Age-Dependent Sexually-Dimorphic Asymmetric Development of the Ferret Cerebellar Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. MRI Measurements
2.3. 3D Volume-Rendered Images
2.4. Volumetric Analysis
2.5. Asymmetry Quotient Analysis
2.6. Statistical Analysis
2.7. Ethics
3. Results
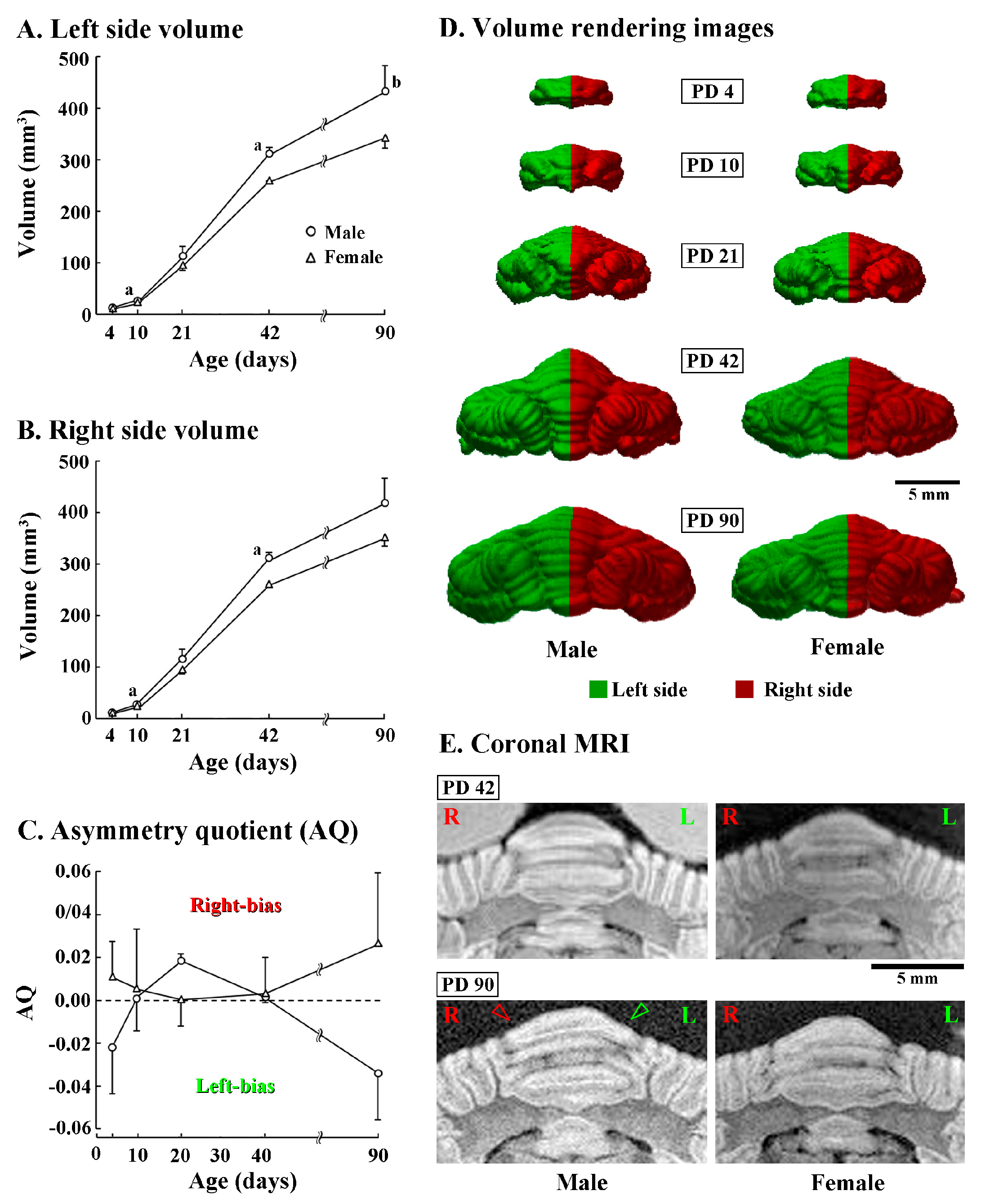
3.1. Developmental Changes in Volume of Entire Cerebellum
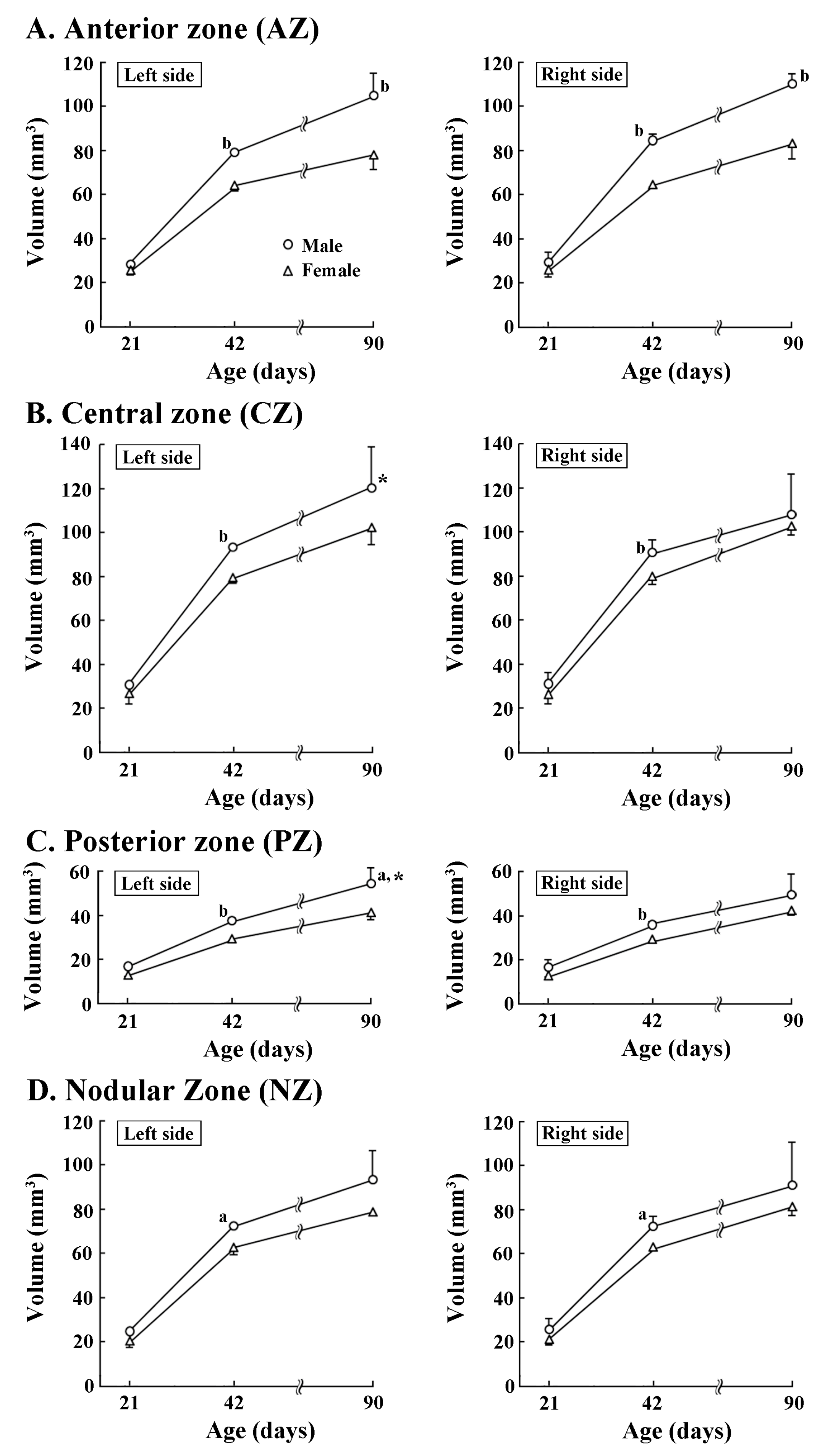
3.2. Asymmetric Development of Cerebellum
3.3. Development of Torque Asymmetry of Cerebellar Cortex
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Synder, P.J.; Bilder, R.M.; Wu, H.; Bogerts, B.; Lieberman, J.A. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia 1995, 33, 407–419. [Google Scholar] [CrossRef]
- Hu, D.; Shen, H.; Zhou, Z. Functional asymmetry in the cerebellum: A brief review. Cerebellum 2008, 7, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Amunts, K. Architecture of the cerebral cortex. In The Human Nervous System, 3rd ed.; Mai, J.K., Paxinos, G., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 836–895. [Google Scholar]
- Ashwell, K.W.S.; Mai, J.K. Fetal development of the central nervous system. In The Human Nervous System, 3rd ed.; Mai, J.K., Paxinos, G., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 31–79. [Google Scholar]
- Alves, N.T.; Fukusima, S.S.; Aznar-Casanova, J.A. Models of brain asymmetry in emotional processing. Psychol. Neurosci. 2008, 1, 63–66. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Buckner, R.L.; Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J. Neurophysiol. 2013, 109, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.A.; Leopold, D.R.; Calhoun, V.D.; Mittal, V.A. Regional cerebellar volume and cognitive function from adolescence to late middle age. Hum. Brain Mapp. 2015, 36, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Sholl, S.A.; Kim, K.L. Androgen receptors are differentially distributed between right and left cerebral hemispheres of the fetal male rhesus monkey. Brain Res. 1990, 516, 122–126. [Google Scholar] [CrossRef]
- Imai, N.; Sawada, K.; Fukunishi, K.; Sakata-Haga, H.; Fukui, Y. Sexual dimorphism of sulcal length asymmetry in cerebrum of adult cynomolgus monkeys (Macaca fascicularis). Congenit. Anom. (Kyoto) 2011, 51, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Collura, R.V.; Ruvolo, M.; Walsh, C.A. Genomic and evolutionary analyses of asymmetrically expressed genes in human fetal left and right cerebral cortex. Cereb. Cortex 2006, 16, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.G.; Dooling, E.C.; Gilles, F.H. Gyral development of the human brain. Ann. Neurol. 