Review of Biohydrometallurgical Metals Extraction from Polymetallic Mineral Resources
Abstract
:1. Introduction
- Ta, In, Ru, Ga, Ge and Pd used in electrical and electronic equipment,
- Ga, Te, In and Ge in photovoltaic cells,
- Co, Li and rare earths in batteries, and
- Pt, Pd and rare earths in catalysts [2].
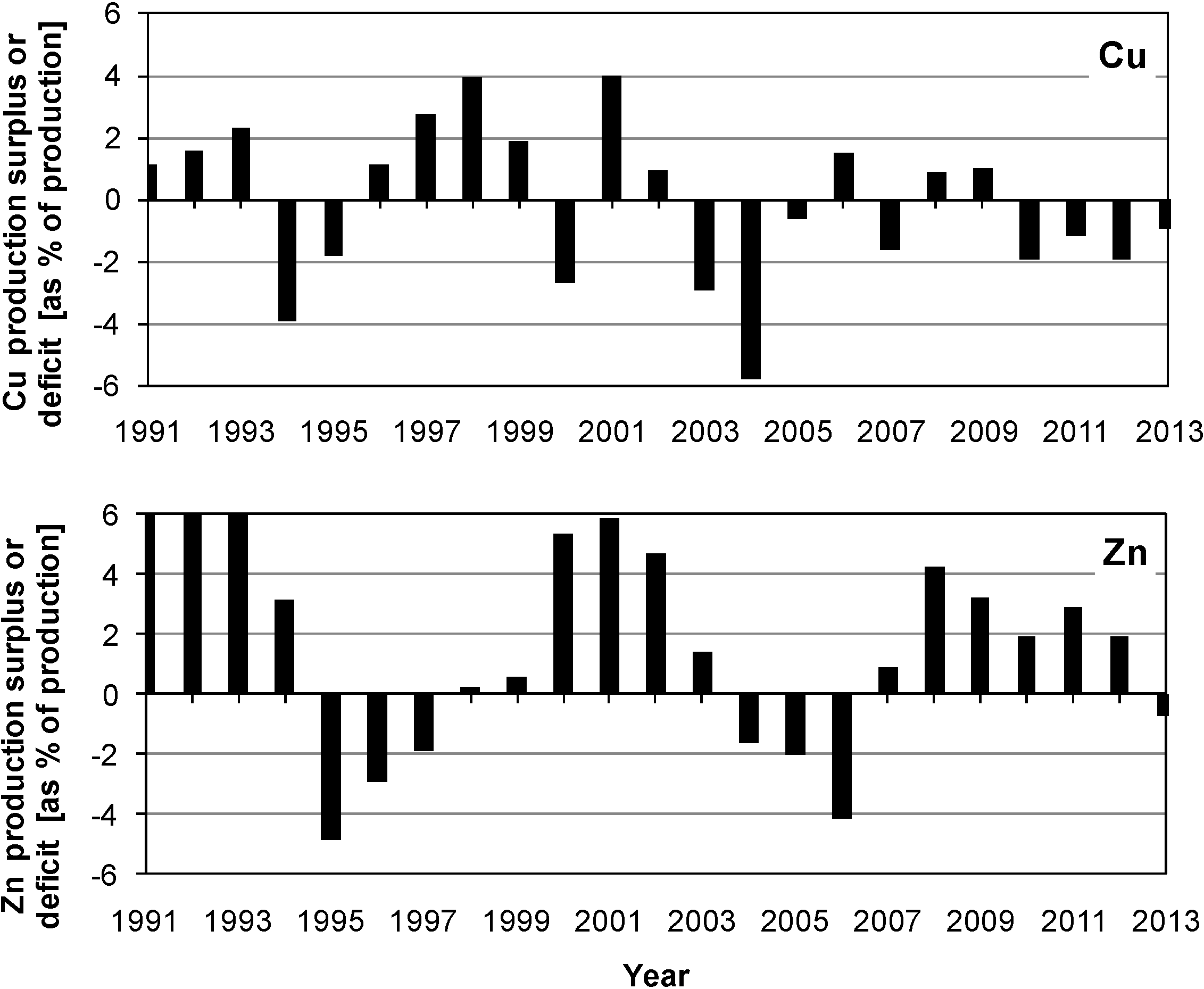

2. The Chemistry and Microbiology of Mineral Dissolution
2.1. Mineral Dissolution in Acidic Environments
2.2. Bio-Generation of Inorganic Acids
2.3. Bio-Generation of Organic Acids and Chelating Agents
2.4. Biodegradation of Organo-Metallic Compounds
2.5. Bio-Participation in Redox Reactions
2.5.1. Fe(II) and RISC Biooxidation in Oxygenated Environments
2.5.2. RISC Biooxidation in Anoxic or Oxygen-Limited Environments
2.5.3. RISC Bioreduction in Anoxic or Oxygen-limited Environments
2.6. Microbial Growth under Element Stress
2.7. Technology Developments



3. Polymetallic Sulfide Concentrates
- extraction of metals;
4. Low Grade Sulfide Ores
4.1. Ores with Low Organic Content
4.2. Sulfidic Schists and Shales with High Organic Content
4.2.1. Microbiological Aspects
| Deposit/Region | Enriched Elements |
|---|---|
| Talvivaara, Finland | Ni, Cu, Co, Zn, Mn, U, Ag, As, |
| Kainuu, Finland | Ni, Cu, Co, Zn, V, Mo |
| Alum shale, Sweden | Ni, Cu, Co, Zn, Pb, V, Mo, U, Cr, Mn, Ba |
| Viken, Sweden | Ni, V, Mo, U |
| Kupferschiefer, Poland | Cu, Ag, Zn, Ni, Co, Pb, Mo, V, U, As, Se, Cd, Bi, Tl, Re, PGE |
| Kamenec, Czech Republic | Ni, Cu, Zn, Mo, Cr, PGE |
| Hromnice, Czech Republic | Ni, Cu, Zn, V, Mo, Au |
| Pyrenées, France | Zn, Pb, P, Ge, Cd |
| Eastern Pyrenées, France | Ni, Cu, Co, Zn, Pb, Au, W, Sb |
| Dauphiné Basin, France | Ni, Cu, Pb, U, Ba |
| Selwyn Basin, YT, Canada | Ni, Zn, Pb, Mo, Ag, Cd, In, Sb, Se, As, Au, Tl, Re, PGE |
| Athabaska region, AB, Canada | Ni, Cu, Co, Zn, V, Mo, U, Ag, Au, Li, Cd, REE |
| Kimberley, BC, Canada | Cu, Zn, Pb, Sn, Ag, Sb, Cd, Bi |
| Red Dog, AK, USA | Zn, Pb, Ag, Se, Ba |
| Carlin region, NV, USA | Ni, Zn, V, Mo, Se, Au, Ag, As |
| Mina Aguilar, Argentina | Zn, Pb, Ag |
| Rajasthan, India | Zn, Pb, Ag |
| KPK Region, Pakistan | Cu, Zn, V, Mn, U, Ti |
| Zunyi, China | Ni, Zn, Mo, Au, Se, As, PGE |
| Changba, China | Zn, Pb |
| Western Yunnan, China | Cu, Zn, Pb, Tl, Cd, Ag, As |
| Okcheon, South Korea | Ni, Cu, Zn, V, Mo, U, Ba, Cr, Y, Au, PGE |
| Mt Isa, Australia | Zn, Pb, Ag |
| Gauteng, RSA | Ni, Cu, Co, Zn, Pb, V, Mo, Cr, Au |
| Konkola, Zambia | Cu, Co, U, Pd, Re |

4.2.2. Prospects for Commercialisation
5. Oxidised Ores
5.1. Leaching with Acidophilic Autotrophs/Inorganic Acid
5.2. Leaching with Acidophilic Heterotrophs/Organic Acid
5.3. Reductive Bioleaching (Organic Carbon Compound as Electron Donor)
5.4. Reductive Bioleaching (RISC as Electron Donor)
6. Biotechnologies for Polymetallic Mine Waste and Tailings
6.1. Waste and Tailings from Sulfide Ores
- the application of heterotrophic microorganisms/organic acids to treat sulfidic tailings comparing direct and indirect bioleaching [376];


6.2. Waste and Tailings from Oxidised Ores
- bioleaching in batch reactors, stirred tanks or columns, including one column leaching experiment in which fungi were applied as inoculum [395];
- a two stage process, in which biological acid production and chemical leaching were separated [399];
- enhanced extraction by applying a surfactant to stimulate microbial growth but reduce carbon-source utilisation [404].
6.3. Towards Responsible Waste Management
- understanding systems that display “natural attenuation” of contaminated water with a view to exploiting them;
- “bioshrouding”—the formation of biofilms around pyrite grains by heterotrophic microorganisms to limit the attachment of pyrite-oxidising acidithiobacilli;
- “ecological engineering” to reverse the reactions that generate ARD—inoculating freshly-deposited tailings with acidophilic algae and heterotrophic bacteria, sustaining the growth of iron(III)- and sulfate-reducing bacteria within the submerged tailings
- iron oxidation and removal from AMD—in a continuous three-stage modular bioreactor comprising (i) an iron(II)-oxidation reactor inoculated with “Ferrovum myxofaciens”; (ii) a schwertmannite precipitation reactor and (iii) a packed bed polishing unit and
- selective precipitation of copper and zinc and other elements as sulfides from mine waters using acidophilic SRB
- using neutral or acidic SRB to convert sulfate to elemental sulfur for removal;
7. Summary
- a longer-term view of what constitutes a mineral resource and a broader approach to what elements can be extracted, co-extracted, and/or separated from resultant process solutions;
- the development of efficient new or modified technologies for the extraction of a suite of elements, rather than the most lucrative element, from complex polymetallic ores, concentrates, mine wastes and tailings, thereby gaining as much benefit as possible from the considerable costs of mining, moving and pre-treating large tonnages of materials;
- discarding waste and tailings that are benign, thus lowering the costs of environmental remediation during mine closure; and
- concomitant development of new separation and purification technologies for the recovery of all elements of interest in the multiple-product mines of the future.
- Heap and dump leaching/bioleaching can be a secondary process or a stand-alone technology applied across a wide range of climatic conditions in terrain that is complex and/or remote. Its flexibility makes it suited to deposits from small to very large and each of the unit processes can be very basic (the use of cementation or precipitation to recover metal products).
- The widespread adoption of stirred tanks for the processing of refractory gold ores and concentrates is one of the success stories of mining biotechnology. Stirred tank processes have been piloted and/or demonstrated for the processing of base metals and in one instance commercialised for cobalt production from tailings.
- Both technologies have benefited from improved SW-EW for the separation and recovery of an increased number of elements through the development of novel reagents.
Acknowledgments
Conflicts of Interest
References
- Zinc Commodity Update. Available online: www.hdrsalva.com/market-news/zinc-commodity-update (accessed on 22 October 2014).
- Critical Metals for Future Sustainable Technologies and Their Recycling Potential. Available online: www.unep.fr/scp/publications/details.asp?id=DTI/1202/PA (accessed on 22 October 2014).
- Graedel, T.E.; Cao, J. Metal spectra as indicators of development. Proc. Natl. Acad. Sci. USA 2010, 107, 20905–20910. [Google Scholar] [CrossRef] [PubMed]
- Reller, A.; Bublies, T.; Staudinger, T.; Oswald, I.; Meissner, S.; Allen, M. The mobile phone: Powerful communicator and potential metal dissipator. GAIA 2009, 18, 127–135. [Google Scholar]
- Dodson, J.R.; Hunt, A.J.; Parker, H.L.; Yang, Y.; Clark, J.H. Element sustainability: Towards the total recovery of scarce metals. Chem. Eng. Process. 2012, 51, 69–78. [Google Scholar] [CrossRef]
- Backman, C.M. Global supply and demand of metals in the future. J. Toxicol. Environ. Health 2008, 71, 1244–1253. [Google Scholar] [CrossRef]
- Prior, T.; Giurco, D.; Mudd, G.; Mason, L.; Behrisch, J. Resource depletion, peak minerals and the implications for sustainable resource management. Glob. Environ. Chang. 2012, 22, 577–587. [Google Scholar] [CrossRef]
- West, J. Decreasing metal ore grades: Are they really being driven by the depletion of high-grade deposits? J. Ind. Ecol. 2011, 15, 165–168. [Google Scholar] [CrossRef]
- Crowson, P. Some observations on copper yields and ore grades. Resour. Policy 2012, 37, 59–72. [Google Scholar] [CrossRef]
- USGS Minerals Yearbooks 1994–2011 and Mineral Commodity Summaries 2012–2014. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/ (accessed on 22 October 2014).
- World Copper Factbook 2013. Available online: http://www.icsg.org/index.php/component/jdownloads/finish/170/1188 (accessed on 22 October 2014).
- The Outlook for Metals Markets; World Bank: Washington, DC, USA, 2006. Available online: http://siteresources.worldbank.org/INTOGMC/Resources/outlook_for_metals_market.pdf (accessed on 22 October 2014).
