REE Incorporation into Calcite Individual Crystals as One Time Spike Addition
Abstract
:1. Introduction
2. Materials and Methods
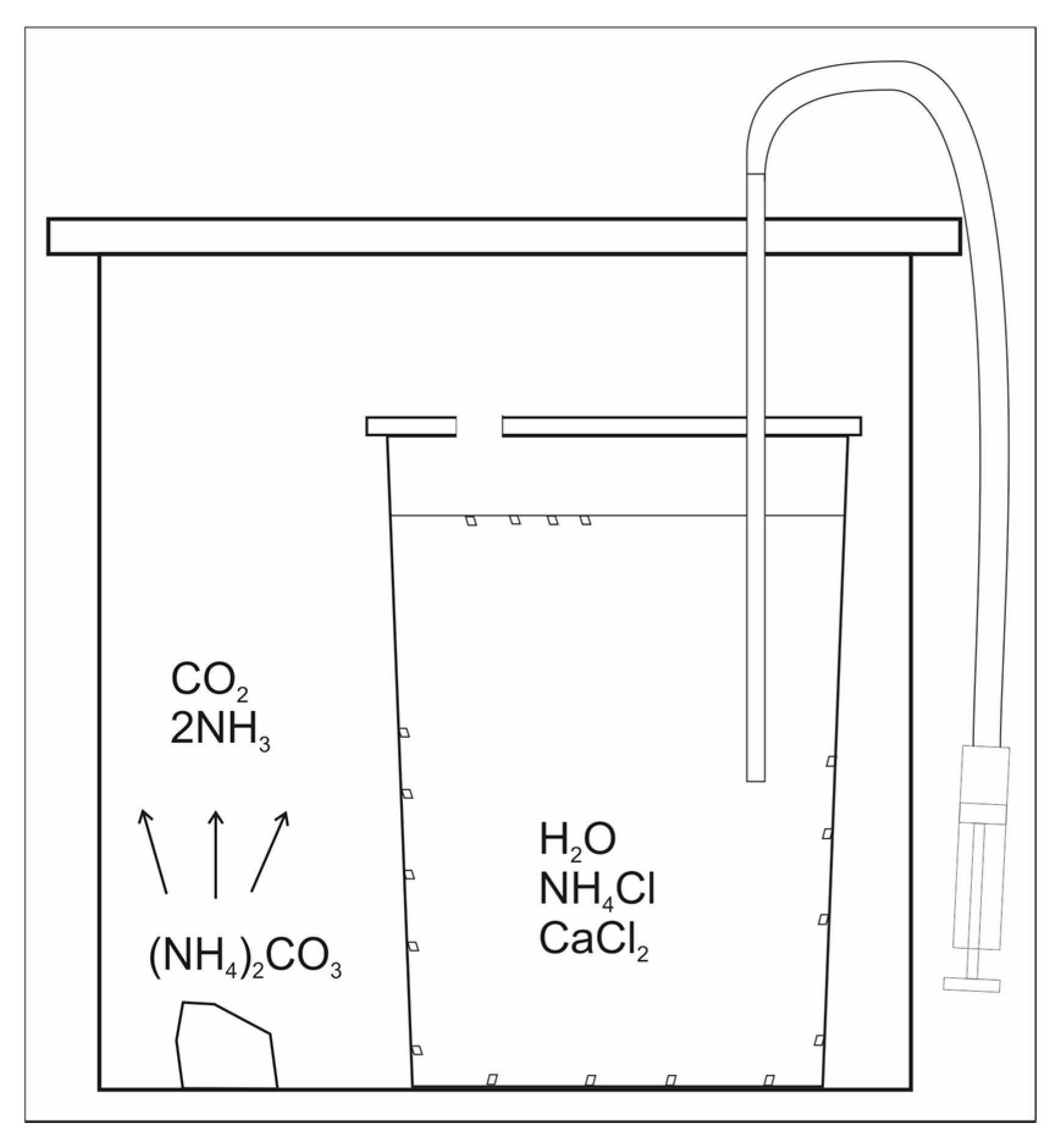
2.1. Calcite Precipitation
2.2. Analyses of Fluids
2.3. Analyses of Solids
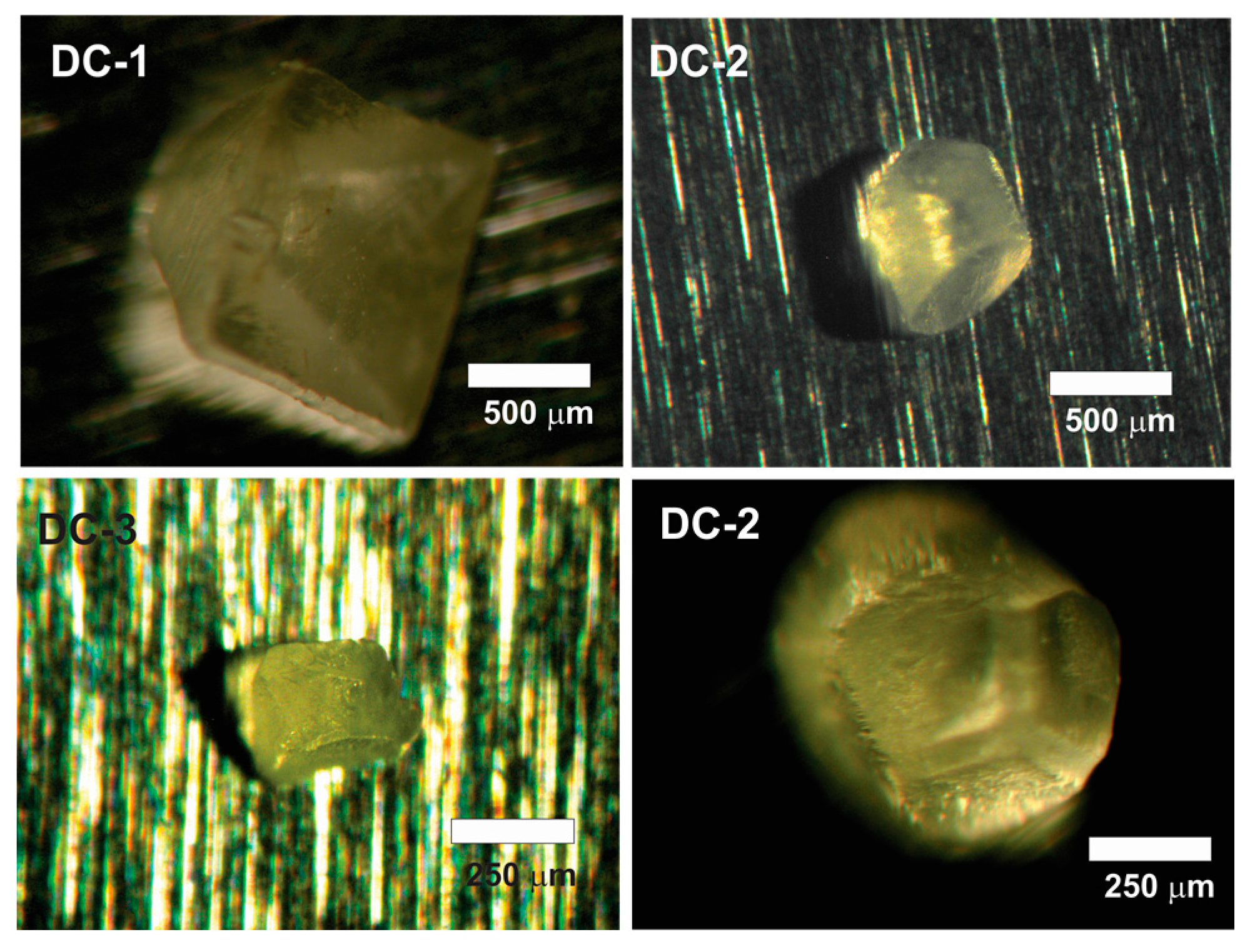
3. Results
4. Discussion
4.1. Calculating of Partition Coefficinets
4.2. Comparison of REE Partitioning Values between Three Experiments
4.3. Comparison with Other Studies
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elderfield, H.; Upstill-Goddard, R.; Sholkovitz, E.R. The rare earth elements in rivers, estuaries, and coastal seas and their significance to the composition of ocean waters. Geochim. Cosmochim. Acta 1990, 54, 971–991. [Google Scholar] [CrossRef]
- Hellebrandt, S.E.; Hofmann, S.; Jordan, N.; Barkleit, A.; Schmidt, M. Incorporation of Eu(III) into Calcite under Recrystallization conditions. Sci. Rep. 2016, 6, 33137. [Google Scholar] [CrossRef] [PubMed]
- Lakshtanov, L.Z.; Stipp, S.L.S. Experimental study of europium(III) coprecipitation with calcite. Geochim. Cosmochim. Acta 2004, 68, 819–827. [Google Scholar] [CrossRef]
- Balboni, E.; Morrison, J.M.; Wang, Z.; Engelhard, M.H.; Burns, P.C. Incorporation of Np(V) and U(VI) in carbonate and sulfate minerals crystallized from aqueous solution. Geochim. Cosmochim. Acta 2015, 151, 133–149. [Google Scholar] [CrossRef]
- Cherniak, D.J. REE diffusion in calcite. Earth Planet. Sci. Lett. 1998, 160, 273–287. [Google Scholar] [CrossRef]
- François, J.L.; Guzmán, J.R.; Martín-del-Campo, C. Study of the radiotoxicity of actinides recycling in boiling water reactors fuel. Nucl. Eng. Des. 2009, 239, 1911–1915. [Google Scholar] [CrossRef]
- Terakado, Y.; Masuda, A. The coprecipitation of rare-earth elements with calcite and aragonite. Chem. Geol. 1988, 69, 103–110. [Google Scholar] [CrossRef]
- Zhong, S.; Mucci, A. Partitioning of rare earth elements (REEs) between calcite and seawater solutions at 25 °C and 1 atm, and high dissolved REE concentrations. Geochim. Cosmochim. Acta 1995, 59, 443–453. [Google Scholar] [CrossRef]
- Toyama, K.; Terakado, Y. Experimental study of rare earth element partitioning between calcite and sodium chloride solution at room temperature and pressure. Geochem. J. 2014, 48, 463–477. [Google Scholar] [CrossRef]
