Occurrence of Chiral Bioactive Compounds in the Aquatic Environment: A Review
Abstract
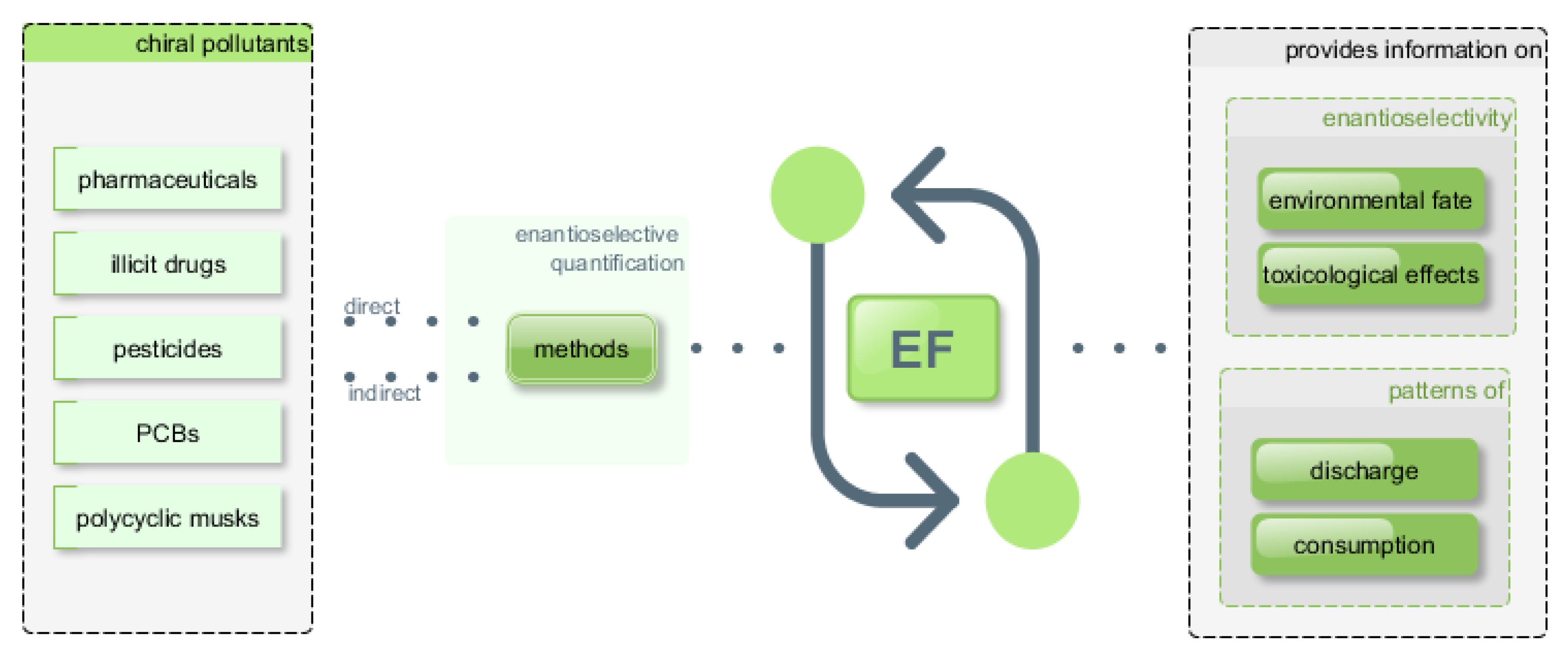
:1. Introduction
2. Basic Concepts of Chirality
3. Analytical Methodologies for Enantioseparation of Chiral Bioactive Compounds
4. Chiral Bioactive Compounds of Environmental Concern
4.1. Illicit Drugs and Pharmaceuticals
Multi-Class Enantioselective Analysis
4.2. Environmental Chiral Analysis of Pesticides, PCBs and PCMs
4.2.1. Pesticides
4.2.2. Polychlorinated Biphenyls (PCBs)
4.2.3. Polycyclic Musks (PCMs)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, K.E.; Thomas, S.M.; Bodour, A.A. Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ. Pollut. 2010, 158, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Currell, M.J. Persistent organic pollutants in China’s surface water systems. Sci. Total Environ. 2017, 580, 602–625. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Ribeiro, A.R.; Tiritan, M.E. Priority substances and emerging organic pollutants in portuguese aquatic environment: A Review. Rev. Environ. Contam. Toxicol. 2015, 238, 1–44. [Google Scholar]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Environmental fate of chiral pharmaceuticals: Determination, degradation and toxicity. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 2, pp. 3–45. [Google Scholar]
- Song, Q.; Zhang, Y.; Yan, L.; Wang, J.; Lu, C.; Zhang, Q.; Zhao, M. Risk assessment of the endocrine-disrupting effects of nine chiral pesticides. J. Hazard. Mater. 2017, 338, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, N.C.P.; Carrão, D.B.; Habenschus, M.D.; Oliveira, A.R.M. Metabolism studies of chiral pesticides: A critical review. J. Pharm. Biomed. Anal. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, M.; Liu, J.; Liu, W. Enantioselectivity in environmental risk assessment of modern chiral pesticides. Environ. Pollut. 2010, 158, 2371–2383. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Lawrence, J.R.; Neu, T.R. Selective degradation of ibuprofen and clofibric acid in two model river biofilm systems. Water Res. 2001, 35, 3197–3205. [Google Scholar] [CrossRef]
- Wong, C.S.; Garrison, A.W.; Foreman, W.T. Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic bed sediment. Environ. Sci. Technol. 2001, 35, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lao, W.; Gan, J. Enantioselective degradation of warfarin in soils. Chirality 2012, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Qi, S.; Li, X.; Peng, X. Concentrations, enantiomeric compositions, and sources of HCH, DDT and chlordane in soils from the Pearl River Delta, South China. Sci. Total Environ. 2006, 372, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, J.; Malia, L.; Lubben, A.; Kasprzyk-Hordern, B. Stereoselective biodegradation of amphetamine and methamphetamine in river microcosms. Water Res. 2013, 47, 5708–5718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, A.S.; Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Analysis of Pesticides and Drugs of Environmental Concern: Biodegradation and Enantiomeric Fraction. Symmetry 2017, 9, 196. [Google Scholar] [CrossRef]
- IUPAC. Basic terminology of stereochemistry. Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar]
- Testa, B.; Caldwell, J.; Kisakürek, M.V. Organic Stereochemistry: Guiding Principles and Biomedicinal Relevance; Wiley-VCH: Berlin, Germany, 2014. [Google Scholar]
- Solomons, T.W.G. Organic Chemistry, 10th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Allenmark, S. Chromatographic Enantioseparation: Methods and Applications, 2nd ed.; Ellis Horwood Ltd.: London, UK, 1991. [Google Scholar]
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; John Wiley & Sons, Inc.: New York, NY, USA, 1994; p. 1267. [Google Scholar]
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M.M.M. Chiral Pharmaceuticals. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Mannschreck, A.; Kiesswetter, R.; von Angerer, E. Unequal activities of enantiomers via biological receptors: Examples of chiral drug, pesticide, and fragrance molecules. J. Chem. Educ. 2007, 84, 2012–2018. [Google Scholar] [CrossRef]
- Lima, V.L.E. Drugs and chirality: A brief overview. Quim. Nova 1997, 20, 657–663. [Google Scholar] [CrossRef]
- Nunez, M.C.; Garcia-Rubino, M.E.; Conejo-Garcia, A.; Cruz-Lopez, O.; Kimatrai, M.; Gallo, M.A.; Espinosa, A.; Campos, J.M. Homochiral drugs: A demanding tendency of the pharmaceutical industry. Curr. Med. Chem. 2009, 16, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Wainer, I.W.; Drayer, D.E. Drug Stereochemistry: Analytical Methods and Pharmacology (Clinical Pharmacology, No 18), 2nd ed.; Marcel Dekker: New York, NY, USA, 1993; p. 432. [Google Scholar]
- Gal, J. Chiral drugs from a historical point of view. In Chirality in Drug Research; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–26. [Google Scholar]
- Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective biodegradation of pharmaceuticals, alprenolol and propranolol, by an activated sludge inoculum. Ecotoxicol. Environ. Saf. 2013, 87, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective HPLC analysis and biodegradation of atenolol, metoprolol and fluoxetine. Environ. Chem. Lett. 2013, 11, 83–90. [Google Scholar] [CrossRef]
- Maia, A.S.; Castro, P.M.L.; Tiritan, M.E. Integrated liquid chromatography method in enantioselective studies: Biodegradation of ofloxacin by an activated sludge consortium. J. Chromatogr. B 2016, 1029–1030, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Amorim, C.L.; Ribeiro, A.R.; Mesquita, R.B.R.; Rangel, A.O.S.S.; van Loosdrecht, M.C.M.; Tiritan, M.E.; Castro, P.M.L. Removal of fluoxetine and its effects in the performance of an aerobic granular sludge sequential batch reactor. J. Hazard. Mater. 2015, 287, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.L.; Moreira, I.S.; Ribeiro, A.R.; Santos, L.H.M.L.M.; Delerue-Matos, C.; Tiritan, M.E.; Castro, P.M.L. Treatment of a simulated wastewater amended with a chiral pharmaceuticals mixture by an aerobic granular sludge sequencing batch reactor. Int. Biodeterior. Biodegrad. 2016, 115, 277–285. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Influence of pH on the stereoselective degradation of the fungicides epoxiconazole and cyproconazole in soils. Environ. Sci. Technol. 2006, 40, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, J.; Zhang, X. Effects of engineered nanoparticles on the enantioselective transformation of metalaxyl agent and commercial metalaxyl in agricultural soils. J. Agric. Food Chem. 2016, 64, 7688–7695. [Google Scholar] [CrossRef] [PubMed]
- Sunsandee, N.; Ramakul, P.; Hronec, M.; Pancharoen, U.; Leepipatpiboon, N. Mathematical model and experimental validation of the synergistic effect of selective enantioseparation of (S)-amlodipine from pharmaceutical wastewater using a HFSLM. J. Ind. Eng. Chem. 2014, 20, 1612–1622. [Google Scholar] [CrossRef]
- Székely, G.; Csordás, B.; Farkas, V.; Kupai, J.; Pogány, P.; Sánta, Z.; Szakács, Z.; Tóth, T.; Hollósi, M.; Nyitrai, J.; et al. Synthesis and Preliminary Structural and Binding Characterization of New Enantiopure Crown Ethers Containing an Alkyl Diarylphosphinate or a Proton-Ionizable Diarylphosphinic Acid Unit. Eur. J. Org. Chem. 2012, 2012, 3396–3407. [Google Scholar]
- Hauser, A.W.; Mardirossian, N.; Panetier, J.A.; Head-Gordon, M.; Bell, A.T.; Schwerdtfeger, P. Functionalized graphene as a gatekeeper for chiral molecules: An alternative concept for chiral separation. Angew. Chem. Int. Ed. 2014, 53, 9957–9960. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of Chirality and macroring in imprinted polymers with enantiodiscriminative power. ACS Appl. Mater. Interfaces 2015, 7, 9516–9525. [Google Scholar]
- Ghazali, N.F.; Ferreira, F.C.; White, A.J.P.; Livingston, A.G. Enantiomer separation by enantioselective inclusion complexation–organic solvent nanofiltration. Tetrahedron Asymmetry 2006, 17, 1846–1852. [Google Scholar] [CrossRef] [Green Version]
- Rukhlenko, I.D.; Tepliakov, N.V.; Baimuratov, A.S.; Andronaki, S.A.; Gun’ko, Y.K.; Baranov, A.V.; Fedorov, A.V. Completely chiral optical force for enantioseparation. Sci. Rep. 2016, 6, 36884. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Schurig, V. Stopped-flow multidimensional gas chromatography: A new method for the determination of enantiomerization barriers. J. Microcolumn Sep. 1999, 11, 475–479. [Google Scholar] [CrossRef]
