The Use of Univariate and Multivariate Analyses in the Geochemical Exploration, Ravanj Lead Mine, Delijan, Iran
Abstract
:1. Introduction
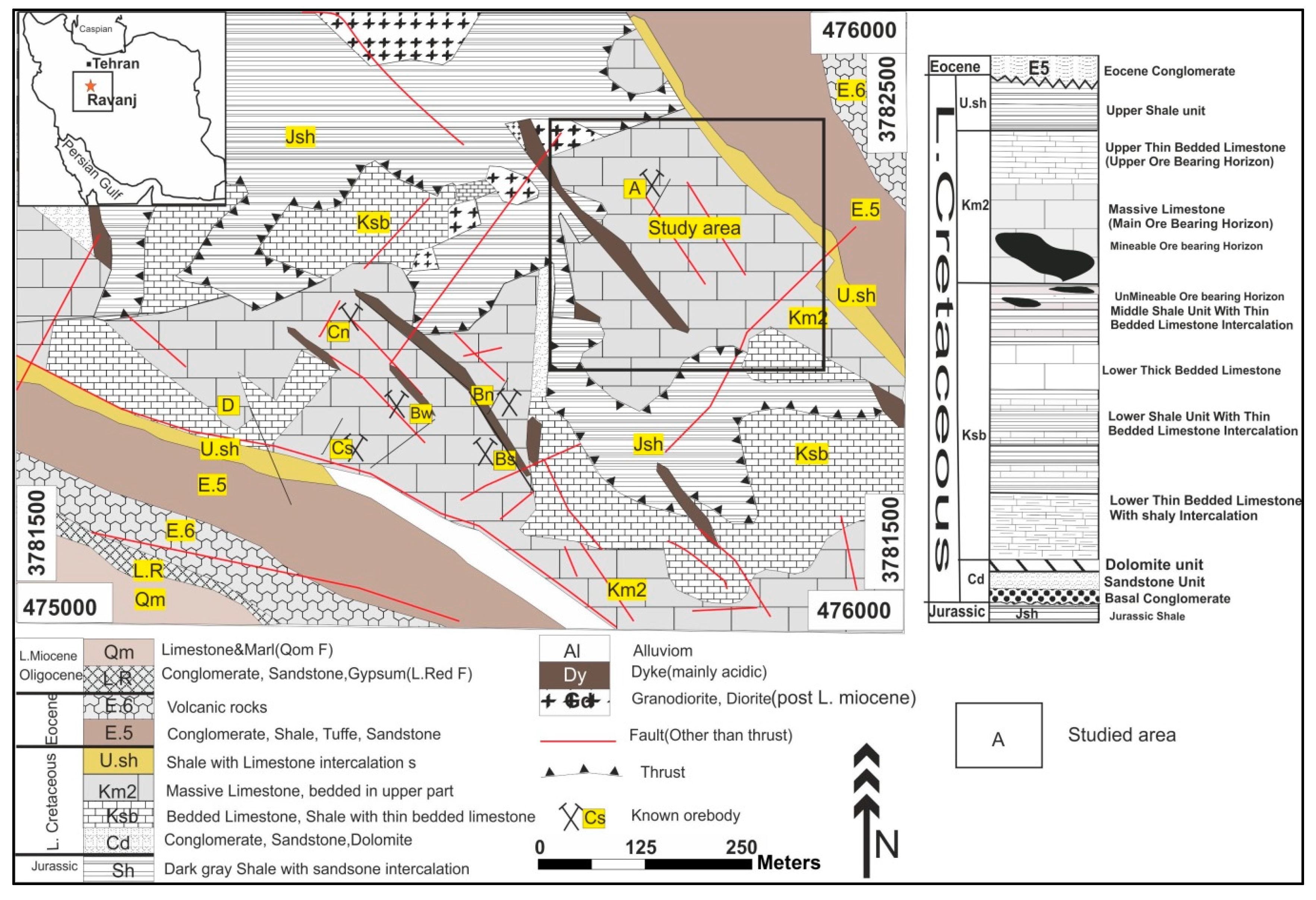
2. The Ravanj Pb Deposit
2.1. Geology
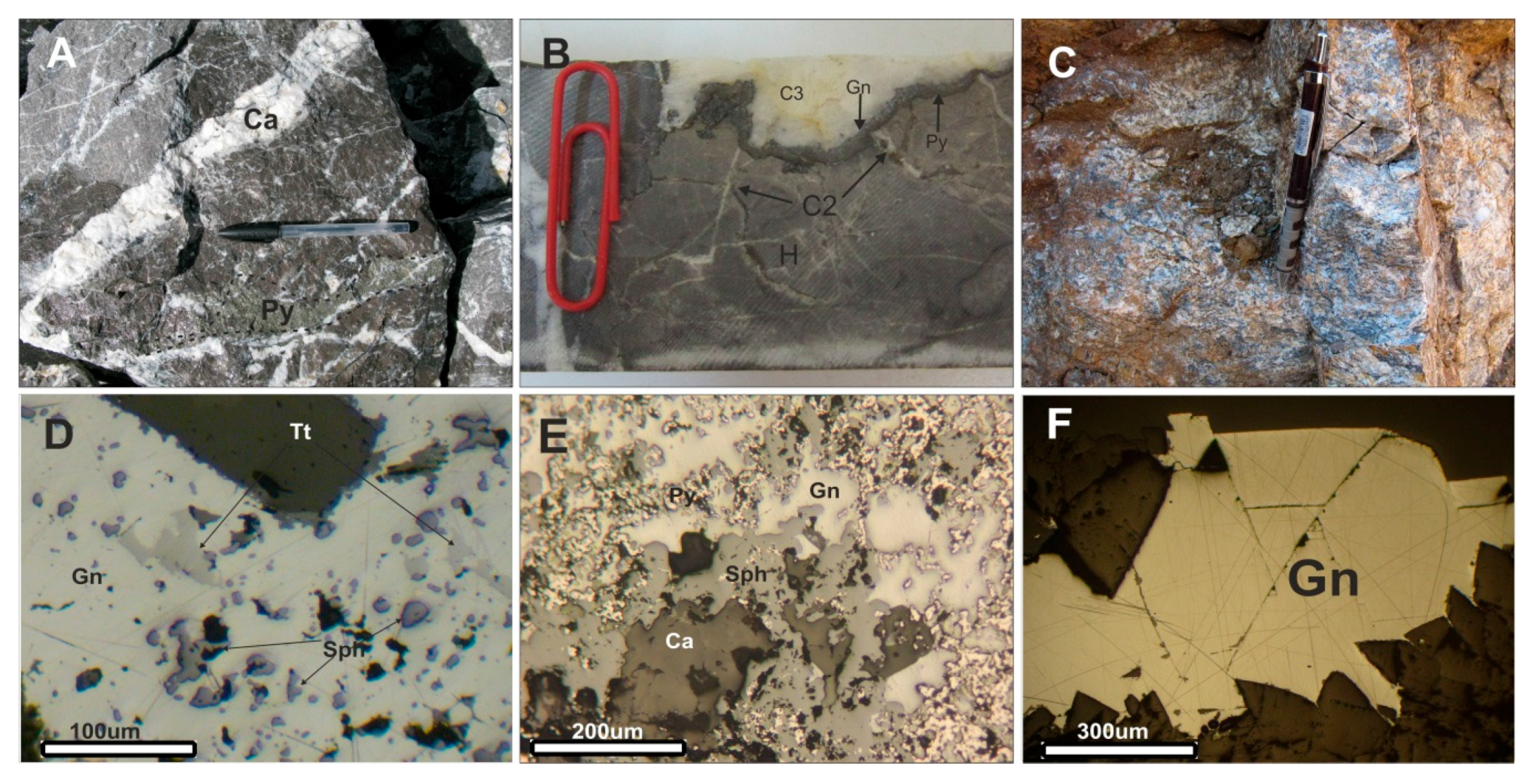
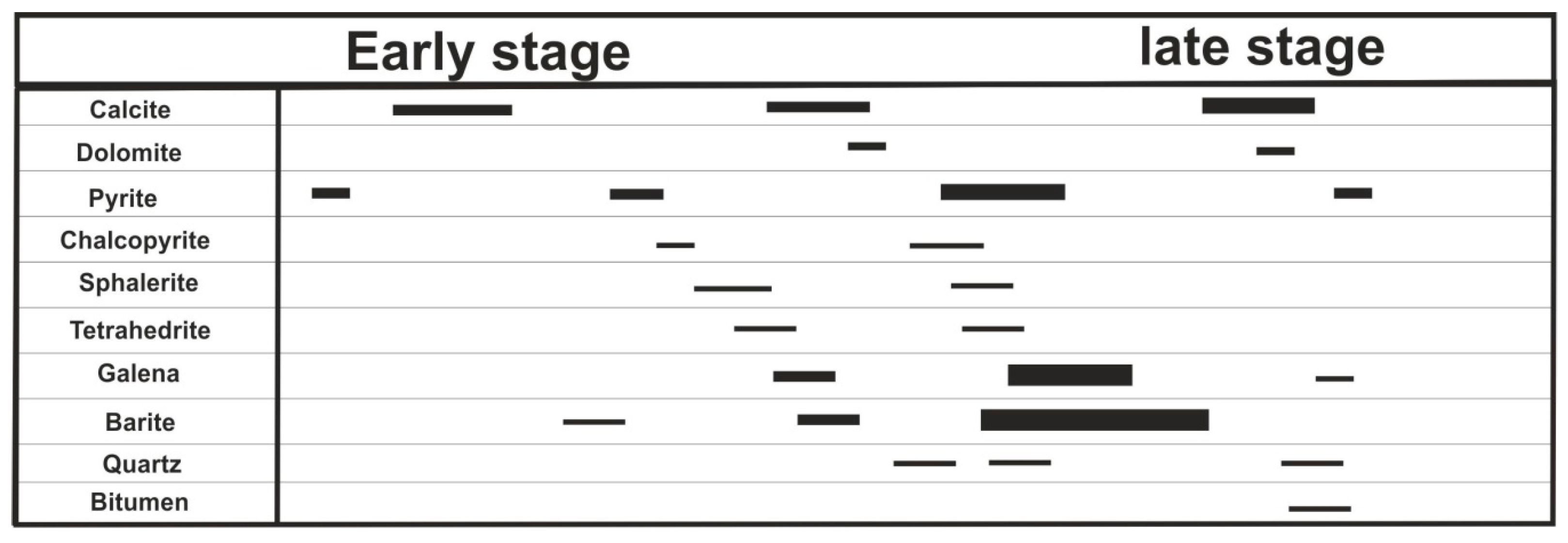
2.2. Mineralization and Ore Zoning
2.3. Ore Genesis
3. Geochemical Analysis
3.1. Sampling and Analytical Method
3.2. Anomaly Recognition Methods
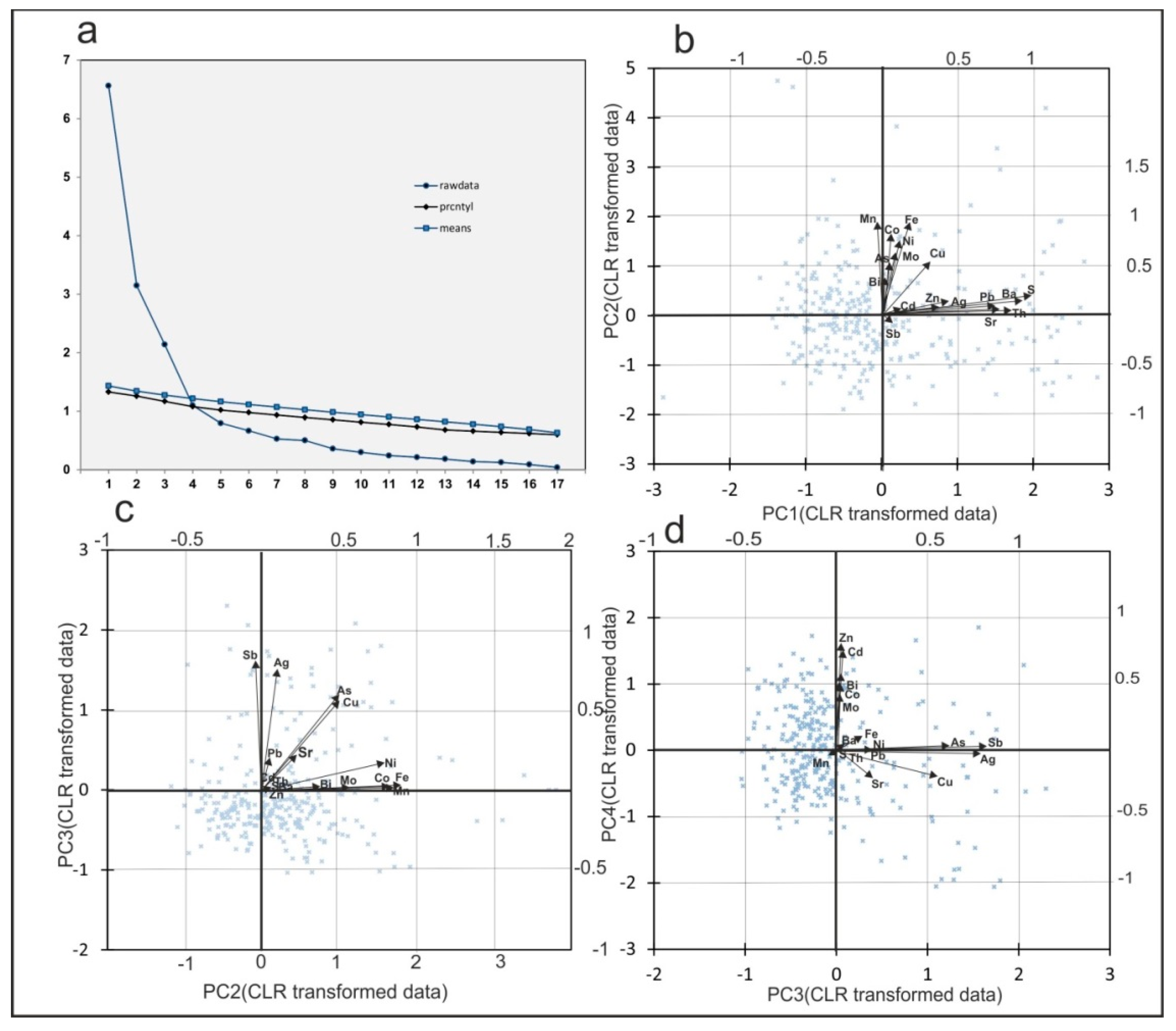
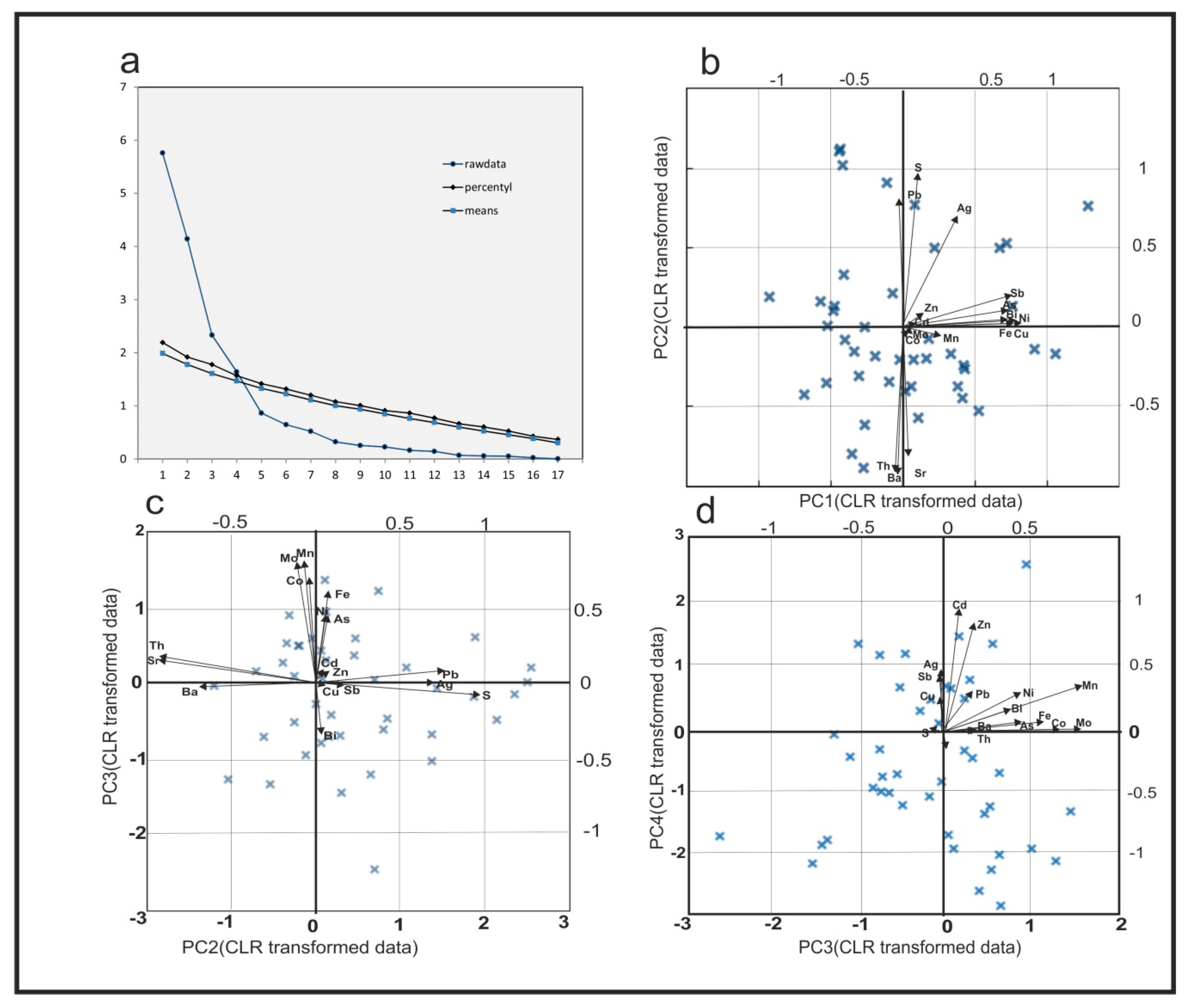
3.3. Results from Principal Component Analysis
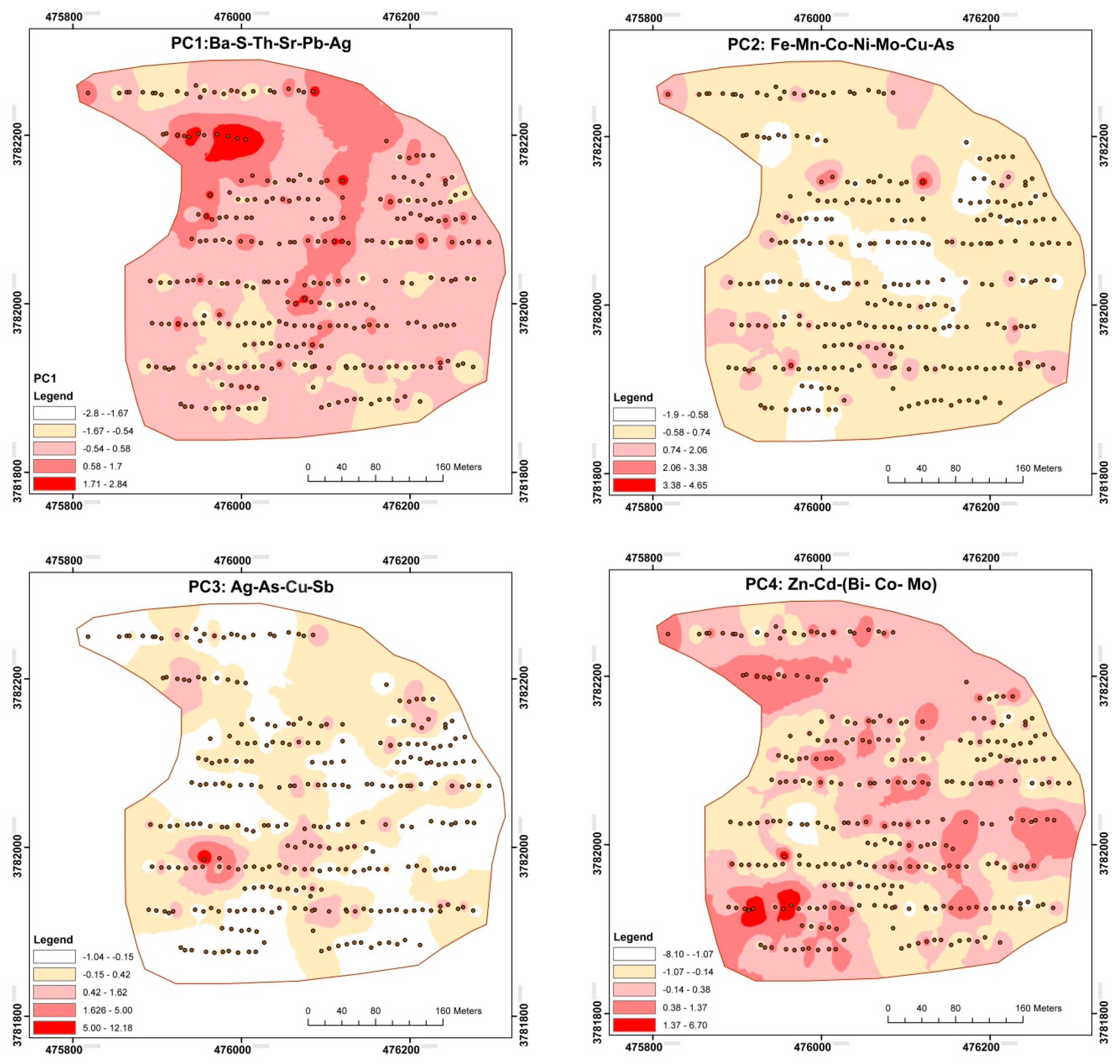
3.4. GIS Application
4. Discussion
4.1. Anomalous Elements
