The Influence of Backwater Al3+ on Diaspore Bauxite Flotation
Abstract
:1. Introduction
2. Materials and Methods
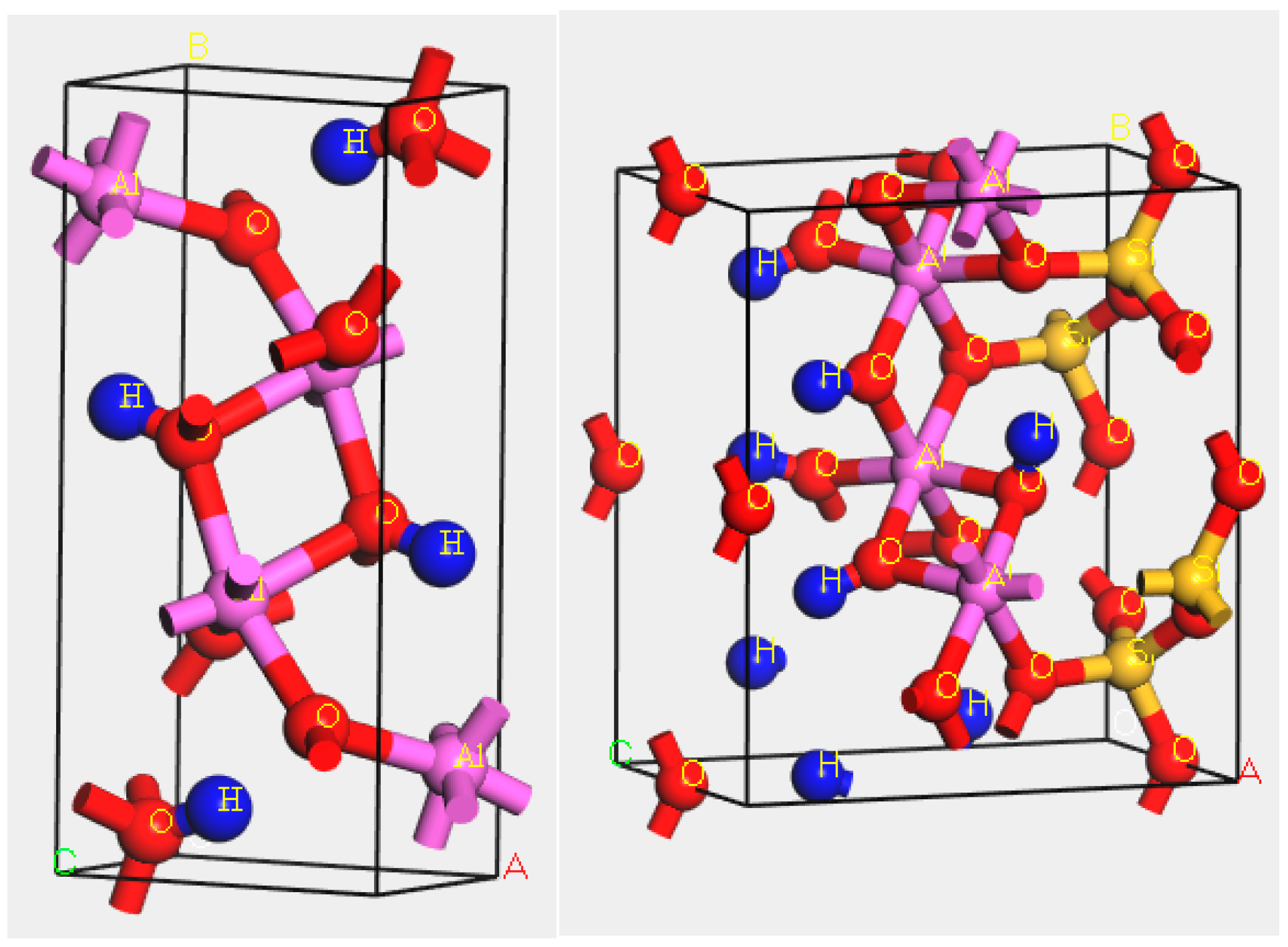
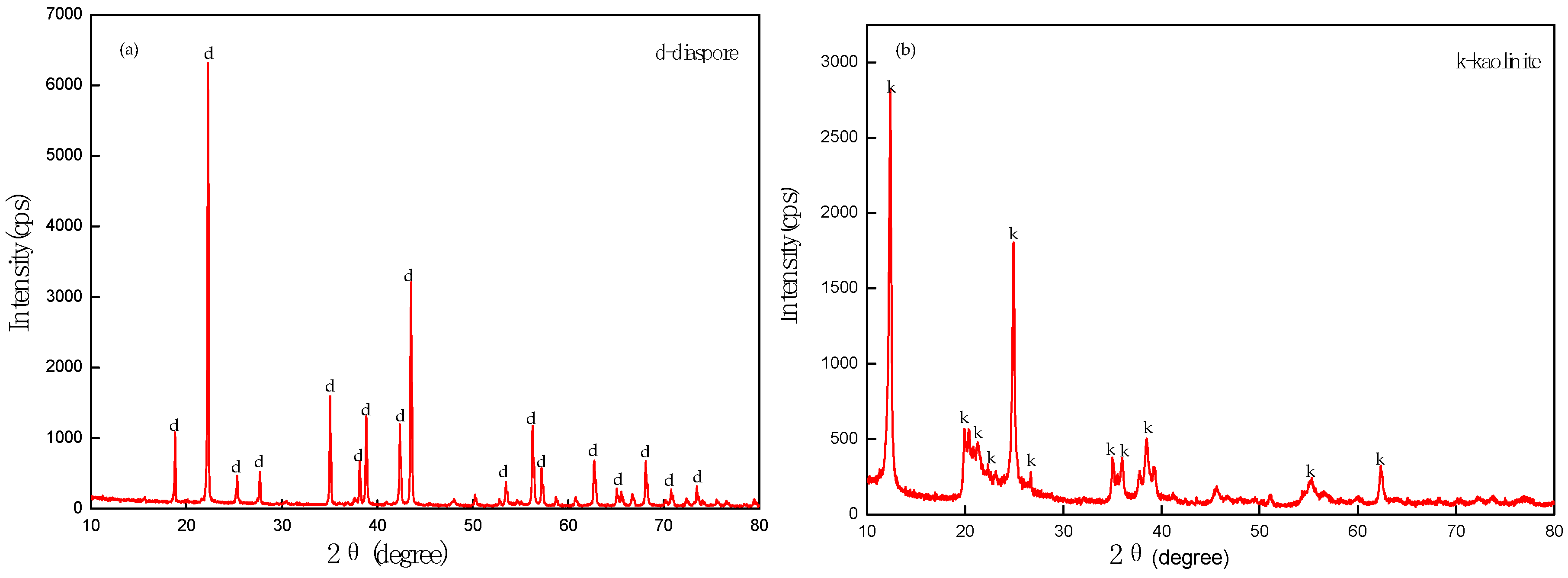
2.1. Minerals
2.2. Reagents
2.3. Micro-Flotation Tests
2.4. Zeta Potential Measurements
2.5. Synchrotron X-ray Absorption Spectroscopy Analyses
3. Results and Discussion
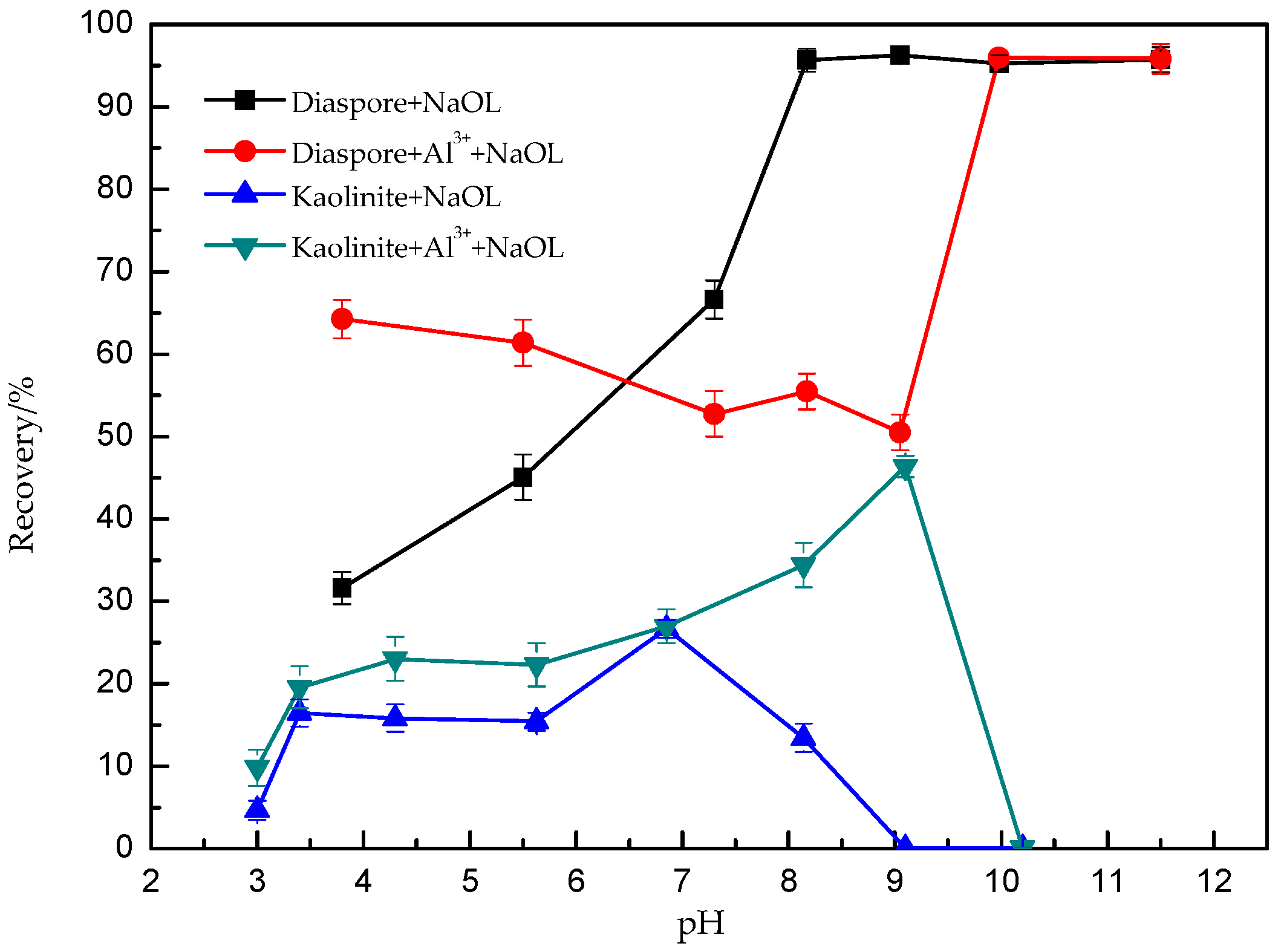
3.1. The Effects of Backwater Al3+ on Diaspore and Kaolinite Flotation Recoveries
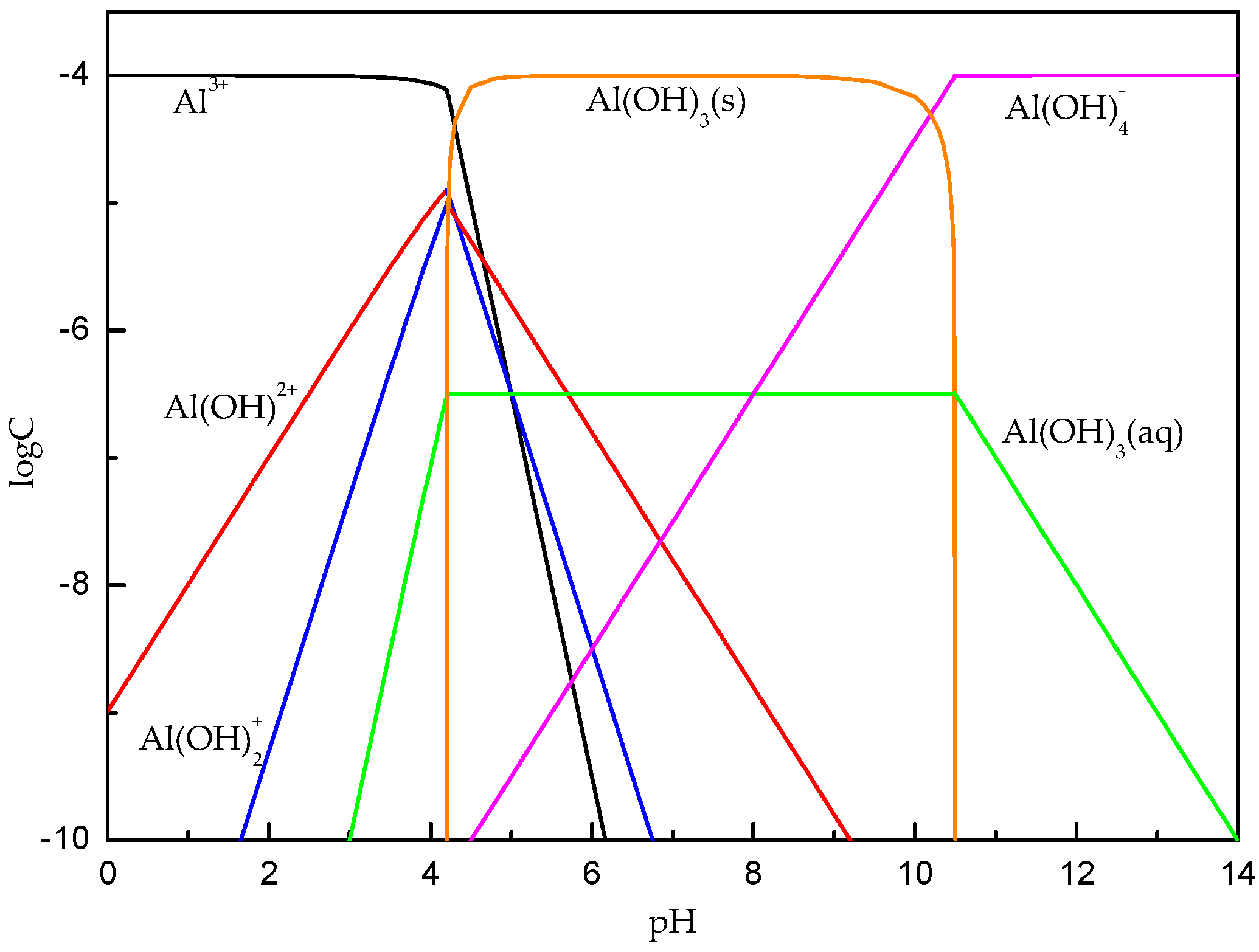
3.2. Solution Chemistry Analyses
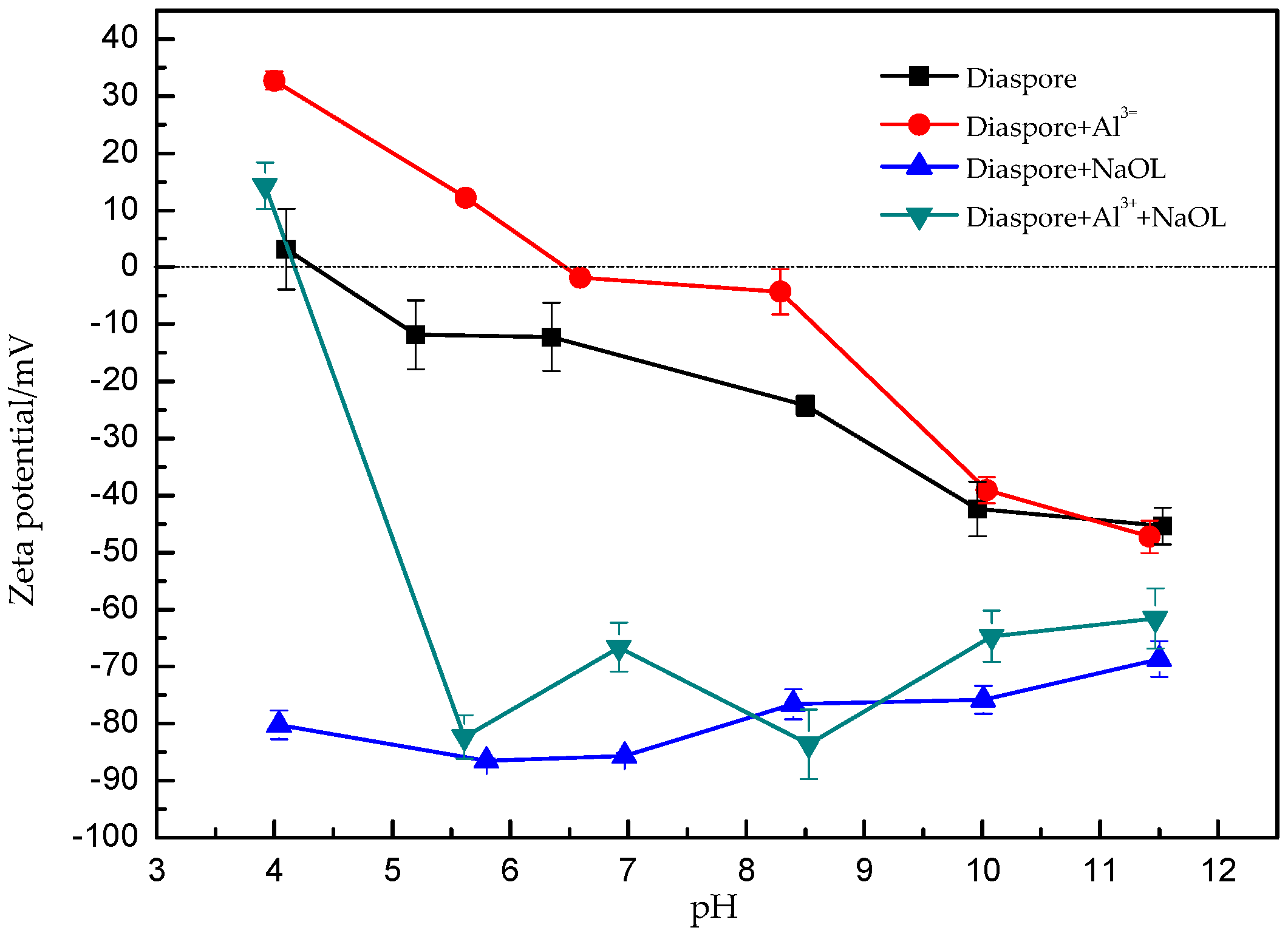
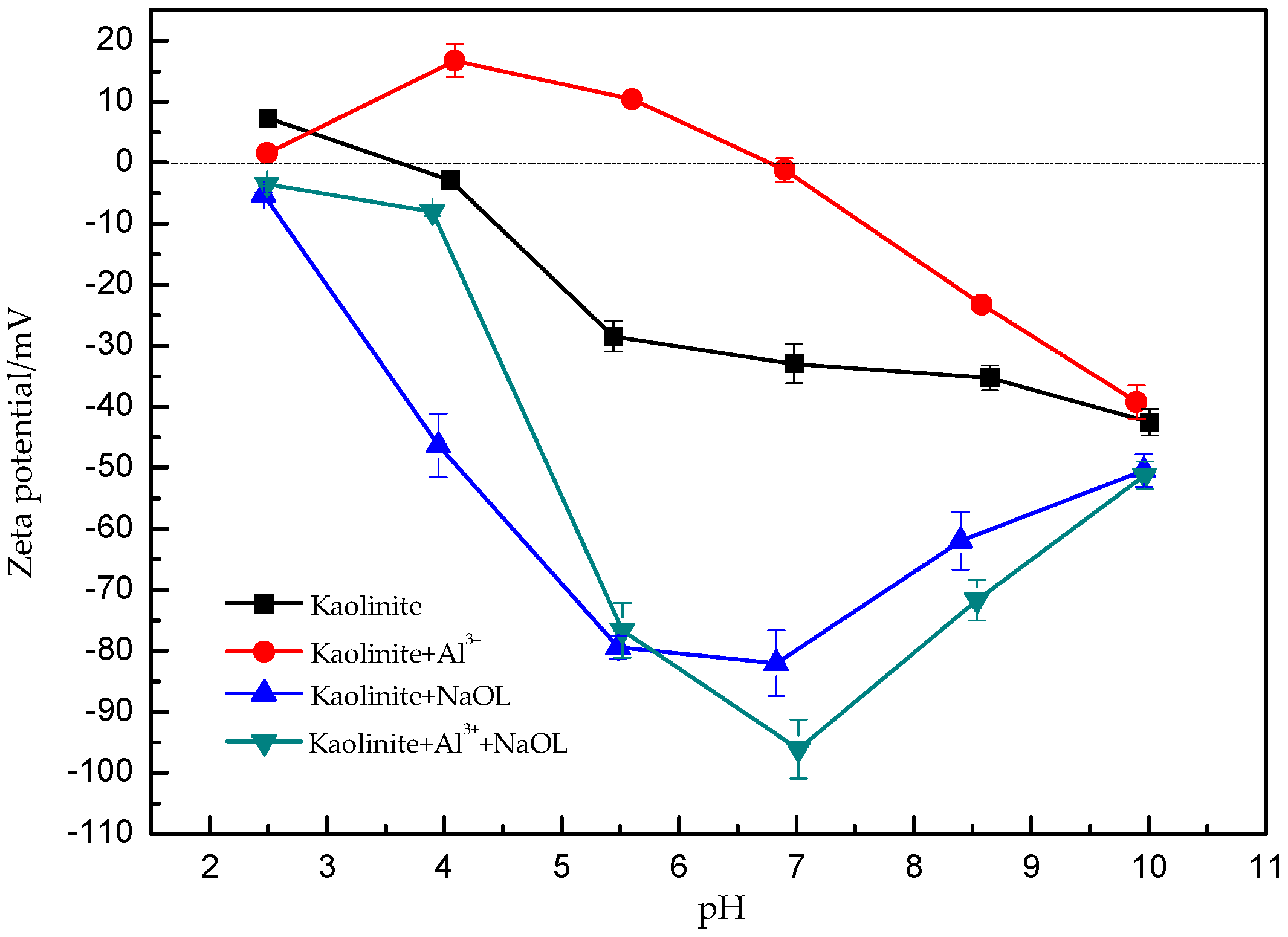
3.3. Zeta Potential Measurements
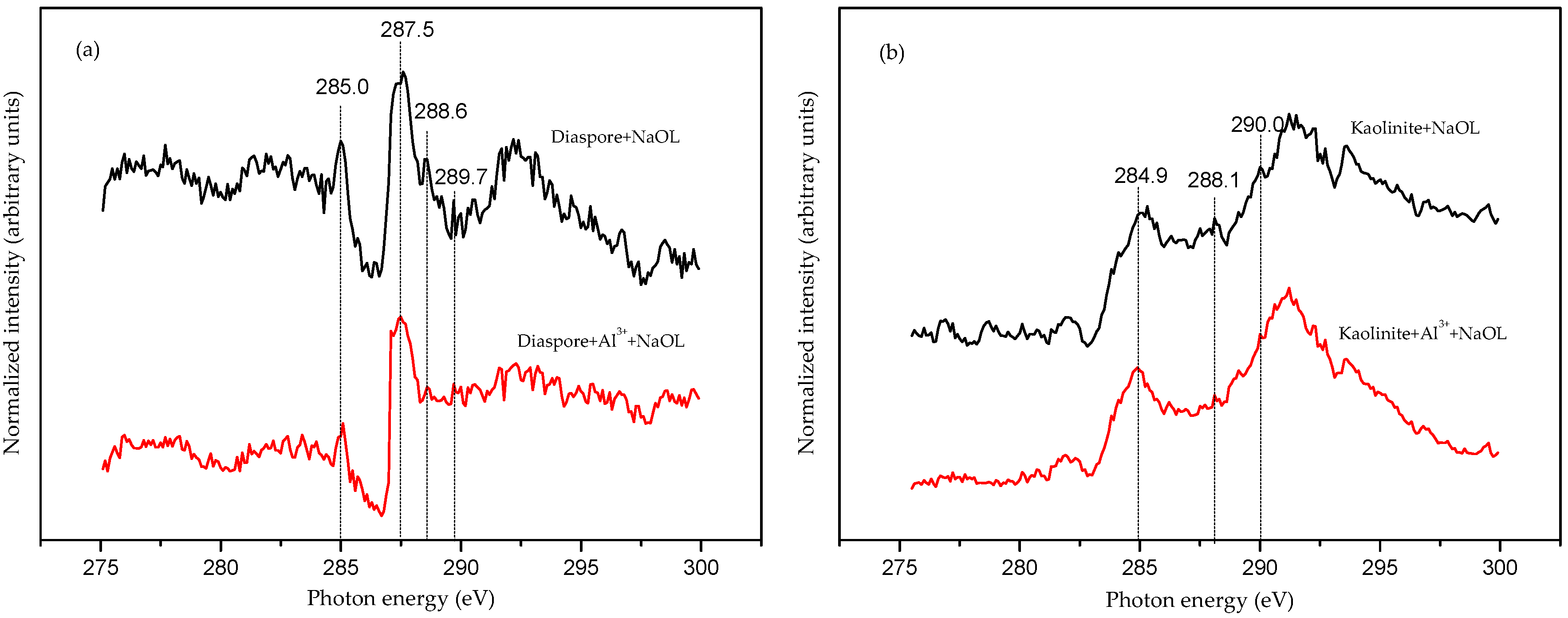
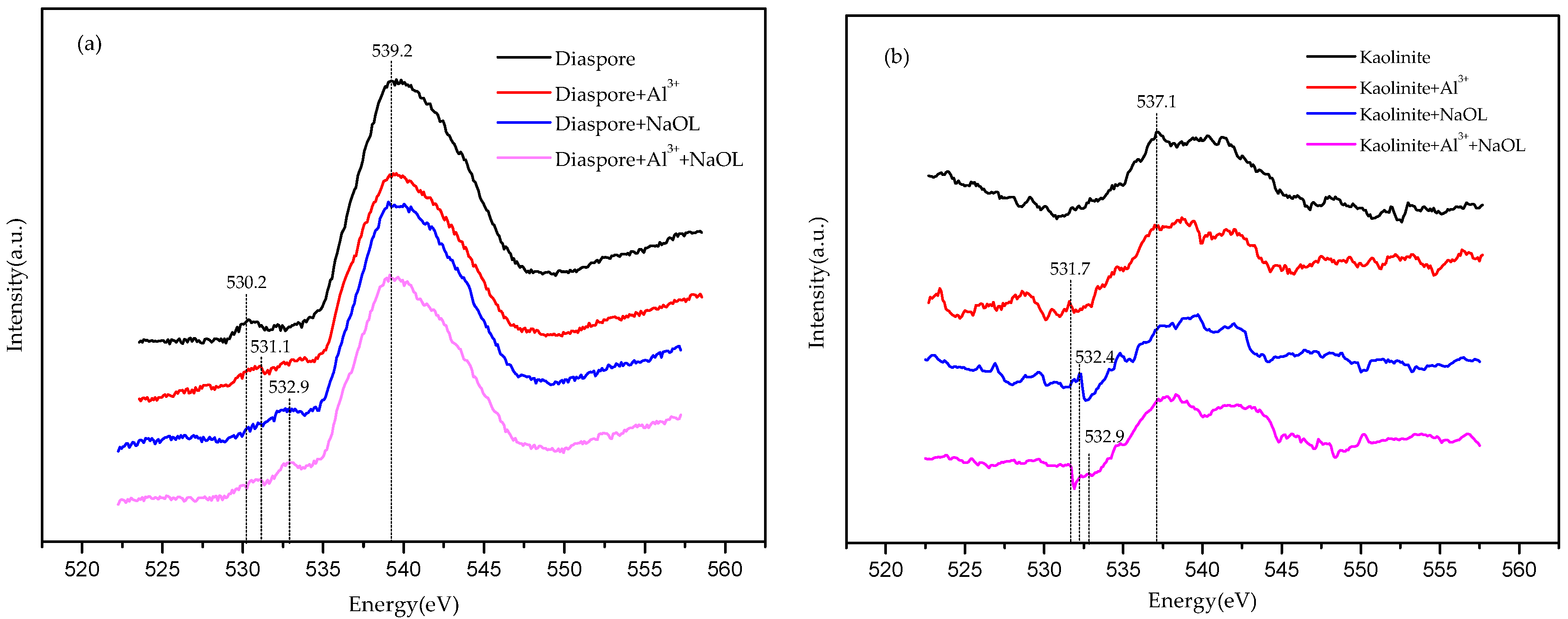
3.4. Synchrotron X-ray Absorption Spectroscopy Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, M.; Peng, Q.M. Chapter 1, China Mineral Resources. In Situation of Mineral Resources; Geological Publishing House: Beijing, China, 2016; p. 5. [Google Scholar]
- Aluminum Corporation of China Limited; Beijing General Research Institute of Mining & Metallurgy; Central South University of Technology. Total Report of Bayer-Mineral Processing Method Producing Alumina; China Nonferrous Metals Industry Association: Beijing, China, 1999. [Google Scholar]
- Friedrich, A.; Wilson, D.J.; Haussuhl, E.; Winkler, B.; Morgenroth, W.; Refson, K.; Milman, V. High-pressure properties of diaspore, AlO(OH). Phys. Chem. Miner. 2007, 34, 145–157. [Google Scholar] [CrossRef]
