Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation
Abstract
:1. Introduction
2. Membranes for Chiral Separation
3. Recent Applications
3.1. Liquid Membranes
3.2. Solid Membranes with Inherent Chiral Polymers
3.3. Solid Membranes Functionalized with Immobilized Chiral Selectors
3.4. Imprinted Membranes
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M. Chiral pharmaceuticals. In Kirk-Othmer Encyclopedia of Chemicl Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Smith, S.W. Chiral toxicology: It’s the same thing only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Agranat, I.; Wainschtein, S.R.; Zusman, E.Z. The predicated demise of racemic new molecular entities is an exaggeration. Nat. Rev. Drug Discov. 2012, 11, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Caner, H.; Groner, E.; Levy, L.; Agranat, I. Trends in the development of chiral drugs. Drug Discov. Today 2004, 9, 105–110. [Google Scholar] [CrossRef]
- Francotte, E.; Lindner, W. Methods and Principles in Medicinal Chemistry: Chirality in Drug Research; Wiley VCH: Weinheim, Germany, 2006; Volume 33. [Google Scholar]
- Francotte, E.R. Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Sousa, M.E.; Tiritan, M.E.; Belaz, K.R.A.; Pedro, M.; Nascimento, M.S.J.; Cass, Q.B.; Pinto, M.M.M. Multimilligram enantioresolution of low-solubility xanthonolignoids on polysaccharide chiral stationary phases using a solid-phase injection system. J. Chromatogr. A 2006, 1120, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M.; Valente, M.J.; Carvalho, M.; de Pinho, P.G.; Remião, F. Chiral enantioresolution of cathinone derivatives present in “legal highs”, and enantioselectivity evaluation on cytotoxicity of 3,4-methylenedioxypyrovalerone (mdpv). Forensic Toxicol. 2016, 34, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Speybrouck, D.; Lipka, E. Preparative supercritical fluid chromatography: A powerful tool for chiral separations. J. Chromatogr. A 2016, 1467, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, L.; Naidu, H.; Asokan, K.; Shaik, K.M.; Kaspady, M.; Arunachalam, P.; Wu, D.R.; Mathur, A.; Sarabu, R. Integrating a post-column makeup pump into preparative supercritical fluid chromatography systems to address stability and recovery issues during purifications. J. Chromatogr. A 2017, 1511, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zehani, Y.; Lemaire, L.; Millet, R.; Lipka, E. Small scale separation of isoxazole structurally related analogues by chiral supercritical fluid chromatography. J. Chromatogr. A 2017, 1505, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Andrushko, V.; Andrushko, N. Stereoselective Synthesis of Drugs and Natural Products; Jonh Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 2, p. 1836. [Google Scholar]
- Bishop, R. Aspects of crystallization and chirality. In Chirality in Supramolecular Assemblies: Causes and Consequences; Wiley: Hoboken, NJ, USA, 2016; pp. 65–93. [Google Scholar]
- Kovalenko, V.N.; Kozyrkov, Y.Y. A simple method for resolution of endo-/exo-monoesters of trans-norborn-5-ene-2,3-dicarboxylic acids into their enantiomers. Chirality 2015, 27, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Ohkuma, T.; Tokunaga, M.; Noyori, R. Dynamic kinetic resolution in binap-ruthenium(ii) catalyzed hydrogenation of 2-substituted 3-oxo carboxylic esters. Tetrahedron Asymmetry 1990, 1, 1–4. [Google Scholar] [CrossRef]
- Francotte, E.; Leutert, T.; Vecchia, L.L.; Ossola, F.; Richert, P.; Schmidt, A. Preparative resolution of the enantiomers of tert-leucine derivatives by simulated moving bed chromatography. Chirality 2002, 14, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Wang, N.H.L. Improvement of the performances of a tandem simulated moving bed chromatography by controlling the yield level of a key product of the first simulated moving bed unit. J. Chromatogr. A 2017, 1488, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Kim, K.M.; Lee, C.H. High-performance strategy of a simulated moving bed chromatography by simultaneous control of product and feed streams under maximum allowable pressure drop. J. Chromatogr. A 2016, 1471, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, H.E.; Mink, D.; WubboLts, M.G. Dispelling the myths—Biocatalysis in industrial synthesis. Science 2003, 299, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Immobilizing enzymes: How to create more suitable biocatalysts. Angew. Chem. Int. Ed. 2003, 42, 3336–3337. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of chirality and macroring in imprinted polymers with enantiodiscriminative power. ACS Appl. Mater. Int. 2015, 7, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Rukhlenko, I.D.; Tepliakov, N.V.; Baimuratov, A.S.; Andronaki, S.A.; Gun’Ko, Y.K.; Baranov, A.V.; Fedorov, A.V. Completely chiral optical force for enantioseparation. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.A.M.; Crespo, J.G. Recent advances in chiral resolution through membrane-based approaches. Angew. Chem. Int. Ed. 2004, 43, 5293–5295. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Peng, X.; Zhang, J.; Yu, K.; Cui, X.; Zhu, J.; Deng, J. Facile resolution of racemic terbutaline and a study of molecular recognition through chiral supramolecules based on enantiodifferentiating self-assembly. Org. Biomol. Chem. 2003, 1, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Mravik, A.; Böcskei, Z.; Simon, K.; Elekes, F.; Izsáki, Z. Chiral recognition of alcohols in the crystal lattice of simple metal complexes of O,O′-dibenzoyltartaric acid: Enantiocomplementarity and simultaneous resolution. Chem. Eur. J. 1998, 4, 1621–1627. [Google Scholar] [CrossRef]
- Xie, R.; Chu, L.Y.; Deng, J.G. Membranes and membrane processes for chiral resolution. Chem. Soc. Rev. 2008, 37, 1243–1263. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Tamai, M.; Ko, Y.A.; Tagawa, Y.I.; Wu, Y.H.; Freeman, B.D.; Bing, J.T.; Chang, Y.; Ling, Q.D. Polymeric membranes for chiral separation of pharmaceuticals and chemicals. Polym. Rev. 2010, 50, 113–143. [Google Scholar] [CrossRef]
- Maier, N.M.; Franco, P.; Lindner, W. Separation of enantiomers: Needs, challenges, perspectives. J. Chromatogr. A 2001, 906, 3–33. [Google Scholar] [CrossRef]
- Gumí, T.; Valiente, M.; Palet, C. Characterization of a supported liquid membrane based system for the enantioseparation of sr-propranolol by N-hexadecyl-l-hydroxyproline. Sep. Sci. Technol. 2004, 39, 431–447. [Google Scholar]
- Gumí, T.; Valiente, M.; Palet, C. Elucidation of sr-propranolol transport rate and enantioselectivity through chiral activated membranes. J. Membr. Sci. 2005, 256, 150–157. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Yonetani, K. Molecularly imprinted polymeric membranes with oligopeptide tweezers for optical resolution. Desalination 2002, 149, 287–292. [Google Scholar] [CrossRef]
- Sueyoshi, Y.; Utsunomiya, A.; Yoshikawa, M.; Robertson, G.P.; Guiver, M.D. Chiral separation with molecularly imprinted polysulfone-aldehyde derivatized nanofiber membranes. J. Membr. Sci. 2012, 401–402, 89–96. [Google Scholar] [CrossRef]
- Teraguchi, M.; Mottate, K.; Kim, S.Y.; Aoki, T.; Kaneko, T.; Hadano, S.; Masuda, T. Synthesis of chiral helical poly(hydroxyl-containing phenylacetylene) membranes by in-situ depinanylsilylation and their enantioselective permeabilities. Macromolecules 2005, 38, 6367–6373. [Google Scholar] [CrossRef]
- Teraguchi, M.; Suzuki, J.I.; Kaneko, T.; Aoki, T.; Masuda, T. Enantioselective permeation through membranes of chiral helical polymers prepared by depinanylsilylation of poly(diphenylacetylene) with a high content of the pinanylsilyl group. Macromolecules 2003, 36, 9694–9697. [Google Scholar] [CrossRef]
- Higuchi, A.; Hayashi, A.; Kanda, N.; Sanui, K.; Kitamura, H. Chiral separation of amino acids in ultrafiltration through DNA-immobilized cellulose membranes. J. Mol. Struct. 2005, 739, 145–152. [Google Scholar] [CrossRef]
- Higuchi, A.; Higuchi, Y.; Furuta, K.; Yoon, B.O.; Hara, M.; Maniwa, S.; Saitoh, M.; Sanui, K. Chiral separation of phenylalanine by ultrafiltration through immobilized DNA membranes. J. Membr. Sci. 2003, 221, 207–218. [Google Scholar] [CrossRef]
- Lee, N.H.; Frank, C.W. Separation of chiral molecules using polypeptide-modified poly(vinylidene fluoride) membranes. Polymer 2002, 43, 6255–6262. [Google Scholar] [CrossRef]
- Singh, K.; Ingole, P.G.; Chaudhari, J.; Bhrambhatt, H.; Bhattacharya, A.; Bajaj, H.C. Resolution of racemic mixture of α-amino acid derivative through composite membrane. J. Membr. Sci. 2011, 378, 531–540. [Google Scholar] [CrossRef]
- Peacock, S.S.; Walba, D.M.; Gaeta, F.C.A.; Helgeson, R.C.; Cram, D.J. Host-guest complexation. 22. Reciprocal chiral recognition between amino acids and dilocular systems. J. Am. Chem. Soc. 1980, 102, 2043–2052. [Google Scholar] [CrossRef]
