Adhesive/Repulsive Codes in Vertebrate Forebrain Morphogenesis
Abstract
: The last fifteen years have seen the identification of some of the mechanisms involved in anterior neural plate specification, patterning, and morphogenesis, which constitute the first stages in the formation of the forebrain. These studies have provided us with a glimpse into the molecular mechanisms that drive the development of an embryonic structure, and have resulted in the realization that cell segregation in the anterior neural plate is essential for the accurate progression of forebrain morphogenesis. This review summarizes the latest advances in our understanding of mechanisms of cell segregation during forebrain development, with and emphasis on the impact of this process on the morphogenesis of one of the anterior neural plate derivatives, the eyes.1. Introduction
Embryonic development is a highly orchestrated process, in which the cells that form the embryo gradually acquire specific fates (patterning) and shapes (morphogenesis) to give rise to tissues and organs of precise organization and function. Intense research in the last few decades has revealed some of the mechanisms involved in the coordination of these two processes. Patterning requires the activity of intercellular signals, secreted molecules called morphogens that confer specific fates in a concentration-dependent manner by controlling the combinatorial activation of fate-promoting transcription factors (TFs) in broad groups of cells [1,2]. Patterning is accompanied by the dynamic reorganization of embryonic tissues, essential to confer the final shape to the organism. During this morphogenetic transformation, mechanisms must be in place to maintain the integrity of domains of cells fated to give rise to one particular structure of the embryo, and to keep them segregated from surrounding domains. A wealth of work has contributed to unravel the molecular mechanisms involved in maintaining the segregation of embryonic domains (reviewed in [3–6]). Briefly, mechanisms involving differential adhesion, cell-cell repulsion, or the generation of mechanical tension at the borders between adjacent domains have been shown to effectively promote cell segregation in a variety of developmental contexts. More recently, it has started to become apparent that cell segregation properties are established downstream of the signals and TFs that confer cell fate, suggesting a functional link between these two processes (e.g., [7]; other examples reviewed in [2]).
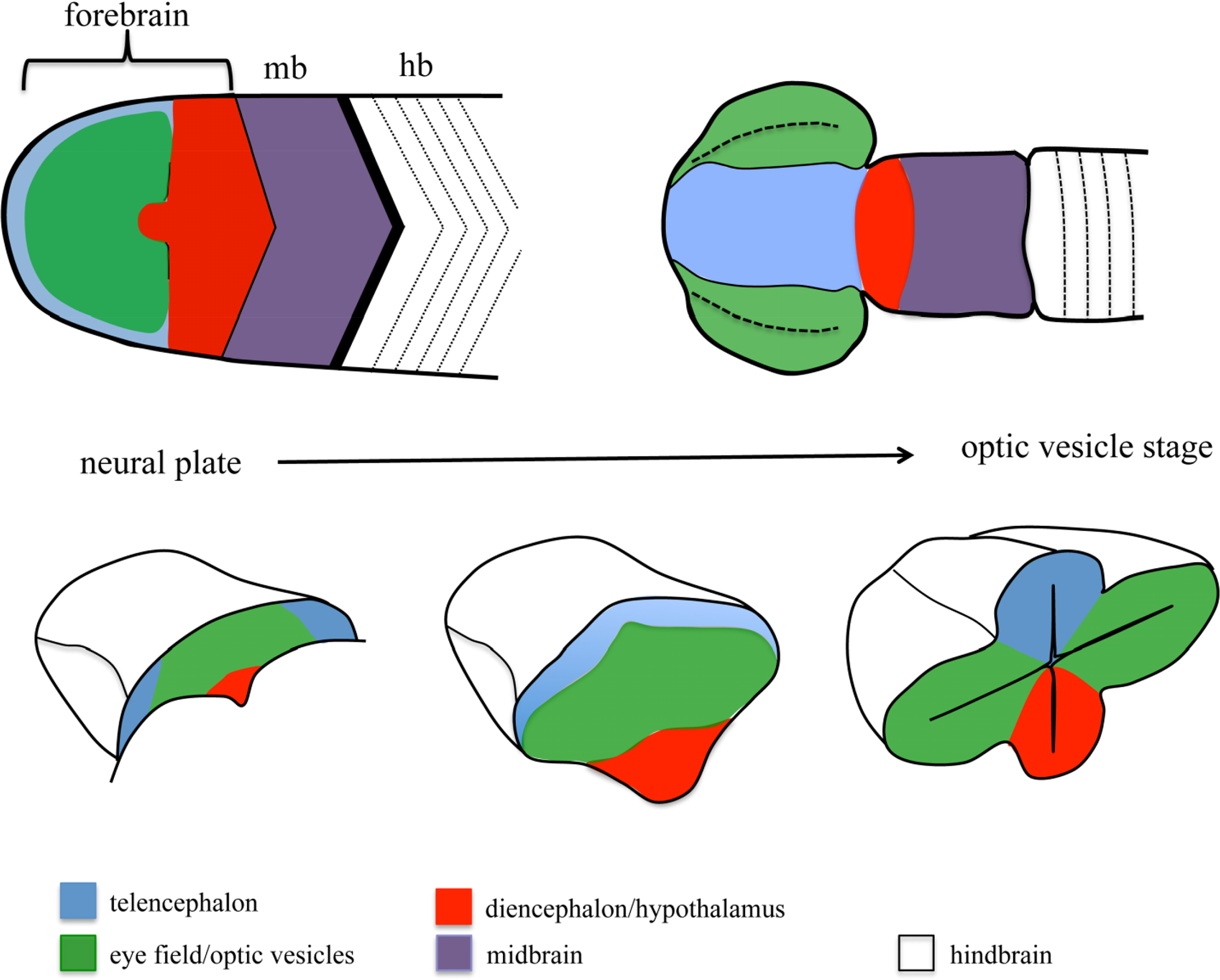
One of the embryonic structures in which more effort has been invested to unravel the mechanisms controlling patterning and morphogenesis is the forebrain. The morphological complexity of the forebrain arises from a simple structure, the anterior neural plate (ANP), which gets gradually subdivided into different domains and extensively reshaped as development proceeds [8–10] (Figure 1). One of the domains specified within the ANP is the eye field. This domain gives rise to the optic vesicles, bilateral extensions of the forebrain that constitute the first morphological manifestation of eye differentiation [11–13]. Over the last 15 years, we have gained extensive knowledge on the signals and TFs involved in eye specification and differentiation. More recently, other works have started to address the mechanisms involved in the segregation of the eye primordium from the rest of the brain. In the first part of this review, I will summarize our current knowledge on general mechanisms of cell segregation, to then discuss the contribution of these mechanisms to the segregation of domains during ANP and eye development. A special emphasis has been put on studies performed in fish models, since they have been instrumental for our understanding of forebrain morphogenesis.
2. Mechanisms of Cell Segregation
The mechanisms promoting cell segregation have been the subject of intense research since more than 50 years ago, when the seminal works from Townes and Holtfreter [14], and later on from Steinberg ([15]; reviewed in [6,16]), led to the formulation of the Differential Adhesion Hypothesis (DAH). The DAH proposes that differential adhesion between cells belonging to different tissues is the cause for their segregation [15]. The molecular machinery responsible to promote this behavior remained elusive until the 1980s, with the discovery of cadherins, a family of transmembrane proteins with homophilic binding properties (an excellent historical review of the path to the discovery of cadherins can be found in [17]). In the last 20 years, it has become apparent that in addition to selective adhesion promoted by cadherins, cell-cell repulsion promoted by the interaction between Eph receptors and their membrane bound ligands the Ephrins, and local mechanical tension promoted by cortical actomyosin activity, can induce cell segregation. As will be discussed below, these molecular mechanisms are functionally interconnected and contribute in an integrated way to the establishment and maintenance of cell segregation during embryonic development. As many excellent reviews have recently covered in detail these processes (see [3–6] for an extensive discussion on the mechanisms of cell segregation), here, I will briefly introduce the general ideas behind each proposed mechanism, to set up the concepts that will be required in the second part of the review.
