Photochirogenesis: Photochemical Models on the Origin of Biomolecular Homochirality
Abstract
:1. Introduction
2. Organic Chiral Molecules in Interstellar Environments
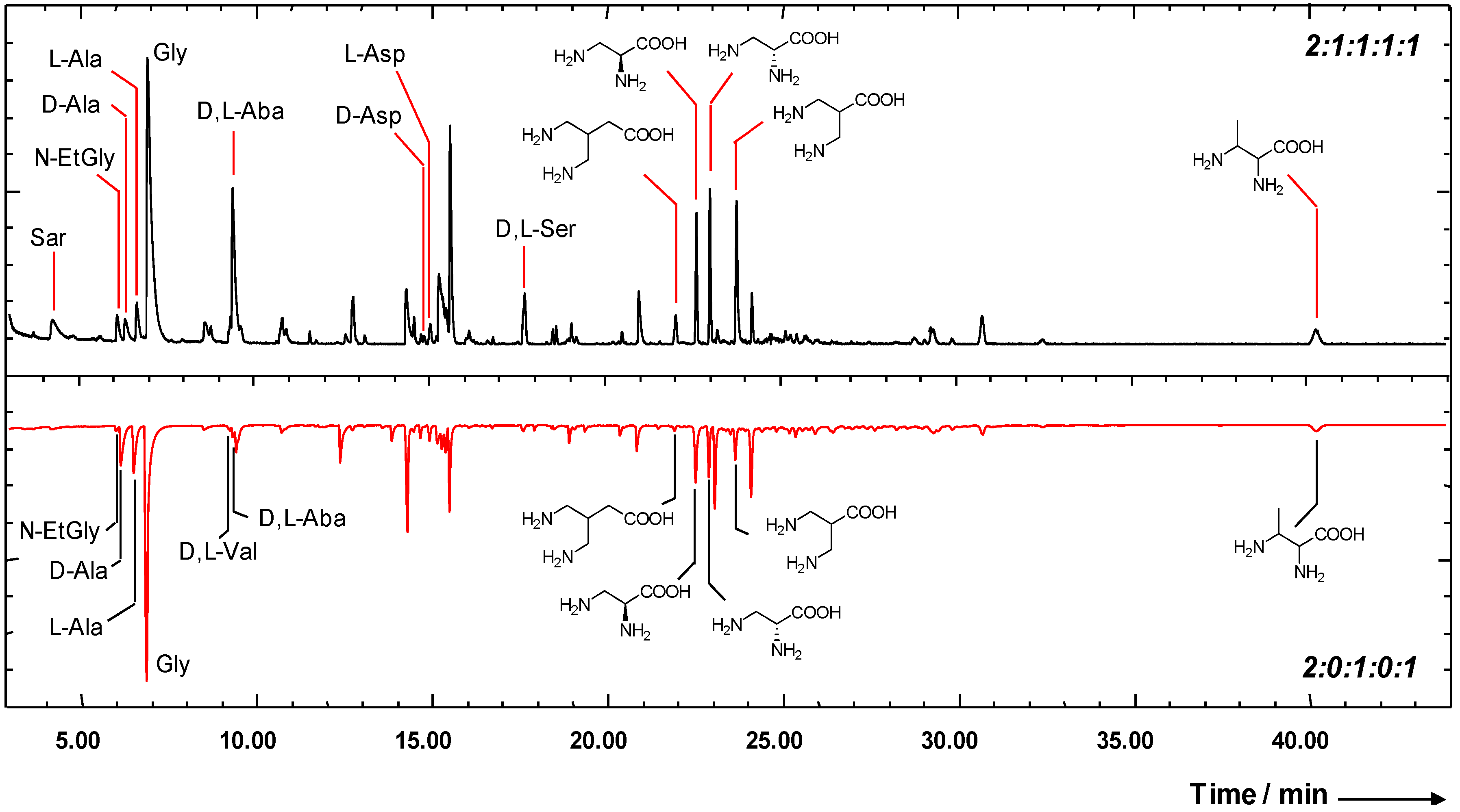
2.1. Amino Acid Formation in Simulated Interstellar Environments
2.2. Enantiomeric Enrichment in Meteoritic Amino Acids
2.3. Reaction Mechanisms for the Formation of Interstellar Amino Acids
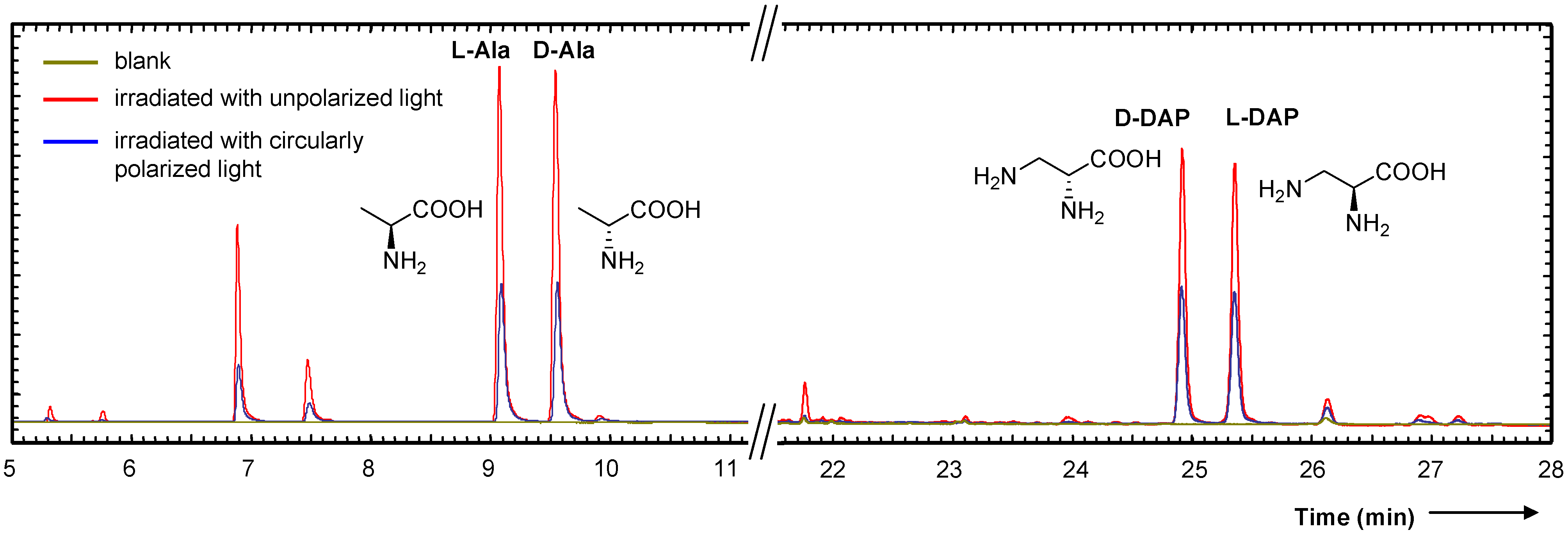
3. Asymmetric Photolysis of Racemic Organic Molecules
4. Enantioselective Photosynthesis
5. Rosetta–The First Landing on a Cometary Nucleus
6. Criticism
7. Conclusions
Acknowledgments
References and Notes
- Bonner, W.A. The origin and amplification of biomolecular chirality. Orig. Life Evol. Biosph. 1991, 21, 59–111. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Sagan, C.; Khare, B.N. Long-wavelength ultraviolet photoproduction of amino acids on the primitive Earth. Science 1971, 173, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.F.; Thomas, P.J.; Brookshaw, L.; Sagan, C. Cometary delivery of organic molecules to the early earth. Science 1990, 249, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F.; Catling, D. Evolution of a habitable planet. Annu. Rev. Astron. Astrophys. 2003, 41, 429–463. [Google Scholar] [CrossRef]
- Stribling, R.; Miller, S.L. Energy yields for hydrogen cyanide and formaldehyde syntheses: The hydrogen cyanide and amino acid concentrations in the primitive ocean. Orig. Life Evol. Biosph. 1987, 17, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U. Amino Acids and the Asymmetry of Life; Springer-Verlag: Berlin-Heidelberg, Germany, 2008; p. 241. [Google Scholar]
- Karagunes, G.; Coumoulos, G. A new method of resolving a racemic compound. Nature 1938, 142, 162–163. [Google Scholar] [CrossRef]
- Bonner, W.A.; Kavasmaneck, P.R.; Martin, F.S.; Flores, J.J. Asymmetric adsorption of alanine by quartz. Science 1974, 186, 143–144. [Google Scholar] [CrossRef]
- Bonner, W.A. The quest for chirality. AIP Conf. Proc. 1996, 379, 17–49. [Google Scholar]
- Yamagata, Y. A hypothesis for the asymmetric appearance of biomolecules on earth. J. Theor. Biol. 1966, 11, 495–498. [Google Scholar] [CrossRef]
- Vester, F.; Ulbricht, T.L.V.; Krauch, H. Optical activity and parity violation in ß-decay. Naturwissenschaften 1959, 46, 68. [Google Scholar] [CrossRef]
- Goldhaber, M.; Grodzins, L.; Sunyar, A.W. Evidence for circular polarization of bremsstrahlung produced by ß-rays. Phys. Rev. 1957, 106, 826–828. [Google Scholar] [CrossRef]
- Bonner, W.A. Experiments on the origin of molecular chirality by parity nonconservation during ß-decay. J. Mol. Evol. 1974, 4, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.D. Fundamental symmetry aspects of chirality. BioSystems 1987, 20, 7–14. [Google Scholar] [CrossRef]
