Subsolidus Evolution of the Magnetite-Spinel-UlvöSpinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia
Abstract
:1. Introduction
Mg-Al-rich magnetite Magnetite Spinel
2(Fe3+0.5Fe2+0.5)(Fe2+Fe3+0.5Ti0.5)O4 → Fe3O4 + Fe2TiO4, etc.
Titanomagnetite Magnetite Ulvöspinel
Ti-rich magnetite Magnetite Ilmenite
20Fe2.4Mg0.3Ti0.3O4 + O2 → 16Fe3O4 + 6MgTiO3
Mg-Ti-rich magnetite Magnetite Geikielite
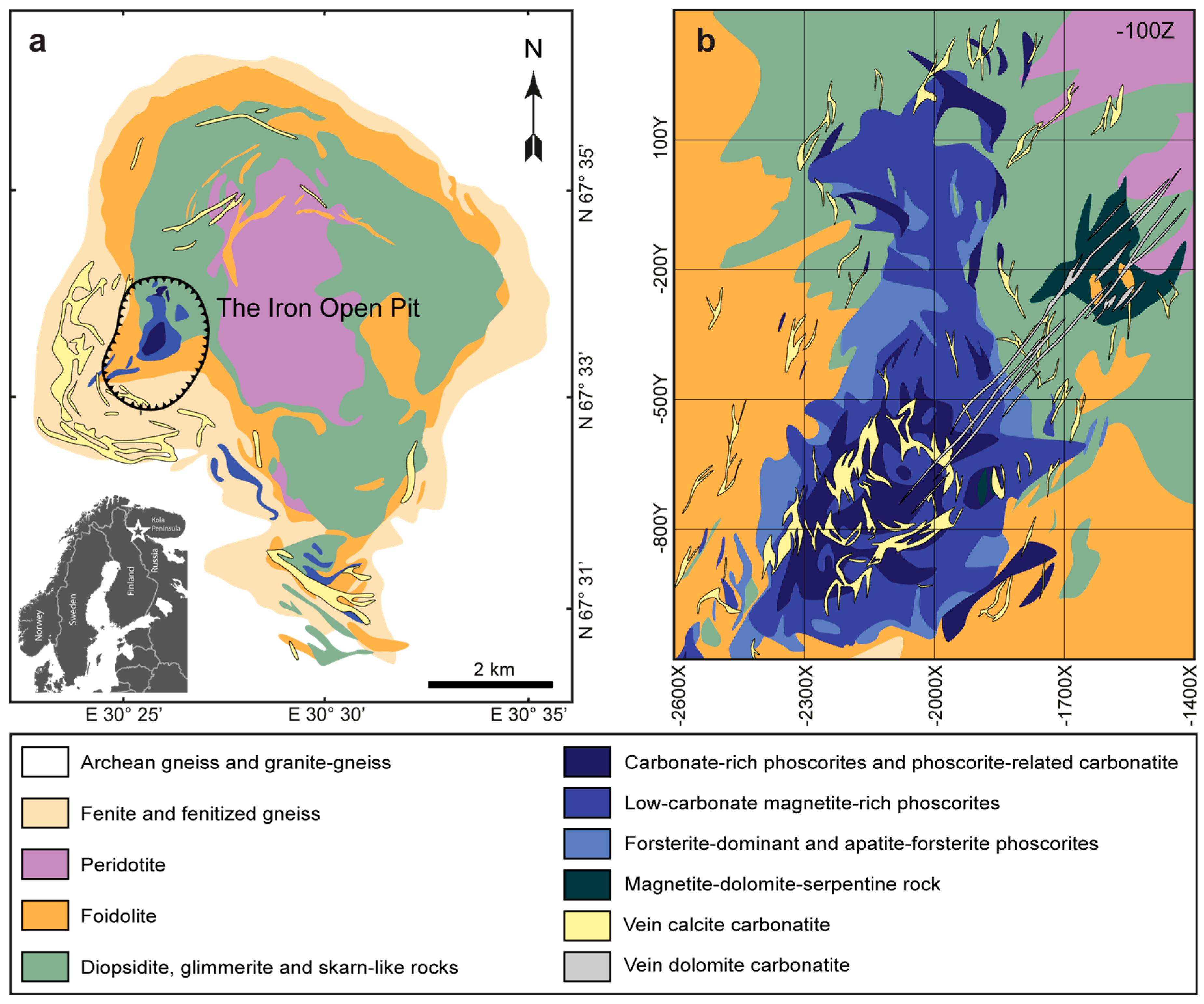
2. Geological Setting
3. Materials and Methods
4. Results
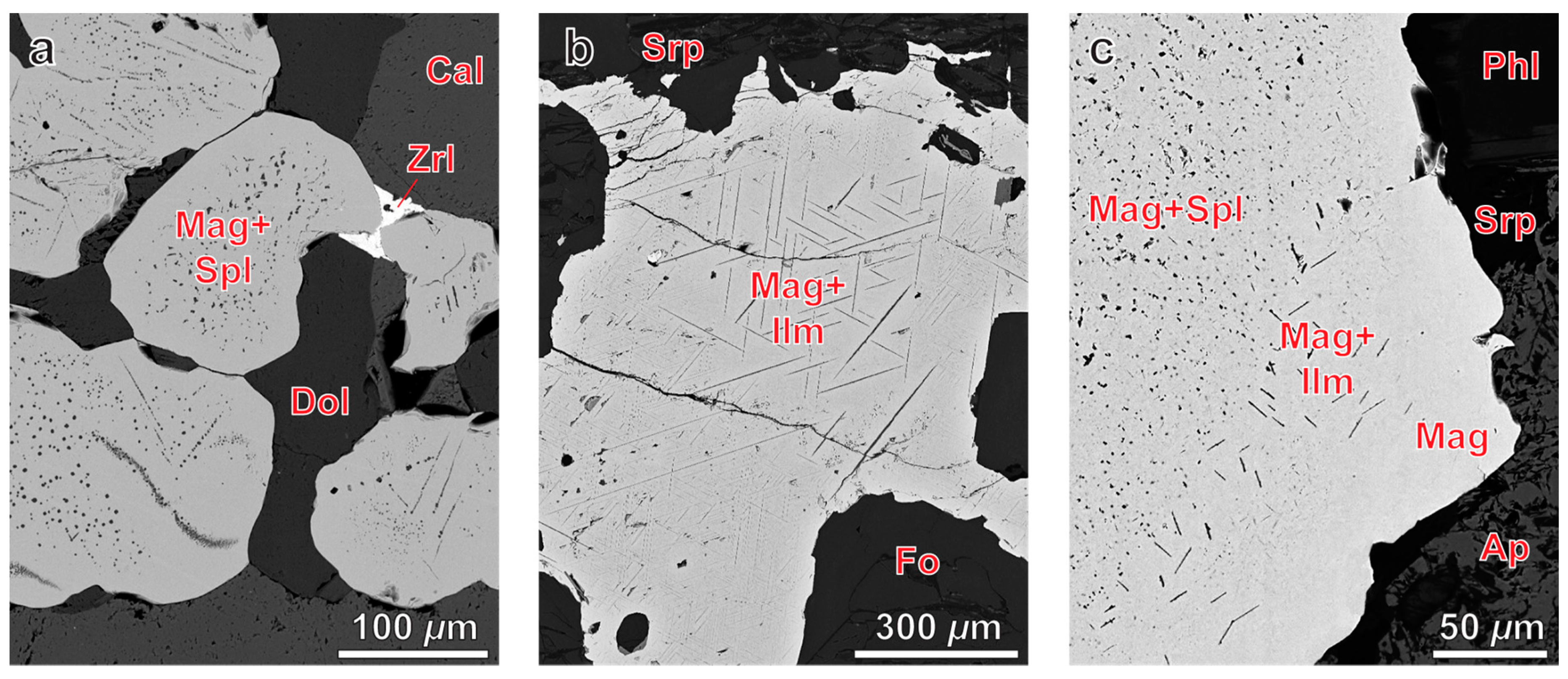
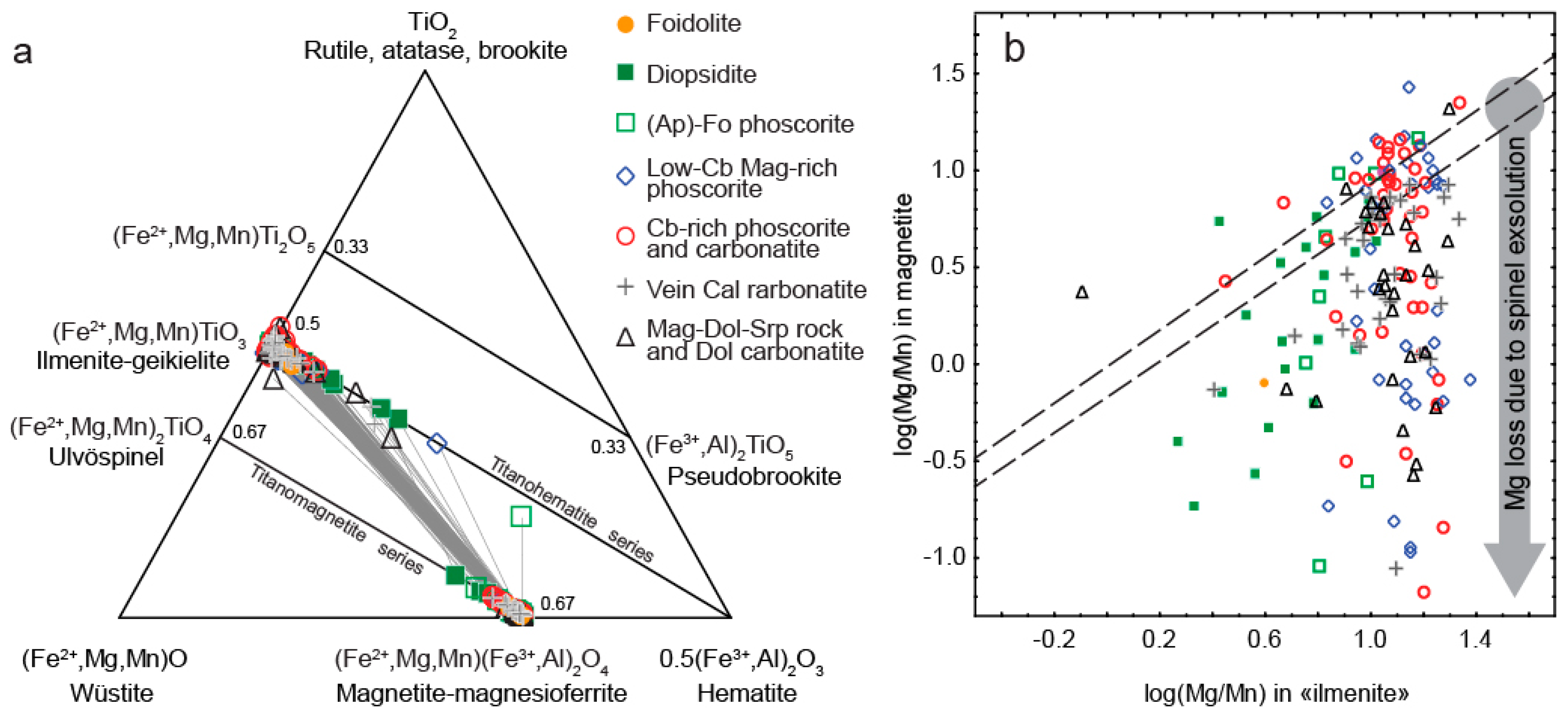
4.1. Spinel
Mg-Al-rich magnetite Magnetite Quintinite
4MgAl2O4 + 10H2O + CO2 → [Mg4Al2(OH)12](CO3)(H2O)4 + 3Al2O3
Spinel Quintinite
4.2. Ilmenite Group Minerals
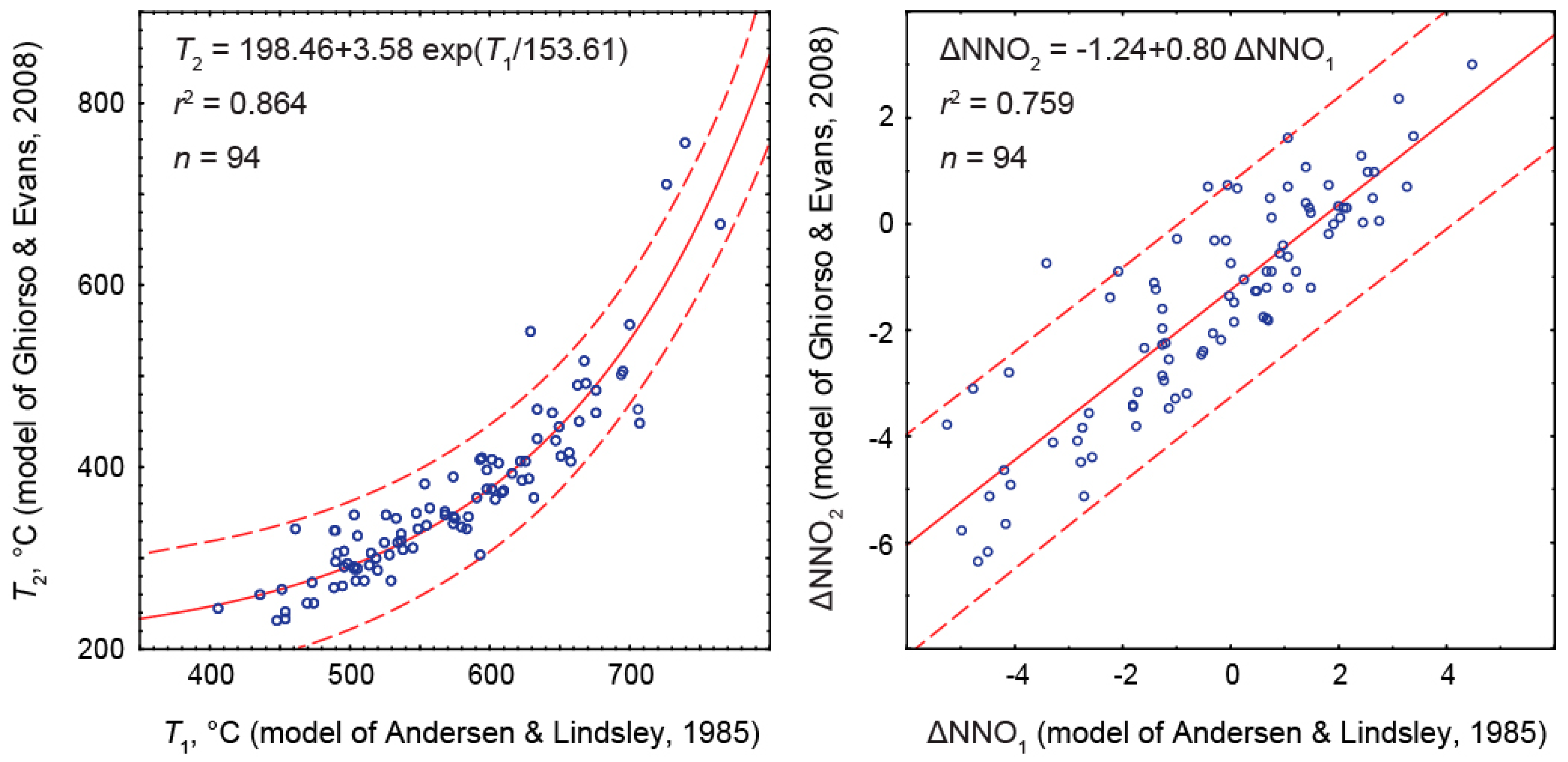
ΔNNO2’ ≈ –1.24 + 0.80 ΔNNO1
5. Discussion
6. Conclusions
- (1)
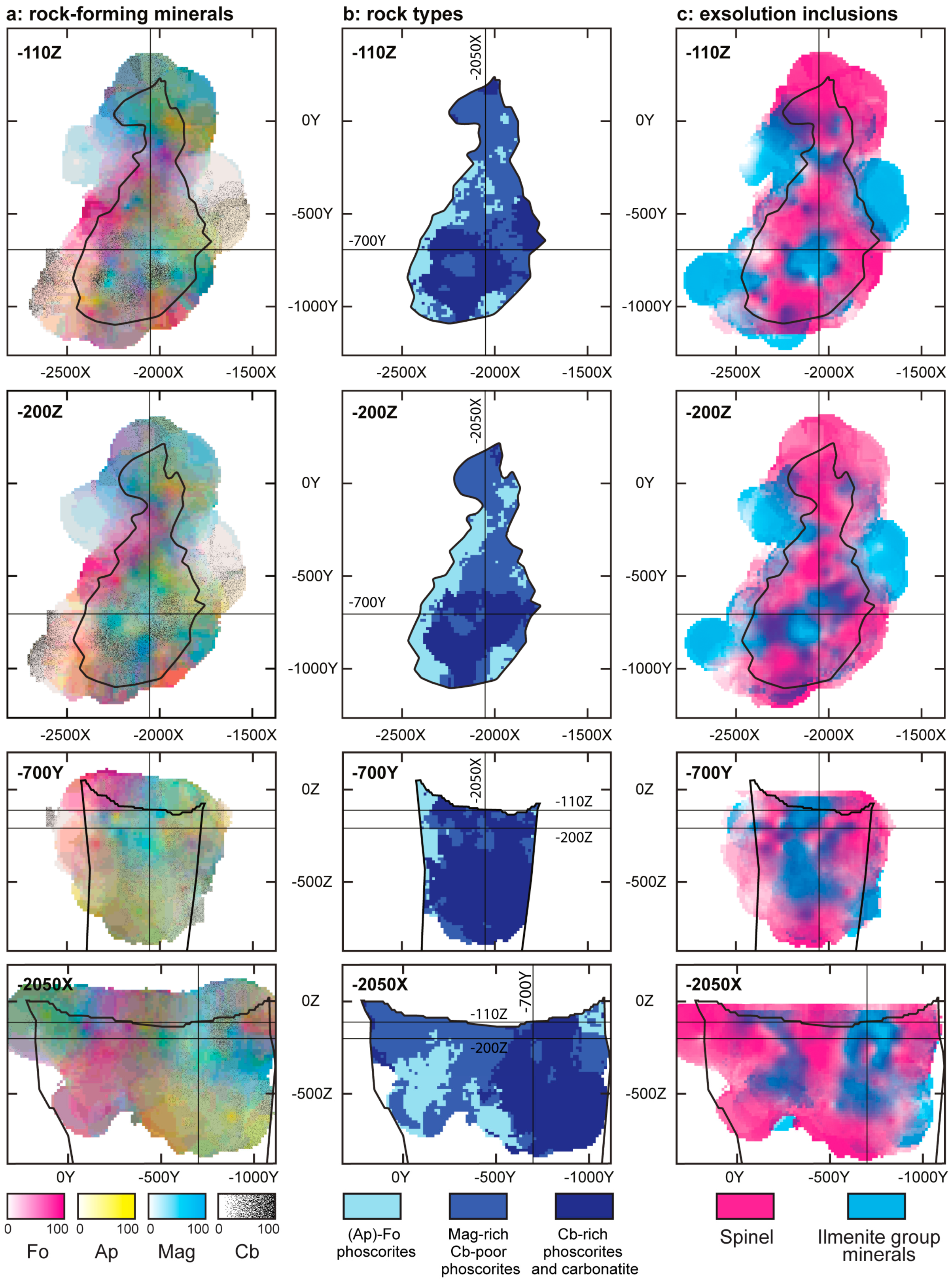
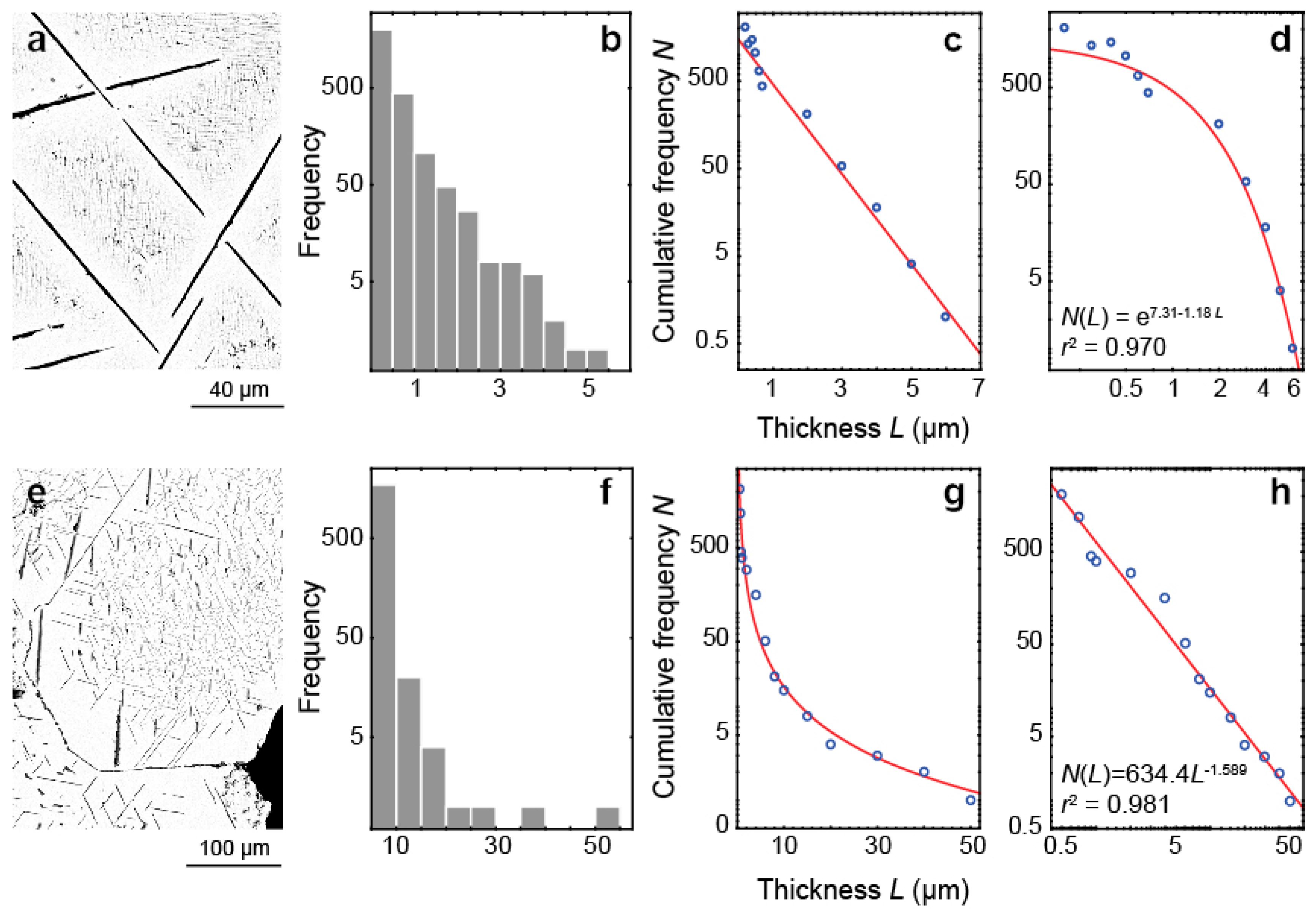
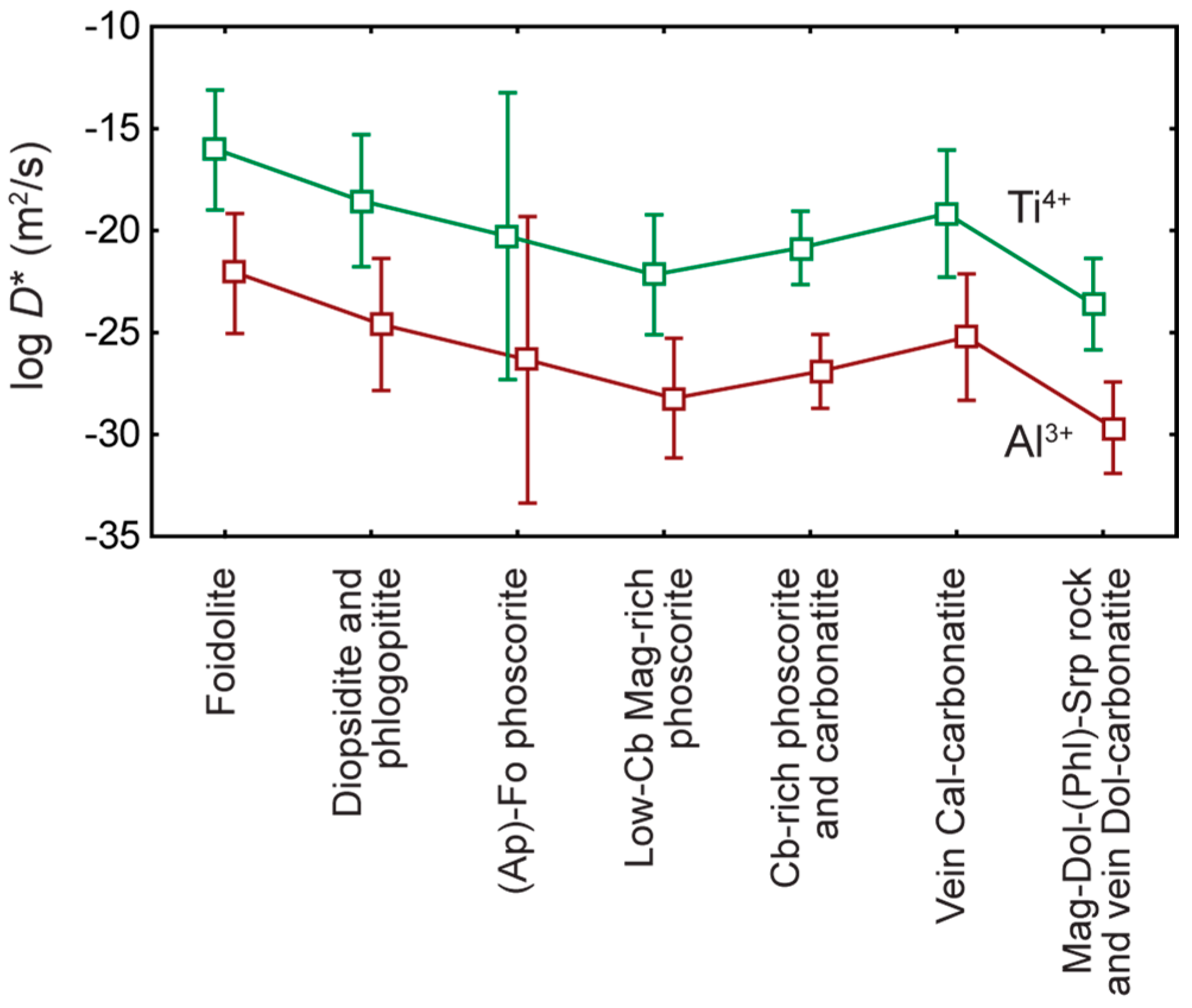
- Exsolution spinel forms spherical grains, octahedral crystals, six-beam (along [100]) and eight-beam (along [111]) skeletal crystals co-oriented with host magnetite and having maximal morphological diversity in magnetite-rich phoscorites of the ore-pipe inner part. The ilmenite group minerals occur usually as thin lamellae on the (111) and (100) planes of host magnetite (respectively, due to direct oxy-exsolution of titanomagnetite and with intermediate ulvöspinel). In accordance with the