Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases
Abstract
:1. Introduction
2. Tissue Factor in Pathological Neovasculature of Cancer, Age-Related Macular Degeneration and Endometriosis
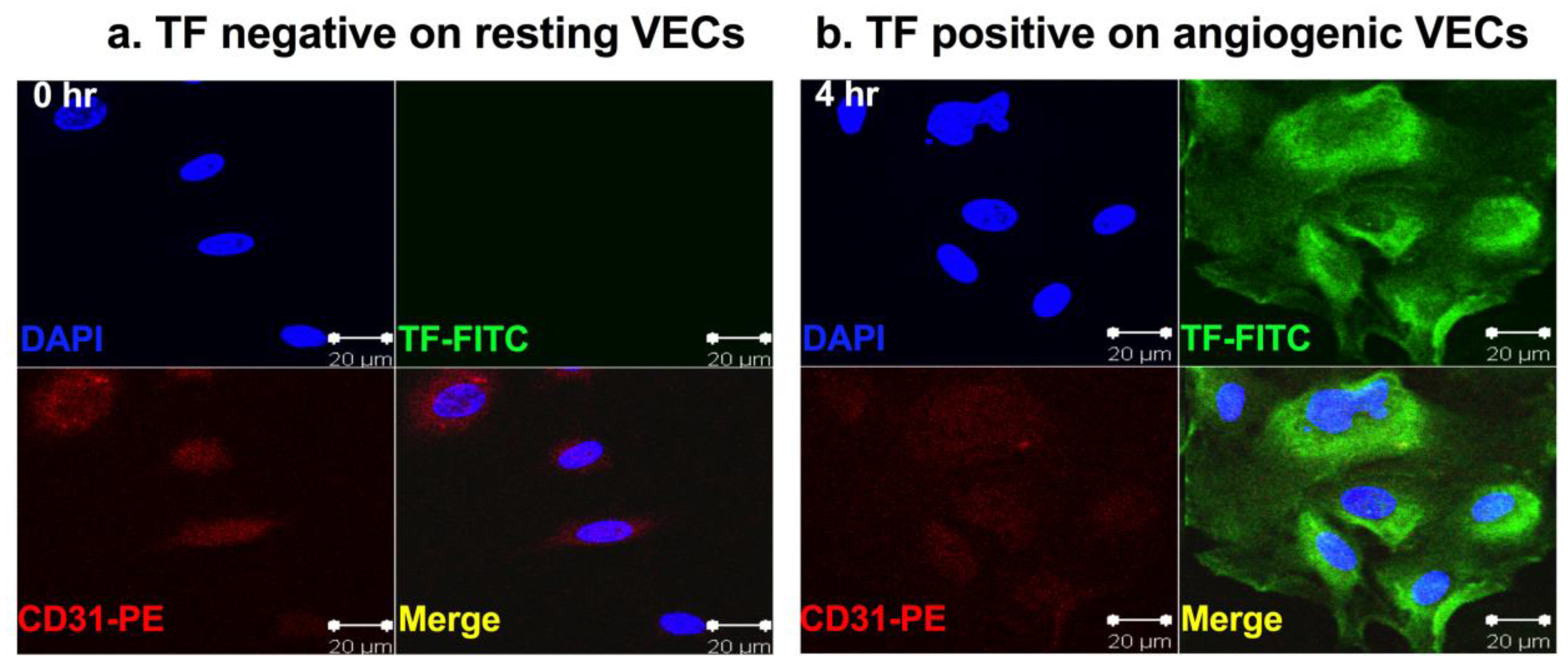
2.1. Tissue Factor Expression in Pathological Neovasculature of Cancer
2.2. Tissue Factor Expression in the Neovasculature of Age-Related Macular Degeneration
2.3. Tissue Factor Expression in the Neovasculature of Endometriosis
3. Tissue Factor Expression in Cancer
3.1. Tissue Factor Expression on the Cancer Cells of Solid Cancers, Leukemia and Sarcoma
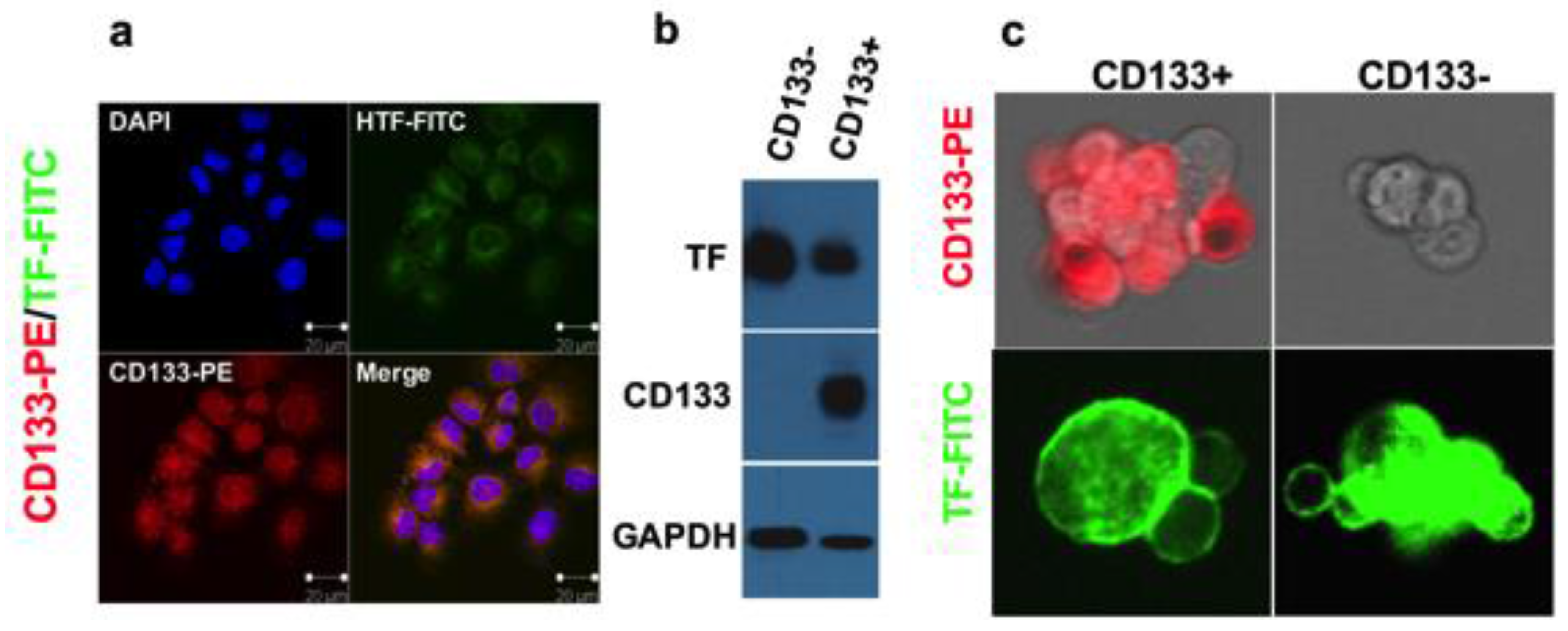
3.2. Tissue Factor Expression on Cancer Stem Cells
4. Tissue Factor in Rheumatoid Arthritis
4.1. TF Expression in Arthritic Joints
4.2. Angiogenesis and Angiogenic Endothelial TF in RA
4.3. Macrophages in RA Expressing TF
4.4. Fibroblasts in RA Expressing TF
4.5. B Cells in RA Expressing TF
5. Cytokines and Growth Factors in RA, Endometriosis and Tumor Microenvironment Contributing to Induction of TF and Angiogenesis
6. Tissue Factor in Macrophage-Involved Human Diseases
6.1. Tissue Factor in Atherosclerosis
6.2. Tissue Factor Expression on HIV-Infected Macrophages
6.3. Tissue Factor Expression in Ebola-Infected Macrophages
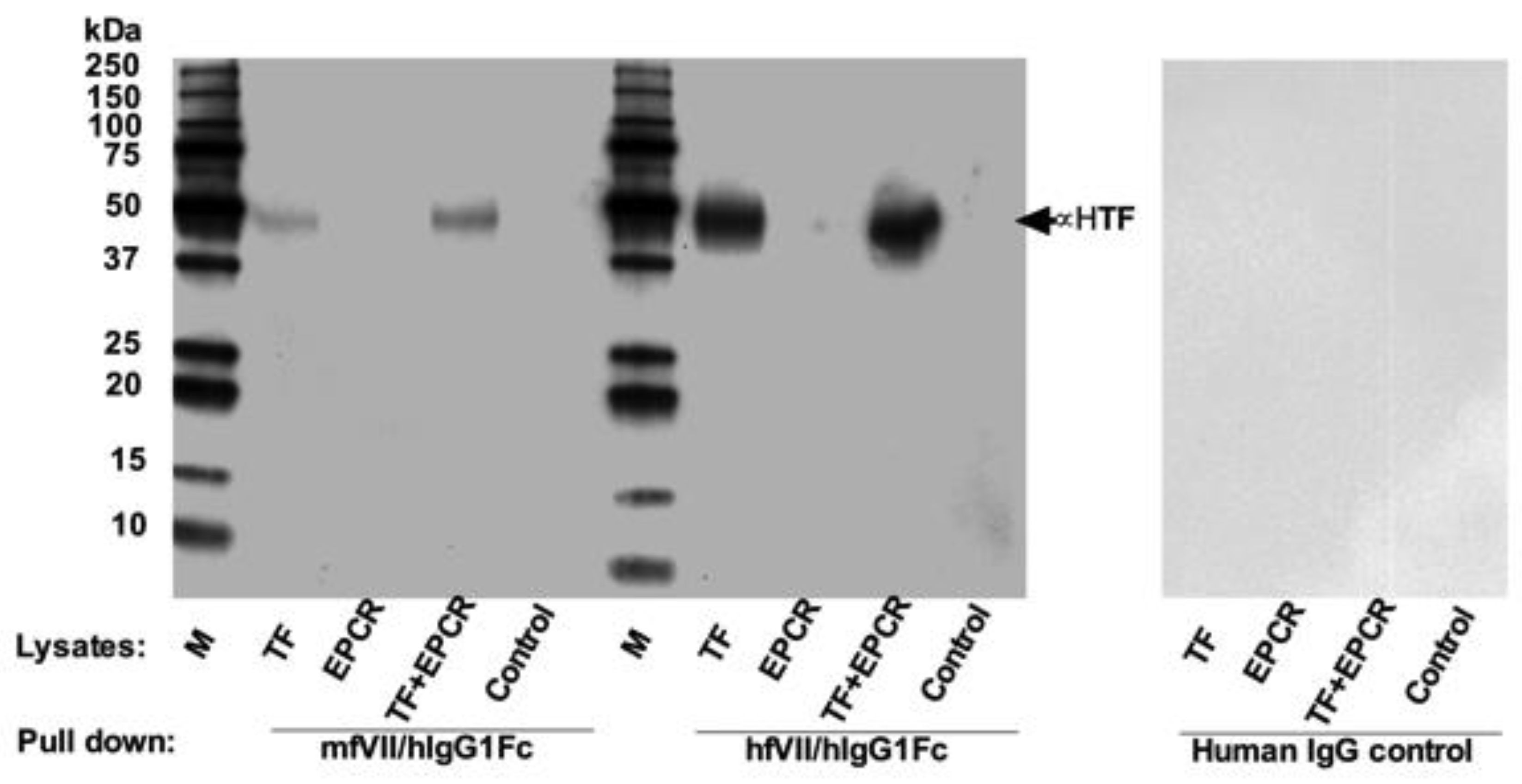
7. Targeting TF Antibodies and Antibody-Like Immunoconjugates in Preclinical Studies
7.1. First Generation of TF-Targeting Antibody-Like Immunoconjugates (Called an ICON or ICON-1)
7.2. Second Generation TF-Targeting Antibody-Like Immunoconjugate (L-ICON1)
7.3. TF-Targeting Antibodies and Antibody-Drug Conjugates (ADC)
8. TF-Targeting ICON and ADC in Clinical Trials
8.1. ICON in Clinical Trials in Patients with Ocular Melanoma and AMD
8.2. ADC in Clinical Trials in Cancer Patients
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Morrissey, J.H.; Fakhrai, H.; Edgington, T.S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell 1987, 50, 129–135. [Google Scholar] [CrossRef]
- Spicer, E.K.; Horton, R.; Bloem, L.; Bach, R.; Williams, K.R.; Guha, A.; Kraus, J.; Lin, T.C.; Nemerson, Y.; Konigsberg, W.H. Isolation of cDNA clones coding for human tissue factor: Primary structure of the protein and cDNA. Proc. Natl. Acad. Sci. USA 1987, 84, 5148–5152. [Google Scholar] [CrossRef] [PubMed]
- Konigsberg, W.H.; Nemerson, Y. Molecular cloning of the cDNA for human tissue factor. Cell 1988, 52, 639–640. [Google Scholar] [CrossRef]
- Schecter, A.D.; Giesen, P.L.; Taby, O.; Rosenfield, C.L.; Rossikhina, M.; Fyfe, B.S.; Kohtz, D.S.; Fallon, J.T.; Nemerson, Y.; Taubman, M.B. Tissue factor expression in human arterial smooth muscle cells. TF is present in three cellular pools after growth factor stimulation. J. Clin. Investig. 1997, 100, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Nemerson, Y. Tissue factor and the initiation of blood coagulation. In The New Dimensions of Warfarin Prophylaxis; Wessler, S., Becker, C.G., Nemerson, Y., Eds.; Plenum Press: New York, NY, USA, 1987; pp. 83–94. [Google Scholar]
- Nemerson, Y. Tissue factor and hemostasis. Blood 1988, 71, 1–8. [Google Scholar] [PubMed]
- Folkman, J. Tumor angiogenesis and tissue factor. Nat. Med. 1996, 2, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrera, C.; Barbarroja, N.; Dorado, G.; Siendones, E.; Velasco, F. Tissue factor as an effector of angiogenesis and tumor progression in hematological malignancies. Leukemia 2006, 20, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Milsom, C.; May, L.; Klement, P.; Yu, J. Tissue factor in cancer and angiogenesis: The molecular link between genetic tumor progression, tumor neovascularization and cancer coagulopathy. Semin. Thromb. Hemost. 2006, 32, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Leppert, U.; Eisenreich, A. The role of tissue factor isoforms in cancer biology. Int. J. Cancer 2015, 137, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Zakrzewicz, A.; Huber, K.; Thierbach, H.; Pepke, W.; Goldin-Lang, P.; Schultheiss, H.-P.; Pries, A.; Rauch, U. Regulation of pro-angiogenic tissue factor expression in hypoxia-induced human lung cancer cells. Oncol. Rep. 2013, 30, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Goldin-Lang, P.; Tran, Q.-V.; Fichtner, I.; Eisenreich, A.; Antoniak, S.; Schulze, K.; Coupland, S.E.; Poller, W.; Schultheiss, H.-P.; Rauch, U. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol. Rep. 2008, 20, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Bogdanov, V.Y.; Zakrzewicz, A.; Pries, A.; Antoniak, S.; Poller, W.; Schultheiss, H.-P.; Rauch, U. Cdc2-like kinases and DNA topoisomerase I regulate alternative splicing of tissue factor in human endothelial cells. Circ. Res. 2009, 104, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Malz, R.; Pepke, W.; Ayral, Y.; Poller, W.; Schultheiss, H.