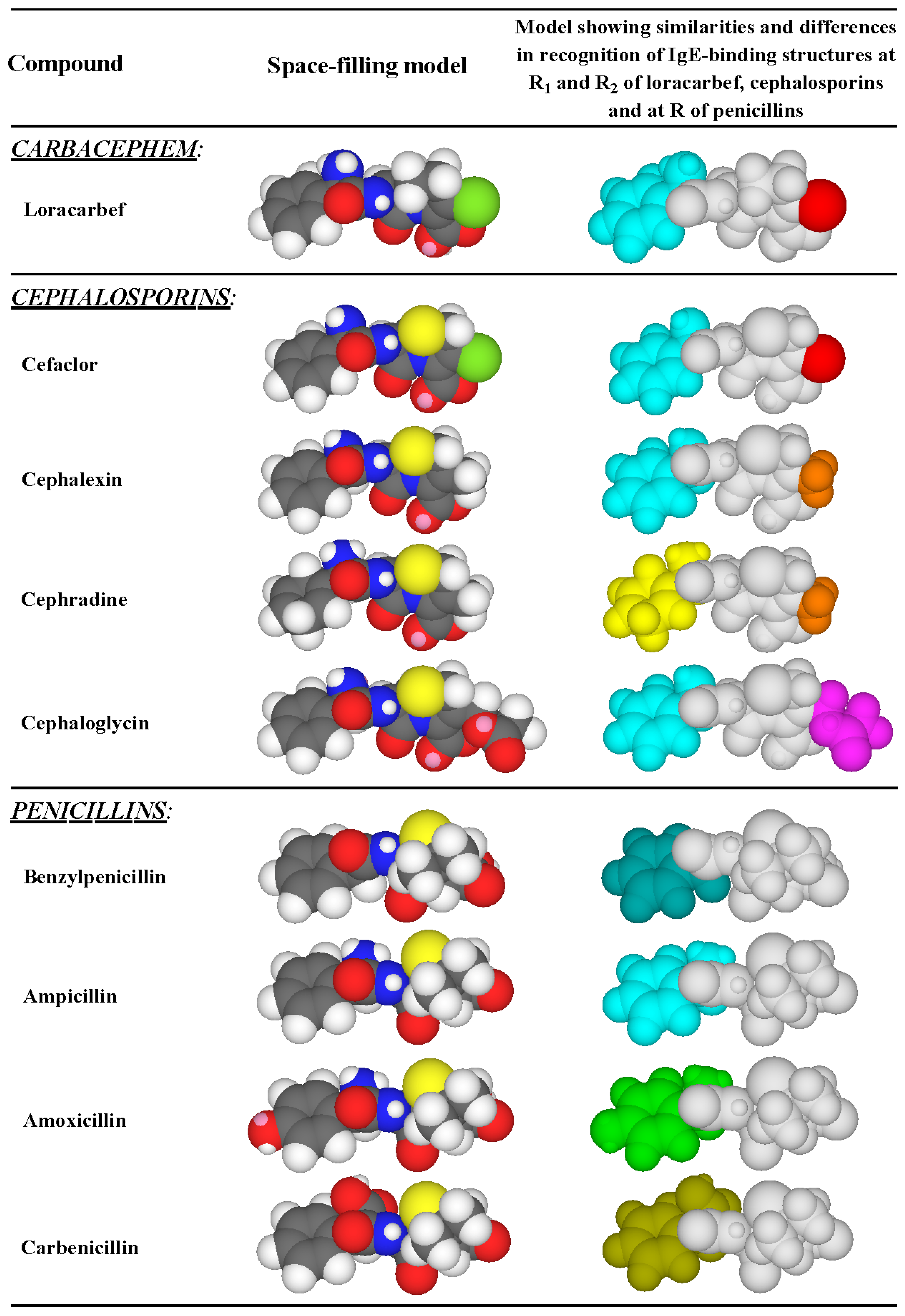
4. Immediate Allergic Reactions to Sulfamethoxazole

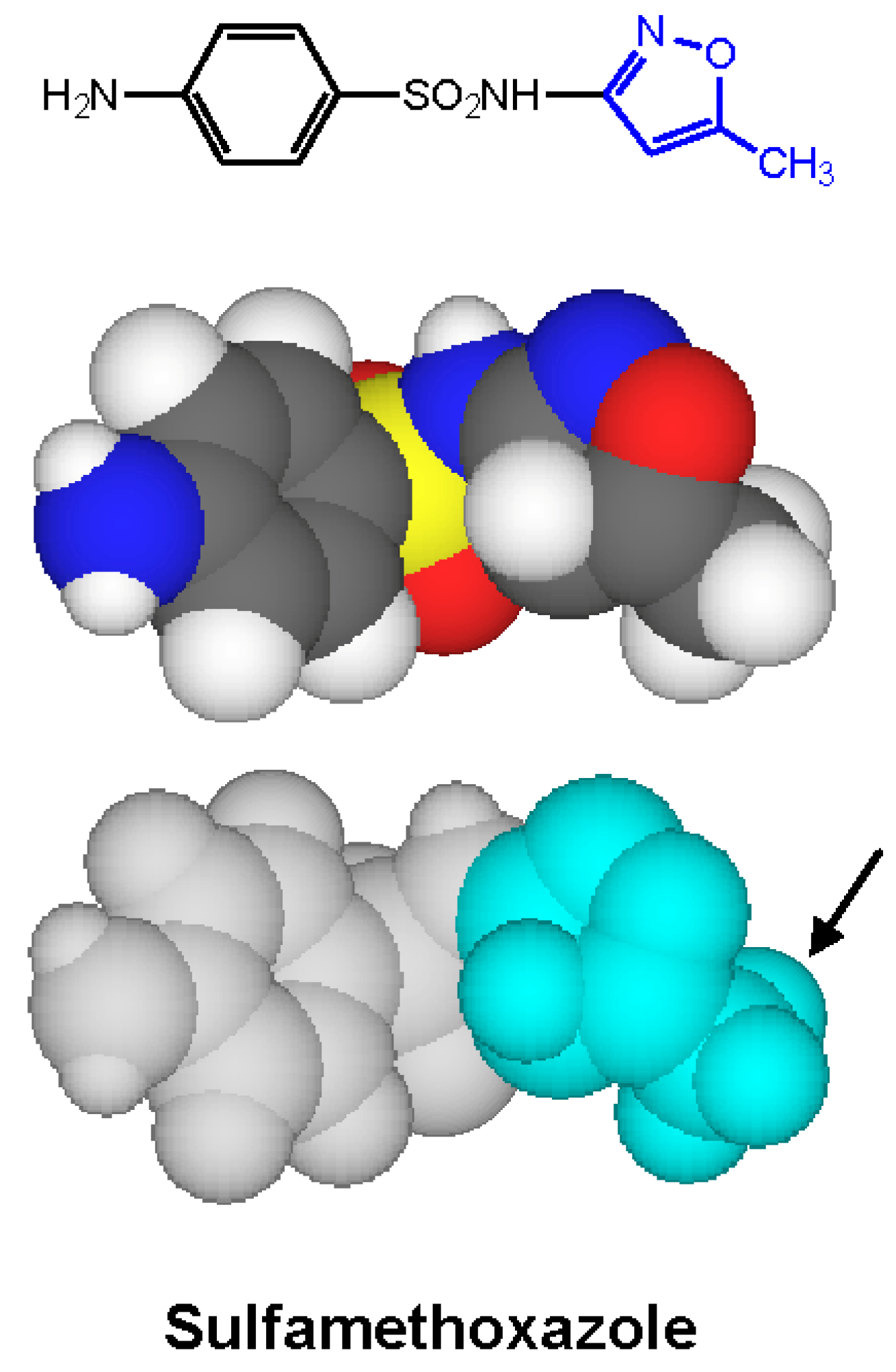
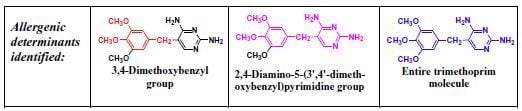
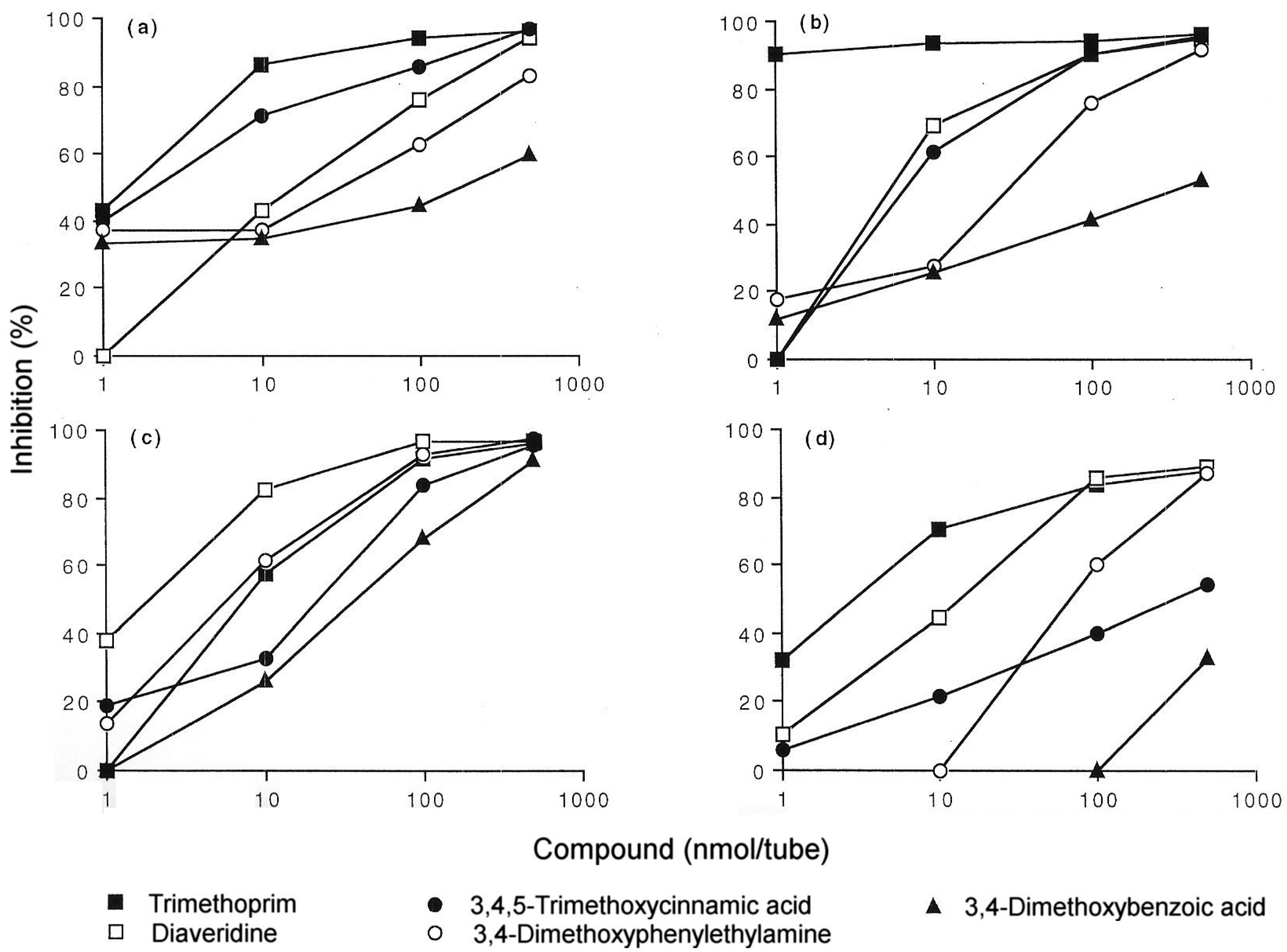
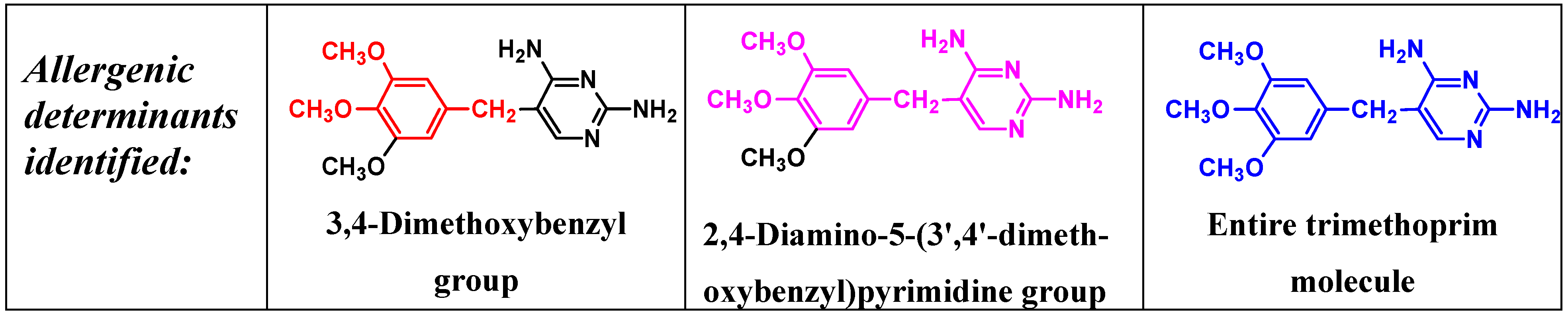
5. IgE antibody-Mediated Reactions to Trimethoprim
6. Anaphylaxis to Neuromuscular Blocking Drugs
6.1. IgE Antibodies, NMBDs and Substituted Ammonium Groups

| Trialkylamines | NR3 |
| Tetraalkylammonium salts | N+R4 , RN+(Rי)3 |
| Choline | (CH3) 3N+CH2CH2OH |
| Acetylcholine | (CH3) 3N+CH2CH2OCOCH3 |
| Promethazine | 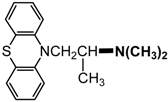 |
| Neostigmine | 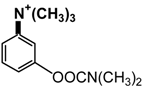 |
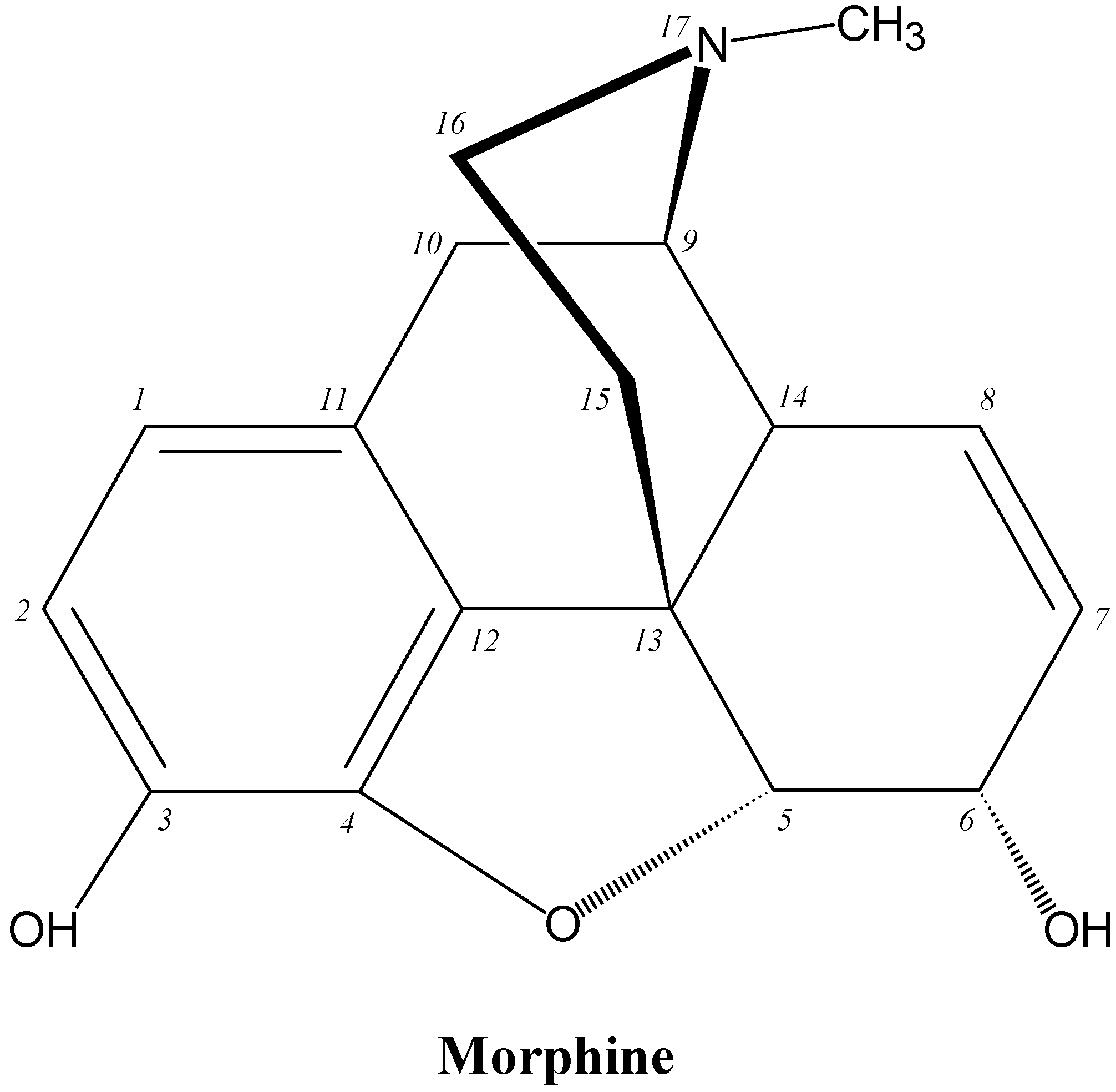
| Morphine | 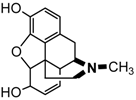 |
6.2. In Vitro Tests to Help Confirm Diagnosis of Anaphylaxis to NMBDs and Cross-Reactivity of NMBDs
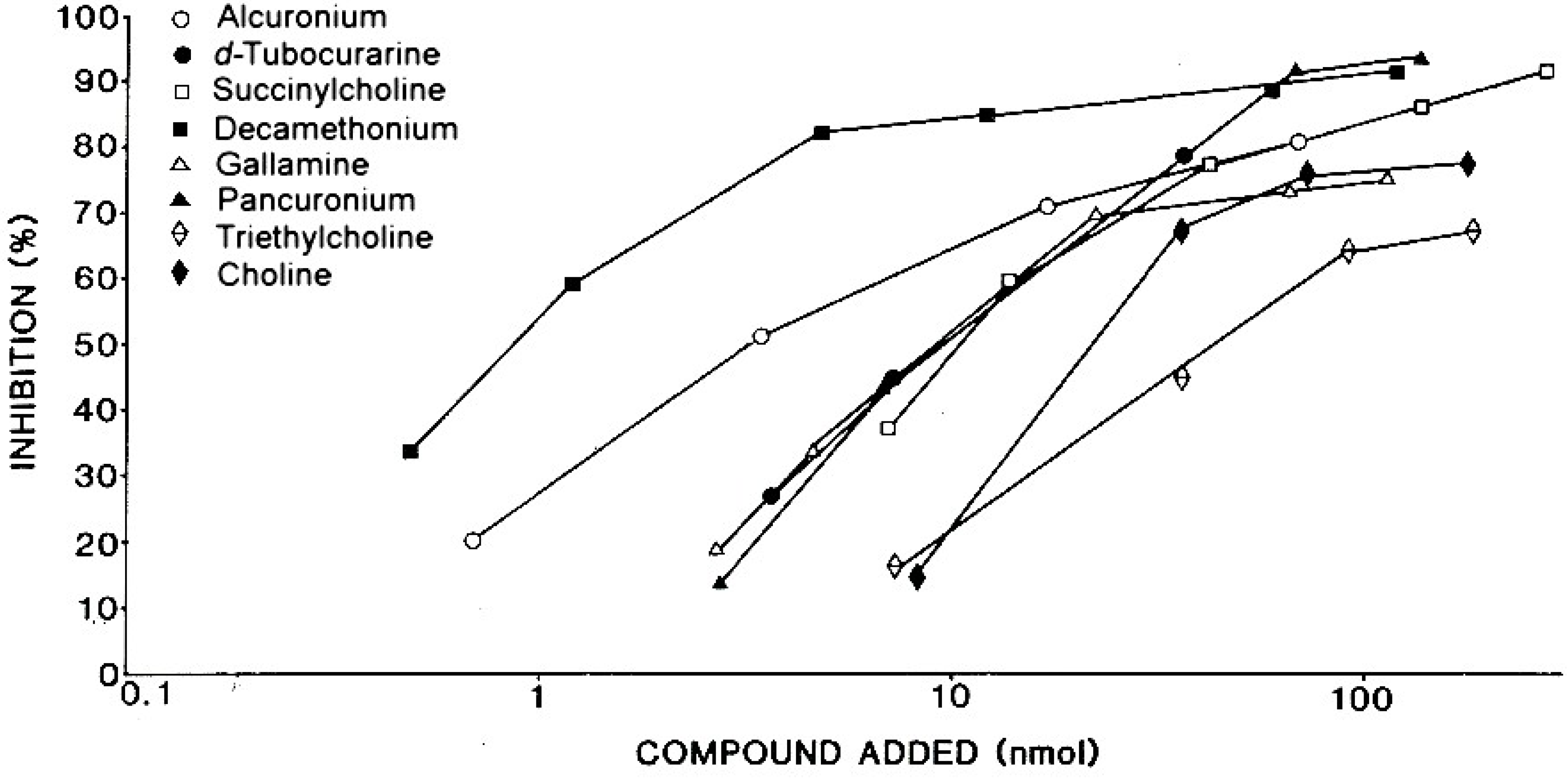
| Patient’s Serum 3 | Amount of drug 1 for 50% inhibition 2 | |||||
|---|---|---|---|---|---|---|
| Succinylcholine | Decamethonium | d-Tubocurarine | Pancuronium | Gallamine | Alcuronium | |
| 1 | 4.7 | 0.5 | 1.3 | 17.0 | 1.6 | 88.0 |
| 2 | 6.0 | 0.9 | 1.8 | 4.7 | 6.8 | 37.0 |
| 3 | 9.8 | 1.9 | 0.7 | 7.7 | 8.4 | 58.0 |
| 4 | 7.3 | 1.3 | 10.0 | 14.0 | 6.9 | 41.0 |
| 5 | 11.0 | 0.8 | 9.7 | 9.8 | 9.6 | 3.3 |
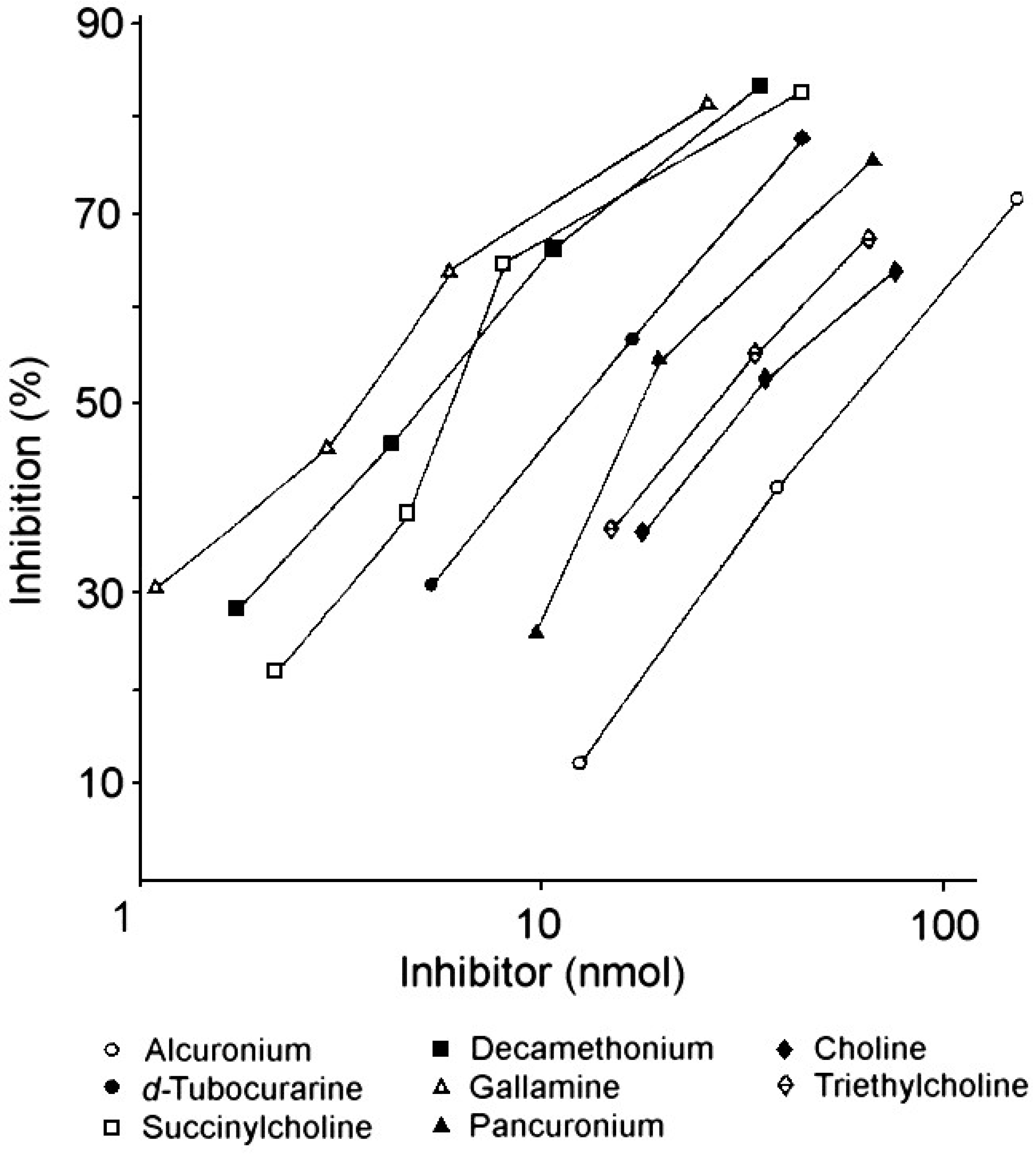
| Drug or analog | Amount (nmol) for 50% inhibition 1 of IgE 2 binding |
|---|---|
| Gallamine | 4.2 |
| Succinylcholine | 8.1 |
| Decamethonium | 7.3 |
| d-Tubocurarine | 12.0 |
| Pancuronium | 14.0 |
| Alcuronium | 74.0 |
| Choline | 27.0 |
| Triethylcholine | 22.0 |
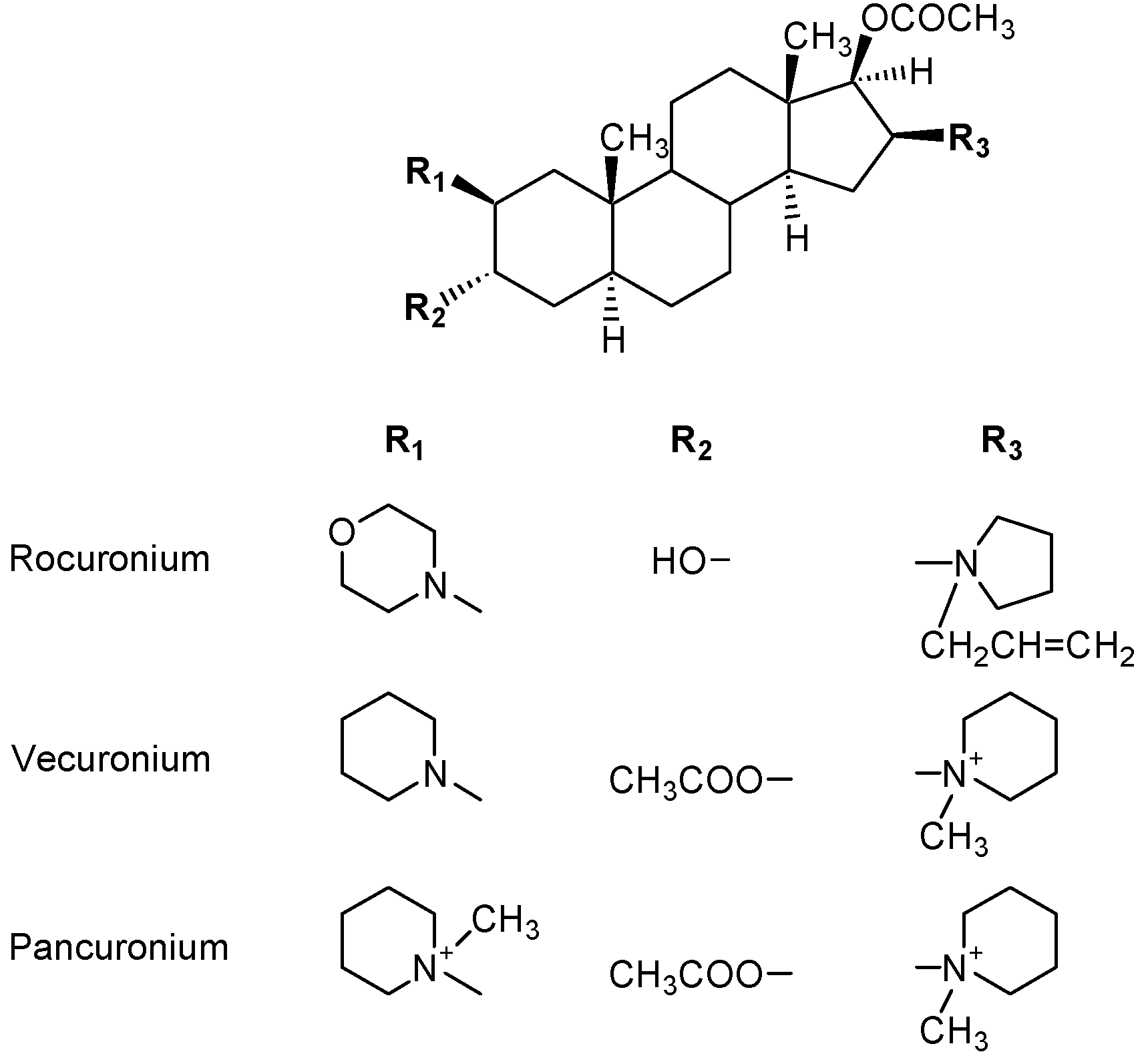
7. Recognition of Polyamines and PrimaryAmines by IgE Antibodies
| Compounds | Structure | IC30 (mM) 1 | IC50 (mM) 1 | Inhibition (%) at 40 mM 1 | |||
|---|---|---|---|---|---|---|---|
| Serum 1 | Serum 2 | Serum 1 | Serum 2 | Serum 1 | Serum 2 | ||
| Poly-L-lysine49 | (L-Lysine)49 | 2 | 3.2 | 93 | |||
| Poly-L-lysine18 | (L-Lysine)18 | 6.1 | 0.18 | 19 | 0.39 | 66 | 94 |
| L-Lysine | NH2(CH2)4CH(NH2)COOH | 3.1 | 90 | 13 | 130 | 76 | 0 |
| D-Lysine | NH2(CH2)4CH(NH2)COOH | 3.1 | 90 | 12 | 140 | 68 | 0 |
| N-Methyl-L-lysine | CH3NH(CH2)4CH(NH2)COOH | 50 | 105 | > 200 | > 200 | 26 | 9 |
| Ethylamine | CH3CH2NH2 | 4.2 | 81 | 19 | 130 | 54 | 5 |
| 1,3-Diaminopropane | NH2(CH2)3NH2 | 7.1 | 18 | 29 | 30 | 56 | 57 |
| 1,5-Diaminopentane | NH2(CH2)5NH2 | 2.6 | 20 | 5.2 | 36 | 62 | 54 |
| 4-Aminomethyl-1,8-octanediamine | NH2(CH2)4CH(CH2NH2)(CH2)3NH2 | 6 | 8.1 | 18 | 13 | 65 | 84 |
| Spermine | NH2(CH2)3NH(CH2)4NH(CH2)3NH2 | 100 | 16 | > 200 | 21 | 19 | 77 |
| Ethanolamine | HO(CH2)2NH2 | 45 | 75 | > 200 | 130 | 28 | 14 |
| N-Methylethanolamine | HO(CH2)2NHCH3 | 90 | 75 | > 200 | 135 | 20 | 13 |
| N,N-Dimethylethanolamine | HO(CH2)2N(CH3)2 | 19 | 80 | 39 | > 200 | 53 | 17 |
