Synthesis of (R)-Modafinil via Organocatalyzed and Non-Heme Iron-Catalyzed Sulfoxidation Using H2O2 as an Environmentally Benign Oxidant
Abstract
:1. Introduction
2. Materials and Methods
2.1. (Methylsulfinyl)Benzene (1a):
2.2. 1-(Methylsulfinyl)-4-Nitrobenzene (1b):
2.3. 2-(Benzhydrylsulfinyl)Acetamide (2):
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pulis, A.P.; Procter, D.J. C–H coupling reactions directed by sulfoxides: Teaching an old functional group new tricks. Angew. Chem. Int. Ed. 2016, 55, 9842–9860. [Google Scholar] [CrossRef] [PubMed]
- Legros, J.; Dehli, J.R.; Bolm, C. Applications of catalytic asymmetric sulfide oxidations to the syntheses of biologically active sulfoxides. Adv. Synth. Catal. 2005, 347, 19–31. [Google Scholar] [CrossRef]
- Bauder, C.; Martínez, J.; Salom-Roig, X. Chiral sulfoxides as building blocks for enantiopure 1,3-diol precursors in the synthesis of natural products. Curr. Org. Synth. 2014, 10, 885–902. [Google Scholar] [CrossRef]
- Pitchen, P.; Dunach, E.; Deshmukh, M.N.; Kagan, H.B. An efficient asymmetric oxidation of sulfides to sulfoxides. J. Am. Chem. Soc. 1984, 106, 8188–8193. [Google Scholar] [CrossRef]
- Di Furia, F.; Modena, G.; Seraglia, R. Synthesis of chiral sulfoxides by metal-catalyzed oxidation witht-butyl hydroperoxide. Synthesis 1984, 1984, 325–326. [Google Scholar] [CrossRef]
- Pitchen, P.; Kagan, H.B. An efficient asymmetric oxidation of sulfides to sulfoxides. Tetrahedron Lett. 1984, 25, 1049–1052. [Google Scholar] [CrossRef]
- Cotton, H.; Elebring, T.; Larsson, M.; Li, L.; Sörensen, H.; von Unge, S. Asymmetric synthesis of esomeprazole. Tetrahedron 2000, 11, 3819–3825. [Google Scholar] [CrossRef]
- Caron, S.; Dugger, R.W.; Ruggeri, S.G.; Ragan, J.A.; Ripin, D.H. Large-scale oxidations in the pharmaceutical industry. Chem. Rev. 2006, 106, 2943–2989. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.T.; Shenvi, A.B.; Mauger, R.C.; Ulatowski, T.G.; Aharony, D.; Buckner, C.K. 4-alkylpiperidines related to SR-48968: Potent antagonists of the neurokinin-2 (NK2) receptor. Bioorg. Med. Chem. Lett. 1998, 8, 1935–1940. [Google Scholar] [CrossRef]
