Rice Leaf Lateral Asymmetry in the Relationship between SPAD and Area-Based Nitrogen Concentration
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Lateral Asymmetry in SPAD Readings
3.2. Lateral Asymmetry in SPAD Differences across Measured Points
3.3. Lateral Asymmetry in the Relationship between SPAD and Na
4. Discussion
4.1. Factors Affecting SPAD Measurement
4.2. Difference in SPAD along the Longitudinal Direction
4.3. Relationship between SPAD and Na
4.4. Conclusions and Future Work
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cassman, K.G.; Grassini, P. Can there be a green revolution in Sub-Saharan Africa without large expansion of irrigated crop production? Glob. Food Secur. 2013, 2, 203–209. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Edmeades, G.O. Breeding and cereal yield progress. Crop Sci. 2010, 50, S85–S98. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q.; et al. Improving nitrogen fertilization in rice by site-specific N management: A review. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Zhang, Q.F. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.F.; Deng, J.S.; Shi, Y.Y.; Chen, L.S.; Wang, K. Investigation of SPAD meter-based indices for estimating rice nitrogen status. Comput. Electron. Agric. 2010, 71, S60–S65. [Google Scholar] [CrossRef]
- Peng, S.B.; Buresh, R.J.; Huang, J.L.; Yang, J.C.; Zou, Y.B.; Zhong, X.H.; Wang, G.H.; Zhang, F.S. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop. Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Field, C.H.; Mooney, H.A. Photosynthesis-nitrogen relationship in wild plants. In On the Economy of Plant Form and Function; Givnish, T.J., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 25–55. [Google Scholar]
- Peng, S.; Cassman, K.G.; Kropff, M.J. Relationship between leaf photosynthesis and nitrogen content of field-grown rice in the tropics. Crop Sci. 1995, 35, 1627–1630. [Google Scholar] [CrossRef]