- Bish, D.L. Rietveld refinement of the kaolinite structure at 1.5 K. Clays Clay Miner. 1993, 41, 738–744. [Google Scholar] [CrossRef]
- Zhang, G.F. Theory and Technology of Flotation on Bauxite Desilicate. Ph.D. Thesis, Central South University, Changsha, China, 2001. [Google Scholar]
- Fatai, I.; Maria, M.; Bjorn, J.; Kota, H.R. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar]
- Morkel, J.; Pistorius, P.C.; Vermaak, M.K.G. Cation exchange behavior of kimberlite in solutions containing Cu2+ and K+. Miner. Eng. 2007, 20, 1145–1152. [Google Scholar] [CrossRef]
- Costa, C.A.; Rubio, J. Deinking flotation: Influence of calcium soap and surface-active substances. Miner. Eng. 2005, 18, 59–64. [Google Scholar] [CrossRef]
- Kirjavainen, V.; Schreithofer, N.; Heiskanen, K. Effect of calcium and thiosulfate ions on flotation selectivity of nickel-copper ores. Miner. Eng. 2002, 15, 1–5. [Google Scholar] [CrossRef]
- Liu, R.Q.; Guo, Y.Z.; Wang, L.; Sun, W.; Tao, H.B.; Hu, Y.H. Effect of calcium hypochlorite on the flotation separation of galena and jamesonite in high-alkali systems. Miner. Eng. 2015, 84, 8–14. [Google Scholar] [CrossRef]