-P.; Rauch, U. Role of the phosphatidylinositol 3-kinase/protein kinase B pathway in regulating alternative splicing of tissue factor mRNA in human endothelial cells. Circ. J. 2009, 73, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cheng, J.; Xu, J.; Ruf, W.; Lockwood, C.J. Tissue factor is an angiogenic-specific receptor for factor VII-targeted immunotherapy and photodynamic therapy. Angiogenesis 2017, 20, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, J.; Cheng, J.; McMichael, E.; Yu, L.; Carson, W.E., III. Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer. Oncotarget 2017, 8, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Bora, P.S.; Hu, Z.; Tezel, T.H.; Sohn, J.-H.; Kang, S.G.; Cruz, J.M.C.; Bora, N.S.; Garen, A.; Kaplan, H.J. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Tezel, T.H.; Bodek, E.; Sonmez, K.; Kaliappan, S.; Kaplan, H.J.; Hu, Z.; Garen, A. Targeting tissue factor for immunotherapy of choroidal neovascularization by intravitreal delivery of factor VII-Fc chimeric antibody. Ocul. Immunol. Inflamm. 2007, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G.; Hu, Z.; Osteen, K.; Bruner-Tran, K.L.; Schatz, F.; Taylor, H.S.; Toti, P.; Arcuri, F.; Konigsberg, W.; Garen, A.; et al. The immunoconjugate “icon” targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am. J. Pathol. 2010, 176, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2002, 2, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Klagsbrun, M.; Sullivan, R.; Smith, S.; Rybka, R.; Shing, Y. Purification of endothelial cell growth factors by heparin affinity chromatography. Methods Enzymol. 1987, 147, 95–105. [Google Scholar] [PubMed]
- Afuwape, A.O.; Kiriakidis, S.; Paleolog, E.M. The role of the angiogenic molecule VEGF in the pathogenesis of rheumatoid arthritis. Histol. Histopathol. 2002, 17, 961–972. [Google Scholar] [PubMed]
- Fujimoto, J.; Sakaguchi, H.; Hirose, R.; Wen, H.; Tamaya, T. Angiogenesis in endometriosis and angiogenic factors. Gynecol. Obstet. Investig. 1999, 48 (Suppl. 1), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Mirza, H.; Conner, C.E.; Lorenz, A.F.; Drews, M.H.; Bahou, W.F.; Jesty, J. Vascular endothelial growth factor induces tissue factor and matrix metalloproteinase production in endothelial cells: Conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int. J. Cancer 1998, 75, 780–786. [Google Scholar] [CrossRef]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer. Breast Cancer Res. Treat. 2011, 126, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice. BMC Cancer 2010, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Brooks, S.A.; Dormoy, V.; Hsu, C.W.; Hsu, H.Y.; Lin, L.T.; Massfelder, T.; Rathmell, W.K.; Xia, M.; Al-Mulla, F.; et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: Focus on the cancer hallmark of tumor angiogenesis. Carcinogenesis 2015, 36 (Suppl. 1), S184–S202. [Google Scholar] [CrossRef] [PubMed]
- Contrino, J.; Hair, G.; Kreutzer, D.L.; Rickles, F.R. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nat. Med. 1996, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sun, Y.; Garen, A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc. Natl. Acad. Sci. USA 1999, 96, 8161–8166. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Garen, A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 12180–12185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xu, J.; Duanmu, J.; Zhou, H.; J. Booth, C.; Hu, Z. Effective treatment of human lung cancer by targeting tissue factor with a factor VII-targeted photodynamic therapy. Curr. Cancer Drug Targets 2011, 11, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, J.; Cheng, J.; Xu, J.; Booth, C.J.; Hu, Z. Effective treatment of chemoresistant breast cancer in vitro and in vivo by a factor VII-targeted photodynamic therapy. Br. J. Cancer 2011, 104, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Garen, A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2000, 97, 9221–9225. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Puklin, J.E.; Frank, R.N. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3178–3188. [Google Scholar]
- Reddy, V.M.; Zamora, R.L.; Kaplan, H.J. Distribution of growth factors in subfoveal neovascular membranes in age-related macular degeneration and presumed ocular histoplasmosis syndrome. Am. J. Ophthalmol. 1995, 120, 291–301. [Google Scholar] [CrossRef]
- Lopez, P.F.; Sippy, B.D.; Lambert, H.M.; Thach, A.B.; Hinton, D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 855–868. [Google Scholar]
- Oh, H.; Takagi, H.; Takagi, C.; Suzuma, K.; Otani, A.; Ishida, K.; Matsumura, M.; Ogura, Y.; Honda, Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1891–1898. [Google Scholar]