- Katoumas, K.; Nikitakis, N.; Perrea, D.; Dontas, I.; Sklavounou, A. In vivo antineoplastic effects of the nsaid sulindac in an oral carcinogenesis model. Cancer Prev. Res. 2015, 8, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Malcolm, R.J.; Markowitz, J.S.; DeVane, C.L. Chiral analysis of d- and l-modafinil in human serum: Application to human pharmacokinetic studies. Ther. Drug Monit. 2003, 25, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Shreeram, S.S.; McDonald, T.; Dennison, S. Psychosis after ultrarapid opiate detoxification. Am. J. Psychiatry 2001, 158, 970. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.T.; Miller, S.C.; Shenvi, A.B.; Ohnmacht, C.J.; Veale, C.A. Substituted piperidinabuty1 nitrogen-containing heterocyclic compounds and analogues thereof as neurokinin antagonist. U.S. Patent US 5,739,149, 4 April 1998. [Google Scholar]
- Simon, P.; Panissaud, C.; Costentin, J. The stimulant effect of modafinil on wakefulness is not associated with an increase in anxiety in mice. Psychopharmacology 1994, 114, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Willie, J.T.; Renthal, W.; Chemelli, R.M.; Miller, M.S.; Scammell, T.E.; Yanagisawa, M.; Sinton, C.M. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience 2005, 130, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Mitler, M.M.; Harsh, J.; Hirshkowitz, M.; Guilleminault, C. Long-term efficacy and safety of modafinil (provigil®) for the treatment of excessive daytime sleepiness associated with narcolepsy. Sleep Med. 2000, 1, 231–243. [Google Scholar] [CrossRef]
- Menza, M.A.; Kaufman, K.R.; Castellanos, A.M. Modafinil augmentation of antidepressant treatment in depression. J. Clin. Psychiatry 2000, 61, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.B.; Russo, J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J. Child Adolesc. Psychopharmacol. 2000, 10, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Zeng, B.Y.; Smith, L.A.; Pearce, R.K.B.; Tel, B.; Chancharme, L.; Moachon, G. Antiparkinsonian and neuroprotective effects of modafinil in the mptp-treated common marmoset. Exp. Brain Res. 2000, 133, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Chatterjie, N.; Stables, J.P.; Wang, H.; Alexander, G.J. Anti-narcoleptic agent modafinil and its sulfone: A novel facile synthesis and potential anti-epileptic activity. Neurochem. Res. 2004, 29, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Approved and investigational uses of modafinil. Drugs 2008, 68, 1803–1839. [Google Scholar] [CrossRef] [PubMed]
- Lafon, L. Acetamide derivatives. U.S. Patent US 4,177,290, 12 April 1979. [Google Scholar]
- Fornaroli, M.; Velardi, F.; Colli, C.; Baima, R. Process for the synthesis of modafinal. U.S. Patent US 20040106829, 2004. [Google Scholar]
- Castaldi, G.; Lucchini, V.; Tarquini, A. Process for the preparation of modafinal. U.S. Patent US 20050154063, 6 June 2006. [Google Scholar]