- Reich, S.; Trapp, O.; Schurig, V. Enantioselective stopped-flow multidimensional gas chromatography: Determination of the inversion barrier of 1-chloro-2,2-dimethylaziridine. J. Chromatogr. A 2000, 892, 487–498. [Google Scholar] [CrossRef]
- Schurig, V.; Reich, S. Determination of the rotational barriers of atropisomeric polychlorinated biphenyls (PCBs) by a novel stopped-flow multidimensional gas chromatographic technique. Chirality 1998, 10, 316–320. [Google Scholar] [CrossRef]
- Welch, C.J. Evolution of chiral stationary phase design in the Pirkle laboratories. J. Chromatogr. A 1994, 666, 3–26. [Google Scholar] [CrossRef]
- Vetter, W. Gas Chromatographic Enantiomer Separation of Polychlorinated Biphenyls (PCBs): Methods, Metabolisms, Enantiomeric Composition in Environmental Samples and their Interpretation. Isr. J. Chem. 2016, 56, 940–957. [Google Scholar] [CrossRef]
- Vetter, W.; Bester, K. Gas chromatographic enantioseparation of chiral pollutants-techniques and results. Chiral Anal. 2006, 131–213. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral separations: A review of current topics and trends. Anal. Chem. 2012, 84, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, D.R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective chromatography in drug discovery. Drug Discov. Today 2005, 10, 571–577. [Google Scholar] [CrossRef]
- Ward, T.J.; Ward, K.D. Chiral Separations: Fundamental Review 2010. Anal. Chem. 2010, 82, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Maher, K. Chiral separations by high-performance liquid chromatography. J. Chromatogr. Sci. 1992, 30, 67. [Google Scholar] [CrossRef]
- Haginaka, J. Pharmaceutical and biomedical applications of enantioseparations using liquid chromatographic techniques. J. Pharm. Biomed. Anal. 2002, 27, 357–372. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Cass, Q.B.; Tiritan, M.E. Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: An overview. J. Chromatogr. B 2014, 968, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small molecules as chromatographic tools for HPLC enantiomeric resolution: Pirkle-Type chiral stationary phases evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Haginaka, J. Recent progresses in protein-based chiral stationary phases for enantioseparations in liquid chromatography. J. Chromatogr. B 2008, 875, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, J.C.; Vanzolini, K.L.; Madureira, T.V.; Tiritan, M.E.; Cass, Q.B. A column-switching method for quantification of the enantiomers of omeprazole in native matrices of waste and estuarine water samples. Talanta 2010, 82, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, J.C.; Vanzolini, K.L.; Cass, Q.B. Direct injection of native aqueous matrices by achiral–chiral chromatography ion trap mass spectrometry for simultaneous quantification of pantoprazole and lansoprazole enantiomers fractions. J. Chromatogr. A 2011, 1218, 2865–2870. [Google Scholar] [CrossRef] [PubMed]
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.-L.; Evard, H.; Herodes, K.; Ravio, P.; Leito, I. Tutorial review on validation of liquid chromatography–mass spectrometry methods: Part II. Anal. Chim. Acta 2015, 870, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Santos, L.H.M.L.M.; Maia, A.S.; Delerue-Matos, C.; Castro, P.M.L.; Tiritan, M.E. Enantiomeric fraction evaluation of pharmaceuticals in environmental matrices by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1363, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhang, Q.; Shi, H.; Gao, B.; Hua, X.; Wang, M. Simultaneous determination of chiral pesticide flufiprole enantiomers in vegetables, fruits, and soil by high-performance liquid chromatography. Anal. Bioanal. Chem. 2015, 407, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.P.A.; Paz, M.S.O.; Cavalcante, R.M.; Nascimento, R.F. Strategy for correction of matrix effect on the determination of pesticides in water bodies using SPME-GC-FID. J. Braz. Chem. Soc. 2017, 28, 1081–1090. [Google Scholar] [CrossRef]
- Buser, H.-R.; Poiger, T.; Müller, M.D. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Fono, L.J.; Sedlak, D.L. Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters. Environ. Sci. Technol. 2005, 39, 9244–9252. [Google Scholar] [CrossRef] [PubMed]
- Barclay, V.K.H.; Tyrefors, N.L.; Johansson, I.M.; Pettersson, C.E. Chiral analysis of metoprolol and two of its metabolites, α-hydroxymetoprolol and deaminated metoprolol, in wastewater using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1269, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Liu, D.; Zhou, Z.; Wang, P. A new chiral residue analysis method for triazole fungicides in water using dispersive liquid-liquid microextraction (DLLME). Chirality 2013, 25, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Deng, M.; Huang, P.; Yu, J.; Guo, X.; Zhao, L. Solid-phase extraction combined with dispersive liquid-liquid microextraction and chiral liquid chromatography-tandem mass spectrometry for the simultaneous enantioselective determination of representative proton-pump inhibitors in water samples. Anal. Bioanal. Chem. 2016, 408, 6381–6392. [Google Scholar] [CrossRef] [PubMed]
- Caballo, C.; Sicilia, M.D.; Rubio, S. Enantioselective determination of representative profens in wastewater by a single-step sample treatment and chiral liquid chromatography-tandem mass spectrometry. Talanta 2015, 134, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashim, N.H.; Stuetz, R.M.; Khan, S.J. Enantiomeric fraction determination of 2-arylpropionic acids in a package plant membrane bioreactor. Chirality 2013, 25, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Baker, D.R. Estimation of community-wide drugs use via stereoselective profiling of sewage. Sci. Total Environ. 2012, 423, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; McDonald, J.A.; Khan, S.J. Enantiomeric analysis of polycyclic musks in water by chiral gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1303, 66–75. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Kasprzyk-Hordern, B.; Petrović, M.; Barceló, D. Multi-residue enantiomeric analysis of pharmaceuticals and their active metabolites in the Guadalquivir River basin (South Spain) by chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 5859–5873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gomez, E.; Fenet, H.; Chiron, S. Chiral signature of venlafaxine as a marker of biological attenuation processes. Chemosphere 2013, 90, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Pankratov, I.; Elhanany, S.; Werner, P.; Gun, J.; Gelman, F.; Lev, O. Field and laboratory studies of the fate and enantiomeric enrichment of venlafaxine and O-desmethylvenlafaxine under aerobic and anaerobic conditions. Chemosphere 2012, 88, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, K.; Wang, Z.; Wang, C.; Peng, X. Enantiomeric determination of azole antifungals in wastewater and sludge by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, U.; Radke, M. Fate of pharmaceuticals in rivers: Deriving a benchmark dataset at favorable attenuation conditions. Water Res. 2012, 46, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, J.P.; Evans, S.E.; Wort, M.T.; Lubben, A.T.; Kasprzyk-Hordern, B. Using chiral liquid chromatography quadrupole time-of-flight mass spectrometry for the analysis of pharmaceuticals and illicit drugs in surface and wastewater at the enantiomeric level. J. Chromatogr. A 2012, 1249, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzyk-Hordern, B.; Baker, D.R. Enantiomeric profiling of chiral drugs in wastewater and receiving waters. Environ. Sci. Technol. 2012, 46, 1681–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morante-Zarcero, S.; Sierra, I. Comparative HPLC methods for β-blockers separation using different types of chiral stationary phases in normal phase and polar organic phase elution modes. Analysis of propranolol enantiomers in natural waters. J. Pharm. Biomed. Anal. 2012, 62, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Morante-Zarcero, S.; Sierra, I. Simultaneous enantiomeric determination of propranolol, metoprolol, pindolol, and atenolol in natural waters by HPLC on new polysaccharide-based stationary phase using a highly selective molecularly imprinted polymer extraction. Chirality 2012, 24, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.H.; Khan, S.J. Enantioselective analysis of ibuprofen, ketoprofen and naproxen in wastewater and environmental water samples. J. Chromatogr. A 2011, 1218, 4746–4754. [Google Scholar] [CrossRef] [PubMed]
