Lateralized Functions in the Dog Brain
Abstract
:1. Introduction
2. Sensory Lateralization
3. Paw Preferences
4. Tail-Wagging as a Tool to Study the Asymmetrical Representation of Emotional Processing in the Dog Brain
- (1)
- Dogs move their tails in an asymmetrical way in response to different emotional stimuli [83].
- (2)
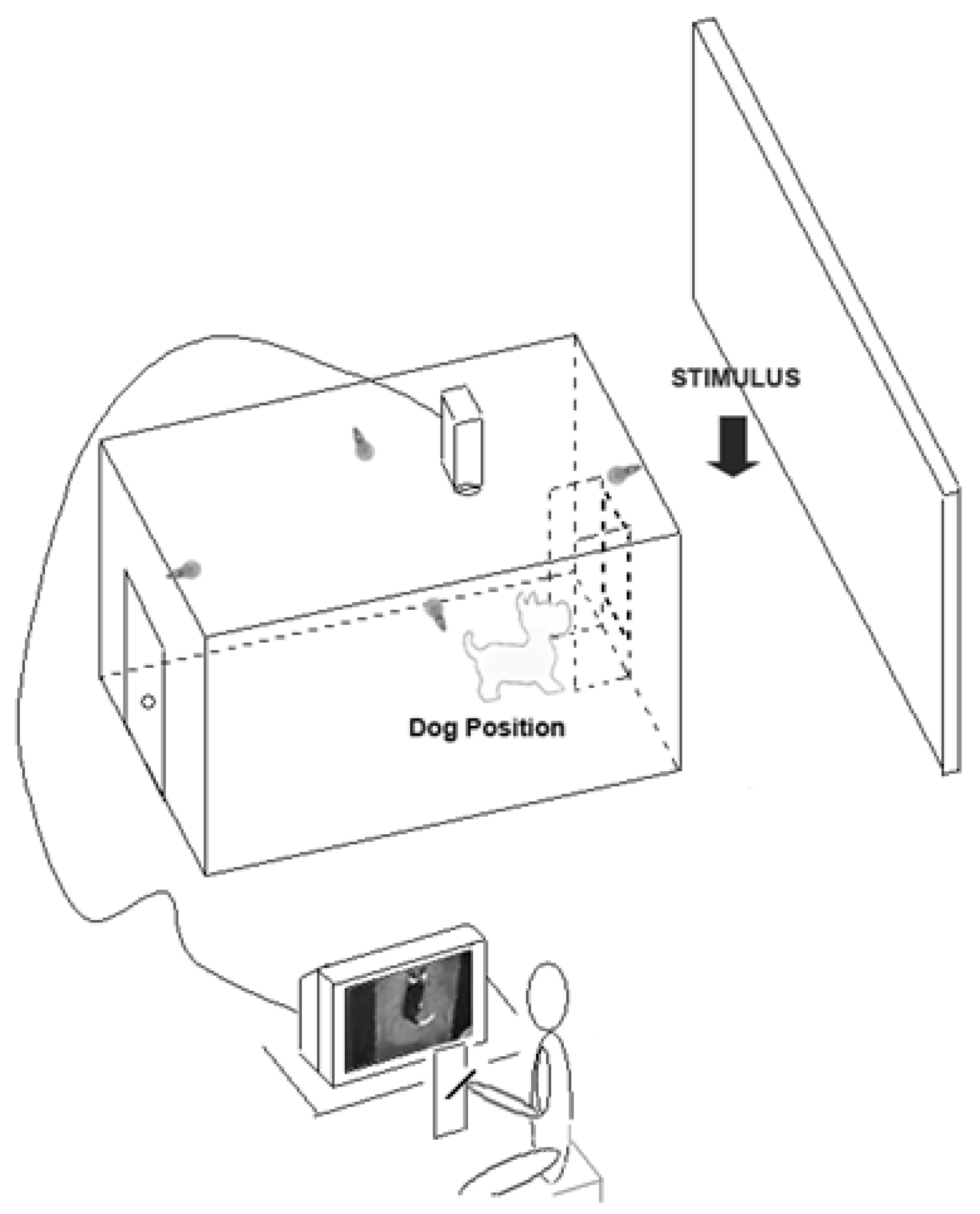
- Studies on behavioural asymmetries associated with lateralized brain functions have usually focused on asymmetric use of paired organs (e.g., forelimbs) but not of a medial organ (i.e., the tail). In order to test asymmetries in tail wagging behaviour, family pet dogs of mixed breeds were placed in a large rectangular wooden box with an opening on the centre of one of its shorter side to allow subjects to view the different stimuli (see Figure 3). Different emotional stimuli were presented as follows: the dog’s owner; an unknown person; an unfamiliar dog with agonistic approach behaviour; and a cat. Tail wagging was analysed frame by frame from video footages recorded through a video camera placed on the ceiling of the box (see Figure 3).