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage-sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef]
- Lee, J.; Pandey, B.D. Bio-processing of solid wastes and secondary resources for metal extraction—A review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Krebs, W.; Brombacher, C.; Bosshard, P.P.; Bachofen, R.; Brandl, H. Microbial recovery of metals from solids. FEMS Microbiol. Rev. 1997, 20, 605–617. [Google Scholar] [CrossRef]
- Hoque, M.E.; Philip, O.J. Biotechnological recovery of heavy metals from secondary sources—An overview. Mater. Sci. Eng. C 2011, 31, 57–66. [Google Scholar] [CrossRef]
- Asghari, I.; Mousavi, I.M.; Amiri, F.; Tavassoli, S. Bioleaching of spent refinery catalysts. J. Ind. Eng. Chem. 2013, 19, 1069–1081. [Google Scholar] [CrossRef]
- Kelly, D.P.; Wood, A.P. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus. gen. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Rudolfs, W.; Helbronner, A. Oxidation of zinc sulfide by microorganisms. Soil Sci. 1922, 14, 459–464. [Google Scholar] [CrossRef]
- Colmer, A.R.; Temple, K.L.; Hinkle, H.E. An iron-oxidizing bacterium from the acid mine drainage of some bituminous coal mines. J. Bacteriol. 1950, 59, 317–328. [Google Scholar] [PubMed]
- Temple, K.L.; Colmer, A.R. The autotrophic oxidation of iron by a new bacterium: Thiobacillus ferrooxidans. J. Bacteriol. 1951, 62, 605–611. [Google Scholar] [PubMed]
- Leathen, W.W.; Braley, S.S., Sr.; McIntyre, L.D. The role of bacteria in the formation of acid from sulfuritic constituents associated with bituminous coal: II. Ferrous iron oxidizing bacteria. Appl. Microbiol. 1953, 1, 65–68. [Google Scholar] [PubMed]
- Bryner, L.C.; Beck, J.V.; Davis, D.B.; Wilson, D.G. Microorganisms in leaching sulfide minerals. Ind. Eng. Chem. 1954, 46, 2587–2592. [Google Scholar] [CrossRef]
- Bryner, L.C.; Anderson, R. Microorganisms in leaching sulfide minerals. Ind. Eng. Chem. 1957, 49, 1721–1724. [Google Scholar] [CrossRef]
- Bryner, L.C.; Jameson, A.K. Microorganisms in leaching sulfide minerals. Appl. Microbiol. 1958, 6, 281–287. [Google Scholar] [PubMed]
- Rawlings, D.E. Mesophilic autotrophic bioleaching bacteria: Description, physiology and role. In Biomining: Theory, Microbes and Industrial Processes; Rawlings, D.E., Ed.; Springer-Verlag: Berlin, Germany, 1997; pp. 229–245. [Google Scholar]
- Norris, P.R. Thermophiles and bioleaching. In Biomining: Theory, Microbes and Industrial Processes; Rawlings, D.E., Ed.; Springer-Verlag: Berlin, Germany, 1997; pp. 247–258. [Google Scholar]
- Hallberg, K.B.; Johnson, D.B. Biodiversity of acidophilic prokaryotes. Adv. Appl. Microbiol. 2001, 49, 37–84. [Google Scholar] [PubMed]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Bosecker, K. Leaching of lateritic nickel ores with heterotrophic microorganisms. In Fundamental and Applied Biohydrometallurgy; Lawrence, R.W., Branion, R.M.R., Ebner, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 367–382. [Google Scholar]
- Tzeferis, P.G. Leaching of a low-grade hematitic laterite ore using fungi and biologically produced acid metabolites. Int. J. Miner. Process. 1994, 42, 267–283. [Google Scholar] [CrossRef]
- Valix, M.; Usai, F.; Malik, R. Fungal bio-leaching of low-grade laterite ores. Miner. Eng. 2001, 14, 197–203. [Google Scholar] [CrossRef]
- Johnson, D.B.; Roberto, F.F. Heterotrophic acidophiles and their roles in the bioleaching of sulfide minerals. In Biomining: Theory, Microbes and Industrial Processes; Rawlings, D.E., Ed.; Springer-Verlag: Berlin, Germany, 1997; pp. 259–279. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv. Microb. Physiol. 2008, 54, 201–255. [Google Scholar]
- Ehrlich, H.L. Beginnings of rational bioleaching and highlights in the development of biohydrometallurgy: A brief history. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 102–112. [Google Scholar]
- Hallberg, K.B.; Johnson, D.B. Novel acidophiles isolated from moderately acidic mine drainage waters. Hydrometallurgy 2003, 71, 139–148. [Google Scholar] [CrossRef]
- Jerez, C.A. The use of genomics, proteomics and other OMICS technologies for the global understanding of biomining organisms. Hydrometallurgy 2008, 94, 162–169. [Google Scholar] [CrossRef]
- Johnson, D.B. Extremophiles: Acidic environments. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Elsevier: Oxford, UK, 2009; pp. 107–126. [Google Scholar]
- Watling, H.R.; Watkin, E.L.J.; Ralph, D.E. The resilience and versatility of acidophiles that contribute to the bio-assisted extraction of metals from mineral sulfides. Environ. Technol. 2010, 31, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.F.; Benjamin, M.M.; Ferguson, J.F. Adsorption and desorption of metals on ferrihydrite: Reversibility of the reaction and sorption properties of the regenerated solid. Environ. Sci. Technol. 1987, 21, 863–869. [Google Scholar] [CrossRef]
- Fuller, C.C.; Davis, J.A.; Waychunas, G.A. Surface chemistry of ferrihydrite: Part 2. Kinetics of arsenate adsorption and coprecipitation. Geochim. Cosmochim. Acta 1993, 57, 2271–2282. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhu, M.; Wei, Y.; Sun, Y. Fe(II)-induced transformation from ferrihydrite to lepidocrocite and goethite. J. Solid State Chem. 2007, 180, 2121–2128. [Google Scholar] [CrossRef]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminium hydroxysulfates from acidic sulfate waters. Rev. Mineral. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Gramp, J.P.; Jones, F.S.; Bigham, J.M.; Tuovinen, O.H. Monovalent cation concentrations determine the types of Fe(III) hydroxysulfate precipitates formed in bioleach solutions. Hydrometallurgy 2008, 94, 29–33. [Google Scholar] [CrossRef]
- Carlson, L.; Bigham, J.M.; Schwertmann, U.; Kyek, A.; Wagner, F. Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: A comparison with synthetic analogues. Environ. Sci. Technol. 2002, 36, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, K.; Sato, T.; Yanase, N.; Minati, J.; Yamada, H. Arsenic sorption on schwertmannite. Am. Mineral. 2004, 89, 1728–1734. [Google Scholar]
- Regenspurg, S.; Peiffer, S. Arsenate and chromate incorporation in schwertmannite. Appl. Geochem. 2005, 20, 1226–1239. [Google Scholar] [CrossRef]
- Acero, P.; Ayora, C.; Torrento, C.; Nieto, J.-M. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim. Cosmochim. Acta 2006, 70, 4130–4139. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Tuovinen, O.H. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidizing microorganisms. Mater. Sci. Eng. 2006, 26, 588–592. [Google Scholar] [CrossRef]
- Sanchez-España, J.; Yusta, I.; López, G.A. Schwertmannite to jarosite conversion in the water column of an acidic mine pit lake. Mineral. Mag. 2012, 76, 2659–2682. [Google Scholar] [CrossRef]
- Daoud, J.; Karamanev, D. Formation of jarosite during Fe2+ oxidation by Acidithiobacillus ferrooxidans. Miner. Eng. 2006, 19, 960–967. [Google Scholar] [CrossRef]
- Sand, W. Microbial mechanisms. In Microbially Influenced Corrosion of Materials; Heitz, E., Flemming, H.-C., Sand, W., Eds.; Springer: Heidelberg, Germany, 1996; pp. 15–25. [Google Scholar]
- Sand, W.; Jozsa, P.-G.; Manasch, R. Weathering microbiology. In Environmental Microbiology; Britton, G., Ed.; Wiley: New York, NY, USA, 2002; Volume 6, pp. 3364–3375. [Google Scholar]
- Williams, T.M.; Unz, R.F. Filamentous sulfur bacteria of activated sludge: Characterization of Thiothrix, Beggiatoa, and Eikelboom Type 021N strains. Appl. Environ. Microbiol. 1985, 49, 887–898. [Google Scholar] [PubMed]
- Grayston, S.J.; Nevell, W.; Wainwright, M. Sulphur oxidation by fungi. Trans. Br. Mycol. Soc. 1986, 87, 193–198. [Google Scholar] [CrossRef]
- Lang, E.; Jagnow, G. Fungi of a forest soil nitrifying at low pH values. FEMS Microbiol. Lett. 1986, 38, 257–265. [Google Scholar] [CrossRef]
- Mansch, R.; Bock, E. Biodeterioration of natural stone with special reference to nitrifying bacteria. Biodegradation 1998, 9, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ikehata, Y.; Ikenaga, Y.; Wakayama, M.; Moriguchi, M. Nitrite oxidation by heterotrophic bacteria under various nutritional and aerobic conditions. J. Ferment. Bioeng. 1996, 82, 613–617. [Google Scholar] [CrossRef]
- Schippers, A.; Rohwerder, T.; Sand, W. Intermediary sulfur compounds in pyrite oxidation: Implications for bioleaching and biodepyritization of coal. Appl. Microbiol. Biotechnol. 1999, 52, 104–110. [Google Scholar] [CrossRef]
- Pronk, J.T.; Meulenberg, R.; Hazeu, W.; Bos, P.; Kuenen, J.G. Oxidation of reduced inorganic sulfur compounds by acidophilic thiobacilli. FEMS Microbiol. Rev. 1990, 75, 293–306. [Google Scholar] [CrossRef]
- McNamara, C.J.; Mitchell, R. Microbial deterioration of historic stone. Front. Ecol. Environ. 2005, 3, 445–451. [Google Scholar] [CrossRef]
- De la Torre, M.A.; Gómez-Alarcón, G.; Vizcaino, C.; Garcia, M.T. Biochemical mechanisms of stone alteration carried out by filamentous fungi living in monuments. Biogeochemistry 1993, 19, 129–147. [Google Scholar] [CrossRef]
- Fischer, K.; Bipp, H.P. Removal of heavy metals from soil components and soils by natural chelating agents: Part II. Soil extraction by sugar acids. Water Air Soil Pollut. 2002, 138, 271–288. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Fley-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Sarniguet, A.; de Boer, W.; Leveau, J.H.J.; Frey-Klett, P. Efficient mineral weathering is a distinctive functional trait of the bacterial genus Collimonas. Soil Biol. Biochem. 2009, 41, 2178–2186. [Google Scholar] [CrossRef]
- Bennett, P.C.; Melcer, M.E.; Siegel, D.I.; Hassett, J.P. The dissolution of quartz in dilute aqueous solutions of organic acids at 25 °C. Geochim. Cosmochim. Acta 1988, 52, 1521–1530. [Google Scholar] [CrossRef]
- Blake, R.E.; Walter, L.M. Effects of organic acids on the dissolution of orthoclase at 80 °C and pH 6. Chem. Geol. 1996, 132, 91–102. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Neaman, A.; Brantley, S.L. Elemental release rates from dissolving basalt and granite with and without organic ligands. Am. J. Sci. 2009, 309, 633–660. [Google Scholar] [CrossRef]
- Welch, S.A.; Taunton, A.E.; Banfield, J.F. Effect of microorganisms and microbial metabolites on apatite dissolution. Geomicrobiol. J. 2002, 19, 343–367. [Google Scholar] [CrossRef]
- Zhang, H.; Bloom, P.R. Dissolution kinetics of hornblende in organic acid solutions. Soil Sci. Soc. Am. J. 1999, 63, 815–822. [Google Scholar] [CrossRef]
- Di Bonaventura, M.P.; Gallo, M.D.; Cacchio, P.; Ercole, C.; Lepidi, A. Microbial formation of oxalate films on monument surfaces: Bioprotection or biodeterioration? Geomicrobiol. J. 1999, 16, 55–64. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [PubMed]
- Sterflinger, K. Fungi as geologic agents. Geomicrobiol. J. 2000, 17, 97–124. [Google Scholar] [CrossRef]
- Alibhai, K.A.K.; Dudeney, A.W.L.; Leak, D.J.; Agatzini, S.; Tzeferis, P. Bioleaching and bioprecipitation of nickel and iron from laterites. FEMS Microbiol. Rev. 1993, 22, 87–93. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S.; Walubita, L.F. The fungal and chemolithotrophic leaching of nickel laterites—Challenges and opportunities. Hydrometallurgy 2010, 103, 150–157. [Google Scholar] [CrossRef]
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Boukhalfa, H.; Reiley, S.D.; Michalczyk, R.; Iyer, S.; Neu, M.P. Iron(III) coordination properties of a pyoverdin siderophore produced by Pseudomonas putida ATCC 33015. Inorg. Chem. 2006, 45, 5607–5616. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, O.W.; Bargar, J.R.; Sposito, G. Coupled biogeochemical cycling of iron and manganese as mediated by microbial siderophores. Biometals 2009, 22, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.M. Iron oxide dissolution and solubility in the presence of siderophores. Aquat. Sci. 2004, 66, 3–18. [Google Scholar] [CrossRef]
