In Situ Investigation of the Adsorption of Styrene Phosphonic Acid on Cassiterite (110) Surface by Molecular Modeling
Abstract
:1. Introduction
2. Experimental and Computational Details
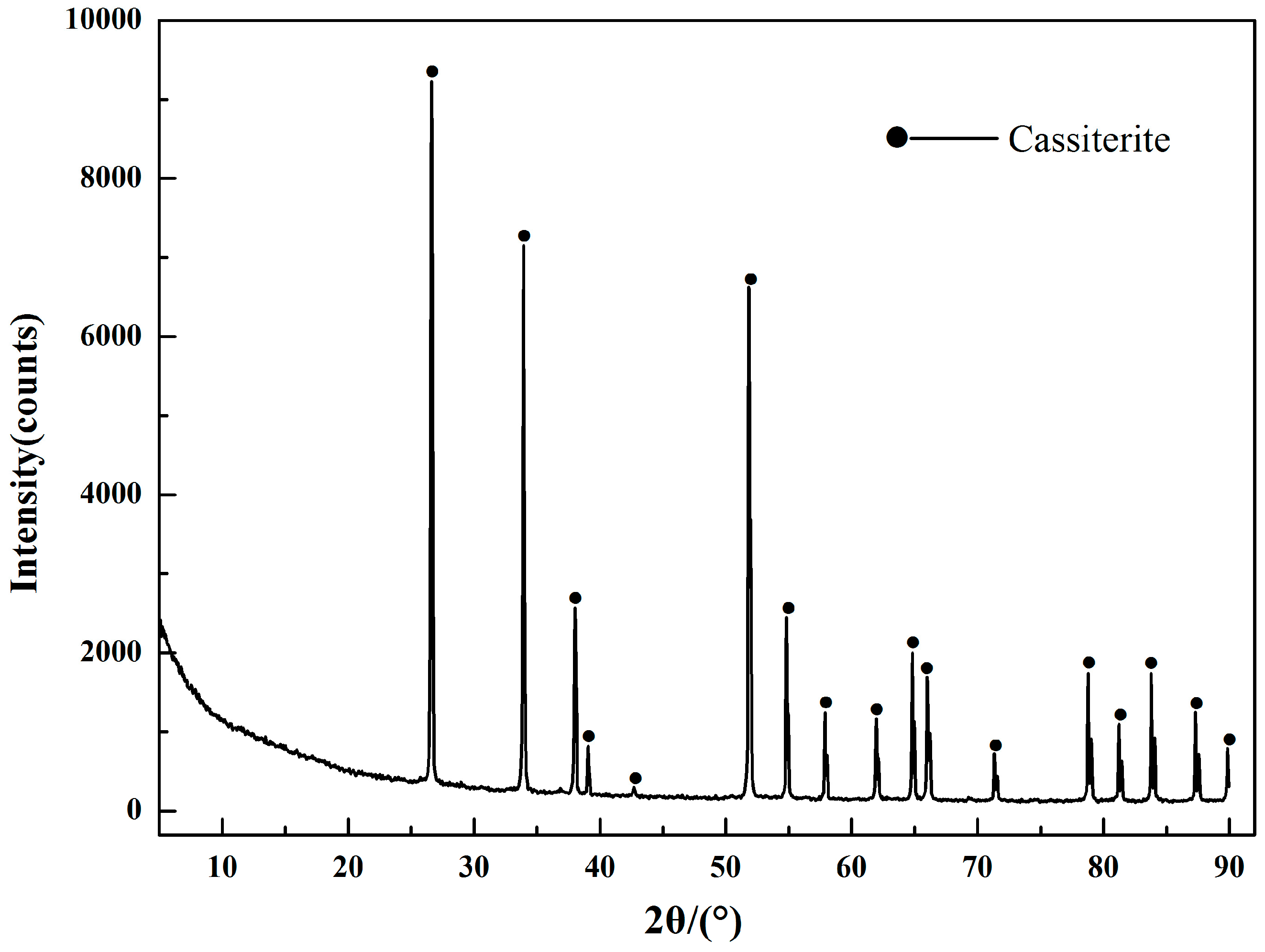
2.1. Minerals and Reagents
2.2. Flotation Tests
2.3. Zeta Potential Measurements
2.4. Computational Details
3. Results and Discussion
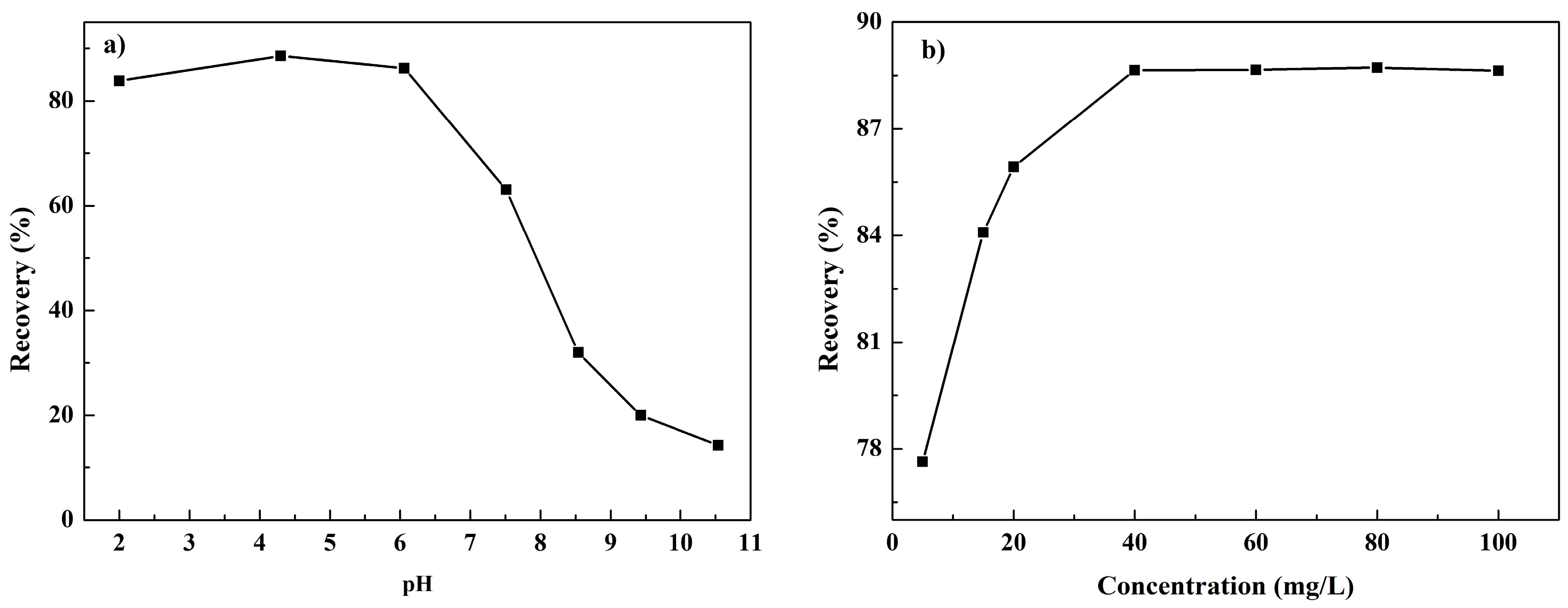
3.1. Single Mineral Flotation Tests
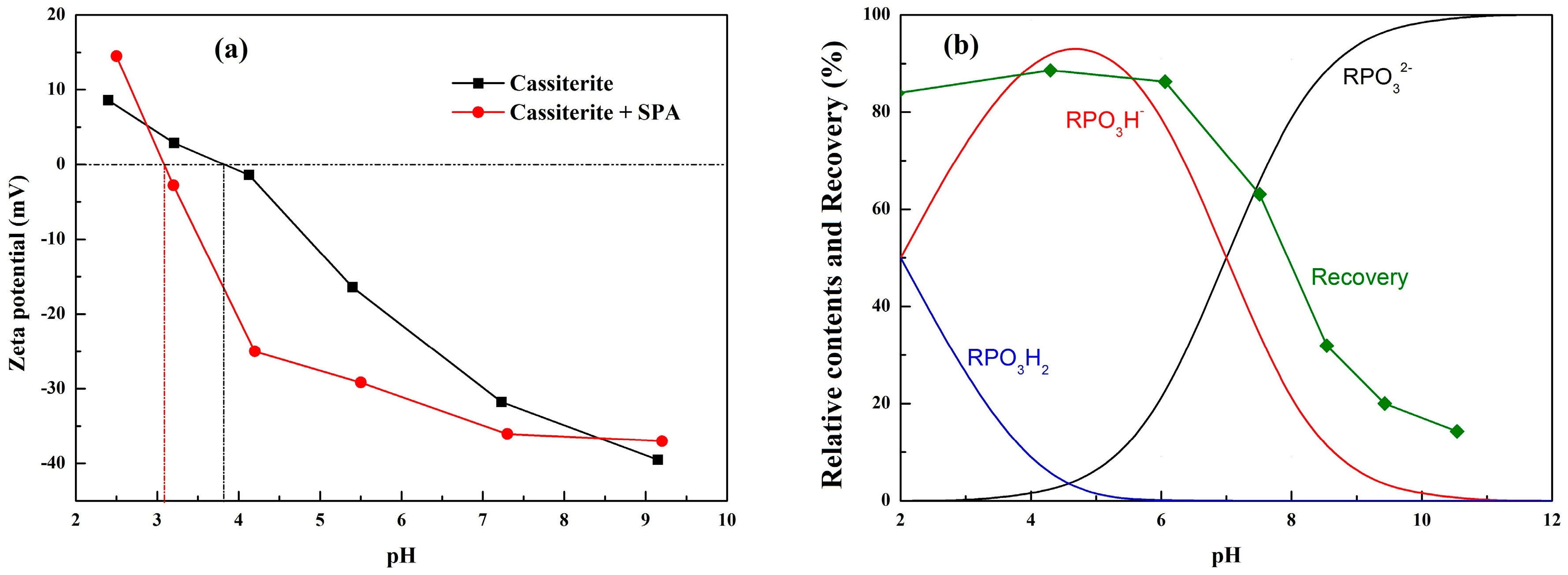
3.2. Zeta Potential Measurements and Solution Chemistry Analysis
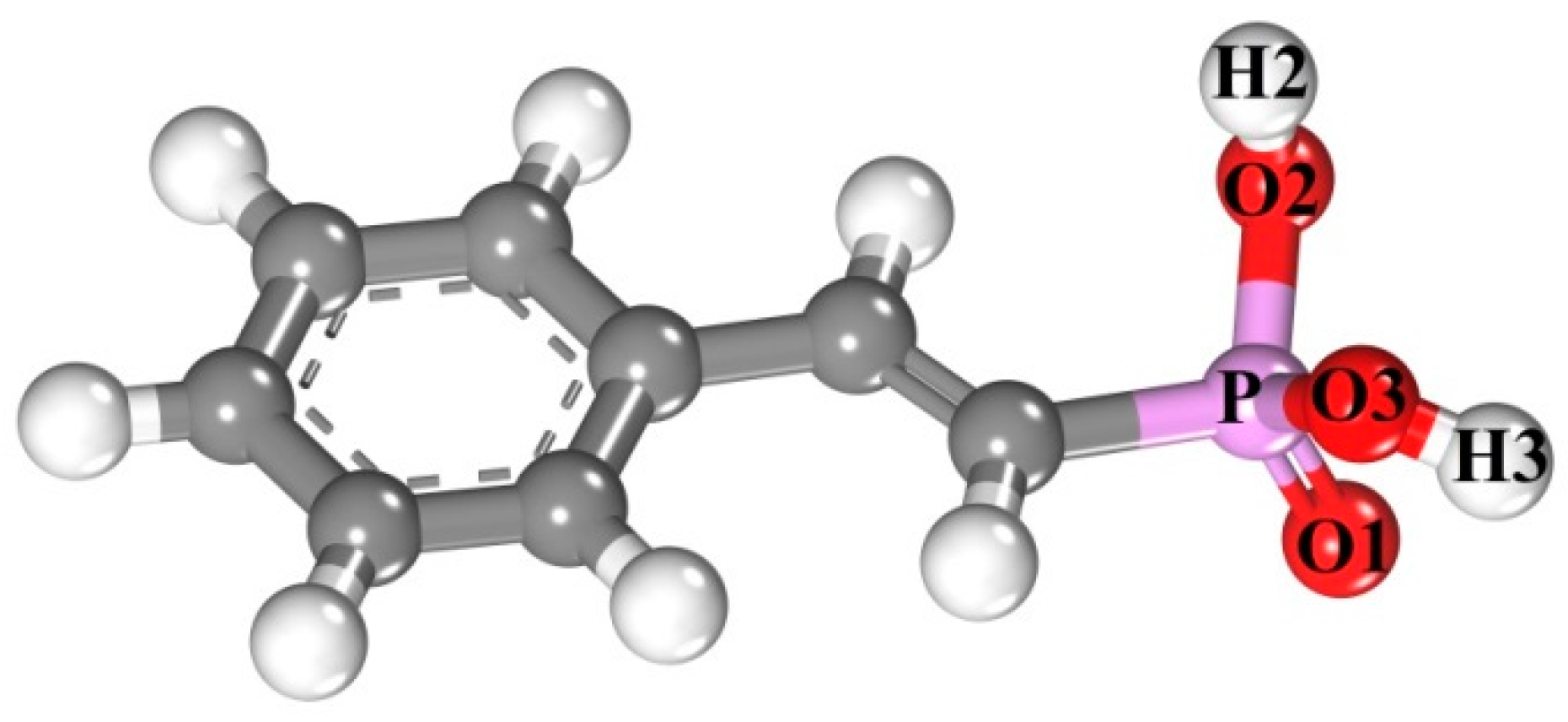
3.3. Structure Details and Mulliken Populations of SPA Species
3.4. Frontier Molecular Orbital Theory Analysis
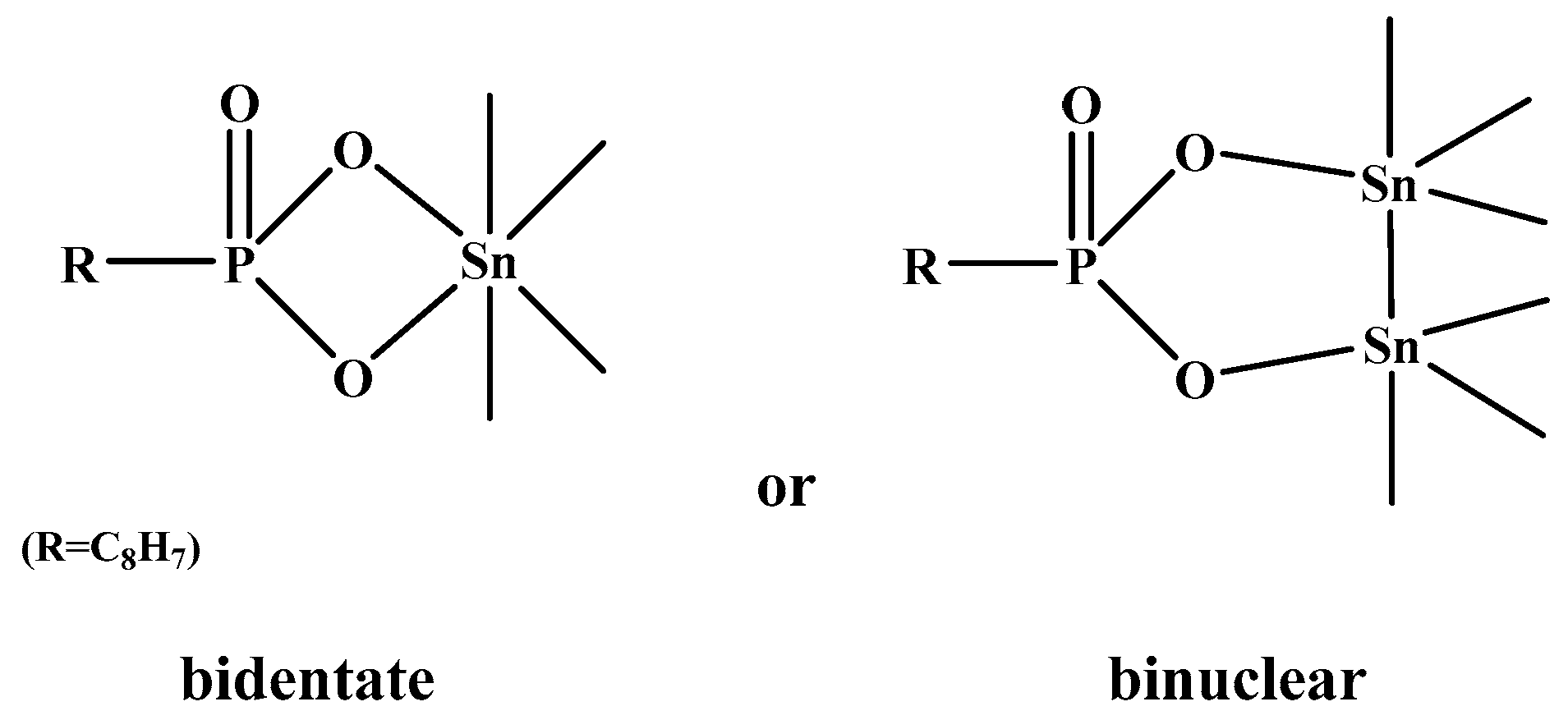
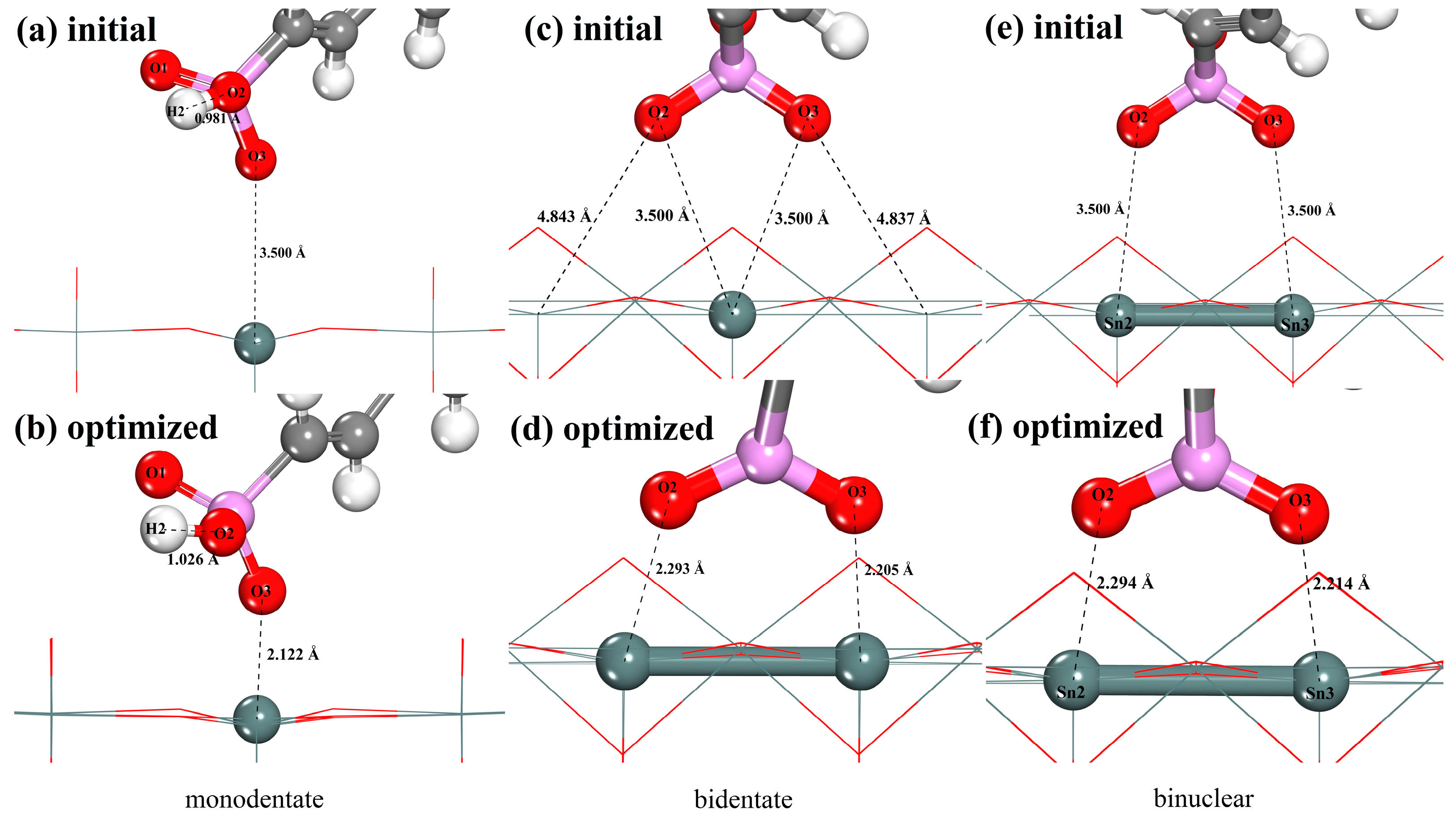
3.5. Adsorption Configurations of SPA on Cassiterite
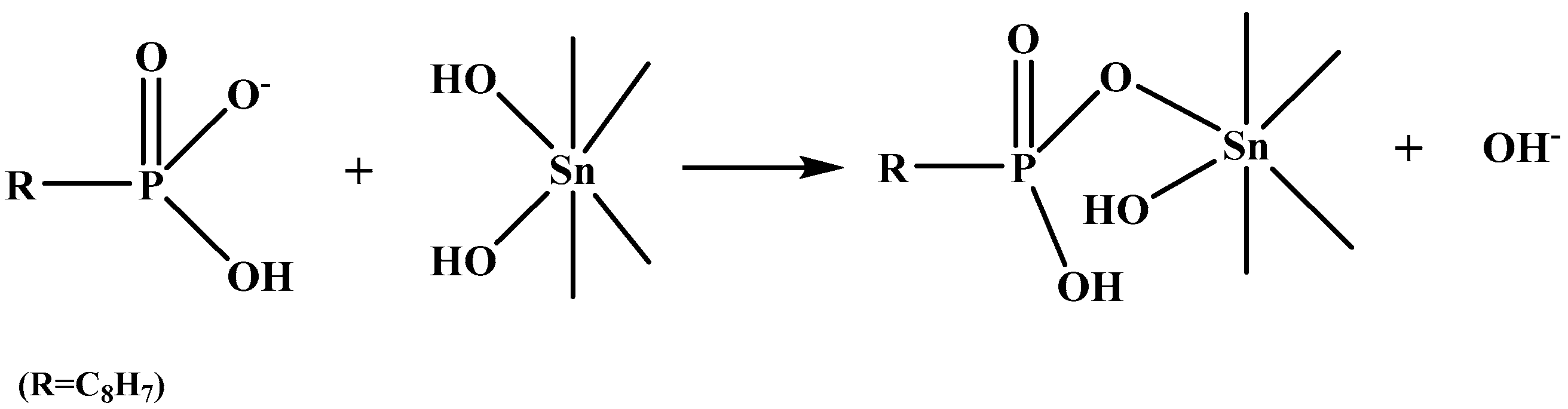
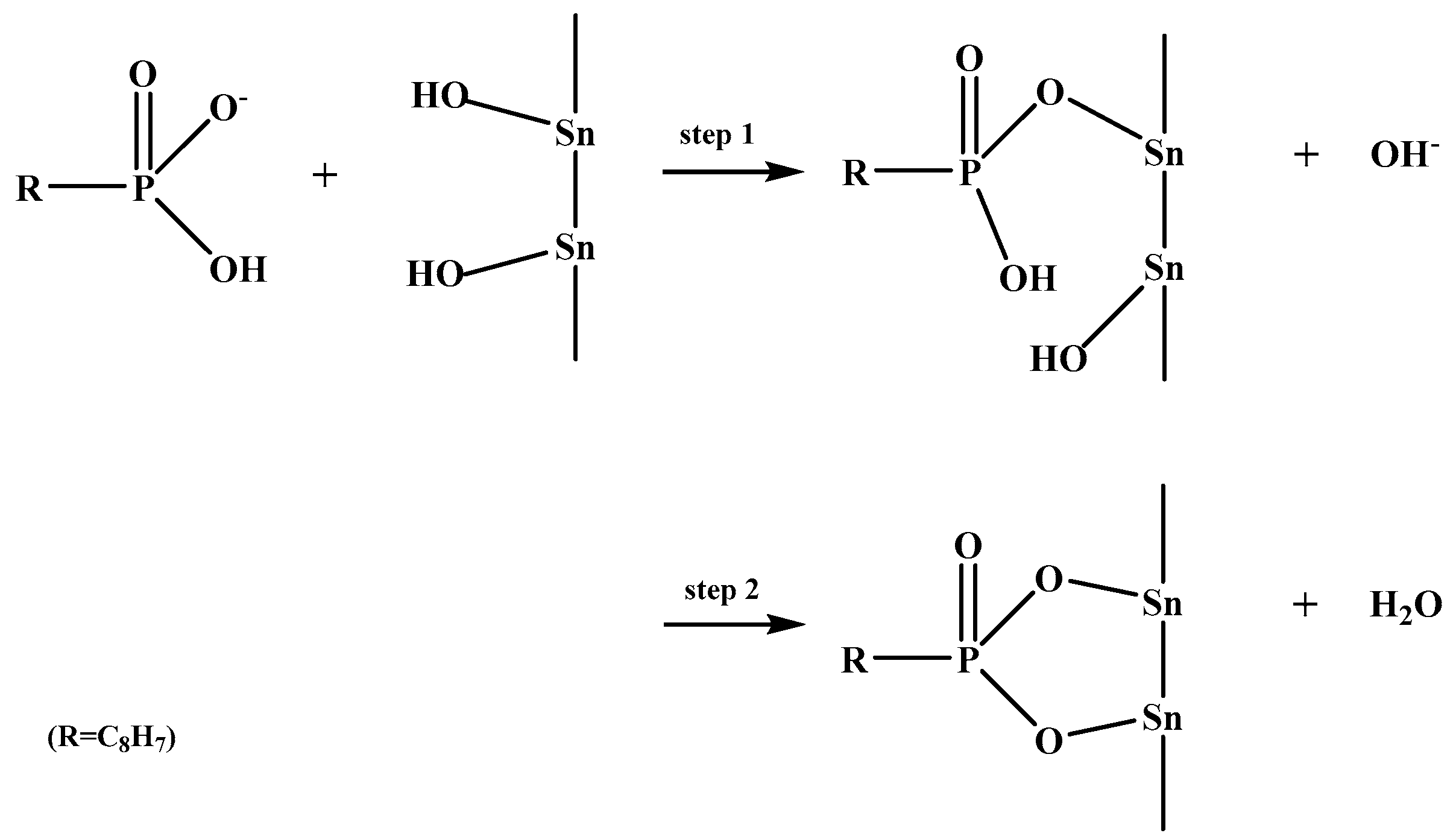
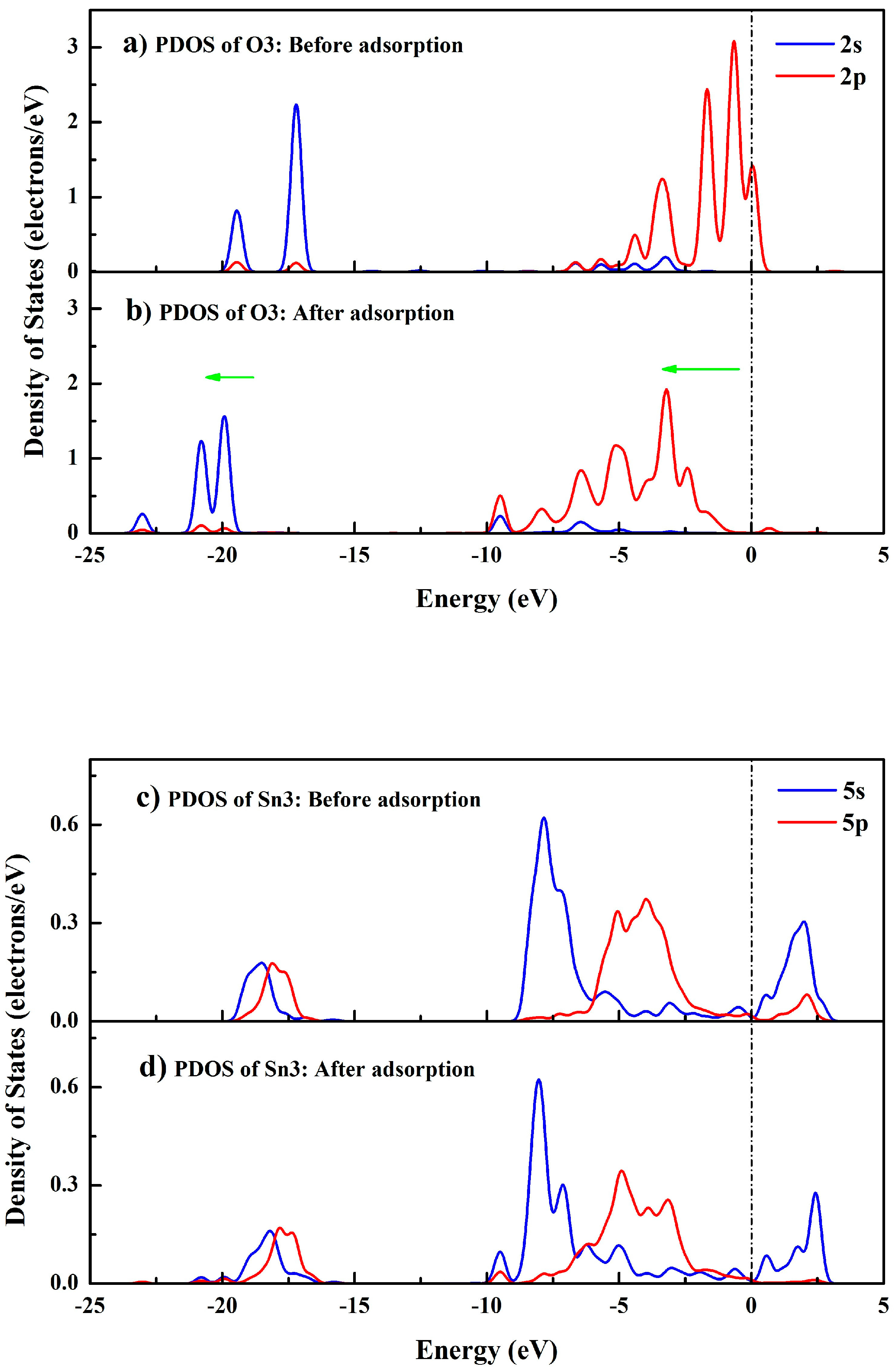
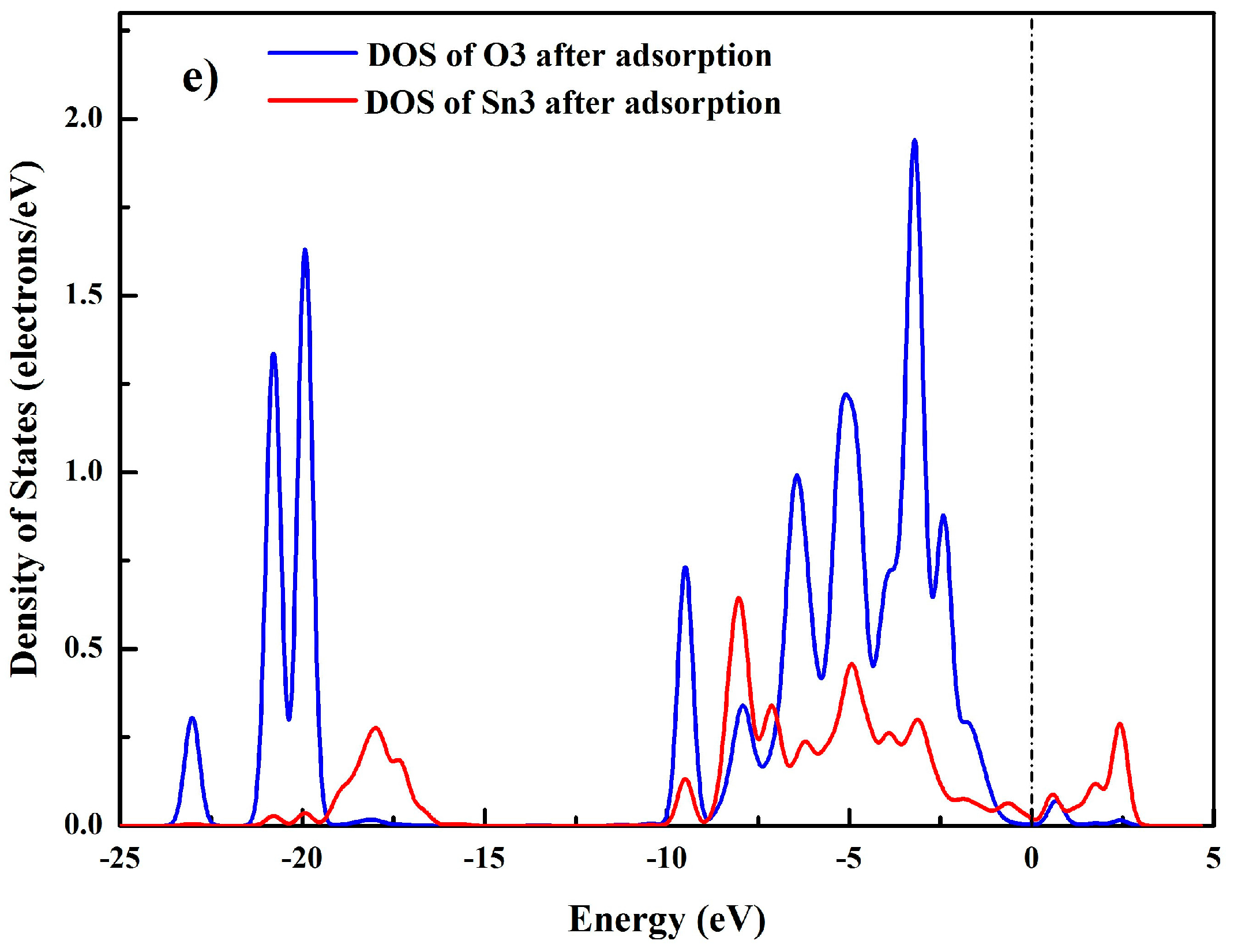
3.6. Bonding Mechanism between SPA and the Cassiterite (110) Surface
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sreevinas, T.; Manohar, C. Adsorption of octyl hydroxamic acid/salt on cassiterite. Miner. Process. Extr. Metall. Rev. 2000, 20, 503–519. [Google Scholar]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Coll. Surf. A Physicochem. Eng. Asp. 