- Grossniklaus, H.E.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.M.; Elner, S.G.; Sternberg, P., Jr. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 2002, 8, 119–126. [Google Scholar] [PubMed]
- Krikun, G.; Schatz, F.; Taylor, H.; Lockwood, C.J. Endometriosis and tissue factor. Ann. N. Y. Acad. Sci. 2008, 1127, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.J.; Runic, R.; Wan, L.; Krikun, G.; Demopolous, R.; Schatz, F. The role of tissue factor in regulating endometrial haemostasis: Implications for progestin-only contraception. Hum. Reprod. 2000, 15 (Suppl. 3), 144–151. [Google Scholar] [CrossRef] [PubMed]
- Schatz, F.; Krikun, G.; Caze, R.; Rahman, M.; Lockwood, C.J. Progestin-regulated expression of tissue factor in decidual cells: Implications in endometrial hemostasis, menstruation and angiogenesis. Steroids 2003, 68, 849–860. [Google Scholar] [CrossRef]
- Krikun, G. Endometriosis, angiogenesis and tissue factor. Scientifica 2012, 2012, 306830. [Google Scholar] [CrossRef] [PubMed]
- Contrino, J.; Hair, G.A.; Schmeizl, M.A.; Rickles, F.R.; Kreutzer, D.L. In situ characterization of antigenic and functional tissue factor expression in human tumors utilizing monoclonal antibodies and recombinant factor VIIa as probes. Am. J. Pathol. 1994, 145, 1315–1322. [Google Scholar] [PubMed]
- Callander, N.S.; Varki, N.; Rao, L.V. Immunohistochemical identification of tissue factor in solid tumors. Cancer 1992, 70, 1194–1201. [Google Scholar] [CrossRef]
- Hu, Z. Factor VII-Targeted Photodynamic Therapy for Breast Cancer and Its Therapeutic Potential for Other Solid Cancers and Leukemia, Breast Cancer-Current and Alternative Therapeutic Modalities. In Breast Cancer-Current and Alternative Therapeutic Modalities; Gunduz, E., Gunduz, M., Eds.; InTech: Rijeka, Croatia, 2011; pp. 175–196. [Google Scholar]
- Sturm, U.; Luther, T.; Albrecht, S.; Flössel, C.; Großmann, H.; Müller, M. Immunohistological detection of tissue factor in normal and abnormal human mammary glands using monoclonal antibodies. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 421, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Toi, M.; Koike, M.; Nakamura, S.; Tominaga, T. Tissue factor expression in breast cancer tissues: Its correlation with prognosis and plasma concentration. Br. J. Cancer 2000, 83, 164–170. [Google Scholar] [PubMed]
- Kageshita, T.; Funasaka, Y.; Ichihashi, M.; Wakamatsu, K.; Ito, S.; Ono, T. Tissue factor expression and serum level in patients with melanoma does not correlate with disease progression. Pigment Cell Melanoma Res. 2001, 14, 195–200. [Google Scholar] [CrossRef]
- Shoji, M.; Hancock, W.W.; Abe, K.; Micko, C.; Casper, K.A.; Baine, R.M.; Wilcox, J.N.; Danave, I.; Dillehay, D.L.; Matthews, E.; et al. Activation of coagulation and angiogenesis in cancer: Immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am. J. Pathol. 1998, 152, 399–411. [Google Scholar] [PubMed]
- Koomagi, R.; Volm, M. Tissue-factor expression in human non-small-cell lung carcinoma measured by immunohistochemistry: Correlation between tissue factor and angiogenesis. Int. J. Cancer 1998, 79, 19–22. [Google Scholar] [CrossRef]
- Sawada, M.; Miyake, S.; Ohdama, S.; Matsubara, O.; Masuda, S.; Yakumaru, K.; Yoshizawa, Y. Expression of tissue factor in non-small-cell lung cancers and its relationship to metastasis. Br. J. Cancer 1999, 79, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Berger, M.; Masters, G.; Albone, E.; Yang, Q.; Sheedy, J.; Kirksey, Y.; Grimm, L.; Wang, B.; Singleton, J.; et al. Radiotherapy of human xenograft NSCLC tumors in nude mice with a 90Y-labeled anti-tissue factor antibody. Cancer Biother. Radiopharm. 2005, 20, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Lau, C.P.-Y.; Ho, J.W.-Y.; Yu, W.-C.; Fan, S.-T.; Wong, J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin. Cancer Res. 2003, 9, 5339–5345. [Google Scholar] [PubMed]
- Kaido, T.; Oe, H.; Yoshikawa, A.; Mori, A.; Arii, S.; Imamura, M. Tissue factor is a useful prognostic factor of recurrence in hepatocellular carcinoma in 5-year survivors. Hepatogastroenterology 2005, 52, 1383–1387. [Google Scholar] [PubMed]
- Kakkar, A.K.; Lemoine, N.R.; Scully, M.F.; Tebbutt, S.; Williamson, R.C. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br. J. Surg. 1995, 82, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Nitori, N.; Ino, Y.; Nakanishi, Y.; Yamada, T.; Honda, K.; Yanagihara, K.; Kosuge, T.; Kanai, Y.; Kitajima, M.; Hirohashi, S. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2005, 11, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Ahrendt, S.A.; Ryan, C.K.; Francis, C.W.; Hruban, R.H.; Hu, Y.C.; Hostetter, G.; Harvey, J.; Taubman, M.B. Tissue factor expression, angiogenesis and thrombosis in pancreatic cancer. Clin. Cancer Res. 2007, 13, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, C.; Wada, H.; Matsumoto, K.; Shiku, H.; Nakamura, S.; Suzuki, H. Tissue factor expression and metastatic potential of colorectal cancer. Thromb. Haemost. 1998, 80, 894–898. [Google Scholar] [PubMed]