- Voigt, M.; Mavromatis, V.; Oelkers, E.H. The experimental determination of REE partition coefficients in the water calcite system. Chem. Geol. 2017, 462, 30–43. [Google Scholar] [CrossRef]
- Schmidt, M.; Stumpf, T.; Marques Fernandes, M.; Walther, C.; Fanghänel, T. Charge Compensation in Solid Solutions. Angew. Chem. Int. Ed. 2008, 47, 5846–5850. [Google Scholar] [CrossRef] [PubMed]
- Marques Fernandes, M.; Stumpf, T.; Rabung, T.; Bosbach, D.; Fanghänel, T. Incorporation of trivalent actinides into calcite: A time resolved laser fluorescence spectroscopy (TRLFS) study. Geochim. Cosmochim. Acta 2008, 72, 464–474. [Google Scholar] [CrossRef]
- Gruzensky, P.M. Growth of calcite crystals. J. Phys. Chem. Solids 1967, S1, 365–367. [Google Scholar]
- Paquette, J.; Reeder, R.J. Relationship between surface structure, growth mechanism, and trace element incorporation in calcite. Geochim. Cosmochim. Acta 1995, 59, 735–749. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Watson, E.B.; Sadekov, A. Oxygen isotope fractionation between calcite and fluid as a function of growth rate and temperature: An in situ study. Chem. Geol. 2012, 306–307, 92–102. [Google Scholar] [CrossRef]
- De Villiers, S.; Greaves, M.; Elderfield, H. An intensity ratio calibration method for the accurate determination of Mg/Ca and Sr/Ca of marine carbonates by ICP-AES. Geochem. Geophys. Geosyst. 2002, 3. [Google Scholar] [CrossRef]
- Johnson, K.M.; Wills, K.D.; Butler, D.B.; Johnson, W.K.; Wong, C.S. Coulometric total carbon dioxide analysis for marine studies, maximizing the performance of an automated gas extraction system and coulometric detector. Mar. Chem. 1993, 44, 167–187. [Google Scholar] [CrossRef]
- Klungness, G.D.; Byrne, R.H. Comparative hydrolysis behavior of the rare earths and yttrium: The influence of temperature and ionic strength. Polyhedron 2000, 19, 99–107. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Comparative carbonate complexation of yttrium and gadolinium at 25 °C and 0.7 mol dm−3 ionic strength. Mar. Chem. 1995, 51, 213–221. [Google Scholar] [CrossRef]