lower diffusivity of Al than Ti in studied magnetites, spinel crystallizes after the ilmenite-group minerals, which is emphasized by the formation of zoned magnetite crystals with spinel-impregnated core, ilmenite-impregnated intermediate zone and inclusion-free marginal zone;
- (2)
- The kinetics of inclusion nucleation and growth depends mainly on the diffusivity of cations in magnetite: comparatively low diffusivities of Al3+ and Ti4+ cations in magnetite- and/or carbonate-rich phoscorite and carbonatite cause size-independent growth of both spinel and ilmenite-group minerals, while higher diffusivities of these cations in surrounding rocks, marginal forsterite-rich phoscorite and vein calcite carbonatite lead to size-dependent growth of corresponding inclusions;
- (3)
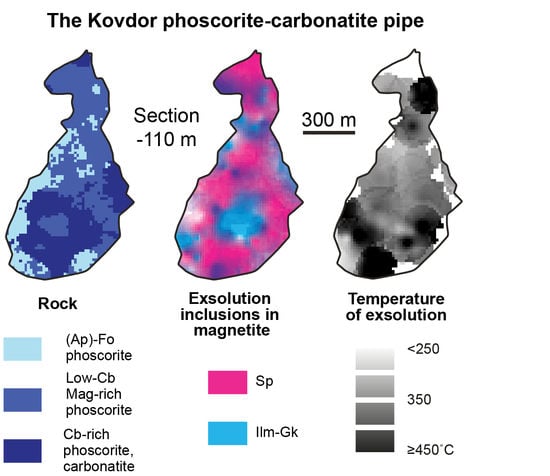
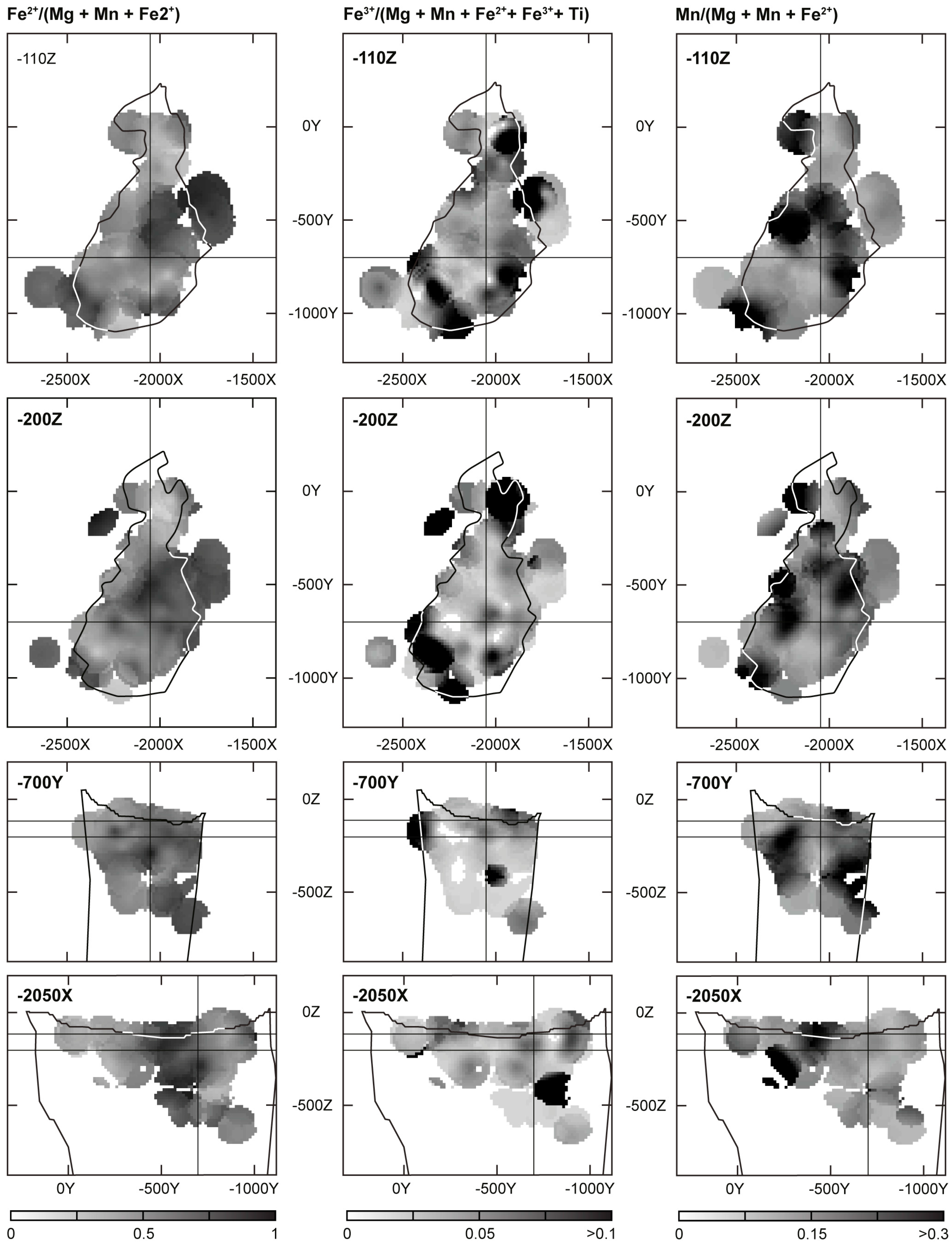
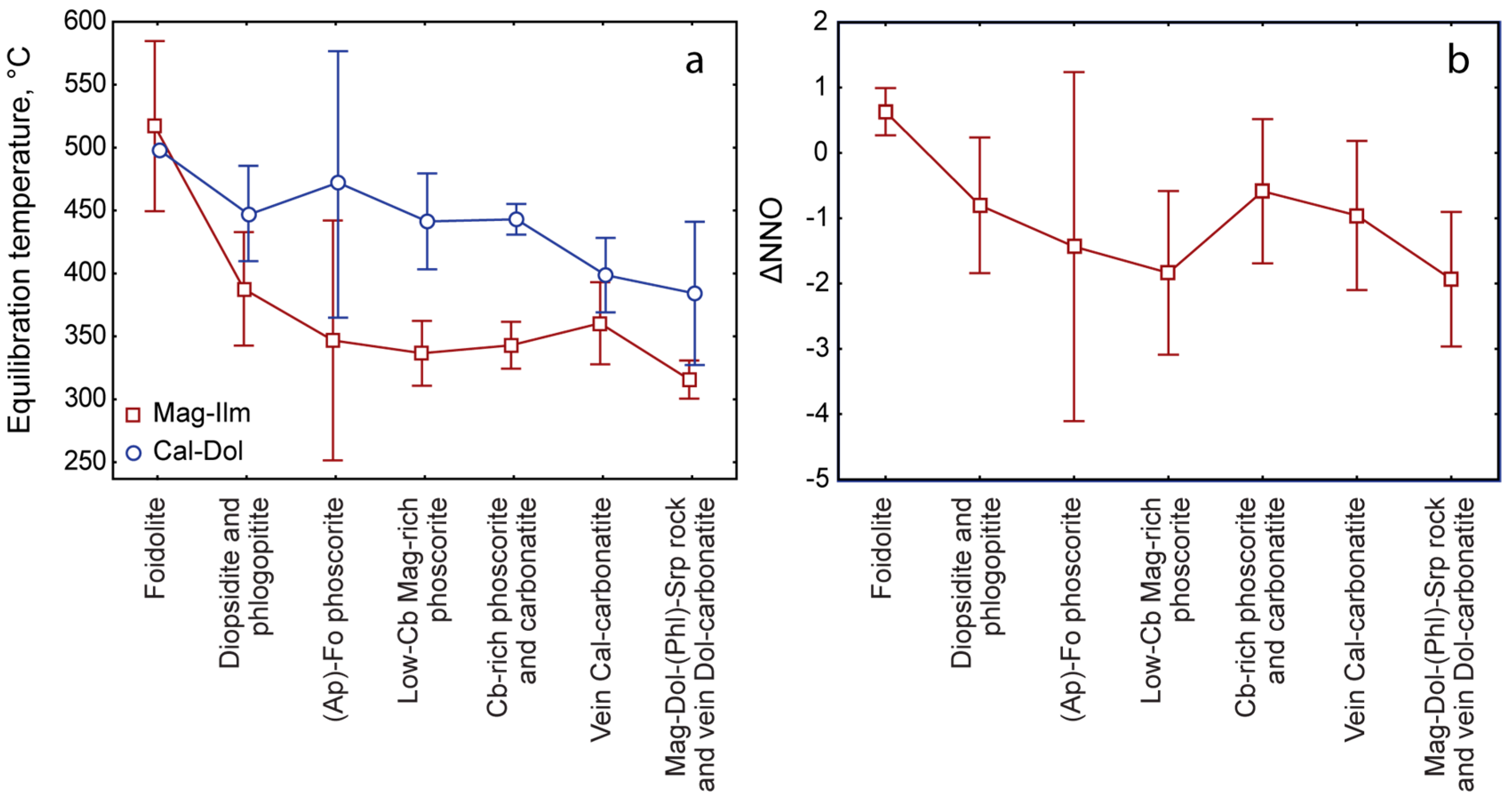
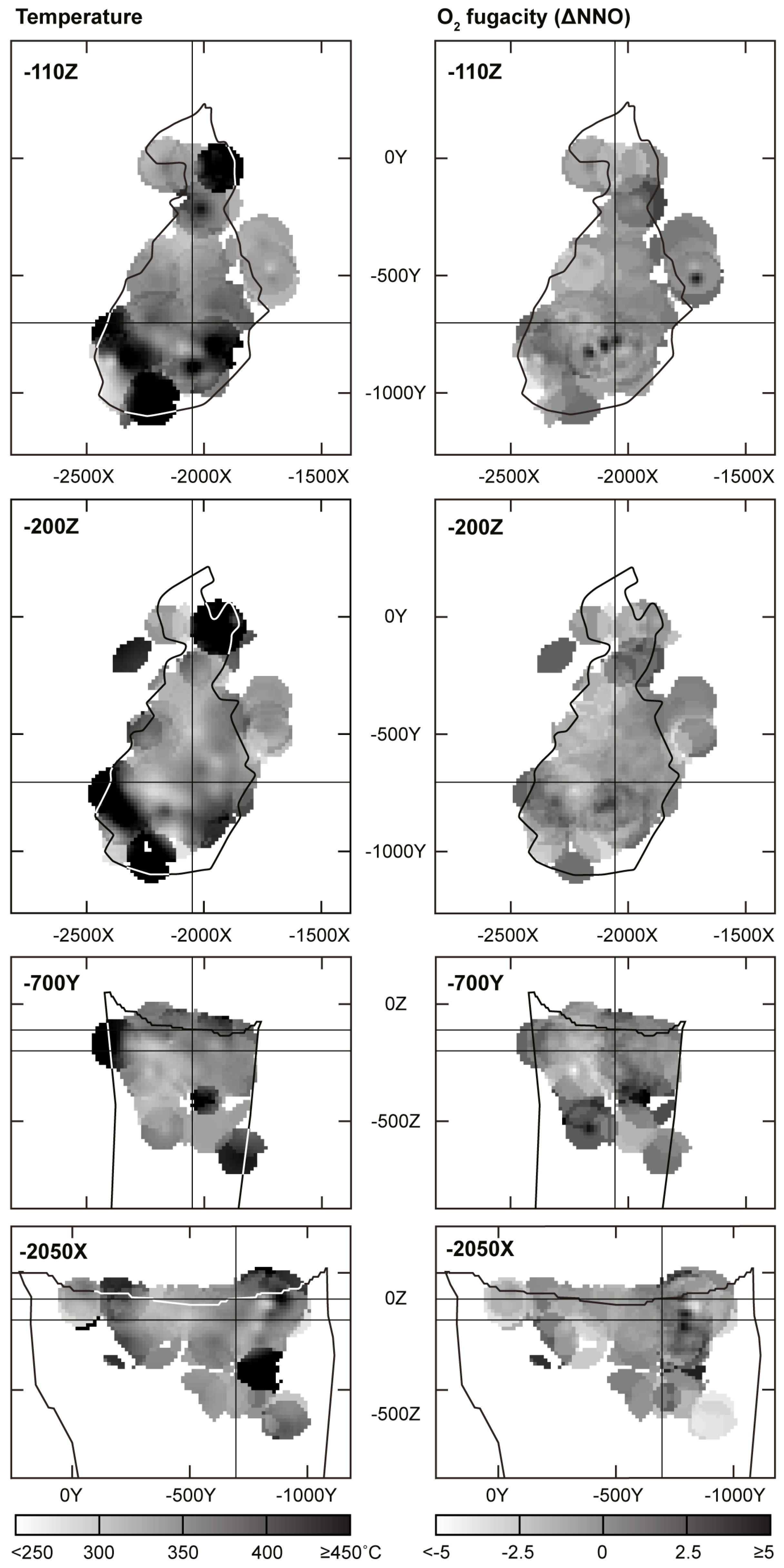
- Three-dimensional mineralogical mapping of the Kovdor phoscorite-carbonatite pipe has shown its concentric (nested) zonation in regard to granulometry, shape, modal and chemical compositions of (oxy)exsolution inclusions in magnetite. In general, this zonation reflects concentric spatial change of host magnetite composition, corresponding in turn to the rock crystallization sequence: surrounding silicate rocks—earlier forsterite-rich phoscorite—intermediate low-carbonate magnetite-rich phoscorite—late carbonate-rich phoscorite and carbonatite;
