Rare Earth Element Fluorocarbonate Minerals from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia
Abstract
:1. Introduction
2. Background
2.1. REE-Fluorocarbonate Minerals
2.2. Olympic Dam Mineralogy
3. Sampling and Analytical Methods
3.1. Sampling
3.2. Analytical Methods
4. Petrography
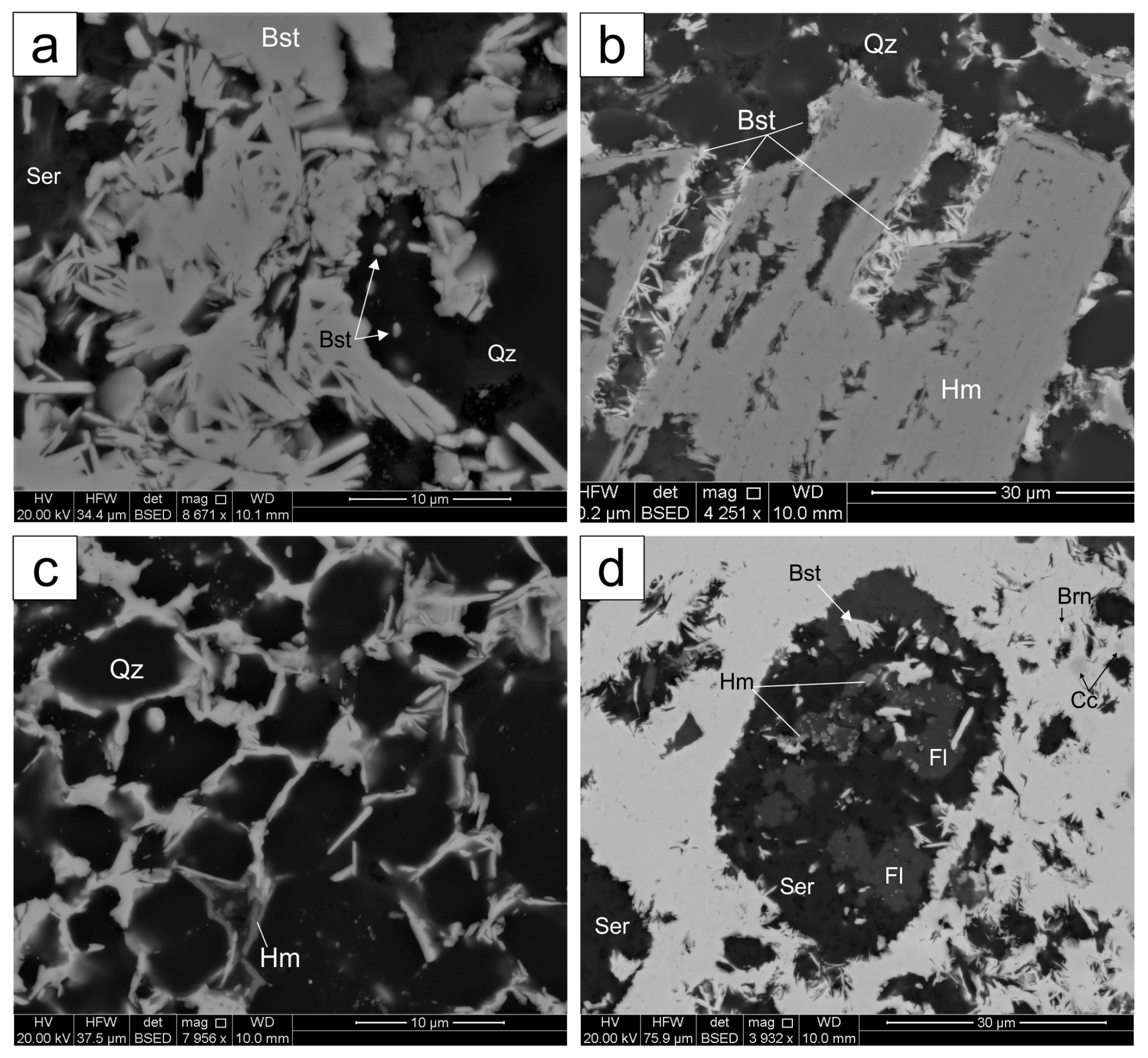
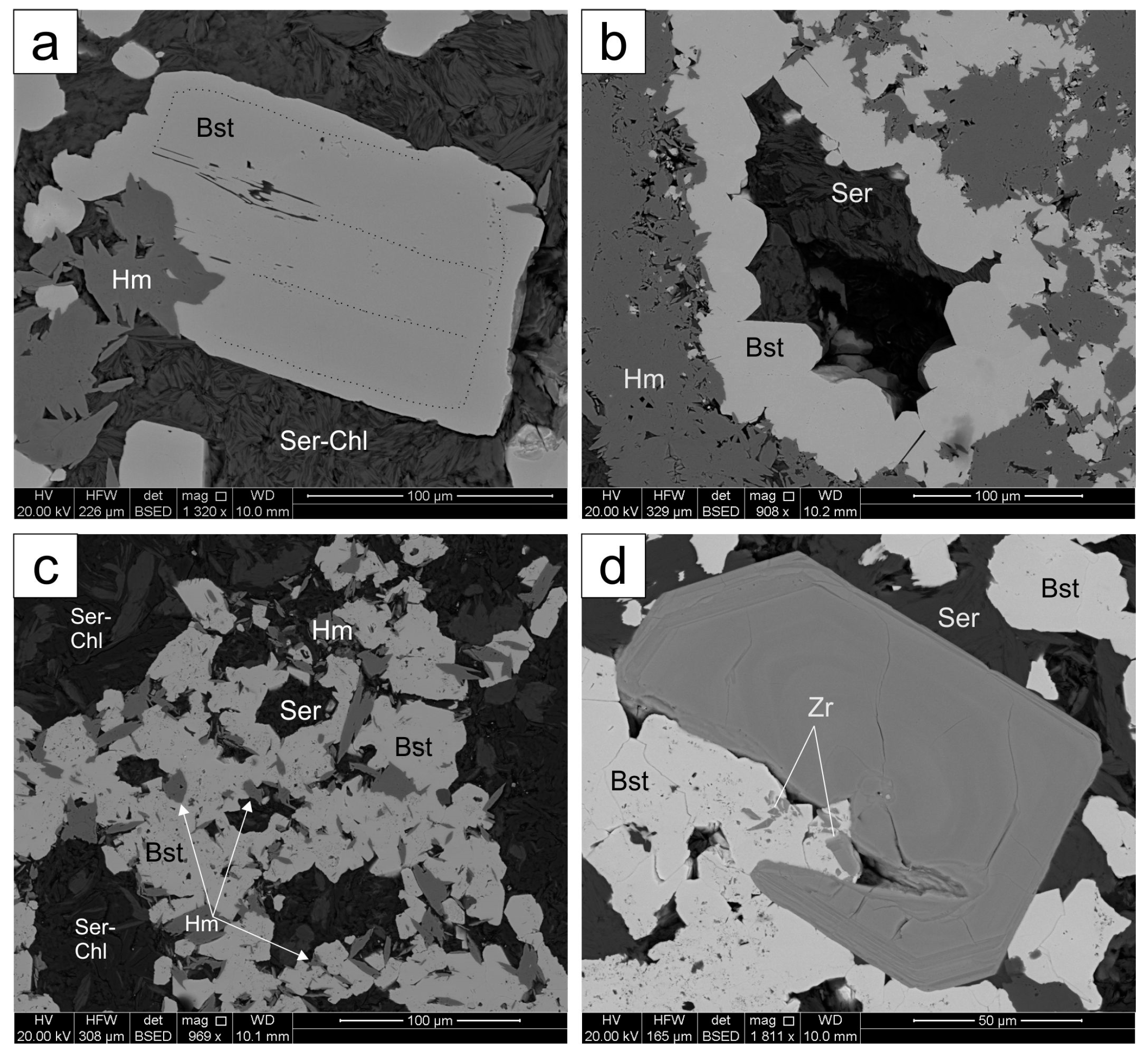
4.1. Bastnäsite
4.1.1. Matrix Bastnäsite
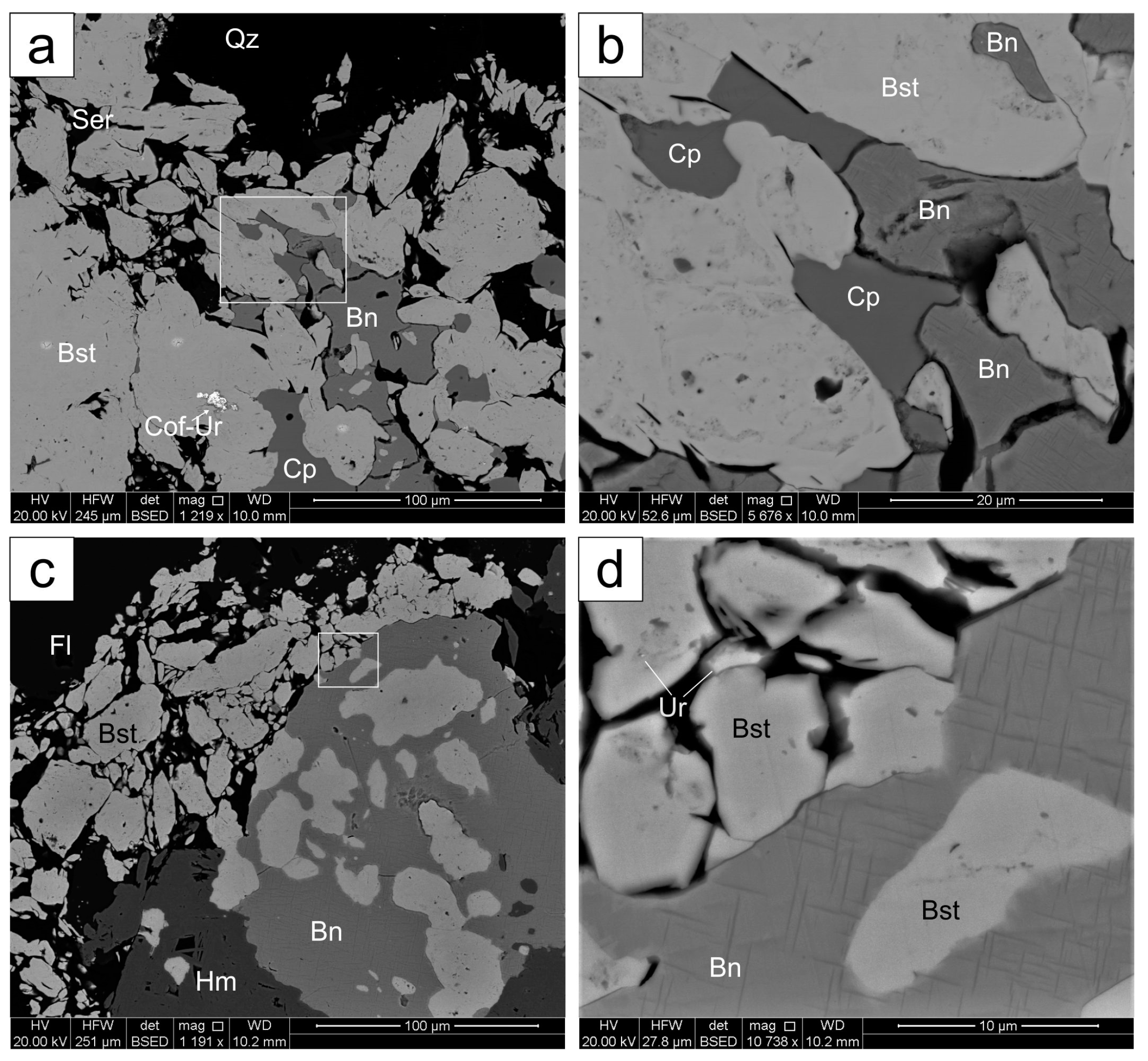
4.1.2. Irregular Bastnäsite Associated with Sulfides
4.1.3. Bastnäsite Replacing Breccia Clasts
4.2. Synchysite
4.3. Other REE-Minerals
5. Compositional Data
5.1. Bastnäsite-(Ce)
5.2. Synchysite-(Ce)
5.3. Trace Element Concentrations
5.4. Compositional Trends and Chondrite-Normalized Fractionation Patterns
6. Discussion
6.1. Data Trends
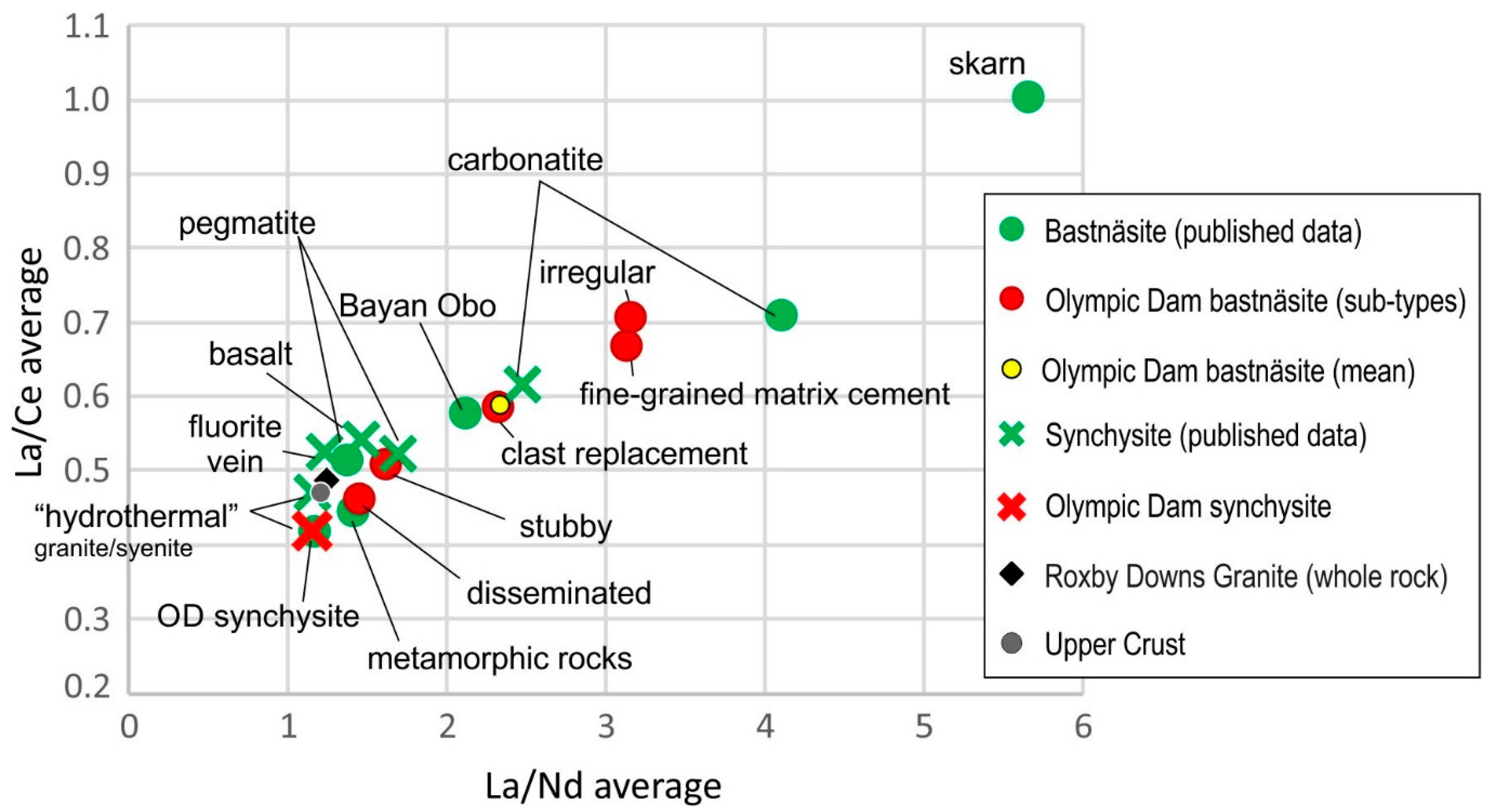
6.2. Comparison with Published Compositional Data
6.3. Formation Conditions
6.4. Implications
7. Conclusions
- Bastnäsite-(Ce) is the most abundant REE-fluorocarbonate across the Olympic Dam deposit; synchysite-(Ce) is subordinate. Representative formulae for Olympic Dam bastnäsite-(Ce) and synchysite-(Ce) are: ((Ca,Sr0.01)La0.31Ce0.49Nd0.12RE*0.08)1.00(F,Cl)0.75(CO3) and (Ca,Sr)1.00(La0.20Ce0.47Nd0.18RE*0.15)1.00(F,Cl)0.55(CO3)2, respectively [RE* = REE other than La, Ce, and Nd]. Both show significant deficiencies in the halogen site, which may possibly be due to F migration under the beam, or met by hydroxl ions.
- Bastnäsite occurs in a range of different textural forms, defined here as matrix, including fine-grained disseminated matrix, fine-grained cement matrix, and stubby matrix, as well as irregular bastnäsite associated with sulfides, and clast replacement bastnäsite. Textures and occurrences of bastnäsite at Olympic Dam are largely driven by the specific location and prevailing mineral assemblage, with morphology and grain size often controlled by the associated minerals (hematite, sulfides). High REE-grade zones formed due to enhanced permeability/porosity and localized conditions that promoted REE deposition.
- Compositionally, REE-fluorocarbonates define a spectrum from relatively La-enriched to (Ce + Nd)-enriched minerals, although Ce is the dominant element in all grains analyzed. The fractionation of REE in bastnäsite and synchysite may reflect two distinct episodes of precipitation during the IOCG-forming event. The earlier, represented by stubby bastnäsite and synchysite may be associated with hydrothermal alteration of granite and Ca release from plagioclase, whereas the latter (bastnäsite only) is contemporaneous with the onset of sulfide deposition.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- BHP Annual Reporting Suite 2016. Available online: http://www.bhp.com/investor-centre/annual-reporting-2016 (accessed on 27 September 2017).