| N,N,N',N'-Tetramethylethylenediamine | (CH3)2NCH2CH2N(CH3)2 | 100 | 90 | > 200 | 190 | 15 | 8 |
| Choline | (CH3)3N(OH)(CH2)2OH | 110 | 190 | > 200 | > 200 | 12 | 17 |
| Glycine | NH2CH2COOH | 115 | > 200 | > 200 | > 200 | 8 | 0 |
| 4-Aminobutyric acid | NH2(CH2)3COOH | 61 | > 200 | 120 | > 200 | 18 | 0 |
| 5-Amino-n-valeric acid | NH2(CH2)4COOH | 10 | > 200 | 35 | > 200 | 52 | 0 |
| 6-Aminocaproic acid | NH2(CH2)5COOH | 21 | > 200 | > 200 | > 200 | 37 | 0 |
| 2,6-Diaminopimelic acid | HOOCCH(NH2)(CH2)3CH(NH2)COOH | 85 | > 200 | 12 | |||
| L-Lysine methyl ester | NH2(CH2)4CH(NH2)COOCH3 | 18 | 45 | 46 | |||
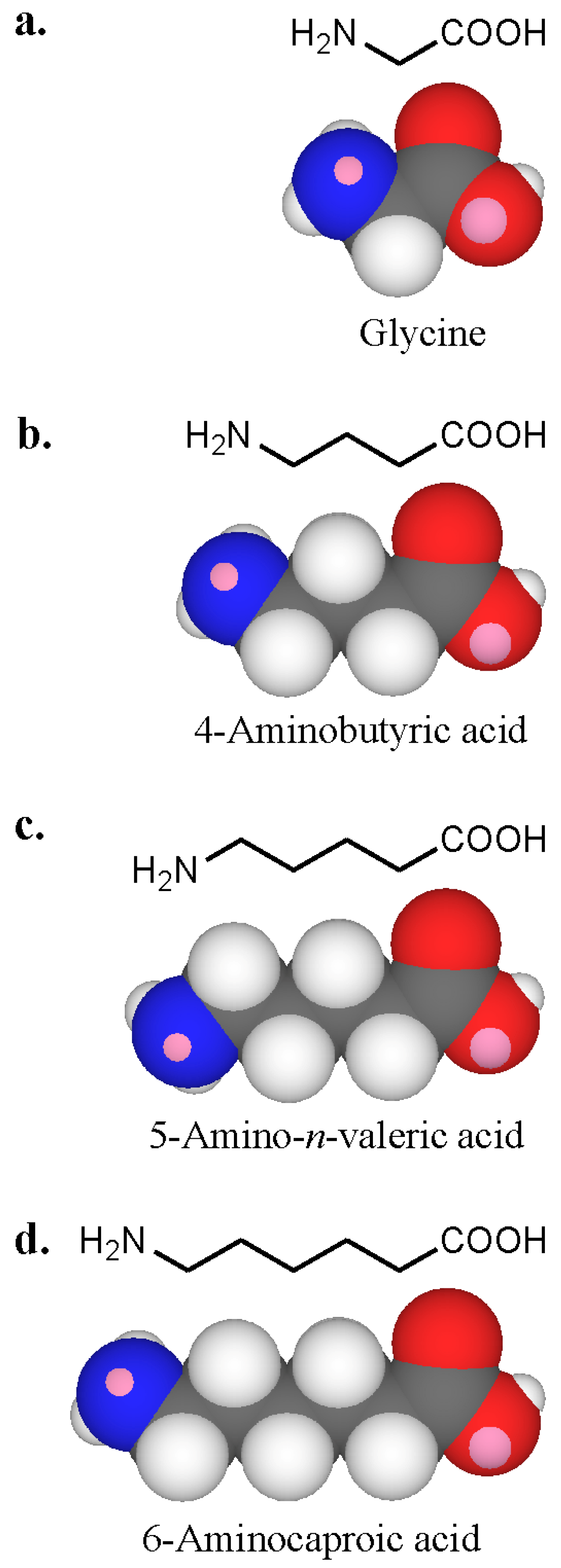
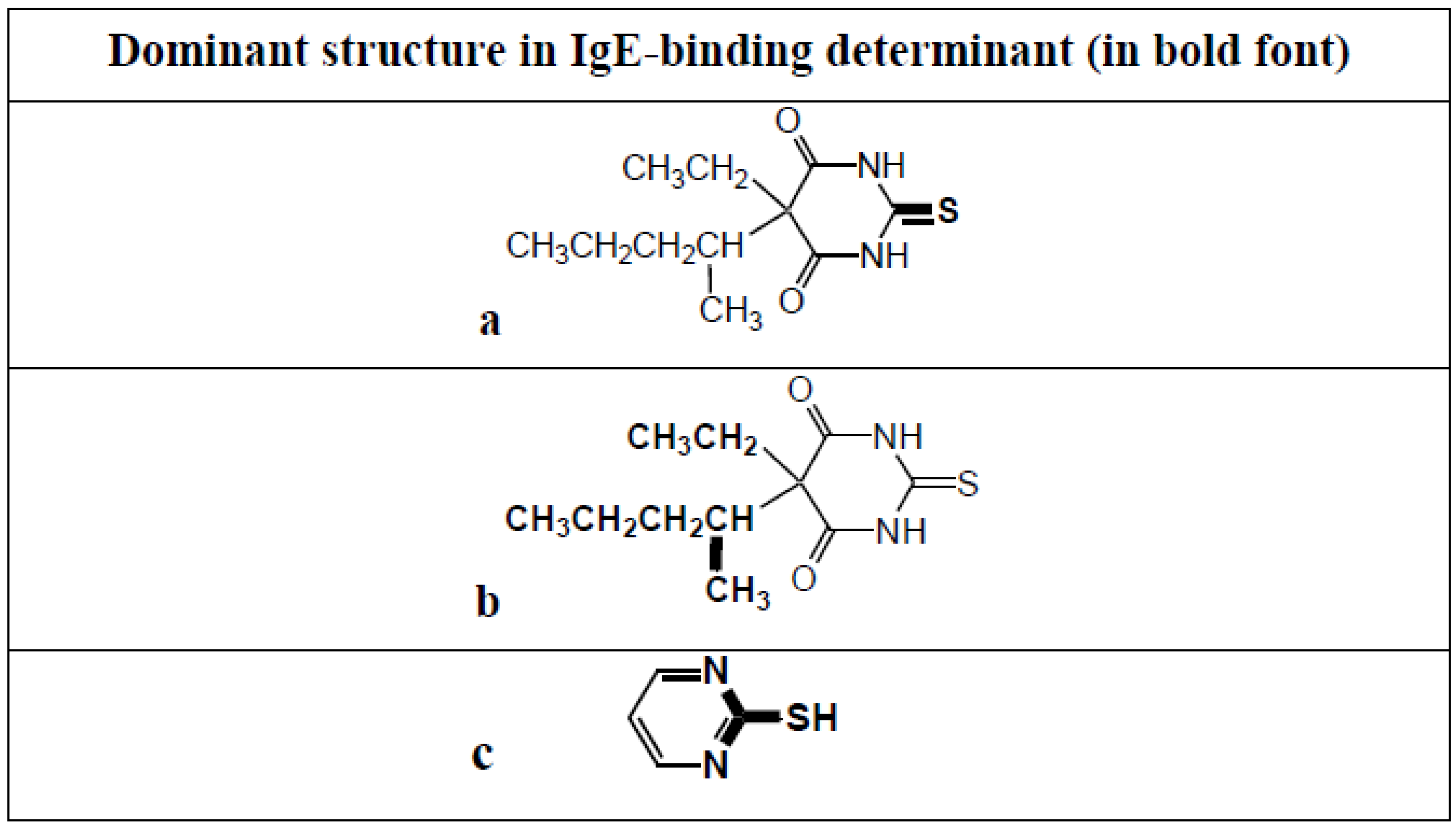
8. Anaphylaxis to the Induction Agent Thiopentone and Identification of Allergenic Determinants
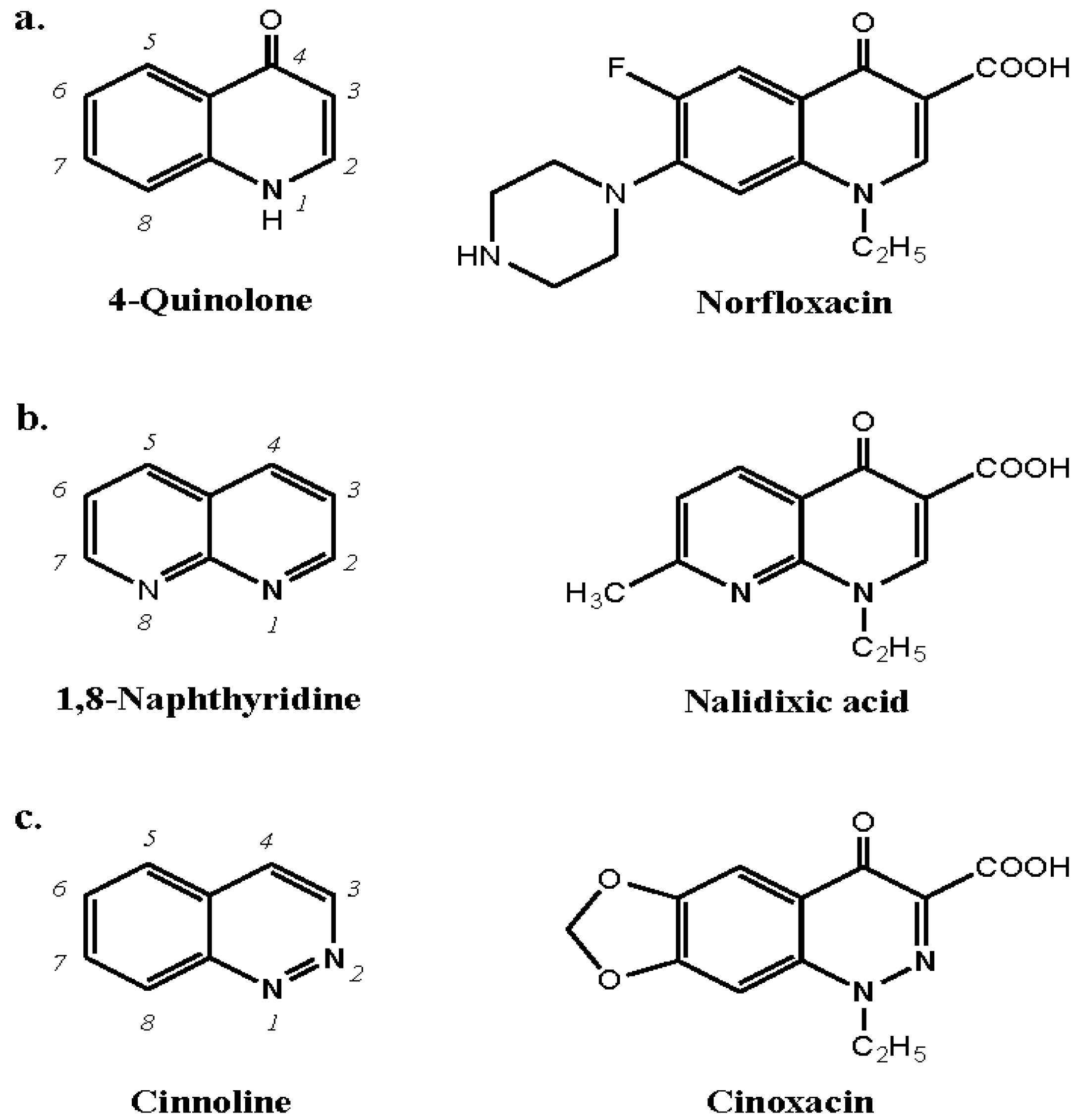
9. Less Well Studied IgE Antibody Responses to Other Drugs
9.1. Quinolones
9.2. Chlorhexidine
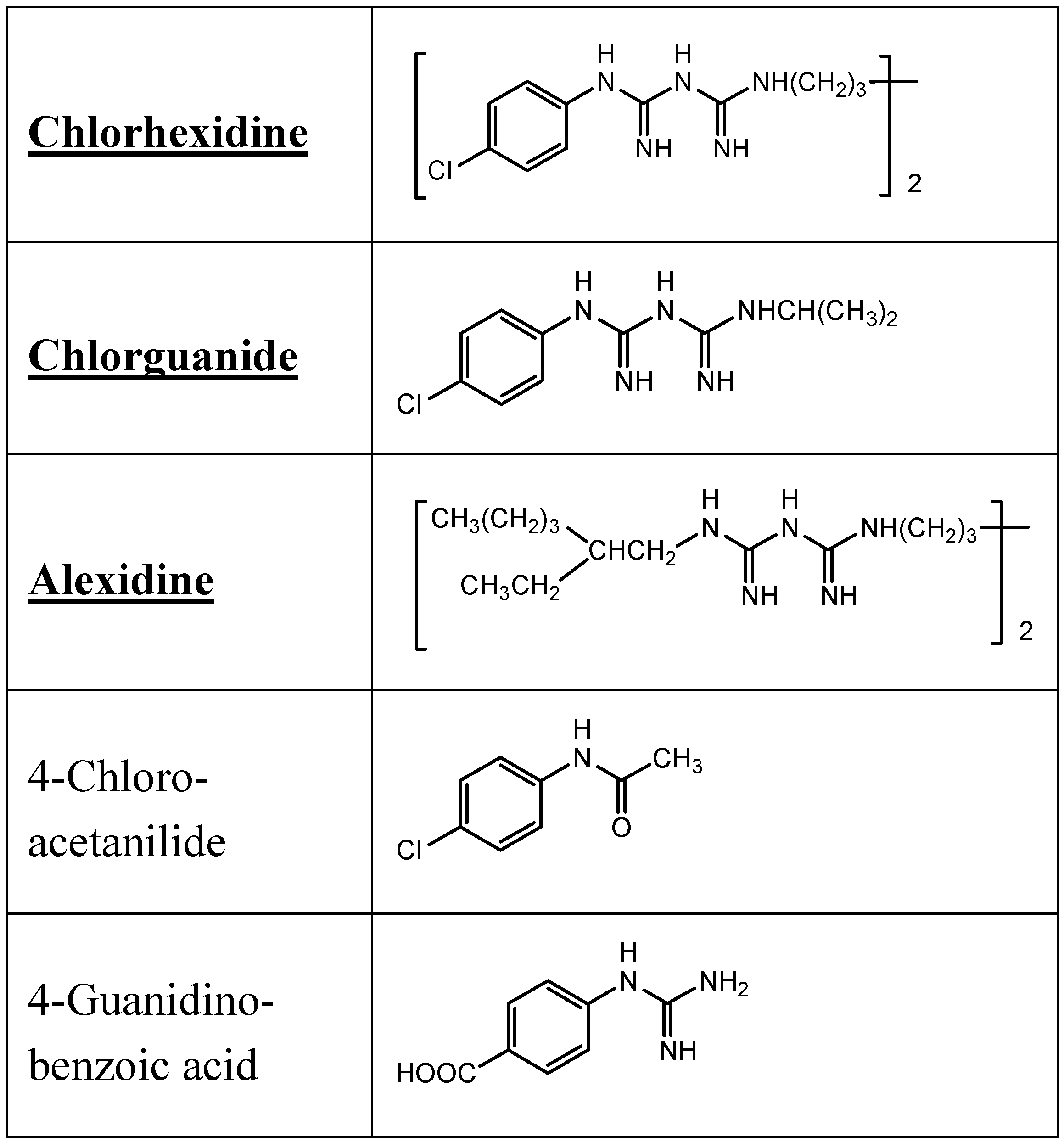
9.3. Opioid Analgesics
9.4. Non-Steroidal Antiinflammatory Drugs
9.5. Macrolide Antibiotics
9.6. Rifamycins
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kabat, E.A.; Mayer, M.M. Experimental Immunochemistry, 2nd ed.; Charles C. Thomas: Springfield, IL, USA, 1961. [Google Scholar]
- Landsteiner, K. The Specificity of Serological Reactions; Dover: New York, NY, USA, 1962. [Google Scholar]
- Trier, N.H.; Hansen, P.R.; Houen, G. Production and characterization of peptide antibodies. Methods 2012, 56, 136–144. [Google Scholar] [CrossRef]
- Baldo, B.A. Immunochemical perspectives of allergy: In the steps of Karl Landsteiner. In Molecular Approaches to the Study of Allergens. Monographs in Allergy; Karger: Basel, Switzerland, 1990; Volume 28. [Google Scholar]
- Baldo, B.A.; Pham, N.H. Drug Allergy: Clinical Aspects, Diagnosis, Mechanisms, Structure-Activity Relationships; Springer: New York, NY, USA, 2013. [Google Scholar]
- Kaliner, M.; Austen, K.F. Immunological release of chemical mediators from human tissues. Ann. Rev. Pharmacol. 1975, 15, 177–189. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; Beavil, R.L.; McCloskey, N.; Coker, H.A.; Fear, D.; Smurthwaite, L. The biology of IgE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef]
- Ogawa, Y.; Grant, J.A. Mediators of anaphylaxis. Immunol. Allergy Clin. North. Am. 2007, 27, 249–260. [Google Scholar] [CrossRef]
- Fisher, M.M.; Baldo, B.A. Acute anaphylactic reactions. Med. J. Aust. 1988, 149, 34–38. [Google Scholar]
- Mendelson, L.M. Adverse reactions to β-lactam antibiotics. Immunol. Allergy Clin. North. Am. 1998, 18, 745–757. [Google Scholar] [CrossRef]
- Salkind, A.R.; Cuddy, P.G.; Foxworth, J.W. The rational clinical examination. Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy. JAMA 2001, 285, 2498–2505. [Google Scholar] [CrossRef]