- Barclay, V.K.H.; Tyrefors, N.L.; Johansson, I.M.; Pettersson, C.E. Trace analysis of fluoxetine and its metabolite norfluoxetine. Part I: Development of a chiral liquid chromatography–tandem mass spectrometry method for wastewater samples. J. Chromatogr. A 2011, 1218, 5587–5596. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Kondakal, V.V.R.; Baker, D.R. Enantiomeric analysis of drugs of abuse in wastewater by chiral liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 4575–4586. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Hijosa, M.; Bayona, J.M. Assessment of the pharmaceutical active compounds removal in wastewater treatment systems at enantiomeric level. Ibuprofen and naproxen. Chemosphere 2009, 75, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Nikolai, L.N.; McClure, E.L.; MacLeod, S.L.; Wong, C.S. Stereoisomer quantification of the -blocker drugs atenolol, metoprolol, and propranolol in wastewaters by chiral high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1131, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fono, L.J.; Kolodziej, E.P.; Sedlak, D.L. Attenuation of Wastewater-Derived Contaminants in an Effluent-Dominated River. Environ. Sci. Technol. 2006, 40, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Moreira, I.S.; Afonso, C.M.; Castro, P.M.L.; Tiritan, M.E. Enantioselective quantification of fluoxetine and norfluoxetine by HPLC in wastewater effluents. Chemosphere 2014, 95, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Depaolini, A.R.; Fattore, E.; Cappelli, F.; Pellegrino, R.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Davoli, E. Source discrimination of drug residues in wastewater: The case of salbutamol. J. Chromatogr. B 2016, 1023–1024, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Z.; Wang, C.; Peng, X. Chiral profiling of azole antifungals in municipal wastewater and recipient rivers of the Pearl River Delta, China. Environ. Sci. Pollut. R. 2013, 20, 8890–8899. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.J.; Wang, L.; Hashim, N.H.; McDonald, J.A. Distinct enantiomeric signals of ibuprofen and naproxen in treated wastewater and sewer overflow. Chirality 2014, 26, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Souchier, M.; Benali-Raclot, D.; Casellas, C.; Ingrand, V.; Chiron, S. Enantiomeric fractionation as a tool for quantitative assessment of biodegradation: The case of metoprolol. Water Res. 2016, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kosugi, Y.; Hosaka, M.; Nishimura, T.; Nakae, D. Occurrence and behavior of the chiral anti-inflammatory drug naproxen in an aquatic environment. Environ. Toxicol. Chem. 2014, 33, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Bagnall, J.; Kasprzyk-Hordern, B. Enantioselective degradation of amphetamine-like environmental micropollutants (amphetamine, methamphetamine, MDMA and MDA) in urban water. Environ. Pollut. 2016, 215, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Bagnall, J.; Kasprzyk-Hordern, B. Enantiomeric profiling of a chemically diverse mixture of chiral pharmaceuticals in urban water. Environ. Pollut. 2017, 230, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, B.; Lu, S.; Zhang, Y.; Yin, L.; Huang, J.; Deng, S.; Wang, Y.; Yu, G. Characterization of pharmaceutically active compounds in Dongting Lake, China: Occurrence, chiral profiling and environmental risk. Sci. Total Environ. 2016, 557–558, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Critical evaluation of monitoring strategy for the multi-residue determination of 90 chiral and achiral micropollutants in effluent wastewater. Sci. Total Environ. 2017, 579, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Ali, I. Analysis of the chiral pollutants by chromatography. Toxicol. Environ. Chem. 2004, 86, 1–22. [Google Scholar] [CrossRef]
- Faller, J.; Huhnerfuss, H.; König, W.A.; Ludwig, P. Gas chromatographic separation of the enantiomers of marine organic pollutants distribution of -HCH enantiomers in the North Sea. Mar. Pollut. Bull. 1991, 22, 82–86. [Google Scholar] [CrossRef]
- Hühnerfuss, H.; Faller, J.; Kallenborn, R.; König, W.A.; Ludwig, P.; Pfaffenberger, B.; Oehme, M.; Rimkus, G. Enantioselective and nonenantioselective degradation of organic pollutants in the marine ecosystem. Chirality 1993, 5, 393–399. [Google Scholar]
- Hühnerfuss, H.; Faller, J.; König, W.A.; Ludwig, P. Gas chromatographic separation of the enantiomers of marine pollutants. 4. Fate of hexachlorocyclohexane isomers in the Baltic and North Sea. Environ. Sci. Technol. 1992, 26, 2127–2133. [Google Scholar]
- Barclay, V.K.H.; Tyrefors, N.L.; Johansson, I.M.; Pettersson, C.E. Trace analysis of fluoxetine and its metabolite norfluoxetine. Part II: Enantioselective quantification and studies of matrix effects in raw and treated wastewater by solid phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1227, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, D.; Kasprzyk-Hordern, B. Multi-residue enantiomeric analysis of human and veterinary pharmaceuticals and their metabolites in environmental samples by chiral liquid chromatography coupled with tandem mass spectrometry detection. Anal. Bioanal. Chem. 2015, 407, 9085–9104. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, D.; Kasprzyk-Hordern, B.; Thomas, K.V. Enantioselective simultaneous analysis of selected pharmaceuticals in environmental samples by ultrahigh performance supercritical fluid based chromatography tandem mass spectrometry. Anal. Chim. Acta 2016, 934, 239–251. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, S.L.; Wong, C.S. Loadings, trends, comparisons, and fate of achiral and chiral pharmaceuticals in wastewaters from urban tertiary and rural aerated lagoon treatments. Water Res. 2010, 44, 533–544. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, S.L.; Sudhir, P.; Wong, C.S. Stereoisomer analysis of wastewater-derived b-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2007, 1170, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Muñoz, D.; Kasprzyk-Hordern, B. Simultaneous enantiomeric analysis of pharmacologically active compounds in environmental samples by chiral LC–MS/MS with a macrocyclic antibiotic stationary phase. J. Mass Spectrom. 2017, 52, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Schurig, V.; Juza, M. Analytical separation of enantiomers by gas chromatography on chiral stationary phases. Adv. Chromatogr. 2015, 52, 117–168. [Google Scholar]
- Hegade, R.S.; De Beer, M.; Lynen, F. Chiral stationary phase optimized selectivity liquid chromatography: A strategy for the separation of chiral isomers. J. Chromatogr. A 2017, 1515, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, E.; Guerra, P.; Barceló, D. Enantiomeric determination of chiral persistent organic pollutants and their metabolites. TrAC Trends Anal. Chem. 2008, 27, 847–861. [Google Scholar] [CrossRef]
- Rykowska, I.; Wasiak, W. Recent advances in gas chromatography for solid and liquid stationary phases containing metal ions. J. Chromatogr. A 2009, 1216, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Garrison, A.W. Enantiomer separation of polychlorinated biphenyl atropisomers and polychlorinated biphenyl retention behavior on modified cyclodextrin capillary gas chromatography columns. J. Chromatogr. A 2000, 866, 213–220. [Google Scholar] [CrossRef]
- Sanganyado, E.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Ridal, J.J.; Bidleman, T.F.; Kerman, B.; Fox, M.E.; Strachan, W.M. Enantiomers of alfa-Hexachlorocyclohexane as Tracers of Air-Water Gas Exchange in Lake Ontario. Environ. Sci. Technol. 1997, 31, 1940–1945. [Google Scholar] [CrossRef]
- Wiberg, K.; Harner, T.; Wideman, J.L.; Bidleman, T.F. Chiral analysis of organochlorine pesticides in Alabama soils. Chemosphere 2001, 45, 843–848. [Google Scholar] [CrossRef]