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rogers, L.J.; Andrew, R.J. Comparative Vertebrate Lateralization; Cambridge University Press: New York, NY, USA, 2002; p. 660. ISBN 0521781612. [Google Scholar]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains. The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: New York, NY, USA, 2013; p. 229. ISBN 0521604850. [Google Scholar]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left and right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Vallortigara, G.; Quaranta, A. Dogs turn left to emotional stimuli. Behav. Brain Res. 2010, 208, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Quaranta, A.; Rogers, L.J. Hemispheric specialization in dogs for processing different acoustic stimuli. PLoS ONE 2008, 3, e3349. [Google Scholar] [CrossRef] [PubMed]
- Reinholz-Trojan, A.; Włodarczyk, E.; Trojan, M.; Kulczyński, A.; Stefańska, J. Hemispheric specialization in domestic dogs (Canis familiaris) for processing different types of acoustic stimuli. Behav. Process. 2012, 91, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, V.F.; Reby, D. Orienting asymmetries in dogs’ responses to different communicatory components of human speech. Curr. Biol. 2014, 24, 2908–2912. [Google Scholar] [CrossRef] [PubMed]
- Andics, A.; Gácsi, M.; Faragó, T.; Kis, A.; Miklósi, A. Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr. Biol. 2014, 24, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Andics, A.; Gábor, A.; Gácsi, M.; Faragó, T.; Szabó, D.; Miklósi, A. Neural mechanisms for lexical processing in dogs. Science 2016, 353, 1030–1032. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Sniffing with right nostril: Lateralization of response to odour stimuli by dogs. Anim. Behav. 2011, 82, 399–404. [Google Scholar] [CrossRef]
- Siniscalchi, M.; d’Ingeo, S.; Quaranta, A. The dog nose “KNOWS” fear: Asymmetric nostril use during sniffing at canine and human emotional stimuli. Behav. Brain Res. 2016, 304, 34–41. [Google Scholar] [CrossRef] [PubMed]
- LoBue, V.; DeLoache, J.S. Detecting the snake in the grass: Attention to fear relevant stimuli by adults and young children. Psychol. Sci. 2008, 19, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Fogle, B. The Dog’s Mind. Pelham Editions: London, UK, 1992; p. 203. ISBN 072071964X. [Google Scholar]
- Wittling, W. Brain asymmetry in the control of autonomic-physiologic activity. In Brain Asymmetry; Davidson, R.J., Hugdahl, K, Eds.; MIT Press: Cambridge, UK, 1995; pp. 305–357. ISBN 9780262041447. [Google Scholar]
- Siniscalchi, M.; Franchini, D.; Pepe, A.M.; Sasso, R.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Volumetric assessment of cerebral asymmetries in dogs. Laterality 2011, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Tan, U.; Caliskan, S. Allometry and asymmetry in the dog brain: The right-hemisphere is heavier regardless of paw preferences. Int. J. Neurosci. 1987, 35, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Evolution of hemispheric specialisation: Advantages and disadvantages. Brain Lang. 2000, 73, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Dharmaretnam, M.; Rogers, L.J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain Res. 2005, 162, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, G.; Bisazza, A.; Rogers, L.J.; Vallortigara, G. Lateralization of predator avoidance responses in three species of toads. Laterality 2002, 7, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Austin, N.P.; Rogers, L.J. Asymmetry of flight and escape turning responses in horses. Laterality 2007, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Scheumann, M.; Zimmermann, E. Sex-specific asymmetries in communication sound perception are not related to hand preference in an early primate. BMC Biol. 2008, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Poremba, A.; Malloy, M.; Saunders, R.C.; Carson, R.E.; Herscovitch, P.; Mishkin, M. Species-specific calls evoke asymmetric activity in the monkey’s temporal lobes. Nature 2004, 427, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.; Boivin, S.; Boutin, A.; Blois-Heulin, C.; Hausberger, M.; Lemasson, A. Socially dependent auditory laterality in domestic horses (Equus caballus). Anim. Cogn. 2009, 12, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Laddago, S.; Quaranta, A. Auditory lateralization of conspecific and heterospecific vocalizations in cats. Laterality 2016, 21, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Böye, M.; Güntürkün, O.; Vauclair, J. Right ear advantage for conspecific calls in adults and subadults, but not infants, California sea lions (Zalophus californianus): Hemispheric specialization for communication? Eur. J. Neurosci. 