- (4)
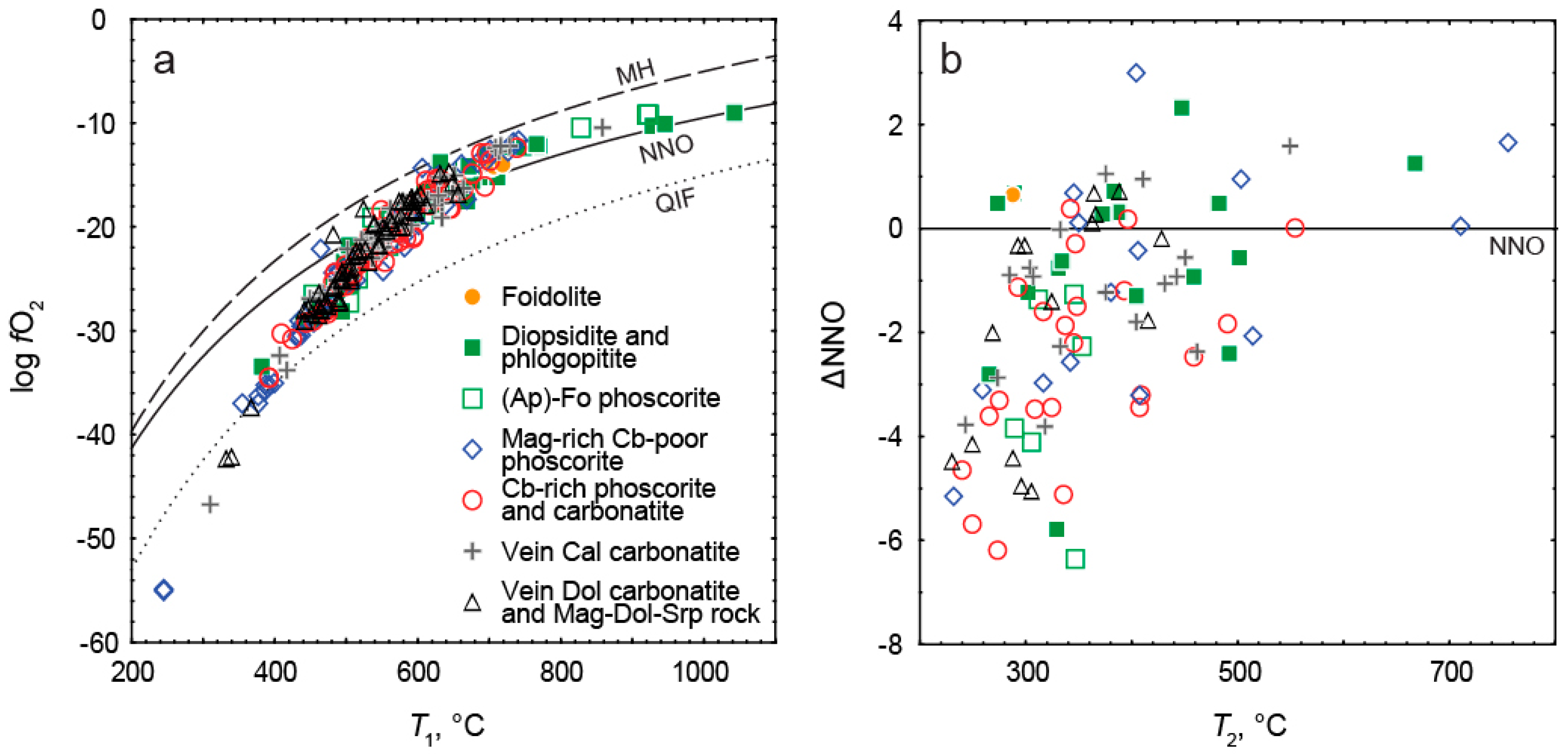
- Temperature and oxygen fugacity of titanomagnetite exsolution decreases in this sequence from about 500 °C to about 300 °C and from NNO + 1 to NNO − 3, with local positive maximums in calcite carbonatite. The temperature of magnetite oxy-exsolution in phoscorite and carbonatites is about 250 °C below the temperature of equilibration of coexisting carbonates;
- (5)
- The intermediate low-carbonate magnetite-rich phoscorite was crystallized under oxidizing conditions resulting in the presence of Fe3+ instead of Fe2+ in melt/fluid. Therefore, oxy-exsolution of titanomagnetite finished here at lower temperature, oxygen fugacity and titanium diffusivity than in marginal and axial zones of the ore-pipe.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vincent, E.A.; Phillips, R. Iron-titanium oxide minerals in layered gabbros of the Skaergaard intrusion, East Greenland. Geochim. Cosmochim. Acta 1954, 6, 1–26. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals Volume 5 Non-Silicates; Longmans: London, UK, 1962. [Google Scholar]
- Turnock, A.C.; Eugster, H.P. Fe-Al Oxides: Phase Relationships below 1000 C. J. Petrol. 1962, 3, 533–565. [Google Scholar] [CrossRef]
- Price, G.D. Subsolidus phase relations in the titanomagnetite solid solution series. Am. Mineral. 1981, 66, 751–758. [Google Scholar]