- Sogah, G.D.Y.; Cram, D.J. Host-guest complexation. 14. Host covalently bound to polystyrene resin for chromatographic resolution of enantiomers of amino acid and ester salts. J. Am. Chem. Soc. 1979, 101, 3035–3042. [Google Scholar] [CrossRef]
- Welch, C.J. Evolution of chiral stationary phase design in the pirkle laboratories. J. Chromatogr. A 1994, 666, 3–26. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small molecules as chromatographic tools for hplc enantiomeric resolution: Pirkle-type chiral stationary phases evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral stationary phases based on small molecules: An update of the last seventeen years. Sep. Purif. Rev. 2017, 1–35. [Google Scholar] [CrossRef]
- Pirkle, W.H. Supported Chiral Liquid Membrane for the Separation of Enantiomers. U.S. Patent 5,080,795, 14 January 1992. [Google Scholar]
- Cheong, W.J.; Ali, F.; Choi, J.H.; Lee, J.O.; Yune Sung, K. Recent applications of molecular imprinted polymers for enantio-selective recognition. Talanta 2013, 106, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Izake, E.L. Chiral discrimination and enantioselective analysis of drugs: An overview. J. Pharm. Sci. 2007, 96, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kaneko, T. New macromolecular architectures for permselective membranes—Gas permselective membranes from dendrimers and enantioselectively permeable membranes from one-handed helical polymers. Polym. J. 2005, 37, 717–735. [Google Scholar] [CrossRef]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.M.; Lindner, W. Chiral recognition applications of molecularly imprinted polymers: A critical review. Anal. Bioanal. Chem. 2007, 389, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.; Rojas, E. Generalized chiral membrane dynamics. Rev. Mex. Fis. 2003, 49, 44–48. [Google Scholar]
- Ingole, P.G.; Ingole, N.P. Methods for separation of organic and pharmaceutical compounds by different polymer materials. Korean J. Chem. Eng. 2014, 31, 2109–2123. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, S.O. Molecularly imprinted membranes: Past, present, and future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, X.; Zhang, Q.; Gu, M.; Zhao, Y.; Feng, X.; Chen, L. Resolution mechanism and preparation methods of chiral separation membrane. Gongneng Cailiao/J. Funct. Mater. 2016, 47, 61–65. [Google Scholar] [CrossRef]
- Keurentjes, J.T.F.; Nabuurs, L.J.W.M.; Vegter, E.A. Liquid membrane technology for the separation of racemic mixtures. J. Membr. Sci. 1996, 113, 351–360. [Google Scholar] [CrossRef]
- Sakaki, K.; Hara, S.; Itoh, N. Optical resolution of racemic 2-hydroxy octanoic acid using biphasic enzyme membrane reactor. Desalination 2002, 149, 247–252. [Google Scholar] [CrossRef]
- Bódalo, A.; Gómez, J.L.; Gómez, E.; Máximo, M.F.; Montiel, M.C. Study of l-aminoacylase deactivation in an ultrafiltration membrane reactor. Enzyme Microb. Technol. 2004, 35, 261–266. [Google Scholar] [CrossRef]
- Long, W.S.; Bhatia, S.; Kamaruddin, A. Modeling and simulation of enzymatic membrane reactor for kinetic resolution of ibuprofen ester. J. Membr. Sci. 2003, 219, 69–88. [Google Scholar] [CrossRef]
- Hazarika, S.; Borthakur, S.; Rao, P.G.; Dutta, N.N. A novel method for synthesis of chiral polymer useful for membrane application. Polym. J. 2009, 41, 1067–1075. [Google Scholar] [CrossRef]
- Demirel, N.; Bulut, Y.; Hoşgören, H. Enantioselective transport and liquid-liquid extraction of amino acids as their potassium and sodium salts by optically active diaza-18-crown-6 ethers. Chirality 2004, 16, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Pickering, P.J.; Chaudhuri, J.B. Enantioselective extraction of (d)-phenylalanine from racemic (d/l)-phenylalanine using chiral emulsion liquid membranes. J. Membr. Sci. 1997, 127, 115–130. [Google Scholar] [CrossRef]
- Edwie, F.; Li, Y.; Chung Tai-Shung, T.S. Exploration of regeneration and reusability of human serum albumin as a stereoselective ligand for chiral separation in affinity ultrafiltration. J. Membr. Sci. 2010, 362, 501–508. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Fu, Y.; Zhang, J. Enantioseparation of chiral ofloxacin using biomacromolecules. Korean J. Chem. Eng. 2013, 30, 1448–1453. [Google Scholar] [CrossRef]
- Skolaut, A.; Rétey, J. Use of enzymes deactivated by site-directed mutagenesis for the preparation of enantioselective membranes. Angew. Chem. Int. Ed. 2002, 41, 2960–2962. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Yoshikawa, M. Polymeric pseudo-liquid membranes from poly(octadecyl methacrylate). J. Membr. Sci. 2013, 445, 8–14. [Google Scholar] [CrossRef]