2005, 21, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Lusito, R.; Sasso, R.; Quaranta, A. Are temporal features crucial acoustic cues in dog vocal recognition? Anim. Cogn. 2012, 15, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M. Olfactory lateralization. In Lateralized Brain Functions; Rogers, L.J., Vallortigara, G., Eds.; Humana press: New York, NY, USA, 2017; pp. 103–120. ISBN 9781493967230. [Google Scholar]
- Siniscalchi, M. Olfaction and the Canine Brain. In Canine Olfaction Science and Law; Jezierski, T., Ensminger, J., Papet, L.E., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 31–37. ISBN 1482260239. [Google Scholar]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). WIREs Cogn. Sci. 2011, 2, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G. Comparative neuropsychology of the dual brain: A stroll through left and right animals’ perceptual worlds. Brain Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O.; Kesh, S. Visual lateralization during feeding in pigeons. Behav. Neurosci. 1987, 101, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Ventolini, N.; Ferrero, E.A.; Sponza, S.; Chiesa, A.D.; Zucca, P.; Vallortigara, G. Laterality in the wild: Preferential hemifield use during predatory and sexual behaviour in the black-winged stilt. Anim. Behav. 2005, 69, 1077–1084. [Google Scholar] [CrossRef]
- Robins, A.; Rogers, L.J. Lateralised prey catching responses in the toad (Bufo marinus): Analysis of complex visual stimuli. Anim. Behav. 2004, 68, 567–575. [Google Scholar] [CrossRef]
- Rogers, L.J. Early experiential effects on laterality: Research on chicks has relevance to other species. Laterality 1997, 2, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Pergola, G.; Quaranta, A. Detour behaviour in attack-trained dogs: Left-turners perform better than right-turners. Laterality 2013, 18, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Tan, U. Paw preferences in dogs. Int. J. Neurosci. 1987, 32, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Siniscalchi, M.; Frate, A.; Vallortigara, G. Paw preference in dogs: Relations between lateralised behaviour and immunity. Behav. Brain Res. 2004, 153, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Poyser, F.; Caldwell, C.; Cobba, M. Dog paw preference shows liability and sex differences. Behav. Process. 2006, 73, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Batt, L.S.; Batt, M.S.; McGreevy, P.D. Two tests for motor laterality in dogs. J. Vet. Behav. 2007, 2, 47–51. [Google Scholar] [CrossRef]
- Batt, L.S.; Batt, M.S.; Baguley, J.A.; McGreevy, P.D. Stability of motor lateralisation in maturing dogs. Laterality 2008, 13, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Batt, L.S.; Batt, M.S.; Baguley, J.A.; McGreevy, P.D. The relationships between motor lateralization, salivary cortisol concentrations and behavior in dogs. J. Vet. Behav. 2009, 4, 216–222. [Google Scholar] [CrossRef]
- Wells, D.L. Lateralised behaviour in the domestic dog, Canis familiaris. Behav. Process. 2003, 61, 27–35. [Google Scholar] [CrossRef]
- Aydınlıoğlu, A.; Arslan, K.; Cengiz, N.; Ragbetli, M.; Erdoğan, E. The relationships of dog hippocampus to sex and paw preference. Int. J. Neurosci. 2006, 116, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Aydınlıoğlu, A.; Arslan, K.; Rıza Erdoğan, A.; Cetin Rağbetli, M.; Keleş, P.; Diyarbakırlı, S. The relationship of callosal anatomy to paw preference in dogs. Eur. J. Morphol. 2000, 38, 128–133. [Google Scholar] [CrossRef]
