Chiral Analysis of Pesticides and Drugs of Environmental Concern: Biodegradation and Enantiomeric Fraction
Abstract
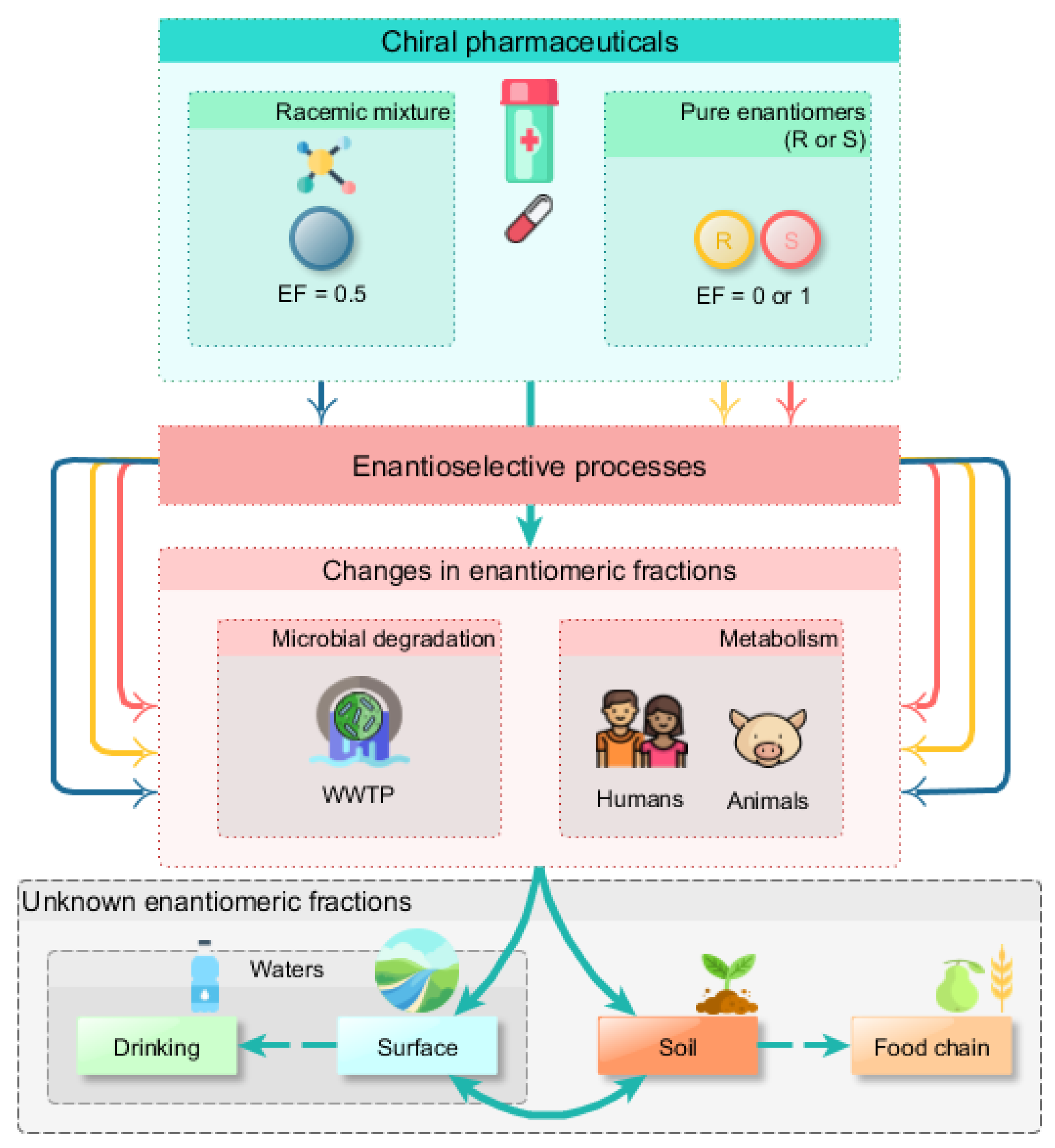
:1. Introduction
2. Chiral Organic Pollutants in the Environment
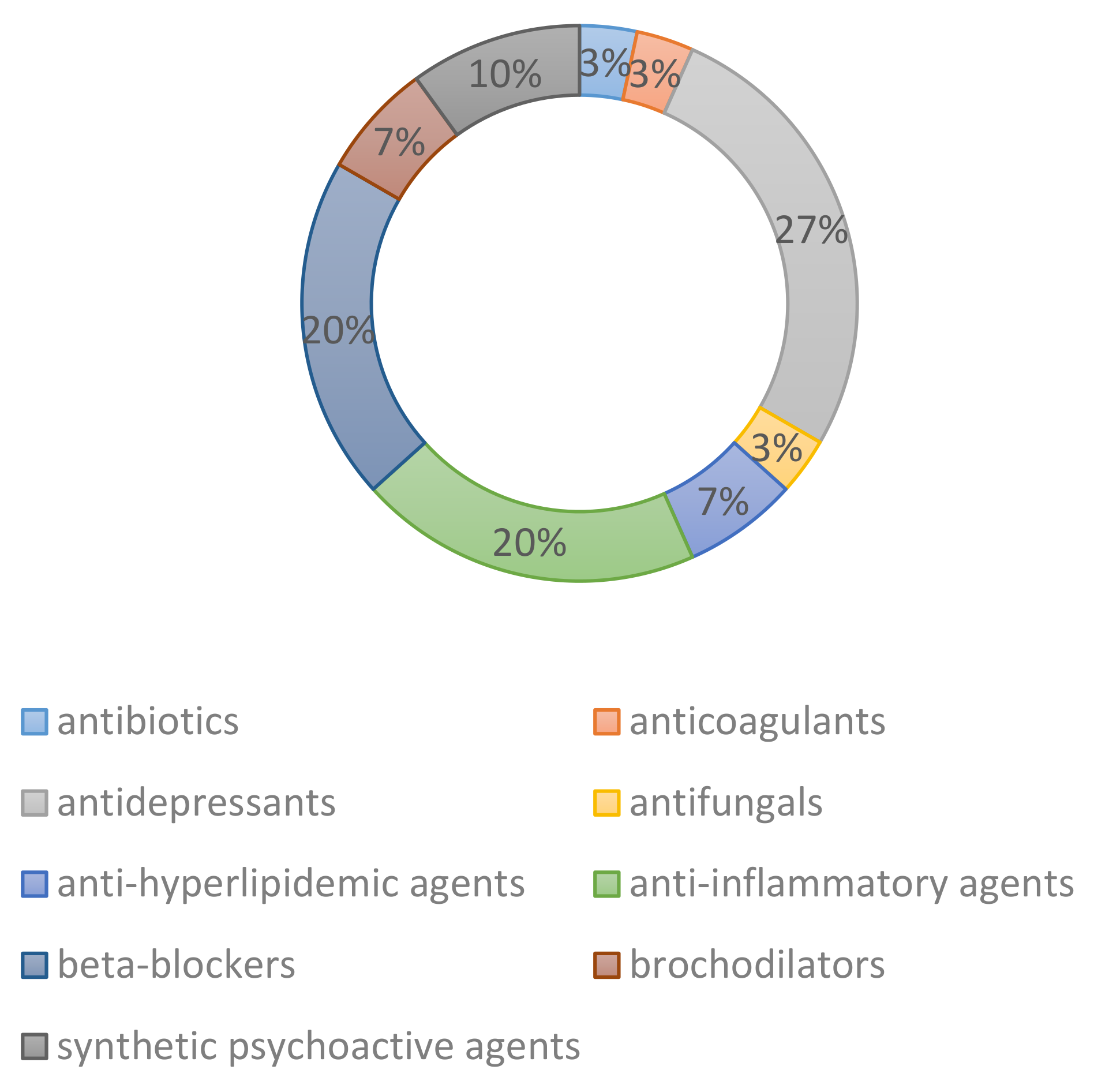
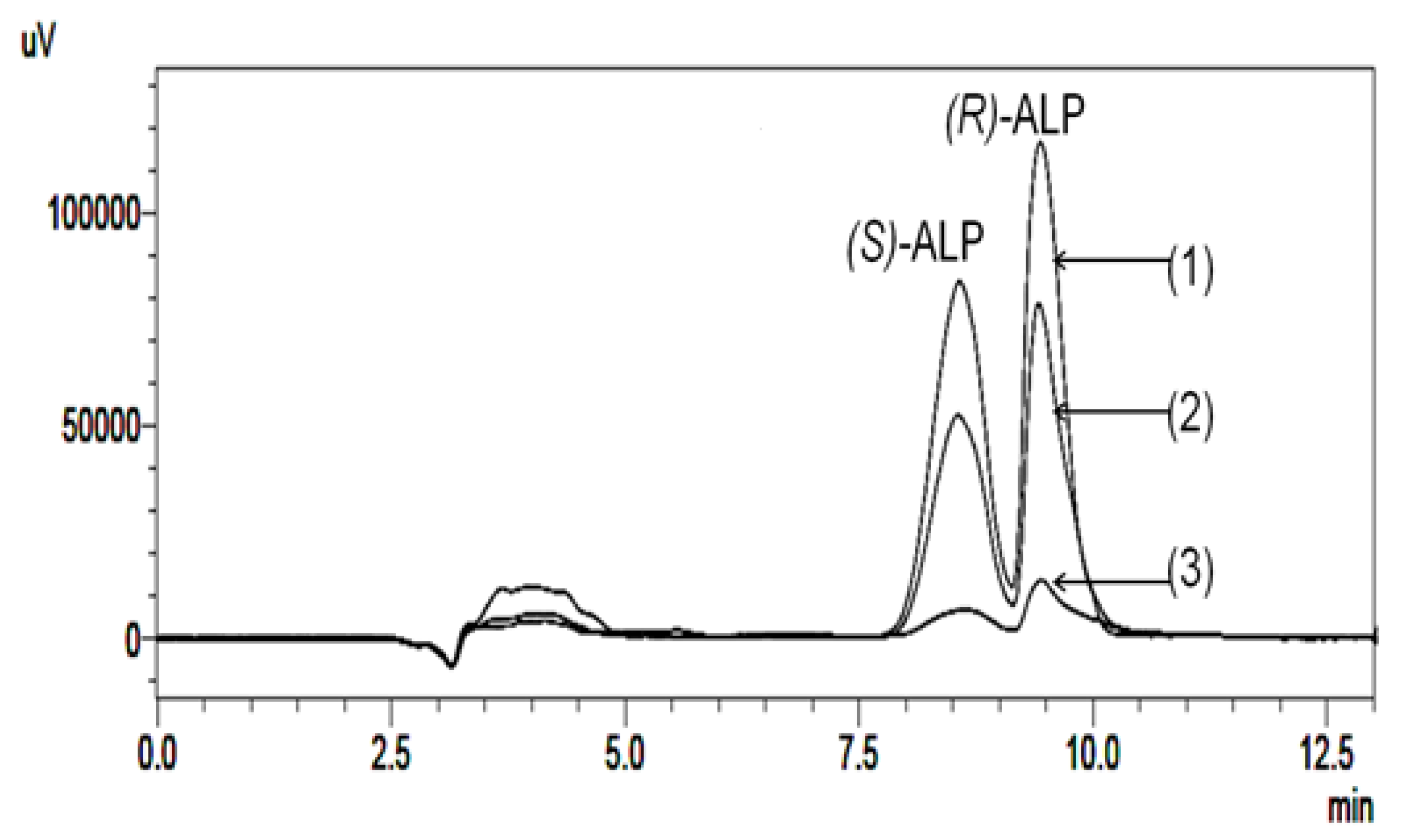
3. Biodegradation Studies of Chiral Drugs
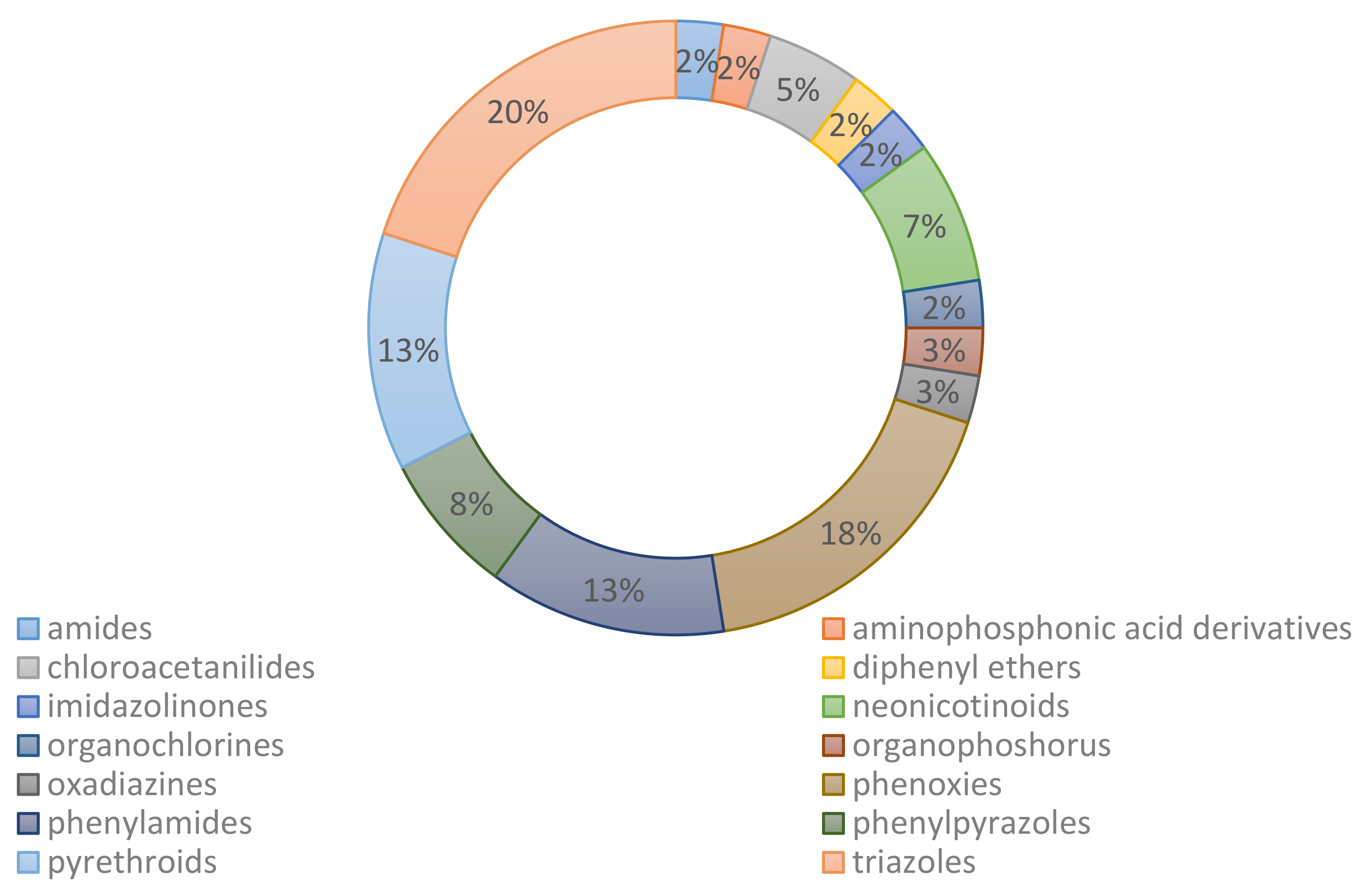
4. Biodegradation Studies of Chiral Pesticides
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, C.S. Environmental fate processes and biochemical transformations of chiral emerging organic pollutants. Anal. Bioanal. Chem. 2006, 386, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Chiral pesticides. J. Pestic. Sci. 2009, 34, 1–12. [Google Scholar] [CrossRef]
- Wainer, I.W.; Drayer, D.E. Drug Stereochemistry: Analytical Methods and Pharmacology (Clinical Pharmacology, no 18), 2nd ed.; Marcel Dekker: New York, NY, USA, 1993; p. 432. [Google Scholar]
- Kurihara, N.; Miyamoto, J.; Paulson, G.D.; Zeeh, B.; Skidmore, M.W.; Hollingworth, R.M.; Kuiper, H.A. Pesticides report 37: Chirality in synthetic agrochemicals: Bioactivity and safety consideration (technical report). Pure Appl. Chem. 1997, 69, 2007–2026. [Google Scholar]
- Ribeiro, A.R.; Maia, A.S.; Cass, Q.B.; Tiritan, M.E. Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: An overview. J. Chromatogr. B 2014, 968, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Korfmacher, W.A.; Hsieh, Y. Chiral liquid chromatography-tandem mass spectrometric methods for stereoisomeric pharmaceutical determinations. J. Chromatogr. B 2005, 820, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, M.; Niu, L.; Liu, W. Enantioselective environmental toxicology of chiral pesticides. Chem. Res. Toxicol. 2015, 28, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Buser, H.-R.; Poiger, T.; Muller, M.D. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Schurig, V. Terms for the quantitation of a mixture of stereoisomers. In Differentiation of Enantiomers I; Schurig, V., Ed.; Springer International Publishing: Cham, Switzerland, 2013; pp. 21–40. [Google Scholar]
- Lao, W.; Gan, J. Enantioselective degradation of warfarin in soils. Chirality 2012, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.; Jan, S.; Andreas, S.; Timm, K.; Burkhard, S. First evidence for a stereoselective incorporation of nonylphenol diastereomers in soil-derived organo-clay complexes. Environ. Chem. Lett. 2011, 9, 293–299. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Zipper, C.; Suter, M.J.F.; Haderlein, S.B.; Gruhl, M.; Kohler, H.P. Changes in the enantiomeric ratio of (r)-to (s)-mecoprop indicate in situ biodegradation of this chiral herbicide in a polluted aquifer. Environ. Sci. Technol. 1998, 32, 2070–2076. [Google Scholar] [CrossRef]
- Liu, W.; Gan, J.; Schlenk, D.; Jury, W.A. Enantioselectivity in environmental safety of current chiral insecticides. Proc. Natl. Acad. Sci. USA 2005, 102, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, J.; Jin, M. Enantioselective phytoeffects of chiral pesticides. J. Agric. Food Chem. 2009, 57, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.W.; Schmitt, P.; Martens, D.; Kettrup, A. Enantiomeric selectivity in the environmental degradation of dichlorprop as determined by high-performance capillary electrophoresis. Environ. Sci. Technol. 1996, 30, 2449–2455. [Google Scholar] [CrossRef]
- Nikolai, L.N.; McClure, E.L.; Macleod, S.L.; Wong, C.S. Stereoisomer quantification of the beta-blocker drugs atenolol, metoprolol, and propranolol in wastewaters by chiral high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1131, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Sanganyado, E.; Fu, Q.; Gan, J. Enantiomeric selectivity in adsorption of chiral β-blockers on sludge. Environ. Pollut. 2016, 214, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Oravec, M.; Šimek, Z.; Holoubek, I. The effect of humic acid and ash on enantiomeric fraction change of chiral pollutants. Colloids Surf. Physicochem. Eng. Aspects 2010, 359, 60–65. [Google Scholar] [CrossRef]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Li, J.; Kong, Z.; Chen, X.; Zheng, Y. Environmental behavior of the chiral triazole fungicide fenbuconazole and its chiral metabolites: Enantioselective transformation and degradation in soils. Environ. Sci. Technol. 2012, 46, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Ariens, E.J.; Van Rensen, J.J.S.; Welling, W. Stereoselectivity of Pesticides: Biological and Chemical Problems; Elsevier: Amsterdam, The Netherlands, 1988; Volume 1. [Google Scholar]
- Wink, O.; Luley, U. Enantioselective transformation of the herbicides diclofop-methyl and fenoxaprop-ethyl in soil. Pest Manage. Sci. 1988, 22, 31–40. [Google Scholar] [CrossRef]
- Hashim, N.H.; Shafie, S.; Khan, S.J. Enantiomeric fraction as an indicator of pharmaceutical biotransformation during wastewater treatment and in the environment--a review. Environ. Technol. 2010, 31, 1349–1370. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, M.; Liu, J.; Liu, W. Enantioselectivity in environmental risk assessment of modern chiral pesticides. Environ. Pollut. 2010, 158, 2371–2383. [Google Scholar] [CrossRef] [PubMed]
- Hühnerfuss, H.; Shah, M.R. Enantioselective chromatography—A powerful tool for the discrimination of biotic and abiotic transformation processes of chiral environmental pollutants. J. Chromatogr. A 2009, 1216, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsdóttir, R.; Jenssen, P.D.; Erland Jensen, P.; Villumsen, A.; Kallenborn, R. A review of wastewater handling in the arctic with special reference to pharmaceuticals and personal care products (ppcps) and microbial pollution. Ecol. Eng. 2013, 50, 76–85. [Google Scholar] [CrossRef]
- Comission, E. Proposal for a directive of the european parliament and of the council amending directives 2000/60/ec and 2008/105/ec as regards priority substances in the field of water policy. Off. J. Eur. Union 2012, 78, 40. [Google Scholar]
- Decision_495. Commission implementing decision (eu) 2015/495 of 20 march 2015 establishing a watch list of substances for union-wide monitoring in the field of water policy pursuant to directive 2008/105/ec of the european parliament and of the council. Off. J. Eur. Union 2015, L78, 40–42.