- Dewdney, J.M. Immunology of the antibiotics. In The Antigens; Sela, M., Ed.; Academic Press: New York, NY, USA, 1977; Volume 4, pp. 73–228. [Google Scholar]
- Levine, B.B. Immunochemical mechanisms of drug allergy. In Textbook of Immunopathology, 2nd ed.; Miescher, P.A., Muller-Eberhard, H.J., Eds.; Grune and Stratton: New York, NY, USA, 1976; Volume 2, pp. 403–419. [Google Scholar]
- Parker, C.W. Drug allergy. In Clinical Immunology; Parker, C.W., Ed.; Saunders: Philadelphia, PA, USA, 1980; Volume 2, pp. 1219–1260. [Google Scholar]
- Levine, B.B.; Redmond, A.P. Minor haptenic determinant-specific reagins of penicillin hypersensitivity in man. Int. Arch. Allergy Appl. Immunol. 1969, 35, 445–455. [Google Scholar] [CrossRef]
- Harle, D.G.; Baldo, B.A. Identification of penicillin allergenic determinants that bind IgE antibodies in the sera of subjects with penicillin allergy. Mol. Immunol. 1990, 27, 1063–1071. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H.; Weiner, J. Detection and side-chain specificity of IgE antibodies to flucloxacillin in allergic subjects. J. Mol. Recognit. 1995, 8, 171–177. [Google Scholar] [CrossRef]
- Zhao, Z.; Baldo, B.A.; Baumgart, K.W.; Mallon, D.F.J. Fine structural recognition specificities of IgE antibodies distinguishing amoxicilloyl and amoxicillanyl determinants in allergic subjects. J. Mol. Recognit. 2001, 14, 300–307. [Google Scholar] [CrossRef]
- Zhao, Z.; Baldo, B.A.; Rimmer, J. β-Lactam allergenic determinants: fine structural recognition of a cross-reacting determinant on benzylpenicillin and cephalothin. Clin. Exp. Allergy 2002, 32, 1644–1650. [Google Scholar] [CrossRef]
- Harle, D.G.; Baldo, B.A. Drugs as allergens: An immunoassay for detecting IgE antibodies to cephalosporins. Int. Arch. Allergy Appl. Immunol. 1990, 92, 439–444. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T.; Newton, G.G.F.; Abraham, E.P. Products of aminolysis and enzymic hydrolysis of the cephalosporins. Biochem. J. 1970, 116, 371–384. [Google Scholar]
- Faraci, W.S.; Pratt, R.F. Elimination of a good leaving group from the 3'-position of a cephalosporin need not be concerted with β-lactam ring opening: TEM-2 β-lactamase-catalysed hydrolysis of pyridine -2-azo-4'-(N',N'-dimethylaniline) cephalosporin (PADAC) and of cephaloridine. J. Am. Chem. Soc. 1984, 106, 1489–1490. [Google Scholar] [CrossRef]
- Pham, N.H.; Baldo, B.A. β-Lactam drug allergens: Fine structural recognition patterns of cephalosporin- reactive IgE antibodies. J. Mol. Recognit. 1996, 9, 287–296. [Google Scholar] [CrossRef]
- Harle, D.G.; Baldo, B.A.; Wells, J.V. Drugs as allergens: Detection and combining site specificities of IgE antibodies to sulfamethoxazole. Mol. Immunol. 1988, 25, 1347–1354. [Google Scholar] [CrossRef]
- Harle, D.G.; Baldo, B.A.; Smal, M.A.; Van Nunen, S.A. An immunoassay for the detection of IgE antibodies to trimethoprim in the sera of allergic patients. Clin. Allergy 1987, 17, 209–216. [Google Scholar] [CrossRef]