4.2. Trace Element Association
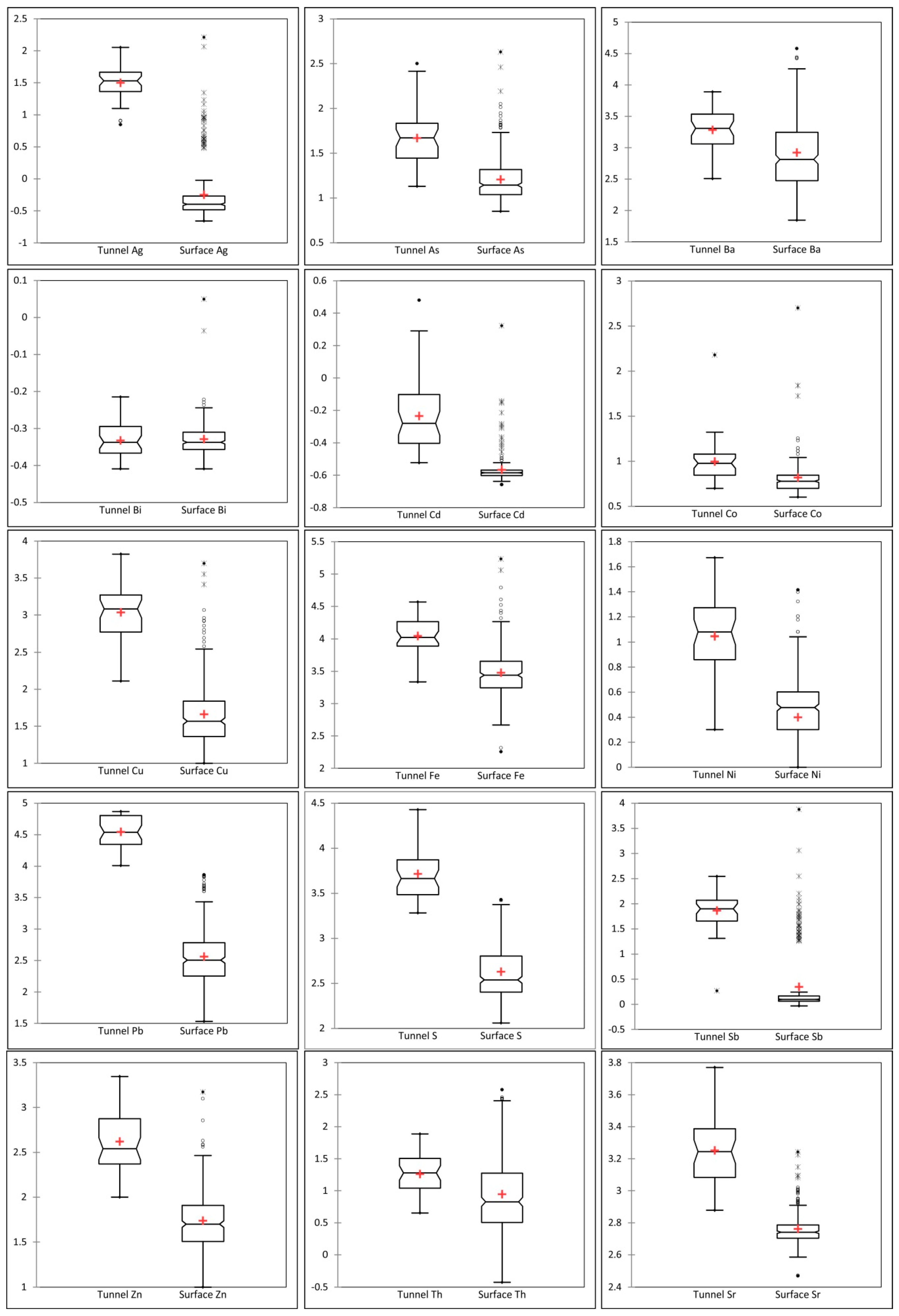
4.3. Vertical Variation of Indicator Elements
Surface Depth
4.4. Anomaly Testing
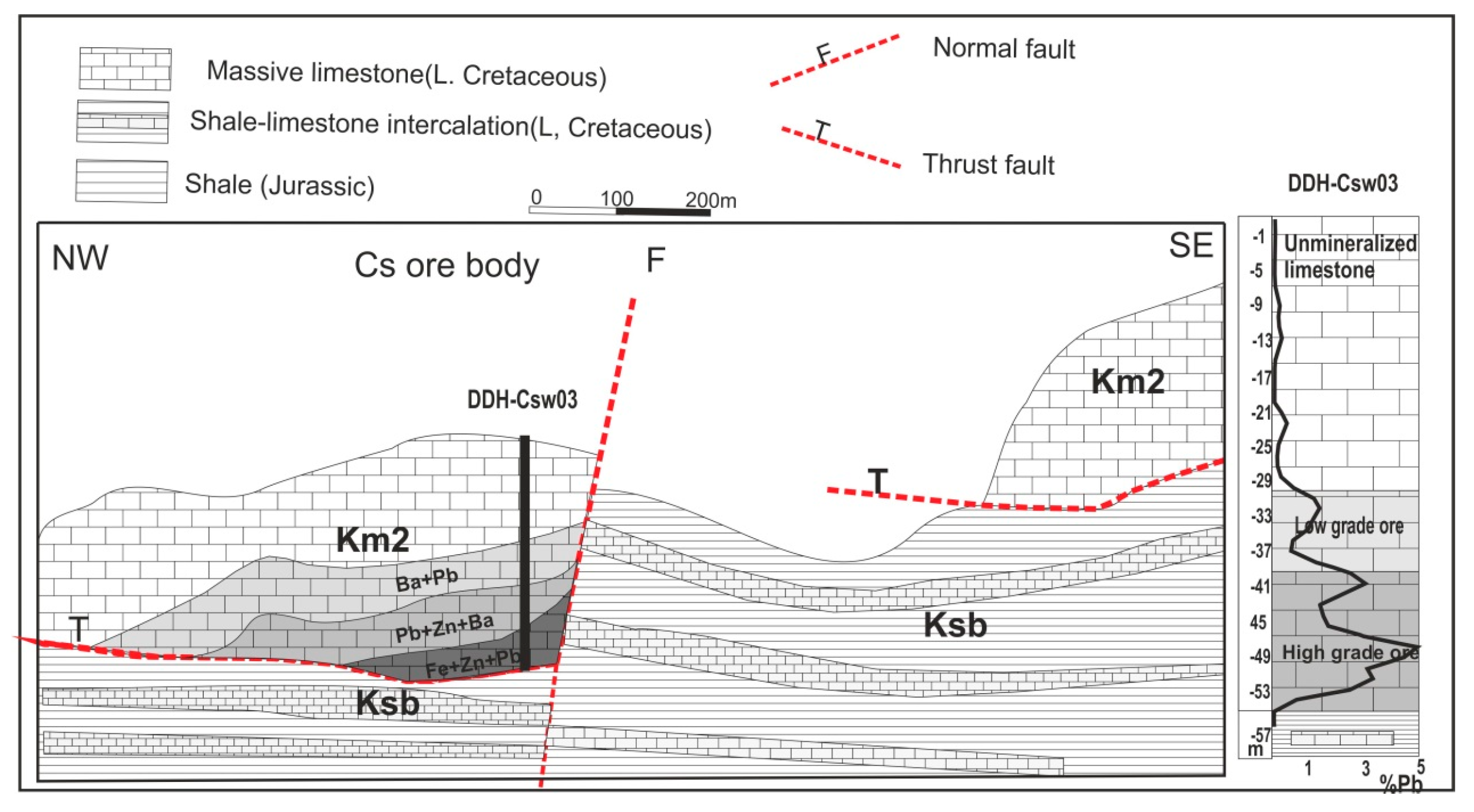
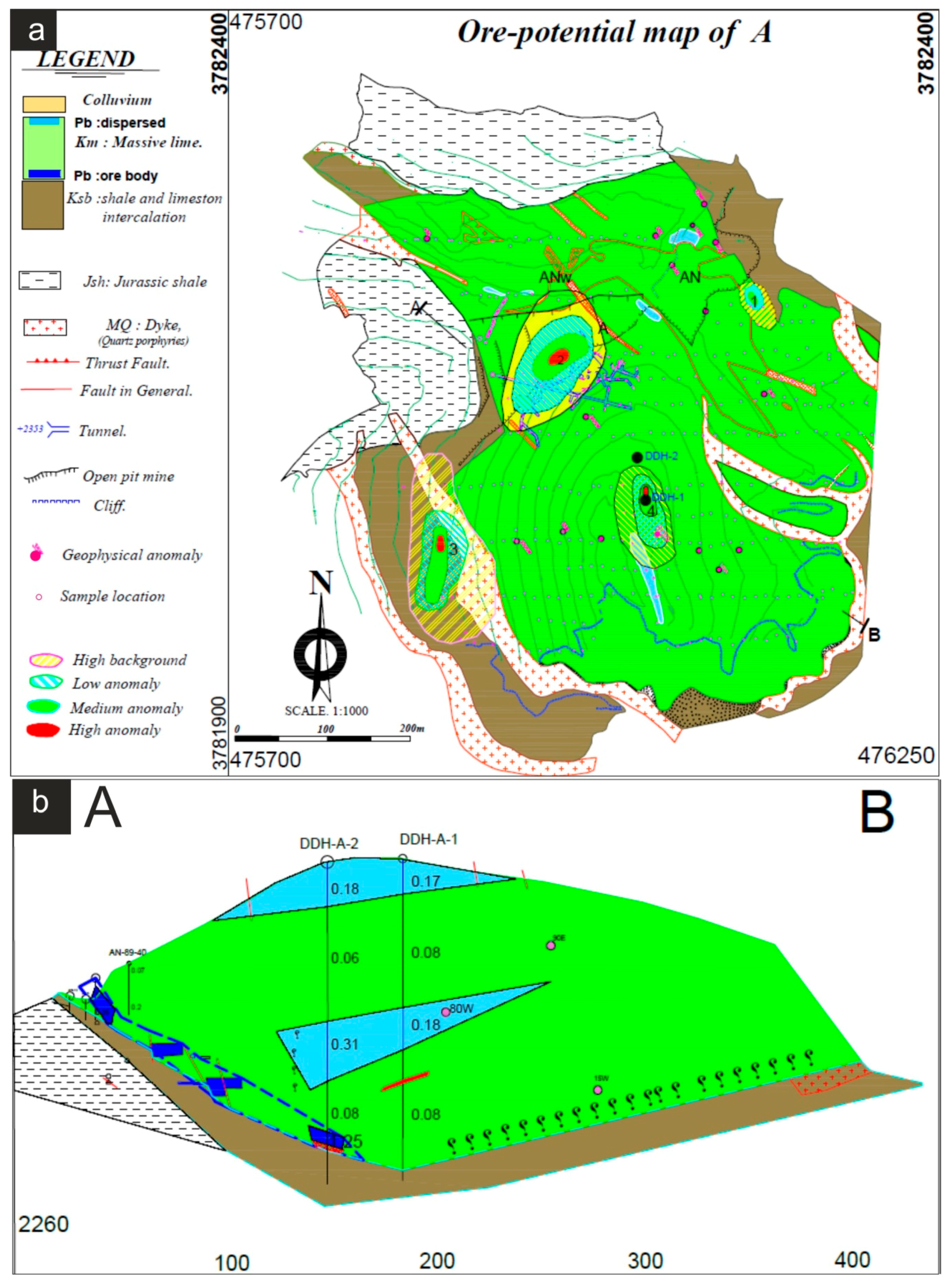
- The first anomaly is located in the northeast margin of open pit A. The existence of this ore is confirmed by previous drilling. This small orebody, dipping 29° SE, is a branch of the previously extracted orebody which is situated at a depth of 16–25 m. The maximum recorded anomaly on this orebody is considered as medium anomaly. Because a medium intensity anomaly represents an ore in depth of approximately 25 m, a low intensity