- Daughton, C.G. Pharmaceutical ingredients in drinking water: Overview of occurrence and significance of human exposure. In Contaminants of Emerging Concern in the Environment: Ecological and Human Health Considerations; American Chemical Society: Washington DC, USA, 2010; Volume 1048, pp. 9–68. [Google Scholar]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. 6), 907–938. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.A.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use--present knowledge and future challenges. J. Environ. Manage. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.; Fatoba, O.; Petrik, L.F. A review of pharmaceuticals and endocrine-disrupting compounds: Sources, effects, removal, and detections. Water, Air, Soil Pollut. 2013, 224, 1–29. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Zambello, E. How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci. Total Environ. 2014, 470–471, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Brar, S.K.; Tyagi, R.D.; Picard, P.; Surampalli, R.Y. Analysis and advanced oxidation treatment of a persistent pharmaceutical compound in wastewater and wastewater sludge-carbamazepine. Sci. Total Environ. 2014, 470–471, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Agranat, I.; Wainschtein, S.R.; Zusman, E.Z. The predicated demise of racemic new molecular entities is an exaggeration. Nat. Rev. Drug Discov. 2012, 11, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral pharmaceuticals in the environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- López-Serna, R.; Kasprzyk-Hordern, B.; Petrović, M.; Barceló, D. Multi-residue enantiomeric analysis of pharmaceuticals and their active metabolites in the guadalquivir river basin (south spain) by chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 5859–5873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.; Santos, L.; Maia, A.S.; Delerue-Matos, C.; Castro, P.M.L.; Tiritan, M.E. Enantiomeric fraction evaluation of pharmaceuticals in environmental matrices by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1363, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiritan, M.E.; Ribeiro, A.R.; Fernandes, C.; Pinto, M.M.M. Chiral pharmaceuticals. Kirk-Othmer Encycl. Chem. Technol. 2016. [Google Scholar] [CrossRef]
- Stanley, J.K.; Brooks, B.W. Perspectives on ecological risk assessment of chiral compounds. Integr. Environ. Assess. Manage. 2009, 5, 364–373. [Google Scholar] [CrossRef]
- De Andrés, F.; Castañeda, G.; Ríos, Á. Use of toxicity assays for enantiomeric discrimination of pharmaceutical substances. Chirality 2009, 21, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, J.P.; Evans, S.E.; Wort, M.T.; Lubben, A.T.; Kasprzyk-Hordern, B. Using chiral liquid chromatography quadrupole time-of-flight mass spectrometry for the analysis of pharmaceuticals and illicit drugs in surface and wastewater at the enantiomeric level. J. Chromatogr. A 2012, 1249, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashim, N.H.; Khan, S.J. Enantioselective analysis of ibuprofen, ketoprofen and naproxen in wastewater and environmental water samples. J. Chromatogr. A 2011, 1218, 4746–4754. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B. Pharmacologically active compounds in the environment and their chirality. Chem. Soc. Rev. 2010, 39, 4466–4503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzyk-Hordern, B.; Baker, D.R. Enantiomeric profiling of chiral drugs in wastewater and receiving waters. Environ. Sci. Technol. 2012, 46, 1681–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzyk-Hordern, B.; Baker, D.R. Estimation of community-wide drugs use via stereoselective profiling of sewage. Sci. Total Environ. 2012, 423, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballo, C.; Sicilia, M.D.; Rubio, S. Enantioselective determination of representative profens in wastewater by a single-step sample treatment and chiral liquid chromatography–tandem mass spectrometry. Talanta 2015, 134, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M. Environmental Fate of Chiral Pharmaceuticals: Determination, Degradation and Toxicity; Springer: Cham, Switzerland, 2012; pp. 3–45. [Google Scholar]
- Barclay, V.K.H.; Tyrefors, N.L.; Johansson, I.M.; Pettersson, C.E. Trace analysis of fluoxetine and its metabolite norfluoxetine. Part ii: Enantioselective quantification and studies of matrix effects in raw and treated wastewater by solid phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1227, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Barclay, V.K.H.; Tyrefors, N.L.; Johansson, I.M.; Pettersson, C.E. Chiral analysis of metoprolol and two of its metabolites, α-hydroxymetoprolol and deaminated metoprolol, in wastewater using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1269, 208–217. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, M.E.; Scott, G.I.; Ross, P.E. Toxicity of pesticides to aquatic microorganisms: A review. Environ. Toxicol. Chem. 2001, 20, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pesticides and Their Application: For the Control of Vectors and Pests of Public Health Importance, 6th ed.; WHO: Geneva, Switzerland, 2006; pp. 1–114. [Google Scholar]
- Magri, A.; Haith, D.A. Pesticide decay in turf: A review of processes and experimental data. J. Environ. Qual. 2009, 38, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Farha, W.; El-Aty, A.M.A.; Rahman, M.M.; Shin, H.C.; Shim, J.H. An overview on common aspects influencing the dissipation pattern of pesticides: A review. Environ. Monit. Assess. 2016, 188, 693. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.; Matthews, G. Effect of different adjuvants on the rainfastness of bendiocarb applied to brussels sprout plants. Crop Protect. 1986, 5, 250–253. [Google Scholar] [CrossRef]
- Pick, F.E.; Van Dyk, L.P.; De Beer, P.R. The effect of simulated rain on deposits of some cotton pesticides. Pest Manage. Sci. 1984, 15, 616–623. [Google Scholar] [CrossRef]
- Barceló, D. Occurrence, handling and chromatographic determination of pesticides in the aquatic environment—A review. Analyst 1991, 16, 681–689. [Google Scholar] [CrossRef]
- Wauchope, R.D. The pesticide content of surface water draining from agricultural fields—A review. J. Environ. Qual. 1978, 7, 459–472. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Thurman, E.M.; Linhart, S.M. The environmental occurrence of herbicides: The importance of degradates in ground water. Arch. Environ. Contam. Toxicol. 1998, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Calderbank, A. The occurrence and significance of bound pesticide residues in soil. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1989; pp. 71–103. [Google Scholar]
- Garrison, A.W. Chiral pesticides and polychlorinated biphenyl congeners in environmental samples, analysis of. In Encyclopedia of Analytical Chemistry; Myers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 6147–6158. [Google Scholar]
- Garrison, A.W. Issues on the enantioselectivity of chiral agrochemicals. Chimica oggi 2002, 20, 28–32. [Google Scholar]
- Dong, F.; Li, J.; Chankvetadze, B.; Cheng, Y. Chiral triazole fungicide difenoconazole: Absolute stereochemistry, stereoselective bioactivity, aquatic toxicity, and environmental behavior in vegetables and soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; Facenda, G.; Celis, R. Evidence for the effect of sorption enantioselectivity on the availability of chiral pesticide enantiomers in soil. Environ. Pollut. 2016, 213, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, W.; Zhan, X.; Liu, W. A comparative study of rac- and s-metolachlor toxicity to daphnia magna. Ecotoxicol. Environ. Saf. 2006, 63, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gan, J.J.; Lee, S.; Werner, I. Isomer selectivity in aquatic toxicity and biodegradation of cypermethrin. J. Agric. Food Chem. 2004, 52, 6233–6238. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Pankratov, I.; Elhanany, S.; Werner, P.; Gun, J.; Gelman, F.; Lev, O. Field and laboratory studies of the fate and enantiomeric enrichment of venlafaxine and o-desmethylvenlafaxine under aerobic and anaerobic conditions. Chemosphere 2012, 88, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gomez, E.; Fenet, H.; Chiron, S. Chiral signature of venlafaxine as a marker of biological attenuation processes. Chemosphere 2013, 90, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.S.; Castro, P.; Tiritan, M. Integrated liquid chromatography method in enantioselective studies: Biodegradation of ofloxacin by an activated sludge consortium. J. Chromatogr. B 2016, 1029, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Bagnall, J.; Kasprzyk-Hordern, B. Enantioselective degradation of amphetamine-like environmental micropollutants (amphetamine, methamphetamine, mdma and mda) in urban water. Environ. Pollut. 2016, 215, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, J.; Malia, L.; Lubben, A.; Kasprzyk-Hordern, B. Stereoselective biodegradation of amphetamine and methamphetamine in river microcosms. Water Res. 2013, 47, 5708–5718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, I.S.; Amorim, C.L.; Ribeiro, A.R.; Mesquita, R.B.R.; Rangel, A.O.S.S.; van Loosdrecht, M.C.M.; Tiritan, M.E.; Castro, P.M.L. Removal of fluoxetine and its effects in the performance of an aerobic granular sludge sequential batch reactor. J. Hazard. Mater. 2015, 287, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.H.; Nghiem, L.D.; Stuetz, R.M.; Khan, S.J. Enantiospecific fate of ibuprofen, ketoprofen and naproxen in a laboratory-scale membrane bioreactor. Water Res. 2011, 45, 6249–6258. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Bagnall, J.; Kasprzyk-Hordern, B. Enantiomeric profiling of a chemically diverse mixture of chiral pharmaceuticals in urban water. Environ. Pollut. 2017, 230, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Hijosa, M.; Bayona, J.M. Assessment of the pharmaceutical active compounds removal in wastewater treatment systems at enantiomeric level. Ibuprofen and naproxen. Chemosphere 2009, 75, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Lawrence, J.R.; Neu, T.R. Selective degradation of ibuprofen and clofibric acid in two model river biofilm systems. Water Res. 2001, 35, 3197–3205. [Google Scholar] [CrossRef]
- Fono, L.J.; Sedlak, D.L. Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters. Environ. Sci. Technol. 2005, 39, 9244–9252. [Google Scholar] [CrossRef] [PubMed]
- Fono, L.J.; Kolodziej, E.P.; Sedlak, D.L. Attenuation of wastewater-derived contaminants in an effluent-dominated river. Environ. Sci. Technol. 2006, 40, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.; Tiritan, M.E. Enantioselective biodegradation of pharmaceuticals, alprenolol and propranolol, by an activated sludge inoculum. Ecotoxicol. Environ. Saf. 2013, 87, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Afonso, C.; Castro, P.M.L.; Tiritan, M. Enantioselective hplc analysis and biodegradation of atenolol, metoprolol and fluoxetine. Environ. Chem. Lett. 2013, 11, 83–90. [Google Scholar] [CrossRef]
- Moreira, I.S.; Ribeiro, A.R.; Afonso, C.M.; Tiritan, M.E.; Castro, P.M.L. Enantioselective biodegradation of fluoxetine by the bacterial strain labrys portucalensis f11. Chemosphere 2014, 111, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Moreira, I.S.; Afonso, C.M. Enantioselective quantification of fluoxetine and norfluoxetine by hplc in wastewater effluents. Chemosphere 2014, 95, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Brienza, M.; Chiron, S. Enantioselective reductive transformation of climbazole: A concept towards quantitative biodegradation assessment in anaerobic biological treatment processes. Water Res. 2017, 116, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.L.; Moreira, I.S.; Ribeiro, A.R.; Santos, L.H.; Delerue-Matos, C.; Tiritan, M.E.; Castro, P.M. Treatment of a simulated wastewater amended with a chiral pharmaceuticals mixture by an aerobic granular sludge sequencing batch reactor. Int. Biodeterior. Biodegrad. 2016, 115, 277–285. [Google Scholar] [CrossRef]
- Khan, S.J.; Wang, L.; Hashim, N.H.; McDonald, J.A. Distinct enantiomeric signals of ibuprofen and naproxen in treated wastewater and sewer overflow. Chirality 2014, 26, 739–746. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, S.L.; Sudhir, P.; Wong, C.S. Stereoisomer analysis of wastewater-derived β-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1170, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Celis, R.; Gámiz, B.; Facenda, G.; Hermosín, M.C. Enantioselective sorption of the chiral fungicide metalaxyl on soil from non-racemic aqueous solutions: Environmental implications. J. Hazard. Mater. 2015, 300, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Z.; Wang, C.; Peng, X. Chiral profiling of azole antifungals in municipal wastewater and recipient rivers of the pearl river delta, China. Environ. Sci. Pollut. Res. 2013, 20, 8890–8899. [Google Scholar] [CrossRef] [PubMed]
- Giesen, A.; De Bruin, L.; Niermans, R.; Van der Roest, H. Advancements in the application of aerobic granular biomass technology for sustainable treatment of wastewater. Water Pract. Technol. 2013, 8, 2013007. [Google Scholar] [CrossRef]
- Li, J.; Ding, L.-B.; Cai, A.; Huang, G.-X.; Horn, H. Aerobic sludge granulation in a full-scale sequencing batch reactor. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.S.; Ribeiro, A.R.; Amorim, C.L.; Barreiro, J.C.; Cass, Q.B.; Castro, P.M.; Tiritan, M.E. Degradation of fluoroquinolone antibiotics and identification of metabolites/transformation products by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1333, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Rodriguez-Roda, I.; Rodriguez-Mozaz, S.; Barceló, D. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, K.; Fryer, J. Degradation of several herbicides in a soil previously treated with mcpa. Weed Res. 1972, 12, 90–95. [Google Scholar] [CrossRef]
- Adkins, A. Degradation of the phenoxy acid herbicide diclofop-methyl by sphingomonas paucimobilis isolated from a canadian prairie soil. J. Ind. Microbiol. Biotechnol. 1999, 23, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Bromilow, R.H.; Evans, A.A.; Nicholls, P.H. Factors affecting degradation rates of five triazole fungicides in two soil types: 1. Laboratory incubations. Pest Manage. Sci. 1999, 55, 1129–1134. [Google Scholar]
- Schneiderheinze, J.; Armstrong, D.; Berthod, A. Plant and soil enantioselective biodegradation of racemic phenoxyalkanoic herbicides. Chirality 1999, 11, 330–337. [Google Scholar] [CrossRef]
- Weijers, C.; De Bont, J. Enantioselective degradation of 1, 2-epoxyalkanes by nocardia h8. Enzyme Microb. Technol. 1991, 13, 306–308. [Google Scholar] [CrossRef]
- Heron, G.; Christensen, T.H. Degradation of the herbicide mecoprop in an aerobic aquifer determined by laboratory batch studies. Chemosphere 1992, 24, 547–557. [Google Scholar] [CrossRef]
- Tett, V.; Willetts, A.; Lappin-Scott, H. Enantioselective degradation of the herbicide mecoprop [2-(2-methyl-4-chlorophenoxy) propionic acid] by mixed and pure bacterial cultures. FEMS Microbiol. Ecol. 1994, 14, 191–199. [Google Scholar] [CrossRef]
- Buser, H.-R.; Mueller, M.D. Isomer and enantioselective degradation of hexachlorocyclohexane isomers in sewage sludge under anaerobic conditions. Environ. Sci. Technol. 1995, 29, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zipper, C.; Nickel, K.; Angst, W.; Kohler, H. Complete microbial degradation of both enantiomers of the chiral herbicide mecoprop [(rs)-2-(4-chloro-2-methylphenoxy) propionic acid] in an enantioselective manner by sphingomonas herbicidovorans sp. Nov. Appl. Environ. Microbiol. 1996, 62, 4318–4322. [Google Scholar] [PubMed]