- Smal, M.A.; Baldo, B.A.; Harle, D.G. Drugs as allergens: The molecular basis of IgE binding to trimethoprim. Allergy 1988, 43, 184–191. [Google Scholar] [CrossRef]
- Pham, N.H.; Baldo, B.A.; Manfredi, M.; Zerboni, R. Fine structural specificity differences of trimethoprim allergenic determinants. Clin. Exp. Allergy 1996, 26, 1155–1160. [Google Scholar] [CrossRef]
- Fisher, M.M.; Baldo, B.A. Anaphylaxis during anaesthesia: Current aspects of diagnosis and prevention. Eur. J. Anaesthesiol. 1994, 11, 263–284. [Google Scholar]
- Mertes, P.M.; Alla, F.; Trechot, P.; Auroy, Y.; Jougla, E. Anaphylaxis during anesthesia in France: An 8-year national survey. J. Allergy Clin. Immunol. 2011, 128, 366–373. [Google Scholar] [CrossRef]
- Fisher, M.M.; Munro, I. Life-threatening anaphylactoid reactions to muscle relaxants. Anesth. Analg. 1983, 62, 559–564. [Google Scholar]
- Baldo, B.A.; Fisher, M.M. Substituted ammonium ions as allergenic determinants in drug allergy. Nature 1983, 306, 262–264. [Google Scholar] [CrossRef]
- Baldo, B.A.; Fisher, M.M. Anaphylaxis to muscle relaxant drugs: Cross-reactivity and molecular basis of binding of IgE antibodies detected by radioimmunoassay. Mol. Immunol. 1983, 20, 1393–1400. [Google Scholar] [CrossRef]
- Baldo, B.A.; Fisher, M.M. Mechanisms of IgE-dependent anaphylaxis to anaesthetic drugs. Ann. Fr. Anesth. Reanim. 1993, 12, 131–140. [Google Scholar] [CrossRef]
- Baldo, B.A.; Fisher, M.M.; Pham, N.H. On the origin and specificity of antibodies to neuromuscular blocking (muscle relaxant) drugs: an immunochemical perspective. Clin. Exp. Allergy 2009, 39, 325–344. [Google Scholar]
- Didier, A.; Cador, D.; Bongrand, P.; Furstoss, R.; Fourneron, P.; Senft, M.; Philip-Joet, F.; Charpin, D.; Charpin, J.; Vervloet, D. Role of quaternary ammonium ion determinants in allergy to muscle relaxants. J. Allergy Clin. Immunol. 1987, 79, 578–584. [Google Scholar] [CrossRef]
- Floorvaag, E.; Johansson, S.G.O.; Oman, H.; Venemalm, L.; Degerbeck, F.; Dybendal, T.; Lundberg, M. Prevalence of IgE antibodies to morphine. Relation to the high and low incidences of NMBA anaphylaxis in Norway and Sweden, respectively. Acta Anaesthesiol. Scand. 2005, 49, 437–444. [Google Scholar] [CrossRef]
- Baldo, B.A.; Fisher, M.M. Detection of serum IgE antibodies that react with alcuronium and tubocurarine after lifethreatening reactions to muscle-relaxant drugs. Anaesth. Intens. Care 1983, 11, 194–197. [Google Scholar]
- Harle, D.G.; Baldo, B.A.; Fisher, M.M. Assays for, and cross-reactivities of, IgE antibodies to the muscle relaxants gallamine, decamethonium and succinylcholine (suxamethonium). J. Immunol. Methods 1985, 78, 293–305. [Google Scholar] [CrossRef]