- Williams, P.J.; Barton, M.D.; Johnson, D.A.; Fontbote, L.; de Haller, A.; Mark, G.; Oliver, N.H.S.; Marschik, R. Iron Oxide Copper-Gold Deposits: Geology, Space-Time Distribution, and Possible Modes of Origin. Econ. Geol. 2005, Anniversary, 371–405. [Google Scholar]
- Groves, D.I.; Bierlein, F.P.; Meinert, L.D.; Hitzman, M.W. Iron Oxide Copper-Gold (IOCG) Deposits through Earth History: Implications for Origin, Lithospheric Setting, and Distinction from Other Epigenetic Iron Oxide Deposits. Econ. Geol. 2010, 105, 641–654. [Google Scholar] [CrossRef]
- Roberts, D.E.; Hudson, G.R.T. The Olympic Dam Copper-Uranium-Gold Deposit, Roxby Downs, South Australia. Econ. Geol. 1983, 78, 799–822. [Google Scholar] [CrossRef]
- Oreskes, N.; Einaudi, M.T. Origin of Rare Earth Element-Enriched Hematite Breccias at the Olympic Dan Cu-U-Au-Ag Deposit, Roxby Downs, South Australia. Econ. Geol. 1990, 85, 1–18. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Rare Earth Element Mineralogy of the Olympic Dam Cu-U-Au-Ag Deposit, Roxby Downs, South Australia: Implications for Ore Genesis. Neues Jahrb. Mineral. Monatshefte 1995, 8, 371–384. [Google Scholar]
- Ehrig, K.; McPhie, J.; Kamenetsky, V.S. Geology and mineralogical zonation of the Olympic Dam iron oxide Cu-U-Au-Ag deposit, South Australia. In Geology and Genesis of Major Copper Deposits and Districts of the World, a Tribute to Richard Sillitoe; Society of Economic Geologists Special Publication; Hedenquist, J.W., Harris, M., Camus, F., Eds.; SEG: Littleton, CO, USA, 2012; Volume 16, pp. 237–268. [Google Scholar]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Mineral. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Pring, A. Chemical and textural interpretation of late stage coffinite and brannerite from the Olympic Dam IOCG-Ag-U deposit. Mineral. Mag. 2017. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Kontonikas-Charos, A. Apatite at Olympic Dam, South Australia: A petrogenetic tool. Lithos 2016, 262, 470–485. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Kontonikas-Charos, A. Rare earth element behaviour in apatite from the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Minerals 2017, 7, 135. [Google Scholar] [CrossRef]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Krneta, S.; Kamenetsky, V.S. Feldspar Evolution in the Roxby Downs Granite, Host to Fe-Oxide Cu-Au-(U) Mineralisation at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 80, 838–859. [Google Scholar] [CrossRef]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Ismail, R.; Krneta, S.; Basak, A. Feldspar mineralogy and rare earth element (re)mobilization in iron-oxide copper gold systems from South Australia: A nanoscale study. Mineral. Mag. 2017. [Google Scholar] [CrossRef]
- Donnay, G.; Donnay, J.D.H. The crystallography of bastnaesite, parisite, roentgenite and synchysite. Am. Mineral. 1953, 38, 932–963. [Google Scholar]
- Van Landuyt, J.; Amelinckx, S. Multiple Beam Direct Lattice Imaging of New Mixed-Layer Compounds of the Bastnaesite-Synchisite Series. Am. Mineral. 1975, 60, 351–358. [Google Scholar]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. The Atomic Arrangement of Bastnäsite-(Ce), Ce(CO3)F, and Structural Elements of Synchysite-(Ce), Rontgenite-(Ce), and Parisite-(Ce). Am. Mineral. 1993, 78, 415–418. [Google Scholar]
- Wu, X.; Dawei, M.; Zhaolu, P.; Guangming, Y. Transmission Electron Microscopic Study of New, Regular, Mixed-Layer Structures in Calcium-Rare-Earth Fluorocarbonate Minerals. Mineral. Mag. 1998, 62, 55–64. [Google Scholar]
- Meng, D.; Wu, X.; Han, Y.; Meng, X. Polytypism and microstructures of the mixed-layer member B2S, CaCe3(CO3)4F3 in the bastnaesite-(Ce)-synchysite-(Ce) series. Earth Planet. Sci. Lett. 2002, 203, 817–828. [Google Scholar] [CrossRef]
- Wang, L.; Ni, Y.; Hughes, J.M.; Bayliss, P.; Drexler, J.W. The atomic arrangement of synchysite-(Ce), CeCaF(CO3)2. Can. Mineral. 1994, 32, 865–871. [Google Scholar]
- Ni, Y.; Post, J.E.; Hughes, J.M. The Crystal Structure of Parisite-(Ce), Ce2CaF2(CO3)3. Am. Mineral. 2000, 85, 251–258. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Wood, S.A. A preliminary petrogenetic grid for REE fluorocarbonates and associated minerals. Geochim. Cosmochim. Acta 1992, 56, 725–738. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E. The Thermodynamic Properties of Bastnäsite-(Ce) and Parisite-(Ce). Chem. Geol. 2015, 392, 87–101. [Google Scholar] [CrossRef]