- Barron, L.D. Can a magnetic field induce absolute asymmetric synthesis? Science 1994, 266, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, W.; Dougherty, R.C. Effects of electric and magnetic fields on prochiral chemical reactions: Macroscopic electric and magnetic fields can cause asymmetric synthesis. J. Am. Chem. Soc. 1978, 100, 6247–6248. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Raupach, E. Pure and cascaded magnetochiral anisotropy in optical absorption. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics 1998, 58, 5081–5084. [Google Scholar] [CrossRef]
- Kuhn, W.; Braun, E. Photochemical preparation of optically active substances. Naturwissenschaften 1929, 17, 227–228. [Google Scholar] [CrossRef]
- Kuhn, W.; Knopf, E. The preparation of optically active compounds by the aid of light. Z. Physik. Chem. 1930, 7, 292–310. [Google Scholar]
- Kuhn, W.; Knopf, E. Photochemical preparation of optically active substances. Naturwissenschaften 1930, 18, 183. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Huang, Y.S. Carbon isotopic analyses of individual Murchison amino acids. Geochim. Cosmochim. Acta 2003, 67, A380–A380. [Google Scholar]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.; Bonner, W.A.; Noyes, H.P.; Brown, G.S. Supernovae and life. Nature 1983, 306, 118. [Google Scholar] [CrossRef]
- Bonner, W.A.; Rubenstein, E. Supernovae, neutron stars and biomolecular chirality. BioSystems 1987, 20, 99–111. [Google Scholar] [CrossRef]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Menard, F.; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef]
- Bailey, J. Astronomical sources of circularly polarized light and the origin of homochirality. Orig. Life Evol. Biosph. 2001, 31, 167–183. [Google Scholar] [CrossRef]
- Buschermoehle, M.; Whittet, D.C.B.; Chrysostomou, A.; Hough, J.H.; Lucas, P.W.; Adamson, A.J.; Whitney, B.A.; Wolff, M.J. An extended search for circularly polarized infrared radiation from the OMC-1 region of orion. Astrophys. J. 2005, 624, 821–826. [Google Scholar] [CrossRef]
- Allamandola, L.J.; Bernstein, M.P.; Sandford, S.A.; Walker, R.L. Evolution of interstellar ices. Space Sci. Rev. 1999, 90, 219–232. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Allamandola, L.J.; Sandford, S.A. Complex organics in laboratory simulations of interstellar/cometary ices. Adv. Space Res. 1997, 19, 991–998. [Google Scholar] [CrossRef]
- Nuevo, M.; Bredehoft, J.H.; Meierhenrich, U.; d’Hendecourt, L.; Thiemann, W. Urea, glycerol and glycolic acid in an organic residue produced by UV irradiation of interstellar/pre-cometary ice analogues. Astrobiology 2010, 10, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Munoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbler, B.; Segovia, A.A.; Rosenbauer, H.; Thiemann, W.H.P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Auger, G.; Blanot, D.; d’Hendecourt, L. A detailed study of the amino acids produced from the vacuum UV irradiation of interstellar ice analogs. Orig. Life Evol. Biosph. 2008, 38, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.M. What are comets made of? A model based on interstellar dust. In Comets; University of Arizona Press: Tucson, AZ, USA, 1982; pp. 131–163. [Google Scholar]
