Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pure Minerals and Reagents
2.2. Flotation Experiment
2.3. Zeta Potential Measurement
3. Results and Discussions
3.1. Single Mineral Flotation Experiment Results
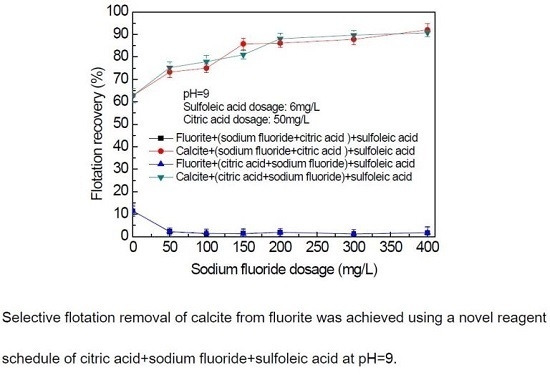
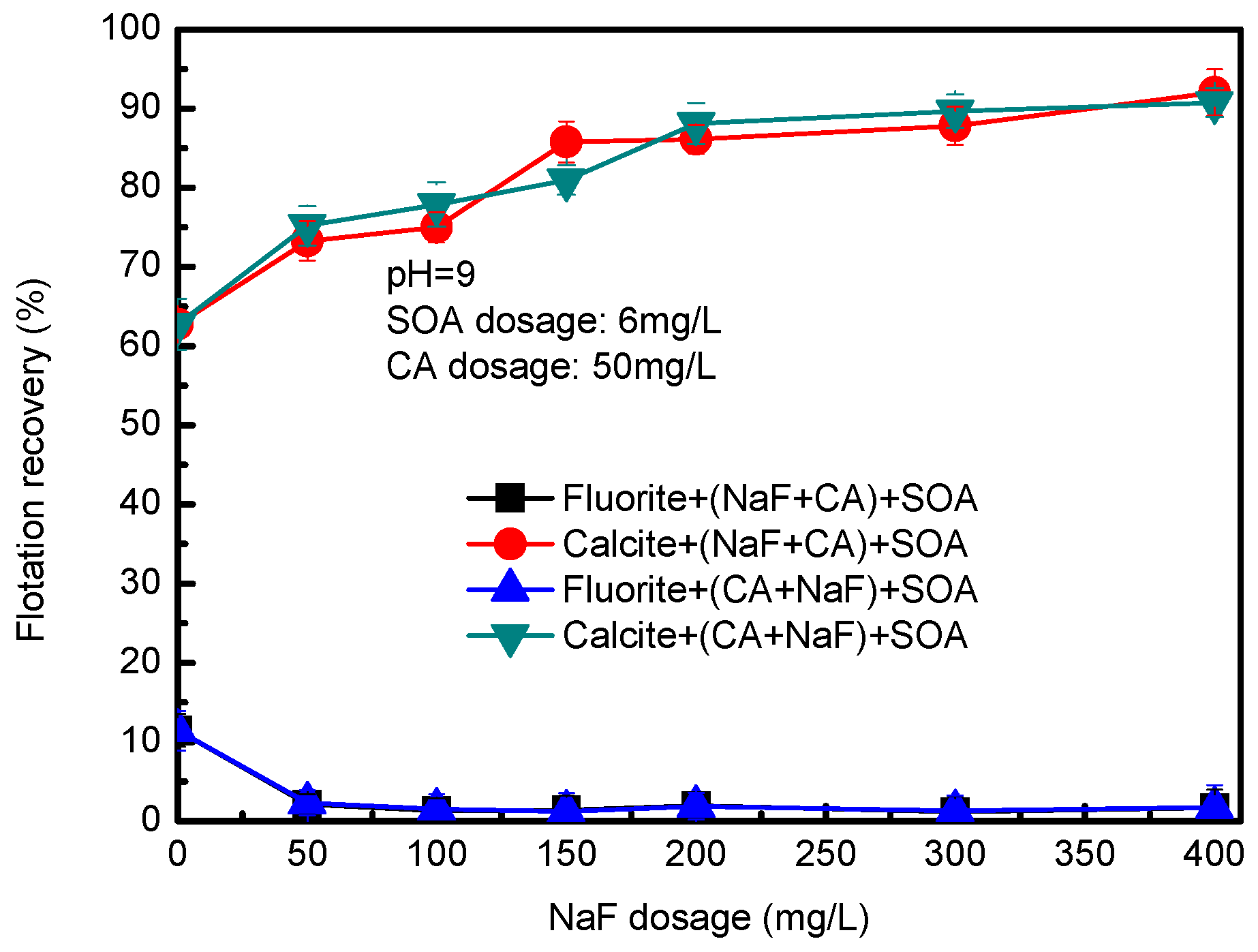
3.2. Mixed Binary Mineral Flotation Experiment Results
3.3. Run-of-Mine Ore Batch Flotation Test Results
3.4. Zeta Potential Measurement Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Q.; Miller, J.D.; Wang, X. Recent developments in the beneficiation of Chinese bauxite. Min. Process. Extr. Metall. Rev. 2010, 31, 111–119. [Google Scholar] [CrossRef]
- Pradip Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J. Molecular modeling of interactions of alkyl hydroxamates with calcium minerals. J. Colloid Interf. Sci. 2002, 256, 106–113. [Google Scholar] [CrossRef]
- Pradip Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J. Molecular modeling of interactions of diphosphonic acid based surfactants with calcium minerals. Langmuir 2002, 18, 932–940. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulfide minerals, I: Flotation of single minerals and mineral mixtures. Miner. Eng. 2004, 17, 855–863. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulfide minerals, II: Mechanisms of flotation depression of sulfide minerals by thioglycollic acid and citric acid. Miner. Eng. 2004, 17, 865–878. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Dos Santos, M.A.; Santana, R.C.; Capponi, F.; Ataíde, C.H.; Barrozo, M. Effect of ionic species on the performance of apatite flotation. Sep. Purif. Technol. 2010, 76, 15–20. [Google Scholar] [CrossRef]
- Miller, J.D.; Misra, M.; Yehia, A.; Hu, J.S. Fluoride activation in oleate flotation of collophanite. Miner. Metall. Process. 1987, 8, 133–140. [Google Scholar]
- Marinakis, K.I.; Shergold, H.L. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 161–176. [Google Scholar] [CrossRef]
- De Leeuw, N.H.; Parker, S.C.; Rao, K.H. Modeling the competitive adsorption of water and methanoic acid on calcite and fluorite surfaces. Langmuir 1998, 14, 5900–5906. [Google Scholar] [CrossRef]