- Yang, H.; Dembowski, R.F.; Conrad, P.G.; Downs, R.T. Crystal Structure and Raman Spectrum of Hydroxyl-Bästnasite-(Ce), CeCO3(OH). Am. Mineral. 2008, 93, 698–701. [Google Scholar] [CrossRef]
- Hsu, L.C. Synthesis and Stability of Bastnaesites in a Part of the System (Ce,La)-F-H-C-O. Mineral. Petrol. 1992, 47, 87–101. [Google Scholar] [CrossRef]
- Shivaramaiah, R.; Anderko, A.; Riman, R.E.; Navrotsky, A. Thermodynamics of Bastnaesite: A Major Rare Earth Ore Mineral. Am. Mineral. 2016, 101, 1129–1134. [Google Scholar] [CrossRef]
- Holtstam, D.; Grins, J.; Nysten, P. Haleniusite-(La) from the Bastnäs deposit, Västmanland, Sweden: A new REE Oxyfluoride mineral species. Can. Mineral. 2004, 42, 1097–1103. [Google Scholar] [CrossRef]
- Allen, S.R.; McPhie, J.; Ferris, G.; Simpson, C. Evolution and Architecture of a Large Felsic Igneous Province in Western Laurentia: The 1.6 Ga Gawler Range Volcanics, South Australia. J. Volcanol. Geotherm. Res. 2008, 172, 132–147. [Google Scholar] [CrossRef]
- Johnson, J.P.; Cross, K.C. U-Pb geochronological constraints on the genesis of the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Econ. Geol. 1995, 90, 1046–1063. [Google Scholar] [CrossRef]
- Creaser, R.A.; Cooper, J.A. U-Pb Geochronology of Middle Proterozoic Felsic Magmatism Surrounding the Olympic Dam Cu-U-Au-Ag and Moonta Cu-Au-Ag Deposits, South Australia. Geology 1993, 88, 186–197. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Wade, B.; Cook, N.J.; Schmidt Mumm, A.; Giles, D. Uranium-bearing hematite from the Olympic Dam Cu-U-Au deposit, South Australia; a geochemical tracer and reconnaissance Pb-Pb geochronometer. Precambrian Res. 2013, 238, 129–147. [Google Scholar] [CrossRef]
- Courtney-Davies, L.; Zhu, Z.; Ciobanu, C.L.; Wade, B.P.; Cook, N.J.; Ehrig, K.; Cabral, A.R.; Kennedy, A. Matrix-matched iron-oxide laser ablation ICP-MS U-Pb geochronology using mixed solutions standards. Minerals 2016, 6, 85. [Google Scholar] [CrossRef]
- Apukhtina, O.B. Distribution, Petrology, Geochemistry and Geochronology of Carbonate Assemblages at the Olympic Dam Deposit. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2016. [Google Scholar]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder inmixed layer compounds A HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 2017. under review. [Google Scholar]
- Diemar, G.A. The Petrology of Hydrothermal Rare Earth Element Bearing Minerals in the Olympic Dam IOCG Deposit Support a 1.3 Ga formation. MSc Thesis, University of Tasmania, Hobart, Australia, 2014. [Google Scholar]
- Smith, M.P.; Henderson, P.; Campbell, L.S. Fractionation of the REE during Hydrothermal Processes: Constraints from the Bayan Obo Fe-REE-Nb Deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. Composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Fleischer, M. Relative proportions of the lanthanides in minerals of the bastnaesite group. Can. Mineral. 1978, 16, 361–363. [Google Scholar]
- Holtstam, D.; Andersson, U.B. The REE minerals of the Bastnäs-type deposits, South-Central Sweden. Can. Mineral. 2007, 45, 1073–1114. [Google Scholar] [CrossRef]
- Forster, H.J. Cerite-(Ce) and thorian synchysite-(Ce) from the Niederbobritzsch Granite, Erzgebirge, Germany: Implications for the differential mobility of the LREE and Th during alteration. Can. Mineral. 2000, 38, 67–79. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Mercer, W.; Gunning, C.; Prout, S. Quantitative characterization of the REE minerals by QEMSCAN from the Nechalacho Heavy Rare Earth Deposit, Thor Lake Project, NWT, Canada. SGS Miner. Serv. Tech. Pap. 2011, 7, 1–11. [Google Scholar]
- Papoutsa, A.; Pe-Piper, G. Variation of REE-Hydrothermal Circulation in Complex Shear Zones: The Cobequid Highlands, Nova Scotia. Can. Mineral. 2014, 52, 943–968. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Wall, F.; Le Bas, M.J. REE-Sr-Ba minerals from the Khibina Carbonatites, Kola Peninsula, Russia: Their mineralogy, paragenesis and evolution. Mineral. Mag. 1998, 62, 225–250. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Brady, A.E.; Wall, F.; Gunn, G.; Dawes, W. REE Minerals at the Songwe Hill Carbonatite, Malawi: HREE-Enrichment in Late-Stage Apatite. Ore Geol. Rev. 2017, 81, 23–41. [Google Scholar] [CrossRef]
- Guastoni, A.; Nestola, F.; Giaretta, A. Mineral Chemistry and Alteration of Rare Earth Element (REE) Carbonates from Alkaline Pegmatites of Mount Malosa, Malawi. Am. Mineral. 2009, 94, 1216–1222. [Google Scholar] [CrossRef]