1977, 1, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rosch, R.E.; Ronan, L.; Cherkas, L.; Gurd, J.M. Cerebellar asymmetry in a pair of monozygotic handedness-discordant twins. J. Anat. 2010, 217, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Hopkins, W.D. Exploring the relationship between cerebellar asymmetry and handedness in chimpanzees (Pan troglodytes) and capuchins (Cebus apella). Neuropsychologia 2007, 45, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. Male prevalent enhancement of leftward asymmetric development of the cerebellar cortex in ferrets (Mustela putorius). Laterality 2015, 20, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.S.; Narr, K.L.; Luders, E.; Szeszko, P.R.; Thompson, P.M.; Bilder, R.M.; Toga, A.W. Asymmetries of cortical thickness: Effects of handedness, sex, and schizophrenia. Neuroreport 2007, 18, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Escalona, P.R.; McDonald, W.M.; Doraiswamy, P.M.; Boyko, O.B.; Husain, M.M.; Figiel, G.S.; Laskowitz, D.; Ellinwood, E.H., Jr.; Krishnan, K.R. In vivo stereological assessment of human cerebellar volume: Effects of gender and age. AJNR Am. J. Neuroradiol. 1991, 12, 927–929. [Google Scholar] [PubMed]
- Filipek, P.A.; Richelme, C.; Kennedy, D.N.; Caviness, V.S., Jr. The young adult human brain: An MRI-based morphometric analysis. Cereb. Cortex 1994, 4, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Dupuis, J.H.; Briggs, S.D.; McGavran, C.; Acker, J.D. Differential effects of age and sex on the cerebellar hemispheres and the vermis: A prospective MR study. AJNR Am. J. Neuroradiol. 1998, 19, 65–71. [Google Scholar] [PubMed]
- Rhyu, I.J.; Cho, T.H.; Lee, N.J.; Uhm, C.S.; Kim, H.; Suh, Y.S. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci. Lett. 1999, 270, 149–152. [Google Scholar] [CrossRef]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Williamson, A.; Acker, J.D. Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. AJNR Am. J. Neuroradiol. 2001, 22, 1161–1167. [Google Scholar] [PubMed]
- Chung, S.C.; Lee, B.Y.; Tack, G.R.; Lee, S.Y.; Eom, J.S.; Sohn, J.H. Effects of age, gender, and weight on the cerebellar volume of Korean people. Brain Res. 2005, 1042, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Raznahan, A.; Mills, K.L.; Lenroot, R.K. Review: Magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Dorr, A.E.; Lerch, J.P.; Spring, S.; Kabani, N.; Henkelman, R.M. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Koyun, N.; Aydinlioğlu, A.; Aslan, K. A morphometric study on dog cerebellum. Neurol. Res. 2011, 33, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Nguon, K.; Ladd, B.; Baxter, M.G.; Sajdel-Sulkowska, E.M. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog. Brain Res. 2005, 148, 341–351. [Google Scholar] [PubMed]