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Tong, X.; Song, S.X.; Wang, X.; Deng, Z.G. Beneficiation of cassiterite fines from a tin tailing slime by froth flotation. Sep. Sci. Technol. 2014, 49, 458–463. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chelgani, S.C.; Osbahr, I. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96, 94–98. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Adamian, R. Physico-chemical factors affecting the separation of cassiterite and fluorite by froth flotation. Miner. Process. Extr. Metall. Rev. 1992, 9, 125–134. [Google Scholar] [CrossRef]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.S.; Baek, S.H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Xu, Y.B.; Qin, W.Q. Surface analysis of cassiterite with sodium oleate in aqueous solution. Sep. Sci. Technol. 2012, 47, 502–506. [Google Scholar] [CrossRef]
- Peng, H.Q.; Luo, W.; Wu, D.; Bie, X.X.; Shao, H.; Jiao, W.Y.; Liu, Y.K. Study on the effect of Fe3+ on zircon flotation separation from cassiterite using sodium oleate as collector. Minerals 2017, 7, 108. [Google Scholar] [CrossRef]
- Senior, G.D.; Poling, G.W. The chemistry of cassiterite flotation. In Advances in Mineral Processing; Somasundaran, P., Ed.; SME Inc.: Littleton, CO, USA, 1986; pp. 229–254. [Google Scholar]
- Collins, D.N.; Kirkup, L.; Davey, M.N.; Arthur, C. Flotation of cassiterite: Development of a flotation process. Trans. Inst. Min. Metall. 1968, 77, C1–C13. [Google Scholar]
- Houot, R.; Desbrosses, Y. Is the cassiterite contained in complex sulfide polymetallic ore recoverable? Int. J. Miner. Process. 1991, 32, 45–57. [Google Scholar] [CrossRef]
- Lepetic, V.M. Cassiterite flotation: A review. In Advances in Mineral Processing; Somasundaran, P., Ed.; SME Inc.: Littleton, CO, USA, 1987; pp. 343–350. [Google Scholar]
- Zambrana, G.Z.; Medina, R.T.; Guierrez, G.B.; Vargas, R.R. Recovery of minus ten micron cassiterite by liquid-liquid extraction. Int. J. Miner. Process. 1974, 1, 335–345. [Google Scholar] [CrossRef]
- Agarwal, Y.K. Hydroxamic acids and their metal complexes. Russ. Chem. Rev. 1979, 48, 948–963. [Google Scholar] [CrossRef]
- Yale, Y.L. The hydroxamic acids. Chem. Rev. 1943, 33, 209–256. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Harris, P.J.; O’Connor, C.T. Synergistic interactions between reagents in sulphide flotation. J. S. Afr. Inst. Min. Metall. 1998, 98, 189–193. [Google Scholar]