- Gan, W.B.; Brendan, C.; Liu, Q. Effect of citric acid on inhibiting hexadecane-quartz coagulation in aqueous solutions containing Ca2+, Mg2+ and Fe3+ ions. Int. J. Miner. Process. 2009, 92, 84–91. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.H.; Sun, W.; Hu, Y.H.; Yin, Z.G.; Lai, X.S. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.M.; Zhang, G.F.; Ma, W.K.; Meng, Q.Y.; Chen, Y.F. Effects of lead ions on the flotation of hemimorphite using sodium oleate. Miner. Eng. 2016, 8, 163–167. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.F.; Feng, Q.M.; Ou, L.M.; Lu, Y.P. Effect of the lattice ions on the calcite flotation in presence of Zn(II). Miner. Eng. 2013, 40, 24–29. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.Q.; Liu, J.; Huang, Y.; Feng, Q.M.; Zhao, H. Fe(III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar]
- Jiang, Y.Q. Research on Dewatering and Water Recycling for Bauxite Direct-Flotation Tailings. Master’s Thesis, Central South University, Changsha, China, 2011. [Google Scholar]
- He, P.B.; Liu, H.; Chen, X.H. Influence of backwater on bauxite flotation desilication. Nonferr. Met. 2011, 3, 25–28. [Google Scholar]
- Ou, L.M.; Feng, Q.M.; Chen, Y.; Lu, Y.P.; Zhang, G.F. Disintegration mode of bauxite and selective separation of Al and Si. Miner. Eng. 2006, 20, 200–203. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, M.C. Flotation: A. M. Gaudin Memorial Volume; American Institute of Mining, Metallurgical, and Petroleum Engineers, Inc.: New York, NY, USA, 1976; Volume 1, pp. 148–150. [Google Scholar]