- Dehner, C.A.; Awaya, J.D.; Maurice, P.A.; du Bois, J.L. Roles of siderophores, oxalate and ascorbate in mobilization of iron from hematite by the aerobic bacterium Pseudomonas mendocina. Appl. Environ. Microbiol. 2010, 76, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Saal, L.B.; Duckworth, O.W. Synergistic dissolution of manganese oxides as promoted by siderophores and small organic acids. Soil Sci. Soc. Am. J. 2010, 74, 2021–2031. [Google Scholar] [CrossRef]
- Loukola-Ruskeeniemi, K.; Heino, T.; Talvitie, J.; Vanne, J. Base-metal-rich metamorphosed black shales associated with Proterozoic ophiolites in the Kainuu schist belt, Finland: A genetic link with the Outokumpu rock assemblage. Miner. Depos. 1991, 26, 143–151. [Google Scholar]
- Loukola-Ruskeeniemi, K. Geochemical evidence for hydrothermal origin of sulphur, base metals and gold in Proterozoic metamorphosed black shales, Kainuu and Outokumpu areas, Finland. Miner. Depos. 1991, 26, 152–164. [Google Scholar]
- Tariq, M.; Aziz, A.; Shafiq, M.; Sajjid, M.; Iqbal, M.M.; Muhammad, S. Characterization of black shale of Chimiari Khyber Pakthunkhawa region of Pakistan for its potential as multiminerals. Int. J. Sci. Res. 2013, 2, 231–238. [Google Scholar]
- Gouin, J.; Auge, T.; Bailly, L.; d’Hugues, P. Organic and mineral characteristics of Kupferschiefer ore from Lubin mine (Poland): Implications for bioleaching of the ore. In Digging Deeper; Andrew, C.J., Ed.; IAEG: Dublin, Ireland, 2007. [Google Scholar]
- Kamradt, A.; Borg, G.; Schaefer, J.; Kruse, S.; Fiedler, M.; Romm, P.; Schippers, A.; Gorny, R.; du Bois, M.; Bieligk, C.; et al. An integrated process for innovative extraction of metals from kupferschiefer mine dumps. Chem. Ing. Tech. 2012, 84, 1694–1703. [Google Scholar] [CrossRef]
- Matlakowska, R.; Ruszkowski, D.; Sklodowska, A. Microbial transformation of fossil organic matter of Kupferschiefer black shale—Elements mobilization from metalloorganic compounds and metalloporphyrins by a community of indigenous microorganisms. Physicochem. Probl. Miner. Process. 2013, 49, 223–231. [Google Scholar]
- Farbiszewska-Kiczma, J.; Farbiszewska, T.; Bak, M. Bioleaching of metals from Polish black shale in neutral medium. Physicochem. Probl. Miner. Process. 2004, 38, 273–280. [Google Scholar]
- Farbiszewska-Kiczma, J.; Farbiszewska, T. Isolation of bacteria that degrade organometallic compounds from metallic wastes. Physicochem. Probl. Miner. Process. 2005, 39, 263–267. [Google Scholar]
- Matlakowska, R.; Hallberg, K.B.; Sklodowska, A. Isolation and characterisation of microorganisms from copper-bearing black shale of Lubin mine, Poland. In Biohydrometallurgy: From Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; p. 580. [Google Scholar]
- Matlakowska, R.; Sklodowska, A. The culturable bacteria isolated from organic-rich black shale potentially useful in biometallurgical procedures. J. Appl. Microbiol. 2009, 107, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Matlakowska, R.; Narkiewicz, W.; Sklodowska, A. Biotransformation of organic-rich copper-bearing black shale by indigenous microorganisms isolated from Lubin mine (Poland). Environ. Sci. Technol. 2010, 44, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Itävaara, M.; Nyyssönen, M.; Kapanen, A.; Nousiainen, A.; Ahonen, L.; Kukkonen, I. Characterization of bacterial diversity to a depth of 1500 m in the Outokumpu deep borehole, Fennoscandian Shield. FEMS Microbiol. Ecol. 2011, 77, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, W.; Wang, S.; Sun, Z.; Lin, S.; Peng, X. Bacteria diversity, distribution and insight into their role in S and Fe biogeochemical cycling during black shale weathering. Environ. Microbiol. 2014, 16, 3533–3547. [Google Scholar] [CrossRef] [PubMed]
- Petsch, S.T.; Edwards, K.J.; Eglinton, T.I. Microbial transformations of organic matter in black shales and implications for global biogeochemical cycles. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 157–170. [Google Scholar] [CrossRef]
- Sadowski, Z.; Szubert, A.; Maliszewska, I.; Jazdzyk, E. A view on the organic matter and metalloporphyrins biodegradation as characteristic component of black shale ore. In Biohydrometallurgy: From Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; pp. 95–98. [Google Scholar]
- Matlakowska, R.; Sklodowska, A. Uptake and degradation of copper and cobalt porphyrins by indigenous microorganisms of Kupferschiefer (Fore-Sudetic Monocline, Poland). Hydrometallurgy 2010, 104, 501–505. [Google Scholar] [CrossRef]
- Matlakowska, R.; Sklodowska, A. Biodegradation of organic matter and release of heavy metals from the copper bearing black shale of Fore Sudetic Monocline (Poland). In Biohydrometallurgy: From Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; pp. 238–239. [Google Scholar]
- Matlakowska, R.; Sklodowska, A. Biodegradation of Kupferschiefer black shale organic matter (Fore-Sudetic Monocline, Poland) by indigenous microorganisms. Chemosphere 2011, 83, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Microbes and metals. Appl. Microbiol. Biotechnol. 1997, 48, 687–692. [Google Scholar] [CrossRef]
- Lyalikova, N.N.; Vedenina, I.Y.; Romanova, A.K. Assimilation of carbon dioxide by a culture of Stibiobacter senarmontii. Microbiology 1976, 45, 476–477. [Google Scholar]
- Ilyaletdinov, A.N.; Abdrashitova, S.A. Autotrophic arsenic oxidation by Pseudomonas arsenitoxidans culture. Mikrobiologiia 1981, 50, 197–204. [Google Scholar] [PubMed]
- Johnson, D.B.; Kanao, T.; Hedrich, S. Redox transformations of iron at extremely low pH: Fundamental and applied aspects. Front. Microbiol. 2012, 3, 96. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Grail, B.M.; Hallberg, K.B. A new direction for biomining: Extraction of metals by reductive dissolution of oxidized ores. Minerals 2013, 3, 49–58. [Google Scholar] [CrossRef]
- Johnson, D.B. Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 1995, 23, 205–218. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Techniques for detecting and identifying acidophilic mineral-oxidising microorganisms. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 237–262. [Google Scholar]
- Valenzuela, L.; Chi, A.; Beard, S.; Orell, A.; Guiliani, N.; Shabanowitz, J.; Hunt, D.F.; Jerez, C.A. Genomics, metagenomics and proteomics in biomining microorganisms. Biotechnol. Adv. 2006, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Shiers, D.W.; Ralph, D.E.; Watling, H.R. A comparative study of substrate utilisation by Sulfobacillus species in mixed ferrous ion and tetrathionate growth medium. Hydrometallurgy 2010, 104, 363–369. [Google Scholar] [CrossRef]
- Shiers, D.W.; Ralph, D.E.; Watling, H.R. The effects of nitrate on substrate utilisation by some iron(II)- and sulfur-oxidising Bacteria and Archaea. Hydrometallurgy 2014, 150, 259–268. [Google Scholar] [CrossRef]
- Suzuki, I.; Takeuchi, T.L.; Yuthasastrakosol, T.D.; Oh, J.K. Ferrous iron and sulfur oxidation and ferric iron reduction activities of Thiobacillus ferrooxidans are affected by growth on ferrous iron, sulfur or a sulfide ore. Appl. Environ. Microbiol. 1990, 56, 1620–1626. [Google Scholar] [PubMed]
- Sugio, T.; Tsujita, Y.; Katagiri, T.; Inagaki, K.; Tano, T. Reduction of Mo6+ with elemental sulfur by Thiobacillus ferrooxidans. J. Bacteriol. 1988, 170, 5956–5959. [Google Scholar] [PubMed]
- Sugio, T.; Tsujita, Y.; Inagaki, K.; Tano, T. Reduction of cupric ions with elemental sulfur by Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1990, 56, 693–696. [Google Scholar] [PubMed]
- Bridge, T.A.M.; Johnson, D.B. Reduction of soluble iron and reductive dissolution of ferric-iron containing minerals by moderately thermophilic iron-oxidizing bacteria. Appl. Environ. Microbiol. 1998, 64, 2181–2186. [Google Scholar] [PubMed]
- Hallberg, K.B.; Grail, B.M.; du Plessis, C.A.; Johnson, D.B. Reductive dissolution of ferric iron minerals: A new approach for Bioprocessing nickel laterites. Miner. Eng. 2011, 24, 620–624. [Google Scholar] [CrossRef]
- Johnson, D.B.; Stallwood, B.; Kimura, S.; Hallberg, K.B. Isolation and characterization of Acidicaldus organivorus, gen. nov., sp. nov.: A novel sulfur-oxidizing, ferric iron-reducing thermoacidophilic heterotrophic Proteobacterium. Arch. Microbiol. 2006, 185, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Coupland, K.; Johnson, D.B. Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol. Lett. 2008, 279, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bridge, T.A.M.; Johnson, D.B. Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol. J. 2000, 17, 193–206. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [PubMed]
- PAQUES Metal and Mining. Available online: http://en.paques.nl/your-sector/other/metal-and-mining (accessed on 22 October 2014).
- Watling, H. Adaptability of biomining organisms in hydrometallurgical processes. In Biohydrometallurgical Processes: A Practical Approach; Santos Sobral, L.G., Monteiro de Oliveira, D., Gomes de Souza, C.E., Eds.; CETEM/MCTI: Rio de Janeiro, Brazil, 2011; pp. 39–70. [Google Scholar]
- Hackl, R.P.; Wright, F.R.; Bruynesteyn, A. Bacteria for Oxidizing Multimetallic Sulphide Ores. US Patent 5,089,412, 18 February 1992. [Google Scholar]
- Van Aswegen, P.C.; Haines, A.K.; Marais, H.J. Design and operation of a commercial bacterial oxidation plant at Fairview. In Proceedings of the Randol Gold Conference, Perth, Australia, 28 October–1 November 1988; pp. 144–147.
- Williams, T.L. Factors affecting bacterial population dynamics at the Youanmi bacterial oxidation plant. In Biotechnology Comes of Age; Australian Minerals Foundation: Glenside, SA, Australia, 1997. [Google Scholar]
- Battaglia, F.; Morin, D.; Ollivier, P. Dissolution of cobaltiferous pyrite by Thiobacillus ferrooxidans and Thiobacillus thiooxidans: Factors influencing bacterial leaching efficiency. J. Biotechnol. 1994, 32, 11–16. [Google Scholar] [CrossRef]
- D’Hughes, P.; Cezac, P.; Cabral, T.; Battaglia, F.; Truong-Meyer, X.M.; Morin, D. Bioleaching of a cobaltiferous pyrite: A continuous laboratory-scale study at high solids concentration. Miner. Eng. 1997, 10, 507–527. [Google Scholar] [CrossRef]
- Heinzle, T.; Miller, D.; Nagel, V. Results of an integrated pilot plant operation using the BioNIC® process to produce nickel metal. In Biomine’ 99 and Water Management in Metallurgical Operations’ 99; AusIMM: Melbourne, Australia, 1999; pp. 16–25. [Google Scholar]
- Du Plessis, C.A.; Batty, J.D.; Dew, D.W. Commercial applications of thermophile bioleaching. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 57–80. [Google Scholar]
- Duncan, D.W.; Landesman, J.; Walden, C.C. Role of Thiobacillus ferrooxidans in the oxidation of sulfide minerals. Can. J. Microbiol. 1967, 13, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shiers, D.W.; Blight, K.R.; Ralph, D.E. Sodium sulphate and sodium chloride effects on batch culture of iron-oxidising bacteria. Hydrometallurgy 2005, 80, 75–82. [Google Scholar] [CrossRef]
- Suzuki, I.; Lee, D.; McKay, B.; Harahuc, L.; Oh, J.K. Effect of various anions, pH, and osmotic pressure on oxidation of elemental sulfur by Thiobacillus thiooxidans. Appl. Environ. Microbiol. 1999, 65, 5163–5168. [Google Scholar] [PubMed]
- Zammit, C.M.; Mutch, L.A.; Watling, H.R.; Watkin, E.L.J. The characterization of salt tolerance in biomining microorganisms and the search for novel salt tolerant strains. In Biohydrometallurgy: A Meeting Point between Microbial Ecology, Metal Recovery Processes and Environmental Remediation; Donati, E.R., Viera, M.R., Tavani, E.L., Giaveno, M.A., Lavalle, T.L., Chiaccharini, P.A., Eds.; TransTech Publications: Zurich, Switzerland, 2009; pp. 283–286. [Google Scholar]
- Gahan, C.S.; Sundkvist, J.E.; Dopson, M.; Sandström, Å. Effect of chloride on ferrous iron oxidation by a Leptospirillum ferriphilum-dominated chemostat culture. Biotechnol. Bioeng. 2010, 106, 422–431. [Google Scholar] [PubMed]
- Williams, T.L. BioHeapTM bacterial leaching of the Sherlock Bay Nickel Mine primary nickel-sulphide ore in saline water. In Proceedings of the ALTA Nickel-Cobalt Conference, Perth, Australia, 15–17 May 2006.