- Padma, T.V.; Dickhut, R.M.; Ducklow, H. Variations in α-hexachlorocyclohexane enantiomer ratios in relation to microbial activity in a temperate estuary. Environ. Toxicol. Chem. 2003, 22, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, T.D.; Müller, S.R.; Heberle, S.; Schwarzenbach, R.P. Occurrence and Behavior of Pesticides in Rainwater, Roof Runoff, and Artificial Stormwater Infiltration. Environ. Sci. Technol. 1998, 32, 3457–3464. [Google Scholar] [CrossRef]
- Schurig, V. Terms for the quantitation of a mixture of stereoisomers. Top. Curr. Chem. 2013, 340, 21–40. [Google Scholar] [PubMed]
- Caballo, C.; Sicilia, M.D.; Rubio, S. Fast, simple and efficient supramolecular solvent-based microextraction of mecoprop and dichlorprop in soils prior to their enantioselective determination by liquid chromatography-tandem mass spectrometry. Talanta 2014, 119, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Madureira, T.V.; Barreiro, J.C.; Rocha, M.J.; Cass, Q.B.; Tiritan, M.E. Pharmaceutical trace analysis in aqueous environmental matrices by liquid chromatography–ion trap tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7033–7042. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.M.J.; Van den Brink, P.J. Review of Recent Literature Concerning Mixture Toxicity of Pesticides to Aquatic Organisms; RIVM Report 601400001/2010; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2010. [Google Scholar]
- Carlsson, P.; Warner, N.A.; Hallanger, I.G.; Herzke, D.; Kallenborn, R. Spatial and temporal distribution of chiral pesticides in Calanus spp. from three Arctic fjords. Environ. Pollut. 2014, 192, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, L.M.; Bidleman, T. Air-water gas exchange of hexachlorocyclohexanes (HCHs) and the enantiomers of c-HCH in arctic regions. J. Geophys. Res. 1996, 101, 28837–28846. [Google Scholar] [CrossRef]
- McBlain, W.A.; Lewin, V.; Wolfe, F.H. Differing estrogenic activities for the enantiomers of o,p′-DDT in immature female rats. Can. J. Physiol. Pharmacol. 1976, 54, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Enantioselective degradation of metalaxyl in soils: Chiral preference changes with soil pH. Environ. Sci. Technol. 2003, 37, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Buser, H.-R.; Müller, M.D. Occurrence and transformation reactions of chiral and achiral phenoxyalkanoic acid herbicides in lakes and rivers in switzerland. Environ. Sci. Technol. 1998, 32, 626–633. [Google Scholar] [CrossRef]
- Gerecke, A.C.; Schärer, M.; Singer, H.P.; Müller, S.R.; Schwarzenbach, R.P.; Sägesser, M.; Ochsenbein, U.; Popow, G. Sources of pesticides in surface waters in Switzerland: pesticide load through waste water treatment plants––current situation and reduction potential. Chemosphere 2002, 48, 307–315. [Google Scholar] [CrossRef]
- Bethan, B.; Bester, K.; Huhnerfuss, H.; Rimkus, G. Bromocyclen contamination of surface water, waste water and fish from northern germany, and gas chromatographic chiral separation. Chemosphere 1997, 34, 2271–2280. [Google Scholar] [CrossRef]
- Harju, M.; Bergman, A.; Olsson, M.; Roos, A.; Haglund, P. Determination of atropisomeric and planar polychlorinated biphenyls, their enantiomeric fractions and tissue distribution in grey seals using comprehensive 2D gas chromatography. J. Chromatogr. A 2003, 1019, 127–142. [Google Scholar] [CrossRef] [PubMed]
- UNEP, U.n.e.p.C. TREATIES-4. Stockholm convention on persistent organic pollutants. Adoption of amendments to Annexes A, B and C by decisions SC-4/10, 4/11, 4/12, 4/13, 4/14, 4/15, 4/16, 4/17 and 4/18. In Proceedings of the Conference of Plenipotentiaries, Stockholm, Sweden, 22 May 2001. [Google Scholar]
- EU-Directive. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, L.226, 1–17. [Google Scholar]
- Wiberg, K.; Letcher, R.; Sandau, C.; Duffe, J.; Norstrom, R.; Haglund, P.; Bidleman, T. Enantioselective gas chromatography/mass spectrometry of methylsulfonyl PCBs with application to arctic marine mammals. Anal. Chem. 1998, 70, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Grimm, F.A.; Hu, D.; Kania-Korwel, I.; Lehmler, H.J.; Ludewig, G.; Hornbuckle, K.C.; Duffel, M.W.; Bergman, A.; Robertson, L.W. Metabolism and metabolites of polychlorinated biphenyls (PCBs). Crit. Rev. Toxicol. 2015, 45, 245–272. [Google Scholar] [CrossRef] [PubMed]
- Mamontova, E.A.; Tarasova, E.N.; Mamontov, A.A. PCBs and OCPs in human milk in Eastern Siberia, Russia: Levels, temporal trends and infant exposure assessment. Chemosphere 2017, 178, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Norström, K.; Czub, G.; McLachlan, M.S.; Hu, D.; Thorne, P.S.; Hornbuckle, K.C. External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ. Int. 2010, 36, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Jimenez, B.; Marsili, L.; Hernández, L.M.; Schurig, V.; González, M.J. Congener specific determination and enantiomeric ratios of chiral polychlorinated biphenyls in striped dolphins (stenella coeruleoalba) from the mediterranean sea. Environ. Sci. Technol. 1999, 33, 1787–1793. [Google Scholar] [CrossRef]
- Wiberg, K.; Bergman, A.; Olsson, M.; Roos, A.; Blomkvist, G.; Haglund, P. Concentrations and enantiomer fractions of organochlorine compounds in baltic species hit by reproductive impairment. Environ. Toxicol. Chem. 2002, 21, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Covaci, A.; Van de Vijver, K.; De Coen, W.; Blust, R.; Schepens, P. Enantiomeric signatures of chiral polychlorinated biphenyl atropisomers in livers of harbour porpoises (Phocoena phocoena) from the southern North Sea. J. Environ. Monit. 2003, 5, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Benická, E.; Novakovsky, R.; Hrouzek, J.; Krupcík, J.; Sandra, P.; Zeeuw, J.D. Multidimensional gas chromatographic separation of selected PCB atropisomers in technical formulations and sediments. J. High Resolut. Chromatogr. 1996, 19, 95–98. [Google Scholar] [CrossRef]
- Glausch, A.; Blanch, G.P.; Schurig, V. Enantioselective analysis of chiral polychlorinated biphenyls in sediment samples by multidimensional gas chromatography-electron-capture detection after steam distillation-solvent extraction and sulfur removal. J. Chromatogr. A 1996, 723, 399–404. [Google Scholar] [CrossRef]
- Lee, I.; Gopalan, A.I.; Lee, K.P. Enantioselective determination of polycyclic musks in river and wastewater by GC/MS/MS. Int. J. Env. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Berset, J.D.; Kupper, T.; Etter, R.; Tarradellas, J. Considerations about the enantioselective transformation of polycyclic musks in wastewater, treated wastewater and sewage sludge and analysis of their fate in a sequencing batch reactor plant. Chemosphere 2004, 57, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.L.; Bidleman, T.F.; Gregorb, D.J. Air-water gas exchange and evidence for metabolism of hexachlorocyclohexanes in Resolute Bay, N.W.T. Sci. Total Environ. 1995, 160–161, 65–74. [Google Scholar] [CrossRef]
- Jantunen, L.M.M.; Bidleman, T.F. Organochlorine pesticides and enantiomers of chiral pesticides in arctic ocean water. Arch. Environ. Contam. Toxicol. 1998, 35, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Karakus, P.B.; Stroud, J.L.; Bidleman, T.; Semple, K.T.; Jantunen, L.M.M.; Jones, K.C. Enantioselective degradation of organochlorine pesticides in background soils: Variability in field and laboratory studies. Environ. Sci. Technol. 2007, 41, 4965–4971. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.; Hunter, S.; Hazrati, S.; Harrad, S. Concentrations and chiral signatures of polychlorinated biphenyls in outdoor and indoor air and soil in a major U.K. conurbation. Environ. Sci. Technol. 2007, 41, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
| Chiral Compounds | Analytical Method | Location/Matrix | Concentration/ER, EF | Ref. |
|---|---|---|---|---|
| Amphetamine Methamphetamine MDMA MDA | UPLC-MS/MS; Chiral-CBH column (100 mm × 2 mm i.d., 5 µm) with a Chiral-CBH guard column (10 mm × 2.0 mm i.d.); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. | United Kingdom Influent (IWW) and effluent (EWW) wastewater; River water. | IWW: EF = 0.6; EWW: EF = 0.4; IWW: EF > 0.5; EWW: EF > 0.5; IWW: EF < 0.5; EWW: EF < 0.5; IWW: EF > 0.5; EWW: EF < 0.5. | [92] |