- Parlar, H.; Fingerling, G.; Angerhöfer, D.; Christ, G.; Coelhan, M. Toxaphene residue composition as an indicator of degradation pathways. In Molecular Markers in Environmental Geochemistry; American Chemical Society: Washington DC, USA, 1997; Volume 671, pp. 346–364. [Google Scholar]
- Zipper, C.; Bunk, M.; Zehnder, A.J.; Kohler, H.-P.E. Enantioselective uptake and degradation of the chiral herbicide dichlorprop [(rs)-2-(2, 4-dichlorophenoxy) propanoic acid] by sphingomonas herbicidovorans mh. J. Bacteriol. 1998, 180, 3368–3374. [Google Scholar] [PubMed]
- Zipper, C.; Fleischmann, T.; Kohler, H.-P.E. Aerobic biodegradation of chiral phenoxyalkanoic acid derivatives during incubations with activated sludge. FEMS Microbiol. Ecol. 1999, 29, 197–204. [Google Scholar] [CrossRef]
- Müller, R.H.; Jorks, S.; Kleinsteuber, S.; Babel, W. Comamonas acidovorans strain mc1: A new isolate capable of degrading the chiral herbicides dichlorprop and mecoprop and the herbicides 2, 4-d and mcpa. Microbiol. Res. 1999, 154, 241–246. [Google Scholar] [CrossRef]
- Buerge, I.J.; Müller, M.D.; Poiger, T. The chiral herbicide beflubutamid (ii): Enantioselective degradation and enantiomerization in soil, and formation/degradation of chiral metabolites. Environ. Sci. Technol. 2012, 47, 6812–6818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-K.; Hu, D.-Y.; Zhu, H.-J.; Yang, J.-C.; Song, B.-A. Enantioselective degradation of dufulin in four types of soil. J. Agric. Food Chem. 2014, 62, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Wei-Ping, L.; Yue-Zhong, W. Enantioselective degradation of rac-metolachlor and s-metolachlor in soil11supported by the national science fund for distinguished young scholars (no. 20225721) and the national natural science foundation of china (no. 30270767). Pedosphere 2006, 16, 489–494. [Google Scholar]
- Diao, J.; Xu, P.; Wang, P.; Lu, D.; Lu, Y.; Zhou, Z. Enantioselective degradation in sediment and aquatic toxicity to daphnia magna of the herbicide lactofen enantiomers. J. Agric. Food Chem. 2010, 58, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, X.; Dong, H.; Zhou, Q.; Zhang, Y. Impact of microorganisms, humidity, and temperature on the enantioselective degradation of imazethapyr in two soils. Chirality 2017, 29, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, X.; Li, C.; Zhang, H.; Fu, Q.; Shao, X.; Ye, Q.; Li, Z. Degradation of chiral neonicotinoid insecticide cycloxaprid in flooded and anoxic soil. Chemosphere 2015, 119, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, Y.; Yang, Y.; Huang, L.; Zhang, H.; Ye, Q.; Wang, H. Non-stereoselective transformation of the chiral insecticide cycloxaprid in aerobic soil. Sci. Total Environ. 2017, 579, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, J.; Ma, J.; Zhu, X.; Cai, J.; Yang, G. Anaerobic degradation pathway of the novel chiral insecticide paichongding and its impact on bacterial communities in soils. J. Agric. Food Chem. 2015, 63, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Karakus, P.B.; Stroud, J.L.; Bidleman, T.; Semple, K.T.; Jantunen, L.; Jones, K.C. Enantioselective degradation of organochlorine pesticides in background soils: Variability in field and laboratory studies. Environ. Sci. Technol. 2007, 41, 4965–4971. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, D.; Zhou, G.; Li, J.; Qiu, X.; Zhou, Z.; Wang, P. Enantioselective degradation and chiral stability of malathion in environmental samples. J. Agric. Food Chem. 2011, 60, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Pang, J.; Qiu, J.; Li, L.; Liu, C.; Jiao, B. Enantioselective degradation and enantiomerization of indoxacarb in soil. J. Agric. Food Chem. 2013, 61, 11273–11277. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, W.; Sheng, G. Enantioselective degradation and ecotoxicity of the chiral herbicide diclofop in three freshwater alga cultures. J. Agric. Food Chem. 2008, 56, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, P.; Liu, D.; Lu, Y.; Zhou, Z. Stereoselective degradation of diclofop-methyl in soil and chinese cabbage. Pestic. Biochem. Physiol. 2008, 92, 1–7. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, C.; Wen, Y.; Liu, W. Enantioselective separation and degradation of the herbicide dichlorprop methyl in sediment. Chirality 2009, 21, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, D.; Luo, M.; Jing, X.; Wang, P.; Zhou, Z. Enantioselective degradation and chiral stability of the herbicide fluazifop-butyl in soil and water. Chemosphere 2016, 146, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Frková, Z.; Johansen, A.; de Jonge, L.W.; Olsen, P.; Gosewinkel, U.; Bester, K. Degradation and enantiomeric fractionation of mecoprop in soil previously exposed to phenoxy acid herbicides–new insights for bioremediation. Sci. Total Environ. 2016, 569, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q.; Cheng, F.; Zhang, W.; Wang, W.; Li, J. Enantioselectivity in degradation and transformation of quizalofop-ethyl in soils. Chirality 2012, 24, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.; Zühlke, S.; Lamshöft, M.; Rosales-Conrado, N.; Spiteller, M. Dissipation and metabolism of 14 c-spiroxamine in soil under laboratory condition. Environ. Pollut. 2010, 158, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, G.; Qiu, J.; Diao, J.; Zhu, W.; Lv, C.; Zhou, Z. Stereoselective degradation of fungicide benalaxyl in soils and cucumber plants. Chirality 2007, 19, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, P.; Liu, D.; Lv, C.; Lu, Y.; Zhou, Z. Stereoselective degradation of benalaxyl in tomato, tobacco, sugar beet, capsicum, and soil. Chirality 2008, 20, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, D.; Diao, J.; Zhou, Z. Enantioselective toxic effects and biodegradation of benalaxyl in scenedesmus obliquus. Chemosphere 2012, 87, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sulimma, L.; Bullach, A.; Kusari, S.; Lamshöft, M.; Zühlke, S.; Spiteller, M. Enantioselective degradation of the chiral fungicides metalaxyl and furalaxyl by brevibacillus brevis. Chirality 2013, 25, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, W. Enantioselective degradation of metalaxyl in anaerobic activated sewage sludge. Bull. Environ. Contam. Toxicol. 2009, 82, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.J.; Mazur, C.S.; Kenneke, J.F.; Garrison, A.W. Enantioselective microbial transformation of the phenylpyrazole insecticide fipronil in anoxic sediments. Environ. Sci. Technol. 2007, 41, 8301–8307. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Cao, Y.; Tang, T.; Qian, K.; Chen, W.L.; Li, J. Biodegradation and chiral stability of fipronil in aerobic and flooded paddy soils. Sci. Total Environ. 2008, 407, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ma, R.X.; Liu, D.H.; Wang, P.; Huang, L.D.; Qiu, X.X.; Zhou, Z.Q. Enantioselective toxicity and degradation of the chiral insecticide fipronil in scenedesmus obliguus suspension system. Environ. Toxicol. Chem. 2014, 33, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Jing, X.; Peng, W.; Liu, X.; Zhou, Z.; Liu, D. Chiral insecticide α-cypermethrin and its metabolites: Stereoselective degradation behavior in soils and the toxicity to earthworm eisenia fetida. J. Agric. Food Chem. 2015, 63, 7714–7720. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Ji, G.D. Enantioselective degradation mechanism of beta-cypermethrin in soil from the perspective of functional genes. Chirality 2015, 27, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-H.; Ji, G.-D. Stereoselective degradation and molecular ecological mechanism of chiral pesticides beta-cypermethrin in soils with different ph values. Environ. Sci. Technol. 2015, 49, 14166–14175. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Budd, R.; Bondarenko, S.; Liu, W.; Gan, J. Enantioselective degradation and chiral stability of pyrethroids in soil and sediment. J. Agric. Food Chem. 2006, 54, 5040–5045. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Zhang, L.; Leng, L. Isomer-and enantioselective degradation and chiral stability of fenpropathrin and fenvalerate in soils. Chemosphere 2009, 76, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Influence of ph on the stereoselective degradation of the fungicides epoxiconazole and cyproconazole in soils. Environ. Sci. Technol. 2006, 40, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.L.; Guo, B.Y.; Peng, Z.; Wang, Z.; Guo, G.; Lin, J.M. Studies on degradation of imazalil enantiomers in soil using capillary electrophoresis. J. Sep. Sci. 2007, 30, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hua, X.; Yang, Y.; Yin, W.; Tian, M.; Shi, H.; Wang, M. Stereoselective degradation of flutriafol and tebuconazole in grape. Environ. Sci. Pollut. Res. 2015, 22, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Li, Q.; Wang, W.; Li, J. Enantioselective degradation, abiotic racemization, and chiral transformation of triadimefon in soils. Environ. Sci. Technol. 2011, 45, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q.; Zhao, J.; Wang, W. Stereoselective degradation and microbial epimerization of triadimenol in soils. Chirality 2013, 25, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhang, H.; Wu, C.; Wang, X.; Xu, H.; Wang, X.; Li, Z. Enantioselective degradation of tebuconazole in cabbage, cucumber, and soils. Chirality 2012, 24, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Han, Y.; Zheng, Y. Enantioselectivity in tebuconazole and myclobutanil non-target toxicity and degradation in soils. Chemosphere 2015, 122, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, O.F.; Maillard, E.; Vuilleumier, S.; Millet, M.; Imfeld, G. Degradation of chloroacetanilide herbicides and bacterial community composition in lab-scale wetlands. Sci. Total Environ. 2015, 520, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, L.; Yang, Y.; Hua, X.; Shi, H.; Wang, M. Study on the stereoselective degradation of three triazole fungicides in sediment. Ecotoxicol. Environ. Saf. 2015, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, D.; Meng, X.; Shi, Q.; Li, P.; Jin, L.; Zhang, K.; Song, B. Enantioselective degradation of indoxacarb from different commercial formulations applied to tea. Chirality 2015, 27, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, P.; Zhu, W.; Gu, X.; Zhou, W.; Zhou, Z. Enantioselective degradation of fipronil in chinese cabbage (brassica pekinensis). Food Chem. 2008, 110, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Liu, X.; Zheng, Y.; Cao, Q.; Li, C. Stereoselective degradation of fungicide triadimenol in cucumber plants. Chirality 2010, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Qiu, J.; Li, L.; Li, W.; Zhou, Z.; Liu, F.; Qiu, L. Stereoselective dissipation of epoxiconazole in grape (vitis viniferacv. Kyoho) and soil under field conditions. Chemosphere 2012, 87, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Q.; Cong, L.; Yin, W.; Wang, M. Enantioselective degradation of metalaxyl in cucumber, cabbage, spinach and pakchoi. Chemosphere 2014, 95, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, D.; Sun, M.; Di, S.; Wang, P.; Zhou, Z. The chiral separation and enantioselective degradation of the chiral herbicide napropamide. Chirality 2014, 26, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dong, F.; Xu, J.; Liu, X.; Wang, Y.; Zheng, Y. Enantioselective degradation of chiral insecticide dinotefuran in greenhouse cucumber and soil. Chirality 2015, 27, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Lu, Y.; Wang, P.; Dang, Z.; Zhou, Z. Enantioselective degradation of diclofop-methyl in cole (brassica chinensis l.). Food Chem. 2010, 121, 264–267. [Google Scholar] [CrossRef]
- Wang, M.; Hua, X.; Zhang, Q.; Yang, Y.; Shi, H.; Wang, M. Enantioselective degradation of metalaxyl in grape, tomato, and rice plants. Chirality 2015, 27, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Zhang, Y.; Casas, M.E.; Carvalho, P.N.; Arias, C.A.; Bester, K.; Brix, H. Phytoremediation of imazalil and tebuconazole by four emergent wetland plant species in hydroponic medium. Chemosphere 2016, 148, 459–466. [Google Scholar] [CrossRef] [PubMed]
- López-Cabeza, R.; Gámiz, B.; Cornejo, J.; Celis, R. Behavior of the enantiomers of the herbicide imazaquin in agricultural soils under different application regimes. Geoderma 2017, 293, 64–72. [Google Scholar] [CrossRef]
- Lv, T.; Carvalho, P.N.; Casas, M.E.; Bollmann, U.E.; Arias, C.A.; Brix, H.; Bester, K. Enantioselective uptake, translocation and degradation of the chiral pesticides tebuconazole and imazalil by phragmites australis. Environ. Pollut. 2017, 229, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Schurig, V.; Reich, S. Determination of the rotational barriers of atropisomeric polychlorinated biphenyls (pcbs) by a novel stopped-flow multidimensional gas chromatographic technique. Chirality 1998, 10, 316–320. [Google Scholar] [CrossRef]
- Vetter, W.; Schurig, V. Enantioselective determination of chiral organochlorine compounds in biota by gas chromatography on modified cyclodextrins. J. Chromatogr. A 1997, 774, 143–175. [Google Scholar] [CrossRef]
- Vetter, W. Gas chromatographic enantiomer separation of polychlorinated biphenyls (pcbs): Methods, metabolisms, enantiomeric composition in environmental samples and their interpretation. Isr. J. Chem. 2016, 56, 940–957. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.R. Enantioselective degradation of metalaxyl in soils: Chiral preference changes with soil ph. Environ. Sci. Technol. 2003, 37, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Kuivila, K.M.; Hladik, M.L.; Ingersoll, C.G.; Kemble, N.E.; Moran, P.W.; Calhoun, D.L.; Nowell, L.H.; Gilliom, R.J. Occurrence and potential sources of pyrethroid insecticides in stream sediments from seven U.S. Metropolitan areas. Environ. Sci. Technol. 2012, 46, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Carvalho, M.F.; Afonso, C.M.M.; Tiritan, M.E.; Castro, P.M. Microbial degradation of 17beta -estradiol and 17alpha -ethinylestradiol followed by a validated hplc-dad method. J. Environ. Sci. Health. Part. B, Pestic. Food Contam. Agric. Waste. 2010, 45, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Gonçalves, V.M.M.; Maia, A.S.; Carvalho, M.F.; Castro, P.M.; Tiritan, M.E. Microbial degradation of pharmaceuticals followed by a simple hplc-dad method. J. Environ. Sci. Health. Part A, Tox./Hazard. Subst. Environ. Eng. 2012, 47, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