- Branson, N.J.; Rogers, L.J. Relationship between paw preference strength and noise phobia in Canis familiaris. J. Comp. Psychol. 2006, 120, 176–183. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Brueckner, A.; Branson, N.J. Motor laterality in four breeds of dog. J. Vet. Behav. 2010, 5, 318–323. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.; Barnard, S. Comparing lateral bias in dogs and humans using the Kong™ ball test. Appl. Anim. Behav. Sci. 2016, 176, 70–76. [Google Scholar] [CrossRef]
- Tomkins, L.M.; Thomson, P.C.; McGreevy, P.D. First-stepping Test as a measure of motor laterality in dogs (Canis familiaris). J. Vet. Behav. 2010, 5, 247–255. [Google Scholar] [CrossRef]
- Schneider, L.A.; Delfabbro, P.H.; Burns, N.R. Temperament and lateralization in the domestic dog (Canis familiaris). J. Vet. Behav. 2013, 8, 124–134. [Google Scholar] [CrossRef]
- Van Alphen, A.; Bosse, T.; Frank, I.; Jonker, C.M.; Koeman, F. Paw preference correlates to task performance in dogs. In 27th Annual Conference of the Cognitive Science Society; Cognitive Science Society: Stresa, Italy, 2005; pp. 2248–2253. [Google Scholar]
- Hackert, R.; Maes, L.D.; Herbin, M.; Libourel, P.A.; Abourachid, A. Limb preference in the gallop of dogs and the halfbound of pikas on flat ground. Laterality 2008, 13, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Gough, W.; McGuire, B. Urinary posture and motor laterality in dogs (Canis lupus familiaris) at two shelters. Appl. Anim. Behav. Sci. 2015, 168, 61–70. [Google Scholar] [CrossRef]
- McManus, I.C. Right Hand, Left Hand: The Origins of Asymmetry in Brains, Bodies, Atoms, and Cultures; Weidenfeld & Nicolson: London, UK, 2002; ISBN 9780674016132. [Google Scholar]
- Diamond, A.C.; McGrew, W.C. True handedness in the cotton-top tamarin (Saguinus oedipus)? Primates 1994, 35, 69–77. [Google Scholar] [CrossRef]
- Laska, M. Manual laterality in spider monkeys (Ateles geoffroyi) solving visually and tactually guided food-reaching tasks. Cortex 1996, 32, 717–726. [Google Scholar] [CrossRef]
- Güven, M.; Elalmis, D.D.; Binokay, S.; Tan, U. Population-level right-paw preference in rats assessed by a new computerized food-reaching test. Int. J. Neurosci. 2003, 113, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Clapham, P.J.; Leimkuhler, E.; Gray, B.K.; Mattila, D.K. Do humpback whales exhibit lateralized behaviour? Anim. Behav. 1995, 50, 73–82. [Google Scholar] [CrossRef]
- Bisazza, A.; Cantalupo, C.; Robins, A.; Rogers, L.J.; Vallortigara, G. Right-pawedness in toads. Nature 1996, 379, 408. [Google Scholar] [CrossRef]
- Hook, M.A.; Rogers, L.J. Development of hand preferences in marmosets (Callithrix jacchus) and effects of ageing. J. Comp. Psychol. 2000, 114, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Murray, L.W. Sheep laterality. Laterality 2013, 18, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Gianesella, M.; Versace, E.; Contalbrigo, L.; Casella, S.; Cannizzo, C.; Piccione, G.; Stelletta, C. Preliminary study on metabolic profile of pregnant and non pregnant ewes with high or low degree of behavioral lateralization. Anim. Sci. J. 2010, 81, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Pike, A.V.L.; Maitland, D.P. Paw preferences in cats (Felis silvestris catus) living in a household environment. Behav. Process. 1997, 39, 241–247. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Lateralization of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Hook, M.A.; Rogers, L.J. Visuospatial reaching preferences of common marmosets (Callithrix jacchus): An assessment of individual biases across a variety of tasks. J. Comp. Psychol. 2008, 122, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Fagot, J.; Vauclair, J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychol. Bull. 1991, 109, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Millsopp, S. Lateralized behaviour in the domestic cat, Felis silvestris catus. Anim. Behav. 2009, 78, 537–541. [Google Scholar] [CrossRef]