| Amphetamine Methamphetamine MDMA MDA MDEA Ephedrine Norephedrine | UPLC-MS/MS; Chiral-CBH column (100 mm × 2 mm i.d., 5 µm) with a Chiral-CBH guard column (10 mm × 2.0 mm i.d.); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. | England Influent (IWW) and effluent (EWW) wastewater River water (RW): 6 locations near the WWTP discharge zone | 17.4–3112.5 ng L−1 (IWW); 4.3–145.2 ng L−1 (EWW); 0.3–4.3 ng L−1 (SW); 0.6–70.3 ng L−1 (IWW); 0.4–1.3 ng L−1 (EWW); 0.3–0.4 ng L−1 (SW); 7.2–32.4 ng L−1 (IWW); 6.3–24.5 ng L−1 (EWW); 0.9–1.9 ng L−1 (SW); 0.7–455.4 ng L−1 (IWW); 0.6–177.7 ng L−1 (EWW); 0.5–24.8 ng L−1 (SW); 1.4 ng L−1 (IWW); n.d. (EWW); n.d. (SW); 8.7–1979.5 ng L−1 (IWW); 5.3–265 ng L−1 (EWW); 6.3–28.9 ng L−1 (SW); 15–99.9 ng L−1 (IWW); n.d. (EWW); n.d. (SW). | [68] |
| Amphetamine Methamphetamine MDMA MDA Ephedrine Pseudoephedrine | UPLC-MS/MS; Chiral-CBH column (100 mm × 2 mm i.d., 5 µm) with a Chiral-CBH guard column (10 mm × 2.0 mm i.d.); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. | England Wastewater influent and effluent | EF = 0.52–0.84; EF ≥ 0.5; EF = 0.68 (mean); EF > 0.5; EF = 0.81–0.96; - | [69] |
| Amphetamine Methamphetamine MDMA MDA Ephedrine Pseudoephedrine Norephedrine Alprenolol Atenolol Citalopram Desmethylcitalopram Desmethylvenlafaxine Fluoxetine Mirtazapine Metoprolol Propranolol Salbutamol Sotalol Tramadol Venlafaxine | UPLC-MS/MS; Chiral-CBH column (100 mm × 2 mm i.d., 5 µm) with a Chiral-CBH guard column (10 mm × 2.0 mm i.d.); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. Chirobiotic V column, (250 × 2.1 mm, i.d. 5 µm) with a Chirobiotic V guard column (20 × 1.0 mm, i.d. 5 µm); Methanol (4 mM ammonium acetate, 0.005% formic acid) | Not referred Influent (IWW) and effluent (EWW) wastewater; Sludge (Sl.). | IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = 0.7; IWW: EF = 0.6; EWW: EF = 0.5; Sl.: EF = 0.5; IWW: EF = 0.7; EWW: EF = 0.9; Sl.: EF = 0.4; IWW: EF = 0.6; EWW: EF = 0.5; Sl.: EF = 0.3; IWW: EF = 0; EWW: EF = 0; Sl.: EF = n.d.; IWW: EF = 1; EWW: EF = 0.2; Sl.: EF = n.d.; IWW: EF = 0; EWW: EF = 0.3; Sl.: EF = 0.1; IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = 0.7; IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = 0.4; IWW: EF = 0.6; EWW: EF = 0.7; Sl.: EF = 0.6; IWW: EF = 1; EWW: EF = n.d.; Sl.: EF = 0.6; IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = 0.5; IWW: EF = 0.7; EWW: EF = 0.7; Sl.: EF = 0.7; IWW: EF = 0.3; EWW: EF = 0.2; Sl.: EF = 0.5; IWW: EF = 0.3; EWW: EF = n.d.; Sl.: EF = 0.4; IWW: EF = 0.4; EWW: EF = 0.4; Sl.: EF = 0.5; IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = n.d.; IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = 0.5; IWW: EF = 0.7; EWW: EF = 0.7; Sl.: EF = 0.7; IWW: EF = 0.5; EWW: EF = 0.5; Sl.: EF = 0.5; | [66] |
| MDMA Atenolol Citalopram Desmethylcitalopram Fluoxetine Mirtazapine Metoprolol Propranolol | UPLC-MS/MS; Chiral-CBH column (100 mm × 2 mm i.d., 5 µm); H2O-2-propanol (85:15, v/v) (1 mM ammonium acetate) Chirobiotic V column, (100 × 2.1 mm, i.d. 5 µm); Methanol (4 mM ammonium acetate, 0.005% formic acid) | Not referred Effluent wastewater. | EF showed temporal changes (0.24–0.38); EF did not show temporal changes; EF did not show temporal changes; EF did not show temporal changes; EF did not show temporal changes; EF did not show temporal changes; EF did not show temporal changes; EF did not show temporal changes. | [95] |
| Amphetamine Methamphetamine MDMA MDA Alprenolol Atenolol Citalopram Desmethylcitalopram Desmethylvenlafaxine Fluoxetine Mirtazapine Metoprolol Propranolol Salbutamol Sotalol Venlafaxine | UPLC-MS/MS; Chiral-CBH column (100 mm × 2 mm i.d., 5 µm) with a Chiral-CBH guard column (10 mm × 2.0 mm i.d.); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. Chirobiotic V column, (250 × 2.1 mm, i.d. 5 µm) with a Chirobiotic V guard column (20 × 1.0 mm, i.d. 5 µm); Methanol (4 mM ammonium acetate, 0.005% formic acid) | Not referred Influent (IWW) and effluent (EWW) wastewater; Sludge (Sl.). | IWW: EF = 0.64; EWW: EF = 0.42; IWW: EF = 0.89; EWW: EF = 0.63; IWW: EF = 0.36; EWW: EF = 0.22; IWW: EF = 0.59; EWW: EF = 0.42; IWW: EF = 0.51; EWW: EF = 0.48; IWW: EF = 0.50; EWW: EF = 0.54; IWW: EF = 1.0; EWW: EF = 1.0; IWW: EF = 0.26; EWW: EF = 0.10; IWW: EF = 0.57; EWW: EF = 0.52; IWW: EF = 0.69; EWW: EF = 0.62; IWW: EF = 0.25; EWW: EF = 0.19; IWW: EF = 0.33; EWW: EF = n.d.; IWW: EF = 0.46; EWW: EF = 0.41; IWW: EF = 0.65; EWW: EF = 0.57; IWW: EF = 0.50; EWW: EF = 0.50. Venlafaxine | [93] |
| Ibuprofen Naproxen Fexofenadine Tetramisole Ketoprofen Aminorex Chloramphenicol 3-N-Dechloroethylifosfamide 10,11-dihydro-10-hydroxycarbamazepine Dihydroketoprofen Ifosfamide Praziquantel | LC-MS/MS Chiral-AGP (100 × 2 mm, i.d. 5 μm) column with a Chiral-AGP (10 × 2.0 mm, i.d. 5 μm) guard column; Aqueous solution of 10 mM ammonium acetate with 1% of acetonitrile, pH 6.7 | United Kingdom Effluent wastewater; River water (South West England). | EF = 0.65 EF = 0.92 EF = 0.55 EF = 0.50 - - - - - - - - | [101] |
| Aminorex 2-hydroxyibuprofen Ibuprofen Imazalil Naproxen Ofloxacin Tetramisole Carprofen Chloramphenicol 3-N-dechloroethylifosfamide Flurbiprofen Ifosfamide Omeprazole Praziquantel Indoprofen | LC-MS/MS Polysaccharide amylose tris-3,5-dimethylphenylcarbamate column and a cellulose tris-(3-chloro-4-methylphenylcarbamate column (150 × 2.1 mm, i.d. 2.5 μm); CO2-methanol/acetonitrile/2-propanol, 1:1:1, v/v with 10 mM ammonium acetate and 0.1% ammonium hydroxide under a gradient program (in positive ionization); Polysaccharide amylose tris-3,5-dimethylphenylcarbamate column (150 × 2.1 mm, i.d. 2.5 μm); CO2-methanol with 0.1% ammonium hydroxide under a gradient program (in negative ionization). | Northern and Western Europe Influent and effluent wastewater. | EF = 0.4 (IWW) EF = 0.2 (IWW) EF = 1.0 (IWW) EF = 0 (IWW) EF = 1.0 (IWW and EWW) EF = 0 (IWW) EF = 0.6 (IWW and EWW) - - - - - - - - | [102] |
| Carboxyibuprofen Chloramphenicol 2-hydroxyibuprofen Ibuprofen Ifosfamide Indoprofen Ketoprofen Naproxen Praziquantel | Chirobiotic T column (250 × 2.1 mm, i.d. 5 μm); Methanol-10 mM ammonium acetate (30/70, v/v), pH 4.2. | United Kingdom Influent and effluent wastewater; River water (South West England). | EF = 0.83 (IWW) - EF = 0.79 (IWW) EF = 1.0 (IWW) - - - EF = 1.0 (IWW) - | [105] |
| MDMA MDA Amphetamine Methamphetamine Ephedrine Venlafaxine Atenolol | LC-MS/MS; Chiral-CBH column (100 mm × 2 mm, 5 μm) with a Chiral-CBH guard column (10 mm × 2.0 mm); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. | Location n.a.; River water; Influent and effluent wastewater (7 WWTPs using mainly activated sludge and trickling filters technologies). | IWW: <LOQ—455 ng L−1; EF = 0.68; EWW: <LOQ—115 ng L−1; EF = 0.78. IWW: 11.8—45.8 ng L−1; EF = 0.26–0.47; EWW: 12.3—19.0 ng L−1; EF = 0.4–0.58. IWW: <LOQ—3112.5 ng L−1; EF = 0.59–0.84; EWW: <LOQ—19.7 ng L−1; EF = 0.68–1.0. IWW: <LOQ—1.8 ng L−1; EF = 0.22–0.53; EWW: <LOQ; EF = 0.70–1.0. IWW: <LOQ—15171 ng L−1; EF = 0.81–1.0; EWW: <LOQ—84.1 ng L−1; EF = 0.72–1.0. IWW: 28.8–325.5 ng L−1; EF = 0.35–0.65; EWW: 25–222 ng L−1; EF = 0.46–0.69. IWW: 4288–19160 ng L−1; EF = 0.30–0.47; EWW: 1480–18831 ng L−1; EF = 0.40–0.61. | [77] |
| Amphetamine Methamphetamine MDA MDMA Propranolol Atenolol Metoprolol Fluoxetine Venlafaxine | LC-MS/MS; Chiral-CBH column (100 × 2 mm, i.d. 5 µm) with a Chiral-CBH µm guard column (10 × 2.0 mm i.d., 5 µm); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 7.0. | United Kingdom River water (River Avon, Salford, Somerset). | <MQL; <MQL; <MQL; <MQL; <MQL; <MQL; <MQL; <MQL; <MQL. | [76] |
| Amphetamine Methamphetamine MDA MDMA Propranolol Atenolol Metoprolol Fluoxetine Venlafaxine | LC-MS/MS; Chirobiotic V column, (250 × 4.6 mm, i.d. 5 µm) with a Chirobiotic V guard column (20 × 4.0 mm, i.d. 5 µm); Methanol containing 4 mM ammonium acetate and 0.005% formic acid. | United Kingdom River water (River Avon, Salford, Somerset); Effluent wastewater | EWW: <MQL; RW: <MQL; EWW: <MQL; RW: <MQL; EWW: <MQL; RW: n.d.; EWW: <MQL; RW: <MQL; EWW: EF = 0.43; RW: EF = 0.45; EWW: EF = 0.55; RW: EF = 0.47; EWW: EF = 0.54; RW: < MQL; EWW: EF = 0.43; RW: EF = 0.58; EWW: <MQL; RW: < MQL. | |