- Womer, F.Y.; Tang, Y.; Harms, M.P.; Bai, C.; Chang, M.; Jiang, X.; Wei, S.; Wang, F.; Barch, D.M. Sexual dimorphism of the cerebellar vermis in schizophrenia. Schizophr. Res. 2016, 176, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.L.; McCarthy, M.M. Steroids, sex and the cerebellar cortex: Implications for human disease. Cerebellum 2008, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ozol, K.; Hayden, J.M.; Oberdick, J.; Hawkes, R. Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 1999, 412, 95–111. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Hawkes, R. Whole-mount immunohistochemistry: A high-throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 2002, 50, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Watanabe, M. Development of cerebral sulci and gyri in ferrets (Mustela putorius). Congenit. Anom. (Kyoto) 2012, 52, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Horiuchi-Hirose, M.; Saito, S.; Aoki, I. MRI-based morphometric characterizations of sexual dimorphism of the cerebrum of ferrets (Mustela putorius). Neuroimage 2013, 83, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Marino, L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI). Neuropsychologia 2000, 38, 493–499. [Google Scholar] [CrossRef]
- Fan, L.; Tang, Y.; Sun, B.; Gong, G.; Chen, Z.J.; Lin, X.; Yu, T.; Li, Z.; Evans, A.C.; Liu, S. Sexual dimorphism and asymmetry in human cerebellum: An MRI-based morphometric study. Brain Res. 2010, 1353, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Häberling, I.; Corballis, M.C. Cerebellar asymmetry, cortical asymmetry and handedness: Two independent networks. Laterality 2015, 19, 1–18. [Google Scholar]
- Geschwind, N.; Galaburda, A.M. Cerebral lateralization: Biological mechanisms, associations, and pathology. 3. A hypothesis and a program for research. Arch. Neurol. Chic. 1985, 42, 634–654. [Google Scholar] [CrossRef]
- Witelson, S.F.; Nowakowski, R.S. Left out axons make men right: A hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia 1991, 29, 327–333. [Google Scholar] [CrossRef]