anomaly and high background values may reflect the presence of ore at depths greater than 25 m (Figure 9).
- The second anomaly is around the old tunnels of the orebody A. Based on the geological section (Figure 9), the thickness of massive limestone increases from NW to SE. As a result, intensity of anomaly is maximum on top of the NW area and it becomes low and finally drops to the background values in the SE area (Figure 9).
- The third anomaly occurs in the thin layered limestone-shale intercalation in the western end of the Block A. Significant orebody has not been detected in these strata. However, rotary air blast drilling does not show an orebody other than minor disseminated galena and barite with the mean Pb of 0.45%.
- The fourth anomaly, located at the center of Block A, is a high intensity anomaly, which coincides with the previously detected geophysical anomaly called 80 W [21]. In the Ravanj deposit, mineralization occurs in the lower part of the massive limestone where it thrusts over the shale strata. The thickness of host limestone in this part was estimated 100 m however, a diamond drilling of 155.6 m hole (DDH-A-1; Figure 9) in this anomaly showed that the limestone is 150 m thick. Mean of Pb in the first 18 m of the hole is 0.17%, which represents a near surface mineralization. In addition, the mean value of Pb is 0.18% in depth interval of 75–86 m. The thickness of limestone in the second drill hole (DDH-A-2; Figure 9) is 133 m. Similar to the first drill hole, the mean value of Pb is 0.18% in the first 20 m of this hole. The mean Pb is 0.31% in the 88–105 m depth interval, which possibly represents extension of the ore zone from depth interval of 75–86 m in the first hole. Stronger Pb mineralization (mean of 1.25%) was encountered at 126–131 m depth interval. This mineralized zone represents the extension of the ore which was previously mined in orebody A (Figure 9). It appears that both of the shallower ore zones are dipping towards the mined orebody A.
5. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Levinson, A.A. Introduction to Exploration Geochemistry, 2nd ed.; Applied Publishing Company: Calgary, AB, Canada, 1980; p. 924. [Google Scholar]
- Wang, C.; Carranza, E.J.M.; Zhang, S.; Zhang, J.; Liu, X.; Zhang, D.; Sun, X.; Duan, C. Characterization of primary geochemical haloes for gold exploration at the Huanxiangwa gold deposit, China. J. Geochem. Explor. 2013, 124, 40–58. [Google Scholar] [CrossRef]
- Leach, D.L.; Sangster, D.F.; Kefiglley, K.D.; Large, R.R.; Garven, G.; Allen, C.R.; Gutzmer, J.; Walters, S. Sediment-hosted lead–zinc deposits: A global perspective. Econ. Geol. 2005, 100th Anniv. Vol., 561–608. [Google Scholar]
- Mavrogenes, J.A.; Hagni, R.D.; Dingess, P.R. Mineralogy, paragenesis, and mineral zoning of the West Fork mine. Viburnum Trend, Southeast Missouri. Econ. Ceol. 1992, 87, 113–124. [Google Scholar]
- Lavery, N.C.; Leach, D.L.; Saunders, J.A. Lithogeochemical investigations applied to exploration for sediment-hosted lead-zinc deposits. In Society for Geology Applied to Mineral Deposits Special Publication; Springer: Berlin/Heidelberg, Germany, 1994; pp. 393–428. [Google Scholar]
- Yusta, I.; Velasco, F.; Herrero, J.-M. Anomaly threshold estimation and data normalization using EDA statistics: Application to lithogeochemical exploration in Lower Cretaceous Zn-Pb carbonate-hosted deposits, Northern Spain. Appl. Geochem. 1998, 13, 421–439. [Google Scholar] [CrossRef]
- Feltrin, L. Predictive modeling of prospectivity for Pb-Zn deposits in the Lawn Hill region, Queensland, Australia. Ore Geol. Rev. 2008, 34, 399–427. [Google Scholar] [CrossRef]
- Decree, S.; Mariganc, C.; De Putter, T.; Deloule, E.; Liegeois, J.P.; Demaiffe, D. Pb-Zn mineralization in a Miocene regional extensional context: The case of the Sidi Driss and the Douahria ore deposits, Nefza mining district, northern Tunisia. Ore Geol. Rev. 2008, 34, 285–303. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer: Berlin, Germany, 2009; p. 1250. [Google Scholar]
- Billstrom, K.; Broman, C.; Schneider, J.; Pratt, W.; Skogsmo, G. Zn-Pb ores of Mississippi valley type in the Lycksele-Storuman District, Northern Sweden: A possible rift-related Cambrian mineralization event. Minerals 2012, 1, 169–207. [Google Scholar] [CrossRef]
- Samimi Namin, M. Geoelectrical report of the Ravanj lead mine. In Unpublished Reports of the Ravanj Mine; Soojmiran Co.: Tehran, Iran, 1997. (In Farsi) [Google Scholar]
- Emami, M.H. Kahak Map 1:100,000; Geological Survey and Mineral Exploration of Iran: Tehran, Iran, 1996. [Google Scholar]
- Modabberi, S. Geology, Facies Analysis, Mineralogy, Geochemistry and Genesis of Ravandje Pb-Ag Deposit, Central Iran. Master’s Thesis, Tarbiat Modarres University, Tehran, Iran, 1995. (In Farsi). [Google Scholar]
- Alavi, M. Tectonics of Zagros Orogenic belt of Iran, new data and interpretation. Tectonophysics 1994, 229, 211–238. [Google Scholar] [CrossRef]
- Golonka, J. Plate tectonic evolution of the southern margin of Eurasia in the Mesozoic and Cenozoic. Tectonophysics 2004, 381, 235–273. [Google Scholar] [CrossRef]
- Nejadhadad, M.; Taghipour, B.; Zarasvandi, A.; Karimzadeh Somarin, A. Geological, geochemical, and fluid inclusion evidences for the origin of the Ravanj Pb-Ba-Ag deposit, north of Delijan city, Markazi Province, Iran. Turkish J. Earth Sci. 2016, 24, 1501–1526. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur Isotope Geochemistry of Sulfide Minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Hron, K.; Templ, M.; Filzmoser, P. Imputation of missing values for compositional data using classical and robust methods. Comput. Stat. Data Anal. 2010, 54, 3095–3107. [Google Scholar] [CrossRef]
- Aitchison, J.; Barceló-Vidal, C.; Martín-Fernandez, J.; Pawlowsky-Glahn, V. Logratio analysis and compositional distance. Math. Geol. 2000, 32, 271–275. [Google Scholar] [CrossRef]
- Buccianti, A.; Grunsky, E. Compositional data analysis in geochemistry: Are we sure to see what really occurs during natural processes? J. Geochem. Explor. 2014, 141, 1–5. [Google Scholar] [CrossRef]
- Blake, S.; Henry, T.; Murray, J.; Flood, R.; Muller, M.R.; Jones, A.G.; Rath, V. Compositional multivariate statistical analysis of thermal groundwater provenance: A hydrogeochemical case study from Ireland. Appl. Geochem. 2016, 75, 171–188. [Google Scholar] [CrossRef]
- Hawkes, H.E.; Webb, J.S. Geochemistry in Mineral Exploration; Harper and Row: New York, NY, USA, 1962. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.G. Factor analysis applied to regional geochemical data: Problems and possibilities. Appl. Geochem. 2002, 17, 185–206. [Google Scholar] [CrossRef]
- Reimann, C.; Garrett, R.G. Geochemical background-concept and reality. Sci. Total Environ. 2005, 350, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Hoaglin, D.C.; Iglewicz, B.; Tukey, J.W. Performance of some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1986, 81, 991–999. [Google Scholar] [CrossRef]
- Wurzer, F. Application of robust statistics in the analysis of geochemical data. In Quantitative Analysis of Mineral and Energy Resources; Chung, C.F., Fabbri, A.G., Sinding-Larsen, R., Eds.; Reidel Publishing Company: Dordrecht, The Netherlands, 1988; pp. 131–143. [Google Scholar]
- Hoaglin, D.; Mosteller, F.; Tukey, J. Understanding Robust and Exploratory Data Analysis, 2nd ed.; Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.G. Background and threshold: Critical comparison of methods of determination. Sci. Total Environ. 2005, 346, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mrvić, V.; Kostić-Kravljanac, L.; Čakmak, D.; Sikirić, B.; Brebanović, B.; Perović, V.; Nikoloski, M. Pedogeochemical mapping and background limit of trace elements in soils of Branicevo Province (Serbia). J. Geochem. Explor. 2011, 109, 18–25. [Google Scholar] [CrossRef]
- Kurzl, H. Exploratory data analysis: Recent advances for the interpretation of geochemical data. J. Geochem. Explor. 1988, 30, 309–322. [Google Scholar] [CrossRef]
