Epitope Sequences in Dengue Virus NS1 Protein Identified by Monoclonal Antibodies
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of NS1-Specific DENV mAbs
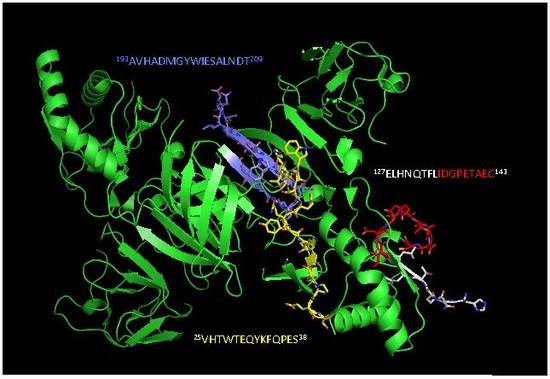
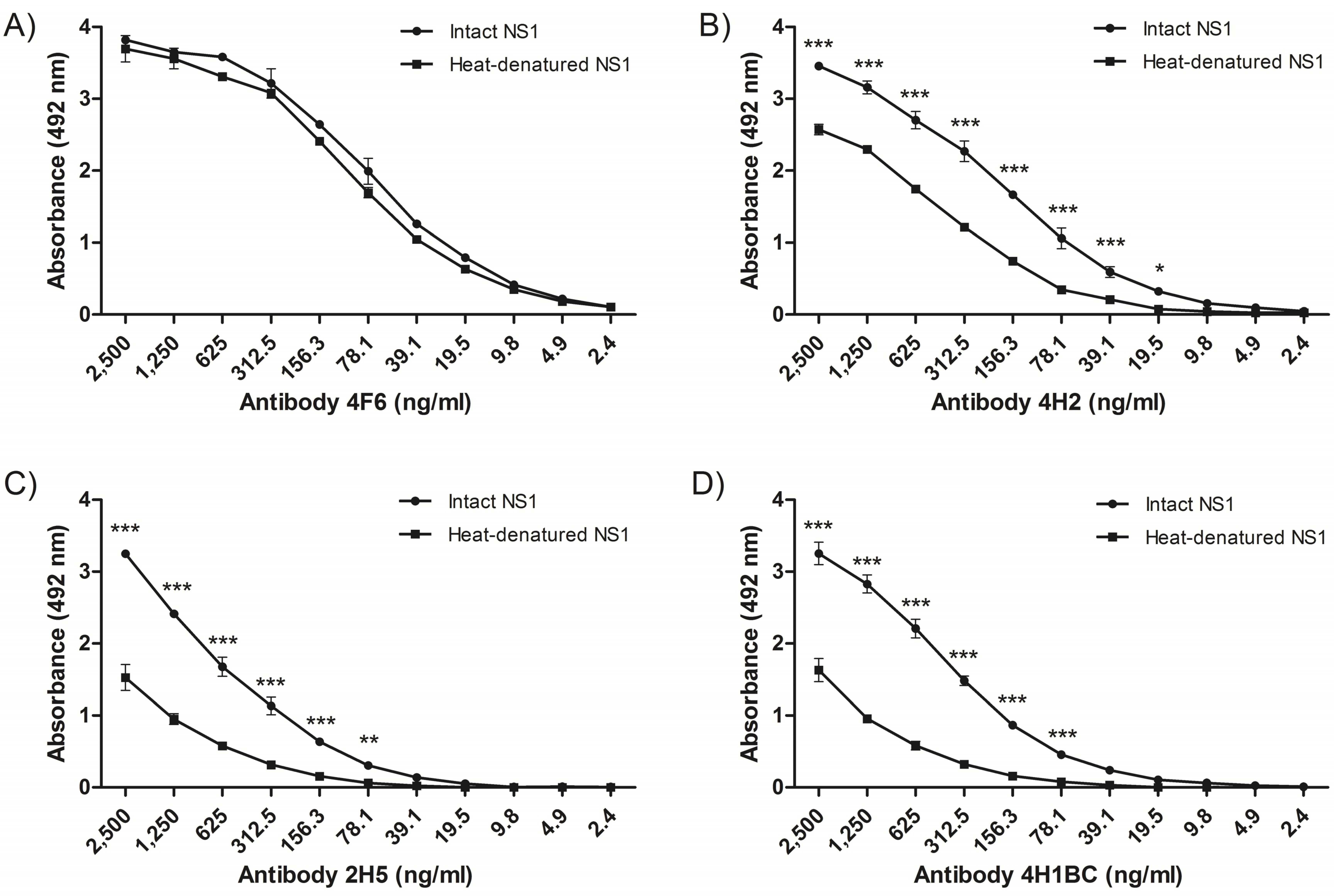