- Mueller, S.C.; Landré, L.; Wierckx, K.; T’Sjoen, G. A structural MRI study in Transgender persons on cross sex hormone therapy. Neuroendocrinology 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Erskine, M.S.; Baum, M.J. Plasma concentrations of testosterone and dihydrotestosterone during perinatal development in male and female ferrets. Endocrinology 1982, 111, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Kästner, R.; Kästner, D.; Apfelbach, R. Developmental patterns of thyroid hormones and testosterone in ferrets. Horm. Metab. Res. 1987, 19, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Lentini, E.; Kasahara, M.; Arver, S.; Savic, I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb. Cortex 2013, 23, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, S.G.; Read, G.; Pan, H.; Fear, C.; Lindenbaum, R.; Crossley, J. Prenatal testosterone levels in XXY and XYY males. Horm. Res. 1994, 42, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Aksglaede, L.; Wikstrom, A.M.; Rajpert-DeMeyts, E.; Dunkel, L.; Skakkebaek, N.E.; Juul, A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum. Reprod. Update 2006, 12, 9–48. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Wierckx, K.; Jackson, K.; T’Sjoen, G. Circulating androgens correlate with resting state MRI in transgender men. Psychoneuroendocrinology 2016, 73, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bloss, C.S.; Courchesne, E. MRI neuroanatomy in young girls with autism: A preliminary study. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Loeber, R.T.; Cintron, C.M.; Yurgelun-Todd, D.A. Morphometry of individual cerebellar lobules in schizophrenia. Am. J. Psychiatry 2001, 158, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Szeszko, P.R.; Gunning-Dixon, F.; Ashtari, M.; Snyder, P.J.; Lieberman, J.A.; Bilder, R.M. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol. Psychiatry 2003, 53, 450–459. [Google Scholar] [CrossRef]
- Kibby, M.Y.; Fancher, J.B.; Markanen, R.; Hynd, G.W. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J. Child Neurol. 2008, 23, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Vaituzis, A.C.; Dickstein, D.P.; Sarfatti, S.E.; Vauss, Y.C.; Snell, J.W.; Lange, N.; et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry 1996, 53, 607–616. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, K.; Aoki, I. Age-Dependent Sexually-Dimorphic Asymmetric Development of the Ferret Cerebellar Cortex. Symmetry 2017, 9, 40. https://doi.org/10.3390/sym9030040
Sawada K, Aoki I. Age-Dependent Sexually-Dimorphic Asymmetric Development of the Ferret Cerebellar Cortex. Symmetry. 2017; 9(3):40. https://doi.org/10.3390/sym9030040
Chicago/Turabian StyleSawada, Kazuhiko, and Ichio Aoki. 2017. "Age-Dependent Sexually-Dimorphic Asymmetric Development of the Ferret Cerebellar Cortex" Symmetry 9, no. 3: 40. https://doi.org/10.3390/sym9030040