- Peng, S.; Garcia, F.V.; Laza, R.C.; Sanico, A.L.; Visperas, R.M.; Cassman, K.G. Increased N-use efficiency using a chlorophyll meter on high-yielding irrigated rice. Field Crop. Res. 1996, 47, 243–252. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K. Leaf Nitrogen Determination Using Non-Destructive Techniques—A Review. J. Plant Nutr. 2016, 40, 928–953. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yang, J.; Wang, H.; Zou, J.; He, J. Effects of nitrogen application rate and leaf age on the distribution pattern of leaf SPAD readings in the rice canopy. PLoS ONE 2014, 9, e88421. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; García, F.V.; Laza, R.C.; Cassman, K.G. Adjustment for specific leaf weight improves chlorophyll meter’s estimate of rice leaf nitrogen concentration. Agron. J. 1993, 85, 987–990. [Google Scholar] [CrossRef]
- Murchie, E.H.; Yang, J.; Hubbart, S.; Horton, P.; Peng, S. Are there associations between grain-filling rate and photosynthesis in the flag leaves of field-grown rice? J. Exp. Bot. 2002, 53, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Sui, R.; Wilkerson, J.B.; Hart, W.E.; Wilhelm, L.R.; Howard, D.D. Multi-spectral sensor for detection of nitrogen status in cotton. Appl. Eng. Agric. 2005, 21, 167–172. [Google Scholar] [CrossRef]
- Dunn, B.L.; Shrestha, A.; Goad, C.; Khoddamzadeh, A.A. Use of optical sensors to assess Gaillardia Foug. nitrogen status. J. Appl. Hortic. 2015, 17, 181–185. [Google Scholar]
- Peng, S.; Laza, M.R.; Garcia, F.V.; Cassman, K.G. Chlorophyll meter estimates leaf area-based nitrogen concentration of rice. Commun. Soil Sci. Plant Anal. 1995, 26, 927–935. [Google Scholar] [CrossRef]
- Esfahani, M.; Abbasi, H.A.; Rabiei, B.; Kavousi, M. Improvement of nitrogen management in rice paddy fields using chlorophyll meter (SPAD). Paddy Water Environ. 2008, 6, 181–188. [Google Scholar] [CrossRef]