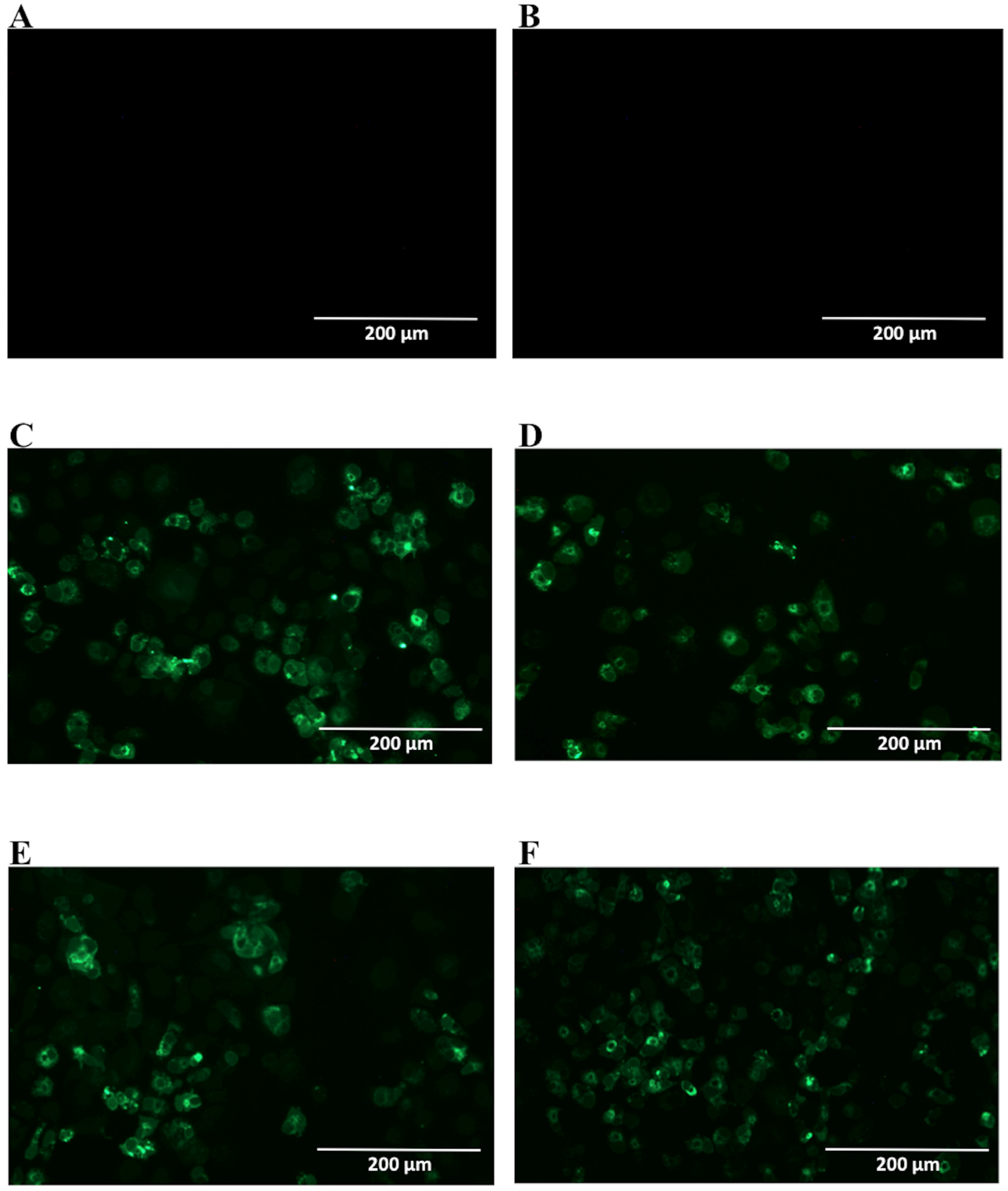
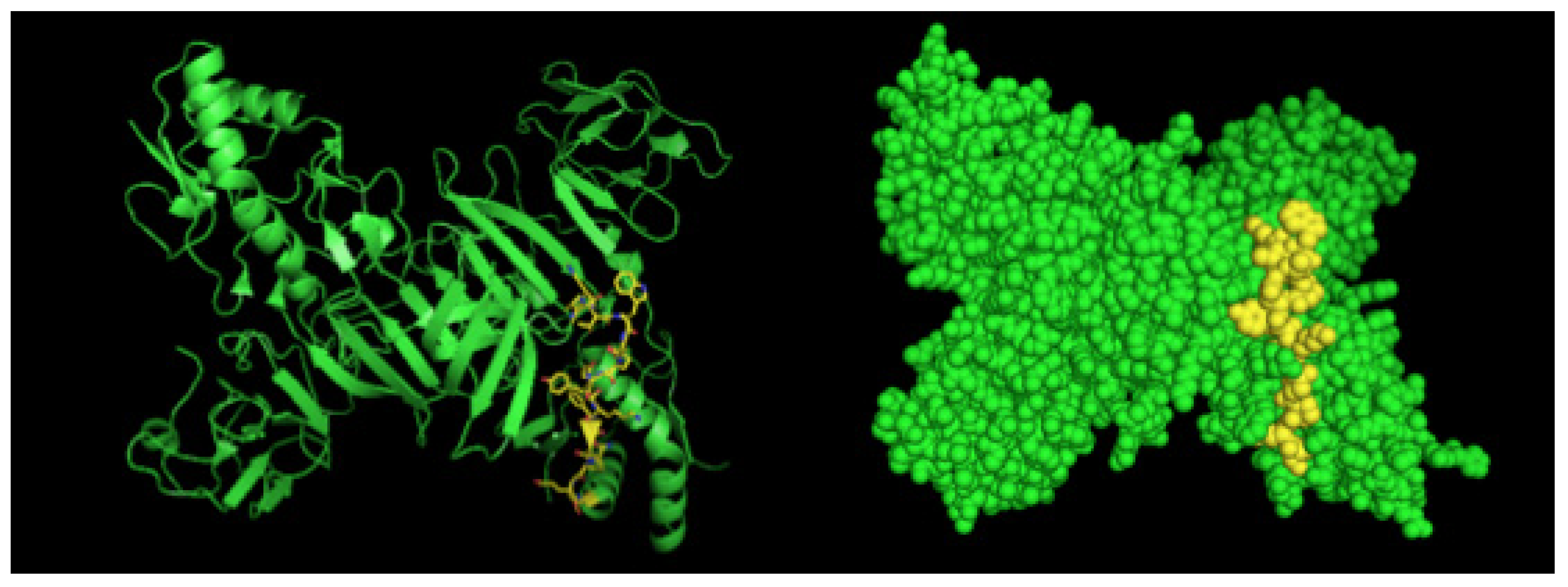
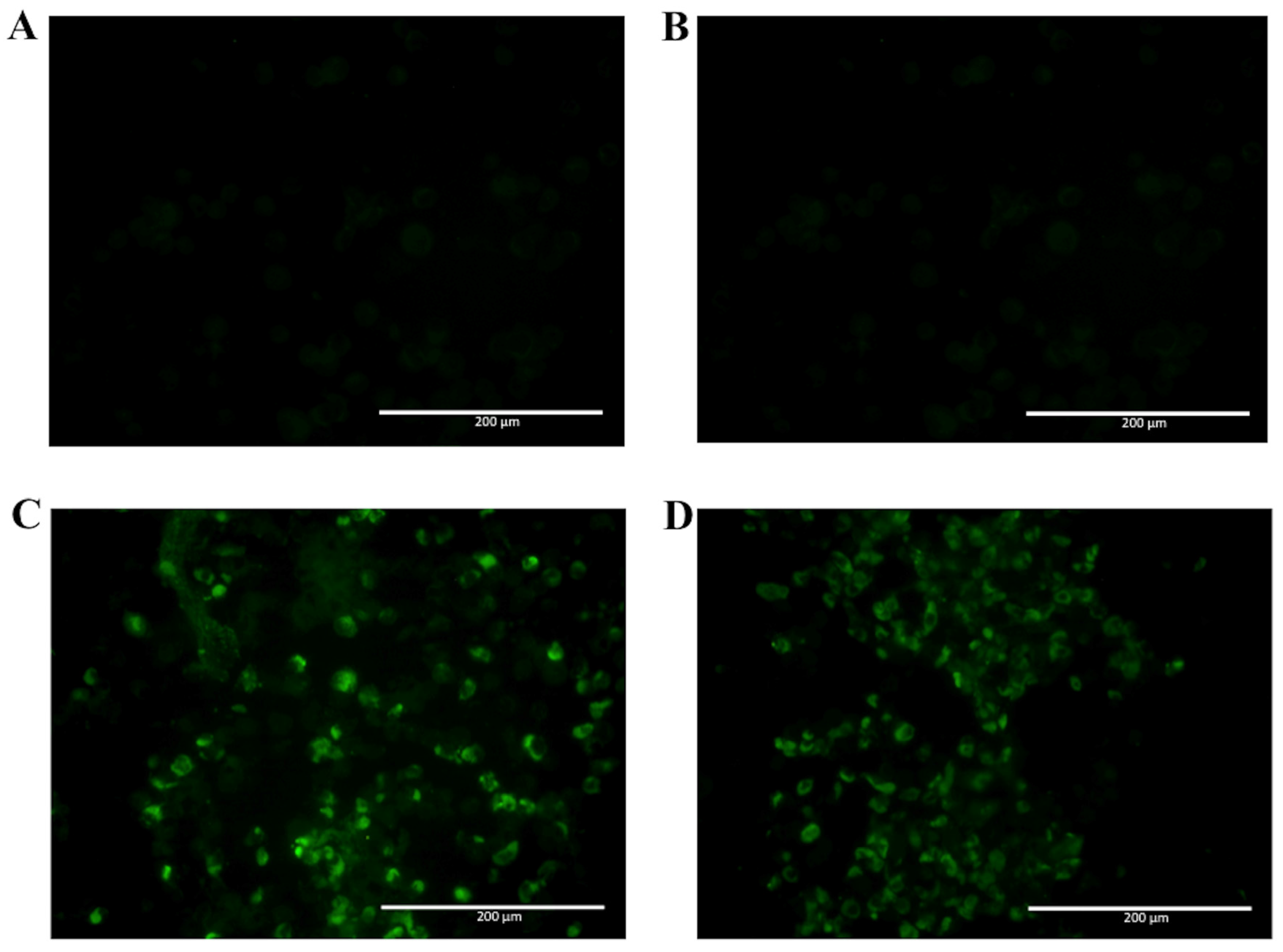
2.2. Detection of Native DENV2 NS1 and Epitope Mapping
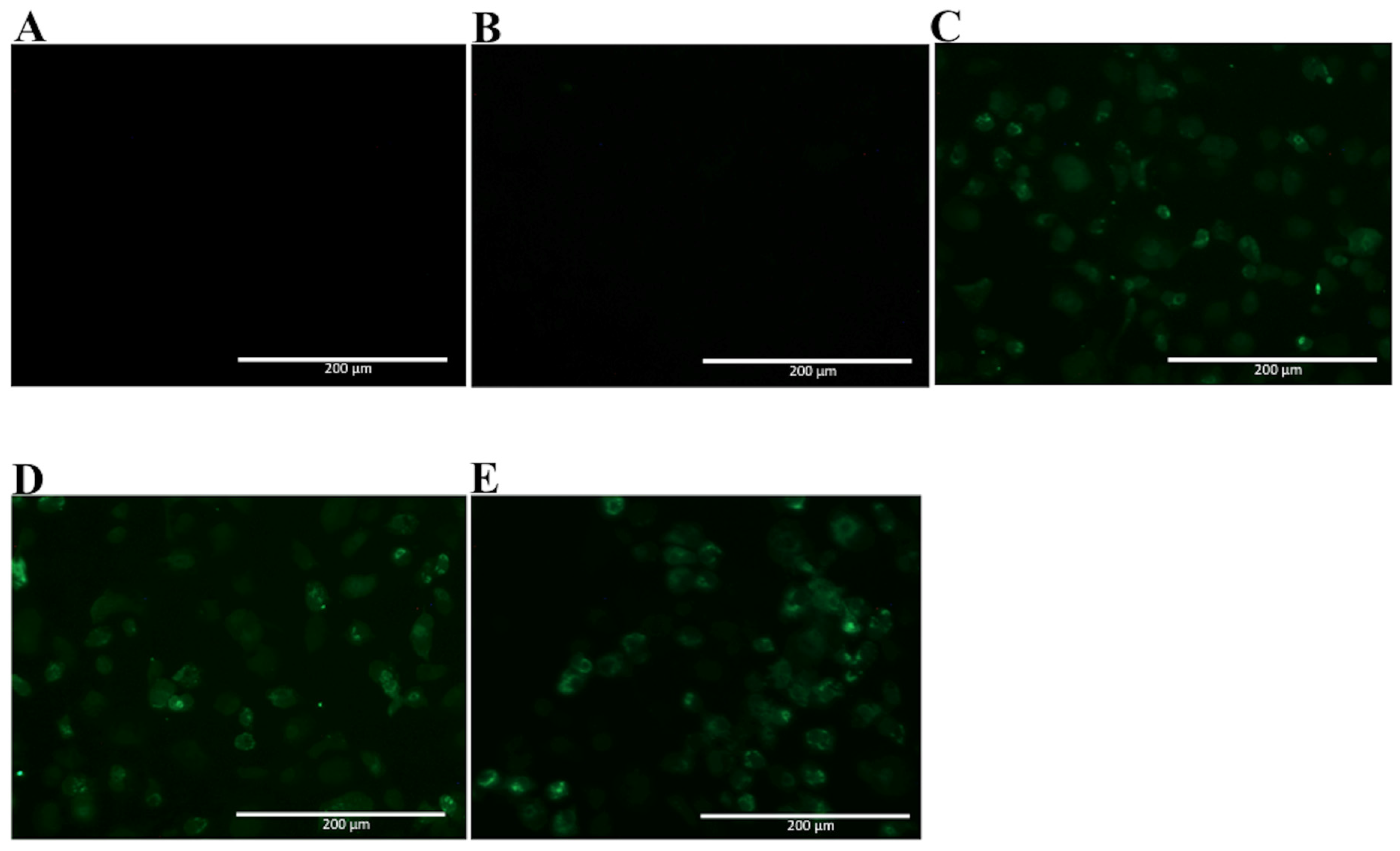
2.3. Analyses of mAbs’ Cross-Reactivity with Different DENV Serotype and ZIKV
3. Discussion
4. Materials and Methods
4.1. Viral Strains and Viral Antigen
4.2. Dengue NS1 Monoclonal Antibody (mAb) Production
4.3. Dengue NS1 mAbs Characterization
4.4. Epitope Characterization and Structure Analysis
4.5. NS1 Sequences Database Building
4.6. Conservancy Analysis
4.7. NS1 mAbs Reactivity to Dengue Virus Serotypes and Zika Virus
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daep, C.A.; Muñoz-Jordán, J.L.; Eugenin, E.A. Flaviviruses, an expanding threat in public health: Focus on dengue, West Nile, and Japanese encephalitis virus. J. Neurovirol. 2014, 6, 539–560. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Vigilância em Saúde, Ministério da Saúde. Bol. Epidemiol. 2017, 48, 1–9.