- Sack, R.O. Spinels as petrogenetic indicators: Activity-composition relations at low pressures. Contrib. Mineral. Petrol. 1982, 79, 169–186. [Google Scholar] [CrossRef]
- Frost, B.; Lindsley, D.; Andersen, D. Fe-Ti oxide-silicate equilibria: Assemblages with fayalitic olivine. Am. Mineral. 1988, 73, 727–740. [Google Scholar]
- Ghiorso, M.S. Thermodynamic properties of hematite- ilmenite-geikielite solid solutions. Contrib. Mineral. Petrol. 1990, 104, 645–667. [Google Scholar] [CrossRef]
- Haggerty, S.E. Oxide textures; a mini-atlas. Rev. Mineral. Geochem. 1991, 25, 129–219. [Google Scholar]
- Frost, B.R.; Lindsley, D.H. Occurrence of iron-titanium oxides in igneous rocks. Rev. Mineral. Geochem. 1991, 25, 433–468. [Google Scholar]
- Zhou, W.; Van der Voo, R.; Peacor, D.R.; Zhang, Y. Variable Ti-content and grain size of titanomagnetite as a function of cooling rate in very young MORB. Earth Planet. Sci. Lett. 2000, 179, 9–20. [Google Scholar] [CrossRef]
- Hammer, J.E. Influence of fO2 and cooling rate on the kinetics and energetics of Fe-rich basalt crystallization. Earth Planet. Sci. Lett. 2006, 248, 618–637. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Kogarko, L.N. Magnetite compositions and oxygen fugacities of the Khibina magmatic system. Lithos 2006, 91, 35–45. [Google Scholar] [CrossRef]
- Ivanyuk, G.; Yakovenchuk, V.; Pakhomovsky, Y.; Kalashnikov, A.; Mikhailova, J.; Goryainov, P. Self-Organization of the Khibiny Alkaline Massif (Kola Peninsula, Russia). In Earth Sciences; Dar, I.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 131–156. ISBN 978-953-307-861-8. [Google Scholar]