| Amphetamine Methamphetamine MDA MDEA MDMA Ephedrine 1R,2S (−) Pseudophedrine 1S,2S (+) Norephedrine Venlafaxine | LC-MS/MS; Chiral-CBH column (100 mm × 2 mm, 5 µm) with a Chiral-CBH guard column (10 mm × 2.0 mm); H2O-2-propanol (90:10, v/v), 1 mM ammonium acetate, pH 5.0. | Location n.a. Wastewater influent and effluent (4 WWTPs). | IWW: (S)-form 24.2–155.2 ng L−1; (R)-form 39.5–212.9 ng L−1; EF = 0.54–0.62; EWW: n.d.; IWW: (S)- and (R)-forms n.d.; EWW: (S)-form n.d. - < MQL; (R)-form n.d.; n.d.; IWW: n.d. - < MQL; EWW: n.d.; IWW: E1 < MQL—5.5 ng L−1; E2 < MQL—13.9 ng L−1; EF = 0.53–0.72; EWW: E1 n.d.–4.0 ng L−1; E2 < MQL—10.0 ng L−1; EF = 0.71 IWW: 14.3–72.3 ng L−1; EWW: <MQL—14.8 ng L−1; IWW: 51.0–329.7 ng L−1; EWW: <MQL—27.7 ng L−1; n.d. IWW: E1 57.2–286.5 ng L−1; E2 56.7–343.8 ng L−1; EF = 0.45–0.50; EWW: E1 80.2–178.2 ng L−1; E2 123.7–248.3 ng L−1; EF = 0.37–0.48. | [82] |
| Metoprolol Propranolol Atenolol Fluoxetine Venlafaxine Ibuprofen Flurbiprofen Naproxen | LC-MS/MS; Chirobiotic V column, (250 × 4.6 mm, i.d. 5 µm) with a Chirobiotic V guard column (20 × 4.0 mm, i.d. 5 µm); Chiralpak AD-RH column, (150 × 4.6 mm, i.d. 5 µm). | China Surface water (Dongting Lake). | 0.48–0.64 0.44–0.56 n.d. - 0.46–0.51 - n.d. - | [94] |
| 16 pharmaceuticals (analgesics, antibiotics, beta-agonists, psychiatric and cardiovascular drugs) and two metabolites | LC-MS/MS; Chirobiotic V (250 × 2.1 mm i.d., 5 μm) with a Chirobiotic V guard column (20 mm × 1.0 mm i.d., 5 μm); Methanol(4 mM ammonium acetate)-formic acid (99.95:0.005, v/v). | Spain Influent and effluent wastewaters; River water (24 sampling locations; Guadalquivir River basin). | [71] | |
| Venlafaxine Fluoxetine Norfluoxetine Alprenolol Bisoprolol Metoprolol Propranolol Salbutamol | LC-MS/MS; Chirobiotic V column (150 mm × 2.1 mm i.d., 5 µm); Ethanol-10 mM ammonium acetate aqueous solution (92.5:7.5, v/v), pH 6.8. | Portugal Effluent wastewater from 3 WWTPs. | EF = 0.54–0.55 - - - - - - | [57] |
| Salbutamol | LC-MS/MS; Chirobiotic V column (250 mm x 2.1 mm i.d., 5 µm); Methanol(4 mM ammonium acetate)-formic acid (99.95:0.005, v/v). | Italy 24-h raw wastewater composite samples from 2 WWTPs (Nosedo and San Rocco, Milan). | EF one-day peaks = 0.484 ± 0.019 EF regular = 0.452 ± 0.018. | [87] |
| Atenolol Metoprolol Propranolol Sotalol Citalopram Paroxetine Naproxen Temazepan | LC-MS/MS; Chirobiotic V column (250 mm × 4.6 mm i.d., 5 µm) and Chiralpak AD-RH column (150 mm × 4.6 mm i.d., 5 µm) for temazepan; Methanol-20 mM ammonium acetate aqueous solution (90:10, v/v), 0.1% formic acid (pH 4). | Canada Wastewater effluents from 1 rural aerated lagoon and 2 urban tertiary WWTP (Alberta). | EF = 0.40–0.52; EF = 0.39–0.52; - EF = 0.34–0.41; EF = 0.44–0.62; - - EF = 0.39–0.49. | [103] |
| Atenolol Citalopram Fluoxetine Metoprolol Nadolol Pindolol Propranolol Salbutamol Sotalol | LC-MS/MS; Inline filter and a Chirobiotic V (250 mm × 4.6 mm i.d., 5 µm) with a nitrile guard cartridge (10 mm × 3 mm i.d.); Methanol-20 mM ammonium acetate aqueous solution (90:10, v/v), 0.1% formic acid (pH 4). | Canada Raw and treated wastewater from a tertiary WWTP (Alberta). | IWW: 971 ± 30 ng L−1; EWW: 664 ± 22 ng L−1; IWW: 307 ± 18 ng L−1; EWW: 207 ± 11 ng L−1; IWW: 18 ± 2 ng L−1; EWW: 14 ± 0.1 ng L−1; IWW: 411 ± 15 ng L−1; EWW: 375 ± 24 ng L−1; IWW: 51 ± 2 ng L−1; EWW: 20 ± 0.5 ng L−1; IWW: <MQL; EWW: <MQL; IWW: 10 ± 1 ng L−1; EWW: 45 ± 1 ng L−1; IWW: 20 ± 3 ng L−1; EWW: 17 ± 1 ng L−1; IWW: 529 ± 10 ng L−1; EWW: 466 ± 24 ng L−1. | [104] |
| Atenolol Metoprolol Propranolol | LC-MS/MS; In-line filter Chirobiotic V (250 mm × 4.6 mm i.d., 5 µm) with a nitrile guard cartridge (10 mm × 3 mm i.d.); Methanol-0.1% TEAA in water (90:10, v/v), acetic acid (pH 4). | Canada Influents and effluents wastewaters from 1 rural aerated lagoon and 2 urban tertiary WWTP (Alberta). | 160–1100 ng L−1; EF ≈ 0.5 (both influent and effluent). 170–520 ng L−1; EF = 0.5 (influent) EF ≠ 0.50 (effluent). 20–92 ng L−1; EF ≈ 0.5 (both influent and effluent). | [84] |
| Propranolol | GC-MS after diastereomer formation with the chiral derivatizing reagent α-methoxy-α-(trifluoromethyl)phenylacetic acid; MDN-5S column (30-m, 0.25-mm i.d., 0.25-µm film thickness), carrier gas helium. | USA Surface water Wastewater influent Wastewater effluent after secondary treatment (7 WWTPs in California and New York). | <0.1–32 ng L−1; EF = 0.42–0.53. 13–250 ng L−1; EF = 0.50 ± 0.02. 3–160 ng L−1; EF ≤ 0.42. | [61]. |
| Metoprolol | GC-MS after diastereomer formation with the chiral derivatizing reagent (-)-α-methoxy-α-(trifluoromethyl)phenylacetic acid); MDN-5S column (30-m, 0.25-mm i.d., 0.25-µm film thickness), carrier gas helium. | USA River water (Trinity River, Dallas, TX); Effluent wastewater. | 10–571 ng L−1; EF = 0.31–0.44. <1–2269 ng L−1; EF = 0.50 ± 0.03. | [85]. |
| Metoprolol | LC-MS/MS; Reprosil AGP column (100 × 2 mm i.d., 5 μm); H2O-acetonitrile (98:2, v/v), containing 10 mM ammonium acetate. | Germany River water (stretch of river Gründlach, Northern Bavaria). | 42–440 ng L−1; EF = 0.43–0.49. | [75] |
| Metoprolol and two of its metabolites: α-Hydroxymetoprolol (α-OH-metoprolol) Deaminated metoprolol (COOH-metoprolol) | LC-MS/MS; enantiomers of metoprolol and four stereoisomers of α-OH-metoprolol: in-line high-pressure filter (4 mm, 0.5 µm) and a Chiral-CBH column (100 × 2.0 mm i.d., 5 µm) with a Chiral-CBH guard column; 2% (v/v) methanol in hydroxylamine (5.0 mM)-acetic acid (0.65 mM) buffer at pH 7.0. | Sweden Treated wastewater samples from a municipal WWTP, Uppsala). | (S)-metoprolol: 1140–1860 pM; (R)-metoprolol: 939–1770 pM; EF metoprolol = 0.51–0.55; EF α-OH-metoprolol = 0.13–0.48. | [62] |
| LC-MS/MS; enantiomers of COOH-metoprolol: in-line high-pressure filter with a replaceable cap frit (4 mm, 0.5 µm) and a Chiral AGP column (100 mm × 2.0 mm, 5 µm) with a Chiral-AGP guard column (10 × 2.0 mm); Methanol-10 mM ammonium acetate buffer at pH 5.0 (5:95, v/v) | n.d. | |||
| Metoprolol and three of its metabolites: α-Hydroxymetoprolol Metoprolol acid O-desmethylmetoprolol | LC-MS/MS; CHIROBIOT V (250 mm × 4.6 mm i.d., 5 µm); Mobile phase not referred. H2O-30 mM ammonium acetate in methanol at pH 6.0 (10:90, v/v). | France Influent and effluent wastewater. | IWW: 0.49–0.52; EWW: 0.57–0.70. | [90] |
| Atenolol Metoprolol Pindolol Propranolol | LC-UV; Chiralpak AD-H, Lux Cellulose-1, Sumichiral OA-4900 and Chirobiotic T, (250 × 4.6 mm i.d., 5 µm); n-hexane-ethanol-DEA (70:30:0.3, v/v/v) | Spain River water (Cega River, Segovia). | Not determined; Not determined; Not determined; (S)-propranolol: 1.22 (±0.07) ng L−1; (R)-propranolol: 1.35 (±0.07) ng L−1. | [78] |
| Atenolol Metoprolol Pindolol Propranolol | LC-UV; Lux Cellulose-1 (250 × 4.6 mm i.d., 5 µm); Gradient elution mobile phase polarity from n-hexane-Ethanol-DEA (90:10:0.5, v/v/v) to (60:40:0.5, v/v/v) | Spain River water (Cega River, Segovia). | <LOQ; <LOQ; <LOQ; <LOQ. | [79] |
| Ibuprofen, and its main metabolites | GC-MS; Homemade OV1701-DMPen (DMPen ) heptakis(2,6-O-dimethyl-3-O-n-pentyl)-â-cyclodextrin; 1:1 diluted with OV1701) fused silica column (16 m, i.d. 0.25 mm) | Switzerland Lake, rivers and sea water (North Sea) Influents wastewaters Effluent wastewaters after secondary treatment. | n.d.—7.8 ng L−1; ER = 0.7–4.2. 990–3300 ng L−1; ER = 5.8–8.0. 2–81 ng L−1; ER 0.9–2. | [60] |
| Ibuprofen Naproxen | GC-MS; Astec Chiraldex chiral column (20-m, 0.25-mm i.d., 0.12-µm film thickness) coated with dimethyl-b-cyclodextrin as CSP, carrier gas helium. | Spain Influent and effluent wastewaters from a conventional WWTP from León (Castilla y León, Spain). | IWW EF = 0.73–0.90, EWW EF = 0.60–0.76; IWW EF = 0.88–0.90, EWW EF = 0.71–0.86. | [83] |
| Ibuprofen Ketoprofen Naproxen | LC-MS/MS Sumichiral OA-2500 (stationary phase:(R)-1-naphthylglycine and 3.5-dinitrobenzoic acid (250 mm × 46 mm i.d., 5 µm); Tetrahydrofuran-50 mM ammonium acetate in methanol (90:10, v/v). | Spain Influents and effluents wastewaters from 2 WWTPs (Córdoba) | EF IWW: 0.79–0.86; EF EWW: 0.63–0.68; EF IWW: 0.54–0.68; EF EWW: 0.61–0.68; EF IWW: 0.99; EF EWW: 0.93–0.96. | [65] |
| Ibuprofen Naproxen | GC-MS after diastereomer formation with the chiral derivatizing reagent (R)-1-phenylethylamine; HP5-MS fused silica capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness), carrier gas helium. | Australia Influent and effluent wastewater from 3 WWTPs | EF IWW: 0.6–0.8; EF EWW: 0.5. EF IWW: 1.0; EF EWW: 0.7–0.9. | [89] |