- Baldo, B.A.; McDonnell, N.J.; Pham, N.H. The cyclodextrin sugammadex and anaphylaxis to rocuronium: Is rocuronium still potentially allergenic in the inclusion complex form? Mini Rev. Med. Chem. 2012, 12, 701–712. [Google Scholar] [CrossRef]
- Ebo, D.G.; Venemalm, L.; Bridts, C.H.; Degerbeck, F.; Hagberg, H.; De Clerck, L.S.; Stevens, W.J. Immunoblobulin E antibodies to rocuronium. A new diagnostic tool. Anesthesiology 2007, 107, 253–259. [Google Scholar] [CrossRef]
- Allen, S.J.; Gallagher, A.; Paxton, L.D. Anaphylaxis to rocuronium. Anaesthesia. 2000, 55, 1223–1224. [Google Scholar] [CrossRef]
- Heier, T.; Guttormsen, A.B. Anaphylactic reactions during induction of anaesthesia using rocuronium for muscle relaxation: A report including 3 cases. Acta. Anesthesiol. Scand. 2000, 44, 775–781. [Google Scholar] [CrossRef]
- Neal, S.M.; Manthri, P.R.; Gadiyar, V.; Wildsmith, J.A.W. Histaminoid reactions associated with rocuronium. Br. J. Anaesth. 2000, 84, 108–111. [Google Scholar] [CrossRef]
- Rose, M.; Fisher, M.M. Rocuronium: high risk for anapylaxis. Br. J. Anaesth. 2001, 86, 678–682. [Google Scholar] [CrossRef]
- Bhananker, S.M.; O’Donnell, J.T.; Salemi, J.R.; Bishop, M.J. The risk of anaphylactic reactions to rocuronium in the United States is comparable to that of vecuronium: An analysis of food and drug administration reporting of adverse events. Anesth. Analg. 2005, 101, 819–822. [Google Scholar] [CrossRef]
- Johansson, S.G.O.; Oman, H.; Degerbeck, F.; Tunelli, J.; Florvaag, E.; Nopp, A. Anaphylaxis to atracurium—a non-QAI-dependent reaction? Acta. Anaesthesiol. Scand. 2012, 56, 262–263. [Google Scholar]
- Zhao, Z.; Baldo, B.A.; O’Brien, R.M.; Plomley, R.F. Reaction with, and fine structural recognition of polyamines by human IgE antibodies. Mol. Immunol. 2000, 37, 233–240. [Google Scholar] [CrossRef]
- Baldo, B.A.; Fisher, M.M.; Harle, D.G. Allergy to thiopentone. Clin. Rev. Allergy 1991, 9, 295–307. [Google Scholar]
- Harle, D.G.; Baldo, B.A.; Smal, M.A.; Wajon, P.; Fisher, M.M. Detection of thiopentone-reactive IgE antibodies following anaphylactoid reactions during anaesthesia. Clin. Allergy 1986, 16, 493–498. [Google Scholar] [CrossRef]
- Harle, D.G.; Baldo, B.A.; Smal, M.A.; Fisher, M.M. Drugs as allergens: The molecular basis of IgE binding to thiopentone. Int. Arch. Allergy Appl. Immunol. 1987, 84, 277–283. [Google Scholar] [CrossRef]
- Harle, D.G.; Baldo, B.A.; Fisher, M.M. The molecular basis of IgE antibody binding to thiopentone. Binding of IgE from thiopentone-allergic and non-allergic subjects. Mol. Immunol. 1990, 27, 853–858. [Google Scholar] [CrossRef]
- Fisher, M.M.; Ross, J.D.; Harle, D.G.; Baldo, B.A. Anaphylaxis to thiopentone: An unusual outbreak in a single hospital. Anaesth. Intens. Care 1989, 17, 361–365. [Google Scholar]
- Fisher, M.M.; Baldo, B.A.; Silbert, B.S. Anaphylaxis during anesthesia: Use of radioimmunoassays to determine etiology and drugs responsible in fatal cases. Anesthesiology 1991, 75, 1112–1115. [Google Scholar] [CrossRef]