- Shiono, H.; Yoshikawa, M. Polymeric pseudo-liquid membranes from poly(N-oleylacrylamide). Membranes 2014, 4, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, L. Chiral adsorption of phenylalanine by α-, β-cyclodextrin modified layered double hydroxides. Korean J. Chem. Eng. 2013, 30, 918–924. [Google Scholar] [CrossRef]
- Miao, L.; Yang, Y.; Tu, Y.; Lin, S.; Hu, J.; Du, Z.; Zhang, M.; Li, Y. Chiral resolution by polysulfone-based membranes prepared via mussel-inspired chemistry. Reac. Funct. Polym. 2017, 115, 87–94. [Google Scholar] [CrossRef]
- Krieg, H.M.; Lotter, J.; Keizer, K.; Breytenbach, J.C. Enrichment of chlorthalidone enantiomers by an aqueous bulk liquid membrane containing β-cyclodextrin. J. Membr. Sci. 2000, 167, 33–45. [Google Scholar] [CrossRef]
- Hadik, P.; Szabó, L.P.; Nagy, E. D,L-lactic acid and D,L-alanine enantioseparation by membrane process. Desalination 2002, 148, 193–198. [Google Scholar] [CrossRef]
- Dzygiel, P.; Wieczorek, P.; Kafarski, P. Supported liquid membrane separation of amine and amino acid derivatives with chiral esters of phosphoric acids as carriers. J. Membr. Sci. 2003, 26, 1050–1056. [Google Scholar] [CrossRef]
- Dzygiel, P.; Wieczorek, P.; Jonsson, J.A.; Milewska, M.; Kafarski, P. Separation of amino acid enantiomers using supported liquid membrane extraction with chiral phosphates and phosphonates. Tetrahedron 1999, 55, 9923–9932. [Google Scholar] [CrossRef]
- Chen, L.X.; Zhang, J.; He, X.W.; Zeng, X.S.; Zhang, Z.Z. A comparison study between liquid-liquid extraction and liquid membrane transport of silver by calix[4]arene derivatives. Acta Chim. Sin. 2003, 61, 104–109. [Google Scholar]
- Okada, Y.; Kasai, Y.; Nishimura, J. The selective extraction and transport of amino acids by calix[4]arene-derived esters. Tetrahedron Lett. 1995, 36, 555–558. [Google Scholar] [CrossRef]
- Clark, J.D.; Han, B.; Bhown, A.S.; Wickramasinghe, S.R. Amino acid resolution using supported liquid membranes. Sep. Purif. Technol. 2005, 42, 201–211. [Google Scholar] [CrossRef]
- Zhang, F.; He, L.; Sun, W.; Cheng, Y.; Liu, J.; Ren, Z. Chiral liquid membrane for enantioselective separation of racemic ibuprofen by l-tartaric acid derivatives. RSC Adv. 2015, 5, 41729–41735. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Zhu, H.; Mao, Y.; Guo, Z.; Zhang, W.; Ren, Z. Selective separation of salbutamol enantiomers with simultaneously synergistic extraction and stripping method. J. Membr. Sci. 2016, 499, 343–351. [Google Scholar] [CrossRef]
- Thoelen, C.; De bruyn, M.; Theunissen, E.; Kondo, Y.; Vankelecom, I.F.J.; Grobet, P.; Yoshikawa, M.; Jacobs, P.A. Membranes based on poly(γ-methyl-l-glutamate): Synthesis, characterization and use in chiral separations. J. Membr. Sci. 2001, 186, 153–163. [Google Scholar] [CrossRef]
- Aoki, T.; Tomizawa, S.; Oikawa, E. Enantioselective permeation through poly[γ-[3-(pentamethyldisiloxanyl)propyl]-l-glutamate] membranes. J. Membr. Sci. 1995, 99, 117–125. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jegal, J.; Lee, K.H. Optical resolution of α-amino acids through enantioselective polymeric membranes based on polysaccharides. J. Membr. Sci. 2003, 213, 273–283. [Google Scholar] [CrossRef]
- Kim, J.H.; Jegal, J.; Kim, J.H.; Lee, K.H.; Lee, Y. Enantioselective permeation of α-amino acid optical isomers through crosslinked sodium alginate membranes. J. Appl. Polym. Sci. 2003, 89, 3046–3051. [Google Scholar] [CrossRef]
- Duri, S.; Tran, C.D. Enantiomeric selective adsorption of amino acid by polysaccharide composite materials. Langmuir 2014, 30, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, X.L.; Jiang, Y.D.; Sun, W.Z.; Wang, W.F.; Yuan, L.M. Enantioseparation of trans-stilbene oxide using a cellulose acetate membrane. J. Membr. Sci. 2009, 336, 149–153. [Google Scholar] [CrossRef]
- Xie, S.M.; Wang, W.F.; Ai, P.; Yang, M.; Yuan, L.M. Chiral separation of (r,s)-2-phenyl-1-propanol through cellulose acetate butyrate membranes. J. Membr. Sci. 2008, 321, 293–298. [Google Scholar] [CrossRef]
- Satoh, T.; Tanaka, Y.; Yokota, K.; Kakuchi, T. Enantioselective permeability of membranes prepared from polyacrylonitrile-graft-(1-6)-2,5-anhydro-d-glucitol. React. Funct. Polym. 1998, 37, 293–298. [Google Scholar] [CrossRef]
- Xiao, Y.; Lim, H.M.; Chung, T.S.; Rajagopalan, R. Acetylation of β-cyclodextrin surface-functionalized cellulose dialysis membranes with enhanced chiral separation. Langmuir 2007, 23, 12990–12996. [Google Scholar] [CrossRef] [PubMed]