2.1. Cadherins and Differential Adhesion
Cadherins constitute a large family of transmembrane proteins with strong homophilic binding properties. Cadherins in opposing cell membranes interact to generate a physical contact, which gets stabilized by the assembly of a multimolecular complex on the cytoplasmic side of both cells and the formation of adherens junctions between them (reviewed in [18,19]). It has been proposed that the strength of the adhesive contact between cells is the product of the amount and the type of cadherins expressed by them (reviewed in [6]). In vitro studies have shown that confronting two populations of cells carefully engineered to express different levels of cadherins results in the segregation of these two populations of cells, with the one expressing higher levels being enveloped by the one expressing lower levels of cadherins [20–22]. Binding between different types of cadherins (heterophilic binding) is presumably weaker than homophilic binding, and can also result in differential strength of adhesion between populations of cells expressing different types of cadherins (i.e., [23–25]).
Despite clear evidence from in vitro studies for a role for cadherins in promoting cell segregation, evidence of their in vivo relevance is scarce. One of the first reports showing this requirement showed that oocyte positioning in the Drosophila follicle is dependent on the levels of DE-cadherin expression [26]. DE-cadherin is expressed at high levels in the oocyte and posterior follicular cells, and this determines the sorting of the oocyte to the posterior part of the follicule. Direct modulation of cadherin adhesive activity has been shown to promote cell segregation in other cases. For example, tissue segregation during gastrulation in Xenopus has been shown to occur as a consequence of the downregulation of C-cadherin adhesive properties by mesoderm-inducing growth factors [27–29]. Regionalized expression of different types of cadherins has also been shown to promote cell segregation. Indeed, cadherins show regionally restricted patterns of expression in a variety of developmental contexts, and their tissue-specific expression often coincides with the presence of sharp boundaries between domains of expression (i.e., [30–36]). In the mouse telencephalon for example, the complementary expression of cadherin-6 and R-cadherin promotes segregation between cortex and striatum [34]. Similarly, the differential expression of a number of cadherins by specific pools of motorneurons in the chick spinal cord promotes their segregation in discrete nuclei [33].
Cell segregation can therefore result from selective adhesion between cells expressing similar levels and/or subtype of cadherins. In addition to selective cell adhesion by cadherins, cell-cell repulsion can also promote cell segregation and result in the establishment of a boundary between two cell populations. In this case, the molecular machinery promoting segregation makes use of a family of membrane bound proteins, the Ephrins, and their receptors, the Ephs.
2.2. Ephrins and Cell-Cell Repulsion
Ephs and Ephrins are subdivided in two families according to their structure. EphrinA ligands are anchored to the membrane by a glycophosphatidyl inositol (GPI) domain, and bind to EphA receptors; EphrinB ligands are transmembrane proteins that bind to EphB and EphA4 receptors [37]. The interaction between Ephs and Ephrins upon cell-cell contact leads to their clustering and to the activation of a bidirectional signal that results in cell repulsion (reviewed in [38,39]). The first evidence for the role of Eph/Ephrins in promoting cell segregation came from the elegant studies performed by the Wilkinson group, to dissect the molecular mechanisms of segmentation during hindbrain development [40–42]. The hindbrain is formed by a number of repetitive units or segments, the rhombomeres, which strictly segregate from each other [43–46]. Odd and even rhombomeres show an alternating pattern of expression of Ephs (EphBs and EphA4) and ephrinBs, with rhombomeres 3 and 5 expressing Ephs while rhombomeres 2, 4, and 6 express ephrins [47–53]. The interaction of Ephs and Ephrins along rhombomere boundaries activates a repulsive signal that maintains the integrity of the boundaries [40–42]. Consistently, loss of function of Ephs/Ephrins leads to a loss of rhombomere boundaries and to defective rhombomeres segregation [40,54]. Ephs and Ephrins show largely complementary expression patterns throughout vertebrate embryos (e.g., [55]) and their interaction at interfaces of expression is similarly involved in the maintenance of intersomitic boundaries [56–59], the boundary between the axial and paraxial mesoderm [60], and the segregation between germinal cell layers during Xenopus gastrulation [61,62].
2.3. Cell Cortex Tension and Cell Segregation
As described above, the DAH was the first one to be proposed to explain cell segregation [15], but other models were subsequently put forward to explain this property of embryonic tissues. One of them relies on the mechanical properties conferred to cells by their cortical actomyosin cytoskeleton, and proposes cell cortex tension and cell-surface contraction as the molecular mechanism promoting cell segregation. Despite being proposed shortly after Steinberg’s DAH [63], experimental evidence in support of this model has started to be provided only recently (reviewed in [5]). The first molecular evidence came from the observation that actomyosin cables concentrated at the compartmental boundaries of Drosophila wing imaginal discs, and at the parasegmental boundaries of Drosophila embryos [64–66]. These actomyosin cables concentrated under the belt of adherens junctions in the cells lining up the compartment, and generated supracellular structures that conferred higher tension properties to these regions of the epithelium, thus preventing the intermingling of cells and generating a straight boundary [64–67]. More definitive evidence of the role of cell cortex tension in promoting cell segregation came from studies in vertebrates, which combined detailed biophysical measurements in vitro with in vivo testing and in silico modeling. A compelling study in the zebrafish by the Heisenberg group, showed that actomyosin-dependent cortex tension needs to be taken into account to explain the pattern of segregation of zebrafish germ layer cells during gastrulation [68]. Similar to the situation at the compartmental borders in Drosphila, cortical actomyosin acummulates at the interfaces between germinal layers, and is reduced at homotypic cell-cell interfaces [69]. In addition to the boundary between germinal layers during early embryogenesis, other boundary regions in vertebrates, such as inter-rhombomeric and intersomitic boundaries [48,59,70,71] the midbrain-hindbrain boundary (MHB; [72]) or the boundary between the eye field and surrounding neural plate domains [73] (see below), are delineated by an accumulation of cortical actomyosin, suggesting a conserved role for these structures in the promotion and/or maintenance of cell segregation during morphogenesis.
2.4. Integration of Cadherin, Eph/Eprins and Cell Cortex Tension Function during Cell Segregation
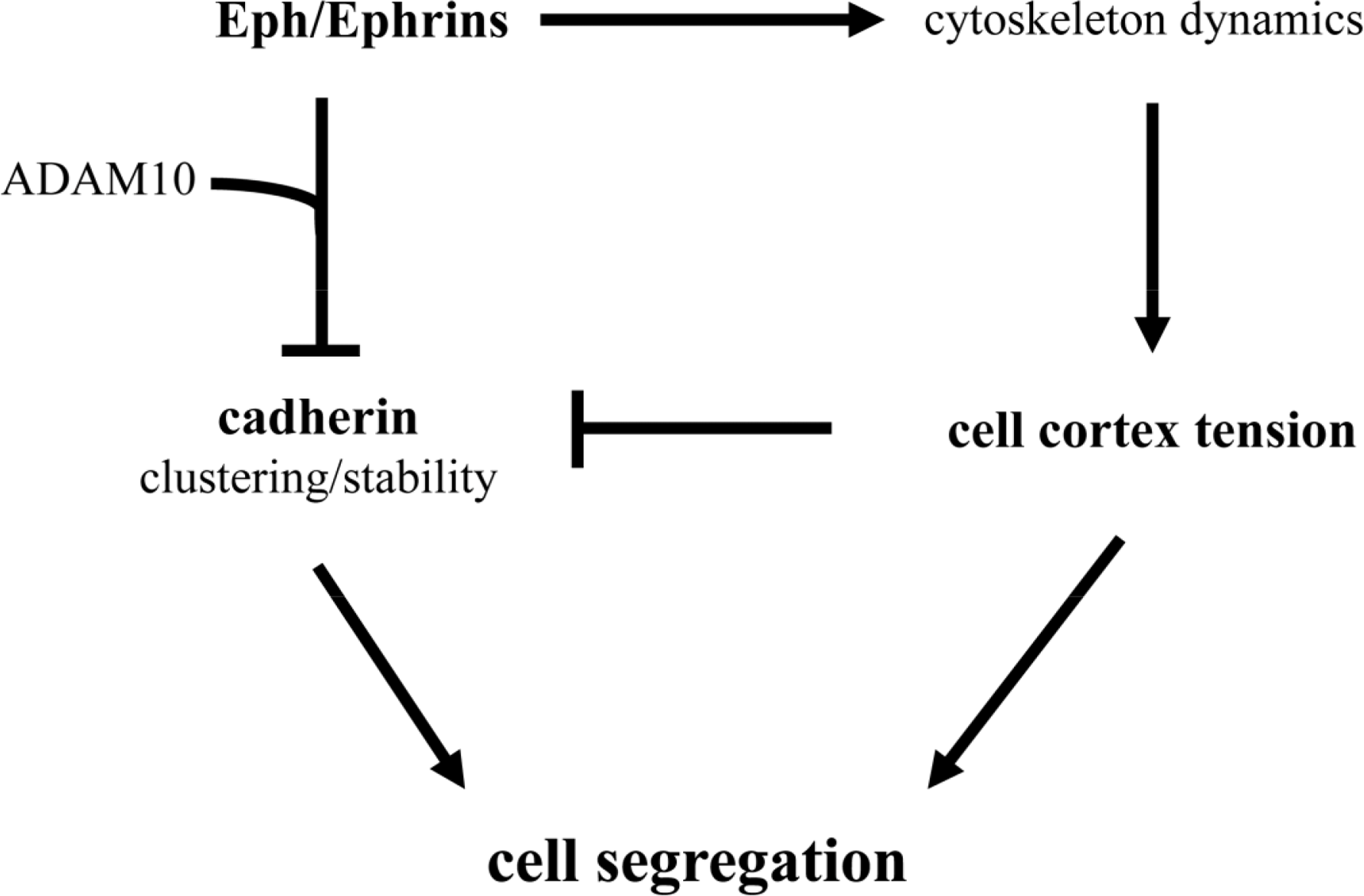