| Ibuprofen Ketoprofen Naproxen | GC-MS after diastereomer formation with the chiral derivatizing reagent (R)-1-phenylethylamine; HP5-MS fused silica capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness), carrier gas helium. | Australia Effluent wastewater from a tertiary wastewater treatment plant (Sydney) | 4.6–120 ng L−1; EF = 0.49–0.62; 3.1–207 ng L−1; EF = 0.54–0.66; 1.6–178.9 ng L−1; EF = 0.66–0.86. | [80] |
| Ibuprofen Ketoprofen Naproxen | GC-MS after diastereomer formation with the chiral derivatizing reagent (R)-1-phenylethylamine; HP5-MS fused silica capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness), carrier gas helium. | Australia Effluent wastewater from MBR of a WWTP (Bega Valley) | EF IWW: 0.88–0.94 EF EWW: 0.38–0.40; EF IWW: 0.56–0.60 EF EWW: 0.54–0.68; EF IWW: 0.99 EF EWW: 0.86–0.94. | [67] |
| Naproxen 6-O-desmethyl desmethyl-naproxen | LC-MS/MS; Chiralpak AD-RH (150 mm × 4.6 mm i.d.); Acetonitrile-0.1% formic acid (50:50, v/v). | Japan Influent and effluent wastewaters (Tokyo); River water (Tama River basin, Tokyo). | EF IWW: 1.0; EF EWW: 0.88–0.91; RW: 0.84–0.98. | [91] |
| Lansoprazole Pantoprazole | LC-MS/MS; Amylose tris-(3,5-dimethoxyphenylcarbamate) (150 mm × 4.6 mm i.d.) coated onto APS-Nucleosil (500 Å, 7 µm, 20%, w/w); Acetonitrile-H2O (35:65, v/v). | Brazil Influent and effluent wastewater; River water (Monjolinho River; São Carlos, SP). | Lansoprazole: n.d.; Pantoprazole: 0.15–0.18 µgL−1 in treated effluents; 0.013 µgL−1 in river water. | [55] |
| Omeprazole | LC-MS/MS; LC-UV; Amylose tris-(3,5-dimethylphenylcarbamate) (150 mm × 4.6 mm i.d.) coated onto APS-Nucleosil (500 Å, 7 µm, 20%, w/w); Acetonitrile-H2O (35:65, v/v). | Brazil Influent and effluent wastewater; River water (Monjolinho River; São Carlos, SP). Portugal Estuarine water samples (Douro River). | Both enantiomers were detected in one influent sample (not quantified); Both enantiomers were detected in one estuarine water sample (not quantified). | [54] |
| Omeprazole Lnsoprazole Pantoprazole Rabeprazole | LC-MS/MS; LC-UV; Chiralpak IC (250 mm × 4.6 mm i.d., 5 μm) Cellulose tris (3,5-dichlorophenylcarbamate) immobilized on silica; Acetonitrile-5 mM ammonium acetate in water (40:60, v/v) | China Influent and effluent wastewater from a municipal WWTP (Shenyang); River water (riverbank from the South Canal of Shenyang). | IWW: 0.70; EWW: 0.53; RW: 0.54. IWW: 0.51; EWW: 0.52; RW: 0.52. IWW: 0.54; EWW: 0.51; RW: 0.53. IWW: 0.52; EWW: <MQL; RW: 0.51. | [64] |
| Venlafaxine | LC-MS/MS; Chirobiotic V column (250 mm × 2.1 mm i.d., 5 µm) with a Chirobiotic guard column (10 mm × 2 mm i.d.); Tetrahydrofuran-8.7 mM ammonium acetate aqueous solution at pH 6.0 (10:90, v/v). | France Wastewater effluent River water | EF = 0.46–0.74. | [72] |
| Venlafaxine and its metabolites O-desmethylvenlafaxine, N-desmethylvenlafaxine,O,N-didesmethylvenlafaxine,N,N-didesmethylvenlafaxine and tridesmethylvenlafaxine | LC-MS/MS; CHIROBIOT V (250 mm × 4.6 mm i.d., 5 µm); LC-MS/MS α1-acid glycoprotein column (100 mm × 4.0 mm i.d., 5 µm) | Israel Six wastewater treatment plants (WWTPs) operating under different conditions. | [73] | |
| Fluoxetine and norfluoxetine | LC-MS/MS; In-line high-pressure filter with a replaceable cap frit (4 mm, 0.5 µm) and a Chiral AGP column (100 mm × 2.0 mm, 5 µm) with a Chiral-AGP guard column (10 mm × 2.0 mm); Acetonitrile-10 mM ammonium acetate buffer, pH 4.4 (3:97, v/v). | Sweden Influent and effluent wastewater from a municipal WWTP (Uppsala). | IWW: (S)-fluoxetine: 52 pM; (R)-fluoxetine: 21 pM; EF = 0.71; EWW: (S)-fluoxetine: 48 pM; (R)-fluoxetine: 19 pM; EF = 0.71; IWW: (S)-norfluoxetine: 27 pM; (R)-norfluoxetine: 12 pM; EF = 0.69; EWW: (S)-norfluoxetine: 9 pM; (R)-norfluoxetine: 4 pM; EF = 0.68. | [81,100] |
| Fluoxetine and norfluoxetine | LC-FD; Chirobiotic V column (150 mm × 4.6 mm i.d., 5 µm); Ethanol-10 mM ammonium acetate buffer (87.5:12.5, v/v), pH 6.8. | Portugal Effluent wastewater from a municipal WWTP. | n.d. | [86] |
| Hexaconazole Triadimefon Tebuconazole Penconazole | LC-DAD Chiralpak IC column 250 mm × 4.6 mm i.d., 5 µm). with the CSPs [cellulose tris-(3,5-dichlorophenylcarbamate)] polymer immobilized on silica; n-hexane/2-propanol (90:10, v/v). | Ground water River water | n.d. | [63] |
| Econazole Miconazole Tebuconazole Ketoconazole | LC-MS/MS; α1-acid glycoprotein column (100 mm × 4.0 mm i.d., 5 µm); Mobile phase not referred. | China Wastewater (dissolved and suspended particulate matter) sludge and river water (Pearl River Delta) | EF (dissolved phase) = 0.47–0.53; EF (suspended particulate matter) = 0.45–0.53; EF (sludge) 0.47–0.53; EF (river water) = 0.47–0.61. | [88] |
| Ketoconazole | LC-MS/MS; HSA column (100 mm × 2 mm i.d., 5.0 μm) with a HSA guard column (10 mm × 2 mm i.d.); Acetonitrile-H2O (10:90, v/v) containing 10 mM ammonium acetate (pH 7.0). | China Influent and effluent wastewater and sludge from a sewage treatment plant (Guangzhou, South China). | IWW: <MQL—91.6 ng L−1; EF = 0.48; EWW: <MQL—12.4 ng L−1; EF = 0.48; Sludge: 230.9–231.9 ng g−1 (dw); EF = 0.49–0.50. | [74] |
| Econazole Miconazole Tebuconazole Propiconazole | LC-MS/MS; AGP column (100 mm × 4 mm i.d., 5.0 μm) with an AGP guard column (10 mm × 4 mm i.d.); Gradient of H2O containing 10 mM ammonium acetate (pH 7.0) and acetonitrile. | IWW: 1–1.2 ng L−1; EF not determined; EWW: 0.29–0.51 ng L−1; EF not determined; Sludge: 8.3–120.8 ng g−1 (dw); EF = 0.50–0.51; IWW: 6.0–11.3 ng L−1; EF = 0.50; EWW: 0.25–0.87 ng L−1; EF = 0.47; Sludge: 87.9–1258.0 ng g−1 (dw); EF = 0.49–0.50. n.d.; n.d. |
| Pesticides | Chiral Compound | Analytical Method | Location/Matrix | Concentration/ER, EF | Ref. |
| α-HCH | GC-ECD heptakis (3-O-butyryl-2,6-di-O-pentyl)-β-CD (60 m), carrier gas hydrogen | North Sea regions | α-HCH: 0.54–2.86 ng L−1 ER (+/-, α-HCH)= 0.88–1.19 γ-HCH: 0.31–2.72 ng L−1 | [97] | |
| α-HCH | GC-ECD β-dex 120 chiral column | USA York river estuary | (+) α-HCH: 11.6–79.3 pg L−1 (-) α-HCH: 20.6–103.0 pg L−1 ER (+/-, α-HCH) = 0.71–1.06 | [114] | |
| α-HCH | GC-ECD γ-DEX 120 column (20%-γ-CD, 20 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas hydrogen | Island Water rivers and lakes | α-HCH: 1.2–5.8 ng L−1 γ-HCH: 0.23–0.65 ng L−1 ER (α/γ, HCH) = 3.5–13.8 | [142] | |
| α-HCH | GC-ECD column A: heptakis (3-O-butyryl-2,6-di-O-pentyl)-β-CD (25 m, i.d. 0.25 mm); column B: 50% heptakis (2,3,6-tri-O-n-pentyl)-β-CD and 50% OV1701(25 m, i.d. 0.25 mm), carrier gas helium | North sea and Baltic sea | γ-HCH: 2.0–7.7 ng L−1 α-HCH: 0.2–5.8 ng L−1 ER (γ/α, HCH) = 0.67–10.0 ER (+/-, α-HCH) = 0.81–0.92 | [99] | |
| α-HCH | GC-MS 30% tert-butyldimethylsilylated-β-CD in PS-086 (20 m, i.d. 0.25 mm, 0.25 µm film thickness) | Arctic regions water from Bering and Chukchi Seas | α-HCH: 0.05–5.32 ng L−1 γ-HCH: 0.10–1.33 ng L−1 ER (α/γ, HCH) = 0.35–12.40 | [121] | |
| α-HCH | GC-MS Beta-DEX (20% permethylated-β-CD in polydimethylsiloxane, (30 m, i.d. 0.25 mm, 0.25 µm film thickness) and BGB-172 (20% tert-butyldimethylsilylated-β-CD in OV-1701, (30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | Arctic Ocean Surface water | ER (+/-,α-HCH) = 0.68–1.09 | [143] | |
| α-HCH | GC-MS Betadex-120 (20% permethylated β-CD in methyl phenylpolysiloxane (30 m, i.d. 0.25 mm) | Canada Lake Ontario and Niagara River Rain water | ER (+/-, α-HCH) = 0.86 ER (+/-, α-HCH) = 0.99 | [112] | |
| α-HCH | Not described | Scotland Kintyre Peninsula Air | EF (α-HCH) = 0.480 | [144] | |
| α-HCH | GC-MS 20% tert-butyldimethylsilylated β-cyclodextrin in OV-1701 | China Pearl River Delta | EF(α-HCH) = 0.104–0.910 | [12] | |
| α-HCH | GC-MS BGB (20% tert-butyldimethylsilylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF(α-HCH) = 0.48–0.53 EF(α-HCH) = 0.50 | [113] | |
| PCCH | GC-ECD column A: heptakis (3-O-butyryl-2,6-di-O-pentyl)-β-CD (25 m, i.d. 0.25 mm); column B: 50% heptakis (2,3,6-tri-O-n- pentyl)-β-CD and 50% OV1701(25 m, i.d. 0.25 mm), carrier gas helium | North sea and Baltic sea | ER (γ1/γ2) PCCH = 1.12–1.17 ER (β1/β2) PCCH = 0.97 | [99] | |
| bromocyclin | GC-ECD 50% heptakis(6-O-tert-butyl-dimethylsilyl-2,3-di-O-methyt)-β-CD and 50% OV-I7O1 ~w/w (25 m, i.d. 0.25 mm, 0.125 µm film thickness) carrier gas hydrogen | Germany River Stör WWTPs | n.d.–261 pg L−1; ER (-/+) = 1.01–1.0 760–11,500 pg L−1 | [126] | |
| MCPP | GC-MS FS 71 PS-086 + 20% Me-β-CD, (15 m, 0.25 mm i.d., 0.13 µm film thickness) | Switzerland Rain water | R-MCPP: up to 50 ng L−1 S-MCPP: up to 19 ng L−1 | [115] | |