- Nakasaki, T.; Wada, H.; Shigemori, C.; Miki, C.; Gabazza, E.C.; Nobori, T.; Nakamura, S.; Shiku, H. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am. J. Hematol. 2002, 69, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Rotelli, M.T.; Pentimone, A.; Rossiello, M.R.; Martinelli, E.; Guglielmi, A.; De Fazio, M.; Marino, F.; Memeo, V.; Colucci, M.; et al. Tissue factor and vascular endothelial growth factor expression in colorectal cancer: Relation with cancer recurrence. Colorectal Dis. 2007, 9, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, S.A.; Carvalhal, G.F.; Kaleem, Z.; Kisiel, W.; Humphrey, P.A.; Catalona, W.J.; Milbrandt, J. Tissue factor expression and angiogenesis in human prostate carcinoma. Hum. Pathol. 2000, 31, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Furuya, Y.; Ohta, S.; Fuse, H. Tissue factor expression and prognosis in patients with metastatic prostate cancer. Urology 2003, 62, 1078–1082. [Google Scholar] [CrossRef]
- Kaushal, V.; Mukunyadzi, P.; Siegel, E.R.; Dennis, R.A.; Johnson, D.E.; Kohli, M. Expression of tissue factor in prostate cancer correlates with malignant phenotype. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Homma, S.; Satoh, T.; Nakanishi, K.; Abe, D.; Matsumoto, K.; Oki, A.; Tsunoda, H.; Yamaguchi, I.; Nagasawa, T.; et al. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br. J. Cancer 2007, 96, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Kuratsu, J.; Saitoh, Y.; Takeshima, H.; Nishi, T.; Ushio, Y. Expression of tissue factor correlates with grade of malignancy in human glioma. Cancer 1996, 77, 1877–1883. [Google Scholar] [CrossRef]
- Takano, S.; Tsuboi, K.; Tomono, Y.; Mitsui, Y.; Nose, T. Tissue factor, osteopontin, alphavbeta3 integrin expression in microvasculature of gliomas associated with vascular endothelial growth factor expression. Br. J. Cancer 2000, 82, 1967–1973. [Google Scholar] [PubMed]
- Guan, M.; Jin, J.; Su, B.; Liu, W.W.; Lu, Y. Tissue factor expression and angiogenesis in human glioma. Clin. Biochem. 2002, 35, 321–325. [Google Scholar] [CrossRef]
- Rickles, F.R.; Hair, G.A.; Zeff, R.A.; Lee, E.; Bona, R.D. Tissue factor expression in human leukocytes and tumor cells. Thromb. Haemost. 1995, 74, 391–395. [Google Scholar] [PubMed]
- Tanaka, H.; Narahara, N.; Kurabayashi, H.; Sadakata, H.; Andoh, K.; Uchiyama, T.; Kobayashi, N.; Maekawa, T. Studies on leukemic cell tissue factor. Thromb. Res. 1989, 53, 535–549. [Google Scholar] [CrossRef]
- Andoh, K.; Kubota, T.; Takada, M.; Tanaka, H.; Kobayashi, N.; Maekawa, T. Tissue factor activity in leukemia cells. Special reference to disseminated intravascular coagulation. Cancer 1987, 59, 748–754. [Google Scholar] [CrossRef]
- Bauer, K.A.; Conway, E.M.; Bach, R.; Konigsberg, W.H.; Griffin, J.D.; Demetri, G. Tissue factor gene expression in acute myeloblastic leukemia. Thromb. Res. 1989, 56, 425–430. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishi, T. Induction of tissue factor by interleukin-2 in acute myelogenous leukemia (AML) cells. Growth Factors 1990, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yamanishi, H. The expression of tissue factor antigen and activity on the surface of leukemic cells. Leuk. Res. 1993, 17, 103–111. [Google Scholar] [CrossRef]
- Nakasaki, T.; Wada, H.; Watanabe, R.; Mori, Y.; Gabazza, E.C.; Kageyama, S.; Nishikawa, M.; Shiku, H. Elevated tissue factor levels in leukemic cell homogenate. Clin. Appl. Thromb. Hemost. 2000, 6, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Andoh, K.; Sadakata, H.; Tanaka, H.; Kobayashi, N. Tissue factor released from leukemic cells. Thromb. Haemost. 1991, 65, 59–63. [Google Scholar] [PubMed]
- Nakasaki, T.; Wada, H.; Mori, Y.; Okugawa, Y.; Watanabe, R.; Nishikawa, M.; Gabazza, E.C.; Masuya, M.; Kageyama, S.; Kumeda, K.; et al. Decreased tissue factor and tissue-plasminogen activator antigen in relapsed acute promyelocytic leukemia. Am. J. Hematol. 2000, 64, 145–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Wendt, T.; Liliensiek, B.; Bierhaus, A.; Greten, J.; He, W.; Chen, B.; Hach-Wunderle, V.; Waldherr, R.; et al. Intravenous somatic gene transfer with antisense tissue factor restores blood flow by reducing tumor necrosis factor-induced tissue factor expression and fibrin deposition in mouse meth-A sarcoma. J. Clin. Investig. 1996, 97, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, J.G.; Slack, S.M. Tissue factor expression by rat osteosarcoma cells adherent to tissue culture polystyrene and selected orthopedic biomaterials. J. Biomater. Sci. Polym. Ed. 1998, 9, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Bachmann, S.; Hemmer, C.; van Lunzen, J.; von Stemm, A.; Kern, P.; Dietrich, M.; Ziegler, R.; Waldherr, R.; Nawroth, P.P. Vascular origin of Kaposi’s sarcoma. Expression of leukocyte adhesion molecule-1, thrombomodulin and tissue factor. Am. J. Pathol. 1994, 144, 51–59. [Google Scholar] [PubMed]