- Augé, T.; Bailly, L.; Wille, G. An Unusual Occurrence of Synchysite-(Ce) in Amygdules from the Esterel Volcanic Rocks, France: Implications for Rare-Earth Element Mobility. Can. Mineral. 2014, 52, 1–19. [Google Scholar] [CrossRef]
- Mondillo, N.; Boni, M.; Balassone, G.; Spoleto, S.; Stellato, F.; Marino, A.; Santoro, L.; Spratt, J. Rare Earth Elements (REE)—Minerals in the Silius Fluorite Vein System (Sardinia, Italy). Ore Geol. Rev. 2016, 74, 211–224. [Google Scholar] [CrossRef]
- Vasyukova, O.; Williams-Jones, A.E. The Evolution of Immiscible Silicate and Fluoride Melts: Implications for REE Ore-Genesis. Geochim. Cosmochim. Acta 2016, 172, 205–224. [Google Scholar] [CrossRef]
- Savko, K.A.; Bazikov, N.S. Phase Equilibria of Bastnaesite, Allanite, and Monazite: Bastnaesite-out Isograde in Metapelites of the Vorontsovskaya Group, Voronezh Crystalline Massif. Petrology 2011, 19, 445–469. [Google Scholar] [CrossRef]
- Groves, D.I.; Vielreicher, N.M. The Phalabowra (Palabora) carbonatite-hosted magnetite-copper sulfide deposit, South Africa: An end-member of the iron-oxide copper-gold-rare earth element deposit group? Miner. Depos. 2001, 36, 189–194. [Google Scholar] [CrossRef]
- Bau, M. Controls on the Fractionation of Isovalent Trace Elements in Magmatic and Aqueous Systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, Y.; Shimizu, H. Local structure of Y and Ho in calcite and its relevance to Y fractionation from Ho in partitioning between calcite and aqueous solution. Chem. Geol. 2008, 248, 104–113. [Google Scholar] [CrossRef]
- Brugger, J.; Liu, W.; Etschmann, B.; Mei, Y.; Sherman, D.M.; Testemale, D. A Review of the Coordination Chemistry of Hydrothermal Systems, or Do Coordination Changes Make Ore Deposits? Chem. Geol. 2016, 447, 219–253. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal Mobilisation of the Rare Earth Elements—A Tale of ‘Ceria’ and ‘Yttria’. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- McPhie, J.; Kamenetsky, V.; Allen, S.; Ehrig, K.; Agangi, A.; Bath, A. The fluorine link between a supergiant ore deposit and silicic large igneous province. Geology 2011, 39, 1003–1006. [Google Scholar] [CrossRef]
- Haas, J.R.; Shock, E.L.; Sassani, D.C. Rare Earth Elements in Hydrothermal Systems: Estimates of Standard Partial Molal Thermodynamic Properties of Aqueous Complexes of the Rare Earth Elements at High Pressures and Temperatures. Geochem. Explor. Environ. Anal. 1995, 59, 4329–4350. [Google Scholar] [CrossRef]
- Didier, A.; Bosse, V.; Boulvais, P.; Bouloton, J.; Paquette, J.-L.; Montel, J.-M.; Devidal, J.-L. Disturbance versus preservation of U-Th-Pb ages in monazite during fluid-rock interaction: Textural, chemical and isotopic in situ study in microgranites (Velay Dome, France). Contrib. Mineral. Petrol. 2013, 165, 1051–1072. [Google Scholar] [CrossRef]
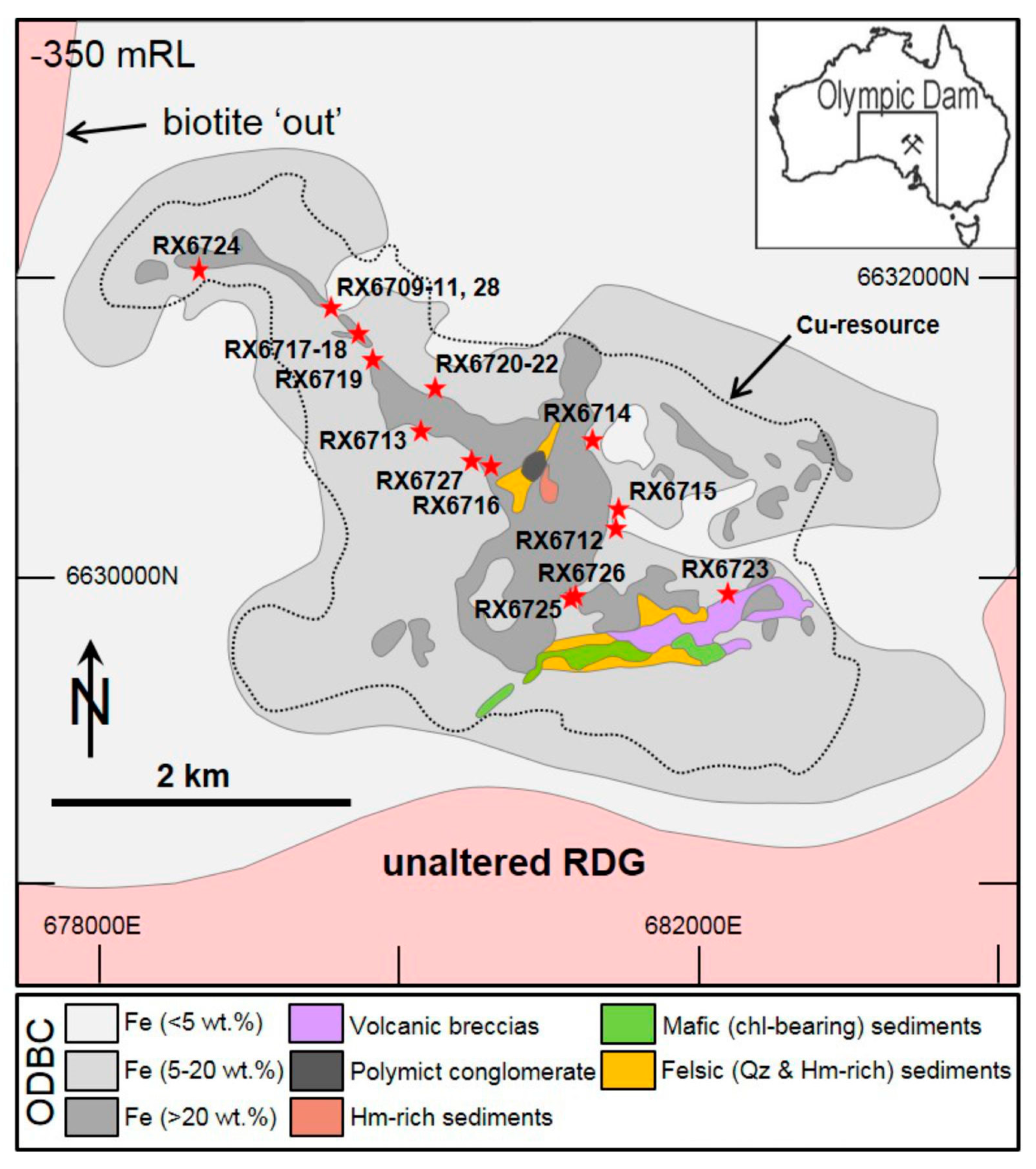
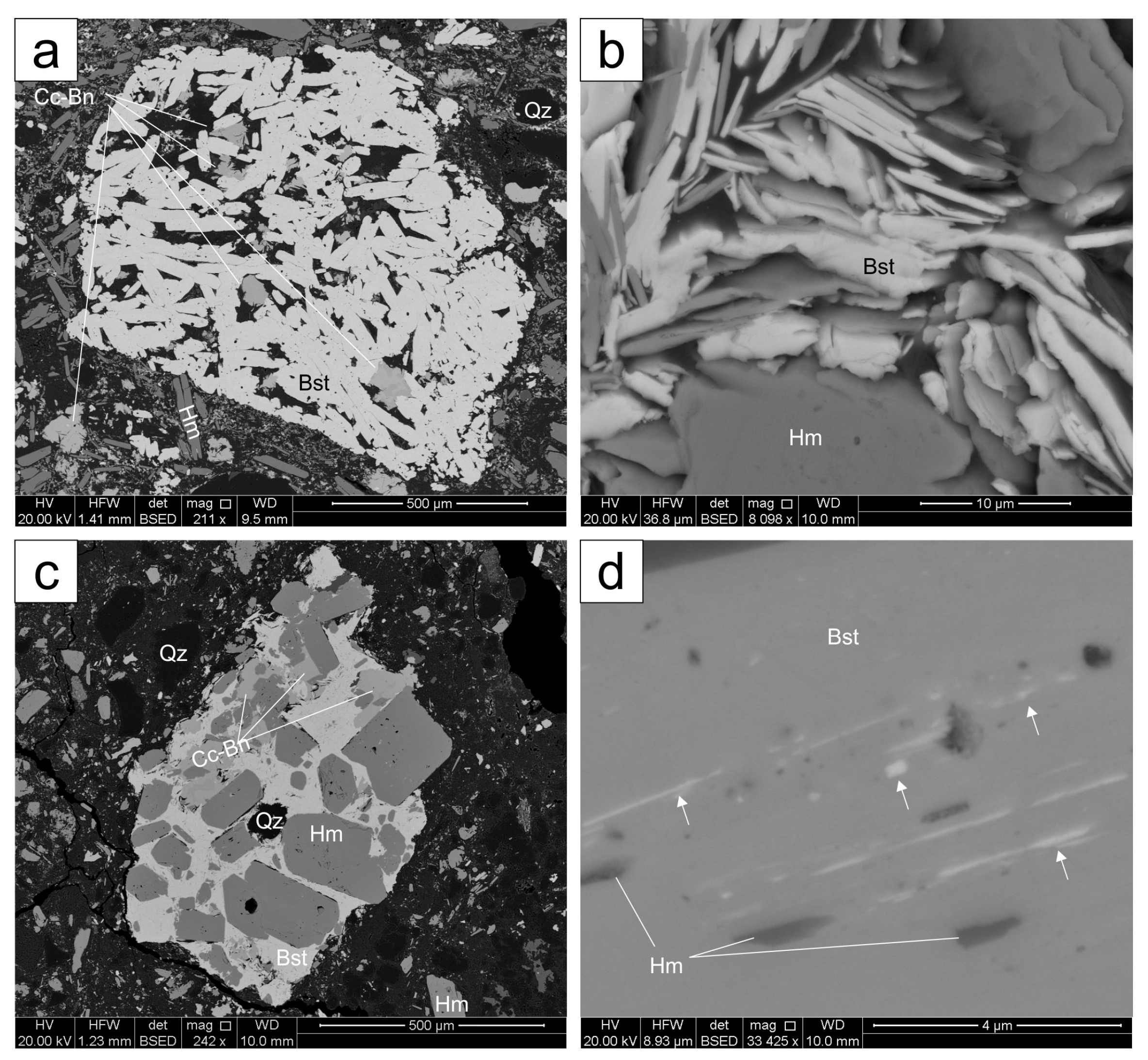
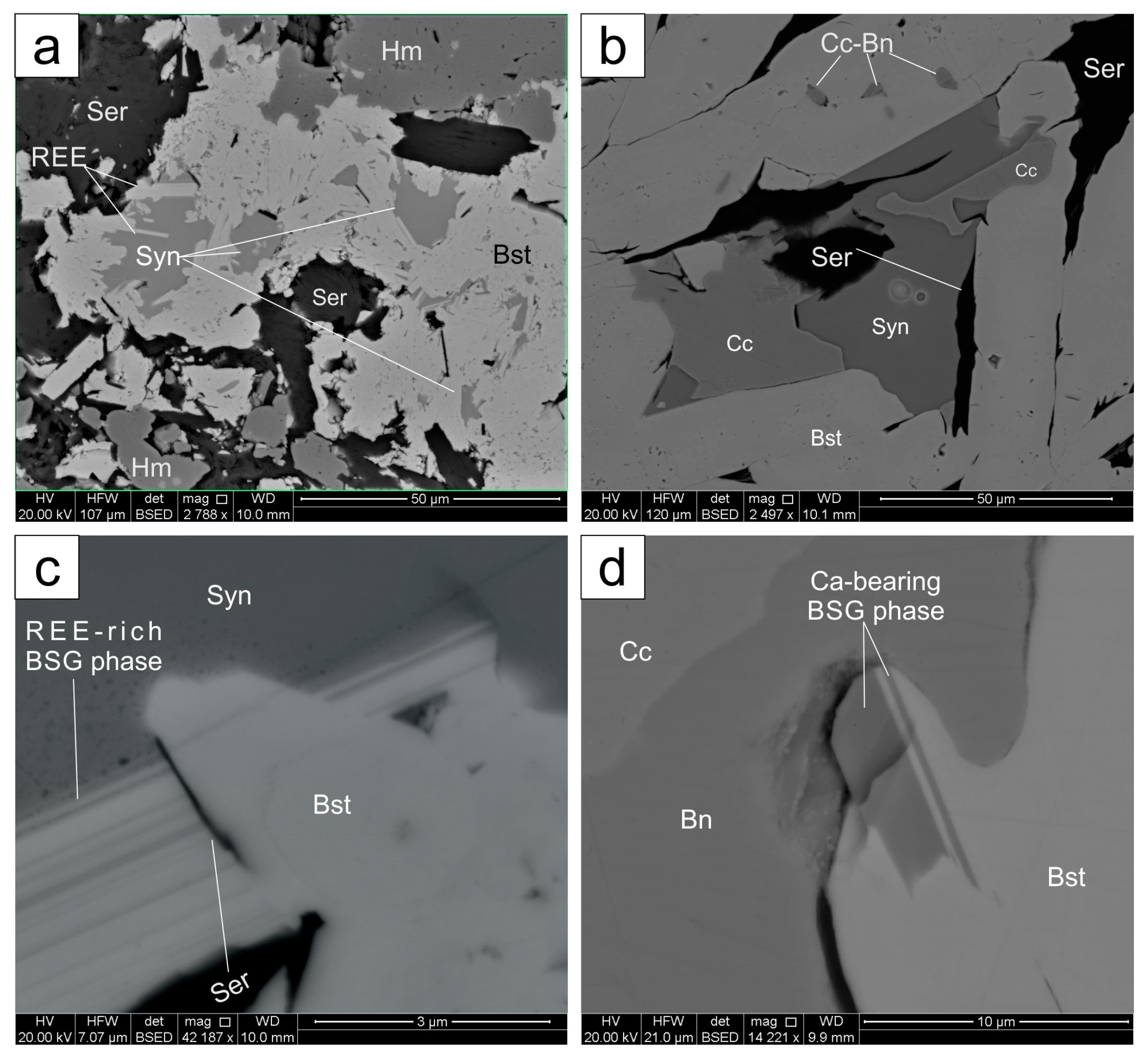
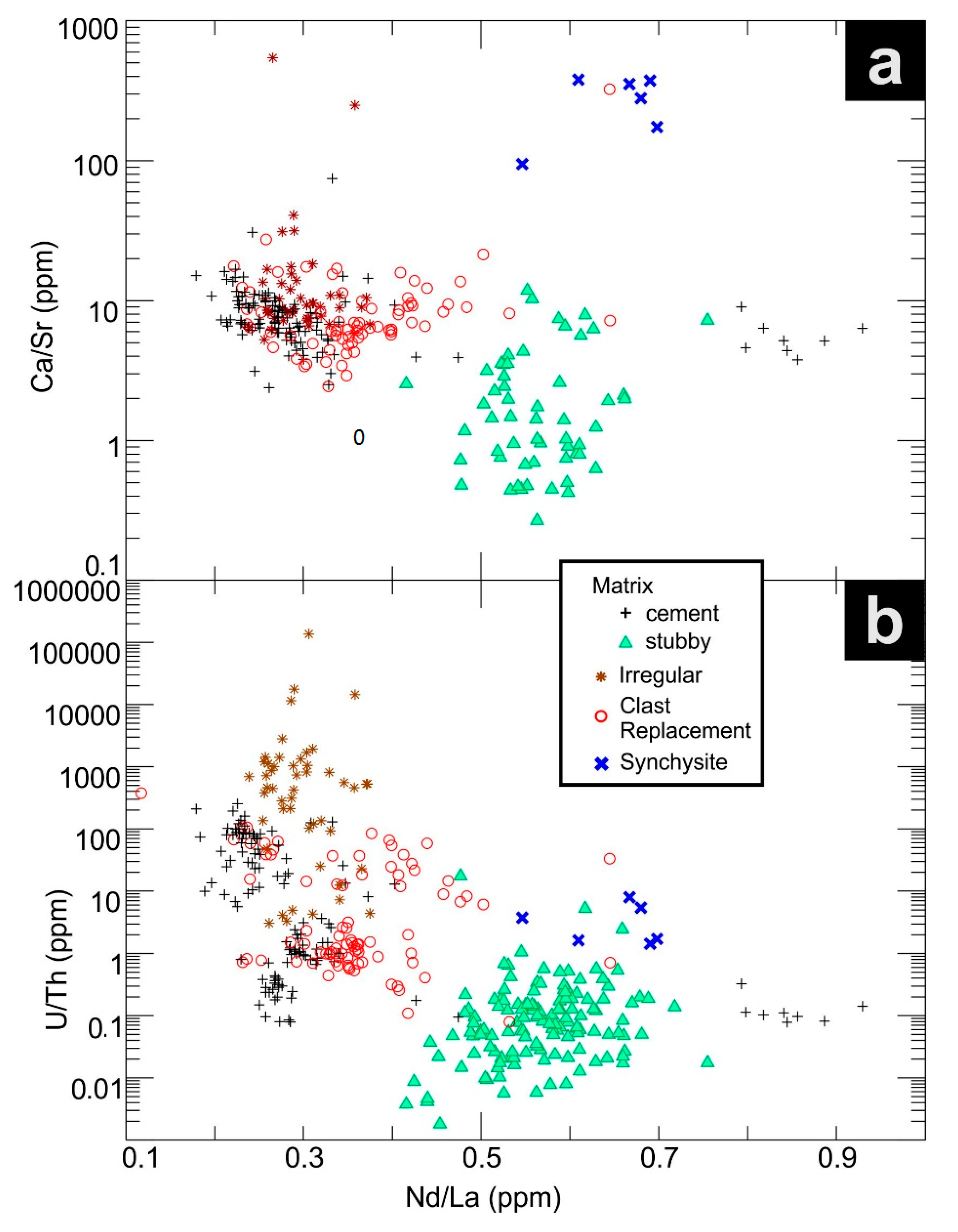
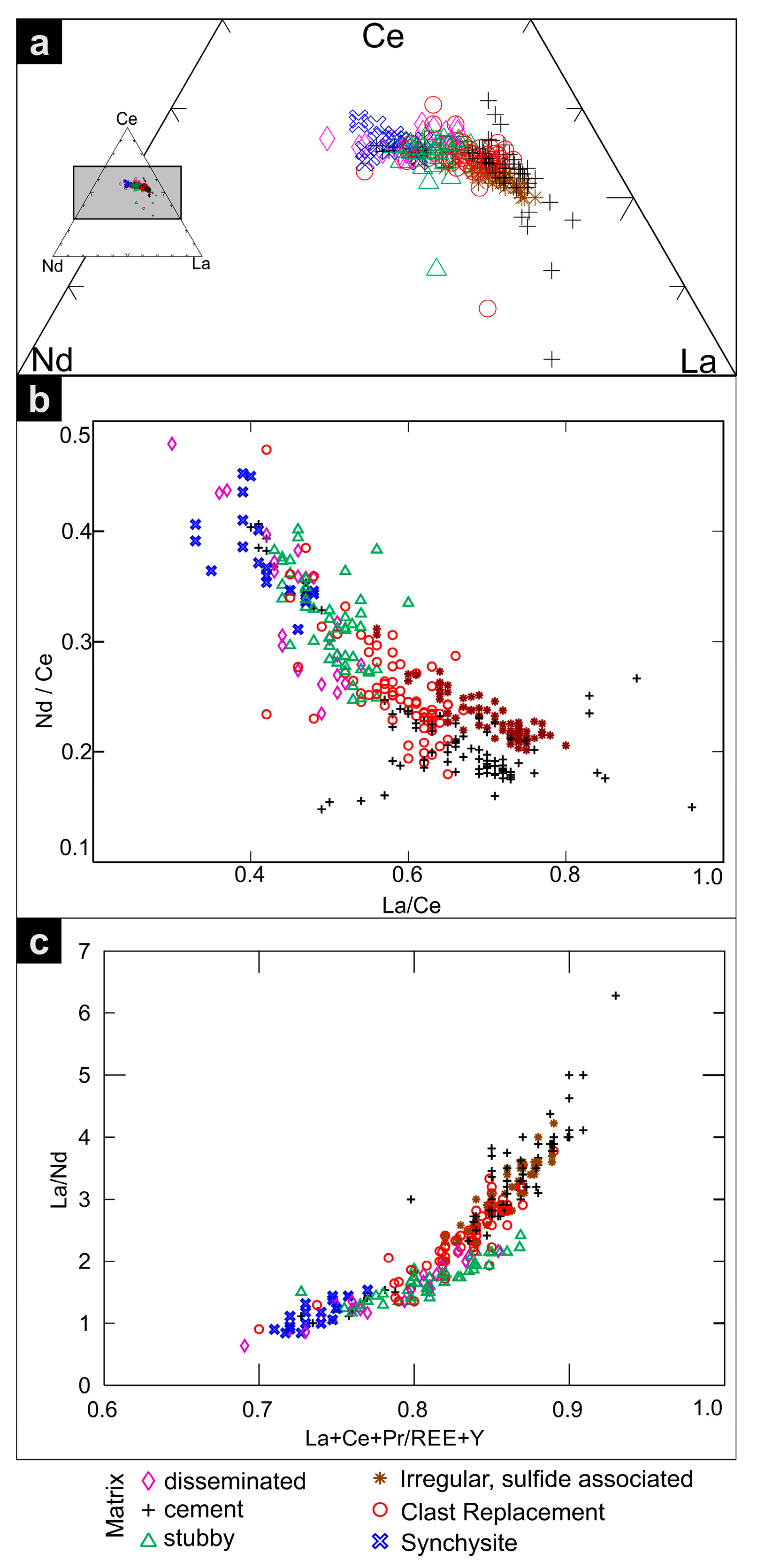

| Lithology | Sample Location | Brief Sample Description 1 | REE Fluorocarbonate Occurances | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bastnasite | Synch-Ysite | ||||||||||
| Matrix | Irregular Sulfide Associated | Clast Replace-Ment | |||||||||
| Area | Drill Hole | Sample No. | Core Depth (m) | Stubby | Cement | Dissem-Inated | |||||
| Breccias containing dyke clasts | NW arm | RU34-3829 | RX6709 | 72.8–73.0 | Strong Bst matrix zone with clasts of Ser and Chl. Around Bst zones are Hm dominate zones with Hm as matrix and clasts with Ser-Chl. Rt and Zr present. | X | |||||
| RX6710 | 73.6–73.7 | Hm is the major mineral, present in clasts and with strong lineaments in matrix. Distinct zones with abundant angular-rounded clasts of Ser-Chl. Zr present. | X | ||||||||
| RX6711 | 74.7–74.8 | Strong foliation across the sample. More fine-grained sample of mostly Ser with Chl, few and small clasts, mostly composed of Hm. Flo occurs along “pressure shadows” around mineral grains. Rt and Zr present. | X | ||||||||
| RU41-2571 | RX6728 | 174.8–174.9 | Mostly Hm with “crackle” Bar veinlets with Bst around clasts and Hm. Some clasts of Hm, Qz, and Ser. | X | |||||||