It should be noted that selective cell adhesion, cell repulsion and cell cortex tension are not exclusive mechanisms. Indeed, from the discussion above it becomes readily apparent that several mechanisms have been described to influence cell segregation in a number of embryonic structures (see Table 1). For example, cadherins, Eph/Ephrins, and cell cortex tension, have been shown to influence germ layer separation, and the establishment of rhombomere and somite boundaries. Recently, molecular mechanisms that functionally integrate the activity of these three systems have been proposed (Figure 2). The compartmentalization of the mouse gut epithelium, for example, requires Eph/Ephrin signaling to modulate E-cadherin localization at the plasma membrane [74,75]. Eph/Ephrin interaction recruits the metalloproteinase ADAM10, and ADAM10 in turn cleaves E-cadherin, reducing the adhesive strength of the intercellular interaction at the Eph/Ephrin interface and promoting in this way cell segregation [75]. The activation of the Eph/Ephrin signalling pathway also leads to the modulation of the cytoskeleton (see [37,38]), and has been proposed to directly control cell cortex tension during germ layer segregation in Xenopus [61,62], and at inter-rhombomeric boundaries in the zebrafish [71]. Recently, Eph/Ephrin control of cell cortex tension has been shown to promote the segregation of axial (notochord) and paraxial (somites) mesoderm and the formation of the notochord boundary [60]. In this case, high cortical tension promoted by Eph/Ephrin activity interferes with cadherin clustering at cell-cell junctions, drastically reducing cell adhesiveness at the notochord boundary and promoting tissue segregation [60].
These studies suggest that the three molecular mechanisms (cell adhesion, cell cortex tension, and Eph/Ephrin signaling) come together into a single molecular pathway (Figure 2). It should be highlighted, though, that the ultimate control of cadherin clustering at the cell membrane by Eph/Ephrin activity, both at the notochord border and in the gut epithelium, generates a boundary of low adhesiveness between two tissues with similar adhesive properties. A similar situation has been described at the antero-posterior boundary (AP) of the wing imaginal disc in Drosophila, where A and P cells show similar affinities [76], and segregation at the AP boundary is thought to rely instead on the generation of a cortical actomyosin cable [65]. This scenario is conceptually different to that proposed by the DAH, where different strengths of adhesion in each tissue would account for their segregation.
3. Cell Segregation during Nervous System Regionalization
Tissue regionalization and cell segregation have been extensively studied in the context of brain development. The primordium of the brain, the neural plate, gets gradually patterned and regionalized to give rise to a number of discrete domains, each of which will develop into a particular brain structure with a specific pattern of connectivity and functionality. The brain is initially subdivided into fore-, mid-, and hindbrain, broad domains specified along the AP axis by a number of secreted signals from the Fibroblast Growth Factor (FGF), Transforming Growth Factor (BMPs), Hedgehog (Hh), retinoic acid (RA), and Wnt families (reviewed in [1,2,10,77]). These broad regions get further subdivided into smaller domains as development proceeds, a process that again requires the function of some of these same signals.
The first boundaries to be uncovered in the brain were the inter-rhombomeric boundaries. As detailed in the previous section, maintenance of cell segregation between rhombomeres seems to involve a combination of selective adhesion by cadherins [44], cell segregation by Eph/Ephrin signaling [41,42,48,54] and modulation of cell cortex tension by actomyosin actvity [71]. In addition, in the rostral part of the brain, similar cell segregation boundaries have been described. For example the MHB, the diencephalon/midbrain boundary, the zona limitans intrathalamica and the pallial/subpallial boundary within the telencephalon, are all of them cell segregation boundaries (reviewed in [2]). The molecular mechanisms involved in their establishment and maintenance are not so well understood as for rhombomere boundaries, but in some cases differential cadherin expression and Eph/ephrin interfaces of expression have been described associated to these structures [30,31,78–80]. Within the ANP, recent insight has shown that strict cell segregation is also observed between the eye primordium and surrounding tissues [73,81,82]. As will be discussed below, a number of molecular mechanisms have been proposed to play a role in the maintenance of cell segregation in this part of the neural plate.
4. Eye-Brain Segregation
4.1. Eye Field Specification and Morphogenesis
The specification of the eye field requires the combined action of a number of transcription factors collectively known as eye field specification transcription factors (EFTFs), whose expression is controlled by the network of secreted signaling molecules controlling AP positional information within the ANP ([83–85] and references within). Once specified, the eye field gets transformed into the optic vesicles, bilateral evaginations from the walls of the forebrain (Figure 1). Initial studies in mouse, chick and Xenopus showed that extensive changes in cell shape and cell intercalation accompany optic vesicle formation [86–89]. Recent work exploiting the imaging advantages of the fish models has allowed us to start building a more precise model of the cell reorganizations that culminate with the formation of the optic vesicles in this model organism. Eye field cells show active behaviors as they get displaced mediolaterally [90–93]. This process is accompanied by a fast change in cell shape, and by the acquisition of apico-basal polarity characteristics by the cells in the eye primordium [94]. These changes in cell shape and polarity occur in a gradual way. The cells lining the edge of the eye field are the first ones to undergo this transformation, which results in the formation of an incipient neuroepithelium; as evagination proceeds, the rest of the eye field cells, located at the core of the eye field, gradually elongate, polarize and integrate in this nascent neuroepithelium by cell intercalation [94]. The molecular mechanisms involved in controlling this whole process are still poorly understood, but recent studies, again making use of the zebrafish, have shown that accurate segregation of the eye field from surrounding tissues at the onset of eye morphogenesis is actually essential for the correct morphogenesis of this ANP domain.
4.2. A Cell Segregation Boundary at the Edge of the Eye Field?
Despite the extensive fate map studies of the ANP performed from the 1980s, in a whole variety of vertebrates from zebrafish to mice ([95–100]; an extensive review can be found in [101]), it seems surprising that the presence of a segregation boundary between the eye field and surrounding ANP domains has only been acknowledged recently. Fate map studies in the zebrafish showed that the eye field anlage is already a coherent domain at mid-gastrulation, but its borders largely overlap with telencephalic and diencephalic domains [99]. Borders between ANP domains quickly resolve during the last phase of gastrulation and by neural plate stage, the edges of the eye field abut the telencephalic and diencephalic domains [99]. As morphogenesis proceeds, virtually no intermingling is observed between ANP domains [73,82,94,102]. A similar mechanism has been recently proposed to refine dorso-ventral domains of neural progenitors in the zebrafish spinal cord [7]. Here, the specification of different domains of neural progenitors is effected by a ventral source of the Shh morphogen. Initially, the specified domains overlap extensively, and during the next few hours of development the boundaries between them get resolved by active cell movements. These observations suggest that fate specification in the ANP and the spinal cord is quickly followed by the strict segregation of specified domains. Recent studies have shown that in the ANP, this strict segregation requires the regionalized expression of adhesion molecules such as Nlcam [81], chemokines receptors such as Cxcr4 [82] and Ephs [73], all of which modulate cell cohesion and migration properties in the eye field.