| Title | Chiral Drugs | Matrix | Biodegradation Experiment | Analytical Method | EF/Observations | Reference |
|---|---|---|---|---|---|---|
| antibiotics | ofloxacin, levofloxacin | minimal salts medium inoculated with activated sludge | laboratory-scale microcosms, under aerobic conditions, with and without an extra carbon source | HPLC-FD; LC-MS/MS | enantioselective biodegradation of ofloxacin observed; (S)-ofloxacin degraded at higher extents; biodegradation of levofloxacin ((S)-enantiomer) led to (R)-enantiomer formation | [77] |
| anticoagulants | warfarin | sterile and nonsterile turfgrass and groundcover soil | aerobic and ambient temperature incubation | HPLC-FD | fast degradation of warfarin in the nonsterile soils while no degradation was observed in the sterile conditions; slightly enantioselective biodegradation with (R)-warfarin being preferentially degraded | [10] |
| antidepressants | fluoxetine | minimal salts medium inoculated with a single microbial strain | batch experiment incubations with an additional carbon source under aerobic conditions, protected from light | HPLC-FD | enantioselective biodegradation of fluoxetine was observed; (R)-fluoxetine preferentially degraded | [89] |
| fluoxetine | synthetic wastewater | laboratory-scale aerobic granular sludge sequencing batch reactor | HPLC-FD | fluoxetine degraded at low extents and following a non-enantioselective pattern | [80] | |
| fluoxetine, norfluoxetine | WWTP effluents | microcosms tests at laboratory scale under aerobic conditions, protected from light | HPLC-FD | fluoxetine degradation followed a non-enantioselective pattern; no formation of the metabolite norfluoxetine was observed | [90] | |
| venlafaxine | river water | laboratory-scale experiments to assess photolysis, sorption and biodegradation | LC-MS/MS | venlafaxine sorption and biotranformation processes were non-enantioselective; venlafaxine biodegradation was enantioselective and formed (O)-desmethylvenlafaxine | [76] | |
| venlafaxine, metabolites | WWTP effluents charged with activated sludge | laboratory-scale incubation of effluents with activated sludge under anaerobic and aerobic conditions | LC-MS/MS | venlafaxine degradation presented slight enantioselectivity; (O)-desmethylvenlafaxine showed (S) to (R)-enantiomer enrichment exclusively under aerobic conditions | [75] | |
| antifungals | climbazole | WWTP effluents charged with activated sludge | biotic and sterile batch anoxic degradation experiments, under dark and light conditions | LC-MS/MS | enantioselective degradation of climbazole observed under biotic conditions; faster degradation of E1-climbazole | [92] |
| anti-hyperlipidemic agents | metoprolol, ibuprofen, naproxen, gemfibrozil | river water | microcosm experiments to assess photolysis and biotransformation | GC-MS/MS | no degradation observed in the dark; metoprolol EF remained unchanged in the microcosms; metoprolol EF decrease along the river flow suggested biological mediated-degradation | [86] |
| anti-hyperlipidemic and anti-inflammatory agents | Ibuprofen clofibric acid | river water | incubation with a river biofilm reactor | GC-MS | ibuprofen and two metabolites were degraded in the biofilm reactor; (R)-ibuprofen, pharmacologically inactive, degraded faster | [84] |
| anti-inflammatory agents | ibuprofen, naproxen, ketoprofen | synthetic wastewater | laboratory-scale membrane bioreactor | GC-MS/MS | ibuprofen EF decreased during biodegradation and (S)-ibuprofen was preferentially degraded; (R)-ketoprofen degraded at a greater extent with minor increase in EF; (S)-naproxen EF significantly decreased during biodegradation, and (R)-naproxen concentration increased, suggesting enantiomeric inversion | [81] |
| ibuprofen | surface waters; WWTP influents and effluents | incubation of fortified lake water; incubation with activated sludge under aerobic conditions | GC-MS/MS | rapid degradation of ibuprofen in the incubation experiments; (S)-ibuprofen exhibited faster degradation rates | [8] | |
| ibuprofen | urban wastewater; synthetic wastewater | aerated batch reactors inoculated with microalgae | GC-MS | enantioselective biodegradation of (S)-ibuprofen observed; EF decreased over degradation time | [91] | |
| ibuprofen, naproxen | synthetic wastewater; real wastewater | removal efficiency in WWTP, pilot and microcosm-scale constructed wetlands | GC-MS | (S)-ibuprofen degraded faster under aerobic conditions; under anaerobic conditions ibuprofen degradation was non-enantioselective; naproxen presented an enantioselective degradation profile both under aerobic and anaerobic conditions | [83] | |
| antidepressants and beta-blockers | atenolol, metoprolol, fluoxetine | minimal salts medium inoculated with activated sludge | batch experiment incubations with and without an extra carbon source under aerobic conditions | HPLC-FD | metoprolol enantioselective biodegradation was observed; (S)-metoprolol degraded at higher extents; atenolol and fluoxetine biodegradation processes were non-enantioselective | [88] |
| beta-blockers | propranolol | WWTP secondary effluents; river water | microcosm experiments to simulate biotransformation in WWTP (activated sludge) and in surface water | GC-MS/MS | EF varied in the incubation with activated sludge but not in the non-inoculated conditions; EF remained unchanged in the surface water experiments | [85] |
| alprenolol, propranolol | minimal salts medium inoculated with activated sludge | batch experiment incubations with and without an extra carbon source under aerobic conditions | HPLC-FD | enantioselective biodegradation of both drugs was observed; (S)-alprenolol and (S)-propranolol slightly higher degraded; enantioselective degradation pattern sustained in the presence of the extra carbon source | [87] | |
| antidepressants, beta-blockers, and bronchodilators | alprenolol, bisoprolol, metoprolol, propranolol, venlafaxine, salbutamol, fluoxetine, norfluoxetine | synthetic wastewater | aerobic granular sludge-sequencing batch reactor | LC-MS/MS | enantioselective biodegradation of norfluoxetine observed; (R)-norfluoxetine preferentially degraded; non-enantioselective removal of the other target compounds | [93] |
| antidepressants, beta-blockers, bronchodilators, and synthetic psychoactive agents | MDMA (3,4-methylenedioxy-methamphetamine), MDA (3,4-methylenedioxyamphetamine), ampethamine, methamphetamine, venlafaxine, fluoxetine, O-desmethylvenlafaxine, atenolol, metoprolol, propranolol, alprenolol, sotalol, salbutamol, mirtazapine, citalopram, desmethylcitalopram | receiving waters (mixture of river water and WWTP effluent); activated sludge | receiving surface waters and activated sludge simulating microcosms systems under light, dark, biotic and abiotic conditions | LC-MS/MS | enantioselective degradation of amphetamines, beta-blockers and antidepressants observed; (S)-forms preferentially degraded for amphetamines and antidepressants and (R)-forms for beta-blockers; metabolites tested showed higher enantioselective degradation rates than parent compounds | [82] |