- Wang, D.Z.; Hu, Y.H. Solution Chemistry of Flotation; Hunan Science and Technology Press: Changsha, China, 1988; Volume 6, pp. 132–179. [Google Scholar]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Hu, Y.H.; Wang, Y.H. Effect of metallic ions on dispersibility of diaspore. Trans. Nonferr. Met. Soc. China 2011, 21, 1166–1171. [Google Scholar] [CrossRef]
- Peng, H.Q.; Luo, W.; Wu, D.; Bie, X.X.; Shao, H.; Jiao, W.Y.; Liu, Y.K. Study on the effect of Fe3+ on zircon flotation separation from cassiterite using sodium oleate as collector. Minerals 2017, 7, 108. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.N.; Peng, H.; Zhao, H.B.; Qin, W.Q.; Qiu, G.Z.; Wang, J. The activation mechanism of Bi3+ ions to rutile flotation in a strong acidic environment. Minerals 2017, 7, 113. [Google Scholar] [CrossRef]
- Li, F.X.; Zhong, H.; Wang, S.; Liu, G.Y. The activation mechanism of Cu(II) to ilmenite and subsequent flotation response to α-hydroxyoctyl phosphinic acid. J. Ind. Eng. Chem. 2016, 37, 123–130. [Google Scholar] [CrossRef]
- Jiang, H. Studies on Solution Chemistry of Interactions between Cationic Collectors and Aliminosilicate Aluminum Minerals in Bauxite Flotation Desilica. Ph.D. Thesis, Central South University, Changsha, China, 2004. [Google Scholar]
- Lu, Y.P. Research on Bauxite Desilication by Selective Grinding-Aggregation Flotation. Ph.D. Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Zhou, Y.L. Investigation of the Influence of Metal Ions on Selective Dispersion of Diaspore and Silicate Minerals. Ph.D. Thesis, Central South University, Changsha, China, 2011. [Google Scholar]