- Luo, Y.-R.; Byrne, R.H. Yttrium and rare earth element complexation by chloride ions at 25 °C. J. Solut. Chem. 2001, 30, 837–845. [Google Scholar] [CrossRef]
- Luo, Y.-R.; Byrne, R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochim. Cosmochim. Acta 2004, 68, 691–699. [Google Scholar] [CrossRef]
- Hemming, N.G.; Reeder, R.J.; Hart, S.R. Growth-step-selective incorporation of boron on the calcite surface. Geochim. Cosmochim. Acta 1998, 62, 2915–2922. [Google Scholar] [CrossRef]
- Wasylenki, L.E.; Dove, P.M.; Wilson, D.S.; De Yoreo, J.J. Nanoscale effects of strontium on calcite growth: An in situ AFM study in the absence of vital effects. Geochim. Cosmochim. Acta 2005, 69, 3017–3027. [Google Scholar] [CrossRef]
- Watson, E.B. Surface enrichment and trace-element uptake during crystal growth. Geochim. Cosmochim. Acta 1996, 60, 5013–5020. [Google Scholar] [CrossRef]
- Watson, E.B. A conceptual model for near-surface kinetic controls on the trace element and stable isotope composition of abiogenic calcite crystals. Geochim. Cosmochim. Acta 2004, 68, 1473–1488. [Google Scholar] [CrossRef]
- Watson, E.B.; Liang, Y. A simple model for sector zoning in slowly growing crystals: Implications for growth rate and lattice diffusion, with emphasis on accessory minerals in crustal rocks. Am. Mineral. 1995, 80, 1179–1187. [Google Scholar] [CrossRef]
- Fenter, P.; Geissbuhler, P.; Dimasi, E.; Srajer, J.; Sorensen, L.B.; Sturchio, N.C. Surface speciation of calcite observed in situ by high-resolution X-ray reflectivity. Geochim. Cosmochim. Acta 2000, 64, 1221–1228. [Google Scholar] [CrossRef]
- Lanzillo, N.A.; Watson, E.B.; Thomas, J.B.; Nayak, S.K.; Curioni, A. Near-surface controls on the composition of growing crystals: Car–Parrinello molecular dynamics (CPMD) simulations of Ti energetics and diffusion in alpha quartz. Geochim. Cosmochim. Acta 2014, 131, 33–46. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Gaetani, G.A.; Watson, E.B.; Cohen, A.L.; Ehrlich, H.L. Experimental determination of growth rate effect on U6+ and Mg2+ partitioning between aragonite and fluid at elevated U6+ concentration. Geochim. Cosmochim. Acta 2008, 72, 4058–4068. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Sadekov, A.; Leinweber, A. Crystal growth rate effect on Mg/Ca and Sr/Ca partitioning between calcite and fluid: An in situ approach. Chem. Geol. 2014, 367, 70–82. [Google Scholar] [CrossRef]