- De Risi, C.; Ferraro, L.; Pollini, G.P.; Tanganelli, S.; Valente, F.; Veronese, A.C. Efficient synthesis and biological evaluation of two modafinil analogues. Bioorg. Med. Chem. 2008, 16, 9904–9910. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Prisinzano, T.E.; Okunola, O.M.; Kopajtic, T.; Shook, M.; Katz, J.L.; Newman, A.H. Structure-activity relationships at the monoamine transporters for a novel series of modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) analogues. ACS Med. Chem. Lett. 2010, 2, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.C.; Lee, Y.; Son, J.Y.; Lim, E.; Jung, M.; Oh, S. Simple synthesis of modafinil derivatives and their anti-inflammatory activity. Molecules 2012, 17, 10446–10458. [Google Scholar] [CrossRef] [PubMed]
- Lari, A.; Karimi, I.; Adibi, H.; Aliabadi, A.; Firoozpour, L.; Foroumadi, A. Synthesis and psychobiological evaluation of modafinil analogs in mice. DARU J. Pharm. Sci. 2013, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Bogolubsky, A.V.; Moroz, Y.S.; Mykhailiuk, P.K.; Ostapchuk, E.N.; Rudnichenko, A.V.; Dmytriv, Y.V.; Bondar, A.N.; Zaporozhets, O.A.; Pipko, S.E.; Doroschuk, R.A.; et al. One-pot parallel synthesis of alkyl sulfides, sulfoxides, and sulfones. ACS Comb. Sci. 2015, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Sturala, J.; Bohacova, S.; Chudoba, J.; Metelkova, R.; Cibulka, R. Electron-deficient heteroarenium salts: An organocatalytic tool for activation of hydrogen peroxide in oxidations. J. Org. Chem. 2015, 80, 2676–2699. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P., Jr.; Hellriegel, E.T. Clinical pharmacokinetic profile of modafinil. Clin. Pharmacokinet. 2003, 42, 123–137. [Google Scholar] [CrossRef]
- Wong, Y.N.; Wang, L.; Hartman, L.; Simcoe, D.; Chen, Y.; Laughton, W.; Eldon, R.; Markland, C.; Grebow, P. Comparison of the single-dose pharmacokinetics and tolerability of modafinil and dextroamphetamine administered alone or in combination in healthy male volunteers. J. Clin. Pharmacol. 1998, 38, 971–978. [Google Scholar] [CrossRef]
- Courvoisier, L.; Duret, G.; Graf, S.; Prat-Lacondemine, L.; Rebiere, F.; Sabourault, N. Process for enantioselective synthesis of signal enantiomers of modafinil by asymmetric oxidation. U.S. Patent 8,759,583, 24 June 2014. [Google Scholar]
- Prisinzano, T.; Podobinski, J.; Tidgewell, K.; Luo, M.; Swenson, D. Synthesis and determination of the absolute configuration of the enantiomers of modafinil. Tetrahedron 2004, 15, 1053–1058. [Google Scholar] [CrossRef]