- Greenberg, J.M. Chemical evolution in space. Orig. Life 1984, 24, 25–36. [Google Scholar] [CrossRef]
- Greenberg, J.M. Chirality in interstellar dust and in comets: Life from dead stars. AIP Conf. Proc. 1996, 379, 185–210. [Google Scholar]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Oro, J. Comets and formation of biochemical compounds on primitive earth. Nature 1961, 190, 389–390. [Google Scholar] [CrossRef]
- Greenberg, J.M. The structure and evolution of interstellar grains. Sci. Am. 1984, 250, 124–154. [Google Scholar] [CrossRef]
- Takenaka, N. Recent Developments of Chemistry and Photochemistry in Ices; Transworld Research Network: Kerala, India, 2008; p. 202. [Google Scholar]
- Agarwal, V.K.; Schutte, W.; Greenberg, J.M.; Ferris, J.P.; Briggs, R.; Connor, S.; Van de Bult, C.P.E.M.; Baas, F. Photochemical reactions in interstellar grains photolysis of carbon monoxide, ammonia, and water. Orig. Life 1985, 16, 21–40. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Charnley, S.B. Organic molecules in the interstellar medium, comets, and meteorites: A voyage from dark clouds to the early earth. Annu. Rev. Astron. Astrophys. 2000, 38, 427–483. [Google Scholar] [CrossRef]
- Irvine, W.M. The molecular composition of dense interstellar clouds. NATO ASI Ser. Ser. C 1991, 323, 89–121. [Google Scholar]
- Chiar, J.E. The nature and evolution of interstellar ices. Orig. Life Evol. Biosph. 1997, 27, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.M. Radical formation, chemical processing, and explosion of interstellar grains. Astrophys. Space Sci. 1976, 39, 9–18. [Google Scholar] [CrossRef]
- Duley, W.W. Chemical evolution of carbonaceous material in interstellar clouds. Astrophys. J. 2000, 528, 841–848. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Kerkhof, O.; Schutte, W.A.; Boogert, A.C.A.; Gerakines, P.A.; Dartois, E.; D’Hendecourt, L.; Tielens, A.G.G.M.; Van Dishoeck, E.F.; Whittet, D.C.B. Laboratory studies of thermally processed H2O-CH3OH-CO2 ice mixtures and their astrophysical implications. Astron. Astrophys. 1999, 350, 240–253. [Google Scholar]
- Gibb, E.L.; Whittet, D.C.B.; Boogert, A.C.A.; Tielens, A.G.G.M. Interstellar ice: The infrared space observatory legacy. Astrophys. J. Suppl. Ser. 2004, 151, 35–73. [Google Scholar] [CrossRef]
- Cosmic rays are charged high energetic particles which are known to create secondary electrons capable of triggering chemical reactions.