- Wang, J.; Cheng, H.W.; Zhao, H.B.; Qin, W.Q.; Qiu, G.Z. Flotation behavior and mechanism of rutile with nonyl hydroxamic acid. Rare Metals 2016, 35, 419–424. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.H. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Bai, D.; Sun, W.; Cao, X.F.; Hu, Y.H. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Gao, Y.S.; Gao, Z.Y.; Sun, W.; Hu, Y.H. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Christopher, E.; Wolfgang, E.S.U.; Peter, S. C K-edge NEXAFS spectra of graphene with physical and chemical defects: A study based on density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14083. [Google Scholar]
- Mikael, T.; Rainer, B.; Karina, T.; Chithra, K.; Ulf, S.; Torbjorn, A.L. Nanomapping and speciation of C and Ca in thermally treated lignocellulosic cell walls using scanning transmission X-ray microscopy and K-edge XANES. Fuel 2016, 167, 149–157. [Google Scholar]
- Akihito, I.; Hidenori, S.; Naomichi, O.; Hiroshi, K.; Toshiaki, O.; Yoshihiro, N. Pretreatment Dependence of Adsorption Properties of Merocyanine Dye at Rutile (110) and 100 TiO2 surface study by C K-edge NEXAFS. J. Phys. Chem. C 2009, 113, 17254–17261. [Google Scholar]