- Turner, F.T.; Jund, M.F. Chlorophyll meter to predict nitrogen topdress requirement for semidwarf rice. Agron. J. 1991, 83, 926–928. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, J.; Yu, T.; Gao, W.; Ling, X.; Li, Y.; Peng, S.; Huang, J. SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics. Sci. Rep. 2015, 5, 13389. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Yang, J.P.; Fei, P.P.; Song, J.; Li, D.S.; Ge, C.S.; Chen, W.Y. Responses of rice leaf thickness, SPAD readings and chlorophyll a/b ratios to different nitrogen supply rates in paddy field. Field Crop. Res. 2009, 114, 426–432. [Google Scholar]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal Leaf Positions for SPAD Meter Measurement in Rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, Y.; Peng, S. Leaf Lateral Asymmetry in Morphological and Physiological Traits of Rice Plant. PLoS ONE 2015, 10, e0129832. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Y.; Jiang, H.; Cao, W. Positional differences in nitrogen and sugar concentrations of upper leaves relate to plant N status in rice under different N rates. Field Crop. Res. 2006, 96, 224–234. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Lv, Y.; He, J. SPAD values and nitrogen nutrition index for the evaluation of rice nitrogen status. Plant Prod. Sci. 2014, 17, 81–92. [Google Scholar] [CrossRef]
- Chapman, S.C.; Barreto, H.J. Using a chlorophyll meter to estimate specific leaf nitrogen of tropical maize during vegetative growth. Agron. J. 1997, 89, 557–562. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Dunn, B.L. Application of canopy sensors for nitrogen assessment management in chrysanthemum. HortScience 2016, 51, 915–920. [Google Scholar]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R. Photosynthesis and respiration in a single leaf. In Science of the Rice Plant: Physiology; Matsuo, T., Kumazawa, K., Ishii, R., Ishihara, K., Hirata, H., Eds.; Food and Agriculture Policy Research Center: Tokyo, Japan, 1995; pp. 491–495. [Google Scholar]