- DHendecourt, L.B.; Allamandola, L.J.; Baas, F.; Greenberg, J.M. Inter-stellar grain explosions–molecule cycling between gas and dust. Astron. Astrophys. 1982, 109, L12–L14. [Google Scholar]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Kaneko, T.; Kobayashi, K.; Hiroishi, D.; Ikeda, H.; Marumo, K. Experimental verification of photostability for free- and bound-amino acids exposed to gamma-rays and UV irradiation. Earth Planets Space 2004, 56, 669–674. [Google Scholar] [CrossRef]
- Elsila, J.E.; Dworkin, J.P.; Bernstein, M.P.; Martin, M.P.; Sandford, S.A. Mechanisms of amino acid formation in interstellar ice analogs. Astrophys. J. 2007, 660, 911–918. [Google Scholar] [CrossRef]
- Briggs, R.; Ertem, G.; Ferris, J.P.; Greenberg, J.M.; McCain, P.J.; Mendoza-Gomez, C.X.; Schutte, W. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Orig. Life Evol. Biosph. 1992, 22, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.-I.; Masuda, H.; Kaneko, T.; Kobayashi, K.; Saito, T.; Hosokawa, T. Photochemical abiotic synthesis of amino-acid precursors from simulated planetary atmospheres by vacuum ultraviolet light. J. Appl. Phys. 2005, 98, 024907.1–024907.6. [Google Scholar] [CrossRef]
- Cottin, H.; Szopa, C.; Moore, M.H. Production of hexamethylenetetramine in photolyzed and irradiated interstellar cometary ice analogs. Astrophys. J. 2001, 561, L139–L142. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kaneko, T.; Saito, T. Characterization of complex organic compounds formed in simulated planetary atmospheres by the action of high energy particles. Adv. Space Res. 1999, 24, 461–464. [Google Scholar] [CrossRef]
- Takahashi, J.-I.; Hosokawa, T.; Masuda, H.; Kaneko, T.; Kobayashi, K.; Saito, T.; Utsumi, Y. Abiotic synthesis of amino acids by x-ray irradiation of simple inorganic gases. Appl. Phys. Lett. 1999, 74, 877–879. [Google Scholar] [CrossRef]
- Takano, Y.; Masuda, H.; Kaneko, T.; Kobayashi, K. Formation of amino acids from possible interstellar media by gamma-rays and UV irradiation. Chem. Lett. 2002, 986–987. [Google Scholar] [CrossRef]
- Takano, Y.; Marumo, K.; Yabashi, S.; Kaneko, T.; Kobayashi, K. Pyrolysis of complex organics following high-energy proton irradiation of a simple inorganic gas mixture. Appl. Phys. Lett. 2004, 85, 1633–1635. [Google Scholar] [CrossRef]
- Cronin, J.R.; Chang, S. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorite. In Chemistry of Life’s Origins (NATO ASI Ser.); Greenberg, J.M., Mendoza-Gomez, C.X., Pirronello, V., Eds.; Kluwer: Dordrecht, The Netherlands, 1993; Volume 416, pp. 209–258. [Google Scholar]
- Most of the components can be obtained by extracting meteorite powders with polar and nonpolar solvents. Nevertheless, the quantities of detectable amino acids increase dramatically when using acid hydrolysis. Hydrolytic breakdown of small polypeptides or acid-labile amino acid precursors are assumed to account for these increases [65].
- Stoks, P.G.; Schwartz, A.W. Uracil in carbonaceous meteorites. Nature 1979, 282, 709–710. [Google Scholar] [CrossRef]
- Stoks, P.G.; Schwartz, A.W. Basic nitrogen-heterocyclic compounds in the Murchison meteorite. Geochim. Cosmochim. Acta 1982, 46, 309–315. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Caro, G.M.M.; Bredehoft, J.H.; Jessberger, E.K.; Thiemann, W.H.P. Identification of diamino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. 2004, 101, 9182–9186. [Google Scholar] [CrossRef] [PubMed]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Orig. Life Evol. Biosph. 2009, 39, 214–214. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.S.; Fuller, M. The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 2004, 68, 4963–4969. [Google Scholar] [CrossRef]