- Bulatovic, S.; De Silvio, E. Process development for impurity removal from a tin gravity concentrate. Miner. Eng. 2000, 13, 871–879. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Yarkevich, A.N.; Safronova, Z.V.; Rodygina, N.I.; Fedoseev, A.M. Extraction of u(vi), th(iv), pu(iv), and am(iii) from nitric acid solutions with polyfunctional organophosphorus acids. Radiochimica 2007, 49, 618–623. [Google Scholar] [CrossRef]
- Gruner, H.; Bilsing, U. Cassiterite flotation using styrene phosphonic acid to produce high-grade concentrates at high recoveries from finely disseminated ores-comparison with other collectors and discussion of effective circuit configurations. Miner. Eng. 1992, 5, 429–434. [Google Scholar] [CrossRef]
- Wottgen, E. Adsorption of phosphonic acids on cassiterite. Trans. Inst. Min. Metall. Sec. C 1969, 78, 91–97. [Google Scholar]
- Kuys, K.J.; Roberts, N.K. In situ investigation of the adsorption of styrene phosphonic acid on cassiterite by FTIR-ATR spectroscopy. Coll. Surf. 1987, 24, 1–17. [Google Scholar] [CrossRef]
- Farrow, J.B.; Warren, L.J. Adsorption of short-chained organic acids on stannic oxide. Coll. Surf. 1988, 34, 255–269. [Google Scholar] [CrossRef]