- Hu, Z.; Li, J. Natural killer cells are crucial for the efficacy of Icon (factor VII/human IgG1 Fc) immunotherapy in human tongue cancer. BMC Immunol. 2010, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Freeburn, J.C.; Gilmore, W.S.; Strain, J.J. The effect of cytokines on tissue factor expression in HL-60 and U937 cell lines. Biochem. Soc. Trans. 1995, 23, 286S. [Google Scholar] [CrossRef] [PubMed]
- Hair, G.A.; Padula, S.; Zeff, R.; Schmeizl, M.; Contrino, J.; Kreutzer, D.L.; de Moerloose, P.; Boyd, A.W.; Stanley, I.; Burgess, A.W.; et al. Tissue factor expression in human leukemic cells. Leuk. Res. 1996, 20, 1–11. [Google Scholar] [CrossRef]
- Tanaka, M. Induction of tissue factor-like activity of human monoblastic leukemia cell line by tumor necrosis factor-alpha. Thromb. Res. 1989, 56, 201–211. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Petrillo, M.; Bonanno, G.; Scambia, G. Targeting CD133 antigen in cancer. Expert Opin. Ther. Targets 2009, 13, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.; Kishimoto, H.; Fuchs, R.K.; Mehrotra, S.; Bhat-Nakshatri, P.; Turner, C.H.; Goulet, R., Jr.; et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006, 8, R59. [Google Scholar] [CrossRef] [PubMed]
- Adorno-Cruz, V.; Kibria, G.; Liu, X.; Doherty, M.; Junk, D.J.; Guan, D.; Hubert, C.; Venere, M.; Mulkearns-Hubert, E.; Sinyuk, M.; et al. Cancer stem cells: Targeting the roots of cancer, seeds of metastasis and sources of therapy resistance. Cancer Res. 2015, 75, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O.; Feldmann, M.; Maini, R.N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 1992, 89, 9784–9788. [Google Scholar] [CrossRef] [PubMed]
- Busso, N.; Morard, C.; Salvi, R.; Péclat, V.; So, A. Role of the tissue factor pathway in synovial inflammation. Arthritis Rheumatol. 2003, 48, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, Y.; Chu, Y.; Xie, J.; Ding, W.; Wang, F. Tissue factor expression in rheumatoid synovium: A potential role in pannus invasion of rheumatoid arthritis. Acta Histochem. 2013, 115, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Koch, A.E. Angiogenesis and its targeting in rheumatoid arthritis. Vasc. Pharmacol. 2009, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Colville-Nash, P.R.; Scott, D.L. Angiogenesis and rheumatoid arthritis: Pathogenic and therapeutic implications. Ann. Rheum. Dis. 1992, 51, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Paleolog, E.M.; Fava, R.A. Angiogenesis in rheumatoid arthritis: Implications for future therapeutic strategies. Springer Semin. Immunopathol. 1998, 20, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Szegedi, G.; Koch, A.E. Angiogenesis in rheumatoid arthritis: Pathogenic and clinical significance. J. Investig. Med. 1998, 46, 27–41. [Google Scholar] [PubMed]
- Stupack, D.G.; Storgard, C.M.; Cheresh, D.A. A role for angiogenesis in rheumatoid arthritis. Braz. J. Med. Biol. Res. 1999, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Paleolog, E.M. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002, 4 (Suppl. 3), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Gaspar, L.; Koch, A.E. Angiogenesis in rheumatoid arthritis. Front. Biosci. 2005, 10, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Cantatore, F.P.; Crivellato, E.; Vacca, A.; Ribatti, D. Angiogenesis in rheumatoid arthritis. Histol. Histopathol. 2006, 21, 557–566. [Google Scholar] [PubMed]
- Szekanecz, Z.; Besenyei, T.; Paragh, G.; Koch, A.E. Angiogenesis in rheumatoid arthritis. Autoimmunity 2009, 42, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, A.; Cipriani, P.; Liakouli, V.; Carubbi, F.; Perricone, C.; Perricone, R.; Giacomelli, R. Angiogenesis in rheumatoid arthritis: A disease specific process or a common response to chronic inflammation? Autoimmun. Rev. 2011, 10, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Koch, A.E. Endothelial cells in inflammation and angiogenesis. Curr. Drug Targets Inflamm. Allergy 2005, 4, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Wakita, Y.; Shiku, H. Tissue factor expression in endothelial cells in health and disease. Blood Coagul. Fibrinolysis 1995, 6 (Suppl. 1), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Osterud, B.; Bjorklid, E. The production and availability of tissue thromboplastin in cellular populations of whole blood exposed to various concentrations of endotoxin. An assay for detection of endotoxin. Scand. J. Haematol. 