- Gumí, T.; Minguillón, C.; Palet, C. Separation of propranolol enantiomers through membranes based on chiral derivatized polysulfone. Polymer 2005, 46, 12306–12312. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Xie, L.; Fan, D.; Huang, S. Β-cyclodextrin modified silica nanochannel membrane for chiral separation. J. Membr. Sci. 2014, 453, 12–17. [Google Scholar] [CrossRef]
- Meng, H.; Li, S.; Xiao, L.; Li, C. Functionalized assembly of solid membranes for chiral separation using polyelectrolytes and chiral ionic liquid. AIChE J. 2013, 59, 4772–4779. [Google Scholar] [CrossRef]
- Krieg, H.M.; Breytenbach, J.C.; Keizer, K. Chiral resolution by β-cyclodextrin polymer-impregnated ceramic membranes. J. Membr. Sci. 2000, 164, 177–185. [Google Scholar] [CrossRef]
- Wang, H.D.; Chu, L.Y.; Song, H.; Yang, J.P.; Xie, R.; Yang, M. Preparation and enantiomer separation characteristics of chitosan/β-cyclodextrin composite membranes. J. Membr. Sci. 2007, 297, 262–270. [Google Scholar] [CrossRef]
- Kakuchi, T.; Takaoka, T.; Yokota, K. Polymeric chiral crown ethers vi optical resolution of amino acid by polymers incorporating 1,3;4,6-Di-O-benzylidene-D-mannitol residues. Polym. J. 1990, 22, 199–205. [Google Scholar] [CrossRef]
- Higuchi, A.; Ishida, Y.; Nakagawa, T. Surface modified polysulfone membranes: Separation of mixed proteins and optical resolution of tryptophan. Desalination 1993, 90, 127–136. [Google Scholar] [CrossRef]
- Lakshmi, B.B.; Martin, C.R. Enantioseparation using apoenzymes immobilized in a porous polymeric membrane. Nature 1997, 388, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Mitchell, D.T.; Trofin, L.; Nevanen, T.K.; Söderlund, H.; Martin, C.R. Antibody-based bio-nanotube membranes for enantiomeric drug separations. Science 2002, 296, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Furuta, K.; Yomogita, H.; Yoon, B.O.; Hara, M.; Maniwa, S.; Saitoh, M. Optical resolution of amino acid by ultrafiltration through immobilized DNA membranes. Desalination 2002, 148, 155–157. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Kanda, N.; Lee, Y.M.; Higuchi, A. Chiral separation of phenylalanine in ultrafiltration through DNA-immobilized chitosan membranes. J. Membr. Sci. 2006, 280, 116–123. [Google Scholar] [CrossRef]
- Tone, S.; Masawaki, T.; Eguchi, K. The optical resolution of amino acids by plasma polymerized terpene membranes. J. Membr. Sci. 1996, 118, 31–40. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Panasyuk, T.L.; Piletskaya, E.V.; Nicholls, I.A.; Ulbricht, M. Receptor and transport properties of imprinted polymer membranes—A review. J. Membr. Sci. 1999, 157, 263–278. [Google Scholar] [CrossRef]
- Ulbricht, M. Membrane separations using molecularly imprinted polymers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 804, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Kyung, H.R. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Lee, W.C.; Cheng, C.H.; Pan, H.H.; Chung, T.H.; Hwang, C.C. Chromatographic characterization of molecularly imprinted polymers. Anal. Bioanal. Chem. 2008, 390, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Spivak, D.A. Optimization, evaluation, and characterization of molecularly imprinted polymers. Adv. Drug Deliv. Rev. 2005, 57, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Mizaikoff, B. Recent advances on noncovalent molecular imprints for affinity separations. J. Membr. Sci. 2007, 30, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Breton, F.; Rouillon, R.; Piletska, E.V.; Guerreiro, A.; Chianella, I.; Piletsky, S.A. How to find effective functional monomers for effective molecularly imprinted polymers? Adv. Drug Deliv. Rev. 2005, 57, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Owens, P.K.; Karlsson, L.; Lutz, E.S.M.; Andersson, L.I. Molecular imprinting for bio- and pharmaceutical analysis. TrAC Trends Anal. Chem. 1999, 18, 146–154. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly imprinted polymers for the identification and separation of chiral drugs and biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef]
- Koter, I.; Ceynowa, J. Kinetic resolution of chiral alcohols in bifunctional membrane exhibiting enzyme activity and enantioselective permeation. J. Mol. Catal. B Enzym. 2003, 24–25, 17–26. [Google Scholar] [CrossRef]