- Tan, X.; He, F.Y.; Shang, Y.B.; Yin, W.Z. Flotation behavior and adsorption mechanism of (1-hydroxy-2-methyl-2-octenyl) phosphonic acid to cassiterite. Trans. Nonferrous Met. Soc. China 2016, 26, 2469–2478. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Luo, B.B.; Sun, C.Y.; Liu, J.; Sun, H.T. Density functional theory study of α-Bromolauric acid adsorption on the α-quartz (1 0 1) surface. Miner. Eng. 2016, 92, 72–77. [Google Scholar] [CrossRef]
- Miller, J.D.; Wang, X.M.; Jin, J.Q.; Shrimali, K. Interfacial water structure and the wetting of mineral surfaces. Int. J. Miner. Process. 2016, 156, 62–68. [Google Scholar] [CrossRef]
- Mulheran, P.A.; Harding, J.H. The stability of SnO2 surfaces. Model. Simul. Mater. Sci. Eng. 1992, 1, 39–43. [Google Scholar] [CrossRef]
- Mulheran, P.A.; Harding, J.H. Thermodynamic calculations of stannic oxide surfaces. J. Phys. IV 1993, 3, C7-1971–C7-1974. [Google Scholar] [CrossRef]
- Oviedo, J.; Gillan, M.J. Energetics and structure of stoichiometric SnO2 surfaces studied by first-principles calculations. Surf. Sci. 2000, 463, 93–101. [Google Scholar] [CrossRef]
- Slater, B.; Catlow, C.R.A.; Gay, D.H.; Williams, D.E.; Dusastre, V. Study of surface segregation of antimony on SnO2 surfaces by computer simulation techniques. J. Phys. Chem. B 1999, 103, 10644–10650. [Google Scholar] [CrossRef]