- Osorio-Lozada, A.; Prisinzano, T.; Olivo, H.F. Synthesis and determination of the absolute stereochemistry of the enantiomers of adrafinil and modafinil. Tetrahedron 2004, 15, 3811–3815. [Google Scholar] [CrossRef]
- Hauck, W.; Adam, P.; Bobier, C.; Landmesser, N. Use of large-scale chromatography in the preparation of armodafinil. Chirality 2008, 20, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Olivo, H.F.; Osorio-Lozada, A.; Peeples, T.L. Microbial oxidation/amidation of benzhydrylsulfanyl acetic acid. Synthesis of (+)-modafinil. Tetrahedron 2005, 16, 3507–3511. [Google Scholar] [CrossRef]
- Ternois, J.; Guillen, F.; Plaquevent, J.-C.; Coquerel, G. Asymmetric synthesis of modafinil and its derivatives by enantioselective oxidation of thioethers: Comparison of various methods including synthesis in ionic liquids. Tetrahedron 2007, 18, 2959–2964. [Google Scholar] [CrossRef]
- Stingl, K.A.; Weiß, K.M.; Tsogoeva, S.B. Asymmetric vanadium- and iron-catalyzed oxidations: New mild (R)-modafinil synthesis and formation of epoxides using aqueous H2O2 as a terminal oxidant. Tetrahedron 2012, 68, 8493–8501. [Google Scholar] [CrossRef]
- Held, F.E.; Grau, D.; Tsogoeva, S.B. Enantioselective cycloaddition reactions catalyzed by BINOL-derived phosphoric acids and N-triflyl phosphoramides: Recent advances. Molecules 2015, 20, 16103–16126. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived bronsted acid and metal catalysis: History and classification by mode of activation; bronsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef] [PubMed]
- Terada, M. Binaphthol-derived phosphoric acid as a versatile catalyst for enantioselective carbon-carbon bond forming reactions. Chem. Commun. 2008, 35, 4097–4112. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T. Stronger bronsted acids. Chem. Rev. 2007, 107, 5744–5758. [Google Scholar] [CrossRef] [PubMed]
- Terada, M. Chiral phosphoric acids as versatile catalysts for enantioselective carbon–carbon bond forming reactions. Bull. Chem. Soc. Jpn. 2010, 83, 101–119. [Google Scholar] [CrossRef]
- Kampen, D.; Reisinger, C.M.; List, B. Chiral bronsted acids for asymmetric organocatalysis. Top. Curr. Chem. 2010, 291, 395–456. [Google Scholar] [CrossRef] [PubMed]
- Zamfir, A.; Schenker, S.; Freund, M.; Tsogoeva, S.B. Chiral BINOL-derived phosphoric acids: Privileged bronsted acid organocatalysts for C–C bond formation reactions. Org. Biomol. Chem. 2010, 8, 5262–5276. [Google Scholar] [CrossRef] [PubMed]