- Versace, E.; Morgante, M.; Pulina, G.; Vallortigara, G. Behavioural lateralization in sheep (Ovis aries). Behav. Brain Res. 2007, 184, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Braccini, S.N.; Caine, N.G. Hand preference predicts reactions to novel foods and predators in marmosets (Callithrix geoffroyi). J. Comp. Psychol. 2009, 123, 18. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. R. Soc. B 2009, 364, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Bennett, A.J. Handedness and approach-avoidance behaviour in chimpanzees (Pan troglodytes). J. Exp. Psychol. 1994, 20, 413–418. [Google Scholar] [CrossRef]
- Cameron, R.; Rogers, L.J. Hand preference of the common marmoset, problem solving and responses in a novel setting. J. Comp. Psychol. 1999, 113, 149–157. [Google Scholar] [CrossRef]
- Gordon, D.J.; Rogers, L.J. Differences in social and vocal behavior between left- and right-handed common marmosets. J. Comp. Psychol. 2010, 124, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.J.; Rogers, L.J. Cognitive bias, hand preference and welfare of common marmosets. Behav. Brain Res. 2015, 287, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Pescini, S.; Barnard, S.; Branson, N.J.; Valsecchi, P. The effect of preferential paw usage on dogs’ (Canis familiaris) performance in a manipulative problem-solving task. Behav. Process. 2013, 100, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, L.M.; Thomson, P.C.; McGreevy, P.D. Associations between motor, sensory and structural lateralisation and guide dog success. Vet. J. 2012, 192, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Bertino, D.; Quaranta, A. Laterality and performance of agility-trained dogs. Laterality 2014, 19, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; d’Ingeo, S.; Fornelli, S.; Quaranta, A. Relationship between visuospatial attention and paw preference in dogs. Sci. Rep. 2016, 6, 31682. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Siniscalchi, M.; Frate, A.; Iacoviello, R.; Buonavoglia, C.; Vallortigara, G. Lateralised behaviour and immune response in dogs: relations between paw preference and interferon-gamma, interleukin-10 and IgG antibodies production. Behav. Brain Res. 2006, 166, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Siniscalchi, M.; Albrizio, M.; Volpe, S.; Buonavoglia, C.; Vallortigara, G. Influence of behavioural lateralization on interleukin-2 and interleukin-6 gene expression in dogs before and after immunization with rabies vaccine. Behav. Brain Res. 2008, 186, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Catecholamine plasma levels following immune stimulation with rabies vaccine in dogs selected for their paw preferences. Neurosci. Lett. 2010, 476, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Siniscalchi, M.; Vallortigara, G. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 2007, 17, R199–R201. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.F.; Goodman, D.C. Motor function and the corticospinal tracts in the dog and raccoon. J. Comp. Neurol. 1967, 129, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Well-being and affective style: Neural substrates and biobehavioural correlates. Phil. Trans. R. Soc. B 2004, 359, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Racca, A.; Guo, K.; Meints, K.; Mills, D.S. Reading faces: differential lateral gaze bias in processing canine and human facial expressions in dogs and 4-year-old children. PLoS ONE 2012, 7, e36076. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Lusito, R.; Vallortigara, G.; Quaranta, A. Seeing left- or right-asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 2013, 23, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Artelle, K.A.; Dumoulin, L.K.; Reimchen, T.E. Behavioral responses of dogs to asymmetrical tail wagging of a robotic dog replica. Laterality 2011, 16, 129–135. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siniscalchi, M.; D’Ingeo, S.; Quaranta, A. Lateralized Functions in the Dog Brain. Symmetry 2017, 9, 71. https://doi.org/10.3390/sym9050071
Siniscalchi M, D’Ingeo S, Quaranta A. Lateralized Functions in the Dog Brain. Symmetry. 2017; 9(5):71. https://doi.org/10.3390/sym9050071
Chicago/Turabian StyleSiniscalchi, Marcello, Serenella D’Ingeo, and Angelo Quaranta. 2017. "Lateralized Functions in the Dog Brain" Symmetry 9, no. 5: 71. https://doi.org/10.3390/sym9050071