- Liese, A.; Kragl, U.; Kierkels, H.; Schulze, B. Membrane reactor development for the kinetic resolution of ethyl 2-hydroxy-4-phenylbutyrate. Enzym. Microb. Technol. 2002, 30, 673–681. [Google Scholar] [CrossRef]
- De Bruin, T.J.M.; Marcelis, A.T.M.; Zuilhof, H.; Rodenburg, L.M.; Niederländer, H.A.G.; Koudijs, A.; Overdevest, P.E.M.; Van Der Padt, A.; Sudhölter, E.J.R. Separation of amino acid enantiomers by micelle-enhanced ultrafiltration. Chirality 2000, 12, 627–636. [Google Scholar] [CrossRef]
- Higuchi, A.; Hara, M.; Horiuchi, T.; Nakagawa, T. Optical resolution of amino acids by ultrafiltration membranes containing serum albumin. J. Membr. Sci. 1994, 93, 157–164. [Google Scholar] [CrossRef]
- Randon, J.; Garnier, F.; Rocca, J.L.; Maïsterrena, B. Optimization of the enantiomeric separation of tryptophan analogs by membrane processes. J. Membr. Sci. 2000, 175, 111–117. [Google Scholar] [CrossRef]
- Bowen, W.R.; Nigmatullin, R.R. Membrane-assisted chiral resolution of pharmaceuticals: Ibuprofen separation by ultrafiltration using bovine serum albumin as chiral selector. Sep. Sci. Technol. 2002, 37, 3227–3244. [Google Scholar] [CrossRef]
- Romero, J.; Zydney, A.L. Staging of affinity ultrafiltration processes for chiral separations. J. Membr. Sci. 2002, 209, 107–119. [Google Scholar] [CrossRef]
- Szabó, T.; Hirsch, E.; Tóth, T.; Müller, J.; Riethmüller, E.; Balogh, G.T.; Huszthy, P. Synthesis and enantioselective transport studies of optically active lipophilic proton-ionizable crown ethers containing a diarylphosphinic acid unit. Tetrahedron Asymmetry 2015, 26, 650–656. [Google Scholar] [CrossRef]
- Székely, G.; Csordás, B.; Farkas, V.; Kupai, J.; Pogány, P.; Sánta, Z.; Szakács, Z.; Tõth, T.; Hollõsi, M.; Nyitrai, J.; et al. Synthesis and preliminary structural and binding characterization of new enantiopure crown ethers containing an alkyl diarylphosphinate or a proton-ionizable diarylphosphinic acid unit. Eur. J. Org. Chem. 2012, 3396–3407. [Google Scholar] [CrossRef]
- Stancu, A.D.; Hillebrand, M.; Tablet, C.; Mutihac, L. Β-cyclodextrin derivative as chiral carrier in membrane transport of some aromatic amino acids. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 71–76. [Google Scholar] [CrossRef]
- Rehn, G.; Adlercreutz, P.; Grey, C. Supported liquid membrane as a novel tool for driving the equilibrium of ω-transaminase catalyzed asymmetric synthesis. J. Biotechnol. 2014, 179, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rehn, G.; Ayres, B.; Adlercreutz, P.; Grey, C. An improved process for biocatalytic asymmetric amine synthesis by in situ product removal using a supported liquid membrane. J. Mol. Catal. B Enzym. 2016, 123, 1–7. [Google Scholar] [CrossRef]
- Börner, T.; Rehn, G.; Grey, C.; Adlercreutz, P. A process concept for high-purity production of amines by transaminase-catalyzed asymmetric synthesis: Combining enzyme cascade and membrane-assisted ispr. Org. Process Res. Dev. 2015, 19, 793–799. [Google Scholar] [CrossRef]
- Sunsandee, N.; Ramakul, P.; Hronec, M.; Pancharoen, U.; Leepipatpiboon, N. Mathematical model and experimental validation of the synergistic effect of selective enantioseparation of (S)-amlodipine from pharmaceutical wastewater using a hfslm. J. Ind. Eng. Chem. 2014, 20, 1612–1622. [Google Scholar] [CrossRef]
- Tabani, H.; Fakhari, A.R.; Shahsavani, A.; Gharari Alibabaou, H. Electrically assisted liquid-phase microextraction combined with capillary electrophoresis for quantification of propranolol enantiomers in human body fluids. Chirality 2014, 26, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ingole, P.G.; Bajaj, H.C.; Singh, K. Optical resolution of α-amino acid derivative through membrane process. Indian J. Chem. Technol. 2011, 18, 197–206. [Google Scholar]
- Ingole, P.G.; Bajaj, H.C.; Singh, K. Optical resolution of racemic lysine monohydrochloride by novel enantioselective thin film composite membrane. Desalination 2012, 305, 54–63. [Google Scholar] [CrossRef]
- Ingole, P.G.; Bajaj, H.C.; Singh, K. Preparation and performance evaluation of enantioselective polymer composite materials. RSC Adv. 2013, 3, 3667–3676. [Google Scholar] [CrossRef]
- Ingole, P.G.; Singh, K.; Bajaj, H.C. Enantioselective polymeric composite membrane for optical resolution of racemic mixtures of α-amino acids. Sep. Sci. Technol. 2011, 46, 1898–1907. [Google Scholar] [CrossRef]