- Tsogoeva, S.; Zamfir, A. Towards a catalytic asymmetric version of the [3+2] cycloaddition between hydrazones and cyclopentadiene. Synthesis 2011, 2011, 1988–1992. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Zamfir, A.; Hampel, F.; Tsogoeva, S.B. Combining in situ generated chiral silicon Lewis acid and chiral brønsted acid catalysts for [3+2] cycloadditions: Cooperative catalysis as a convenient enantioselective route to pyrazolidines. Adv. Synth. Catal. 2012, 354, 3115–3121. [Google Scholar] [CrossRef]
- Zamfir, A.; Tsogoeva, S.B. Asymmetric hydrocyanation of hydrazones catalyzed by in situ formed o-silylated BINOL-phosphate: A convenient access to versatile alpha-hydrazino acids. Org. Lett. 2010, 12, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Enantioselective mannich-type reaction catalyzed by a chiral bronsted acid. Angew. Chem. Int. Ed. 2004, 43, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Uraguchi, D.; Terada, M. Chiral bronsted acid-catalyzed direct mannich reactions via electrophilic activation. J. Am. Chem. Soc. 2004, 126, 5356–5357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Zhao, H.; Li, M.-Q.; Lan, Y.-B.; Yao, Q.-B.; Tao, J.-C.; Wang, X.-W. Chiral phosphoric acid-catalyzed asymmetric oxidation of aryl alkyl sulfides and aldehyde-derived 1,3-dithianes: Using aqueous hydrogen peroxide as the terminal oxidant. Adv. Synth. Catal. 2012, 354, 1012–1022. [Google Scholar] [CrossRef]
- List, B.; Coric, I.; Liao, S. Process for the asymmetric oxidation of organic compounds with peroxides in the presence of a chiral acid catalyst. U.S. Patent WO 2013104605, 17 July 2013. [Google Scholar]
- Liao, S.; Coric, I.; Wang, Q.; List, B. Activation of H2O2 by chiral confined bronsted acids: A highly enantioselective catalytic sulfoxidation. J. Am. Chem. Soc. 2012, 134, 10765–10768. [Google Scholar] [CrossRef]
- Tsogoeva, S.; Wei, S.; Stingl, K.; Weiß, K. Thieme chemistry journal awardees—Where are they now? Bifunctional organocatalysis with N-formyl-l-proline: A novel approach to epoxide ring opening and sulfide oxidation. Synlett 2010, 2010, 707–711. [Google Scholar] [CrossRef]
- Stingl, K.A.; Tsogoeva, S.B. Recent advances in sulfoxidation reactions: A metal-free approach. Tetrahedron 2010, 21, 1055–1074. [Google Scholar] [CrossRef]