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.K.; DelProposto, J.; Ogata, C.W.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014, 343, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Jones, M.K.; Young, P.R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology 1996, 1, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.K.; Young, P.R.; Miles, M.A. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch. Virol. 1994, 137, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M.; Conde, J.N.; Allonso, D.; Nogueira, M.L.; Mohana-Borges, R. Mapping the interactions of dengue virus NS1 protein with human liver proteins using a yeast two-hybrid system: Identification of C1q as an interacting partner. PLoS ONE 2013, 8, e57514. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir. Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, N.; Bradbury, R.S.; Taylor-Robinson, A.W. The global spread of Zika virus: Is public and media concern justified in regions currently unaffected? Infect. Dis. Poverty 2016, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Kochakarn, T.; Kotanan, N.; Kümpornsin, K.; Loesbanluechai, D.; Thammasatta, M.; Auewarakul, P.; Wilairat, P.; Chookajorn, T. Comparative genome analysis between Southeast Asian and South American Zika viruses. Asian Pac. J. Trop. Med. 2016, 9, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sheng, J.; Plevka, P.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc. Natl. Acad. Sci. USA 2013, 110, 6795–6799. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Legge, F.S.; Zeng, X.; Treutlein, H.R.; Zeng, J. Antibody recognition of Shiga toxins (Stxs): Computational identification of the epitopes of Stx2 subunit A to the antibodies 11E10 and S2C4. PLoS ONE 2014, 2, e88191. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Legge, F.S.; Lebani, K.; Mahler, S.M.; Young, P.R.; Watterson, D.; Treutlein, H.R.; Zeng, J. Computational Identification of Antibody Epitopes on the Dengue Virus NS1 Protein. Molecules 2017, 22, 607. [Google Scholar] [CrossRef] [PubMed]
- Lebani, K.; Jones, M.L.; Watterson, D.; Ranzoni, A.; Traves, R.J.; Young, P.R.; Mahler, S.M. Isolation of serotype-specific antibodies against dengue virus non-structural protein 1 using phage display and application in a multiplexed serotyping assay. PLoS ONE 2017, 12, e0180669. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Gromowski, G.D.; Ripoll, D.R.; Khavrutskii, I.V.; Desai, V.; Wallqvist, A. Dengue virus antibody database: Systematically linking serotype-specificity with epitope mapping in dengue virus. PLoS Negl. Trop. Dis. 2017, 11, e0005395. [Google Scholar] [CrossRef] [PubMed]