- Kotz, S.; Johnson, N.L. Encyclopedia of Statistical Sciences; John Wiley and Sons: New York, NY, USA, 1985; pp. 136–137. [Google Scholar]
- Cook, S.J.; Fletcher, W.K. Distribution and behavior of platinum in soils, sediments and waters or the Tulameen ultramafic complex, southern British Columbia, Canada. J. Geochem. Explor. 1993, 46, 279–308. [Google Scholar] [CrossRef]
- Templ, M.; Filzmoser, P.; Reimann, C. Cluster analysis applied to regional geochemical data: Problems and possibilities. Appl. Geochem. 2008, 23, 2198–2213. [Google Scholar] [CrossRef]
- Fresia, B.; Ross, P.S.; Gloaguen, E.; Bourke, A. Lithological discrimination based on statistical analysis of multi-sensor drill core logging data in the Matagami VMS district, Quebec, Canada. Ore Geol. Rev. 2016. [Google Scholar] [CrossRef]
- Reimann, C.; Filzmoser, P. Normal and lognormal data distribution in geochemistry: Death of a myth. Consequences for the statistical treatment of geochemical and environmental data. Environ. Geol. 2000, 39, 1001–1014. [Google Scholar] [CrossRef]
- Howarth, R.J.; Sinding-Larsen, R. Multivariate analyses. In Statistics and Data Analysis in Geochemical Prospecting: Handbook of Exploration Geochemistry; Howarth, R.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 207–289. [Google Scholar]
- Day, S.J.A.; McCurdy, M.W.; Friske, P.W.B.; McNeil, R.J.; Hornbrook, E.H.W.; Lynch, J.J. Regional lake sediment and water geochemical data, Melville Peninsula, Nunavut (parts of NTS 046 N, O, P, 047A and B). Geol. Surv. Can. Open File 2009, 12, 6269. [Google Scholar]
- Filzmoser, P.; Hron, K.; Reimann, C. Principal component analysis for compositional data with outliers. Environmetrics 2009, 20, 621–632. [Google Scholar] [CrossRef]
- Grunsky, E.C.; Mueller, U.A.; Corrigan, D. A study of the lake sediment geochemistry of the Melville Peninsula using multivariate methods: Applications for predictive geological mapping. J. Geochem. Explor. 2014, 141, 15–41. [Google Scholar] [CrossRef]
- Filzmoser, P.; Hron, K.; Reimann, C. Univariate statistical analysis of environmental (compositional) data: Problems and possibilities. Sci. Total Environ. 2009, 407, 6100–6108. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, J.A.; Thió-Henestrosa, S. Compositional Data Analysis. In Springer Proceedings in Mathematics & Statistics; Springer: London, UK, 2015; Volume 187, p. 211. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: London, UK, 2002. [Google Scholar]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. Giving meaningful interpretation to ordination axes: Assessing loading significance in principal component analysis. Ecology 2003, 84, 2347–2363. [Google Scholar] [CrossRef]
- Hronsky, J.M.A. The science of exploration targeting. In SEG 2004, Predictive Mineral Discovery under Cover: Publication, 33; Muhling, J., Ed.; University of Western Australia: Crawley, Australia, 2004; pp. 129–133. [Google Scholar]
- Carranza, E.J.M. Geochemical Anomaly and Mineral Prospectivity Mapping in GIS; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Grunsky, E.C. The interpretation of geochemical survey data. Geochem. Explor. Environ. Anal. 2010, 10, 27–74. [Google Scholar] [CrossRef]
- Hosseini-Dinani, H.; Aftabi, A. Vertical lithogeochemical halos and zoning vectors at Goushfil Zn-Pb deposit, Irankuh district, southwestern Isfahan, Iran: Implications for concealed ore exploration and genetic models. Ore Geol. Rev. 2016, 72, 1004–1021. [Google Scholar] [CrossRef]
- Cameron, E.M.; Hamilton, S.M.; Leybourne, M.I.; Hall, G.E.M.; McClenaghan, M.B. Finding deeply buried deposits using geochemistry. Geochem. Explor. Environ. Anal. 2004, 4, 7–32. [Google Scholar] [CrossRef]
- Carranza, E.J.M.; Owusu, E.A.; Hale, M. Mapping of prospectivity and estimation of number of undiscovered prospects for lode gold, southwestern Ashanti Belt, Ghana. Miner. Depos. 2009, 44, 915–938. [Google Scholar] [CrossRef]
- Viets, J.G.; Leach, D.L. Genetic implications of regional and temporal trends in ore fluid geochemistry of Mississippi Valley-type deposits in Ozark region. Econo. Geol. 1990, 85, 842–861. [Google Scholar] [CrossRef]
- Klein, C.; Hurlbut, C.S. Manual of Mineralogy; John Wiley & Sons: Hoboken, NJ, USA, 1993; p. 681. [Google Scholar]
- Beus, A.A.; Grigorian, S.V. Geochemical Exploration Methods for Mineral Deposits; Applied publishing Company: Wilmette, IL, USA, 1977; 287 p. [Google Scholar]
- Hanor, J.S. Barite-celestine geochemistry and environments of formation. Rev. Miner. Geochem. 2000, 40, 193–275. [Google Scholar] [CrossRef]
- Scheffer, F.; Schachtschabel, P. Lehrbuch der Bodenkunde; Spektrum Akademischer Verlag: Heidelberg, Germany, 2002; 494p. (In German) [Google Scholar]
- Boni, M. Non-sulfide Zinc Deposits: A new-(old) type of economic mineralization. SGA News 2003, 15, 6–11. [Google Scholar]
- Reichert, J.; Borg, G. Numerical simulation and a geochemical model of supergene carbonate-hosted non-sulfide zinc deposits. Ore Geol. Rev. 2008, 33, 134–151. [Google Scholar] [CrossRef]
| Host Mineral | Inclusion Type | Tm, Carb | Tm, Clath (°C) | Te (°C) | Tm, Ice (°C) | Th (°C) | Salinity (wt % NaCl Equiv.) | N |
|---|---|---|---|---|---|---|---|---|
| Stage 2 calcite | L + V | - | - | - | −3.3/−13.8 | 123.7–204.8 | 5.2–17.9 | 55 |
| Stage 3 calcite | L + V | - | - | −37.2/−52.8 | −0.4/−19.8 | 120.7–220.4 | 0.66–22.2 | 21 |
| Barite | L + V | - | - | - | −1.8/11.9 | 141–200.8 | 2.95–15.95 | 17 |
| Stage 2 calcite | L1 + L2 + V | −56.7/−58.1 | 4.2/7.3 | - | - | 173–194.6 | 5.2–10.2 | 5 |
| Stage 3 calcite | L1 + L2 + V | −56.7/−57.8 | 1.9/6.3 | - | - | 177.1–202 | 6.87–13.2 | 3 |
| n = 302 | Minimum | Q1 | Median | Q3 | Maximum | Mean | Std.dev. | MAD | Skewness | EF |
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | 0.22 | 0.33 | 0.40 | 0.54 | 162.8 | 2.06 | 11.63 | 0.09 | 11.91 | 1.41 |
| As | 7.10 | 10.83 | 13.85 | 20.80 | 426.7 | 21.50 | 32.74 | 4.15 | 8.59 | 1.30 |
| Ba | 70.00 | 314.00 | 693.50 | 1876.25 | 28047 | 2235.88 | 4018.39 | 485.50 | 3.22 | 2.46 |
| Be | 0.2 | 0.28 | 0.31 | 0.35 | 0.52 | 0.32 | 0.05 | 0.03 | 0.75 | 1.2 |