- Bredehoft, J.H.; Meierhenrich, U. Amino Acid Structures from UV Irradiation of Simulated Interstellar Ices; Transworld Research Network: Kerala, India, 2008; pp. 175–202. [Google Scholar]
- Balavoine, G.; Moradpour, A.; Kagan, H.B. Preparation of chiral compounds with high optical purity by irradiation with circularly polarized light, A model reaction for the prebiotic generation of optical activity. J. Am. Chem. Soc. 1974, 96, 5152–5158. [Google Scholar] [CrossRef]
- Nishino, H.; Kosaka, A.; Hembury, G.A.; Matsushima, K.; Inoue, Y. The pH dependence of the anisotropy factors of essential amino acids. J. Chem. Soc. Perkin Trans. 2002, 582–590. [Google Scholar] [CrossRef]
- Brückner, H.; Maisch, J.; Reinecke, C.; Kimonyo, A. Use of α-aminoisobutyric acid and isovaline as marker amino acids for the detection of fungal polypeptide antibiotics. Screen. Hypocrea. Amino Acids 1991, 1, 251–257. [Google Scholar] [CrossRef]
- Bruckner, H.; Becker, D.; Gams, W.; Degenkolb, T. Aib and Iva in the biosphere: Neither rare nor necessarily extraterrestrial. Chem. Biodivers. 2009, 6, 38–56. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A.; Silfer, J.A. Carbon isotope composition of individual amino-acids in the Murchison meteorite. Natsure 1990, 348, 47–49. [Google Scholar] [CrossRef]
- Epstein, S.; Krishnamurthy, R.V.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Unusual stable isotope ratios in amino-acid and carboxylic-acid extracts from the Murchison meteorite. Nature 1987, 326, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.; Blair, N.; Desmarais, D.J.; Chang, S. Carbon isotope composition of low-molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 1984, 307, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Feng, X.; Epstein, S.; Cronin, J.R. Isotopic analyses of nitrogenous compounds from the Murchison meteorite—Ammonia, Amines, Amino-Acids, and Polar Hydrocarbons. Geochim. Cosmochim. Acta 1994, 58, 5579–5587. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.S. The deuterium enrichment of individual amino acids in carbonaceous meteorites: A case for the presolar distribution of biomolecule precursors. Geochim. Cosmochim. Acta 2005, 69, 599–605. [Google Scholar] [CrossRef]
- Pizzarello, S.; Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R. Isotopic analyses of amino-acids from the Murchison meteorite. Geochim. Cosmochim. Acta 1991, 55, 905–910. [Google Scholar] [CrossRef]
- Cohen, B.A.; Chyba, C.F. Racemization of meteoritic amino acids. Icarus 2000, 145, 272–281. [Google Scholar] [CrossRef]
- Cronin, J.R.; Moore, C.B. Amino acid analyses of Murchison, Murray, and Allende carbonaceous chondrites. Science 1971, 172, 1327–1328. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of Cl type carbonaceous chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef]
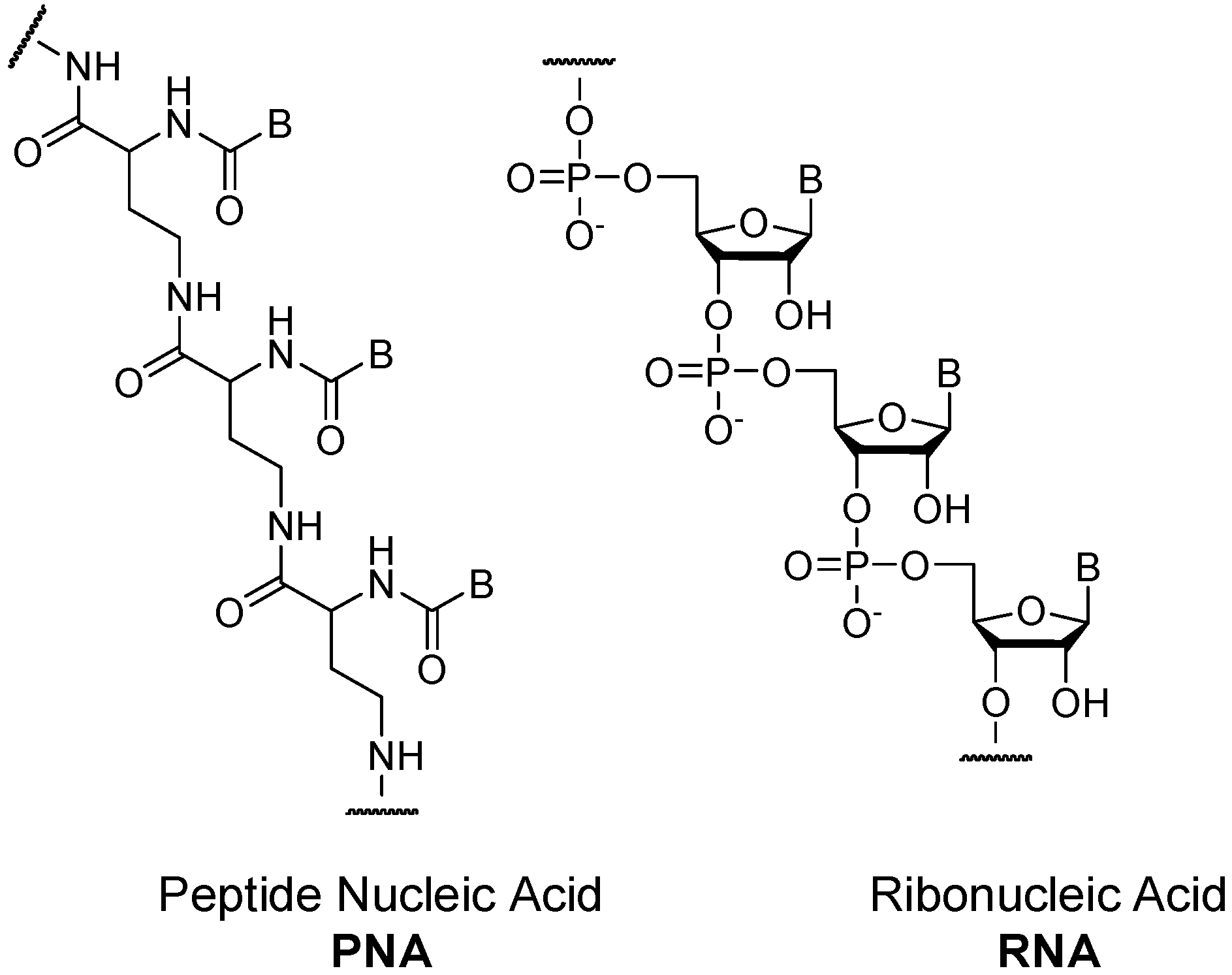
- Nielsen, P.E. Peptide Nucleic-Acid (PNA)–A model structure for the primordial genetic material. Orig. Life Evol. Biosph. 1993, 23, 323–327. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Weber, A.L. Stereoselective syntheses of pentose sugars under realistic prebiotic conditions. Orig. Life Evol. Biosph. 2010, 40, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.L.; Pizzarello, S. The peptide-catalyzed stereospecific synthesis of tetroses: A possible model for prebiotic molecular evolution. Proc. Natl. Acad. Sci. USA 2006, 103, 12713–12717. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L.; Miller, S.L. Racemization and the origin of optically-active organic-compounds in living organisms. Biosystems 1987, 20, 21–26. [Google Scholar] [CrossRef]
- Abe, I.; Fujimoto, N.; Nishiyama, T.; Terada, K.; Nakahara, T. Rapid analysis of amino acid enantiomers by chiral-phase capillary gas chromatography. J. Chromatogr. A 1996, 722, 221–227. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Bada, J.L.; Schlesinger, G.; Miller, S.L. The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res. 1984, 4, 69–74. [Google Scholar] [CrossRef]