- Rautenbach, G.F.; Davis-Belmar, C.S.; Demergasso, C.S. A Method of Treating Sulphide Mineral. World Patent WO2010/009481-A, 21 January 2010. [Google Scholar]
- Patel, B.C.; Tipre, D.R.; Dave, S.R. Optimization of copper and zinc extraction from polymetallic bulk concentrate and ferric iron bioregeneration under metallic stress. Hydrometallurgy 2012, 117–118, 18–23. [Google Scholar] [CrossRef]
- Corbillon, M.S.; Olazabal, M.A.; Madariaga, J.M. Potentiometric study of aluminium fluoride complexation equilibria and definition of the thermodynamic model. J. Solut. Chem. 2008, 37, 567–579. [Google Scholar] [CrossRef]
- Radic, N.; Bralic, M. Aluminium fluoride complexation and its ecological importance in the aquatic environment. Sci. Total Environ. 1995, 172, 237–243. [Google Scholar] [CrossRef]
- Sicupira, L.; Veloso, T.; Reis, F.; Leao, V. Assessing metal recovery from low-grade copper ores containing fluoride. Hydrometallurgy 2011, 109, 202–210. [Google Scholar] [CrossRef]
- Soli, A.L.; Byrne, R.H. The hydrolysis and fluoride complexation behaviour of Fe(III) at 25 °C and 0.68 molal ionic strength. J. Solut. Chem. 1996, 25, 773–785. [Google Scholar] [CrossRef]
- Sundkvist, J.E.; Sandström, Å.; Gunneriusson, L.; Lindstrom, E.B. Fluorine toxicity in bioleaching systems. In Proceedings of the 16th International Biohydrometallurgy Symposium, Cape Town, South Africa, 25–29 September 2005; Harrison, S.T.L., Rawlings, D.E., Petersen, J., Eds.; IBS: Cape Town, South Africa, 2005; pp. 19–28. [Google Scholar]
- Razzell, W.E.; Trussell, P.C. Isolation and properties of an iron-oxidizing Thiobacillus. J. Bacteriol. 1963, 85, 595–603. [Google Scholar] [PubMed]
- Harahuc, L.; Lizama, H.; Suzuki, I. Effect of anions on selective solubilisation of zinc and copper in bacterial leaching of sulfide ores. Biotechnol. Bioeng. 2000, 69, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.F.; Noah, K.S.; Glenn, A.Q.; Stevens, C.J. Combined physical/microbial beneficiation of coal using the flood/drain bioreactor. Fuel Process. Technol. 1994, 40, 283–296. [Google Scholar] [CrossRef]
- Andrews, G.F.; Noah, K.S. The flood/drain bioreactor for coal and mineral processing. In Mineral Bioprocessing II; Holmes, D.S., Smith, R.W., Eds.; TMS: Warrendale, PA, USA, 1995; pp. 219–230. [Google Scholar]
- Andrews, G.F.; Stevens, C.J.; Glenn, A.; Noah, K.S. Microbial coal depyritization: Why and how? In Biohydrometallurgical Technologies; Torma, A.E., Wey, J.E., Lakshmanan, V.L., Eds.; TMS: Warrendale, PA, USA, 1993; pp. 381–391. [Google Scholar]
- Andrews, G. The optimal design of bioleaching processes. Miner. Process. Extr. Metall. Rev. 1998, 19, 149–165. [Google Scholar] [CrossRef]
- Tyagi, R.D.; Couillard, D.; Tran, F.T. Comparative study of bacterial leaching of metals from sewage sludge in continuous stirred tank and airlift reactors. Process Biochem. 1991, 26, 47–54. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lin, J.G. Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: Effects of sulfur concentration. Water Res. 2004, 38, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Puhakka, J.; Tuovinen, O.H. Biological leaching of sulfide minerals with the use of shake flask, aerated column, airlift reactor, and percolation techniques. Acta Biotechnol. 1986, 6, 345–354. [Google Scholar] [CrossRef]
- Miller, P.C.; Huberts, R.; Livesey-Goldblatt, E. The semicontinuous bacterial agitated leaching of nickel sulphide material. In Fundamental and Applied Biohydrometallurgy; Lawrence, R.W., Branion, R.M.R., Ebner, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 23–42. [Google Scholar]
- Sethurajan, M.; Aruliah, R.; Karthikeyan, O.P.; Balasubramanian, R. Bioleaching of copper from black shale ore using mesophilic mixed populations in an airlift reactor. Environ. Eng. Manag. J. 2012, 11, 1839–1848. [Google Scholar]
- Slavkina, O.V.; Fomchencko, N.V.; Biriukov, V.V.; Arkhipov, M.Y. Study on bacterial leaching of copper-zinc ore concentrate. 3. Experimental trial of two-stage recirculation technology of copper-zinc concentrate leaching. Biotekhnologiya 2005, 3, 48–54. [Google Scholar]
- Li, D.; Li, D.W.; Zhang, S.J. The study of toxic elements removal and valuable metals recovery from mine tailings in gas-liquid-solid internal circulation bioreactor. Res. J. Chem. Environ. 2011, 15, 990–993. [Google Scholar]
- Loi, G.; Mura, A.; Trois, P.; Rossi, G. Bioreactor performance vs. Solids concentration in coal biodepyritization. Fuel Process. Technol. 1994, 40, 251–260. [Google Scholar] [CrossRef]
- Loi, G.; Trois, P.; Rossi, G. Biorotor®: A new development for biohydrometallurgical processing. In Biohydrometallurgical Processing; Vargas, T., Jerez, C.A., Wiertz, J.V., Toledo, H., Eds.; University of Chile: Santiago, Chile, 1995; Volume I, pp. 263–271. [Google Scholar]
- Rossi, G. The design of bioreactors. Hydrometallurgy 2001, 59, 217–231. [Google Scholar] [CrossRef]
- Loi, G.; Rossi, A.; Trois, P.; Rossi, G. Continuous revolving barrel bioreactor tailored to the bioleaching microorganisms. Miner. Metall. Process. 2006, 23, 196–202. [Google Scholar]
- Groudev, S.; Groudeva, V.I. Microbial communities in four industrial copper dump leaching operations in Bulgaria. FEMS Microbiol. Rev. 1993, 11, 261–267. [Google Scholar] [CrossRef]
- Brierley, J.A.; Brierley, C.L. Present and future commercial applications of biohydrometallurgy. Hydrometallurgy 2001, 59, 233–239. [Google Scholar] [CrossRef]
- Duncan, D.W.; Bruynesteyn, A. Enhancing bacterial activity in a uranium mine. Can. Min. Metall. Bull. 1971, 64, 32–36. [Google Scholar]
- McCready, R.G.L.; Gould, W.D. Bioleaching of uranium at Denison Mines. In Biohydrometallurgy; Sally, J., McCready, R.G.L., Wichlacz, P.L., Eds.; Canmet: Ottawa, ON, Canada, 1989; pp. 477–485. [Google Scholar]
- Domic, E. A review of the development and current status of copper bioleaching operations in Chile: 25 years of successful commercial implementation. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 81–95. [Google Scholar]
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.; Seal, T.; Brierley, J.A. Whole-ore heap biooxidation of sulfidic gold-bearing ores. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 113–138. [Google Scholar]
- Readett, D.; Fox, F. Commercialisation of Ni heap leaching at Murrin Murrin operations. In Proceedings of the XXV International Mineral Processing Congress, Brisbane, Australia, 6–10 September 2010; AusIMM: Melbourne, Australia, 2010; pp. 3611–3616. [Google Scholar]
- Fernandes, H.M.; Lamego Simoes Filho, F.F.; Perez, V.; Ramalho Franklin, M.; Gomiero, L.A. Radioecological characterization of a uranium mining site located in a semi-arid region of Brazil. J. Environ. Radioact. 2006, 88, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Lizama, H.M.; Harlamovs, J.R.; Belanger, S.; Brienne, S.H. The Teck Cominco HydrozincTM Process. In Hydrometallurgy 2003; Young, C., Alfantazi, A.M., Anderson, C.G., Dreisinger, D.B., Harris, B., James, A., Eds.; TMS: Warrendale, PA, USA, 2003; Volume 2, pp. 1503–1516. [Google Scholar]
- Harvey, T.J.; Bath, M. The Geobiotics GEOCOAT® technology—Progress and challenges. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 97–112. [Google Scholar]
- Gunn, M.; Tittes, P.; Harvey, P.; Carretero, E.; da Silva, P.M.; de Souza, J.P. Laboratory and demonstration-scale operation of the Caraiba heap leach using GEOCOAT®. In Hydrocopper 2009; Casas, J., Domic, E., Eds.; Gecamin: Santiago, Chile, 2009; pp. 352–364. [Google Scholar]
- Williams, T.L.; Gunn, M.J.; Jaffer, A.; Harvey, P.I.; Tittes, P.R. The application of Geobiotics LLC GEOCOAT® technology to the bacterial oxidation of a refractory arsenopyrite gold concentrate. In Hydrometallurgy 2008; Young, C.A., Taylor, P.R., Anderson, C.G., Choi, Y., Eds.; SME: Littleton, CO, USA, 2008; pp. 474–483. [Google Scholar]
- Sampson, M.I.; van der Merwe, J.W.; Harvey, T.J.; Bath, M.D. Testing the ability of a low-grade sphalerite concentrate to achieve autothermality during biooxidation heap leaching. Miner. Eng. 2005, 18, 427–437. [Google Scholar] [CrossRef]
- Soleimani, M.; Petersen, J.; Roostaazad, R.; Hosseini, S.; Mousavi, S.M.; Najafi, A.; Kazemi Vasiri, A. Leaching of a zinc ore and concentrate using the Geocoat™ technology. Miner. Eng. 2011, 24, 64–69. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Competitive bioleaching of pyrite and chalcopyrite. Hydrometallurgy 2006, 83, 40–49. [Google Scholar] [CrossRef]
- Mwase, J.M.; Petersen, J.; Eksteen, J.J. A conceptual flow-sheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate. Hydrometallurgy 2012, 111–112, 129–135. [Google Scholar] [CrossRef]
- Cope, L.W. Vat leaching—An overlooked process. Eng. Min. J. 1999, 200, 17–24. [Google Scholar]
- Mackie, D.; Trask, F. Continuous vat leaching—First copper pilot trials. In ALTA Copper-X, Perth, Australia; ALTA Metallurgical Services: Melbourne, Australia, 2009. [Google Scholar]
- Schlitt, J.; Johnston, A. The Marcobre vat leach system: A new look at an old process. In Proceedings of Copper 2010, Hamburg, Germany, 6–10 June 2010; GDMB: Clausthal-Zellerfeld, Germany, 2010; Volume 5, pp. 2039–2057. [Google Scholar]
- Van Aswegen, P.C.; van Niekerk, J.; Olivier, W. The BIOX™ process for the treatment of refractory gold concentrates. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 1–33. [Google Scholar]
- Miller, P.C. The design and operating practice of bacterial oxidation plant using moderate thermophiles (The BacTech Process). In Biomining: Theory, Microbes and Industrial Processes; Rawlings, D.E., Ed.; Springer-Verlag: Berlin, Germany, 1997; pp. 81–102. [Google Scholar]
- Rorke, G.V.; Basson, P.; Miller, D.M. Advancements in thermophile bioleaching technology. In ALTA Nickel/Cobalt-7 Technical Proceedings; ALTA: Melbourne, Australia, 2001. [Google Scholar]
- Batty, J.D.; Rorke, G.V. Development and commercial demonstration of the BioCOP™ thermophile process. Hydrometallurgy 2006, 83, 83–89. [Google Scholar] [CrossRef]
- Wang, S. Copper leaching from chalcopyrite concentrates. JOM 2005, 57, 48–51. [Google Scholar] [CrossRef]
- Miller, D.M.; Dew, D.W.; Norton, A.E.; Johns, M.W.; Cole, P.M.; Benetis, G.; Dry, M. The BioNIC Process: Description of the process and presentation of pilot plant results. In Nickel/Cobalt 97; Cooper, W.C., Mihaylov, I., Eds.; CIM: Montreal, QC, Canada, 1997; Volume 1, pp. 97–110. [Google Scholar]
- Gilbertson, B.P. Creating value through innovation: Biotechnology in mining. Miner. Process. Extr. Metall. 2000, 109, 61–67. [Google Scholar] [CrossRef]
- Morin, D.H.R.; d’Hugues, P. Bioleaching of a cobalt-containing pyrite in stirred tank reactors: A case study from laboratory scale to industrial application. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer-Verlag: Berlin, Germany, 2007; pp. 35–55. [Google Scholar]
- Romero, R.; Palencia, I.; Carranza, F. Silver catalysed IBES process: Application to a Spanish copper-zinc sulphide concentrate: Part 3. Selection of the operational parameters for a continuous pilot plant. Hydrometallurgy 1998, 49, 75–86. [Google Scholar] [CrossRef]
- Frias, C.; Carranza, F.; Sanchez, F.; Mazuelos, A.; Frades, M.; Romero, R.; Diaz, G.; Iglesias, N. New developments in indirect bioleaching of zinc and lead sulphide concentrates. In Hydrometallurgy 2008; Young, C.A., Taylor, P.R., Anderson, C.G., Choi, Y., Eds.; SME: Littleton, CO, USA, 2008; pp. 497–505. [Google Scholar]
- Zafiratos, J.G.; Agatzini-Leonardou, S. Aerobic and anaerobic leaching of manganese. In Biohydrometallurgy: A Sustainable Technology in Evolution; Tsezos, M., Hatzikioseyian, A., Remoundaki, E., Eds.; National Technical University of Athens: Athens, Greece, 2004; pp. 41–54. [Google Scholar]
- Ritcey, G.M. Solvent extraction in hydrometallurgy: Present and future. Tsinghua Sci. Technol. 2006, 11, 137–152. [Google Scholar] [CrossRef]
- Alexandros, S.D. Ion-exchange resins: A Retrospective from Industrial and Engineering Chemistry Research. Ind. Eng. Chem. Res. 2009, 48, 388–398. [Google Scholar] [CrossRef]
- Sole, K.C.; Cole, P.M.; Feather, A.M.; Kotze, M.H. Solvent extraction and ion exchange applications in Africa’s resurging uranium industry: A review. Solvent Extr. Ion Exch. 2011, 29, 868–899. [Google Scholar] [CrossRef]
- Van Deventer, J. Selected ion exchange applications in the hydrometallurgical industry. Solvent Extr. Ion Exch. 2011, 29, 695–718. [Google Scholar] [CrossRef]
- Torma, A.E. Microbiological oxidation of synthetic cobalt, nickel and zinc sulfides by Thiobacillus ferrooxidans. Rev. Can. Biol. 1971, 30, 209–216. [Google Scholar] [PubMed]
- Third, K.A.; Cord-Ruwisch, R.; Watling, H.R. The role of iron-oxidizing bacteria in stimulation or inhibition of chalcopyrite bioleaching. Hydrometallurgy 2000, 57, 225–233. [Google Scholar] [CrossRef]
- Fowler, T.A.; Crundwell, F.K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: Bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentrations of ferrous ions. Appl. Environ. Microbiol. 1999, 65, 5285–5292. [Google Scholar] [PubMed]
- Zhang, G.; Fang, Z. The contribution of direct and indirect actions in bioleaching of pentlandite. Hydrometallurgy 2005, 80, 59–66. [Google Scholar] [CrossRef]
- King, A.J.; Budden, J.R. The bacterial oxidation of nickel and cobalt polymetallic concentrates. In Proceedings of the Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, Perth, Australia, 13–14 May 1996; ALTA Metallurgical Services: Melbourne, Australia, 1996. [Google Scholar]
- Gómez, C.; Blázquez, M.L.; Ballester, A. Bioleaching of a Spanish complex sulphide ore bulk concentrate. Miner. Eng. 1999, 12, 93–106. [Google Scholar] [CrossRef]
- Pradhan, D.; Kim, D.J.; Chaudhury, G.R. Dissolution kinetics of complex sulfides acidophilic microorganisms. Jpn. Inst. Met. Mater. Trans. 2010, 51, 413–419. [Google Scholar]
- Kim, D.J.; Pradhan, D.; Roy Chaudhury, G.; Ahn, J.G.; Lee, S.W. Bioleaching of complex sulfides concentrate and correlation of leaching parameters using multivariate data analysis technique. Jpn. Inst. Met. Mater. Trans. 2009, 50, 2318–2322. [Google Scholar]
- Wang, Y.; Su, L.; Zeng, W.; Qiu, G.; Wan, L.; Chen, X.; Zhou, H. Optimization of copper extraction for bioleaching of complex Cu-polymetallic concentrate by moderate thermophiles. Trans. Nonferr. Met. Soc. China 2014, 24, 1161–1170. [Google Scholar] [CrossRef]
- Uryga, A.; Sadowski, Z.; Grotowski, A. Bioleaching of cobalt from mineral products. Physicochem. Probl. Miner. Process. 2004, 38, 291–299. [Google Scholar]
- Tasa, A.; Garcia, O.; Bigham, J.M.; Vuorinen, A.; Tuovinen, O.H. Acid and biological leaching of a black shale from Toolse, Estonia. In Biohydrometallurgical Processing; Vargas, T., Jerez, C.A., Wiertz, J.V., Toledo, H., Eds.; University of Chile: Santiago, Chile, 1995; Volume I, pp. 229–238. [Google Scholar]
- Abdollahi, H.; Shaefaei, S.Z.; Noaparast, M.; Manafi, Z.; Aslan, N. Bio-dissolution of Cu, Mo and Re from molybdenite concentrate using a mix mesophilic microorganism in shake flask. Trans. Nonferr. Met. Soc. China 2013, 23, 219–230. [Google Scholar] [CrossRef]
- Gericke, M.; Muller, H.H.; van Staden, P.J.; Pinches, A. Development of a tank bioleaching process for the treatment of complex Cu-polymetallic concentrates. Hydrometallurgy 2008, 94, 23–28. [Google Scholar] [CrossRef]
- Mehta, K.D.; Pandey, B.D. Bioleaching of a copper sulphide concentrate by two different strains of acidophilic bacteria. Int. J. Metall. Eng. 2012, 1, 83–86. [Google Scholar]
- Norris, P.R.; Burton, N.P.; Clark, D.A. Mineral sulfide concentrate leaching in high temperature bioreactors. Miner. Eng. 2013, 48, 10–19. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Biryukov, V.V. A two-stage technology for bacterial and chemical leaching of copper-zinc raw materials by Fe3+ ions with their subsequent regeneration by chemolithotrophic bacteria. Appl. Biochem. Microbiol. 2009, 1, 56–60. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Muravyev, M.I.; Kondrat’eva, T.F. Two-stage bacterial-chemical oxidation of refractory gold-bearing sulfidic concentrates. Hydrometallurgy 2010, 101, 28–34. [Google Scholar] [CrossRef]
- Patel, B.C.; Tipre, D.R.; Dave, S.R. Development of Leptospirillum ferriphilum dominated consortium for ferric iron regeneration and metal bioleaching under extreme stresses. Bioresour. Technol. 2012, 118, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joulian, C.; Gouin, J.; Ibáñez, A.; Augé, T.; Morin, D.; d’Hugues, P. Bioleaching of an organic-rich polymetallic concentrate using stirred-tank technology. Hydrometallurgy 2009, 99, 137–143. [Google Scholar] [CrossRef]
- Conic, V.T.; Cvetkovski, V.B.; Stanojevich Simsic, Z.S.; Dragulovic, S.S.; Ljubomirovic, Z.S.; Cvetkovska, M.; Vukovic, M.N. Bioleaching of Zn-Pb-Ag sulphidic concentrate. In Proceedings of the 15th International Research/Expert Conference “Trends in the Development of Machinery and Associated Technology” (TMT 2011), Prague, Czech Republic, 12–18 September 2011.