| synthetic psychoactive agents | amphetamine, methamphetamine | river water | microcosm bioreactors in the light (microbial degradation) and in the dark (photochemical processes) | LC-MS/MS | EF variations observed exclusively under biotic conditions; non-racemic by-products formation during the biodegradation | [79] |
| amphetamine, methamphetamine, MDMA, MDA | WWTP effluents; river water | receiving surface waters and activated sludge simulating microcosms systems | LC-MS/MS | enantioselective biodegradation of all compounds observed in activated sludge simulating microcosms with the (S)-enantiomers being preferentially degraded; (R)-enantiomers limited or non-degraded; racemic MDMA enantioselective biodegradation resulted in (R)-enantiomer enrichment and formed (S)-MDA; MDMA slight enantioselective degradation observed in river water | [78] |
| Chiral Pesticide | Matrix | Biodegradation Experiment | Analytical Method | EF/Observations | Reference |
|---|---|---|---|---|---|
| amides | - | - | - | - | - |
| beflubutamid | soil | laboratory incubation experiments under aerobic conditions with acidic and alkaline matrices | GC-MS | enantioselective degradation of beflubutamid observed in alkaline soil; (-)-beflubutamid degraded slower in alkaline soil; both enantiomers degraded similarly in acidic soil; highly enantioselective degradation of the metabolite phenoxybutanamide observed | [116] |
| aminophosphonic acid derivatives | - | - | - | - | - |
| dufulin | soil | laboratory incubation experiments under sterile and non-sterile conditions with racemic mixture and individual enantiomers | HPLC-DAD | faster degradation of enantiopure (S)-dufulin compared to its antipode; enantiomerization not observed during incubation of individual enantiomers | [117] |
| chloroacetanilides | - | - | - | - | - |
| metolachlor | soil | laboratory incubation experiments under sterile and non-sterile conditions | GC-ECD | enantioselectivity observed during degradation; (S)-metolachlor degraded faster than the racemic mixture | [118] |
| metolachlor | runoff waters | laboratory scale wetlands; column wetlands | GC-MS | enantioselective degradation of metolachlor observed; EF variations detected along the wetland distinct zones | [154] |
| diphenyl ethers | - | - | - | - | - |
| lactofen and metabolites | sediment | laboratory incubation experiments with racemic mixture and individual enantiomers | HPLC-VWD | enantioselective degradation observed with (S)-lactofen and (S)-desethyl lactofen being preferentially degraded and enrichment of the (R)-forms. | [119] |
| imidazolinones | - | - | - | - | - |
| imazethapyr | soil | laboratory incubation experiments under aerobic, sterile and non-sterile conditions with variable pH, humidity and temperature settings | HPLC-UV-CD | (R)-imazethapyr preferentially degraded in all samples; average (R)-imazethapyr half-lives significantly shorter than its antipode; EF values significantly higher in less acidic soil | [120] |
| neonicotinoids | - | - | - | - | - |
| cycloxaprid | soil | laboratory incubation experiments under anoxic and flooded conditions with racemic mixture and individual enantiomers | HPLC-LSC; LC-MS/MS | enantioselective abiotic and biotic cycloxaprid degradation not observed; non-enantioselective transformation could be related to the absence of oxabridged ring in the transformation products | [121] |
| cycloxaprid | soil | laboratory incubation experiments under aerobic conditions | HPLC-DAD | non-enantioselective degradation of racemic-cycloxaprid and its (1S2R)- and (1R2S)-enantiomers observed in the soil samples tested | [122] |
| paichongding | soil | laboratory incubation experiments under anaerobic conditions | HPLC-DAD; LC-MS/MS | enantioselective degradation of paichongding observed; types of soil influenced enantiomers degradation rates; degradation process originated three achiral transformation products | [123] |
| organochlorines | - | - | - | - | - |
| α-HCH, cis- and trans-chlordane, o,p'-DDT | woodland and grassland background soil | laboratory incubation experiments under aerobic conditions | GC-ECNI-MS | enantioselectivity degradation observed in field and laboratory experiments | [124] |
| organophoshorus | - | - | - | - | - |
| malathion | soil, environmental waters | laboratory incubation experiments | HPLC-VWD | (S)-malathion degraded faster than the active (R)-malathion in all environmental samples; biodegradation of pure enantiomers of malathion showed enantiomeric inversion in soil and water samples | [125] |
| oxadiazines | - | - | - | - | - |
| indoxacarb | soil | laboratory incubation experiments under sterile and non-sterile conditions with acidic and alkaline matrices | HPLC-DAD | enantioselective degradation of indoxacarb observed under non-sterile conditions; (R)-indoxacarb degraded faster in acidic soil; (S)-indoxacarb preferentially degraded in alkaline soil; enantiomerization observed in both acidic and alkaline soils | [126] |
| phenoxies | - | - | - | - | - |
| diclofop-methyl, diclofop | algae cultures | laboratory incubation experiments | HPLC-FD | enantioselective degradation of diclofop and diclofop-methyl observed and influenced by temperature | [127] |
| diclofop-methyl | agricultural soil, Chinese cabbage | laboratory incubation experiments; field experiments in spiked plants | HPLC-DAD | enantioselective degradation of diclofop-methyl observed in two of the tested soil samples, where (-)-enantiomer degraded faster; (+)-enantiomer preferentially degraded in cabbage | [128] |
| dichlorprop-methyl | sediment | laboratory incubation experiments with bacterial strain isolated from activated sludge | HPLC-UV-CD; GC-ECD | (R)-dichlorprop-methyl preferentially degraded at different pH values; enantioselectivity more evident at neutral pH conditions | [129] |
| fluazifop-butyl | soil, water | laboratory incubation experiments under different pH conditions (water) with racemic mixture and individual enantiomers | LC-MS/MS | enantioselective degradation of fluazifop-butyl observed in two soil samples but not on water; enantiomeric form preferentially degraded varied within soil samples | [130] |
| mecoprop | soil sampled at different depths | laboratory incubation experiments under aerobic and anaerobic conditions | LC-MS/MS | (R)-mecoprop preferentially degraded under aerobic conditions in soils from 3 and 6 m depth when using nM mecoprop concentrations; (S)-mecoprop preferentially degraded in all samples when using higher mecoprop concentrations (μM) | [131] |
| quizalofop-ethyl, quizalofop-acid (metabolite) | soil | laboratory incubation experiments with racemic mixture and individual enantiomers | HPLC-UV | enantioselective degradation of quizalofop-ethyl observed; (S)-quizalofop-ethyl degraded faster both in acidic and alkaline soils; quizalofop-acid degraded faster in acidic soil; quizalofop-acid enantiomerization observed with enrichment of the (R)-enantiomer | [132] |
| spiroxamine | soil | laboratory incubation experiments under anaerobic conditions | LC-MS; GC-MS | non-enantioselective degradation of spiroxamine observed | [133] |
| phenylamides | - | - | - | - | - |