- Ondrejka, M.; Broska, I.; Uher, P. The late magmatic to subsolidus T-fO2 evolution of the Lower Triassic A-type rhyolites (Silicic Superunit, Western Carpathians, Slovakia): Fe–Ti oxythermo- metry and petrological implications. Acta Geol. Slovaca 2015, 7, 51–61. [Google Scholar]
- Liao, M.; Tao, Y.; Song, X.; Li, Y.; Xiong, F. Study of oxygen fugacity during magma evolution and ore genesis in the Hongge mafic–ultramafic intrusion, the Panxi region, SW China. Acta Geochim. 2016, 35, 25–42. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Kalashnikov, A.O.; Sokharev, V.A.; Pakhomovsky, Y.A.; Konopleva, N.G.; Yakovenchuk, V.N.; Bazai, A.V.; Goryainov, P.M.; Ivanyuk, G.Y. 3D mineralogical mapping of the Kovdor phoscorite–carbonatite complex (Russia). Miner. Depos. 2016, 51, 131–149. [Google Scholar] [CrossRef]
- Kawai, N. Exsolution of titanomagnetites and its effect on rock-magnetism III. Proc. Jpn. Acad. 1956, 32, 464–468. [Google Scholar]
- Nagata, T. Rock Magnetism, 2nd ed.; Maruzen: Tokyo, Japan, 1961. [Google Scholar]
- Akimoto, S. Magnetic properties of FeO-Fe2O3-TiO2 system as a basis of rock magnetism. J. Phys. Soc. Jpn 1962, 17, 706–710. [Google Scholar]
- Marshall, M.; Cox, A. Magnetism of Pillow Basalts and Their Petrology. Geol. Soc. Am. Bull. 1971, 82, 537. [Google Scholar] [CrossRef]