| East edge | RU33-159 | RX6714 | 66.3–66.4 | Sample dominated by Hm as clasts and matrix with Ser. Hm makes rounded thick rims around cores of Ser and Bar. Bst concentrated in light-colored zone on edge. | x | X | X | ||||
| RU37-7354 | RX6723 | 4.3–4.4 | Bst occurs as blades in the matrix, both disseminated and abundant in distinct zones, and in relict clasts. Abundant clasts of mostly Qz in sample, rounded-angular, all sizes. Orange areas on hand sample are mostly Qz + Hm. | x | X | ||||||
| Central | RU34-454 | RX6716 | 82.9–83.0 | Very abundant Bst throughout sample, concentrated in more fine-grained conduit, as matrix and clast replacement. Around Bst zone is mostly Hm as clasts and matrix. | X | x | |||||
| RU45-2661 | RX6727 | 51.8–51.9 | Chaotic sample with blocky zones of fine grained Ser and Hm matrix. Other areas of breccia conduits with abundant clasts of Qz, Hm, sulfides, and Bar. | X | x | ||||||
| Granite-rich breccias | East edge | RU3-558 | RX6712 | 23.7–23.8 | Clasts are mostly Qz with some Bar and Hm, rounded to angular. Ser matrix. Rt and Zr present. | x | |||||
| RU4-105 | RX6715 | 12.2–12.3 | Abundant clasts, rounded to angular, matrix supported heterolithic breccia but clasts mostly of Qz and Hm, matrix is microbreccia and Ser. | x | |||||||
| Central | RU3-954 | RX6713 | 70.1–70.2 | Hm abundant as matrix and clasts. Other clats of sulfide, Qz, and Ser. Bn vein. Lacking pervasive disseminated bast in the matrix, Flo is present instead. Rt present. | X | ||||||
| NW arm | RU31-751 | RX6717 | 90.3–90.4 | Large cross cutting vein of Fl Abundant Qz clasts of ranging size, rounded to angular. Bst in matrix concentrated in localized zones, otherwise Ser and Hm. Rt and Zr present. | X | x | |||||
| RX6718 | 90.8–91.0 | Similar to above. Bst mostly occurs as fine-grained blades concentrated in zones as the matrix. More abundant irregular Bst “clumps”. Zr and Rt present. | X | x | |||||||
| RU31-767 | RX6719 | 5.3–5.4 | Zone of Qz clasts with strong veinlet features. Other zone dominated by Hm with less defined directional features. Fine grained blade Bst as matrix around fine-grained rounded Qz clasts. | X | x | ||||||
| Hematite-rich breccia | Central | RU40-1407 | RX6720 | 19.0–19.2 | Primarily composed of coarse-grained Hm with a range of textures. Cluster of clasts of rounded to angular Fl and minor Qz and Cp/Bn. Bst matrix in distinct veinlet zone with Cp and Bn. | X | |||||
| RX6721 | 19.4–19.5 | Strong linear zones from veins of Hm and Ser with Cp-Bn between bands of Fl clasts with matrix of Ser, Hm, Bst, and Cp-Bn. | X | ||||||||
| RX6722 | 19.7–19.8 | Matrix supported; abundant clasts, rounded to angular, composed primarily of either Fl, Qz, and Cu-(Fe) sulfides in matrix of blade Hm. Rare irregular Bst. Fl clasts are the largest. | X | ||||||||
| East edge | RU38-2994 | RX6725 | 92.8–92.9 | Heterolithic clasts and clasts of mostly Qz and Hm in microbreccia of mostly Ser with also fine-grained bladed Hm and Bst. | x | X | |||||
| RU38-2685 | RX6726 | 77.1–77.3 | Clasts of mostly Qz, Ser, Hm, and Bst. Microbreccia matrix of fine-grained Hm blades and Ser with veinlets and lineation around clasts. Rt and Zr are present. | x | X | X | |||||
| Sericitized dyke | NW arm | RU41-5181 | RX6724 | 183.7–183.8 | Bst in distinct zones in darker red areas. Fine grained Bst in matrix to massive clumps. Clumps are dirty with possible faint zoning from inclusions of Hm and Ser but mostly irregular. Some distinct zones of Flo with distinct hexagonal textures with Ser in shape. Fine grained irregular Bst in Ser around Flo. | X | x | ||||
| Mineral/Type | Bastnäsite | Synchysite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | Clast Replacement | Irregular | ||||||||||
| Stubby | Fine-Grained Cement | Disseminated | ||||||||||
| (n = 46) | (n = 93) | (n = 22) | (n = 66) | (n = 79) | (n = 20) | |||||||
| RX: 6709, 6710, 6728 | RX: 6714, 6716, 6717, 6718, 6719, 6723, 6724, 6727 | RX: 6711, 6712, 6715, 6723, 6725 | RX: 6713, 6714, 6716, 6726 | RX: 6720, 6721, 6722 | RX: 6714, 6726 | |||||||
| (wt %) | Mean | St.dev. | Mean | St.dev. | Mean | St.dev. | Mean | St.dev. | Mean | St.dev. | Mean | St.dev. |
| Ca | 0.04 | 0.02 | 0.35 | 0.21 | 0.29 | 0.33 | 0.23 | 0.14 | 0.32 | 0.19 | 12.30 | 0.58 |
| Sr | 0.03 | 0.03 | 0.07 | 0.04 | 0.08 | 0.06 | 0.05 | 0.02 | 0.05 | 0.02 | 0.05 | 0.01 |
| La | 17.03 | 1.65 | 21.97 | 3.63 | 15.38 | 2.27 | 19.93 | 2.38 | 22.49 | 1.86 | 9.88 | 1.23 |