- Cronin, J.R.; Cooper, G.W.; Pizzarello, S. Characteristics and formation of amino acids and hydroxy acids of the Murchison meteorite. Adv. Space Res. 1995, 15, 91–97. [Google Scholar] [CrossRef]
- Botta, O.; Glavin, D.P.; Kminek, G.; Bada, J.L. Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 143–163. [Google Scholar] [CrossRef]
- Crovisier, J.; Bockelee-Morvan, D. Remote observations of the composition of cometary volatiles. Space Sci. Rev. 1999, 90, 19–32. [Google Scholar] [CrossRef]
- Podolak, M.; Mekler, Y. Dirty ice grains in the protoplanetary nebula. Planet Space Sci. 1997, 45, 1401–1406. [Google Scholar] [CrossRef]
- Blagojevic, V.; Petrie, S.; Bohme, D.K. Gas-phase syntheses for interstellar carboxylic and amino acids. Mon. Not. R. Astron. Soc. 2003, 339, L:7–L:11. [Google Scholar] [CrossRef]
- Largo, A.; Redondo, P.; Barrientos, C. Theoretical study of possible ion-molecule reactions leading to precursors of glycine in the interstellar medium. Int. J. Quantum Chem. 2004, 98, 355–360. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chakrabarti, S.K. Can DNA bases be produced during molecular cloud collapse? Astron. Astrophys. 2000, 354, L6–L8. [Google Scholar]
- Millar, T.J.; Bennett, A.; Herbst, E. Deuterium fractionation in dense interstellar clouds. Astrophys. J. 1989, 340, 906–920. [Google Scholar] [CrossRef]
- Inoue, Y. Asymmetric photochemical reactions in solution. Chem. Rev. 1992, 92, 741–770. [Google Scholar] [CrossRef]
- Flores, J.J.; Bonner, W.A.; Massey, G.A. Asymmetric photolysis of (RS)-leucine with circularly polarized ultraviolet light. J. Am. Chem. Soc. 1977, 99, 3622–3625. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Kosaka, A.; Hembury, G.A.; Shitomi, H.; Onuki, H.; Inoue, Y. Mechanism of pH-dependent photolysis of aliphatic amino acids and enantiomeric enrichment of racemic leucine by circularly polarized light. Org. Lett. 2001, 3, 921–924. [Google Scholar] [CrossRef]
- Naito, Y.; Nishino, H.; Inoue, Y. The absolute asymmetric synthesis by two-photon excitation using femtosecond circularly polarized pulse. In Proceedings of the Photochirogenesis Symposium, Osaka, Japan, September 2001. [Google Scholar]
- Bonner, W.A.; Bean, B.D. Asymmetric photolysis with elliptically polarized light. Orig. Life Evol. Biosph. 2000, 30, 513–517. [Google Scholar] [CrossRef]
- Donovan, J.W.; Mapes, C.J.; Davis, J.G.; Hamburg, R.D. Dissociation of chicken egg-white macroglobulin into subunits in acid. Hydrodynamic, spectrophotometric, and optical rotatory measurements. Biochemistry 1969, 8, 4190–4199. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Nahon, L.; Alcaraz, C.; Bredehoft, J.H.; Hoffmann, S.V.; Barbier, B.; Brack, A. Asymmetric vacuum UV photolysis of the amino acid leucine in the solid state. Angew. Chem. Int. Ed. 2005, 44, 5630–5634. [Google Scholar] [CrossRef]
- Meierhenrich, U.; Filippi, J.J.; Meinert, C.; Hoffmann, S.V.; Bredehoft, J.H.; Nahon, L. Photolysis of rac-leucine with circularly polarized synchrotron radiation. Chem. Biodivers. 2010, 7. in press. [Google Scholar] [CrossRef] [PubMed]
- Kagan, H.; Moradpour, A.; Nicoud, J.F.; Balavoine, G.; Martin, R.H.; Cosyn, J.P. Photochemistry with circularly polarized light. II) Asymmetric synthesis of octa and nonahelicene. Tetrahedron Lett. 1971, 12, 2479–2482. [Google Scholar] [CrossRef]
- Rau, H. Asymmetric photochemistry in solution. Chem. Rev. 1983, 83, 535–547. [Google Scholar] [CrossRef]
- Takano, Y.; Takahashi, J.; Kaneko, T.; Marumo, K.; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett. 2007, 254, 106–114. [Google Scholar] [CrossRef]