- Evans, M.E.; Wayman, M.L. An Investigation of the Role of Ultra-fine Titanomagnetite Intergrowths in Palaeomagnetism. Geophys. J. Int. 1974, 36, 1–10. [Google Scholar] [CrossRef]
- Gee, J.; Kent, D.V. Magnetization of axial lavas from the southern East Pacific Rise (14°–23°S): Geochemical controls on magnetic properties. J. Geophys. Res. Solid Earth 1997, 102, 24873–24886. [Google Scholar] [CrossRef]
- Ivanyuk, G.I.; Tyuremnov, V.A.; Balabonin, N.L. On the origin of magnetic heterogeneity of magnetites from ferroginous quartzites. Izv. Phys. Solid Earth 1994, 30, 266–272. [Google Scholar]
- Dunlop, D.; Özdemir, Ö. Rock Magnetism: Fundamentals and Frontiers; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Harrison, R.J.; Putnis, A. Magnetic properties of the magnetite-spinel solid solution: Saturation magnetization and cation distributions. Am. Mineral. 1995, 80, 213–221. [Google Scholar] [CrossRef]
- Evans, M.E.; Krása, D.; Williams, W.; Winklhofer, M. Magnetostatic interactions in a natural magnetite-ulvöspinel system. J. Geophys. Res. Solid Earth 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- McEnroe, S.A.; Robinson, P.; Langenhorst, F.; Frandsen, C.; Terry, M.P.; Boffa Ballaran, T. Magnetization of exsolution intergrowths of hematite and ilmenite: Mineral chemistry, phase relations, and magnetic properties of hemo-ilmenite ores with micron- to nanometer-scale lamellae from Allard Lake, Quebec. J. Geophys. Res. 2007, 112, B10103. [Google Scholar] [CrossRef]