- Zhou, Q.; Wang, J. Comparison of upper leaf and lower leaf of rice plants in response to supplemental nitrogen levels. J. Plant Nutr. 2003, 26, 607–617. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Yao, X.; Liu, X.; Cao, W.; Zhu, Y. Development of critical nitrogen dilution curve of Japonica rice in Yangtze River Reaches. Field Crop. Res. 2013, 149, 149–158. [Google Scholar] [CrossRef]
- Matsunaka, T.; Watanabe, Y.; Miyawaki, T.; Ichikawa, N. Prediction of grain protein content in winter wheat through leaf color measurements using a chlorophyll meter. Soil Sci. Plant Nutr. 1997, 43, 127–134. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Fox, R.H.; Piekielek, W.P.; Macneal, K.E. Comparison of late-season diagnostic tests for predicting nitrogen status of corn. Agron. J. 2001, 93, 590–597. [Google Scholar] [CrossRef]
- Víg, R.; Huzsvai, L.; Dobos, A.; Nagy, J. Systematic measurement methods for the determination of the SPAD values of maize (Zea mays L.) canopy and potato (Solanum tuberosum L.). Commun. Soil Sci. Plant Anal. 2012, 43, 684–1693. [Google Scholar] [CrossRef]
- Yoshida, S. Physiological analysis of rice yield. In Fundamentals of Rice Crop Science; International Rice Research Institute: Makita City, Philippines, 1981; pp. 231–251. [Google Scholar]
- Mae, T. Physiological nitrogen efficiency in rice: Nitrogen utilization, photosynthesis, and yield potential. Plant Soil 1997, 196, 201–210. [Google Scholar] [CrossRef]
- Dunn, B.L.; Goad, C. Effect of foliar nitrogen and optical sensor sampling method and location for determining ornamental cabbage fertility status. HortScience 2015, 50, 74–77. [Google Scholar]