- Baldo, B.A.; Fisher, M.M. Diagnosis of IgE-dependent anaphylaxis to neuromuscular blocking drugs, thiopentone and opioids. Ann. Fr. Anesth. Reanim. 1993, 12, 173–181. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H.; Zhao, Z. Chemistry of drug allergenicity. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 327–335. [Google Scholar]
- Vieluf, D.; Russwurm, R.; Przybilla, B.; Ring, J. Anaphylactic reactions to quinolones. J. Allergy Clin. Immunol. 1991, 87, 228. [Google Scholar]
- Colas, C.; Pola, J.; Zapata, C.; Dominguez, A.; Gimeno, A.; Duce, F. Quinolones hypersensitivity: Clinical and immunological aspects. J. Allergy Clin. Immunol. 1993, 91, 365. [Google Scholar]
- Dávila, I.; Diez, M.L.; Quirce, S.; Fraj, J.; de La Hoz, B.; Lázaro, M. Cross-reactivity between quinolones. Report of three cases. Allergy 1993, 48, 388–390. [Google Scholar] [CrossRef]
- Diaz, M.V.; Labairu, T.L.; del Pozo Gil, M.D.; Sarramián, A.B.; Mahave, I.G. In vivo diagnostic tests in adverse reactions to quinolones. J. Invest. Allergol. Clin. Immunol. 2007, 17, 393–398. [Google Scholar]
- Manfredi, M.; Severino, M.; Testi, S.; Macchia, D.; Ermini, G.; Pichler, W.J.; Campi, P. Detection of specific IgE to quinolones. J. Allergy Clin. Immunol. 2004, 113, 155–160. [Google Scholar] [CrossRef]
- Aranda, A.; Mayorga, C.; Ariza, A.; Doña, I.; Rosado, A.; Blanca-Lopez, N.; Andreu, I.; Torres, M.J. In vitro evaluation of IgE-mediated hypersensitivity to quinolones. Allergy 2011, 66, 247–254. [Google Scholar] [CrossRef]
- Rouzaire, P.; Nosbaum, A.; Mullet, C.; Diot, N.; Dubost, R.; Bienvenu, F.; Guilloux, L.; Piriou, V.; Bienvenu, J.; Bérard, F. Immediate allergic hypersensitivity to quinolones associates with neuromuscular blocking agent sensitization. J. Allergy Clin. Immunol. In Pract. 2013, 1, 273–279. [Google Scholar] [CrossRef]
- Pham, N.H. Immunochemical and diagnostic approaches to drug allergy. Ph.D. thesis, University of Sydney, Sydney, Australia, 1999. [Google Scholar]
- Pham, N.H.; Baldo, B.A.; Puy, R.M. Studies on the mechanism of multiple drug allergies. Structural basis of drug recognition. J. Immunoass. Immunoch. 2001, 22, 47–73. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. On the question of the association between immediate hypersensitivity to quinolones and neuromuscular blocking drug sensitization. J. Allergy Clin. Immunol. In Pract. 2013, 1, 709–710. [Google Scholar] [CrossRef]
- Okano, M.; Nomura, M.; Hata, S.; Okada, N.; Sato, K.; Kitano, Y.; Tashiro, M.; Yoshimoto, Y.; Hama, R.; Aoki, T. Anaphylactic symptoms due to chlorhexidine gluconate. Arch. Dermatol. 1989, 125, 50–52. [Google Scholar] [CrossRef]
- Lim, K.S.; Kam, P.C.A. Chlorhexidine–pharmacology and clinical applications. Anaesth. Intens. Care 2008, 36, 502–512. [Google Scholar]