- Friguet, B.; Chaffotte, A.F.; Djavadi-Ohaniance, L.; Goldberg, M.E. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 1985, 77, 305–319. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Rothman, A.L.; Ennis, F.A.; Nisalak, A. Dengue in the early febrile phase: Viremia and antibody responses. J. Infect. Dis. 1997, 76, 322–330. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, J.M.; Yin, Y.; Fang, D.Y.; Tang, Y.X.; Jiang, L.F. Selection and identification of B-cell epitope on NS1 protein of dengue virus type 2. Virus Res. 2010, 150, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gelanew, T.; Poole-Smith, B.K.; Hunsperger, E. Development and characterization of mouse monoclonal antibodies against monomeric dengue virus non-structural glycoprotein 1 (NS1). J. Virol. Methods 2015, 222, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.H.; Porchia, B.F.M.M.; Balan, A.; Cavalcante, R.C.M.; da Costa, S.M.; de Barcelos Alves, A.M.; de Souza Ferreira, L.C. Refolded dengue virus type 2 NS1 protein expressed in Escherichia coli preserves structural and immunological properties of the native protein. J. Virol. Methods 2010, 167, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.S.; Amorim, J.H.; Alves, R.P.S.; Pereira, S.A.; Ferreira, L.C.S.; Romano, C.M. Complete Genome Sequence of an Atypical Dengue Virus Serotype 2 Lineage Isolated in Brazil. Genome Announc. 2015, 3, e00779-15. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.H.; Pereira Bizerra, R.S.; dos Santos Alves, R.P.; Sbrogio-Almeida, M.E.; Levi, J.E.; Capurro, M.L.; de Souza Ferreira, L.C. A genetic and pathologic study of a DENV2 clinical isolate capable of inducing encephalitis and hematological disturbances in immunocompetent mice. PLoS ONE 2012, 7, e44984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, S.S.; Falgout, B.; Hanley, K.A.; Blaney, J.E., Jr.; Markoff, L.; Murphy, B.R. A live, attenuated Dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3′ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 2003, 77, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.E., Jr.; Sathe, N.S.; Goddard, L.; Hanson, C.T.; Romero, T.A.; Hanley, K.A.; Murphy, B.R.; Whitehead, S.S. Dengue virus type 3 vaccine candidates generated by introduction of deletions in