| Bi | 0.22 | 0.44 | 0.46 | 0.49 | 1.12 | 0.47 | 0.06 | 0.02 | 5.09 | 0.93 |
| Ca | 324,579 | 367,052 | 380,302 | 391,574 | 405,603 | 377,820 | 16,769 | 11,682 | −0.76 | - |
| Cd | 0.12 | 0.25 | 0.26 | 0.27 | 2.10 | 0.28 | 0.12 | 0.01 | 11.07 | 1.17 |
| Ce | 6 | 7 | 8 | 9 | 12 | 8.2 | 1.40 | 1 | 0.55 | 0.78 |
| Co | 3.00 | 5.00 | 6.00 | 7.00 | 502 | 8.74 | 29.06 | 1.00 | 16.35 | 1.00 |
| Cr | 2 | 4 | 5 | 6 | 8 | 5.34 | 1.136 | 1.00 | 0.56 | 0.43 |
| Cu | 10.00 | 23.00 | 37.00 | 68.75 | 4971 | 114.67 | 396.4857 | 18.00 | 9.24 | 0.75 |
| Fe | 180.00 | 1750.75 | 2754.50 | 4510.25 | 170,931 | 5165.87 | 12,757.74 | 1150.50 | 9.83 | 0.93 |
| K | 1039 | 1600 | 1931 | 2409 | 4177 | 2061 | 658 | 414 | 0.93 | - |
| Mg | 2013 | 2332 | 2695 | 2974 | 4325 | 2713 | 477.5 | 334 | 0.89 | - |
| Mn | 194.00 | 481.00 | 669.00 | 941.00 | 5698 | 811.15 | 629.44 | 203.50 | 4.22 | 0.73 |
| Mo | 0.58 | 1.20 | 1.26 | 1.34 | 5.50 | 1.32 | 0.37 | 0.07 | 8.50 | 1.06 |
| Ni | 1.00 | 1.25 | 3.00 | 4.00 | 26 | 3.29 | 3.18 | 1.00 | 3.78 | 1.25 |
| P | 64 | 105 | 123 | 147 | 256 | 129 | 33.73 | 21 | 0.87 | 0.57 |
| Pb | 28.00 | 177.50 | 319.50 | 606.25 | 7272 | 754.21 | 1296.57 | 185.50 | 3.37 | 8.52 |
| Rb | 77 | 100 | 109 | 124 | 148 | 111 | 15.6 | 11 | 0.09 | 1.05 |
| S | 115.00 | 253.25 | 346.00 | 635.50 | 2690 | 588.73 | 558.24 | 131.00 | 1.85 | 1.67 |
| Sb | 0.93 | 1.15 | 1.25 | 1.46 | 7527 | 37.90 | 437.49 | 0.13 | 16.75 | 1.01 |
| Sr | 295.00 | 505.00 | 550.00 | 610.75 | 1747 | 594.53 | 173.59 | 51.50 | 3.06 | 1.11 |
| Th | 0.38 | 3.20 | 6.80 | 18.73 | 275 | 21.47 | 39.24 | 4.55 | 3.68 | 2.52 |
| Ti | 22 | 43 | 54 | 66.75 | 120 | 56 | 17.2 | 12 | 0.66 | 0.85 |
| V | 6 | 9 | 11 | 12 | 19 | 10.9 | 2.37 | 2 | 0.76 | 0.76 |
| W | 1.15 | 1.3 | 1.34 | 1.37 | 1.61 | 1.34 | 0.056 | 0.04 | 0.76 | 0.97 |
| Zn | 10.00 | 32.00 | 50.00 | 81.00 | 1487 | 79.62 | 129.23 | 21.00 | 7.50 | 2.88 |
| n = 42 | Minimum | Q1 | Median | Q3 | Maximum | Mean | Std.dev. | MAD | Skewness | EF |
|---|---|---|---|---|---|---|---|---|---|---|
| Ag | 7.04 | 23.27 | 33.56 | 46.11 | 112.94 | 37.58 | 23.00 | 12.12 | 1.47 | 235.9 |
| As | 13.5 | 28.1 | 49.2 | 69.65 | 317 | 68.77 | 69.74 | 21 | 2.36 | 4.0 |
| Ba | 322 | 1149 | 2107 | 3477.5 | 7753 | 2470.88 | 1622.93 | 1087 | 1.12 | 24.8 |
| Bi | 0.39 | 0.43 | 0.46 | 0.51 | 0.61 | 0.47 | 0.05 | 0.04 | 0.55 | 19.1 |
| Cd | 0.3 | 0.4 | 0.53 | 0.78 | 3.02 | 0.69 | 0.50 | 0.16 | 2.85 | 118.6 |
| Co | 5 | 7 | 10 | 12 | 151 | 13.34 | 21.58 | 2 | 6.25 | 1.1 |
| Cu | 129 | 622 | 1250 | 1849 | 6654 | 1643.88 | 1500.45 | 636 | 1.70 | 4.0 |
| Fe | 2165 | 7747 | 10,717 | 18,731 | 36,970 | 13,993.6 | 8783.16 | 5853 | 0.89 | 2.9 |
| Mn | 444 | 1046 | 1404 | 1774 | 4169 | 1592.46 | 783.56 | 368 | 1.34 | 1.1 |
| Mo | 1.24 | 1.67 | 1.99 | 5.2 | 16 | 3.97 | 3.85 | 0.46 | 1.89 | 1.1 |
| Ni | 2 | 7.5 | 12 | 19 | 47 | 14.67 | 10.03 | 6 | 1.26 | 6.0 |
| Pb | 10,208 | 22,174.5 | 31,953 | 63,297.5 | 73,763 | 40,793.93 | 20,879.95 | 17,046 | 0.11 | 1665.8 |
| S | 1917 | 3013 | 4567 | 7386 | 26,833 | 6429.58 | 5303.43 | 1851 | 2.25 | 27.1 |
| Sb | 1.85 | 46.335 | 80.73 | 122.035 | 350.22 | 100.42 | 74.61 | 36.92 | 1.47 | 69.0 |
| Sr | 755 | 1218.5 | 1737 | 2408 | 5891 | 2006.11 | 1105.60 | 563 | 1.96 | 1.9 |
| Th | 4.5 | 11.4 | 19.5 | 32.45 | 77 | 23.23 | 15.54 | 10.7 | 1.31 | 14.8 |
| Zn | 100 | 236.5 | 354 | 753.5 | 2213 | 557.86 | 442.33 | 183 | 1.70 | 211.0 |
| Rotated Component Matrix of Surficial Samples | ||||
|---|---|---|---|---|
| Component | 1 | 2 | 3 | 4 |
| Ag | 0.451 | 0.161 | 0.749 | −0.149 |
| As | 0.193 | 0.559 | 0.626 | 0.138 |
| Ba | 0.905 | 0.104 | 0.064 | 0.083 |
| Bi | 0.038 | 0.380 | 0.085 | 0.473 |
| Cd | 0.142 | 0.056 | 0.089 | 0.714 |
| Co | 0.098 | 0.778 | 0.095 | 0.475 |
| Cu | 0.329 | 0.565 | 0.570 | −0.214 |
| Fe | 0.154 | 0.865 | 0.135 | 0.207 |
| Mn | −0.070 | 0.829 | -0.019 | −0.012 |
| Mo | 0.125 | 0.634 | 0.004 | 0.443 |
| Ni | 0.127 | 0.752 | 0.214 | 0.011 |
| Pb | 0.715 | 0.069 | 0.231 | 0.076 |
| S | 0.945 | 0.078 | 0.095 | -0.006 |
| Sb | −0.147 | −0.072 | 0.789 | 0.144 |
| Sr | 0.757 | 0.253 | 0.266 | −0.283 |
| Th | 0.823 | 0.052 | 0.104 | −0.041 |
| Zn | 0.299 | 0.119 | 0.053 | 0.772 |
| Component | Eigenvalues | Percent of Variance | Cumulative Percent | |
| 1 | 6.56 | 38.6 | 38.6 | |
| 2 | 3.15 | 18.5 | 57.1 | |
| 3 | 2.14 | 12.6 | 69.7 | |
| 4 | 1.13 | 5.67 | 75.3 | |
| Rotated Component Matrix of Underground Tunnel Samples | ||||
|---|---|---|---|---|
| Component | 1 | 2 | 3 | 4 |
| Cu | 0.81 | 0.09 | −0.07 | 0.24 |
| Ni | 0.78 | 0.16 | 0.45 | 0.25 |
| Fe | 0.75 | 0.04 | 0.60 | 0.10 |
| As | 0.74 | 0.15 | 0.46 | 0.11 |
| Sr | 0.11 | −0.73 | −0.02 | −0.17 |
| Bi | 0.72 | −0.13 | 0.35 | −0.18 |
| Sb | 0.70 | 0.20 | −0.07 | 0.42 |
| S | 0.15 | 0.95 | −0.13 | 0.05 |
| Ba | −0.11 | −0.95 | 0.18 | 0.03 |
| Th | −0.07 | −0.94 | 0.20 | 0.02 |
| Pb | −0.16 | 0.77 | 0.18 | 0.31 |
| Ag | 0.39 | 0.70 | −0.01 | 0.45 |
| Mn | 0.24 | −0.11 | 0.79 | 0.31 |
| Mo | 0.04 | −0.13 | 0.78 | 0.03 |
| Co | 0.09 | −0.08 | 0.68 | 0.05 |
| Cd | 0.09 | 0.05 | 0.14 | 0.92 |
| Zn | 0.20 | 0.21 | 0.23 | 0.84 |
| Component | Eigenvalues | Percent of Variance | Cumulative Percent | |
| 1 | 4.25 | 25.02 | 25.02 | |
| 2 | 3.97 | 23.38 | 48.40 | |
| 3 | 3.07 | 18.06 | 66.46 | |
| 4 | 2.39 | 14.04 | 80.50 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejadhadad, M.; Taghipour, B.; Karimzadeh Somarin, A. The Use of Univariate and Multivariate Analyses in the Geochemical Exploration, Ravanj Lead Mine, Delijan, Iran. Minerals 2017, 7, 212. https://doi.org/10.3390/min7110212
Nejadhadad M, Taghipour B, Karimzadeh Somarin A. The Use of Univariate and Multivariate Analyses in the Geochemical Exploration, Ravanj Lead Mine, Delijan, Iran. Minerals. 2017; 7(11):212. https://doi.org/10.3390/min7110212
Chicago/Turabian StyleNejadhadad, Mostafa, Batoul Taghipour, and Alireza Karimzadeh Somarin. 2017. "The Use of Univariate and Multivariate Analyses in the Geochemical Exploration, Ravanj Lead Mine, Delijan, Iran" Minerals 7, no. 11: 212. https://doi.org/10.3390/min7110212