1982, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.C.; Jones, N.L.; Hermanns, M.I.; Röhrig, O.; Klein, C.L.; Kirkpatrick, C.J. Tissue factor expression during coculture of endothelial cells and monocytes. Exp. Mol. Pathol. 1995, 62, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Luther, T.; Flössel, C.; Hietschhold, V.; Koslowski, R.; Müller, M. Flow cytometric analysis of tissue factor (TF) expression on stimulated monocytes-comparison to procoagulant activity of mononuclear blood cells. Blut 1990, 61, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.M.; Savi, P.; Laplace, M.C.; Lale, A. IL-4 inhibits LPS-, IL-1 beta- and TNF alpha-induced expression of tissue factor in endothelial cells and monocytes. FEBS Lett. 1992, 310, 31–33. [Google Scholar] [CrossRef]
- Herbert, J.-M.; Savi, P.; Lalé, A.; Laplace, M.-C.; Baudry, N.; Pereillo, J.-M.; Emonds-Alt, X. Malformin-A1 inhibits the binding of interleukin-1 beta (IL1 beta) and suppresses the expression of tissue factor in human endothelial cells and monocytes. Biochem. Pharmacol. 1994, 48, 1211–1217. [Google Scholar] [CrossRef]
- Camerer, E.; Kolsto, A.B.; Prydz, H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb. Res. 1996, 81, 1–41. [Google Scholar] [CrossRef]
- Pendurthi, U.R.; Alok, D.; Rao, L.V. Binding of factor VIIa to tissue factor induces alterations in gene expression in human fibroblast cells: Up-regulation of poly(A) polymerase. Proc. Natl. Acad. Sci. USA 1997, 94, 12598–12603. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, E.F.; Zuckerman, D.B.; Nemerson, Y. The functional expression of tissue factor by fibroblasts and endothelial cells under flow conditions. Blood 1993, 81, 3265–3270. [Google Scholar] [PubMed]
- Juarez, M.; Filer, A.; Buckley, C.D. Fibroblasts as therapeutic targets in rheumatoid arthritis and cancer. Swiss Med. Wkly. 2012, 142, w13529. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.; Burmester, G.R. The role of B cells in rheumatoid arthritis: Mechanisms and therapeutic targets. Curr. Opin. Rheumatol. 2003, 15, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.; Lipsky, P.E. B-cell targeting: A novel approach to immune intervention today and tomorrow. Expert Opin. Biol. Ther. 2007, 7, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.; Isenberg, D.; Jayne, D.; Wiendl, H.; Zillikens, D.; Burmester, G. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmun. Rev. 2009, 9, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.; Kinnman, N.; Tak, P.P. Targeting B cells in immune-mediated inflammatory disease: A comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol. Ther. 2010, 125, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Mechiche, H.; Cornillet-Lefebvre, P.; Nguyen, P. A subpopulation of human B lymphocytes can express a functional Tissue Factor in response to phorbol myristate acetate. Thromb. Haemost. 2005, 94, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Mechiche, H.; Nguyen, P. IL-4 modulates tissue factor expression by human B lymphocytes in response to phorbol myristate acetate. Thromb. Haemost. 2007, 97, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Badolato, R.; Oppenheim, J.J. Role of cytokines, acute-phase proteins and chemokines in the progression of rheumatoid arthritis. Semin. Arthritis Rheum. 1996, 26, 526–538. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Pakozdi, A.; Szentpetery, A.; Besenyei, T.; Koch, A.E. Chemokines and angiogenesis in rheumatoid arthritis. Front. Biosci. 2009, 1, 44–51. [Google Scholar]
- Deane, K.D.; O'Donnell, C.I.; Hueber, W.; Majka, D.S.; Lazar, A.A.; Derber, L.A.; Gilliland, W.R.; Edison, J.D.; Norris, J.M.; Robinson, W.H.; et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheumatol. 2010, 62, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, H.; Söderström, I.; Rocklöv, J.; Hallmans, G.; Lejon, K.; Dahlqvist, S.R. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheumatol. 2010, 62, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Puhlmann, M.; Bartlett, D.L.; Libutti, S.K.; Turner, E.N.; Gnant, M.F.X.; Alexander, H.R. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood 2002, 100, 1334–1339. [Google Scholar] [PubMed]
- Wilcox, J.N.; Smith, K.M.; Schwartz, S.M.; Gordon, D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA 1989, 86, 2839–2843. [Google Scholar] [CrossRef] [PubMed]
- Thiruvikraman, S.V.; Guha, A.; Roboz, J.; Taubman, M.B.; Nemerson, Y.; Fallon, J.T. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab. Investig. 1996, 75, 451–461. [Google Scholar] [PubMed]