- Bandura, A.V.; Sofo, J.O.; Kubicki, J.D. Derivation of force field parameters for SnO2−H2O surface systems from plane-wave density functional theory calculations. J. Phys. Chem. B 2006, 110, 8386–8397. [Google Scholar] [CrossRef] [PubMed]
- Vlcek, L.; Zhang, Z.; Machesky, M.L.; Fenter, P.; Rosenqvist, J.; Wesolowski, D.J.; Anovitz, L.M.; Predota, M.; Cummings, P.T. Electric double layer at metal oxide surfaces: Static properties of the cassiterite–water interface. Langmuir 2007, 23, 4925–4937. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.G.; Cohen, R.E. A more accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Rai, B.; Sathish, P.; Tanwar, J.; Pradip; Moon, K.S. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Coll. Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H.; Sinha, N.; Rath, S.S.; Das, B. Ionic liquids as novel quartz collectors: Insights from experiments and theory. Chem. Eng. J. 2015, 273, 46–54. [Google Scholar] [CrossRef]
- Li, F.X.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G.Y. Flotation performances and adsorption mechanism of α-hydroxyoctyl phosphinic acid to cassiterite. Appl. Surf. Sci. 2015, 353, 856–864. [Google Scholar] [CrossRef]
- Feng, Q.C.; Zhao, W.J.; Wen, S.M.; Cao, Q.B. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Sauer, J.; Sustmann, R. Mechanistic aspects of Diels-Alder reactions: A critical survey. Angew. Chem. Int. Ed. Engl. 1980, 19, 779–807. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.Y.; Wang, S.; Gao, Y.D.; Dai, Z.L.; Huang, J.P.; Liu, G.Y. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Sun, L.H.; Li, G.P.; Chen, W.; Hu, J.F. Adsorption of CO on the oxygen defective LaCoO3 (001) surface: A first-principles study. Comp. Mater. Sci. 2016, 115, 154–157. [Google Scholar] [CrossRef]
| SnO2 | SiO2 | CaCO3 | MgO | Al2O3 | TFe | Pb | Zn | Cu |
|---|---|---|---|---|---|---|---|---|
| 97.77 | <0.001 | <0.001 | 0.075 | <0.01 | 0.007 | <0.001 | 0.0027 | <0.001 |
| Species | Bond Lengths/Å | Bond Angles/° | Mulliken Charges | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P=O1 | P–O2 | P–O3 | O1–P–O2 | O2–P–O3 | O1–P–O3 | P | O1 | O2 | O3 | |
| Molecular | 1.456 | 1.590 | 1.595 | 111.614 | 104.880 | 114.671 | 2.230 | −1.040 | −1.020 | −1.030 |
| Monoanion | 1.493 | 1.600 | 1.487 | 110.643 | 109.927 | 117.591 | 2.320 | −0.900 | −1.030 | −0.960 |
| Dianion | 1.510 | 1.503 | 1.507 | 111.747 | 113.658 | 110.868 | 2.410 | −0.810 | −0.870 | −0.860 |
| Adsorbates | RPO3H2 | RPO3H− | RPO32− | H2O | OH− |
|---|---|---|---|---|---|
| ELUMO-HOMO (eV) | 3.389 | 0.448 | 0.624 | 7.647 | 7.717 |