4.3. Molecular Mechanisms of Eye Field Integrity
The regionalised expression of Ephs and Ephrins in the ANP has been acknowledged for more than 15 years (e.g., [55]). In the zebrafish, EphB4 and EphA4 are expressed in the telencephalic and diencephalic ANP domains, while EphrinB1 and EphrinB2 are expressed in the eye field, thus generating a border of Eph/Ephrin interaction at the edge of the eye field [47,50,73,103]. Indeed, early studies showed that the segregation of eye and brain tissues was compromised upon interference with EphA4 activity [103], and more recent work in Xenopus showed that EphrinB1 misexpression was able to direct the progeny of a specific blastomere to populate the eye field, suggesting a role for this molecule in promoting eye field integrity [104,105]. A recent study, by us, in the zebrafish has contributed extensive evidence towards this idea, by carefully analyzing the ability of cells manipulated to express high levels of Ephs or Ephrins to segregate into different ANP domains [73]. By generating small transplants of Eph-expressing or Ephrin-expressing cells in the ANP, we showed that the expression of Ephs or Ephrins determines the forebrain domain cells will populate: EphA4/EphB4-expressing cells were excluded from the eye field, while EphrinB2-expressing cells were invariably integrated into this domain. The segregation behavior of transplanted cells occurred at the end of gastrulation and could be followed in vivo, clearly showing an active process “pushing” the cells into the right domain of the ANP. In the wild type situation the complementary expression of Ephs and Ephrins starts precisely at the end of gastrulation, shortly after the specification of the eye field. These observations support a model in which regional specification of the ANP leads to the complementary expression of Ephs and Ephrins and promotes in this way the segregation of the eye field from surrounding tissues. Indeed, in the absence of the EFTF Rx3 the expression of Ephs, usually excluded from the eye field, expands into this domain, further indicating the existence of a hierarchical cascade that goes from eye fate specification to the control of Eph expression and the implementation of a cell segregation mechanism [73]. The molecular mechanisms involved downstream of Eph/Ephrin activity, to promote cell segregation at the edge of the eye field are unclear. However, a strong acummulation of actomyosin is observed at this interface [73], suggesting that a mechanism similar to that proposed to account for germ layer segregation and notochord boundary formation in Xenopus (see above) may be in place.
Other molecules likely to promote differential adhesion are expressed in the ANP, in addition to Ephrins. That is the case of the Ig-domain adhesion molecule Nlcam, which is expressed at low levels in the eye field and at high levels in surrounding tissues. The fact that Nlcam is able to promote homophilic cell-cell adhesion suggests that differential adhesion modulated by different levels of Nlcam may be controlling the segregation of the eye field from the telencephalon; however, cell segregation upon Nlcam misexpression has not been observed [81]. Nlcam has a range of functions beyond cell-cell adhesion [106], and during eye field morphogenesis it has been proposed that, instead of controlling adhesion, it differentially controls the migration properties of the forebrain cells [81]. In the wild type situation low levels of Nlcam in eye field correlate with the reduced convergence of eye field cells as compared to that of high-expressing telencephalic cells. High resolution imaging of eye field morphogenesis in embryos misexpressing high levels of Nlcam in the eye field showed a fast convergence of these cells to the midline, similar to the behavior of telencephalic cells. Consistently, the phenotypic outcome of these manipulations was the lack of optic vesicle evagination. These observations suggested that Nlcam controls the cellular behavior underlying the first phases of telencephalic and eye field morphogenesis [81].
If it may seem unexpected that a typical cell-adhesion molecule is involved in modulating cell migration during eye morphogenesis, it is also surprising that a molecular modulator of cell migration such as the chemokine receptor Cxcr4 is involved in maintaining eye field cell coherence. Cxcr4 has been shown to control cell migration as well as cohesion in many other contexts (reviewed in [107]), and it is expressed in the eye field at neural plate stages [82]. Loss of Cxcr4 leads to an intermingling of eye and telencephalic precursors in the neural plate, suggesting a direct requirement for Cxcr4 to generate the eye field/telencephalon segregation boundary. Cxcr4 is a chemokine receptor, and as such it needs to interact with a chemokine to exert its function. The Cxcr4 ligand Sdf1/Cxcl12 is expressed in the mesendodermal tissues underlying the eye field, and thus high levels of Sdf1/Cxcl12 are probably present in the ANP [82]. However, it is unlikely that the chemokine generates a chemoattractive gradient. Rather, the mechanism that can be envisioned to control eye field integrity may be more similar to that described during cerebellar and cortex formation in the mouse (reviewed in [108,109]). Here, a homogeneous field of chemokine, secreted at the periphery of the brain, traps Cxcr4-positive migrating neurons in the most external layer of the brain, effectively influencing their positioning during cerebellar and cortex development. Similarly, during fish, chick and mouse gonad formation, primordial germ cells colonizing the gonads are kept in place by a high concentration of Cxcl12 (reviewed in [107]). Eye field cells may thus be “kept in place” by a comparable mechanism. Cxcr4 expression is maintained in the eye field by Rx3 and repressed in the telencephalon by the BMP signalling pathway, a network of signals responsible to establish telencephalic versus eye fates, thus, linking, once again, fate specification in the forebrain to the implementation of a cell segregation mechanism.
5. Downstream of Eye-Brain Segregation
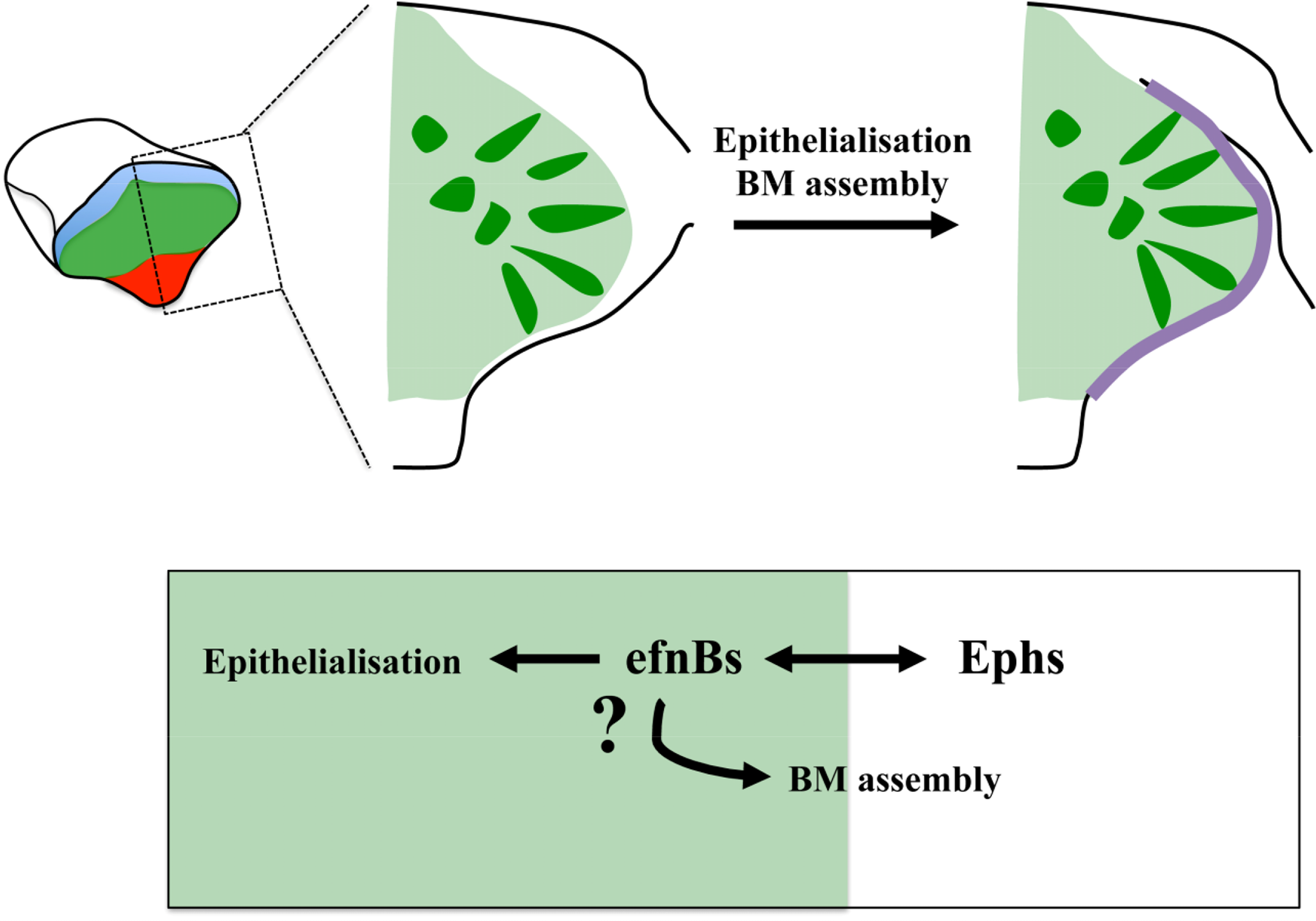
Much needs to be done to unravel how the molecular mechanisms downstream of Ephrins, Cxcr4 and Nlcam impact on eye morphogenesis. Eye field specification and segregation from surrounding tissues is rapidly followed by the apico-basal polarization of the cells at the edge of the eye field, the assembly of a laminin-rich basement membrane (BM) around this primordium, and extensive cell intercalation concomitant to the lateral expansion of the evaginating optic vesicles [94]. The Eph/Ephrin signaling pathway is a particularly suggestive candidate to control at least part of these processes. Indeed, recent studies of somite formation in the chick and the zebrafish have shown a requirement for this pathway in the modulation of cell polarity and extracellular matrix (ECM) assembly. Eph/Ephrin activity at the future somite boundary is followed by the generation of a physical cleft [56,57,59]. Cells at the boundary detach and undergo a mesenchymal-to-epithelial transition, a process controlled by Eph/Ephrin signaling [56]. In addition, cell detachment at the intersomitic boundary relieves integrin trans-inhibition thus promoting the assembly of a fibrillar fibronectin matrix [70,110] that is eventually replaced by a laminin-rich BM [111]. Thus, Eph/Ephrin signalling seems to be enough to trigger the changes in cell shape and tissue organization that accompany somite boundary formation. Eph/Ephrins do so by modulating actin dynamics and integrin activity, processes that may also be controlled by this pathway during eye morphogenesis to ultimately regulate ECM assembly and changes in cell shape and polarity in this structure (Figure 3).