- Uchida, H. Single-crystal X-ray diffraction of spinels from the San Carlos Volcanic Field, Arizona: Spinel as a geothermometer. Am. Mineral. 2005, 90, 1900–1908. [Google Scholar] [CrossRef]
- Lavina, B.; Cesare, B.; Alvarez-Valero, A.M.; Uchida, H.; Downs, R.T.; Koneva, A.; Dera, P. Closure temperatures of intracrystalline ordering in anatectic and metamorphic hercynite, Fe2+Al2O4. Am. Mineral. 2009, 94, 657–665. [Google Scholar] [CrossRef]
- Essene, E.; Peacor, D. Crystal chemistry and petrology of coexisting galaxite and jacobsite and other spinel solutions and solvi. Am. Mineral. 1983, 68, 449–455. [Google Scholar]
- Bosi, F.; Skogby, H.; Fregola, R.A.; Hålenius, U. Crystal chemistry of spinels in the system MgAl2O4-MgV2O4-Mg2VO4. Am. Mineral. 2016, 101, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Radtke, A.S. Coulsonite, FeV2O4, a spinel-type mineral from Lovelock, Nevada. Am. Mineral. 1962, 47, 1284–1291. [Google Scholar]
- Bosi, F.; Halenius, U.; Skogby, H. Crystal chemistry of the magnetite-ulvospinel series. Am. Mineral. 2009, 94, 181–189. [Google Scholar] [CrossRef]
- O’Neil, H.S.C.; Annersten, H.; Virgo, D. The temperature dependence of the cation distribution in magnesioferrite (MgFe2O4) from powder XRD structural refinements and Moessbauer spectroscopy. Am. Mineral. 1992, 77, 725–740. [Google Scholar]
- Bosi, F.; Halenius, U.; Skogby, H. Crystal chemistry of the ulvospinel-qandilite series. Am. Mineral. 2014, 99, 847–851. [Google Scholar] [CrossRef]