- Singh, K.; Ingole, P.G.; Bajaj, H.C.; Bhattacharya, A.; Brahmbhatt, H.R. Optical resolution of α-amino acids by reverse osmosis using enantioselective polymer membrane containing chiral metal-schiff base complex. Sep. Sci. Technol. 2010, 45, 1374–1384. [Google Scholar] [CrossRef]
- Singh, K.; Ingole, P.G.; Bhrambhatt, H.; Bhattachayra, A.; Bajaj, H.C. Preparation, characterization and performance evaluation of chiral selective composite membranes. Sep. Purif. Technol. 2011, 78, 138–146. [Google Scholar] [CrossRef]
- Singh, K.; Ingole, P.G.; Bajaj, H.C.; Gupta, H. Preparation, characterization and application of β-cyclodextrin-glutaraldehyde crosslinked membrane for the enantiomeric separation of amino acids. Desalination 2012, 298, 13–21. [Google Scholar] [CrossRef]
- Ingole, P.G.; Bajaj, H.C.; Singh, K. Membrane separation processes: Optical resolution of lysine and asparagine amino acids. Desalination 2014, 343, 75–81. [Google Scholar] [CrossRef]
- Yuan, L.M.; Ma, W.; Xu, M.; Zhao, H.L.; Li, Y.Y.; Wang, R.L.; Duan, A.H.; Ai, P.; Chen, X.X. Optical resolution and mechanism using enantioselective cellulose, sodium alginate and hydroxypropyl-β-cyclodextrin membranes. Chirality 2017, 29, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, L.Z.; Caloca, J.; Rogel-Hernández, E.; Espinoza-Gomez, H. Development of an enantioselective membrane from cellulose acetate propionate/cellulose acetate, for the separation of trans-stilbene oxide. Cellulose 2014, 21, 1987–1995. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Msayib, K.J.; McKeown, N.B.; Harris, K.D.M.; Pan, Z.; Budd, P.M.; Butler, A.; Selbie, J.; Book, D.; Walton, A. A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption. Chem. Commun. 2007, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (pims): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 10, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M.; Dose, M.E.; Freeman, B.D.; Paul, D.R. Analysis of the transport properties of thermally rearranged (tr) polymers and polymers of intrinsic microporosity (pim) relative to upper bound performance. J. Membr. Sci. 2017, 525, 18–24. [Google Scholar] [CrossRef]
- Weng, X.; Baez, J.E.; Khiterer, M.; Hoe, M.Y.; Bao, Z.; Shea, K.J. Chiral polymers of intrinsic microporosity: Selective membrane permeation of enantiomers. Angew. Chem. Int. Ed. 2015, 54, 11214–11218. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, F.; Luo, Y. Preparation and evaluation of chiral selective cation-exchange pmma-pnipam thermal-sensitive membranes. Iranian Polym. J. 2014, 23, 679–687. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Q.; Liang, X.; Li, J.; Zhao, Y.; Chen, L. Preparation, characterization and application of a chiral thermo-sensitive membrane for phenylalanine separation of the racemic mixture. J. Polym. Res. 2014, 21. [Google Scholar] [CrossRef]
- Hovorka, Š.; Randová, A.; Borbášová, T.; Sysel, P.; Vychodilová, H.; Červenková-Š͗astná, L.; Brožová, L.; Žitka, J.; Storch, J.; Kačírková, M.; et al. Permeability and diffusion coefficients of single methyl lactate enantiomers in nafion® and cellophane membranes measured in diffusion cell. Sep. Purif. Technol. 2016, 158, 322–332. [Google Scholar] [CrossRef]
- Meng, C.; Sheng, Y.; Chen, Q.; Tan, H.; Liu, H. Exceptional chiral separation of amino acid modified graphene oxide membranes with high-flux. J. Membr. Sci. 2017, 526, 25–31. [Google Scholar] [CrossRef]
- Ignacio-De Leon, P.A.; Abelow, A.E.; Cichelli, J.A.; Zhukov, A.; Stoikov, I.I.; Zharov, I. Silica colloidal membranes with enantioselective permeability. Israel J. Chem. 2014, 54, 767–773. [Google Scholar] [CrossRef]
- Yuan, L.; Su, Y.; Duan, A.; Zheng, Y.; Ai, P.; Chen, X. Optical resolution of D,L-phenylglycine and chiral separation mechanism using an enantioselective membrane of vancomycin. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ. 2016, 37, 1960–1965. [Google Scholar] [CrossRef]
- Singh, K.; Devi, S.; Bajaj, H.C.; Ingole, P.; Choudhari, J.; Bhrambhatt, H. Optical resolution of racemic mixtures of amino acids through nanofiltration membrane process. Sep. Sci. Technol. 2014, 49, 2630–2641. [Google Scholar] [CrossRef]
- Shah, N.; Rehan, T.; Park, J.K. Adsorptive molecularly imprinted composite membranes for chiral separation of phenylalanine. Pol. J. Chem. Technol. 2016, 18, 22–29. [Google Scholar] [CrossRef]
- Zhou, Z.; He, L.; Mao, Y.; Chai, W.; Ren, Z. Green preparation and selective permeation of d-tryptophan imprinted composite membrane for racemic tryptophan. Chem. Eng. J. 2017, 310, 63–71. [Google Scholar] [CrossRef]