The molecular mechanisms, downstream of Nlcam and Cxcr4, controlling cell cohesion and migration have been partially explored in other contexts. Cxcr4 controls cytoskeleton dynamics by modulating the activity of RhoGTPases [112–115], and interacts with components of the cell-cell and cell-matrix adhesion machinery [107,116], suggesting that similarly to Ephs/Ephrins, Cxcr4 has the potential to modulate cell motility, cell shape, and cell-ECM interactions during eye morphogenesis. The Nlcam ortholog ALCAM has also been shown to interact with the cell cytoskeleton machinery [106], suggesting a potential control of cytoskeleton dynamics also for this molecule. These observations also suggest a functional integration of the Eph/Ephrin, Cxcr4 and Nlcam outcomes during eye morphogenesis, a possibility that would be worth to explore. Finally, other molecular mechanisms are likely to work in concert with these. Indeed, the Wnt/βcatenin-independent pathway, a potent modulator of integrin function [117], cytoskelton dynamics, and cell shape/polarity [118], seems to be involved as well in promoting eye field cell cohesion [119]. However, the putative existence of a functional interaction with any of the previously described mechanisms is largely unknown.
6. Perspective
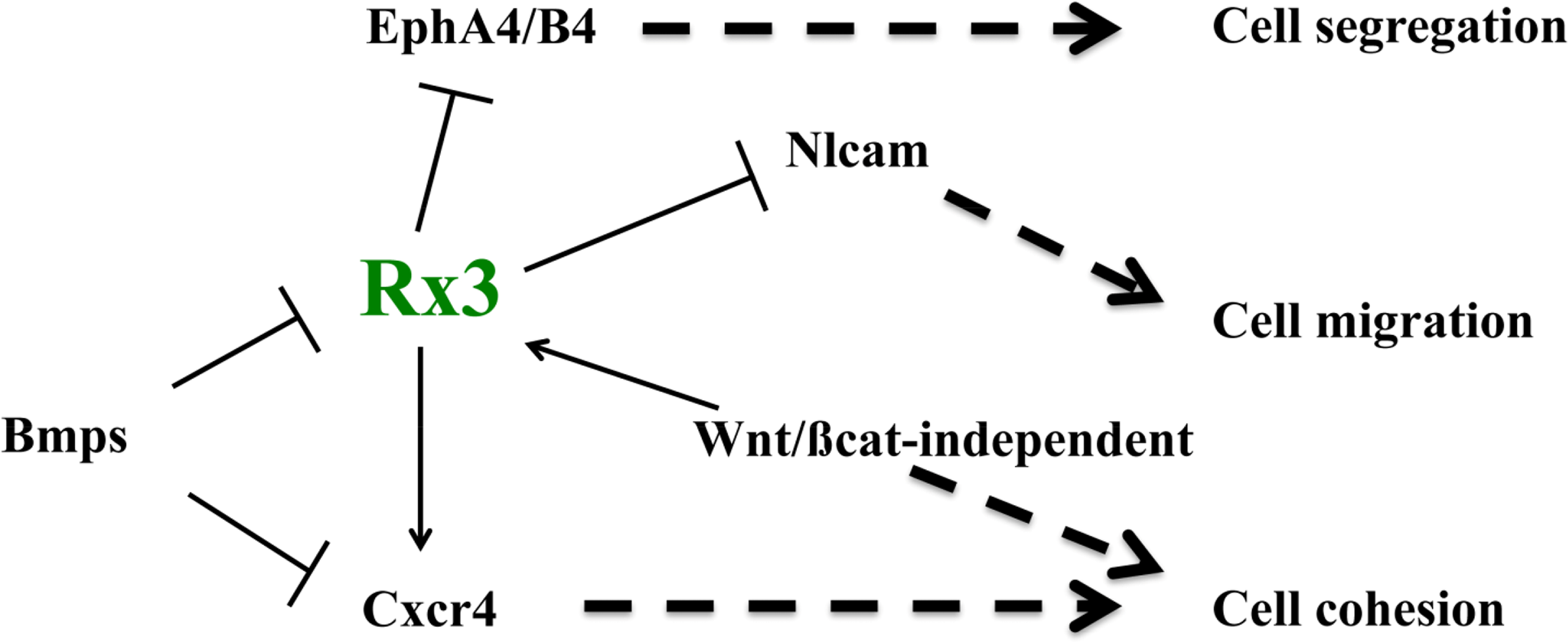
The last few years have seen the identification of some of the mechanisms involved in ANP specification, patterning and morphogenesis, which constitute the first stages in the formation of the forebrain. As I have discussed here, some of the signals and factors that promote ANP regionalization and fate acquisition also control the expression of molecules that implement particular adhesion, repulsion and migration properties to cells in different domains within the ANP. For example, the EFTF Rx3 controls the regionalized expression of Ephs, Nlcam and Cxcr4 [73,81,82], and thus it may be promoting the integrity of the eye field by a combination of mechanisms, including the control of cell cortex tension at the eye field boundary, cell cohesion and differential cell migration (Figure 4). In addition, the BMP and the Wnt/βcatenin-independent pathways control not only eye fate specification, but also eye field integrity by promoting cell cohesion in this domain and controlling some of the same molecules controlled by Rx3 [82,119] (Figure 4). A very recent study has added a further player to this list of adhesion/repulsion mechanisms, by identifying the Semaphorin/Plexin ligand-receptor pair as a critical controller of optic vesicle integrity [120]. In their study, the authors show that these two molecules generate a code of “attractive” and “repulsive” regions crucial for the maintenance of the regionalization of the evaginating optic vesicle as morphogenesis proceeds, and for its overall integrity. Again, we are still far from having a full picture of the mechanisms by which these molecules control eye patterning and morphogenesis, something that will need to await more in depth studies.
In summary, a model starts to emerge in which eye-brain segregation can be seen as arising from a combination of very diverse molecular mechanisms. The challenge for the future will be to get a full picture of the process by which the tissue responds in an integrated way to all these inputs, to build up the final, mature structure.