- Baudequin, C.; Zamfir, A.; Tsogoeva, S.B. Highly enantioselective organocatalytic formation of a quaternary carbon center via chiral bronsted acid catalyzed self-coupling of enamides. Chem. Commun. 2008, 38, 4637–4639. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Itoh, J.; Fuchibe, K.; Akiyama, T. Chiral bronsted acid catalyzed enantioselective mannich-type reaction. J. Am. Chem. Soc. 2007, 129, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Goeddel, D.; Shu, L.; Yuan, Y.; Wong, O.A.; Wang, B.; Shi, Y. Effective asymmetric epoxidation of styrenes by chiral dioxirane. J. Org. Chem. 2006, 71, 1715–1717. [Google Scholar] [CrossRef]
- Held, F.E.; Wei, S.; Eder, K.; Tsogoeva, S.B. One-pot route to β-adrenergic blockers via enantioselective organocatalysed epoxidation of terminal alkenes as a key step. RSC Adv. 2014, 4, 32796–32801. [Google Scholar] [CrossRef]
- Aoki, M.; Seebach, D. Preparation of tadooh, a hydroperoxide from taddol, and use in highly enantioface- and enantiomer-differentiating oxidations. Helv. Chim. Acta 2001, 84, 187–207. [Google Scholar] [CrossRef]


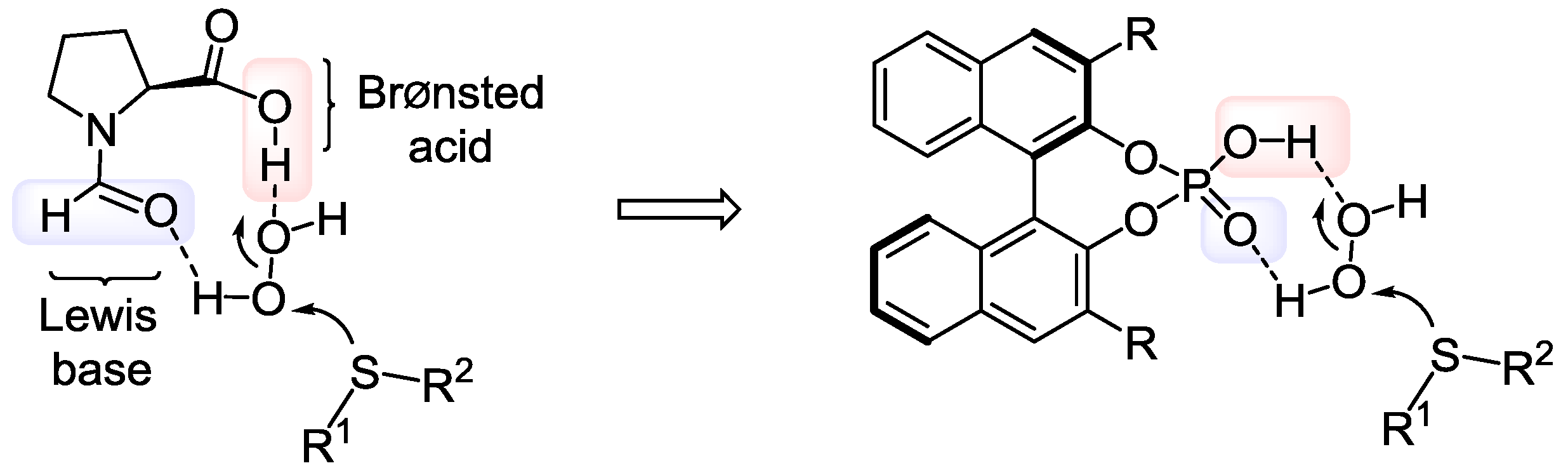

| Entry | Product | BINOL-Phosphate | Yield, a % | ee, % (S) |
|---|---|---|---|---|
| 1 | 1a | II | 44 | 10 b |
| 2 | 1a | II d | 26 | 10 b |
| 3 | 1a | III | 98 | 20 b |
| 4 | 1a | I | 68 | 36 b |
| 5 | 1a | IV | Traces | n.d. |
| 6 | 1a | V | 73 | 30 b |
| 7 | 1a | – | Traces | n.d. |
| 8 | 1b | VI | 11 | 33 c |
| 9 | 1b | VI e | 14 | 59 c |
| 10 | 1b | VII | Traces | 18 c |

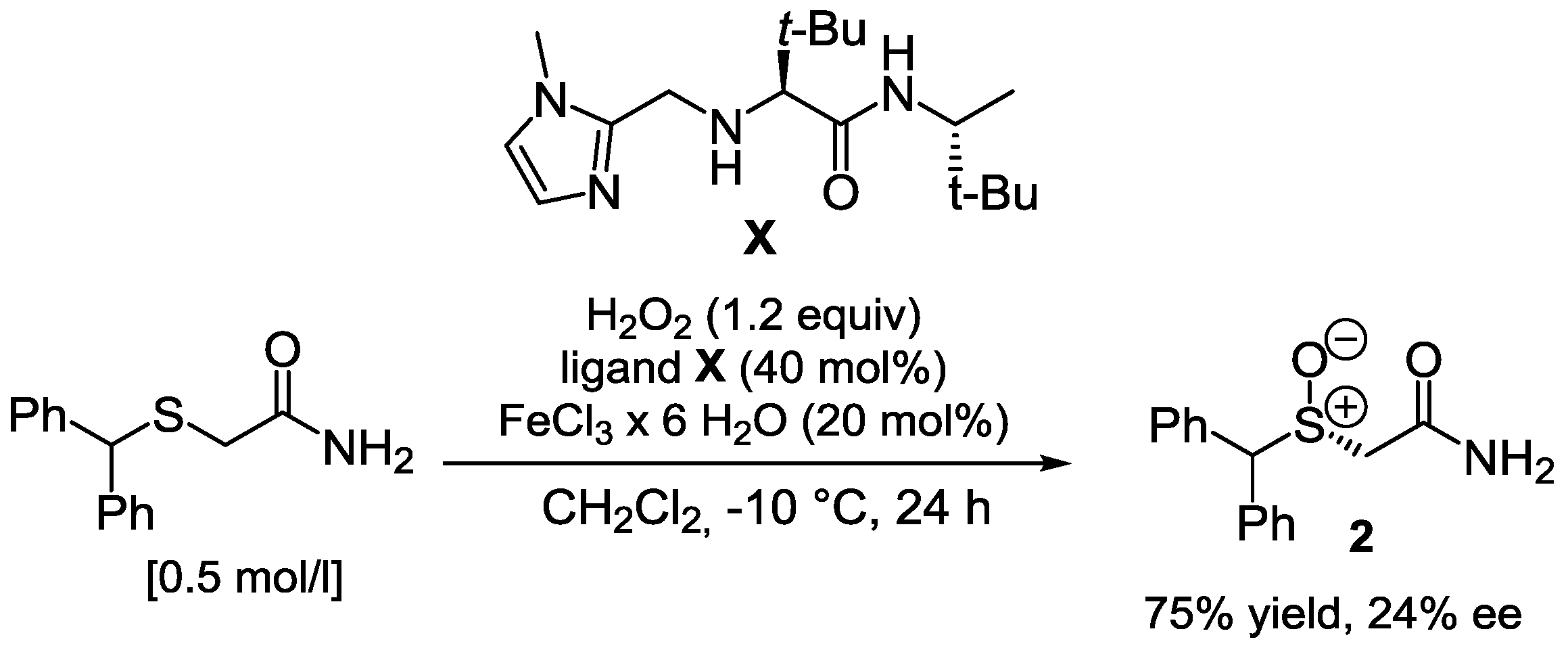
| Entry | Catalyst | Time, h | Yield, a % | ee, b % (R) |
|---|---|---|---|---|
| 1 | V | 24 | >99 | 10 |
| 2 | V c | 24 | >99 | 7 |
| 3 | V | 12 | 61 | rac |
| 4 d | V | 12 | >99 | 13 |
| 5 | VIII | 24 | 38 | 26 |
| 6 | IX e | 96 | 66 | 16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Held, F.E.; Stingl, K.A.; Tsogoeva, S.B. Synthesis of (R)-Modafinil via Organocatalyzed and Non-Heme Iron-Catalyzed Sulfoxidation Using H2O2 as an Environmentally Benign Oxidant. Symmetry 2017, 9, 88. https://doi.org/10.3390/sym9060088
Held FE, Stingl KA, Tsogoeva SB. Synthesis of (R)-Modafinil via Organocatalyzed and Non-Heme Iron-Catalyzed Sulfoxidation Using H2O2 as an Environmentally Benign Oxidant. Symmetry. 2017; 9(6):88. https://doi.org/10.3390/sym9060088
Chicago/Turabian StyleHeld, Felix E., Kerstin A. Stingl, and Svetlana B. Tsogoeva. 2017. "Synthesis of (R)-Modafinil via Organocatalyzed and Non-Heme Iron-Catalyzed Sulfoxidation Using H2O2 as an Environmentally Benign Oxidant" Symmetry 9, no. 6: 88. https://doi.org/10.3390/sym9060088