| Ce | 33.77 | 2.00 | 33.13 | 2.80 | 33.49 | 1.63 | 34.34 | 2.06 | 32.00 | 1.22 | 23.76 | 1.20 |
| Pr | 3.61 | 0.18 | 2.74 | 0.44 | 3.56 | 0.49 | 3.14 | 0.36 | 2.67 | 0.19 | 2.67 | 0.15 |
| Nd | 10.91 | 1.18 | 7.29 | 1.97 | 11.06 | 2.11 | 8.91 | 1.67 | 7.39 | 0.74 | 8.91 | 0.79 |
| Sm | 0.95 | 0.31 | 0.57 | 0.33 | 1.16 | 0.38 | 0.84 | 0.34 | 0.55 | 0.15 | 1.18 | 0.18 |
| Eu | 0.44 | 0.14 | 0.28 | 0.12 | 0.48 | 0.10 | 0.43 | 0.16 | 0.25 | 0.08 | 0.56 | 0.10 |
| Gd | 0.36 | 0.21 | 0.33 | 0.21 | 0.60 | 0.29 | 0.43 | 0.24 | 0.30 | 0.11 | 0.77 | 0.13 |
| Tb | 0.07 | 0.02 | 0.07 | 0.02 | 0.09 | 0.03 | 0.08 | 0.03 | 0.07 | 0.02 | 0.09 | 0.02 |
| Dy | 0.18 | 0.09 | 0.15 | 0.09 | 0.27 | 0.14 | 0.19 | 0.08 | 0.14 | 0.05 | 0.34 | 0.08 |
| Y | 0.38 | 0.30 | 0.60 | 0.29 | 0.67 | 0.35 | 0.64 | 0.29 | 0.59 | 0.13 | 0.93 | 0.32 |
| Ho | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.14 | 0.00 | 0.13 | 0.00 |
| Er | 0.084 | 0.02 | 0.08 | 0.02 | 0.09 | 0.03 | 0.08 | 0.03 | 0.08 | 0.02 | 0.09 | 0.02 |
| Tm | b.d.l. | b.d.l. | 0.07 | 0.01 | b.d.l. | b.d.l. | 0.05 | 0.00 | 0.064 | 0.01 | b.d.l. | b.d.l. |
| Yb | 0.07 | 0.00 | 0.07 | 0.02 | b.d.l. | b.d.l. | 0.49 | 0.43 | 0.08 | 0.01 | b.d.l. | b.d.l. |
| F | 7.35 | 0.14 | 7.19 | 0.30 | 7.24 | 0.34 | 7.25 | 0.23 | 7.15 | 0.17 | 3.62 | 0.46 |
| Cl | 0.02 | 0.01 | 0.04 | 0.02 | 0.03 | 0.02 | 0.03 | 0.01 | 0.03 | 0.02 | 0.02 | 0.01 |
| Na | b.d.l. | b.d.l. | 0.10 | 0.11 | 0.11 | 0.05 | b.d.l. | b.d.l. | 0.03 | 0.00 | b.d.l. | b.d.l. |
| Al | 0.18 | 0.23 | 0.20 | 0.26 | 0.10 | 0.09 | 0.34 | 0.62 | 0.67 | 1.80 | 0.30 | 0.36 |
| K | 0.01 | 0.00 | 0.05 | 0.09 | 0.06 | 0.04 | 0.03 | 0.05 | 0.06 | 0.24 | 0.07 | 0.08 |
| Fe3+ | 0.25 | 0.21 | 0.33 | 0.41 | 0.57 | 0.37 | 0.30 | 0.32 | 0.47 | 0.74 | 0.11 | 0.06 |
| S | 0.04 | 0.02 | 0.03 | 0.02 | 0.05 | 0.03 | 0.07 | 0.21 | 0.02 | 0.02 | 0.01 | 0.00 |
| U | b.d.l. | b.d.l. | 0.33 | 1.11 | 0.06 | 0.03 | 0.06 | 0.03 | 0.17 | 0.09 | 0.03 | 0.00 |
| Th | 0.08 | 0.07 | 0.11 | 0.09 | 0.43 | 0.32 | 0.16 | 0.12 | 0.07 | 0.04 | 0.05 | 0.00 |
| Pb | b.d.l. | b.d.l. | 0.03 | 0.01 | 0.04 | 0.01 | b.d.l. | b.d.l. | 0.05 | 0.02 | b.d.l. | b.d.l. |
| Measured Total | 75.30 | 2.12 | 75.30 | 3.28 | 75.41 | 3.66 | 76.98 | 2.64 | 74.66 | 2.76 | 65.32 | 2.04 |
| ∑REE + Y | 67.62 | 2.15 | 67.18 | 3.75 | 66.79 | 3.61 | 69.06 | 2.82 | 66.48 | 2.50 | 48.20 | 1.95 |
| La/Ce | 0.51 | 0.06 | 0.67 | 0.15 | 0.46 | 0.06 | 0.58 | 0.09 | 0.70 | 0.05 | 0.42 | 0.05 |
| CO2 wt % | 21.20 | 0.68 | 21.16 | 1.17 | 20.96 | 1.13 | 21.71 | 0.88 | 20.94 | 0.78 | 28.98 | 0.99 |
| Calc. Total | 96.24 | 2.81 | 95.98 | 4.78 | 95.38 | 4.84 | 98.32 | 3.76 | 94.96 | 3.30 | 94.09 | 2.92 |
| (a.p.f.u.) | ||||||||||||
| Ca | 0.002 | 0.001 | 0.018 | 0.010 | 0.014 | 0.016 | 0.011 | 0.007 | 0.016 | 0.009 | 0.998 | 0.001 |
| Sr | 0.001 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.002 | 0.001 |
| Total 2+ | 0.002 | 0.001 | 0.020 | 0.011 | 0.016 | 0.017 | 0.012 | 0.008 | 0.017 | 0.010 | 1.000 | 0.000 |
| La | 0.254 | 0.023 | 0.329 | 0.050 | 0.233 | 0.034 | 0.291 | 0.032 | 0.340 | 0.022 | 0.202 | 0.021 |
| Ce | 0.500 | 0.026 | 0.492 | 0.031 | 0.502 | 0.018 | 0.497 | 0.024 | 0.480 | 0.006 | 0.483 | 0.011 |
| Pr | 0.053 | 0.003 | 0.040 | 0.006 | 0.053 | 0.006 | 0.045 | 0.005 | 0.040 | 0.003 | 0.054 | 0.003 |
| Nd | 0.157 | 0.016 | 0.105 | 0.027 | 0.161 | 0.027 | 0.125 | 0.022 | 0.108 | 0.011 | 0.176 | 0.017 |
| Sm | 0.013 | 0.004 | 0.008 | 0.004 | 0.016 | 0.005 | 0.011 | 0.004 | 0.008 | 0.002 | 0.022 | 0.004 |
| Eu | 0.006 | 0.002 | 0.004 | 0.002 | 0.007 | 0.001 | 0.006 | 0.002 | 0.003 | 0.001 | 0.011 | 0.002 |
| Gd | 0.005 | 0.003 | 0.004 | 0.003 | 0.008 | 0.004 | 0.005 | 0.003 | 0.004 | 0.001 | 0.014 | 0.002 |
| Tb | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.002 | 0.000 |
| Dy | 0.002 | 0.001 | 0.002 | 0.001 | 0.003 | 0.002 | 0.002 | 0.001 | 0.002 | 0.001 | 0.006 | 0.001 |
| Y | 0.009 | 0.007 | 0.014 | 0.007 | 0.016 | 0.008 | 0.014 | 0.007 | 0.014 | 0.003 | 0.030 | 0.010 |
| Ho | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.002 | 0.000 | 0.000 | 0.000 |
| Er | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 |
| Tm | b.d.l. | b.d.l. | 0.001 | 0.000 | b.d.l. | b.d.l. | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| Yb | 0.001 | 0.000 | 0.001 | 0.000 | b.d.l. | b.d.l. | 0.006 | 0.005 | 0.001 | 0.000 | 0.000 | 0.000 |
| Total 3+ | 0.998 | 0.001 | 0.980 | 0.011 | 0.984 | 0.017 | 0.988 | 0.008 | 0.983 | 0.010 | 1.000 | 0.000 |