- Askari Zamani, M.A.; Hiroyoshi, N.; Tsunekawa, M.; Vaghar, R.; Oliazadeh, M. Bioleaching of Sarcheshmeh molybdenite concentrate for extraction of rhenium. Hydrometallurgy 2005, 80, 23–31. [Google Scholar] [CrossRef]
- Askari Zamani, M.A.; Vaghar, R.; Oliazadeh, M. Selective copper dissolution during bioleaching of molybdenite concentrate. Int. J. Miner. Process. 2006, 81, 105–112. [Google Scholar] [CrossRef]
- Langhans, D.L.; Baglin, E.G. Biological oxidation of a platinum-group metal flotation concentrate and converter matte. In Biohydrometallurgical Technologies; Torma, A.E., Wey, J.E., Lakshmanan, V.L., Eds.; TMS: Warrendale, PA, USA, 1993; pp. 315–325. [Google Scholar]
- Romano, P.; Blázquez, M.L.; Alguacil, F.J.; Muñoz, J.A.; Ballester, A.; González, F. Comparative study on the selective chalcopyrite bioleaching of a molybdenite concentrate with mesophilic and thermophilic bacteria. FEMS Microbiol. Lett. 2001, 196, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dymov, I.; Ferron, C.J.; Philips, W. The development of a hybrid biological leaching—Pressure oxidation process for auriferous arsenopyrite/pyrite feedstocks. In Biohydrometallurgy: A Sustainable Technology in Evolution; Tsezos, M., Hatzikioseyian, A., Remoundaki, E., Eds.; National Technical University of Athens: Athens, Greece, 2004; pp. 377–386. [Google Scholar]
- Tipre, D.R.; Vora, S.B.; Dave, S.R. Comparison of air-lift and stirred tank batch and semicontinuous bioleaching of polymetallic bulk concentrate. In Biohydrometallurgy: A Sustainable Technology in Evolution; Tsezos, M., Hatzikioseyian, A., Remoundaki, E., Eds.; National Technical University of Athens: Athens, Greece, 2004; pp. 211–218. [Google Scholar]
- Tipre, D.R.; Dave, S.R. Bioleaching process for Cu-Pb-Zn bulk concentrate at high pulp density. Hydrometallurgy 2004, 75, 37–43. [Google Scholar] [CrossRef]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl. Environ. Microbiol. 2003, 69, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, T.A.; Melamud, V.S.; Savari, E.E.; Sedel’nikova, G.V.; Kondrat’eva, T.F. Species and strain composition of microbial associations oxidizing different types of gold-bearing concentrates. Appl. Microbiochem. Microbiol. 2012, 46, 497–504. [Google Scholar] [CrossRef]
- D’Hugues, P.; Joulian, C.; Spolaore, P.; Michel, C.; Garrido, F.; Morin, D. Continuous bioleaching of a pyrite concentrate in stirred reactors: Population dynamics and exopolysaccharides production vs. Bioleaching performances. Hydrometallurgy 2008, 94, 34–41. [Google Scholar] [CrossRef]
- Hao, C.; Wang, L.; Dong, H.; Zhang, H. Succession of acidophilic bacterial community during bio-oxidation of refractory gold-containing sulfides. Geomicrobiol. J. 2010, 27, 683–691. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Zhuang, T.; Qin, W.; Zhu, S.; Qiu, G. Bioleaching of Pb-Zn-Sn chalcopyrite concentrate in tank bioreactor and microbial community succession analysis. Trans. Nonferr. Met. Soc. China 2013, 23, 3758–3762. [Google Scholar] [CrossRef]
- Attia, Y.A.; El-Zeky, M. Effects of galvanic interactions on sulfides on extraction of precious metals from refractory complex sulfides by bioleaching. Int. J. Miner. Process. 1990, 30, 99–111. [Google Scholar] [CrossRef]
- D’Hugues, P.; Norris, P.R.; Hallberg, K.B.; Sanchez, F.; Langwaldt, J.; Grotowski, A.; Chmielewski, T.; Groudev, S.; Bioshale Consortium. Bioshale FP6 European project: Exploiting black shale ores using biotechnologies? Miner. Eng. 2008, 21, 111–120. [Google Scholar] [CrossRef]
- Langwaldt, J. Bioleaching of multimetal black shale by thermophilic micro-organisms. In Biohydrometallurgy: From Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; p. 167. [Google Scholar]
- Spolaore, P.; Joulian, C.; Gouin, J.; Sanchez, F.; Auge, T.; Morin, D.; d’Hugues, P. Continuous bioleaching of a polymetallic black shale concentrate using the stirred tank technology. In Proceedings of the XXIV International Mineral Processing Congress, Beijing, China, 24–28 September 2008; Wang, D., Sun, C., Wang, F., Zhan, L., Han, L., Eds.; Science Press: Beijing, China, 2008; pp. 2576–2584. [Google Scholar]
- Spolaore, P.; Joulian, C.; Ménard, Y.; d’Hugues, P. Non-traditional operating conditions for a copper concentrate continuous bioleaching. In BioHydromet 10; MEI: Falmouth, UK, 2010; pp. 156–174. [Google Scholar]
- Beane, R.; Ramey, D. In-situ leaching at the San Manuel porphyry copper deposit, Arizona, USA. In Copper 95—Cobre 95; Cooper, W.C., Dreisinger, D.B., Dutrizac, J.E., Hein, H., Ugarte, G., Eds.; MetSoc CIM: Montreal, QC, Canada, 1995; Volume III, pp. 363–375. [Google Scholar]
- Rossi, G. Potassium recovery through leucite bioleaching: Possibilities and limitations. In Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena; Murr, L.E., Torma, A.E., Brierley, J.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 297–319. [Google Scholar]
- Gallant, A.; Wadden, D.G. The in-place leaching or uranium at Denison Mines. Can. Metall. Q. 1984, 24, 127–134. [Google Scholar]
- Groudeva, V.I.; Groudev, S.N. Combined bacterial and chemical leaching of a polymetallic sulfide ore. In Mineral Bioprocessing; Smith, R.W., Misra, M., Eds.; TMS: Warrendale, PA, USA, 1991; pp. 153–161. [Google Scholar]
- Korehi, H.; Schippers, A. Bioleaching of a marine hydrothermal sulfide ore with mesophiles, moderate thermophiles and thermophiles. In Integrating Scientific and Industrial Knowledge on Biohydrometallurgy; Guiliani, N., Demergasso, C., Quatrini, R., Remonsellez, F., Davis-Belmar, C., Levican, G., Parada, P., Barahona, C., Zale, R., Eds.; TransTech Publications: Zurich, Switzerland, 2013; pp. 229–232. [Google Scholar]
- Hunter, C. BioHeap™ leaching of a primary nickel-copper sulphide ore. In Nickel-Cobalt-8 Technical Proceedings, Perth, Australia; ALTA Metallurgical Services: Melbourne, Australia, 2002. [Google Scholar]
- Riekkola-Vanhanen, M. Talvivaara Sotkamo mine—Bioleaching of a polymetallic nickel ore in subarctic climate. Nova Biotechnol. 2010, 10, 7–14. [Google Scholar]
- Wen, J.K.; Ruan, R.; Guo, X.J. Heap leaching—An option of treating nickel sulfide ore and laterite. In Proceedings of the Nickel/Cobalt Conference, Perth, Australia, 15–17 May 2006; ALTA Metallurgical Services: Melbourne, Australia, 2006. [Google Scholar]
- Norton, A.E.; Coetzee, J.J.; Barnett, C.C. BioNIC®: An economically competitive process for the biological extraction of nickel. In Proceedings of the Nickel/Cobalt Pressure Leaching and Hydrometallurgy Forum, Perth, Australia, 25–27 May 1998; Metallurgical Services: Melbourne, Australia, 1998. [Google Scholar]
- Watling, H.R.; Elliot, A.D.; Maley, M.; van Bronswijk, W.; Hunter, C. Leaching of a low-grade, copper-nickel sulfide ore. 1. Key parameters impacting on Cu recovery during column bioleaching. Hydrometallurgy 2009, 97, 204–212. [Google Scholar] [CrossRef]
- Maley, M.; van Bronswijk, W.; Watling, H.R. Leaching of a low-grade, copper-nickel sulfide ore. 2. Impact of aeration and pH on Cu recovery during abiotic leaching. Hydrometallurgy 2006, 98, 66–72. [Google Scholar] [CrossRef]
- Maley, M.; van Bronswijk, W.; Watling, H.R. Leaching of a low-grade, copper-nickel sulfide ore. 3. Interactions of Cu with selected sulfide minerals. Hydrometallurgy 2009, 98, 73–80. [Google Scholar] [CrossRef]
- Elliot, A.D.; Watling, H.R. Chalcopyrite formation through the metathesis of pyrrhotite with aqueous copper. Geochim.Cosmochim. Acta 2011, 75, 2103–2118. [Google Scholar] [CrossRef]
- Cameron, R.A.; Lastra, R.; Mortazavi, S.; Bedard, P.L.; Morin, L.; Gould, W.D.; Kennedy, K.J. Bioleaching of a low-grade ultramafic nickel sulphide ore in stirred-tank reactors at elevated pH. Hydrometallurgy 2009, 97, 213–220. [Google Scholar] [CrossRef]
- Cameron, R.A.; Lastra, R.; Mortazavi, S.; Gould, W.D.; Thibault, Y.; Bédard, P.L.; Morin, L.; Kennedy, K.J. Elevated-pH bioleaching of a low-grade ultramafic nickel sulphide ore in stirred-tank reactors at 5 to 45 °C. Hydrometallurgy 2009, 99, 77–83. [Google Scholar] [CrossRef]
- Cameron, R.A.; Yeung, C.W.; Greer, C.W.; Gould, W.D.; Mortazavi, S.; Bédard, PL.; Morin, L.; Lortie, L.; Dinardo, O.; Kennedy, K.J.; et al. The bacterial structure during bioleaching of a low-grade nickel sulphide ore in stirred tank reactors at different combinations of temperature and pH. Hydrometallurgy 2010, 104, 207–215. [Google Scholar] [CrossRef]
- Cameron, R.A.; Lastra, R.; Gould, W.D.; Mortazavi, S.; Thibault, Y.; Bédard, P.L.; Morin, L.; Koren, D.W.; Kennedy, K.J. Bioleaching of six nickel sulphide ores with differing mineralogies in stirred tank reactors at 30 °C. Miner. Eng. 2013, 49, 172–183. [Google Scholar] [CrossRef]
- Qin, W.; Zhen, S.; Yan, Z.; Campbell, M.; Wang, J.; Liu, K.; Zhang, Y. Heap bioleaching of a low-grade nickel-bearing sulfide ore containing high levels of magnesium as olivine, chlorite and antigorite. Hydrometallurgy 2009, 98, 58–65. [Google Scholar] [CrossRef]
- Miller, P.C. Large scale bacterial leaching of a copper-zinc ore in situ. In Fundamental and Applied Biohydrometallurgy; Lawrence, R.W., Branion, R.M.R., Ebner, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 215–239. [Google Scholar]
- Oros, V.; Peterfi, M.; Bivolaru, M.; Kovacs, S.; Straut, I.; Jelea, M.; Hudrea, I. Production of copper and zinc by microbial in situ stope leaching at Ilba mine, (Romania). In Proceedings of the 9th International Symposium on Biohydrometallurgy, Troia, Portugal, 9–13 September 1991; Duarte, J.C., Lawrence, R.W., Eds.; Forbitec: Queluz, Portugal, 1991. [Google Scholar]
- Sand, W.; Hallmann, R.; Rohde, K.; Sobotke, B.; Wentzien, S. Controlled microbiological in-situ stope leaching of a sulphidic ore. Appl. Microbiol. Biotechnol. 1993, 40, 421–426. [Google Scholar] [CrossRef]
- Krafft, C.; Hallberg, R.O. Bacterial leaching of two Swedish zinc sulfide ores. FEMS Microbiol. Rev. 1993, 11, 121–128. [Google Scholar] [CrossRef]
- Brauckmann, B.; Poppe, W.; Beyer, W.; Lerche, R.; Steppke, H.D. Investigations of increased biological in-situ leaching of the “Old Deposit” of the Preussag Rammelsberg ore mine. In Biohydrometallurgy; Norris, P.R., Kelly, D.P., Eds.; Science and Technology Letters: Kew, UK, 1988; pp. 521–523. [Google Scholar]
- Burton, C.; Cowman, S.; Heffernan, J.; Thorne, B. In-situ bioleaching of sulphide ores at Avoca, Ireland: Part I. Development, characterization and operation of a medium-scale (6000 t) experimental leach site. In Recent Progress in Biohydrometallurgy; Rossi, G., Torma, A.E., Eds.; Associazone Mineraria Sarda: Iglesias, Italy, 1983; pp. 243–264. [Google Scholar]