- Toschi, V.; Gallo, R.; Lettino, M.; Fallon, J.T.; Gertz, S.D.; Ferna´ndez-Ortiz, A.; Chesebro, J.H.; Badimon, L.; Nemerson, Y.; Fuster, V.; et al. Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation 1997, 95, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Mackman, N. Tissue Factor and Atherothrombosis. J. Atheroscler. Thromb. 2015, 22, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Grainger, D.; Mayr, U.; Leroyer, A.S.; Leseche, G.; Sidibe, A.; Herbin, O.; Yin, X.; Gomes, A.; Madhu, B.; Griffiths, J.R.; et al. Proteomics, metabolomics and immunomics on microparticles derived from human atherosclerotic plaques. Circ. Genom. Precis. Med. 2009, 2, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Badimon, J.J. Atherothrombosis: The role of tissue factor. Int. J. Biochem. Cell Biol. 2004, 36, 25–30. [Google Scholar] [CrossRef]
- Saha, D.; Saha, S.; Sergeeva, E.G.; Ionova, Z.I.; Gorbach, A.V. Tissue factor and atherothrombosis. Curr. Pharm. Des. 2015, 21, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Camera, M.; Toschi, V.; Brambilla, M.; Lettino, M.; Rossetti, L.; Canzano, P.; Di Minno, A.; Tremoli, E. The Role of Tissue Factor in Atherothrombosis and Coronary Artery Disease: Insights into Platelet Tissue Factor. Semin. Thromb. Hemost. 2015, 41, 737–746. [Google Scholar] [PubMed]
- Owens, A.P., III; Mackman, N. Role of tissue factor in atherothrombosis. Curr. Atheroscler. Rep. 2012, 14, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Basili, S.; Alessandri, C.; Mantovani, B.; Cordova, C.; Violi, F. Simvastatin reduces monocyte-tissue-factor expression type IIa hypercholesterolaemia. Lancet 1997, 350, 1222. [Google Scholar] [CrossRef]
- Matetzky, S.; Tani, S.; Kangavari, S.; Dimayuga, P.; Yano, J.; Xu, H.; Chyu, K.-Y.; Fishbein, M.C.; Shah, P.K.; Cercek, B. Smoking increases tissue factor expression in atherosclerotic plaques: Implications for plaque thrombogenicity. Circulation 2000, 102, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P., III; Passam, F.H.; Antoniak, S.; Marshall, S.M.; McDaniel, A.L.; Rudel, L.; Williams, J.C.; Hubbard, B.K.; Dutton, J.-A.; Wang, J.; et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J. Clin. Investig. 2012, 122, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Benaroch, P.; Billard, E.; Gaudin, R.; Schindler, M.; Jouve, M. HIV-1 assembly in macrophages. Retrovirology 2010, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, Z.F.; Fauci, A.S. Immunopathogenesis of HIV infection. FASEB J. 1991, 5, 2382–2390. [Google Scholar] [PubMed]
- Funderburg, N.T.; Mayne, E.; Sieg, S.F.; Asaad, R.; Jiang, W.; Kalinowska, M.; Luciano, A.A.; Stevens, W.; Rodriguez, B.; Brenchley, J.M.; et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: Relationship to in vivo coagulation and immune activation. Blood 2010, 115, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Borgstrom, P.; Maynard, J.; Koziol, J.; Hu, Z.; Garen, A.; Deisseroth, A. Mapping of angiogenic markers for targeting of vectors to tumor vascular endothelial cells. Cancer Gene Ther. 2007, 14, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Young, H.A.; Jahrling, P.B.; Davis, K.J.; Kagan, E.; Hensley, L.E. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: Overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 2003, 188, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Hensley, L.E.; Jahrling, P.B.; Larsen, T.; Geisbert, J.B.; Paragas, J.; Young, H.A.; Fredeking, T.M.; Rote, W.E.; Vlasuk, G.P. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: A study in rhesus monkeys. Lancet 2003, 362, 1953–1958. [Google Scholar] [CrossRef]
- Hu, Z. Overcome the Impairment of NK Cells for Icon and Antibody Immunotherapy of Cancer. J. Immune Based Ther. Vaccines Antimicrob. 2013, 2, 1–8. [Google Scholar] [CrossRef]
- Cocco, E.; Hu, Z.; Richter, C.E.; Bellone, S.; Casagrande, F.; Bellone, M.; Todeschini, P.; Krikun, G.; Silasi, D.-A.; Azodi, M.; et al. hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor for immunotherapy of uterine serous papillary carcinoma. Br. J. Cancer 2010, 103, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A. Role of current and emerging antithrombotics in thrombosis and cancer. Timely Top Med. Cardiovasc. Dis. 2006, 10, E19. [Google Scholar] [CrossRef] [PubMed]
- Waxman, E.; Ross, A.J.B.; Laue, T.M.; Guha, A.; Thiruvikraman, S.V.; Lin, T.C.; Konigsberg, W.H.; Nemerson, Y. Tissue factor and its extracellular soluble domain: The relationship between intermolecular association with factor VIIa and enzymatic activity of the complex. Biochemistry 1992, 31, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Presta, L.; Sims, P.; Meng, Y.G.; Moran, P.; Bullens, S.; Bunting, S.; Schoenfeld, J.; Lowe, D.; Lai, J.; Rancatore, P.; et al. Generation of a humanized, high affinity anti-tissue factor antibody for use as a novel antithrombotic therapeutic. Thromb. Haemost. 2001, 85, 379–389. [Google Scholar] [PubMed]