- Ohtoshi, T.; Yamauchi, N.; Tadokoro, K.; Miyachi, S.; Suzuki, S.; Miyamoto, T.; Muranaka, M. IgE antibody-mediated shock reaction caused by topical application of chlorhexidine. Clin. Allergy 1986, 16, 155–161. [Google Scholar] [CrossRef]
- Pham, N.H.; Weiner, J.M.; Reisner, G.S.; Baldo, B.A. Anaphylaxis to chlorhexidine. Case report. Implication of immunoglobulin E antibodies and identification of an allergenic determinant. Clin. Exp. Allergy 2000, 30, 1001–1007. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Histamine-releasing and allergenic properties of opioid analgesic drugs: Resolving the two. Anaesth. Intens. Care 2012, 40, 216–235. [Google Scholar]
- Harle, D.G.; Baldo, B.A.; Coroneos, N.J.; Fisher, M.M. Anaphylaxis following administration of papaveretum. Case report: Implication of IgE antibodies that react with morphine and codeine, and identification of an allergenic determinant. Anesthesiology 1989, 71, 489–494. [Google Scholar] [CrossRef]
- Zhu, D.; Becker, W.M.; Schulz, K.H.; Schubeler, K.; Schlaak, M. The presence of specific IgE to salicyloyl and O-methylsalicyloyl in aspirin-sensitive patients. Asian Pac. J. Allergy Immunol. 1992, 10, 25–32. [Google Scholar]
- Zhu, D.; Becker, W.M.; Schulz, K.H.; Schubeler, K.; Schlaak, M. Detection of IgE antibodies specific for 1-phenyl-2,3-dimethyl-3-pyrazoline-5-one by RAST: A serological diagnostic method for sensitivity to pyrazoline drugs. Asian Pac. J. Allergy Immunol. 1992, 10, 95–101. [Google Scholar]
- Himly, M.; Jahn-Schmid, B.; Pittertschatscher, K.; Bohle, B.; Grubmayr, K.; Ferreira, F.; Ebner, H.; Ebner, C. IgE-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J. Allergy. Clin. Immunol. 2003, 111, 882–888. [Google Scholar] [CrossRef]
- Davies, R.J.; Pepys, J. Asthma due to inhaled chemical agents: The macrolide antibiotic spiramycin. Clin. Allergy 1975, 1, 99–107. [Google Scholar] [CrossRef]
- Pascual, C.; Crespo, J.F.; Quiralte, J.; Lopez, C.; Wheeler, G.; Martin-Esteban, M. In vitro detection of specific IgE antibodies to erythromycin. J. Allergy Clin. Immunol. 1995, 95, 668–671. [Google Scholar] [CrossRef]
- Jorro, G.; Morales, C.; Brasó, J.V.; Peláez, A. Anaphylaxis to erythromycin. Ann. Allergy Asthma Immunol. 1996, 77, 456–458. [Google Scholar] [CrossRef]
- Martinez, E.; Collazos, J.; Mayo, J. Hypersensitivity reactions to rifampin. Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine 1999, 78, 361–369. [Google Scholar] [CrossRef]
- Magnan, A.; Venemalm, L.; Porro, F.; Vervloet, D. Anaphylactic reaction to rifamycin SV: Presence of specific IgE antibodies. J. Allergy Clin. Immunol. 1999, 103, 954–956. [Google Scholar] [CrossRef]
- Bassi, L.; Di Bernardino, L.; Silvestri, L.G. IgE antibodies in patients allergic to rifampicin. Int. Arch. Allergy Appl. Immunol. 1976, 51, 390–394. [Google Scholar] [CrossRef]