| F | 0.802 | 0.030 | 0.774 | 0.055 | 0.790 | 0.060 | 0.765 | 0.031 | 0.777 | 0.031 | 0.545 | 0.079 |
| Cl | 0.001 | 0.000 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.001 | 0.001 | 0.001 |
| F + Cl | 0.803 | 0.030 | 0.776 | 0.055 | 0.791 | 0.059 | 0.767 | 0.031 | 0.779 | 0.031 | 0.546 | 0.079 |
| Mineral/Type | Bastnäsite | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | Clast Replacement | Irregular | Synchysite | ||||||||||||
| Stubby | Fine-Grained Cement | ||||||||||||||
| (n = 137) | (n = 123) | (n = 84) | (n = 49) | (n = 6) | |||||||||||
| RX: 6709, 6710, 6728 | RX: 6714, 6716, 6717, 6718, 6719, 6723, 6724, 6727 | RX: 6713, 6714, 6716, 6726 | RX: 6720, 6721, 6722 | RX: 6714 | |||||||||||
| Mean | Min. | Max. | Mean | Min. | Max. | Mean | min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | |
| La | 180,000 | 139,700 | 237,000 | 233,000 | 102,800 | 320,000 | 235,000 | 159,000 | 395,000 | 241,000 | 213,100 | 271,000 | 117,900 | 114,200 | 123,000 |
| Pr | 35,300 | 29,380 | 40,600 | 26,900 | 11,800 | 37,960 | 30,700 | 21,800 | 58,100 | 26,600 | 24,000 | 29,400 | 25,000 | 21,700 | 26,140 |
| Nd | 101,400 | 78,000 | 124,000 | 67,600 | 29,140 | 129,000 | 81,200 | 43,200 | 163,000 | 71,200 | 64,400 | 86,900 | 76,500 | 62,400 | 83,500 |
| Sm | 9170 | 5520 | 13,200 | 5760 | 2860 | 16,600 | 7500 | 1860 | 16,800 | 6230 | 4670 | 8520 | 9140 | 6550 | 10,500 |
| Eu | 2790 | 1460 | 4820 | 1710 | 435 | 4820 | 2510 | 347 | 7200 | 1700 | 870 | 2690 | 3160 | 2110 | 3680 |
| Gd | 3290 | 1470 | 5720 | 2870 | 1190 | 8420 | 3480 | 627 | 9820 | 3220 | 2270 | 4700 | 5660 | 3680 | 6550 |
| Tb | 289 | 93.2 | 561 | 302 | 95.4 | 819 | 343 | 41.4 | 830 | 320 | 218 | 455 | 684 | 376 | 835 |
| Dy | 1180 | 278 | 2340 | 1430 | 335 | 3510 | 1480 | 153 | 3290 | 1440 | 1020 | 1890 | 3230 | 1630 | 3890 |
| Y | 2990 | 664 | 6450 | 5170 | 1030 | 10,600 | 4230 | 378 | 11,600 | 5320 | 3860 | 7370 | 9050 | 3430 | 11,500 |
| Ho | 161 | 29.0 | 324 | 216 | 39.1 | 456 | 210 | 19.2 | 460 | 225 | 156 | 309 | 420 | 189 | 545 |
| Er | 313 | 44.4 | 682 | 466 | 55.9 | 920 | 430 | 36.7 | 935 | 512 | 309 | 757 | 695 | 297 | 1003 |
| Tm | 28.2 | 3.0 | 66.0 | 44.9 | 3.8 | 106 | 42.0 | 2.7 | 108 | 53.6 | 25.6 | 91.9 | 49.9 | 21.5 | 74.0 |
| Yb | 129 | 13.0 | 322 | 219 | 12.6 | 620 | 211 | 11.4 | 617 | 284 | 105 | 623 | 178 | 75.0 | 290 |
| Lu | 13.3 | 1.0 | 34.9 | 23.5 | 1.2 | 72.3 | 21.7 | 1.1 | 60.8 | 33.6 | 12.5 | 90.0 | 13.3 | 5.9 | 26.2 |
| Ca | 897 | 158 | 3100 | 3980 | 820 | 9950 | 2290 | 790 | 6100 | 3920 | 1440 | 9800 | 111,000 | 20,200 | 155,000 |
| Sr | 531 | 27.1 | 6500 | 591 | 26.9 | 2940 | 294 | 24.1 | 752 | 377 | 217 | 872 | 427 | 214 | 890 |
| Th | 213 | 0.14 | 4960 | 533 | 0.23 | 3760 | 272 | 0.08 | 5290 | 78.3 | 0.88 | 619 | 20.2 | 8.3 | 29.5 |
| U | 28.1 | 0.07 | 1510 | 388 | 7.4 | 7200 | 266 | 1.5 | 1040 | 1840 | 781 | 10,100 | 62.3 | 29.0 | 114 |
| Pb | 36.2 | 1.8 | 606 | 352 | 5.2 | 1370 | 196 | 1.0 | 2840 | 751 | 422 | 2150 | 23.7 | 3.0 | 69.5 |
| Ba | 108 | 0.67 | 7000 | 266 | 0.53 | 2320 | 82.7 | 0.5 | 1560 | 47.4 | 5.0 | 205 | 182 | 22.9 | 690 |
| Mn | 10.8 | 0.50 | 126 | 21.4 | 3.9 | 142 | 19.5 | 2.7 | 450 | 19.2 | 3.5 | 147 | 48.6 | 18.6 | 78.5 |
| Na | 229 | 8.5 | 2910 | 710 | 71.0 | 3250 | 449 | 69.0 | 3740 | 260 | 83 | 1100 | 59.0 | 59.0 | 59.0 |
| Nb | 17.0 | 0.1 | 250 | 6.1 | 0.09 | 150 | 19.0 | 0.07 | 560 | 2.4 | 0.22 | 10.2 | 3.6 | 0.66 | 7.4 |
| P | 1730 | 52.0 | 31,400 | 1450 | 69.0 | 9700 | 260 | 260 | 260 | 275 | 75.0 | 1100 | b.d.l. | b.d.l. | b.d.l. |
| Zr | 20.7 | 0.09 | 1220 | 5.0 | 0.11 | 110 | 5.1 | 0.10 | 155 | 0.42 | 0.10 | 0.84 | 258 | 4.5 | 690 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kamenetsky, V.S. Rare Earth Element Fluorocarbonate Minerals from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia. Minerals 2017, 7, 202. https://doi.org/10.3390/min7100202
Schmandt DS, Cook NJ, Ciobanu CL, Ehrig K, Wade BP, Gilbert S, Kamenetsky VS. Rare Earth Element Fluorocarbonate Minerals from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia. Minerals. 2017; 7(10):202. https://doi.org/10.3390/min7100202
Chicago/Turabian StyleSchmandt, Danielle S., Nigel J. Cook, Cristiana L. Ciobanu, Kathy Ehrig, Benjamin P. Wade, Sarah Gilbert, and Vadim S. Kamenetsky. 2017. "Rare Earth Element Fluorocarbonate Minerals from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia" Minerals 7, no. 10: 202. https://doi.org/10.3390/min7100202