- Singh, B.; Singh, V.; Singh, Y.; Thind, H.S.; Kumar, A.; Gupta, R.K.; Kaul, A.; Vashistha, M. Fixed-time adjustable dose site-specific fertilizer nitrogen management in transplanted irrigated rice (Oryza sativa L.) in South Asia. Field Crop. Res. 2012, 126, 63–69. [Google Scholar] [CrossRef]
- Ramesh, K.; Chandrasekaran, B.; Balasubramanian, T.N.; Bangarusamy, U.; Sivasamy, R.; Sankaran, N. Chlorophyll dynamics in rice (Oryza sativa) before and after flowering based on spad (chlorophyll) meter monitoring and its relation with grain yield. J. Agron. Crop Sci. 2002, 188, 102–105. [Google Scholar] [CrossRef]
- Ali, A.M.; Thind, H.S.; Sharma, S.; Singh, V. Prediction of dry direct-seeded rice yields using chlorophyll meter, leaf color chart and greenseeker optical sensor in northwestern India. Field Crop. Res. 2014, 161, 11–15. [Google Scholar] [CrossRef]
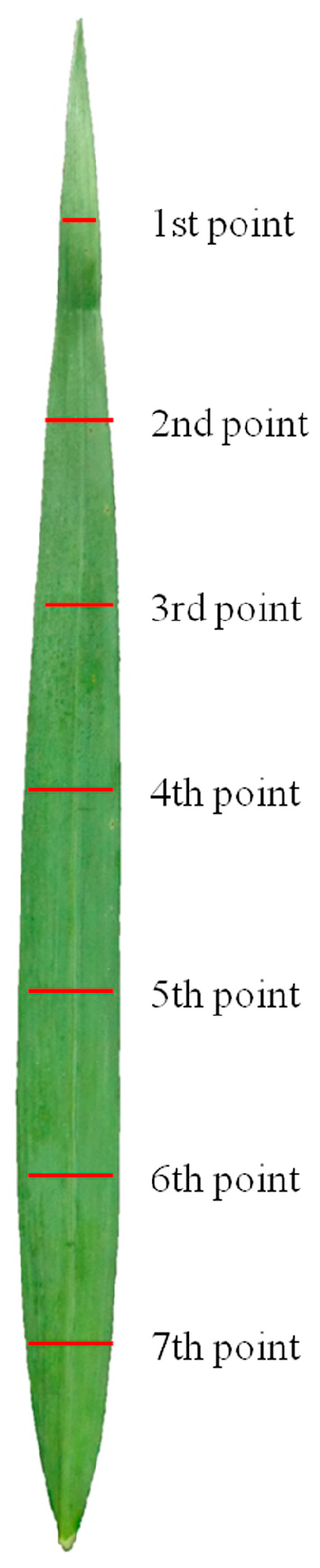
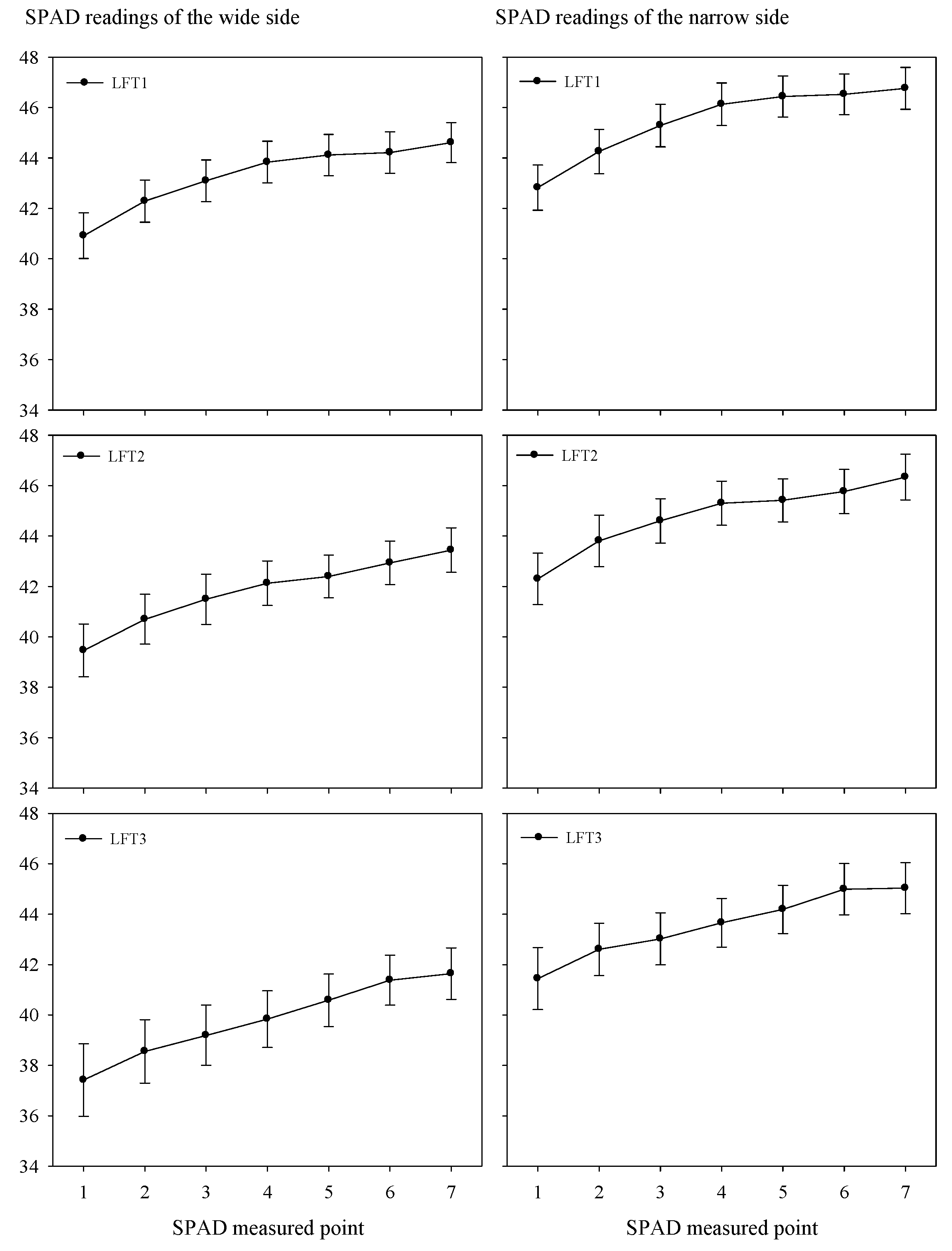
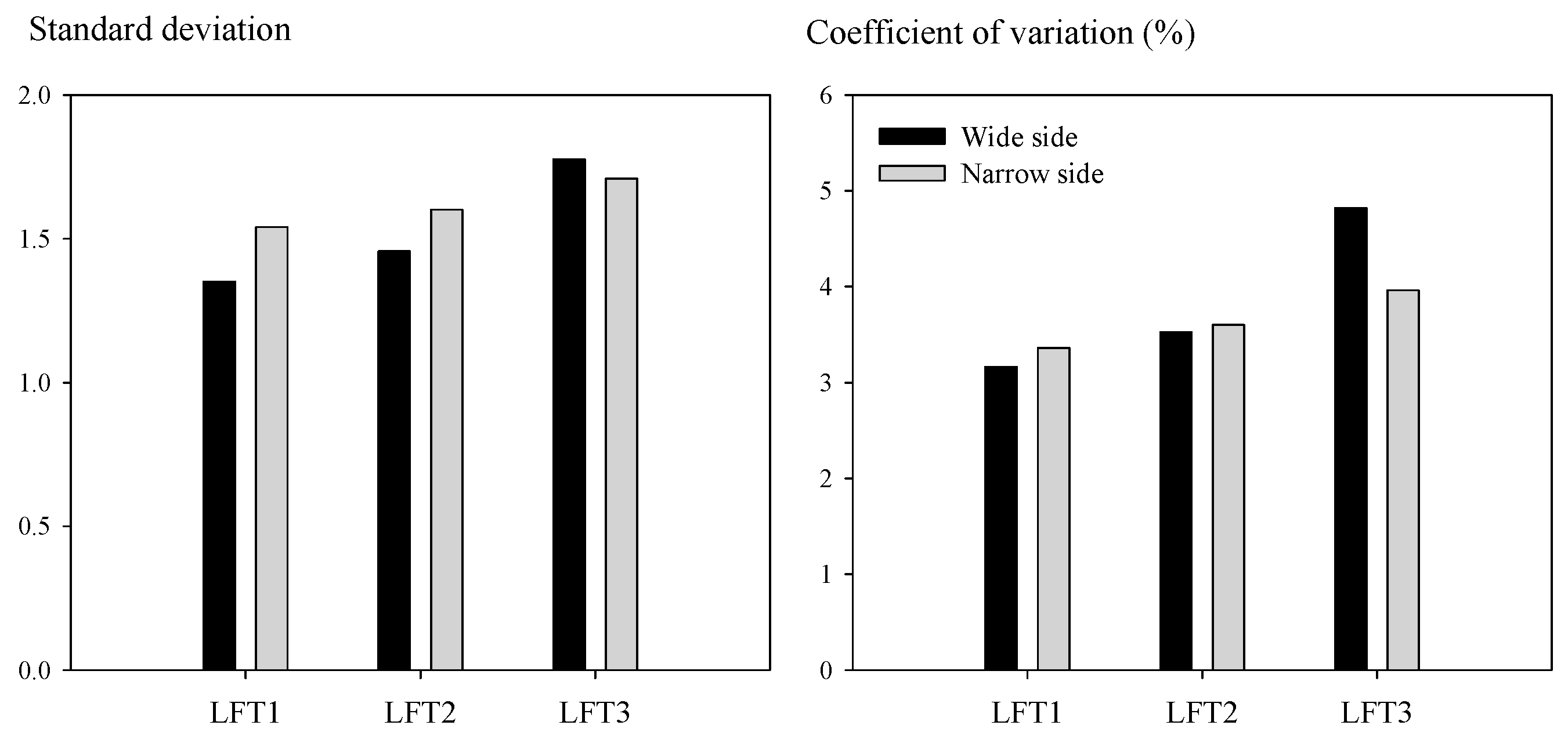
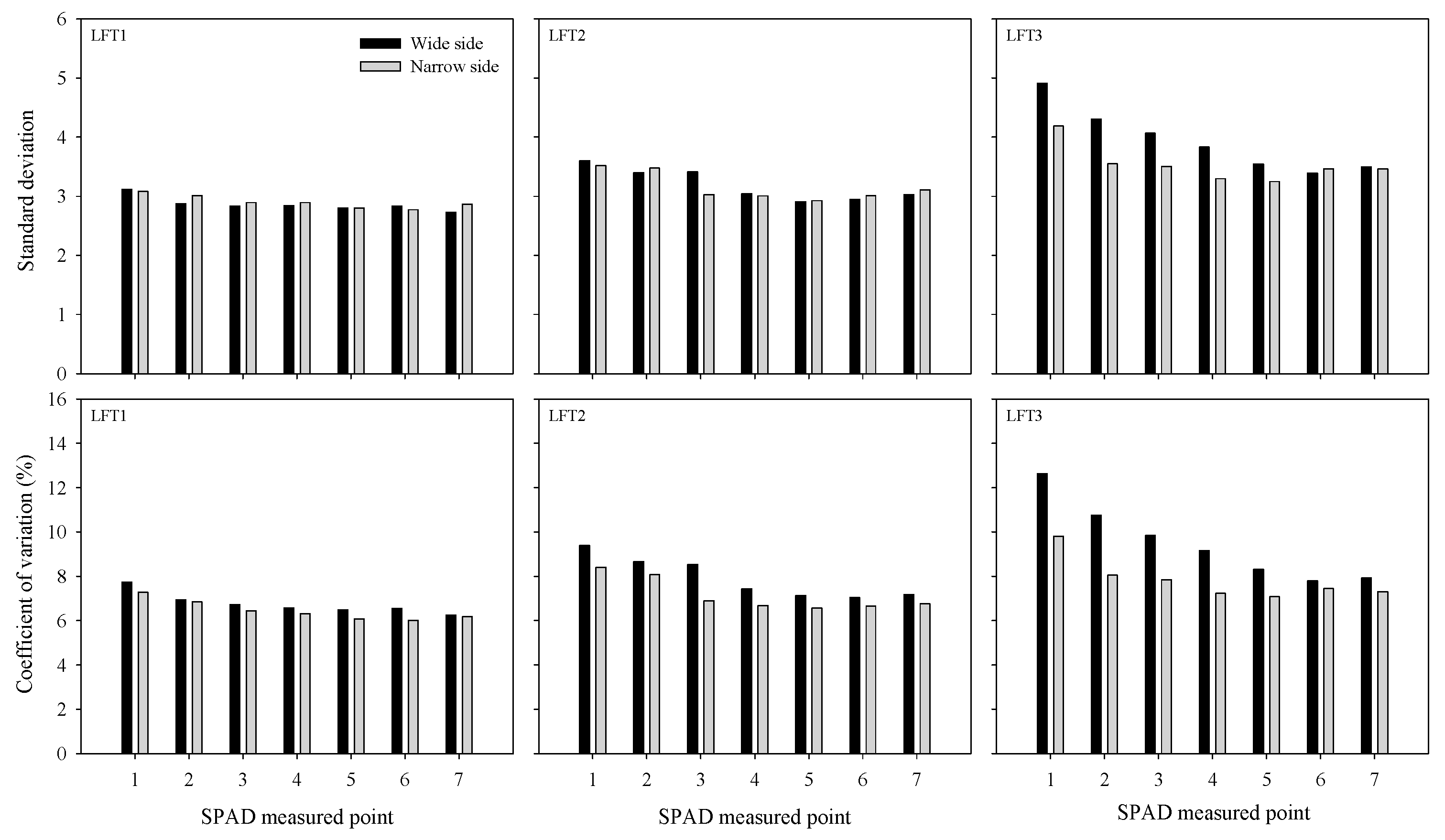
| Location | Season | pH | Organic Matter | Total N | Olsen-P | Available K |
|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | |||
| Wuxue | Early | 5.29 | 22.57 | 2.08 | 18.84 | 111.6 |
| Middle | 5.60 | 27.18 | 1.83 | 4.91 | 105.8 | |
| Late | 5.36 | 21.19 | 1.93 | 13.35 | 63.2 | |
| Wuhan | Middle | 6.40 | 10.12 | 0.81 | 7.56 | 146.4 |
| Number | Cultivar | Location | Season | Type |
|---|---|---|---|---|
| 1 | Liangyou287 | Wuxue | Early/Late | Hybrid |
| 2 | Zhongjiazao17 | Wuxue | Early/Late | Inbred |
| 3 | Ezao17 | Wuxue | Early/Late | Inbred |
| 4 | Ezao18 | Wuxue | Early/Late | Inbred |
| 5 | Huanghuazhan | Wuxue/Wuhan | Middle | Inbred |
| 6 | Zhuliangyou35 | Wuxue/Wuhan | Middle | Hybrid |
| 7 | Zhongzu14 | Wuxue/Wuhan | Middle | Hybrid |