- Antonicelli, L.; Micucci, C.; Bilò, M.B.; Manfredi, M.; Valentini, M.; Campi, P. IgE-mediated reactions to rifaximin and rifamycin SV and cross-reactivity among rifamycins. Allergy 2009, 64, 1232–1233. [Google Scholar] [CrossRef]
- Baldo, B.A.; Zhao, Z.; Pham, N.H. Antibiotic allergy: Immunochemical and clinical considerations. Curr. Allergy Asthma Rep. 2008, 8, 49–55. [Google Scholar] [CrossRef]
- Montañez, M.I.; Mayorga, C.; Torres, M.J.; Ariza, A.; Blanca, M.; Perez-Inestrosa, E. Synthetic approach to gain insight into antigenic determinants of cephalosporins: In vitro studies of chemical structure-IgE molecular recognition relationships. Chem. Res. Toxicol. 2011, 24, 706–717. [Google Scholar] [CrossRef]
- Romano, A.; Viola, M.; Guéant-Rodriguez, R.-M.; Gaeta, F.; Valluzzi, R.; Guéant, J.L. Brief communication: Tolerability of menopenem in patients with IgE-mediated hypersensitivity to penicillins. Ann. Intern. Med. 2007, 146, 266–269. [Google Scholar] [CrossRef]
- Cribb, A.E.; Miller, M.; Leeder, J.S.; Hill, J.; Spielberg, S.P. Reactions of the nitroso and hydroxylamine metabolites of sulfamethoxazole with reduced glutathione. Implications for idiosyncratic toxicity. Drug Metab. Dispos. 1991, 19, 900–906. [Google Scholar]
- Cribb, A.E.; Lee, B.L.; Trepanier, L.A.; Spielberg, S.P. Adverse reactions to sulfonamide and sulfonamide-trimethoprim antimicrobials: Clinical syndromes and pathogenesis. Adverse Drug React. Toxicol. Rev. 1996, 15, 9–50. [Google Scholar]
- Adams, J.M.; Bennett, D.J.; Bom, A.; Clark, J.K.; Feilden, H.; Hutchinson, E.J.; Palin, R.; Prosser, A.; Rees, D.C.; Rosair, G.M.; et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: Synthesis and structure-activity relationships. J. Med. Chem. 2002, 45, 1806–1816. [Google Scholar] [CrossRef]
- Zhang, M.-Q. Drug-specific cyclodextrins: The future of rapid neuromuscular block reversal? Drugs Future 2003, 28, 347–354. [Google Scholar] [CrossRef]
- Baldo, B.A.; McDonnell, N.J.; Pham, N.H. Drug-specific cyclodextrins with emphasis on sugammadex, the neuromuscular blocker rocuronium and perioperative anaphylaxis: Implications for drug allergy. Clin. Exp. Allergy 2011, 41, 1663–1678. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baldo, B.A. IgE and Drug Allergy: Antibody Recognition of ‘Small’ Molecules of Widely Varying Structures and Activities. Antibodies 2014, 3, 56-91. https://doi.org/10.3390/antib3010056
Baldo BA. IgE and Drug Allergy: Antibody Recognition of ‘Small’ Molecules of Widely Varying Structures and Activities. Antibodies. 2014; 3(1):56-91. https://doi.org/10.3390/antib3010056
Chicago/Turabian StyleBaldo, Brian A. 2014. "IgE and Drug Allergy: Antibody Recognition of ‘Small’ Molecules of Widely Varying Structures and Activities" Antibodies 3, no. 1: 56-91. https://doi.org/10.3390/antib3010056