- Zhen, S.; Qin, W.; Yan, Z.; Zhang, Y.; Wang, J.; Ren, L. Bioleaching of low-grade nickel sulfide mineral in column reactor. Trans. Nonferr. Met. Soc. China 2008, 18, 1480–1484. [Google Scholar] [CrossRef]
- Giaveno, A.; Lavalle, L.; Chiacchiarini, P.; Donati, E. Bioleaching of zinc from low-grade complex sulfide ores in an airlift by isolated Leptospirillum ferrooxidans. Hydrometallurgy 2007, 89, 117–126. [Google Scholar] [CrossRef]
- Chen, J.W.; Gao, C.J.; Zhang, Q.X.; Xiao, L.S.; Zhang, G.Q. Leaching of nickel-molybdenum sulfide ore in membrane biological reactor. Trans. Nonferr. Met. Soc. China 2011, 21, 1395–1401. [Google Scholar] [CrossRef]
- Lizama, H.M.; Oh, J.K.; Takeuchi, T.L.; Suzuki, I. Bacterial leaching of copper and zinc from a sulfide ore by a mixed culture of Thiobacillus ferrooxidans and Thiobacillus thiooxidans in laboratory scale and pilot plant scale columns. In Biohydrometallurgy; Salley, J., McCready, R.G.L., Wichlacz, P.L., Eds.; Canmet: Ottawa, ON, Canada, 1989; pp. 519–531. [Google Scholar]
- Groudev, S.N. Complex utilization of polymetallic sulphide ores by means of combined bacterial and chemical leaching. In Harnessing Biotechnology for the 21st Century; Ladsich, M.R., Bose, A., Eds.; American Chemical Society: Washington, DC, USA, 1992; pp. 454–457. [Google Scholar]
- Sandström, Å.; Petersson, S. Bioleaching of a complex sulphide ore with moderate thermophilic and extreme thermophilic microorganisms. Hydrometallurgy 1997, 46, 181–190. [Google Scholar] [CrossRef]
- Liao, M.X.; Deng, T.L. Zinc and lead from complex sulfides by sequential bioleaching and acidic brine leach. Miner. Eng. 2004, 17, 17–22. [Google Scholar] [CrossRef]
- Radio Hill and Sholl Heap Leaching Project. Available online: www.foxresources.com.au/radio-hill-sholl-heap.asp (accessed on 22 October 2014).
- Lippmaa, E.; Maremae, E.; Pihlak, A.T. Resources, production and processing of Baltoscandian multimetal black shales. Oil Shale 2011, 28, 68–77. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Watling, H.R.; Collinson, D.M.; Shiers, D.W.; Bryan, C.G.; Watkin, E.L.J. Effects of temperature and solids loading on microbial community structure during batch culture on a polymetallic ore. Miner. Eng. 2013, 48, 68–76. [Google Scholar] [CrossRef]
- Watling, H.R.; Collinson, D.M.; Fjastad, S.; Kaksonen, A.H.; Li, J.; Morris, C.; Perrot, F.A.; Rea, S.M.; Shiers, D.W. Column bioleaching of a polymetallic ore: Effects of pH and temperature on metal extraction and microbial community structure. Miner. Eng. 2014, 58, 90–99. [Google Scholar] [CrossRef]
- Choi, M.S.; Cho, K.S.; Kim, D.S.; Ryu, H.W. Bioleaching of uranium from low-grade schists by Acidithiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 2005, 21, 377–380. [Google Scholar] [CrossRef]
- Anjum, F.; Bhatti, H.N.; Ambreen, A. Bioleaching of black shale by Acidithiobacillus ferrooxidans. Asian J. Chem. 2009, 21, 5251–5266. [Google Scholar]
- Pal, S.; Pradhan, D.; Das, T.; Sukla, L.B.; Roy Chaudhury, G. Bioleaching of low-grade uranium ore using Acidithiobacillus ferrooxidans. Indian J. Microbiol. 2010, 50, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Riekkola Vanhanen, M.; Heimala, S. Study of the bioleaching of a nickel-containing black-schist ore. In Biohydrometallurgy and the Environment toward the Mining of the 21st Century; Amils, R., Ballester, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 533–542. [Google Scholar]
- Technical Report on the Polymetallic Black Shale SBH Property, Birch Mountains, Athabasca Region, Alberta, Canada. Available online: www.dnimetals.com/pdf/TechRpt_SBH-pty-AB_Dumont-2008.pdf (accessed on 22 October 2014).
- Anjum, F.; Shahid, M.; Akcil, A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 2012, 117–118, 1–12. [Google Scholar] [CrossRef]
- Aziz, A.; Sajjad, M.; Mohammad, B. Elemental characterization of black shales of Khyber Pakthunkhawa (KPK) region of Pakistan using AAS. Chin. J. Geochem. 2013, 32, 248–251. [Google Scholar] [CrossRef]
- Developing High Margin Uranium Projects. Available online: www.auraenergy.com.au/assets/Aura_Roadshow_Presentation_January_2014.pdf (accessed on 22 October 2014).
- Jowitt, S.M.; Keays, R.R. Shale-hosted Ni–(Cu–PGE) mineralisation: A global overview. Trans. Inst. Min. Metall. Appl. Earth Sci. 2011, 120, 187–197. [Google Scholar] [CrossRef]
- Major Base Metal Districts Favourable for Future Bioleaching Technologies. Final Report to the Bioshale Project WP6. Available online: http://infoterre.brgm.fr/rapports/RP-55610-FR.pdf (accessed 22 October 2014).
- Pašava, J.; Hladiková, J.; Dobeš, P. Origin of Proterozoic metal-rich black shales from the Bohemian Massif, Czech Republic. Econ. Geol. 1996, 91, 63–79. [Google Scholar] [CrossRef]
- Pašava, J.; Zaccharini, F.; Aiglsperger, T.; Vymazalová, A. Platinum group elements (PGE) and their principal carriers in metal-rich black shales: An overview with new data from Mo-Ni-PGE black shales (Zunyi region, Guizhou Province, south China). J. Geosci. 2013, 58, 209–216. [Google Scholar] [CrossRef]
- Norris, P.R.; Brown, C.F.; Caldwell, P.E. Ore column leaching with thermophiles: II, polymetallic sulfide ore. Hydrometallurgy 2012, 127–128, 70–76. [Google Scholar] [CrossRef]
- Norris, P.R.; Gould, O.; Ogden, T. Anaerobic microbial growth to enhance iron removal from a polymetallic sulfide ore from 30 to 75 °C. In Proceedings of Biohydrometallurgy 2014, Falmouth, UK, 9–11 June 2014.
- Kalinowski, B.E.; Oskarsson, A.; Albinsson, Y.; Arlinger, J.; Ödegaard-Jensen, A.; Andlid, T.; Pederson, K. Microbial leaching of uranium and other trace elements from shale mine tailings at Ranstad. Geoderma 2004, 122, 177–194. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, N.; Kar, R.N.; Sukla, L.B.; Mishra, B.K. Microbial recovery of uranium using native fungal strains. Hydrometallurgy 2009, 95, 175–177. [Google Scholar] [CrossRef]
- Abd El Wahab, G.M.; Amin, M.M.; Aita, S.K. Bioleaching of uranium-bearing material from Abu Thor area, West Central Sinai, Egypt, for recovering uranium. Arab J. Nucl. Sci. Appl. 2012, 45, 169–178. [Google Scholar]
- Anjum, F.; Bhatti, H.N.; Asgher, M.; Shahid, M. Leaching of metal ions from black shale by organic acids produced by Aspergillus niger. Appl. Clay Sci. 2010, 47, 356–361. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Sarwar, S.; Ilyas, S. Effect of organic acids produced by Penicillium notatum on the extraction of metals ions from brown shale. J. Chem. Soc. Pak. 2012, 34, 1040–1047. [Google Scholar]
- Nouren, S.; Bhatti, H.N.; Ilyas, S. Bioleaching of copper, aluminium, magnesium and manganese from brown shale by Ganoderma lucidum. Afr. J. Biotechnol. 2011, 10, 10664–10673. [Google Scholar]
- Anjum, F.; Bhatti, H.N.; Ghauri, M.A.; Bhatti, I.A.; Asgher, M.; Asi, M.R. Bioleaching of copper, cobalt and zinc from black shale by Penicillium notatum. Afr. J. Biotechnol. 2009, 8, 5038–5045. [Google Scholar]
- Anjum, F.; Bhatti, H.N.; Ghauri, M.A. Enhanced bioleaching of metals from black shale using ultrasonics. Hydrometallurgy 2010, 100, 122–128. [Google Scholar] [CrossRef]
- Sjoberg, V.; Grandin, A.; Karlsson, L.; Karlsson, S. Bioleaching of shale—Impact of carbon source. In The New Uranium Mining Boom; Merkel, B., Schipek, M., Eds.; Springer-Verlag: Berlin, Germany, 2011; pp. 449–454. [Google Scholar]
- Hsu, K.J.; Sun, S.; Li, J.L.; Chen, H.H.; Pen, H.P.; Sengor, A.M.C. Mesozoic overthrust tectonics in south China. Geology 1988, 16, 418–421. [Google Scholar] [CrossRef]
- Morin, D.H.R.; Pinches, T.; Huisman, J.; Frias, C.; Norberg, A.; Forssberg, E. Progress after three years of BioMinE-Research and Technological Development project for a global assessment of biohydrometallurgical processes applied to European non-ferrous metal resources. Hydrometallurgy 2008, 94, 58–68. [Google Scholar] [CrossRef]
- Loukola-Ruskeeniemi, K. Geochemistry and genesis of the black-shale hosted Ni-Cu-Zn deposit at Talvivaara, Finland. Econ. Geol. 1996, 91, 80–110. [Google Scholar] [CrossRef]
- Puhakka, J.A.; Kaksonen, A.H.; Riekkola-Vanhanen, M. Heap leaching of black schist. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin, Germany, 2007; pp. 139–151. [Google Scholar]
- Salo-Zieman, V.L.A.; Kinnunen, P.H.M.; Puhakka, J.A. Bioleaching of acid-consuming low-grade nickel ore with elemental sulfur addition and subsequent acid generation. J. Chem. Technol. Biotechnol. 2006, 81, 34–40. [Google Scholar] [CrossRef]
- Halinen, A.K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore. Part I: Effect of pH on metal extraction and microbial composition in pH controlled columns. Hydrometallurgy 2009, 98, 92–100. [Google Scholar] [CrossRef]
- Halinen, A.K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore. Part II: Effect of temperature on base metal extraction and bacterial compositions. Hydrometallurgy 2009, 98, 101–107. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Bigham, J.M.; Riekkola-Vanhanen, M.; Tuovinen, O.H. Altered mineralogy associated with stirred tank bioreactor leaching of a black schist ore. Hydrometallurgy 2010, 100, 181–184. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Bigham, J.M.; Vuorinen, A.; Tuovinen, O.H. Chemical and bacterial leaching of metals from black schist sulfide minerals in shake flasks. Int. J. Miner. Process. 2012, 110–111, 25–29. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Vuorinen, A.; Tuovinen, O.H. Dissolution of non-sulfide phases during the chemical and bacterial leaching of a sulfidic black schist. Hydrometallurgy 2012, 117–118, 32–35. [Google Scholar] [CrossRef]
- Wakeman, K.; Auvinen, H.; Johnson, D.B. Microbiological and geochemical dynamics in simulated-heap leaching of a polymetallic sulfide ore. Biotech. Bioeng. 2008, 101, 739–750. [Google Scholar] [CrossRef]
- Halinen, A.K.; Beecroft, N.J.; Määttä, K.; Nurmi, P.; Laukkanen, K.; Kaksonen, A.H.; Riekkola-Vanhanen, M.; Puhakka, J.A. Microbial community dynamics during a demonstration-scale bioheap leaching operation. Hydrometallurgy 2013, 125–126, 34–41. [Google Scholar]
- Updated Resource Estimate and Preliminary Economic Assessment Estimate Viken Project NPV at US $1 Billion. Available online: www.CZQminerals.com/news-releases/page/2/ (accessed on 22 October 2014).