Acknowledgments
I apologize to those authors whose work has not been cited. I am grateful to Kenzo Ivanovitch and Luisa Sánchez-Arrones for critical reading of the manuscript, and to the anonymous reviewers for their comments and suggestions. My research is funded by a National Project Grant of the Spanish Government (BFU2011-24701) and the EU (PCIG11-GA-2012-321788), and by an Institutional Grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kiecker, C.; Lumsden, A. The role of organizers in patterning the nervous system. Annu. Rev. Neurosci 2012, 35, 347–367. [Google Scholar]
- Cavodeassi, F.; Houart, C. Brain regionalization: Of signaling centers and boundaries. Dev. Neurobiol 2012, 72, 218–233. [Google Scholar]
- Batlle, E.; Wilkinson, D.G. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol 2014, 4. [Google Scholar] [CrossRef]
- Dahmann, C.; Oates, A.C.; Brand, M. Boundary formation and maintenance in tissue development. Nat. Rev. Genet 2011, 12, 43–55. [Google Scholar]
- Amack, J.D.; Manning, M.L. Knowing the boundaries: Extending the differential adhesion hypothesis in embryonic cell sorting. Science 2012, 338, 212–215. [Google Scholar]
- Foty, R.A.; Steinberg, M.S. Differential adhesion in model systems. Wiley Interdiscip. Rev. Dev. Biol 2013, 2, 631–645. [Google Scholar]
- Xiong, F.; Tentner, A.R.; Huang, P.; Gelas, A.; Mosaliganti, K.R.; Souhait, L.; Rannou, N.; Swinburne, I.A.; Obholzer, N.D.; Cowgill, P.D.; et al. Specified neural progenitors sort to form sharp domains after noisy Shh signaling. Cell 2013, 153, 550–561. [Google Scholar]
- Wilson, S.W.; Houart, C. Early steps in the development of the forebrain. Dev. Cell 2004, 6, 167–181. [Google Scholar]
- Hoch, R.V.; Rubenstein, J.L.; Pleasure, S. Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin. Cell Dev. Biol 2009, 20, 378–386. [Google Scholar]
- Andoniadou, C.L.; Martinez-Barbera, J.P. Developmental mechanisms directing early anterior forebrain specification in vertebrates. Cell Mol. Life Sci 2013, 20, 3739–3750. [Google Scholar]
- Adler, R.; Canto-Soler, M.V. Molecular mechanisms of optic vesicle development: Complexities, ambiguities and controversies. Dev. Biol 2007, 305, 1–13. [Google Scholar]
- Fuhrmann, S. Eye morphogenesis and patterning of the optic vesicle. Curr. Top. Dev. Biol 2010, 93, 61–84. [Google Scholar]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The visual system of zebrafish and its use to model human ocular diseases. Dev. Neurobiol 2012, 72, 302–327. [Google Scholar]
- Townes, P.L.; Holtfreter, J. Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool 1955, 128, 53–120. [Google Scholar]
- Steinberg, M.S. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 1963, 141, 401–408. [Google Scholar]
- Steinberg, M.S. Adhesion in development: An historical overview. Dev. Biol 1996, 180, 377–388. [Google Scholar]
- Okada, T.S. The path leading to the discovery of cadherin: In retrospect. Dev. Growth Differ 1996, 38, 583–596. [Google Scholar]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev 2006, 20, 3199–3214. [Google Scholar]
- Takeichi, M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol 2014, 15, 397–410. [Google Scholar]
- Steinberg, M.S.; Takeichi, M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA 1994, 91, 206–209. [Google Scholar]
- Duguay, D.; Foty, R.A.; Steinberg, M.S. Cadherin-mediated cell adhesion and tissue segregation: Qualitative and quantitative determinants. Dev. Biol 2003, 253, 309–323. [Google Scholar]
- Foty, R.A.; Steinberg, M.S. The differential adhesion hypothesis: A direct evaluation. Dev. Biol 2005, 278, 255–263. [Google Scholar]
- Nose, A.; Nagafuchi, A.; Takeichi, M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell 1988, 54, 993–1001. [Google Scholar]
- Takeichi, M.; Hatta, K.; Nose, A.; Nagafuchi, A. Identification of a gene family of cadherin cell adhesion molecules. Cell Differ. Dev 1988, 25, 91–94. [Google Scholar]
- Tepass, U.; Godt, D.; Winklbauer, R. Cell sorting in animal development: Signalling and adhesive mechanisms in the formation of tissue boundaries. Curr. Opin. Genet. Dev 2002, 12, 572–582. [Google Scholar]
- Godt, D.; Tepass, U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature 1998, 395, 387–391. [Google Scholar]
- Zhong, Y.; Brieher, W.M.; Gumbiner, B.M. Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J. Cell Biol 1999, 144, 351–359. [Google Scholar]
- Wacker, S.; Grimm, K.; Joos, T.; Winklbauer, R. Development and control of tissue separation at gastrulation in Xenopus. Dev. Biol 2000, 224, 428–439. [Google Scholar]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol 2005, 6, 622–634. [Google Scholar]
- Redies, C. Cadherins in the central nervous system. Prog. Neurobiol 2000, 61, 611–648. [Google Scholar]
- Yoon, M.S.; Puelles, L.; Redies, C. Formation of cadherin-expressing brain nuclei in diencephalic alar plate divisions. J. Comp. Neurol 2000, 421, 461–480. [Google Scholar]
- Redies, C.; Neudert, F.; Lin, J. Cadherins in cerebellar development: Translation of embryonic patterning into mature functional compartmentalization. Cerebellum 2011, 10, 393–408. [Google Scholar]
- Price, S.R.; de Marco Garcia, N.V.; Ranscht, B.; Jessell, T.M. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell 2002, 109, 205–216. [Google Scholar]
- Inoue, T.; Tanaka, T.; Takeichi, M.; Chisaka, O.; Nakamura, S.; Osumi, N. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development 2001, 128, 561–569. [Google Scholar]
- Redies, C.; Takeichi, M. Cadherins in the developing central nervous system: An adhesive code for segmental and functional subdivisions. Dev. Biol 1996, 180, 413–423. [Google Scholar]
- Suzuki, S.C.; Inoue, T.; Kimura, Y.; Tanaka, T.; Takeichi, M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol. Cell Neurosci 1997, 9, 433–447. [Google Scholar]
- Lackmann, M.; Boyd, A.W. Eph, a protein family coming of age: More confusion, insight, or complexity? Sci. Signal 2008, 1. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph-Ephrin bidirectional signaling in physiology and disease. Cell 2008, 133, 38–52. [Google Scholar]
- Halloran, M.C.; Wolman, M.A. Repulsion or adhesion: Receptors make the call. Curr. Opin. Cell Biol 2006, 18, 533–540. [Google Scholar]
- Xu, Q.; Alldus, G.; Holder, N.; Wilkinson, D.G. Expression of truncated Sek-1 receptor tyrosine kinase disrupts the segmental restriction of gene expression in the Xenopus and zebrafish hindbrain. Development 1995, 121, 4005–4016. [Google Scholar]
- Xu, Q.; Mellitzer, G.; Robinson, V.; Wilkinson, D.G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 1999, 399, 267–271. [Google Scholar]
- Mellitzer, G.; Xu, Q.; Wilkinson, D.G. Eph receptors and ephrins restrict cell intermingling and communication. Nature 1999, 400, 77–81. [Google Scholar]
- Guthrie, S.; Prince, V.; Lumsden, A. Selective dispersal of avian rhombomere cells in orthotopic and heterotopic grafts. Development 1993, 118, 527–538. [Google Scholar]
- Wizenmann, A.; Lumsden, A. Segregation of rhombomeres by differential chemoaffinity. Mol. Cell Neurosci 1997, 9, 448–459. [Google Scholar]
- Fraser, S.; Keynes, R.; Lumsden, A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature 1990, 344, 431–435. [Google Scholar]