- Nuevo, M.; Meierhenrich, U.J.; d’Hendecourt, L.; Caro, G.M.M.; Dartois, E.; Deboffle, D.; Thiemann, W.H.P.; Bredehoft, J.H.; Nahon, L. Enantiomeric separation of complex organic molecules produced from irradiation of interstellar/circumstellar ice analogs. Adv. Space Res. 2007, 39, 400–404. [Google Scholar] [CrossRef]
- Nuevo, M.; Meierhenrich, U.J.; Caro, G.M.M.; Dartois, E.; d’Hendecourt, L.; Deboffle, D.; Auger, G.; Blanot, D.; Bredehoft, J.H.; Nahon, L. The effects of circularly polarized light on amino acid enantiomers produced by the UV irradiation of interstellar ice analogs. Astron. Astrophys. 2006, 457, 741–751. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino Acid Enantiomer Excesses in Meteorites: Origin and Significance. In Life Sciences: Exobiology; Raulin, F., Kobayashi, K., Brack, A., Eds.; Pergamon Press Ltd.: Oxford, UK, 1999; Volume 23, pp. 293–299. [Google Scholar]
- Takahashi, J.; Shinojima, H.; Seyama, M.; Ueno, Y.; Kaneko, T.; Kobayashi, K.; Mita, H.; Adachi, M.; Hosaka, M.; Katoh, M. Chirality emergence in thin solid films of amino acids by polarized light from synchrotron radiation and free electron laser. Int. J. Mol. Sci. 2009, 10, 3044–3064. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.; Lauretta, D. Extraterrestrial flux of potentially prebiotic C, N, and P to the early earth. Orig. Life Evol. Biosph. 2008, 38, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Huebner, W.F.; Boice, D.C. Comets as a possible source of prebiotic molecules. Orig. Life Evol. Biosph. 1992, 21, 299–315. [Google Scholar] [CrossRef]
- Brownlee, D.; Tsou, P.; Aleon, J.; Alexander, C.M.O.D.; Araki, T.; Bajt, S.; Baratta, G.A.; Bastien, R.; Bland, P.; Bleuet, P.; et al. Comet 81P/Wild 2 under a microscope. Science 2006, 314, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Goesmann, F.; Rosenbauer, H.; Roll, R.; Szopa, C.; Raulin, F.; Sternberg, R.; Israel, G.; Meierhenrich, U.; Thiemann, W.; Munoz-Caro, G. COSAC, The cometary sampling and composition experiment on philae. Space Sci. Rev. 2007, 128, 257–280. [Google Scholar] [CrossRef]
- Thiemann, W.H.P.; Meierhenrich, U. ESA mission ROSETTA will probe for chirality of cometary amino acids. Orig. Life Evol. Biosph. 2001, 31, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.M.; Yencha, A.J.; Corbett, J.W.; Frisch, H.L. Ultraviolet effects on the chemical composition and optical properties of interstellar grains. Mem. Soc. Roy. Sci. 1972, 3, 425–436. [Google Scholar]
- Caro, G.M.M.; Meierhenrich, U.; Schutte, W.A.; Thiemann, W.H.P.; Greenberg, J.M. UV-photoprocessing of interstellar ice analogs: Detection of hexamethylenetetramine-based species. Astron. Astrophys. 2004, 413, 209–216. [Google Scholar] [CrossRef]
- Kuan, Y.-J.; Charnley, S.B.; Huang, H.-C.; Tseng, W.-L.; Kisiel, Z. Interstellar glycine. Astrophys. J. 2003, 593, 848–867. [Google Scholar] [CrossRef]
- Snyder, L.E.; Lovas, F.J.; Hollis, J.M.; Friedel, D.N.; Jewell, P.R.; Remijan, A.; Ilyushin, V.V.; Alekseev, E.A.; Dyubko, S.F. A rigorous attempt to verify interstellar glycine. Astrophys. J. 2005, 619, 914–930. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Allamandola, L.J. The photostability of amino acids in space. Astrophys. J. 2001, 550, L95–L99. [Google Scholar] [CrossRef]
- Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 2009, 44, 1323–1330. [Google Scholar] [CrossRef]
- Fukue, T.M.T.; Kandori, R.; Kusukabe, N.; Hough, J.H.; Bailey, J.; Whittet, D.C.B.; Lucas, P.W.; Nakajima, Y.; Hashimoto, J. Extended high circular polarization in the orion massive star forming region: Implications for the origin of homochirality in the solar system. Orig. Life Evol. Biosph. 2010, 40, 335–346. [Google Scholar] [CrossRef]
- Menard, F.; Chrysostomou, A.; Gledhill, T.; Hough, J.H.; Bailey, J. High circular polarization in the star forming region NGC 6334: Implications for biomoleclar homochirality. Astron. Soc. Pac. Conf. Ser. 2000, 213, 355–358. [Google Scholar]