- Francesca, G.; Paola, Z.; Alain, J.C.; Marco, N.; Enrico, T.; Davide, B.; Maria, G.P. Stability and extreme ultraviolet photo-reduction of graphene during C-K-edge characteraction. Surf. Coat. Technol. 2016, 296, 211–215. [Google Scholar]
- Alberto, B.; Marco, M.; Vincenzo, B.; Giovanna, F.; Gustavo, A.C.J.; Mauro, S.; Cesare, G.; de Monica, S.; Marcello, C. Vibrationally resolved NEXAFS at C and N K-edges of pyridine, 2-fluoropyridine and 2,6-difluoropyridine: A combined experimental and theoretical assessment. J. Chem. Phys. 2015, 143, 204102. [Google Scholar] [Green Version]
- Masaaki, Y.; Yosuke, M.; Takehiro, M.; Masanari, N.; Hayato, Y.; Nobuhiro, K.; Hiroshi, K. Direct Observation of Active Nickel Oxide Cluster in Nickel−Borate electrocatalyst for water oxidation by in situ O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 2015, 119, 19279–19286. [Google Scholar]
- Natalia, P.; Anil, A.; Teguh, C.A.; Diao, C.Z.; Yu, X.J.; Mark, B.H.B.; Venkatesan, T.; Ariando; Andrivo, R. Electronic defect states at the LaAlO3/SrTiO3 heterointerface revealed by O K-edge X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 13844. [Google Scholar]
- Benjamin, J.A.M.; Grant, S.H.; Hiroshi, F.; Nozomu, H.; de Dominique, L.; Camille, S.; Masami, K. In situ structural changes of amorphous diopside (CaMgSi2O6) up to 20 GPa: A Raman and O K-edge X-ray Roman spectroscopic study. Geochim. Cosmochim. Acta 2016, 178, 41–61. [Google Scholar]