| 8 | 9you6 | Wuxue/Wuhan | Middle | Hybrid |
| 9 | Guangliangyou5 | Wuxue/Wuhan | Middle | Hybrid |
| 10 | Quanyou982 | Wuxue/Wuhan | Middle | Hybrid |
| 11 | Rongfengyou41 | Wuxue/Wuhan | Middle | Hybrid |
| 12 | Wuyouhang1573 | Wuxue/Wuhan | Middle | Hybrid |
| 13 | Huiliangyou630 | Wuxue/Wuhan | Middle | Hybrid |
| 14 | Huiliangyou858 | Wuhan | Middle | Hybrid |
| 15 | Xiangzao45 | Wuxue | Late | Inbred |
| 16 | Wandao143 | Wuxue | Late | Inbred |
| 17 | Xiangzaoxian6 | Wuxue | Late | Inbred |
| Leaf | Point | Regression Equation | R2 | ||||
|---|---|---|---|---|---|---|---|
| Wide | Narrow | Whole | Wide | Narrow | Whole | ||
| 1 | 1 | y = 0.049x − 0.250 | y = 0.050x − 0.259 | y = 0.051x − 0.300 | 0.58 * | 0.44 * | 0.57 * |
| 2 | y = 0.050x − 0.343 | y = 0.050x − 0.347 | y = 0.051x − 0.405 | 0.64 * | 0.53 * | 0.65 * | |
| 3 | y = 0.049x − 0.321 | y = 0.051x − 0.456 | y = 0.050x − 0.409 | 0.67 * | 0.61 * | 0.70 * | |
| 4 | y = 0.051x − 0.454 | y = 0.050x − 0.458 | y = 0.051x − 0.481 | 0.71 * | 0.64 * | 0.74 * | |
| 5 | y = 0.049x − 0.393 | y = 0.047x − 0.361 | y = 0.049x − 0.408 | 0.75 * | 0.66 * | 0.77 * | |
| 6 | y = 0.051x − 0.477 | y = 0.050x − 0.499 | y = 0.052x − 0.567 | 0.74 * | 0.66 * | 0.78 * | |
| 7 | y = 0.053x − 0.579 | y = 0.051x − 0.574 | y = 0.052x − 0.599 | 0.69 * | 0.69 * | 0.74 * | |
| Ave4–6 | y = 0.051x − 0.480 | y = 0.050x − 0.476 | y = 0.051x − 0.512 | 0.75 * | 0.66 * | 0.77 * | |
| 2 | 1 | y = 0.047x − 0.432 | y = 0.039x − 0.067 | y = 0.044x − 0.292 | 0.51 * | 0.41 * | 0.47 * |
| 2 | y = 0.046x − 0.433 | y = 0.046x − 0.461 | y = 0.048x − 0.522 | 0.58 * | 0.47 * | 0.58 * | |
| 3 | y = 0.050x − 0.639 | y = 0.049x − 0.650 | y = 0.051x − 0.694 | 0.68 * | 0.57 * | 0.66 * | |
| 4 | y = 0.057x − 0.956 | y = 0.059x − 1.136 | y = 0.060x − 1.115 | 0.71 * | 0.67 * | 0.73 * | |