- Burton, E.A.; Walter, L.M. Relative precipitation rates of aragonite and Mg calcite from seawater: Temperature or carbonate ion control? Geology 1987, 15, 111–114. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Rollion-Bard, C.; Tripati, A.; Sadekov, A. In situ study of boron partitioning between calcite and fluid at different crystal growth rates. Geochim. Cosmochim. Acta 2014, 137, 81–92. [Google Scholar] [CrossRef]
- Watson, E.B.; Müller, T. Non-equilibrium isotopic and elemental fractionation during diffusion-controlled crystal growth under static and dynamic conditions. Chem. Geol. 2009, 267, 111–124. [Google Scholar] [CrossRef]
- Li, Y.-H.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar]
- Voigt, M.; Rodriguez-Blanco, J.D.; Vallina, B.; Benning, L.G.; Oelkers, E.H. An experimental study of hydroxylbastnasite solubility in aqueous solutions at 25 °C. Chem. Geol. 2016, 430, 70–77. [Google Scholar] [CrossRef]
- Kesler, D.R.; Duedall, I.W.; Connors, D.N.; Pitkowicz, R.M. Preparation of artificial seawater. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar]
- Marriott, C.S.; Henderson, G.M.; Crompton, R.; Staubwasser, M.; Shaw, S. Effect of mineralogy, salinity, and temperature on Li/Ca and Li isotope composition of calcium carbonate. Chem. Geol. 2004, 212, 5–15. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ichikuni, M. Uptake of sodium and potassium by calcite. Chem. Geol. 1984, 42, 137–146. [Google Scholar] [CrossRef]

| t | REE | pH | Volume (spike) | REE (spike) | Volume (gr.med.) | REE * (gr.med.) | REE (gr.med.) | Ca# (gr.med.) |
|---|---|---|---|---|---|---|---|---|
| days | mL | ppm | mL | ppm | mol/kg H2O | mol/kg H2O | ||
| DC-1, 24.6 °C | ||||||||
| 0 | Sm | 8.01 | 1 | 0.2 | 1940 | 1.03 × 10−4 | 6.86 × 10−10 | 5.90 × 10−3 |
| 24 | La | 8.02 | 1 | 0.2 | 1895 | 1.06 × 10−4 | 7.60 × 10−10 | 5.11 × 10−3 |
| 36 | Nd | 8.05 | 1 | 0.2 | 1721 | 1.16 × 10−4 | 8.06 × 10−10 | 4.76 × 10−3 |
| 86 | Tb | 8.15 | 1 | 0.5 | 1514 | 3.30 × 10−4 | 2.08 × 10−9 | 3.52 × 10−3 |
| DC-2, 2 °C | ||||||||
| 32 | La | 8.76 | 1 | 0.2 | 465 | 4.30 × 10−4 | 3.10 × 10−9 | n/a |