- Chudasama, V.; Maruani, A.; Caddick, S. Recent advances in the construction of antibody-drug conjugates. Nat. Chem. 2016, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Breij, E.C.W.; de Goeij, B.E.C.G.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Zhao, H.; Ma, L.; Meng, T.; Qian, J.; Jin, R.; Shen, J.; Yu, K. Pathological expression of tissue factor confers promising antitumor response to a novel therapeutic antibody SC1 in triple negative breast cancer and pancreatic adenocarcinoma. Oncotarget 2017, 8, 59086–59102. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Bolbrinker, J.; Leppert, U. Tissue Factor: A Conventional or Alternative Target in Cancer Therapy. Clin Chem, 2016, 62, 563–570. [Google Scholar] [CrossRef] [PubMed]
- De Goeij, B.E.; Satijn, D.; Freitag, C.M.; Wubbolts, R.; Bleeker, W.K.; Khasanov, A.; Zhu, T.; Chen, G.; Miao, D.; van Berkel, P.H.C.; et al. High turnover of tissue factor enables efficient intracellular delivery of antibody-drug conjugates. Mol. Cancer Ther. 2015, 14, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Pecen, P.E.; Kaiser, P.K. Current phase 1/2 research for neovascular age-related macular degeneration. Curr. Opin. Ophthalmol. 2015, 26, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Christmas, N.J. A Phase 2 Study (EMERGE) Evaluating Repeated Intravitreal Administration of ICON-1 in Patients with Choroidal Neovascularization (CNV) Secondary to Age-related Macular Degeneration (AMD). Investig. Ophthalmol. Vis. Sci. 2016, 57, 4434. [Google Scholar]

| Type of Tumor | Case Number | % on TC | % on TVEC | References |
|---|---|---|---|---|
| Breast cancer | 115 | 81% | ND | [50] |
| 7 | 100% | 100% | [32] | |
| 213 | 91% | 98.6% (stromal cells) | [51] | |
| Human chemoresistant breast tumor xenograft from mice * | + | + | [36] | |
| Melanoma | 41 primary 42 metastatic | 95% 100% | ND | [52] |
| Human melanoma xenograft from mice * | + | + | [33] | |
| Lung cancer | 25 | 28% | 78% (stromal macrophages, VECs) | [53] |
| 191 (NSCLC) | 43% | ND | [54] | |
| 55 | 80% | ND | [55] | |
| 50 | 88% | ND | [56] | |
| 12 (snap-frozen adenocarcinoma NSCLC tissues) | 66.7% (8/12) moderately positive for flTF and 91.7% (11/12) for asTF ** vs. the overall negative control healthy tissue | ND | [12] | |
| Hepatocellular carcinoma (HCC) | 58 | 100% | ND | [57] |
| 62 | 63% | ND | [58] | |
| Pancreatic cancer | 55 | 53% | TF negative in normal pancreas | [59] |
| 113 | 88.4% | ND | [60] | |
| 240 (10 normal pancreas 70 intraductal papillary mucinous neoplasms 40 pancreatic intraepithelial neoplasia, 130 resected or metastatic pancreatic adenocarcinomas) | 87.9% overall (77% pancreatic intraepithelial neoplasias 91% intraductal papillary mucinous neoplasms 89% pancreatic cancers) | ND (TF negative in normal pancreas) | [61] | |
| Colorectal cancer | 67 primary, of which 18 with liver metastasis | 46% of primary, 88.9% of liver metastasis | ND | [62] |
| 100 | 57.0% | ND | [63] | |
| 50 | 100% | ND | [64] | |
| Prostate cancer | 67 | 73% | ND | [65] |
| 73 | 75.3% | ND | [66] | |
| 32 early stage 22 advanced stage | 78% early-stage 60% advanced stage | ND (TF negative in benign prostate gland) | [67] | |
| Human prostate tumor in mice *** | + | + | [34] | |
| Ovarian cancer | 32 | 84% | ND | [68] |
| Glioma | 44 (10 benign gliomas 14 anaplastic astrocytomas 20 glioblastomas) | 75% overall (10% in Grade I-II, 86% in grade III 95% in grade IV) | ND | [69] |
| 68 (23 glioblastomas 13 anaplastic astrocytomas 32 low-grade astrocytomas) | 47% overall (91.3% glioblastomas, 46.2% anaplastic astrocytomas and 15.6% low-grade astrocytomas) | 44% overall (73.9% glioblastomas, 53.8% anaplastic astrocytomas, 0% low grade astrocytomas) | [70] | |
| 34 gliomas 5 normal brain tissues | 58.8% overall (20% of grade I 43% of grade II, 58% of grade III 90% of grade IV) | ND (TF negative in normal brain tissues) | [71] | |
| Leukemia | Human AML lines and leukemic cells from patients with AML | + | TF negative on the normal peripheral mononuclear cells unless stimulated by endotoxin or other cytokines [72] | [73,74,75,76,77,78] |
| Human ALL lines and leukemic cells from patients with ALL | + | TF negative on myeloid precursor cells [75] | [79,80] | |
| Sarcoma | Mouse Meth-A sarcoma cells | + | [81] | |
| Rat osteosarcoma cells | + | [82] | ||
| Kaposi’s sarcoma **** | + | [83] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z. Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases. Antibodies 2018, 7, 8. https://doi.org/10.3390/antib7010008
Hu Z. Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases. Antibodies. 2018; 7(1):8. https://doi.org/10.3390/antib7010008
Chicago/Turabian StyleHu, Zhiwei. 2018. "Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases" Antibodies 7, no. 1: 8. https://doi.org/10.3390/antib7010008