- Guglieri, C.; Chaboy, J. O K-Edge X-ray Absorption Spectroscopy in Al-Doped ZnO Materials: Structural vs Electronic Effects. J. Phys. Chem. 2014, 118, 25779–25785. [Google Scholar] [CrossRef]
- Liu, X.; Dean, M.P.; Liu, J.; Chluzbalan, S.G.; Jaouen, N.; Nicolaou, A.; Yin, W.G.; Rayan Serrao, C.; Ramesh, R.; Ding, H.; et al. Probing single magnon excitations in Sr2IrO4 using O K-edge resonant inelastic X-ray scattering. J. Phys. Condens. Matter 2015, 27, 202202. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.A.M.; Grant, S.H.; Camille, S.; Cedrick, O.S.; Lucia, Z.; Tom, R.; de Dominique, L. The structure of haplobasaltic glasses investigated using X-ray absorption near edge structure (XANES) spectroscopy at the Si, Al, Mg, and O K-edges and Ca, Si, and Al L2, 3-edges. Chem. Geol. 2016, 420, 213–230. [Google Scholar]
- Karen, H.; Johannes, L.; Dawit, S.; Michael, W.I.S.; Thomas, R. C 1s K-edge near edge X-ray absorption fine structure (NEXAFS) spectroscopy for characterizing functional group chemistry of black carbon. Org. Geochem. 2011, 42, 1055–1064. [Google Scholar]

| Sample | Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Diaspore | 80.86 | 0.82 | 0.24 | 2.81 | 0.01 | 0.049 | 0.008 | 0.029 | 14.62 |
| Kaolinite | 39.61 | 43.55 | 0.34 | 1.86 | 0.015 | 0.071 | 0.01 | 0.026 | 14.18 |
| Sample | Ca2+ (mmol/L) | Mg2+ (mmol/L) | Al3+ (mmol/L) | Fe3+ (mmol/L) |
|---|---|---|---|---|
| Fresh water | 1.793 | 0.838 | 0.004 | 0.018 |
| Backwater | 0.215 | 0.077 | 0.130 | 0.008 |