| 63 | Nd | 8.74 | 1 | 0.2 | 455 | 4.40 × 10−4 | 3.05 × 10−9 | n/a |
| DC-3, 21.7 °C | ||||||||
| 0 | Sm | 7.83 | 1 | 0.2 | 840 | 2.38 × 10−4 | 1.58 × 10−9 | 2.09 × 10−3 |
| 1.2 | La | 7.70 | 1 | 0.2 | 820 | 2.44 × 10−4 | 1.76 × 10−9 | 1.90 × 10−3 |
| REE | (REE/Ca)fluid | (REE/Ca)calcite | KREE | DREE |
|---|---|---|---|---|
| DC-1, 24.6 °C (2 crystals) | ||||
| Sm | 1.16 × 10−4 | (0.93–4.1) × 10−3 | 8.0.2–35.34 | 13,418–59,153 |
| La | 1.49 × 10−4 | (1.4–3.2) × 10−3 | 10.74–21.48 | 20,867–41,734 |
| Nd | 1.69 × 10−4 | (1.5–2.2) × 10−3 | 8.88–13.02 | 18,446–27,055 |
| Tb | 5.90 × 10−4 | (1.4–4.1) × 10−3 | 2.03–3.81 | 6671–19,538 |
| DC-2, 2 °C (2 crystals) | ||||
| La | n/a | (5.32–5.51) × 10−3 | n/a | 17,010–17,617 |
| Nd | n/a | (4.38–5.79) × 10−3 | n/a | 14,234–18,816 |
| DC-3, 21.7 °C (3 crystals) | ||||
| Sm | 7.57 × 10−4 | (5.82–5.90) × 10−4 | 0.769–0.0779 | 8241–8354 |
| La | 9.22 × 10−4 | 6.58 × 10−4 | 0.714 | 2104 |
| Gr.med. | Ca, mol/kg | REE, mol/kg | pH | pCO2 | Ω | T °C | KREE | Study |
|---|---|---|---|---|---|---|---|---|
| 0.5 M NH4Cl | (2.7–5.9) × 10−3 | (6.8–8.1) × 10−10 | 8.01–8.05 | n/a | 1.8 ± 0.29 | 24.6 | 8.02–35.34 | 1 |
| 0.11 M NaCl | 8.73 × 10−3 | (0.6–3) × 10−10 | 7.5–8.5 | n/a | n/a | 21–26 | 3.5–10 | 2 |
| ASW | 1.06 × 10−2 | (0.0001–1) × 10−7 | 7.4–7.8 | 0.0031 | 3.5–15 | 25 | 1585–2512 | 3 |
| 0.27 M NaCl | 2.25 × 10−3 | (1.8–2.0) × 10−11 | 8.4–8.5 | n/a | n/a | 25 | 86–94 | 4 |
| 0.1 M NaCl | 2.08 × 10−2 | (0.01–1) × 10−7 | 6.0 | 0.91 | 1–5 | 25 | 100–1000 | 5 |
| 0.1 M NaClO4 | 1.00 × 10−2 | (0.1–7) × 10−9 | 6.0 | 1 | 1 | 25 | 477–1575 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabitov, R.I.; Sadekov, A.; Migdisov, A. REE Incorporation into Calcite Individual Crystals as One Time Spike Addition. Minerals 2017, 7, 204. https://doi.org/10.3390/min7110204
Gabitov RI, Sadekov A, Migdisov A. REE Incorporation into Calcite Individual Crystals as One Time Spike Addition. Minerals. 2017; 7(11):204. https://doi.org/10.3390/min7110204
Chicago/Turabian StyleGabitov, Rinat I., Aleksey Sadekov, and Artas Migdisov. 2017. "REE Incorporation into Calcite Individual Crystals as One Time Spike Addition" Minerals 7, no. 11: 204. https://doi.org/10.3390/min7110204




_Migdisov.png)