| Adsorption Complex | H2O | OH− | Monodentate | Bidentate | Binuclear |
|---|---|---|---|---|---|
| Interaction energy, kJ/mol | −76.74 | −115.22 | −201.06 | −363.24 | −369.52 |
| Atom | Status | s | p | Total | Charge/e |
|---|---|---|---|---|---|
| Sn3 | Before adsorption | 1.00 | 1.20 | 2.20 | 1.80 |
| After adsorption | 0.93 | 1.22 | 2.15 | 1.85 | |
| O3 | Before adsorption | 1.92 | 4.94 | 6.86 | −0.86 |
| After adsorption | 1.88 | 5.07 | 6.95 | −0.95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, G.; Han, Y.; Liu, J.; Zhu, Y.; Li, Y.; Yuan, S. In Situ Investigation of the Adsorption of Styrene Phosphonic Acid on Cassiterite (110) Surface by Molecular Modeling. Minerals 2017, 7, 181. https://doi.org/10.3390/min7100181
Gong G, Han Y, Liu J, Zhu Y, Li Y, Yuan S. In Situ Investigation of the Adsorption of Styrene Phosphonic Acid on Cassiterite (110) Surface by Molecular Modeling. Minerals. 2017; 7(10):181. https://doi.org/10.3390/min7100181
Chicago/Turabian StyleGong, Guichen, Yuexin Han, Jie Liu, Yimin Zhu, Yanfeng Li, and Shuai Yuan. 2017. "In Situ Investigation of the Adsorption of Styrene Phosphonic Acid on Cassiterite (110) Surface by Molecular Modeling" Minerals 7, no. 10: 181. https://doi.org/10.3390/min7100181