- Lucas, P.W.; Hough, J.H.; Bailey, J.; Chrysostomou, A.; Gledhill, T.M.; McCall, A. UV circular polarisation in star formation regions: The origin of homochirality? Orig. Life Evol. Biosph. 2005, 35, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.F. Extraterrestrial handedness. Nature 1997, 389, 804. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.K.; Kondrashov, F.A.; Adzhubei, I.A.; Wolf, Y.I.; Koonin, E.V.; Kondrashov, A.S.; Sunyaev, S. A universal trend of amino acid gain and loss in protein evolution. Nature 2005, 433, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Cerf, C.; Jorissen, A. Is amino-acid homochirality due to asymmetric photolysis in space? Space Sci. Rev. 2000, 92, 603–612. [Google Scholar] [CrossRef]
- Sephton, M.A.; Verchovsky, A.B.; Bland, P.A.; Gilmour, I.; Grady, M.M.; Wright, I.P. Investigating the variations in carbon and nitrogen isotopes in carbonaceous chondrites. Geochim. Cosmochim. Acta 2003, 67, 2093–2108. [Google Scholar] [CrossRef]
- Engrand, C.; Maurette, M. Carbonaceous micrometeorites from Antarctica. Meteorit. Planet. Sci. 1998, 33, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, G.; Pizzarello, S.; Taylor, S.; Brownlee, D. Concentration and variability of the AIB amino acid in polar micrometeorites: Implications for the exogenous delivery of amino acids to the primitive earth. Meteorit. Planet. Sci. 2004, 39, 1849–1858. [Google Scholar] [CrossRef]
- Botta, O.; Glavin, D.P.; Dworkin, J.P.; Matrajt, G.; Harvey, R.P. Detection of AIB in antarctic ice samples: Implications for exogenous delivery of prebiotic organic compounds. Orig. Life Evol. Biosph. 2009, 39, 225–226. [Google Scholar]
- Fenwick, D.R.; Kagan, H.B. Asymmetric amplification. Top. Stereochem. 1999, 22, 257–296. [Google Scholar]
- Shibata, T.; Yamamoto, J.; Matsumoto, N.; Yonekubo, S.; Osanai, S.; Soai, K. Amplification of a slight enantiomeric imbalance in molecules based on asymmetric autocatalysis: The first correlation between high enantiomeric enrichment in a chiral molecule and circularly polarized light. J. Am. Chem. Soc. 1998, 120, 12157–12158. [Google Scholar] [CrossRef]
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Soai, K.; Shibata, T.; Sato, I. Enantioselective automultiplication of chiral molecules by asymmetric autocatalysis. Acc. Chem. Res. 2000, 33, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Reiner, C.; Nicholson, G.J.; Nagel, U.; Schurig, V. Evaluation of enantioselective gas chromatography for the determination of minute deviations from racemic composition of amino acids with emphasis on tyrosine: accuracy and precision of the method. Chirality 2007, 19, 401–414. [Google Scholar] [CrossRef] [PubMed]



© 2010 by the authors. licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meinert, C.; Filippi, J.-J.; Nahon, L.; Hoffmann, S.V.; D’Hendecourt, L.; De Marcellus, P.; Bredehöft, J.H.; Thiemann, W.H.-P.; Meierhenrich, U.J. Photochirogenesis: Photochemical Models on the Origin of Biomolecular Homochirality. Symmetry 2010, 2, 1055-1080. https://doi.org/10.3390/sym2021055
Meinert C, Filippi J-J, Nahon L, Hoffmann SV, D’Hendecourt L, De Marcellus P, Bredehöft JH, Thiemann WH-P, Meierhenrich UJ. Photochirogenesis: Photochemical Models on the Origin of Biomolecular Homochirality. Symmetry. 2010; 2(2):1055-1080. https://doi.org/10.3390/sym2021055
Chicago/Turabian StyleMeinert, Cornelia, Jean-Jacques Filippi, Laurent Nahon, Søren V. Hoffmann, Louis D’Hendecourt, Pierre De Marcellus, Jan Hendrik Bredehöft, Wolfram H.-P. Thiemann, and Uwe J. Meierhenrich. 2010. "Photochirogenesis: Photochemical Models on the Origin of Biomolecular Homochirality" Symmetry 2, no. 2: 1055-1080. https://doi.org/10.3390/sym2021055