| MCPP | GC-MS OV1701-TBDM (TBDM, heptakis-(6-O-tert-butyldimethylsilyl-2,3-di-O-methyl)-β-CD) fused silica (20 m, i.d. 0.25 mm) column with 35% of the chiral selector (amount relative to OV1701) | Switzerland Lake and rivers | R-MCPP: <0.2 to 25 ng L−1 S-MCPP: <0.2 to 121 ng L−1 ER (R/S) = 0.21–4.36 | [124] | |
| MCPP | GC-MS Not described | Switzerland WWTPs and Lake Greifensee | ER (R/S )= ~1 to 2 | [125] | |
| DCPP | GC-MS FS 71 PS-086 + 20% Me-β-CD, (15 m, 0.25 mm i.d., 0.13 µm film thickness) | Switzerland Rain water | R-dichlorprop: up to 106 ng L−1 S-dichlorprop: up to 11 ng L−1 | [115] | |
| DCPP | GC-MS OV1701-TBDM (TBDM, heptakis-(6-O-tert-butyldimethylsilyl-2,3-di-O-methyl)-β-CD) fused silica (20 m, i.d. 0.25 mm) column with 35% of the chiral selector (amount relative to OV1701) | Switzerland Lake and rivers | R-DCPP: <0.2 to 2.7 ng L−1 S-DCPP: <0.2 to 2.7 ng L−1 | [124] | |
| TC | GC-MS BGB-172 (20% tert-butyldimethylsilylated-β-CD in OV-1701, (30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | Arctic Ocean Surface water | ER (+/- TC): 0.97–1.03 | [143] | |
| TC | Not described | Scotland Kintyre Peninsula Air | EF (TC) = 0.476 | [144] | |
| TC | GC-MS 20% tert-butyldimethylsilylated β-cyclodextrin in OV-1701 | China Pearl River Delta | EF (TC) = 0.112–0.734 | [12] | |
| TC | GC-MS Betadex (20% permethylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF (TC) = 0.47–0.49 EF (TC) = 0.40–0.50 | [113] | |
| CC | GC-MS BGB-172 (20% tert-butyldimethylsilylated-β-CD in OV-1701, (30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | Arctic Ocean Surface water | ER (+/- CC) = 0.94–1.06 | [143] | |
| CC | Not described | Scotland Kintyre Peninsula Air | EF (CC) = 0.511 | [144] | |
| CC | GC-MS 20% tert-butyldimethylsilylated β-cyclodextrin in OV-1701 | China Pearl River Delta | EF (CC) = 0.043–0.813 | [12] | |
| CC | GC-MS Betadex (20% permethylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF (CC) = 0.50–0.56 EF (CC) = 0.48–0.53 | [113] | |
| OXY | GC-MS BGB (20% tert-butyldimethylsilylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF (OXY) = 0.55–0.60 EF (OXY) = 0.550 | [113] | |
| HEPX | GC-MS BGB (20% tert-butyldimethylsilylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF (HEPX) = 0.69–0.73 EF (HEPX) = 0.50–0.76 | [113] | |
| MC5 | GC-MS Betadex (20% permethylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF (MC5) = 0.25–0.46 EF (MC5) = 0.41–0.47 | [113] | |
| DDT | GC-MS 20% tert-butyldimethylsilylated β-cyclodextrin in OV-1701 | China Pearl River Delta | EF (o,p’-DDT) = 0.102–0.801 | [12] | |
| DDT | GC-MS BGB (20% tert-butyldimethylsilylated β-CD, 30 m, i.d. 0.25 mm, 0.25 µm film thickness), carrier gas helium | USA Alabama Agricultural soil Cemeteries | EF (o,p’-DDT) = 0.41–0.55 EF (o,p’-DDT) = 0.50–0.57 | [113] | |
| PBs | PCB 91 | GC-ECD and GC-MS Chirasil-Dex | USA Lake Hartwell sediment | ER (first/second enantiomer) = 0.56 | [110] |
| PCBs 95, 132, and 149 | GC-ECD Chirasil-Dex (10 m, i.d. 0.25 mm, 0.2 µm film thickness), carrier gas hydrogen | Germany River Elsenz | ER (PCBs 95, 132, and 149) ~ 1 | [138] | |
| PCB 95 | GC-ECD Chirasil-Dex CB (25 m, i.d. 0.25 mm 0.25 µm film thickness), carrier gas hydrogen | USA Sediments Hudson River in New York State | ER = 0.5–0.6 | [137] | |
| PCBs 91, 95, 132, 136, 149, 174, and 176 | GC-MS Chirasil-Dex Cyclosil-B | USA Sediments Hudson and Housatonic Rivers | ER (E1/E2, PCB 91) = 0.56–1.28 ER (E1/E2, PCB 95) = 0.67–1.02 ER (+/-, PCB 132) = n.d.–1.32 ER (+/-, PCB 136) = n.d.–5.33 ER (+/-, PCB 149) = 0.91–2.31 ER (+/-, PCB 174) = n.d.–3.71 ER (+/-, PCB 176) = n.d.–1.02 ER (+/-, PCB 183) = n.d.–1.04 | [10] | |
| PCBs 95, 136, 149 | GC-MS Chirasil-Dex (10% permethylated 2,3,6-tri-O-methyl-β-CD (25 m × 0.25 mm × 0.25 µm film thickness) | U.K West Midlands Air Soil | EF (95) = 0.488–0.499 EF (136) = 0.495–0.503 EF (149) = 0.495–0.500 EF (95) = 0.444–0.496 EF (136) = 0.472–0.522 EF (149) = 0.490–0.544 | [145] | |
| Polycyclic musk | HHCB | GC-MS/MS Cyclosil-B: heptakis (2,3-di-O-methyl-6-O-tert-butyldimethylsilyl-β-CD in DV-1701 (25 m, i.d.0.25 mm, 0.25 µm film thickness), carrier gas helium | Korea Nakdong River WWTPs Influent Effluent | <18.0–342.0 ng L−1; ER (trans-HHCB) = 0.86–1.09 ER (cis-HHCB) = 0.95–1.10 <785.0–3491 ng L−1; ER (trans-HHCB) = 0.91–1.01 ER (cis-HHCB) = 1.03–1.14 <284.0–576.0 ng L−1; ER (trans-HHCB) = 0.74–1.04 ER (cis-HHCB) = 0.69–1.25 | [139] |
| HHCB | GC-MS/MS 14% cyanopropylphenyl/86% dimethyl polysiloxane) doped with proprietary amounts of cyclodextrin material (30 m, i.d. 0.25 mm, 0.25 µm film thickness) OV 1701 capillary column, carrier gas helium | Switzerland WWTPs Influent Effluent Sewage sludge Aerobic Anaerobic | ER (trans-HHCB) = 1.0 ER (cis-HHCB) = 0.97 ER (trans-HHCB) = 0.81 ER (cis-HHCB) = 1.00 ER (trans-HHCB) = 0.93 ER (cis-HHCB) = 0.98 | [140] | |
| HHCB | GC-MS; Chiral heptakis (2,3-di-O-methyl-6-O-t-butyl dimethylsilyl)-a-cyclodextrin column combined with a (non-chiral) HP-5MS column. | Effluent wastewater biologically treated; Advanced treated recycled water. | 1679 ng L−1; EF = 0.25/0.25/0.26; 28.1 ng L−1; EF = 0.24/0.24/0.25; | [70] | |
| AHTN | GC-MS/MS 14% cyanopropylphenyl/86% dimethyl polysiloxane) doped with proprietary amounts of cyclodextrin material (30 m, i.d. 0.25 mm, 0.25 µm film thickness) OV 1701 capillary column, carrier gas helium | Switzerland WWTPs Influent Effluent Sewage sludge Aerobic Anaerobic | ER = 0.94 ER = 0.96 ER = 1.17 ER = 0.99 | [140] | |
| AHTN | GC-MS; Chiral heptakis (2,3-di-O-methyl-6-O-t-butyl dimethylsilyl)-a-cyclodextrin column combined with a (non-chiral) HP-5MS column. | Effluent wastewater biologically treated; Advanced treated recycled water. | 31.2 ng L−1; EF = 0.50; 4.6 ng L−1; EF = 0.50; | [70] | |
| AHDI | GC-MS/MS Cyclosil-B: heptakis (2,3-di-O-methyl-6-O-tert-butyldimethylsilyl-β-CD in DV-1701 (25 m, i.d.0.25 mm, 0.25 µm film thickness), carrier gas helium | Korea Nakdong River WWTPs Influent Effluent | <69.0 ng L−1 <69.0 ng L−1 | [139] | |
| AHDI | GC-MS/MS 14% cyanopropylphenyl/86% dimethyl polysiloxane) doped with proprietary amounts of CD material (30 m, i.d. 0.25 mm, 0.25 µm film thickness) OV 1701 capillary column, carrier gas helium | Switzerland WWTPs Influent Effluent Sewage sludge Aerobic Anaerobic | ER = 0.97 ER = 1.19 ER = 1.16 ER = 0.95 | [140] | |
| ATII | GC-MS/MS Cyclosil-B: heptakis (2,3-di-O-methyl-6-O-tert-butyldimethylsilyl-β-CD in DV-1701 (25 m, i.d.0.25 mm, 0.25 µm film thickness), carrier gas helium | Korea Nakdong River WWTPs Influent Effluent | <107.0 ng L−1 <107.0 ng L−1 | [139] | |
| ATII | GC-MS/MS 14% cyanopropylphenyl/86% dimethyl polysiloxane) doped with proprietary amounts of CD material (30 m, i.d. 0.25 mm, 0.25 µm film) OV 1701 capillary column, carrier gas helium | Switzerland WWTPs Influent Effluent Sewage sludge Aerobic Anaerobic | ER = 0.86 ER = 2.94 ER = 0.92 ER = 0.79 | [140] | |
| ATII | GC-MS; Chiral heptakis (2,3-di-O-methyl-6-O-t-butyl dimethylsilyl)-a-cyclodextrin column combined with a (non-chiral) HP-5MS column. | Effluent wastewater biologically treated; Advanced treated recycled water. | 5.0 ng L−1; EF = 0.55; n.d. | [70] | |
| DPMI | GC-MS/MS Cyclosil-B: heptakis (2,3-di-O-methyl-6-O-tert-butyldimethylsilyl-β-CD in DV-1701 (25 m, i.d.0.25 mm, 0.25 µm film thickness), carrier gas helium | Korea Nakdong River WWTPs Influent Effluent | <79.0 ng L−1 <79.0 ng L−1 | [139] | |
| DPMI | GC-MS; Chiral heptakis (2,3-di-O-methyl-6-O-t-butyl dimethylsilyl)-a-cyclodextrin column combined with a (non-chiral) HP-5MS column. | Effluent wastewater biologically treated; Advanced treated recycled water. | 66.6 ng L−1; EF = 0.48. 2.2 ng L−1; EF = 0.51. | [70] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, C.; Ribeiro, A.R.; Maia, A.S.; Tiritan, M.E. Occurrence of Chiral Bioactive Compounds in the Aquatic Environment: A Review. Symmetry 2017, 9, 215. https://doi.org/10.3390/sym9100215
Ribeiro C, Ribeiro AR, Maia AS, Tiritan ME. Occurrence of Chiral Bioactive Compounds in the Aquatic Environment: A Review. Symmetry. 2017; 9(10):215. https://doi.org/10.3390/sym9100215
Chicago/Turabian StyleRibeiro, Cláudia, Ana Rita Ribeiro, Alexandra S. Maia, and Maria Elizabeth Tiritan. 2017. "Occurrence of Chiral Bioactive Compounds in the Aquatic Environment: A Review" Symmetry 9, no. 10: 215. https://doi.org/10.3390/sym9100215