- Investment Presentation: High Impact Exploration in a World-Class Producing Copper-Nickel Region. Available online: www.finnaustmining.com/ (accessed on 22 October 2014).
- Alberta Black Shale Metals Projects. Available online: www.dnimetals.com/properties/black_shales.htm (accessed on 22 October 2014).
- Vaughan, D.J.; Sweeney, M.; Friedrich, G.; Diedel, R.; Haranczyk, C. The Kupferschiefer: An overview with an appraisal of the different types of mineralization. Econ. Geol. 1989, 84, 1003–1027. [Google Scholar] [CrossRef]
- Sadowski, Z.; Szubert, A. Comparison of kinetics of black shale bioleaching process using stationary and agitated systems. Physicochem. Probl. Miner. Process. 2007, 41, 387–395. [Google Scholar]
- Grobelski, T.; Farbiszewska-Kiczma, J.; Farbiszewska, T. Bioleaching of Polish black shale. Physicochem. Probl. Miner. Process. 2007, 41, 259–264. [Google Scholar]
- Groudev, S.N.; Spasova, I.I.; Nicolova, M.V.; Georgiev, P.S. Bacterial leaching of black shale copper ore. Adv. Mater. Res. 2007, 50, 187–190. [Google Scholar]
- Farbiszewska, T.; Farbiszewska-Kiczma, J.; Bak, M. Biological extraction of metals from a Polish black shale. Physicochem. Probl. Miner. Process. 2003, 37, 51–56. [Google Scholar]
- BioMOre: An Alternative Mining Concept—Raw Materials Commitment. Available online: https://ec.europa.eu/eip/raw-materials/en/content/biomore-alternative-mining-concept-raw-materials-commitment (accessed on 22 October 2014).
- Taylor, A. Laterites—Still a Frontier of Nickel Process Development. Paper Presented at TMS2013, San Antonio, Texas, USA, 3–7 March 2013; Available online: http://www.altamet.com.au/wp-content/uploads/2013/04/laterites-still-a-frontier-of-nickel-process-development1.pdf (accessed on 22 October 2014).
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part 1: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy 2007, 89, 137–159. [Google Scholar] [CrossRef]
- Das, A.P.; Sukla, L.B.; Pradhan, N.; Nayak, S. Manganese biomining: A review. Bioresour. Technol. 2011, 102, 7381–7387. [Google Scholar] [CrossRef] [PubMed]
- Bacon, W.G.; Dalvi, A.D.; Rochon, B.A.; Selby, M. Nickel outlook—2000–2010. Cim Bull. 2002, 95, 47–52. [Google Scholar]
- Tzeferis, P.G.; Agatzini-Leonardou, S. Leaching of nickel and iron from Greek non-sulphide nickeliferous ores by organic acids. Hydrometallurgy 1994, 36, 345–360. [Google Scholar] [CrossRef]
- Tzeferis, P.G.; Agatzini, S.; Nerantzis, E.T. Mineral leaching of non-sulphide nickel ores using heterotrophic microorganisms. Lett. Appl. Microbiol. 1994, 18, 209–213. [Google Scholar] [CrossRef]
- Agatzini, S.; Tzeferis, P. Bioleaching of nickel-cobalt oxide ores. AusIMM Proc. 1997, 1, 9–15. [Google Scholar]
- Sukla, L.B.; Panchanadikar, V.V.; Kar, R.N. Microbial leaching of lateritic nickel ore. World J. Microbiol. Biotechnol. 1993, 9, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Sukla, L.B.; Panchanadikar, V.V. Bioleaching of lateritic nickel ore using an indigenous microflora. In Biohydrometallurgical Technologies; Torma, A.E., Wey, J.E., Lakshmanan, V.L., Eds.; TMS: Warrendale, PA, USA, 1993; Volume 1, pp. 373–380. [Google Scholar]
- Sukla, L.B.; Panchanadikar, V. Bioleaching of lateritic nickel ore using a heterotrophic micro-organism. Hydrometallurgy 1993, 32, 373–379. [Google Scholar] [CrossRef]
- Sukla, L.B.; Swamy, K.M.; Narayana, K.L.; Kar, R.N.; Panchanadikar, V.V. Bioleaching of Sukinda laterite using ultrasonics. Hydrometallurgy 1995, 37, 387–391. [Google Scholar] [CrossRef]
- Sukla, L.B.; Kar, R.N.; Panchanadikar, V.V.; Choudhury, S.; Mishra, R.K. Bioleaching of lateritic nickel ore using Penicillium sp. Trans. Indian Inst. Met. 1995, 48, 103–106. [Google Scholar]
- Valix, M.; Tang, J.Y.; Cheung, W.H. The effects of mineralogy on the biological leaching of nickel laterite ores. Miner. Eng. 2001, 14, 1629–1635. [Google Scholar] [CrossRef]
- Valix, M.; Tang, J.Y.; Malik, R. Heavy metal tolerance of fungi. Miner. Eng. 2001, 14, 499–505. [Google Scholar] [CrossRef]
- Valix, M.; Loon, L.O. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Valix, M.; Thangavalu, V.; Ryan, D.; Tang, J. Using halotolerant Aspergillus foetidus in bioleaching nickel laterite ore. Int. J. Environ. Waste Manag. 2009, 3, 253–264. [Google Scholar] [CrossRef]
- Tang, J.A.; Valix, M. Leaching of low grade limonite and nontronite ores by fungi metabolic acids. Miner. Eng. 2006, 19, 1274–1279. [Google Scholar] [CrossRef]
- Tang, J.; Valix, M. Leaching kinetics of limonite and nontronite ores. Int. J. Environ. Waste Manag. 2009, 3, 244–252. [Google Scholar] [CrossRef]
- Thangavalu, V.; Tang, J.; Ryan, D.; Valix, M. Effect of saline stress on fungi metabolism and biological leaching of weathered saprolite ores. Miner. Eng. 2006, 19, 1266–1273. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Characterisation of factors in the bacterial leaching of nickel laterites using statistical design of experiments. In Biohydrometallurgy: From Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; pp. 66–69. [Google Scholar]
- Simate, G.S.; Ndlovu, S.; Gericke, M. Bacterial leaching of nickel laterites using chemolithotrophic microorganisms: Process optimisation using response surface methodology and central composite rotatable design. Hydrometallurgy 2009, 98, 241–246. [Google Scholar] [CrossRef]
- Watling, H.R.; Elliot, A.D.; Fletcher, H.M.; Robinson, D.J.; Sully, D.M. Ore mineralogy of nickel laterites: Controls on processing characteristics under simulated heap-leach conditions. Aust. J. Earth Sci. 2011, 58, 725–744. [Google Scholar] [CrossRef]
- Imai, K. On the mechanism of bacterial leaching. In Metallurgical Applications of Bacterial Leaching and Related Phenomena; Murr, L.E., Torma, A.E., Brierley, J.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 275–295. [Google Scholar]
- Nakazawa, H.; Sato, H. Bacterial leaching of cobalt-rich ferromanganese crusts. Int. J. Miner. Process. 1995, 43, 255–265. [Google Scholar] [CrossRef]
- Belyi, A.V.; Pustochilov, P.P.; Gurevich, Y.L.; Kadochnikova, G.G.; Ladygina, V.P. Bacterial leaching of manganese ores. Appl. Biochem. Microbiol. 2006, 42, 289–292. [Google Scholar] [CrossRef]
- Kai, T.; Taniguchi, S.; Ikeda, S.I.; Takahashi, T. Effect of Thiobacillus ferrooxidans during redox leaching of manganese nodules and nickel sulfide. Resour. Environ. Biotechnol. 1996, 1, 99–1009. [Google Scholar]
- Konishi, Y.; Asai, S. Leaching of marine manganese nodules by acidophilic bacteria growing on elemental sulfur. Metall. Mater. Trans. B 1997, 28, 25–32. [Google Scholar] [CrossRef]
- Ndlovu, S.; Simate, G.S.; Gericke, M. The microbial assisted leaching of nickel laterites using a mixed culture of chemolithotrophic microorganisms. In Biohydrometallurgy: A Meeting Point between Microbial Ecology, Metal Recovery Processes and Environmental Remediation; Donati, E.R., Viera, M.R., Tavani, E.L., Giaveno, M.A., Lavalle, T.L., Chiaccharini, P.A., Eds.; TransTech Publications: Zurich, Switzerland, 2009; pp. 493–496. [Google Scholar]
- Kumari, A.; Natarajan, K.A. Bioleaching of ocean manganese nodules in the presence of reducing agents. Eur. J. Miner. Process. Environ. Prot. 2001, 1, 10–24. [Google Scholar]
- Jain, N.; Sharma, D.K. Biohydrometallurgy for nonsulfidic minerals—A review. Geomicrobiol. J. 2004, 21, 135–144. [Google Scholar] [CrossRef]
- Dave, S.R.; Natarajan, K.A.; Bhat, J.V. Leaching of copper and zinc from oxidised ores by fungi. Hydrometallurgy 1981, 7, 235–242. [Google Scholar] [CrossRef]
- Groudev, S.N.; Groudeva, V.I. Biological leaching of aluminium from clays. In Biotechnology for the Mining, Metal-Refining, and Fossil Fuel Processing Industries; Ehrlich, H.L., Holmes, D.S., Eds.; John Wiley and Sons: New York, NY, USA, 1986; pp. 91–96. [Google Scholar]
- Mohapatra, S.; Bohidar, S.; Pradhan, N.; Kar, R.N.; Sukla, L.B. Microbial extraction of nickel from Sukinda chromite overburden by Acidithiobacillus ferrooxidans and Aspergillus strains. Hydrometallurgy 2007, 85, 1–8. [Google Scholar] [CrossRef]
- Das, C.; Mehta, K.D.; Pandey, B.D. Biochemical leaching of metals from Indian Ocean nodules by Aspergillus niger. Miner. Process. Technol. 2005, 8, 460–467. [Google Scholar]
- McKenzie, D.I.; Denys, L.; Buchanan, A. The solubilisation of nickel, cobalt and iron from laterites by means of organic chelating agents at low pH. Int. J. Miner. Process. 1987, 21, 275–292. [Google Scholar] [CrossRef]
- Das, A.P.; Swain, S.; Panda, S.; Pradhan, N.; Sukla, L.B. Reductive acid leaching of low-grade manganese ores. Geomaterials 2012, 2, 70–72. [Google Scholar] [CrossRef]
- Burgstaller, W.; Schinner, F. Leaching of metals with fungi. J. Biotechnol. 1993, 27, 91–116. [Google Scholar] [CrossRef]
- Le, L.; Tang, J.; Ryan, D.; Valiz, M. Bioleaching nickel laterite ores using multi-metal tolerant Aspergillus foetidus organism. Miner. Eng. 2006, 19, 1259–1265. [Google Scholar] [CrossRef]
- Veglio, F.; Beolchini, F.; Gasbarro, A.; Toro, L.; Ubaldini, S.; Abbruzzese, C. Batch and semi-continuous tests in the bioleaching of manganiferous minerals by heterotrophic mixed microorganisms. Int. J. Miner. Process. 1997, 50, 255–273. [Google Scholar] [CrossRef]
- Mukherjee, A.; Raichur, A.M.; Modak, J.M.; Natarajan, K.A. A novel bio-leaching process to recover valuable metals from Indian Ocean nodules using a marine isolate. In Biohydrometallurgy: A Sustainable Technology in Evolution; Tsezos, M., Hatzikioseyian, A., Remoundaki, E., Eds.; National Technical University of Athens: Athens, Greece, 2004; pp. 25–33. [Google Scholar]
- Lovley, D.R. Organic matter mineralization with the reduction of ferric iron: A review. Geomicrobiol. J. 1987, 5, 375–399. [Google Scholar] [CrossRef]
- Lovley, D.R. Microbial reduction of iron, manganese and other metals. Adv. Agron. 1995, 54, 175–231. [Google Scholar]
- Lovley, D.R.; Coates, J.D. Novel forms of anaerobic respiration of environmental relevance. Curr. Opin. Microbiol. 2000, 3, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Eisele, T.C.; Gabby, K.L. Review of reductive leaching of iron by anaerobic bacteria. Miner. Process. Extr. Metall. Rev. 2014, 35, 75–105. [Google Scholar] [CrossRef]
- Lee, E.Y.; Noh, S.R.; Cho, K.S. Leaching of Mn, Co and Ni from manganese nodules using an anaerobic bioleaching method. J. Biosci. Bioeng. 2001, 92, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Agate, A.D. Recent advances in microbial mining. World J. Microbiol. Biotechnol. 1996, 12, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Ocean manganese nodules: Biogenesis and bioleaching possibilities. Miner. Metall. Process. 2000, 17, 121–128. [Google Scholar]
- Urrutia, M.M.; Roden, E.E.; Fredrickson, J.K.; Zachara, J.M. Microbial and surface chemistry controls on reduction of synthetic Fe(III) oxide minerals by the dissimilatory iron-reducing bacterium Shewanella alga. Geomicrobiol. J. 1998, 15, 269–291. [Google Scholar] [CrossRef]
- Cummings, D.E.; Caccavo, F.; Fendorf, S.; Rosenzweig, R.F. Arsenic mobilization by the dissimilatory Fe(III) reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 1999, 33, 723–729. [Google Scholar] [CrossRef]
- Liu, C.; Gorby, Y.A.; Zachara, J.M.; Fredrickson, J.K.; Brown, C.F. Reduction kinetics of Fe(III), Co(III), U(VI) and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol. Bioeng. 2002, 80, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Saitoh, N.; Ogi, T. A new biohydrometallurgical method for processing of deep-sea mineral resources. In Proceedings of the ASME 2009 28th International Conference on Ocean, Offshore and Arctic Engineering (OMAE2009), Honolulu, HI, USA, 31 May–5 June 2009; ASME: New York, NY, USA, 2009; Volume 4B, pp. 1319–1324. [Google Scholar]
- Brand, N.W.; Butt, C.R.M.; Elias, M. Nickel laterites: Classification and features. AGSO J. Aust. Geol. Geophys. 1998, 17, 81–88. [Google Scholar]
- Freyssinet, P.; Butt, C.R.M.; Morris, R.C.; Piantone, P. Ore-forming processes related to laterite weathering. In Economic Geology 100th Anniversary Volume; Hedenquist, J.W., Thomson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Economic Geology Publishing Company: New Haven, CT, USA, 2005; pp. 681–722. [Google Scholar]
- Du Plessis, C.A.; Slabbert, W.; Hallberg, K.B.; Johnson, D.B. Ferredox: A biohydrometallurgical processing concept for limonitic nickel laterites. Hydrometallurgy 2011, 109, 221–229. [Google Scholar] [CrossRef]
- Dold, B. Sustainability in metal mining: From exploration, over processing to mine waste management. Rev. Environ. Sci. Biotechnol. 2008, 7, 275–285. [Google Scholar] [CrossRef]
- Mudd, G.M. Sustainability and Mine Waste Management—A Snapshot of Mining Waste Issues. Available online: http://users.monash.edu.au/~gmudd/files/2007-WasteMment-Sustainability-v-MineWastes.pdf (accessed on 22 October 2014).