- Jimenez-Guri, E.; Udina, F.; Colas, J.F.; Sharpe, J.; Padron-Barthe, L.; Torres, M.; Pujades, C. Clonal analysis in mice underlines the importance of rhombomeric boundaries in cell movement restriction during hindbrain segmentation. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Cooke, J.E.; Xu, Q.; Wilson, S.W.; Holder, N. Characterisation of five novel Eph-related receptor tyrosine kinases suggests roles in patterning the neural plate. Dev. Genes Evol 1997, 206, 515–531. [Google Scholar]
- Cooke, J.; Moens, C.; Roth, L.; Durbin, L.; Shiomi, K.; Brennan, C.; Kimmel, C.; Wilson, S.; Holder, N. Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Development 2001, 128, 571–580. [Google Scholar]
- Cooke, J.E.; Moens, C.B. Boundary formation in the hindbrain: Eph only it were simple. Trends Neurosci 2002, 25, 260–267. [Google Scholar]
- Nieto, M.A.; Gilardi-Hebenstreit, P.; Charnay, P.; Wilkinson, D.G. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development 1992, 116, 1137–1150. [Google Scholar]
- Becker, N.; Seitanidou, T.; Murphy, P.; Mattei, M.G.; Topilko, P.; Nieto, M.A.; Wilkinson, D.G.; Charnay, P.; Gilardi-Hebenstreit, P. Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mech. Dev 1994, 47, 3–17. [Google Scholar]
- Bergemann, A.D.; Cheng, H.J.; Brambilla, R.; Klein, R.; Flanagan, J.G. ELF-2, a new member of the Eph ligand family, is segmentally expressed in mouse embryos in the region of the hindbrain and newly forming somites. Mol. Cell Biol 1995, 15, 4921–4929. [Google Scholar]
- Flenniken, A.M.; Gale, N.W.; Yancopoulos, G.D.; Wilkinson, D.G. Distinct and overlapping expression patterns of ligands for Eph-related receptor tyrosine kinases during mouse embryogenesis. Dev. Biol 1996, 179, 382–401. [Google Scholar]
- Cooke, J.E.; Kemp, H.A.; Moens, C.B. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol 2005, 15, 536–542. [Google Scholar]
- Gale, N.W.; Holland, S.J.; Valenzuela, D.M.; Flenniken, A.; Pan, L.; Ryan, T.E.; Henkemeyer, M.; Strebhardt, K.; Hirai, H.; Wilkinson, D.G.; et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 1996, 17, 9–19. [Google Scholar]
- Barrios, A.; Poole, R.J.; Durbin, L.; Brennan, C.; Holder, N.; Wilson, S.W. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr. Biol 2003, 13, 1571–1582. [Google Scholar]
- Durbin, L.; Brennan, C.; Shiomi, K.; Cooke, J.; Barrios, A.; Shanmugalingam, S.; Guthrie, B.; Lindberg, R.; Holder, N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev 1998, 12, 3096–3109. [Google Scholar]
- Durbin, L.; Sordino, P.; Barrios, A.; Gering, M.; Thisse, C.; Thisse, B.; Brennan, C.; Green, A.; Wilson, S.; Holder, N. Anteroposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development 2000, 127, 1703–1713. [Google Scholar]
- Watanabe, T.; Sato, Y.; Saito, D.; Tadokoro, R.; Takahashi, Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc. Natl. Acad. Sci. USA 2009, 106, 7467–7472. [Google Scholar]
- Fagotto, F.; Rohani, N.; Touret, A.S.; Li, R. A molecular base for cell sorting at embryonic boundaries: Contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev. Cell 2013, 27, 72–87. [Google Scholar]
- Park, E.C.; Cho, G.S.; Kim, G.H.; Choi, S.C.; Han, J.K. The involvement of Eph-Ephrin signaling in tissue separation and convergence during Xenopus gastrulation movements. Dev. Biol 2011, 350, 441–450. [Google Scholar]
- Rohani, N.; Canty, L.; Luu, O.; Fagotto, F.; Winklbauer, R. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol 2011, 9. [Google Scholar] [CrossRef]
- Harris, A.K. Is cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J. Theor. Biol 1976, 61, 267–285. [Google Scholar]
- Major, R.J.; Irvine, K.D. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev. Dyn 2006, 235, 3051–3058. [Google Scholar]
- Landsberg, K.P.; Farhadifar, R.; Ranft, J.; Umetsu, D.; Widmann, T.J.; Bittig, T.; Said, A.; Julicher, F.; Dahmann, C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol 2009, 19, 1950–1955. [Google Scholar]
- Monier, B.; Pelissier-Monier, A.; Brand, A.H.; Sanson, B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol 2010, 12, 60–65. [Google Scholar]
- Aliee, M.; Roper, J.C.; Landsberg, K.P.; Pentzold, C.; Widmann, T.J.; Julicher, F.; Dahmann, C. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr. Biol 2012, 22, 967–976. [Google Scholar]
- Krieg, M.; Arboleda-Estudillo, Y.; Puech, P.H.; Kafer, J.; Graner, F.; Muller, D.J.; Heisenberg, C.P. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol 2008, 10, 429–436. [Google Scholar]
- Maitre, J.L.; Berthoumieux, H.; Krens, S.F.; Salbreux, G.; Julicher, F.; Paluch, E.; Heisenberg, C.P. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 2012, 338, 253–256. [Google Scholar]
- Julich, D.; Mould, A.P.; Koper, E.; Holley, S.A. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development 2009, 136, 2913–2921. [Google Scholar]
- Calzolari, S.; Terriente, J.; Pujades, C. Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J 2014, 33, 686–701. [Google Scholar]
- Filas, B.A.; Oltean, A.; Majidi, S.; Bayly, P.V.; Beebe, D.C.; Taber, L.A. Regional differences in actomyosin contraction shape the primary vesicles in the embryonic chicken brain. Phys. Biol 2012, 9. [Google Scholar] [CrossRef]
- Cavodeassi, F.; Ivanovitch, K.; Wilson, S.W. Eph/Ephrin signalling maintains eye field segregation from adjacent neural plate territories during forebrain morphogenesis. Development 2013, 140, 4193–4202. [Google Scholar]
- Cortina, C.; Palomo-Ponce, S.; Iglesias, M.; Fernandez-Masip, J.L.; Vivancos, A.; Whissell, G.; Huma, M.; Peiro, N.; Gallego, L.; Jonkheer, S.; et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet 2007, 39, 1376–1383. [Google Scholar]
- Solanas, G.; Cortina, C.; Sevillano, M.; Batlle, E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat. Cell Biol 2011, 13, 1100–1107. [Google Scholar]
- Fausto-Sterling, A.; Hsieh, L. In vitro culture of Drosophila imaginal disc cells: Aggregation, sorting out, and differentiative abilities. Dev. Biol 1987, 120, 284–293. [Google Scholar]
- Beccari, L.; Marco-Ferreres, R.; Bovolenta, P. The logic of gene regulatory networks in early vertebrate forebrain patterning. Mech. Dev 2012, 130, 95–111. [Google Scholar]
- Redies, C.; Puelles, L. Modularity in vertebrate brain development and evolution. Bioessays 2001, 23, 1100–1111. [Google Scholar]
- Scholpp, S.; Brand, M. Integrity of the midbrain region is required to maintain the diencephalic-mesencephalic boundary in zebrafish no isthmus/pax2.1 mutants. Dev. Dyn 2003, 228, 313–322. [Google Scholar]
- Rhinn, M.; Dierich, A.; le Meur, M.; Ang, S. Cell autonomous and non-cell autonomous functions of Otx2 in patterning the rostral brain. Development 1999, 126, 4295–4304. [Google Scholar]
- Brown, K.E.; Keller, P.J.; Ramialison, M.; Rembold, M.; Stelzer, E.H.; Loosli, F.; Wittbrodt, J. Nlcam modulates midline convergence during anterior neural plate morphogenesis. Dev. Biol 2010, 339, 14–25. [Google Scholar]
- Bielen, H.; Houart, C. BMP signaling protects telencephalic fate by repressing eye identity and its Cxcr4-dependent morphogenesis. Dev. Cell 2012, 23, 812–822. [Google Scholar]
- Zuber, M.E.; Gestri, G.; Viczian, A.S.; Barsacchi, G.; Harris, W.A. Specification of the vertebrate eye by a network of eye field transcription factors. Development 2003, 130, 5155–5167. [Google Scholar]
- Agathocleous, M.; Harris, W.A. From progenitors to differentiated cells in the vertebrate retina. Annu. Rev. Cell Dev. Biol 2009, 25, 45–69. [Google Scholar]
- Cavodeassi, F. Integration of anterior neural plate patterning and morphogenesis by the Wnt signaling pathway. Dev. Neurobiol 2013, 74, 759–771. [Google Scholar]