- Fa, K.; Jiang, T.; Nalaskowski, J.; Miller, J.D. Interaction forces between a calcium dioleate sphere and calcite/fluorite surfaces and their significance in flotation. Langmuir 2003, 19, 10523–10530. [Google Scholar] [CrossRef]
- Chee, K.K.; Lan, W.G.; Wong, M.K.; Lee, H.K. Optimization of liquid chromatographic parameters for the separation of priority phenols by using mixed-level orthogonal array design. Anal. Chim. Acta 1995, 312, 271–280. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hu, Y.; Chi, R.; Xu, Z. Solution chemistry study of salt-type mineral flotation systems: Role of inorganic dispersants. Ind. Eng. Chem. Res. 2003, 42, 1641–1647. [Google Scholar]
- Miller, J.D.; Fa, K.; Calara, J.V.; Paruchuri, V.K. The surface charge of fluorite in the absence of surface carbonation. Colloids Surf. A Physicochem. Metall. Eng. 2004, 238, 91–97. [Google Scholar]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Gao, Y.; Hu, Y.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]






| Weight (g) | Grade (%) | Recovery (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) | (b) | (a) | (b) | (a) | (b) | |||||
| Fluorite | Calcite | Fluorite | Calcite | Fluorite | Calcite | Fluorite | Calcite | |||
| Concentrate | 0.4 (0.02) | 121.6 (2.3) | 27.07 (1.7) | 72.93 (2.1) | 18.59 (1.1) | 66.67 (1.3) | 6.5 (0.2) | 88.1 (1.5) | 11.2 (0.9) | 85.2 (1.9) |
| Tailings | 1.6 (0.08) | 373.4 (3.4) | 97.57 (2.3) | 2.43 (0.12) | 48.01 (1.5) | 3.78 (0.4) | 93.5 (2.1) | 11.9 (0.8) | 88.8 (2.0) | 14.8 (0.7) |
| Total | 2.0 | 495 (2.8) | 66.7 | 33.3 | 40.78 (1.4) | 19.23 (0.6) | 100.00 | 100.00 | 100.00 | 100.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Gao, Y.; Zhu, Y.; Hu, Y.; Sun, W. Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule. Minerals 2016, 6, 114. https://doi.org/10.3390/min6040114
Gao Z, Gao Y, Zhu Y, Hu Y, Sun W. Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule. Minerals. 2016; 6(4):114. https://doi.org/10.3390/min6040114
Chicago/Turabian StyleGao, Zhiyong, Yuesheng Gao, Yiyang Zhu, Yuehua Hu, and Wei Sun. 2016. "Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule" Minerals 6, no. 4: 114. https://doi.org/10.3390/min6040114