the 3′ untranslated region (3′-UTR) or by exchange of the DENV-3 3′-UTR with that of DENV-4. Vaccine 2008, 26, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Blaney, J.E., Jr.; Johnson, D.H.; Manipon, G.G.; Firestone, C.Y.; Hanson, C.T.; Murphy, B.R.; Whitehead, S.S. Genetic basis of attenuation of Dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology 2002, 300, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Khöler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Biotechnology 1975, 24, 524–526. [Google Scholar]
- Rocha, L.B.; Luz, D.E.; Moraes, C.T.P.; Caravelli, A.; Fernandes, I.; Guth, B.E.C.; Horton, D.S.P.Q.; Piazza, R.M.F. Interaction between Shiga toxin and monoclonal antibodies: Binding characteristics and in vitro neutralizing abilities. Toxins (Basel) 2012, 4, 729–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
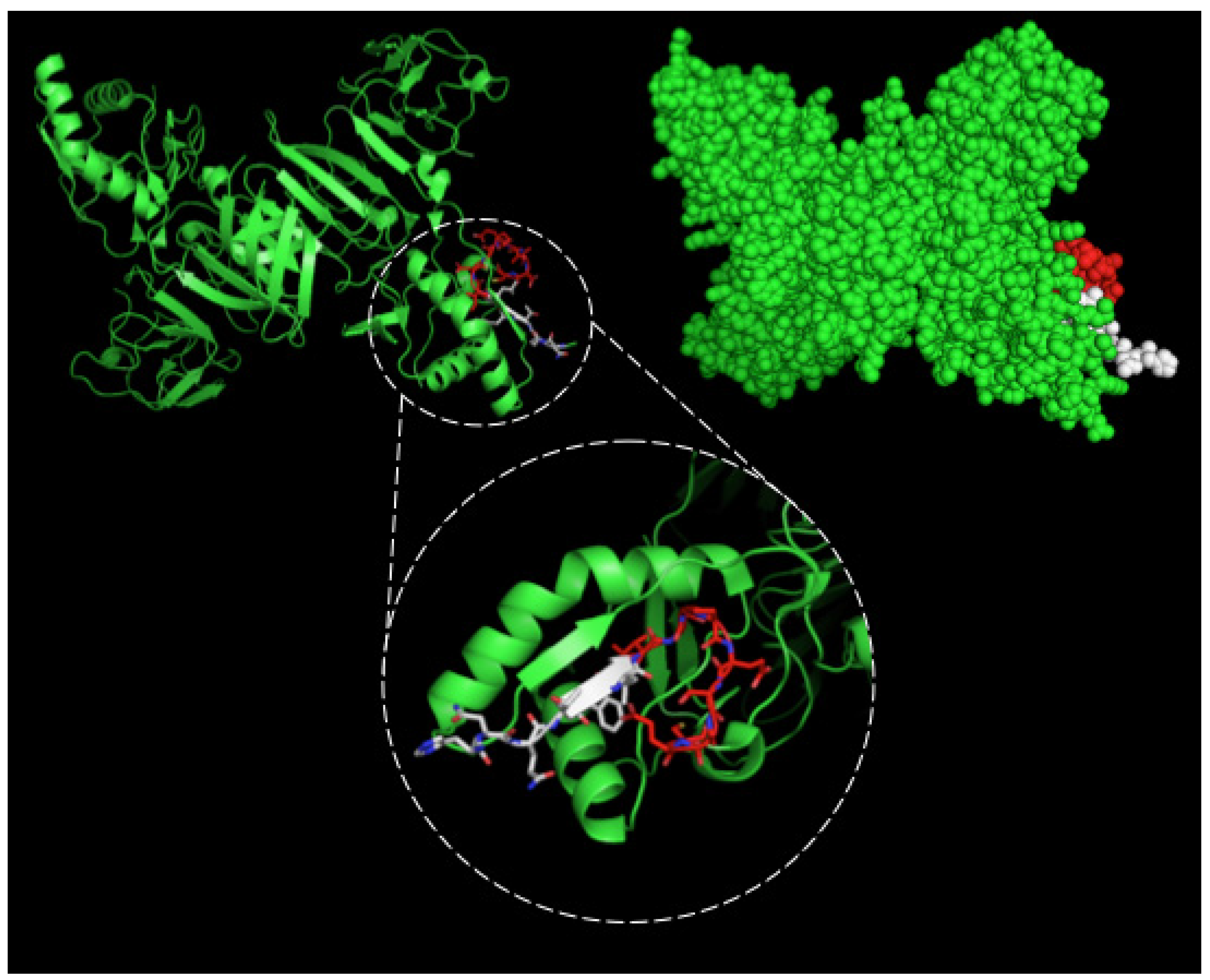

| Name | 4F6 | 4H2 | 2H5 | 4H1BC |
|---|---|---|---|---|
| IgG Subtype a | IgG2a | IgG2a | IgG1 | IgG1 |
| DENV2 NS1 reactivity b | Yes | Yes | Yes | Yes |
| Dissociation Constant (KD) c | 1.1 × 10−8 M | 6.2 × 10−8 M | 7.3 × 10−8 M | 8.4 × 10−8 M |
| Detection limit d | 16 ng/mL | 32 ng/mL | 32 ng/mL | 32 ng/mL |
| Epitope sequence e | 25VHTWTEQYKFQPES38 | 127ELHNQTFLIDGPETAEC143 | 193AVHADMGYWIESALNDT209 | 193AVHADMGYWIESALNDT209 |
| DENV (1–4) reactivity f | No | Yes | No | No |
| ZIKV reactivity g | No | No | Yes | Yes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, L.B.; Alves, R.P.d.S.; Caetano, B.A.; Pereira, L.R.; Mitsunari, T.; Amorim, J.H.; Polatto, J.M.; Botosso, V.F.; Gallina, N.M.F.; Palacios, R.; et al. Epitope Sequences in Dengue Virus NS1 Protein Identified by Monoclonal Antibodies. Antibodies 2017, 6, 14. https://doi.org/10.3390/antib6040014
Rocha LB, Alves RPdS, Caetano BA, Pereira LR, Mitsunari T, Amorim JH, Polatto JM, Botosso VF, Gallina NMF, Palacios R, et al. Epitope Sequences in Dengue Virus NS1 Protein Identified by Monoclonal Antibodies. Antibodies. 2017; 6(4):14. https://doi.org/10.3390/antib6040014
Chicago/Turabian StyleRocha, Leticia Barboza, Rubens Prince dos Santos Alves, Bruna Alves Caetano, Lennon Ramos Pereira, Thais Mitsunari, Jaime Henrique Amorim, Juliana Moutinho Polatto, Viviane Fongaro Botosso, Neuza Maria Frazatti Gallina, Ricardo Palacios, and et al. 2017. "Epitope Sequences in Dengue Virus NS1 Protein Identified by Monoclonal Antibodies" Antibodies 6, no. 4: 14. https://doi.org/10.3390/antib6040014