| benalaxyl | agricultural soil, cucumber plant | laboratory incubation experiments in the dark | HPLC-DAD | enantioselective degradation of benalaxyl observed; (S)-benalaxyl degraded faster in plants and (R)-benalaxyl degraded faster in soils | [134] |
| benalaxyl | soil, vegetables | laboratory incubation experiments with soil; growth of plants in controlled environment with fungicide application | HPLC-DAD | enantioselective degradation observed in soil where (R)-benalaxyl dissipated faster; (S)-benalaxyl preferentially degraded in all vegetables with resulting enrichment of (R)-benalaxyl. | [135] |
| benalaxyl | freshwater algae cultures | laboratory incubation experiments | HPLC-UV | enantioselective degradation of benalaxyl observed; (S)-benalaxyl half-life slightly smaller and relative enrichment of the (R)-enantiomer occurred | [136] |
| furalaxyl, metalaxyl | microbial liquid cultures | laboratory incubation experiments with the individual compounds and its mixture | HPLC-MS | enantioselective degradation of furalaxyl and metalaxyl observed with one of the isolated microorganisms; (R)-enantiomers of both compounds preferentially degraded | [137] |
| metalaxyl | sewage sludge | laboratory incubation experiments under anaerobic conditions | HPLC-UV-CD | (S)-metalaxyl from the racemic mixture degraded faster, presenting a T1/2 much lower than the (R)-metalaxyl; racemic mixture T1/2 lower than the (R)-enantiomer | [138] |
| phenylpyrazoles | - | - | - | - | |
| fipronil | sediment | laboratory incubation experiments under anaerobic conditions | GC-MS | enantioselective degradation of fipronil observed; fipronil EF varied during incubation period in sulfidogenic sediments and the (S)-enantiomer was preferentially degraded | [139] |
| fipronil | soil | laboratory incubation experiments under aerobic and anaerobic conditions | HPLC-DAD | almost non-enantioselective degradation of racemic fipronil observed; (S)-fipronil preferentially degraded under anaerobic conditions with flooded soil; no enantiomerization of fipronil observed | [140] |
| fipronil | algae cultures | laboratory incubation experiments with racemic mixture and individual enantiomers | HPLC-UV | enantioselective degradation of fipronil observed; EF varied from 0.5 to 0.65 in 17 days; longer half-life values observed for (S)-fipronil | [141] |
| pyrethroids | - | - | - | - | - |
| alpha-cypermethrin | soil | laboratory incubation experiments | HPLC-VWD; GC-ECD | enantioselective degradation of α-cypermethrin observed; EF varied from 0.55 to 0.61 in 42 days; (+)-(1R,cis,αS)-cypermethrin preferentially degraded | [142] |
| beta-cypermethrin | soil | laboratory incubation experiments under sterile and non-sterile conditions | HPLC-VWD | enantioselective degradation of beta-cypermethrin observed; different degradation rates observed for the four beta-cypermethrin isomers; EF variation noticed during the degradation process | [143] |
| beta-cypermethrin- | soil | laboratory incubation experiments under sterile and non-sterile conditions with acidic and alkaline matrices, and with racemic mixture and individual enantiomers | HPLC-UV | enantioselective degradation of racemic-beta-cypermethrin observed only in non-sterile soils; different degradation rates and half-lives observed for the four beta-cypermethrin isomers; no enantiomeric enrichment observed during degradation of individual enantiomers | [144] |
| (Z)-cis-bifenthrin, cis-permethrin, cyfluthrin, cypermethrin | soil, sediment | laboratory incubation experiments under aerobic and anaerobic conditions | GC-ECD | enantioselective degradation of cis-bifemthrin, pemethrin and cyfluthrin observed | [145] |
| fenpropathrin, fenvalerate | soil | laboratory incubation experiments with acidic and alkaline matrices | HPLC-UV | slightly enantioselective degradation of fenpropathrin and fenvalerate in alkaline samples where (S)-fenpropathrin and (αS,2R)-fenvalerate were degraded faster; racemization observed in alkaline samples but not on acidic soils | [146] |
| triazoles | - | - | - | - | - |
| epoxiconazole, cyproconazole | soil | laboratory incubation experiments under different pH conditions | GC-MS | soil pH affected degradation enantioselectivity; enantioselective degradation of epoxiconazole observed at higher pH values | [147] |
| enilconazole | soil | laboratory incubation experiments under different conditions of light and UV irradiation | CE | enantioselective degradation of enilconazole not observed in alkaline soil | [148] |
| fenbuconazole, RH-9129 (metabolite), RH-9130 (metabolite) | soil | laboratory incubation experiments under aerobic and anaerobic conditions | LC-MS/MS | enantioselective degradation of fenbuconazole observed under aerobic and anaerobic conditions; (-)-fenbuconazole preferentially degraded; enantioselective degradation of the metabolites differed with aeration and pH conditions | [21] |
| flutriafol, hexaconazole, tebuconazole | sediment | laboratory incubation experiments under sterile and non-sterile conditions | HPLC-UV | enantioselective degradation of the three triazole fungicides observed; (-)-enantiomers preferentially degraded in native conditions; no significant enantioselective degradation observed under sterilized conditions | [155] |
| triadimefon | soil | laboratory incubation experiments in sterile and non-sterile conditions | HPLC-UV | (R)-triadimefon preferentially degraded in acidic and alkaline soils; racemization observed in the abiotic degradation of enantiopure triadimefon enantiomers | [150] |
| triadimenol | soil | laboratory incubation experiments in sterile and non-sterile conditions | HPLC-UV | relative enantioselective degradation of triadimenol observed; epimerization observed in incubations with enantiopure triadimenol enantiomers | [151] |
| tebuconazole | agricultural soil, vegetables | laboratory incubation experiments in sterile and non-sterile conditions | HPLC-DAD, LC-MS/MS | tebuconazole EF varied slightly during biodegradation in soil samples; (R)-tebuconazole degraded faster than the (S)-enantiomer in tested soils | [152] |
| tebuconazole, myclobutanil | soil | laboratory incubation experiments under aerobic and anaerobic conditions with racemic mixture and individual enantiomers | LC-MS/MS | enantioselective degradation of tebuconazole observed in aerobic and anaerobic soils; (S)-tebuconazole preferentially degraded; enantioselectivity correlated with the soils organic carbon content; (+)-myclobutanil preferentially degraded in aerobic soils; similar degradation rates of myclobutanil enantiomers in anaerobic soils | [153] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, A.S.; Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Analysis of Pesticides and Drugs of Environmental Concern: Biodegradation and Enantiomeric Fraction. Symmetry 2017, 9, 196. https://doi.org/10.3390/sym9090196
Maia AS, Ribeiro AR, Castro PML, Tiritan ME. Chiral Analysis of Pesticides and Drugs of Environmental Concern: Biodegradation and Enantiomeric Fraction. Symmetry. 2017; 9(9):196. https://doi.org/10.3390/sym9090196
Chicago/Turabian StyleMaia, Alexandra S., Ana R. Ribeiro, Paula M. L. Castro, and Maria Elizabeth Tiritan. 2017. "Chiral Analysis of Pesticides and Drugs of Environmental Concern: Biodegradation and Enantiomeric Fraction" Symmetry 9, no. 9: 196. https://doi.org/10.3390/sym9090196