| 5 | y = 0.053x − 0.823 | y = 0.051x − 0.782 | y = 0.054x − 0.881 | 0.74 * | 0.69 * | 0.76 * | |
| 6 | y = 0.058x − 1.035 | y = 0.054x − 0.913 | y = 0.057x − 1.061 | 0.72 * | 0.71 * | 0.74 * | |
| 7 | y = 0.054x − 0.919 | y = 0.050x − 0.745 | y = 0.054x − 0.916 | 0.69 * | 0.63 * | 0.70 * | |
| Ave4–6 | y = 0.058x − 1.012 | y = 0.057x − 1.052 | y = 0.059x − 1.100 | 0.75 * | 0.72 * | 0.77 * | |
| 3 | 1 | y = 0.032x + 0.004 | y = 0.031x + 0.063 | y = 0.033x − 0.001 | 0.63 * | 0.53 * | 0.56 * |
| 2 | y = 0.031x + 0.016 | y = 0.033x − 0.062 | y = 0.032x − 0.024 | 0.67 * | 0.52 * | 0.62 * | |
| 3 | y = 0.033x − 0.074 | y = 0.038x − 0.299 | y = 0.036x − 0.196 | 0.72 * | 0.57 * | 0.69 * | |
| 4 | y = 0.036x − 0.223 | y = 0.048x − 0.769 | y = 0.041x − 0.437 | 0.79 * | 0.70 * | 0.76 * | |
| 5 | y = 0.042x − 0.485 | y = 0.051x − 0.918 | y = 0.046x − 0.679 | 0.82 * | 0.69 * | 0.79 * | |
| 6 | y = 0.046x − 0.674 | y = 0.053x − 1.004 | y = 0.049x − 0.852 | 0.79 * | 0.73 * | 0.80 * | |
| 7 | y = 0.048x − 0.781 | y = 0.049x − 0.820 | y = 0.049x − 0.823 | 0.74 * | 0.65 * | 0.73 * | |
| Ave4–6 | y = 0.042x − 0.470 | y = 0.052x − 0.968 | y = 0.046x − 0.675 | 0.81 * | 0.73 * | 0.80 * | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Goron, T.L.; Huang, L.; Wu, L.; Wang, F. Rice Leaf Lateral Asymmetry in the Relationship between SPAD and Area-Based Nitrogen Concentration. Symmetry 2017, 9, 83. https://doi.org/10.3390/sym9060083
Yuan S, Goron TL, Huang L, Wu L, Wang F. Rice Leaf Lateral Asymmetry in the Relationship between SPAD and Area-Based Nitrogen Concentration. Symmetry. 2017; 9(6):83. https://doi.org/10.3390/sym9060083
Chicago/Turabian StyleYuan, Shen, Travis Luc Goron, Liying Huang, Lilian Wu, and Fei Wang. 2017. "Rice Leaf Lateral Asymmetry in the Relationship between SPAD and Area-Based Nitrogen Concentration" Symmetry 9, no. 6: 83. https://doi.org/10.3390/sym9060083