- Shaw, R.A.; Petravratzi, E.; Bloodworth, A.J. Resource recovery from mine waste. In Waste as a Resource; Hester, R.M., Harrison, R.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 44–65. [Google Scholar]
- Olson, G.J.; Brierley, C.L.; Briggs, A.P.; Calmet, E. Biooxidation of thiocyanate-containing refractory gold tailings from Minacalpa, Peru. Hydrometallurgy 2006, 81, 159–166. [Google Scholar] [CrossRef]
- Thomas, J.; Subramanian, S.; Riyaz Ulla, M.S.; Louis, K.T.; Gundewar, C.S. Studies on the biodissolution of cobaltic pyrite from copper tailings. In Proceedings of the XXIII International Mineral Processing Congress, Istanbul, Turkey, 3–8 September 2006; Önal, G., Acarkan, N., Çelik, M.S., Arslan, F., Ateşok, G., Güney, A., Sirkeci, A.A., Yüce, A.E., Perek, K.T., Eds.; Promed Advertising Agency: Istanbul, Turkey, 2006; pp. 1329–1333. [Google Scholar]
- Kitobo, W.; Ilunga, A.; Frenay, J.; Gaydardzhiev, S.; Basti, D. Bacterial leaching of complex sulphides from mine tailings altered by acid drainage. In Hydrocopper 2009; Casas, J., Domic, E., Eds.; Gecamin: Santiago, Chile, 2009; pp. 365–373. [Google Scholar]
- Muñoz, A.; Bevilaqua, D.; Garcia, O. Leaching of Ni and Cu from mine wastes (tailings and slags) using acid solutions of A. ferrooxidans. In Biohydrometallurgy: A Meeting Point between Microbial Ecology, Metal Recovery Processes and Environmental Remediation; Donati, E.R., Viera, M.R., Tavani, E.L., Giaveno, M.A., Lavalle, T.L., Chiaccharini, P.A., Eds.; TransTech Publications: Zurich, Switzerland, 2009; pp. 425–428. [Google Scholar]
- Zhu, J. Bacterial leaching research of Cu-Ni flotation tailing. In Biohydrometallurgy: Biotech Key to Unlock Mineral Resources Values; Qiu, G., Jiang, T., Qin, W., Liu, X., Yang, Y., Wang, H., Eds.; Central South University Press: Changsha, China, 2011; pp. 821–823. [Google Scholar]
- Kondrat’eva, T.F.; Pivovarova, T.A.; Bulaev, A.G.; Melamud, V.S.; Muravyov, M.I.; Usoltsev, A.V.; Vasil’ev, E.A. Percolation bioleaching of copper and zinc and gold recovery from flotation tailings of the sulfide complex ores of the Ural region, Russia. Hydrometallurgy 2012, 111–112, 82–86. [Google Scholar] [CrossRef]
- Bulaev, A.G.; Muravyov, M.I.; Pivovarova, T.A.; Fomochenko, N.V. Bioprocessing of mining and metallurgical wastes containing non-ferrous and precious metals. In Integrating Scientific and Industrial Knowledge on Biohydrometallurgy; Guiliani, N., Demergasso, C., Quatrini, R., Remonsellez, F., Davis-Belmar, C., Levican, G., Parada, P., Barahona, C., Zale, R., Eds.; TransTech Publications: Zurich, Switzerland, 2013; pp. 301–304. [Google Scholar]
- Galvez-Cloutier, R.; Mulligan, C.N.; Ouattra, A. Biolixiviation of Cu, Ni, Pb and Zn using organic acids by Aspergillus niger and Penicillium simplicissinum. In Biohydrometallurgy: A Sustainable Technology in Evolution; Tsezos, M., Hatzikioseyian, A., Remoundaki, E., Eds.; National Technical University of Athens: Athens, Greece, 2004; pp. 175–184. [Google Scholar]
- Bryan, C.G.; Hallberg, K.B.; Johnson, D.B. Mobilisation of metals in mineral tailings at the abandoned Sao Domingos copper mine (Portugal) by indigenous acidophilic bacteria. Hydrometallurgy 2006, 83, 184–194. [Google Scholar] [CrossRef]
- Willscher, S.; Pohle, C.; Sitte, J.; Werner, P. Solubilization of heavy metals from a fluvial AMD generating tailings sediment by heterotrophic microorganisms. Part I: Influence of pH and solid content. J. Geochem. Explor. 2007, 92, 177–185. [Google Scholar] [CrossRef]
- Tan, G.L.; Shu, W.S.; Hallberg, K.B.; Li, F.; Lan, C.Y.; Zhou, W.H.; Huang, L.N. Culturable and molecular phylogenetic diversity of microorganisms in an open-dumped, extremely acidic Pb/Zn mine tailings. Extremophiles 2008, 12, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.L.; Shu, W.S.; Hallberg, K.B.; Li, F.; Lan, C.Y.; Huang, L.N. Cultivation-dependent and cultivation-independent characterization of the microbial community in acid mine drainage associated with acidic Pb/Zn tailings at Lechang, Guandong, China. FEMS Microbiol. Ecol. 2007, 59, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kock, D.; Schippers, A. Quantitative microbial community analysis of three different sulfidic mine tailing dumps generating acid mine drainage. Appl. Environ. Microbiol. 2008, 74, 5211–5219. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Carvalho, N.; Iglesias, N.; Palencia, I. Bacterial leaching studies of a Portuguese flotation tailing. In Biohydrometallurgy: A Sustainable Technology in Evolution; Tsezos, M., Hatzikioseyian, A., Remoundaki, E., Eds.; National Technical University of Athens: Athens, Greece, 2004; pp. 75–84. [Google Scholar]
- Rivera-Santillan, R.E.; Becerril-Reyes, V. Comparison of the bioleaching effect of mesophilic (35 °C) and thermophilic (45 °C) bacteria on the Tizapa tailings. In Biohydrometallurgy: From the Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; pp. 34–37. [Google Scholar]
- Nagy, A.A.; Gock, E.D.; Melcher, F.; Atmaca, T.; Hahn, L.; Schippers, A. Biooxidation and cyanidation for gold and silver recovery from acid mine drainage generating tailings (Ticapampa, Peru). In Biohydrometallurgy: From Single Cell to the Environment; Schippers, A., Sand, W., Glombitza, F., Willscher, S., Eds.; TransTech Publications: Zurich, Switzerland, 2007; pp. 91–94. [Google Scholar]
- Schippers, A.; Nagy, A.A.; Kock, D.; Melcher, F.; Gock, E.D. The use of FISH and real time PCR to monitor the biooxidation and cyanidation for gold and silver recovery from mine tailings concentrate (Ticapampa, Peru). Hydrometallurgy 2008, 94, 77–81. [Google Scholar] [CrossRef]
- Tong, L.; Yang, H.; Liu, C. Bioleaching of Cu-Zn tailings. In Biohydrometallurgy: Biotech Key to Unlock Mineral Resources Values; Qiu, G., Jiang, T., Qin, W., Liu, X., Yang, Y., Wang, H., Eds.; Central South University Press: Changsha, China, 2011; pp. 793–797. [Google Scholar]
- Liu, Y.G.; Zhou, M.; Zeng, G.M.; Li, X.; Xu, W.H.; Fan, T. Effect of solids concentration on removal of heavy metals from mine tailings via bioleaching. J. Hazard. Mater. 2007, 141, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Zhou, M.; Zeng, G.M.; Wang, X.; Li, X.; Fan, T.; Xu, W.H. Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: Effects of substrate concentration. Bioresour. Technol. 2008, 99, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Lee, J.U. Catalytic effect of activated charcoal on microbial extraction of arsenic and heavy metals from mine tailings. Geosci. J. 2014, 18, 355–363. [Google Scholar] [CrossRef]
- Mugire, F. Changing Lives: Chinese Investment in Uganda’s Copper Mining Gives Hope to Thousands. Available online: http://english.people.com.cn/business/8568960.html (accessed on 22 October 2014).
- Tollinsky, N. Bioleaching Plant Proposed for Cobalt. Available online: www.Sudburyminingsolutions.com/bioleaching-plant-proposed-for-Cobalt.html (accessed on 22 October 2014).
- Miller, P. The use of bioleaching for cobalt/arsenic tailings remediation in Ontario Canada. In ALTA Ni-Co 2009, Perth, Australia; ALTA Metallurgical Services: Melbourne, Australia, 2009. [Google Scholar]
- McEwan, K.N.; Ralph, D.E.; Savage, C.J. In-Situ Bio-Oxidation of Low-Grade Refractory Sulphide Minerals. World Patent 2002061155 A1, 30 January 2014. [Google Scholar]
- McEwan, K.; Ralph, D. The i-BOTM process and related treatments for mine waste remediation. In Brownfield Sites; Brebbia, C.A., Almorza, D., Klapperich, H., Eds.; WIT Press: Southampton, UK, 2002; pp. 525–532. [Google Scholar]
- Seh-Bardan, B.J.; Othman, R.; Ab Wahib, S.; Husin, A.; Sadegh-Zadeh, F. Column bioleaching of arsenic and heavy metals from gold mine tailings by Aspergillus fumigatus. Clean Soil Air Water 2012, 40, 607–614. [Google Scholar] [CrossRef]
- Hernández, I.; Galizia, F.; Coto, O.; Donati, E. Improvement in metal recovery from laterite tailings by bioleaching. In Biohydrometallurgy: A Meeting Point between Microbial Ecology, Metal Recovery Processes and Environmental Remediation; Donati, E.R., Viera, M.R., Tavani, E.L., Giaveno, M.A., Lavalle, T.L., Chiaccharini, P.A., Eds.; TransTech Publications: Zurich, Switzerland, 2009; pp. 489–492. [Google Scholar]
- Coto, O.; Galizia, F.; Hernandez, I.; Marrero, J.; Donati, E. Cobalt and nickel recoveries from laterite tailings by organic and inorganic bio-acids. Hydrometallurgy 2008, 94, 18–22. [Google Scholar] [CrossRef]
- Coto, O.; Schippers, A. Influence of sulfur source on nickel and cobalt bioleaching from laterite tailings using Acidithiobacillus thiooxidans. In Biohydrometallurgy: Biotech Key to Unlock Mineral Resources Values; Qiu, G., Jiang, T., Qin, W., Liu, X., Yang, Y., Wang, H., Eds.; Central South University Press: Changsha, China, 2011; pp. 579–583. [Google Scholar]
- Cabrera, G.; Gomez, J.M.; Hernandez, I.; Coto, O.; Cantero, D. Different strategies for recovering metals from CARON process residue. J. Hazard. Mater. 2011, 189, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Hernández Diaz, I.; Galizia, F.; Coto Perez, O. Reduction of heavy-metal content in overburden material by bacterial action. In Biohydrometallurgy: A Meeting Point between Microbial Ecology, Metal Recovery Processes and Environmental Remediation; Donati, E.R., Viera, M.R., Tavani, E.L., Giaveno, M.A., Lavalle, T.L., Chiaccharini, P.A., Eds.; TransTech Publications: Zurich, Switzerland, 2009; pp. 653–656. [Google Scholar]
- Mulligan, C.N.; Galvez-Cloutier, R. Bioremediation of metal contamination. Environ. Monit. Assess. 2003, 84, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Kamali, M.; Gibbs, B.F. Bioleaching of copper and other metals from low-grade oxidized mining ores by Aspergillus niger. J. Chem. Technol. Biotechnol. 2003, 78, 497–503. [Google Scholar] [CrossRef]
- Behera, S.K.; Panda, P.P.; Singh, S.; Pradhan, N.; Sukla, L.B.; Mishra, B.K. Study on reaction mechanism of bioleaching of nickel and cobalt from lateritic chromite overburdens. Int. Biodeterior. Biodegrad. 2011, 65, 1035–1042. [Google Scholar] [CrossRef]
- Behera, S.K.; Sukla, L.B. Microbial extraction of nickel from chromite overburdens in the presence of surfactant. Trans. Nonferr. Met. Soc. China 2012, 22, 2840–2845. [Google Scholar] [CrossRef]
- Behera, S.K.; Panda, S.K.; Pradhan, N.; Sukla, L.B.; Mishra, B.K. Extraction of nickel by microbial reduction of laterite chromite overburden of Sukinda, India. Bioresour. Technol. 2012, 125, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Recent developments in microbiological approaches for securing mine wastes and for recovering metals from mine waters. Minerals 2014, 4, 279–292. [Google Scholar] [CrossRef]
© 2014 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watling, H.R. Review of Biohydrometallurgical Metals Extraction from Polymetallic Mineral Resources. Minerals 2015, 5, 1-60. https://doi.org/10.3390/min5010001
Watling HR. Review of Biohydrometallurgical Metals Extraction from Polymetallic Mineral Resources. Minerals. 2015; 5(1):1-60. https://doi.org/10.3390/min5010001
Chicago/Turabian StyleWatling, Helen R. 2015. "Review of Biohydrometallurgical Metals Extraction from Polymetallic Mineral Resources" Minerals 5, no. 1: 1-60. https://doi.org/10.3390/min5010001