- Camatini, M.; Ranzi, S. Ultrastructural analysis of the morphogenesis of the neural tube, optic vesicle and optic cup in chick embryo. Acta Embryol. Exp. Palermo 1976, 1, 81–113. [Google Scholar]
- Svoboda, K.K.; O’Shea, K.S. An analysis of cell shape and the neuroepithelial basal lamina during optic vesicle formation in the mouse embryo. Development 1987, 100, 185–200. [Google Scholar]
- Jacobson, M.; Hirose, G. Origin of the retina from both sides of the embryonic brain: A contribution to the problem of crossing at the optic chiasma. Science 1978, 202, 637–639. [Google Scholar]
- Eagleson, G.; Ferreiro, B.; Harris, W.A. Fate of the anterior neural ridge and the morphogenesis of the Xenopus forebrain. J. Neurobiol 1995, 28, 146–158. [Google Scholar]
- Rembold, M.; Loosli, F.; Adams, R.J.; Wittbrodt, J. Individual cell migration serves as the driving force for optic vesicle evagination. Science 2006, 313, 1130–1134. [Google Scholar]
- England, S.J.; Blanchard, G.B.; Mahadevan, L.; Adams, R.J. A dynamic fate map of the forebrain shows how vertebrate eyes form and explains two causes of cyclopia. Development 2006, 133, 4613–4617. [Google Scholar]
- Picker, A.; Cavodeassi, F.; Machate, A.; Bernauer, S.; Hans, S.; Abe, G.; Kawakami, K.; Wilson, S.W.; Brand, M. Dynamic coupling of pattern formation and morphogenesis in the developing vertebrate retina. PLoS Biol 2009, 7. [Google Scholar] [CrossRef]
- Kwan, K.M.; Otsuna, H.; Kidokoro, H.; Carney, K.R.; Saijoh, Y.; Chien, C.B. A complex choreography of cell movements shapes the vertebrate eye. Development 2012, 139, 359–372. [Google Scholar]
- Ivanovitch, K.; Cavodeassi, F.; Wilson, S.W. Precocious acquisition of neuroepithelial character in the eye field underlies the onset of eye morphogenesis. Dev. Cell 2013, 27, 293–305. [Google Scholar]
- Eagleson, G.W.; Harris, W.A. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J. Neurobiol 1990, 21, 427–440. [Google Scholar]
- Couly, G.F.; le Douarin, N.M. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: Implications for the genesis of cephalic human congenital abnormalities. Dev. Biol 1987, 120, 198–214. [Google Scholar]
- Inoue, T.; Nakamura, S.; Osumi, N. Fate mapping of the mouse prosencephalic neural plate. Dev. Biol 2000, 219, 373–383. [Google Scholar]
- Puelles, L.; Rubenstein, J.L. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 2003, 26, 469–476. [Google Scholar]
- Woo, K.; Fraser, S.E. Order and coherence in the fate map of the zebrafish nervous system. Development 1995, 121, 2595–2609. [Google Scholar]
- Sanchez-Arrones, L.; Ferran, J.L.; Rodriguez-Gallardo, L.; Puelles, L. Incipient forebrain boundaries traced by differential gene expression and fate mapping in the chick neural plate. Dev. Biol 2009, 335, 43–65. [Google Scholar]
- Garcia-Lopez, R.; Pombero, A.; Martinez, S. Fate map of the chick embryo neural tube. Dev. Growth Differ 2009, 51, 145–165. [Google Scholar]
- Staudt, N.; Houart, C. The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol 2007, 5. [Google Scholar] [CrossRef]
- Xu, Q.; Alldus, G.; Macdonald, R.; Wilkinson, D.G.; Holder, N. Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature 1996, 381, 319–322. [Google Scholar]
- Moore, K.B.; Mood, K.; Daar, I.O.; Moody, S.A. Morphogenetic movements underlying eye field formation require interactions between the FGF and ephrinB1 signaling pathways. Dev. Cell 2004, 6, 55–67. [Google Scholar]
- Lee, H.S.; Bong, Y.S.; Moore, K.B.; Soria, K.; Moody, S.A.; Daar, I.O. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat. Cell Biol 2006, 8, 55–63. [Google Scholar]
- Swart, G.W. Activated leukocyte cell adhesion molecule (CD166/ALCAM): Developmental and mechanistic aspects of cell clustering and cell migration. Eur. J. Cell Biol 2002, 81, 313–321. [Google Scholar]
- Raz, E.; Mahabaleshwar, H. Chemokine signaling in embryonic cell migration: A fisheye view. Development 2009, 136, 1223–1229. [Google Scholar]
- Tiveron, M.C.; Rossel, M.; Moepps, B.; Zhang, Y.L.; Seidenfaden, R.; Favor, J.; Konig, N.; Cremer, H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J. Neurosci 2006, 26, 13273–13278. [Google Scholar]
- Li, M.; Ransohoff, R.M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol 2008, 84, 116–131. [Google Scholar]
- Watanabe, T.; Takahashi, Y. Tissue morphogenesis coupled with cell shape changes. Curr. Opin. Genet. Dev 2010, 20, 443–447. [Google Scholar]
- Crawford, B.D.; Henry, C.A.; Clason, T.A.; Becker, A.L.; Hille, M.B. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol. Biol. Cell 2003, 14, 3065–3081. [Google Scholar]
- Kardash, E.; Reichman-Fried, M.; Maitre, J.L.; Boldajipour, B.; Papusheva, E.; Messerschmidt, E.M.; Heisenberg, C.P.; Raz, E. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat. Cell Biol 2010, 12, 47–53. [Google Scholar]
- Kullander, K.; Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol 2002, 3, 475–486. [Google Scholar]
- Salvucci, O.; de la Luz Sierra, M.; Martina, J.A.; McCormick, P.J.; Tosato, G. EphB2 and EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling. Blood 2006, 108, 2914–2922. [Google Scholar]
- Sharfe, N.; Freywald, A.; Toro, A.; Dadi, H.; Roifman, C. Ephrin stimulation modulates T cell chemotaxis. Eur. J. Immunol 2002, 32, 3745–3755. [Google Scholar]
- Nair, S.; Schilling, T.F. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science 2008, 322, 89–92. [Google Scholar]
- Dzamba, B.J.; Jakab, K.R.; Marsden, M.; Schwartz, M.A.; DeSimone, D.W. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev. Cell 2009, 16, 421–432. [Google Scholar]
- Tada, M.; Heisenberg, C.P. Convergent extension: Using collective cell migration and cell intercalation to shape embryos. Development 2012, 139, 3897–3904. [Google Scholar]
- Cavodeassi, F.; Carreira-Barbosa, F.; Young, R.M.; Concha, M.L.; Allende, M.L.; Houart, C.; Tada, M.; Wilson, S.W. Early stages of Zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-Catenin pathway. Neuron 2005, 47, 43–56. [Google Scholar]
- Ebert, A.M.; Childs, S.J.; Hehr, C.L.; Cechmanek, P.B.; McFarlane, S. Sema6a and Plxna2 mediate spatially regulated repulsion within the developing eye to promote eye vesicle cohesion. Development 2014, 141, 2473–2482. [Google Scholar]
| Mechanisms | Molecules | Examples |
|---|---|---|
| Differential adhesion | Cadherins | Drosophila oocyte positioning |
| Xenopus gastrulation | ||
| Mouse telencephalon regionalisation | ||
| Chick spinal cord motorneuron pools segregation | ||
| Cell-cell repulsion | Eph/Ephrins | Zebrafish hindbrain segmentation |
| Xenopus axial/paraxial mesoderm segregation | ||
| Xenopus germ layers segregation | ||
| Zebrafish ANP regionalisation | ||
| Cell cortex tension | Actomyosin | Drosophila compartmental borders |
| Zebrafish germ layer segregation | ||
| Zebrafish hindbrain segmentation | ||
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cavodeassi, F. Adhesive/Repulsive Codes in Vertebrate Forebrain Morphogenesis. Symmetry 2014, 6, 704-721. https://doi.org/10.3390/sym6030704
Cavodeassi F. Adhesive/Repulsive Codes in Vertebrate Forebrain Morphogenesis. Symmetry. 2014; 6(3):704-721. https://doi.org/10.3390/sym6030704
Chicago/Turabian StyleCavodeassi, Florencia. 2014. "Adhesive/Repulsive Codes in Vertebrate Forebrain Morphogenesis" Symmetry 6, no. 3: 704-721. https://doi.org/10.3390/sym6030704