| C Form | Bond | Peak Energy (eV) | Fit Position (eV) |
|---|---|---|---|
| Aromatic C | C=O | 283–284.5 | 284.5 |
| Aromatic C | C=C | 284.9–285.5 | 285.4 |
| Alkyl C | C–H | 287–287.6 | 287.6 |
| Carboxylic C | R–COOH | 288–288.7 | 288.4 |
| O-alkyl C/carbonyl | COO− | 289.5–290.2 | 289.9 |
| O Form | Bond | Peak Energy (eV) | Fit Position (eV) |
|---|---|---|---|
| –Al–O–H | –Al–O–H | 529.6–530.6 | 530.2 |
| –Al–O–Al–(OH)2 | –Al–(OH)2 | 530.7–531.2 | 531.1 |
| –Al/Si–O–Al–(OH)2 | –Al–(OH)2 | 531.3–531.9 | 531.7 |
| –Al/Si–O–H⋯RCOO− | –Al/Si–O–H⋯O | 532.0–532.4 | 532.4 |
| –Al–O–H⋯RCOO− | –Al–O–H⋯O | 532.5–533.8 | 532.9 |
| –Al–O–Al–(OH)2⋯RCOO− | –Al–O–H⋯O | 532.5–533.3 | 532.9 |
| –Al/Si–O–Al–(OH)2⋯RCOO− | –Al–O–H⋯O | 532.5–533.0 | 532.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; Chang, Z.; Feng, Q.; Xiao, W.; Yu, S.; Qiu, G.; Wang, J. The Influence of Backwater Al3+ on Diaspore Bauxite Flotation. Minerals 2017, 7, 195. https://doi.org/10.3390/min7100195
Fang C, Chang Z, Feng Q, Xiao W, Yu S, Qiu G, Wang J. The Influence of Backwater Al3+ on Diaspore Bauxite Flotation. Minerals. 2017; 7(10):195. https://doi.org/10.3390/min7100195
Chicago/Turabian StyleFang, Chaojun, Ziyong Chang, Qiming Feng, Wei Xiao, Shichao Yu, Guanzhou Qiu, and Jun Wang. 2017. "The Influence of Backwater Al3+ on Diaspore Bauxite Flotation" Minerals 7, no. 10: 195. https://doi.org/10.3390/min7100195