- Singh, K.; Bajaj, H.C.; Ingole, P.; Bhattacharya, A. Comparative study of enantioseparation of racemic tryptophan by ultrafiltration using bsa-immobilized and bsa-interpenetrating network polysulfone membranes. Sep. Sci. Technol. 2010, 45, 346–354. [Google Scholar] [CrossRef]
- Zhou, Z.; Cheng, J.H.; Chung, T.S.; Hatton, T.A. The exploration of the reversed enantioselectivity of a chitosan functionalized cellulose acetate membranes in an electric field driven process. J. Membr. Sci. 2012, 389, 372–379. [Google Scholar] [CrossRef]
- Wu, Y.; Meng, M.; Liu, X.; Li, C.; Zhang, M.; Ji, Y.; Sun, F.; He, Z.; Yan, Y. Efficient one-pot synthesis of artemisinin-imprinted membrane by direct surface-initiated aget-atrp. Sep. Purif. Technol. 2014, 131, 117–125. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Brovko, O.O.; Piletska, E.V.; Piletsky, S.A.; Goncharova, L.A.; Karabanova, L.V.; Sergeyeva, L.M.; El’skaya, A.V. Porous molecularly imprinted polymer membranes and polymeric particles. Anal. Chim. Acta 2007, 582, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, L.; Li, Y. Designing and preparation of novel alkaloid-imprinted membrane with grafting type and its molecular recognition characteristic and permselectivity. Mater. Sci. Eng. C 2016, 66, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Cui, K.; Li, Y. Preparation of molecule imprinted membrane of single enantiomer of amino acid with an innovative strategy and study on its chiral recognition and resolution properties. J. Chem. Technol. Biotechnol. 2017, 92, 1566–1576. [Google Scholar] [CrossRef]
- Ingole, P.G.; Bajaj, H.C.; Singh, K. Enantiomeric separation of α-amino acids by imprinted terpolymer membrane. Arab. J. Chem. 2016, 9, S960–S965. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. Preparation of imprinted cryogel cartridge for chiral separation of l-phenylalanine. Artif. Cells Nanomed. Biotechnol. 2017, 45, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Tang, S.; Xie, J.; Cai, C.; Chen, X.; Chen, C. Highly efficient chiral separation of amlodipine enantiomers via triple recognition hollow fiber membrane extraction. J. Chromatogr. A 2017, 1490, 63–73. [Google Scholar] [CrossRef] [PubMed]
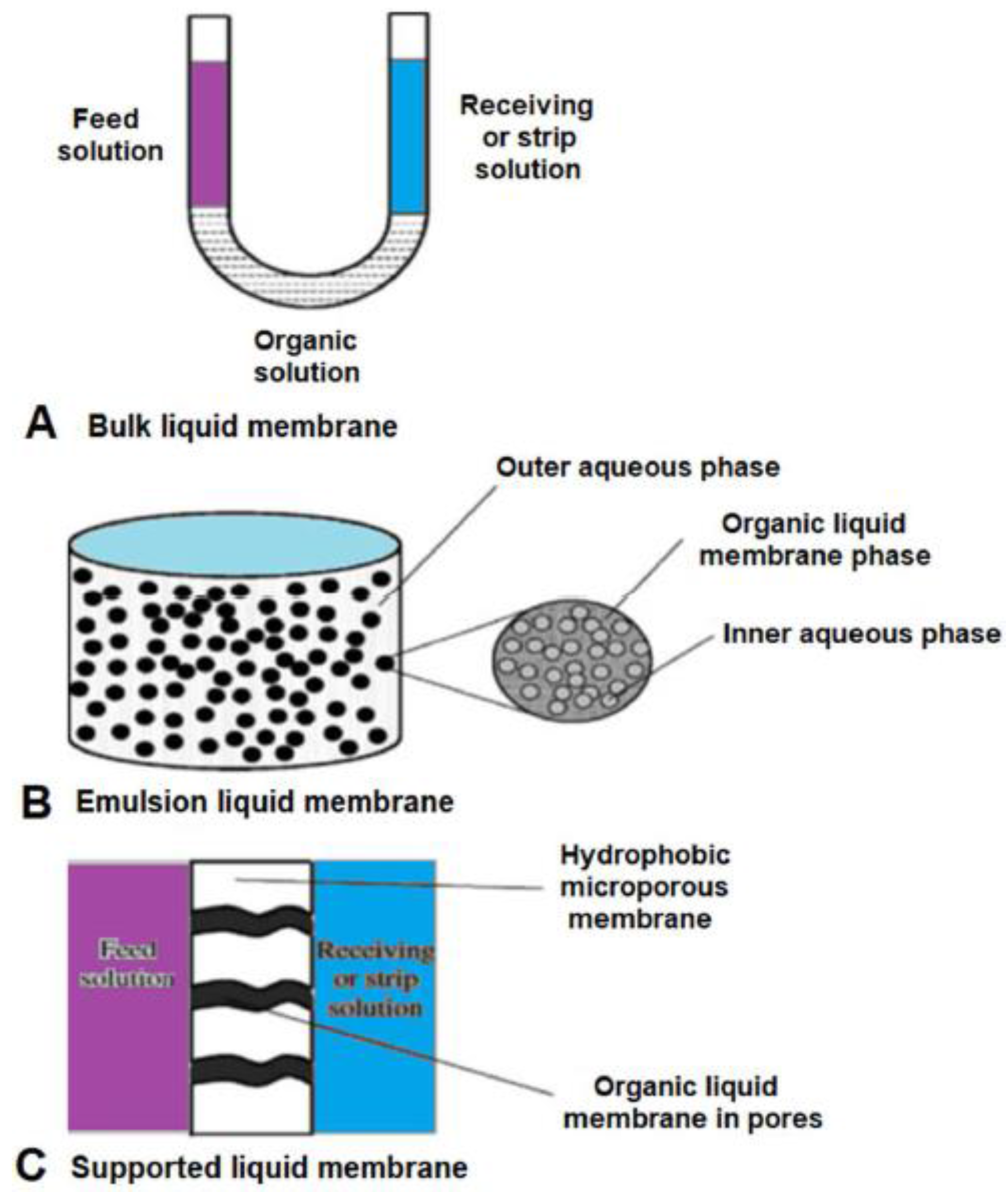
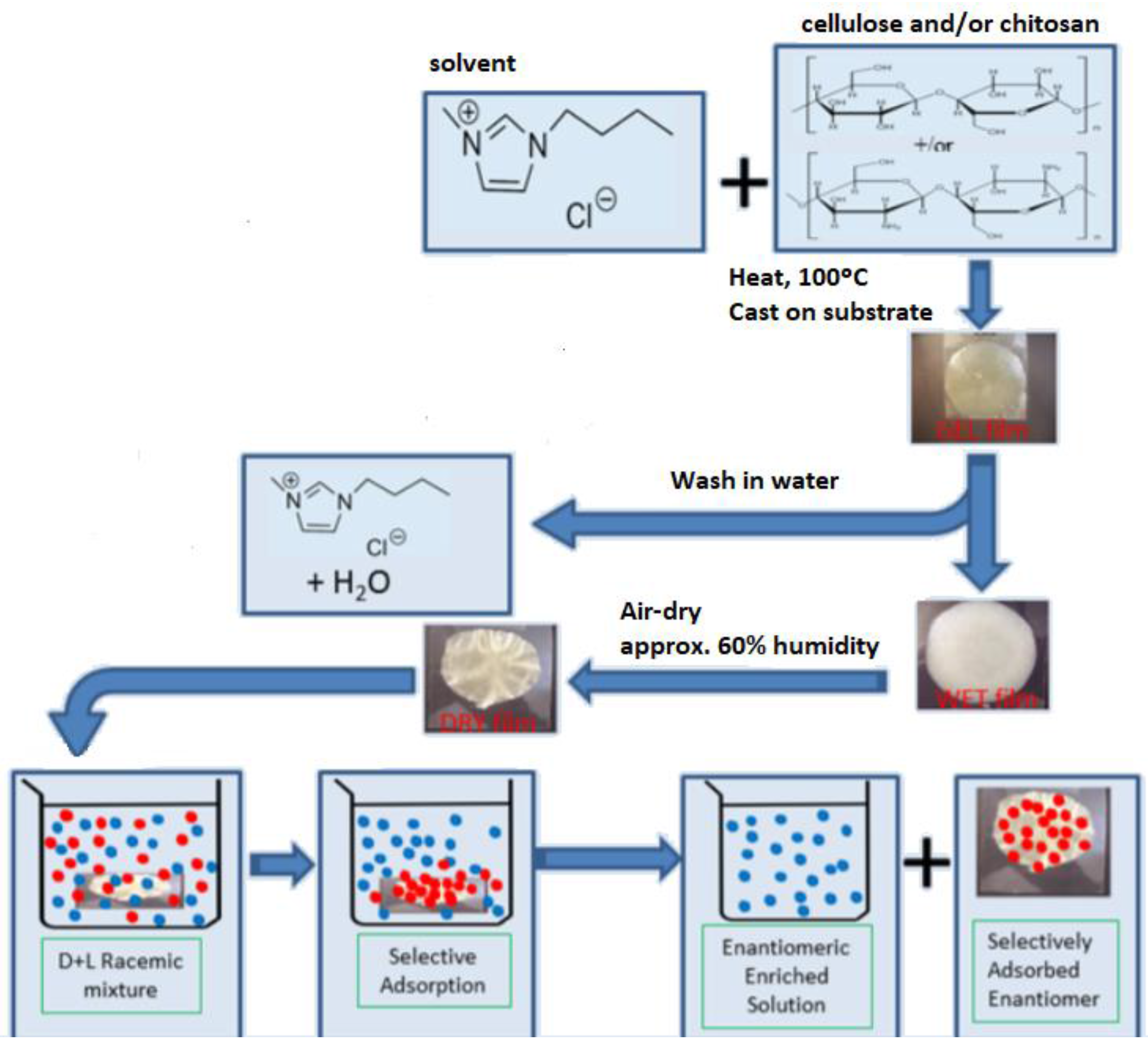

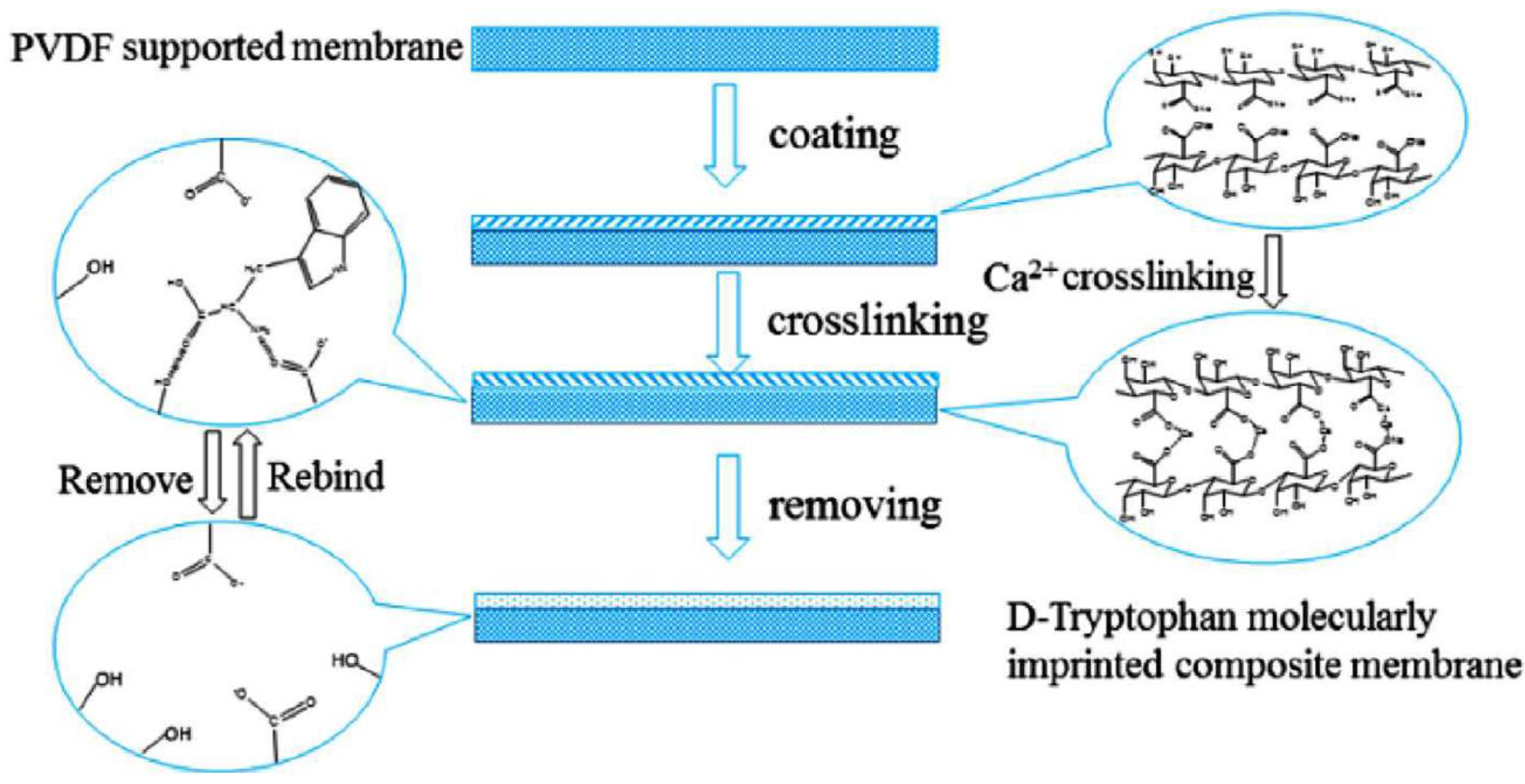

| Chiral Membrane | Advantages | Drawbacks |
|---|---|---|
| Bulk liquid membrane | Easy to design and operate. Good for preliminary studies in the evaluation of possible carriers for a given separation. | Low of long-term stability. The transporter and/or transporter/target molecule complex may be washed out during operation. Low reproducibility of results. Mass transfer rate is very low. |
| Emulsion liquid membrane | The fastest mass transfer rates of all liquid membrane systems. Highs separation factor and mass transfer flux can be achieved. | Complicated operation procedure. Leakage in the procedures. Low of long-term stability. |
| Supported liquid membrane | Easy to scaling-up and cascade design. Different type of supported can be used. Compared with emulsion and bulk liquid membranes, it could save the consumption and processing costs. | Leakage in the procedures. Low long-term stability. |
| Solid membrane with inherent chiral polymers | Several types of material are available: natural and synthetic polymers. High range of applicability. | Low efficiency in chiral separation. |
| Solid membrane functionalized with immobilized chiral selectors | Different type of support and material are available: natural and synthetic polymers. High range of applicability. | The synthetic strategies for development of chiral selectors to be immobilized on solid membranes could involve multi-step pathways with time-consuming and laborious work. |
| Imprinted membrane | High stability and reproducibility. High efficiency in chiral separation. | High specificity with consequent low range of applicability. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation. Symmetry 2017, 9, 206. https://doi.org/10.3390/sym9100206
Fernandes C, Tiritan ME, Pinto MMM. Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation. Symmetry. 2017; 9(10):206. https://doi.org/10.3390/sym9100206
Chicago/Turabian StyleFernandes, Carla, Maria Elizabeth Tiritan, and Madalena M. M. Pinto. 2017. "Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation" Symmetry 9, no. 10: 206. https://doi.org/10.3390/sym9100206