- Nestola, F.; Ballaran, T.B.; Balic-Zunic, T.; Princivalle, F.; Secco, L.; Dal Negro, A. Comparative compressibility and structural behavior of spinel MgAl2O4 at high pressures: The independency on the degree of cation order. Am. Mineral. 2007, 92, 1838–1843. [Google Scholar] [CrossRef]
- Lucchesi, S.; Russo, U.; Della Giusta, A. Crystal chemistry and cation distribution in some Mn-rich natural and synthetic spinels. Eur. J. Mineral. 1996, 9, 31–42. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D.H. Iron-Titanium Oxide Minerals and Synthetic Equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Tan, W.; Liu, P.; He, H.; Wang, C.Y.; Liang, X. Mineralogy and Origin of Exsolution In Ti-Rich Magnetite From Different Magmatic Fe-Ti Oxide-Bearing Intrusions. Can. Mineral. 2016, 54, 539–553. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Mikhailova, J.A.; Yakovenchuk, V.N.; Konopleva, N.G.; Sokharev, V.A.; Bazai, A.V.; Goryainov, P.M. Economic minerals of the Kovdor baddeleyite-apatite-magnetite deposit, Russia: Mineralogy, spatial distribution and ore processing optimization. Ore Geol. Rev. 2016, 77, 279–311. [Google Scholar] [CrossRef]
- Rimskaya-Korsakova, O.M. A question about regular intergrowths of spinel with magnetite. Zap. Vsesoyuznogo Mineral. Obs. 1950, 89, 178–190. (In Russian) [Google Scholar]
- Krasnova, N.I.; Krezer, Y.L. New data on the nature of fine and ultrafine lamellae in titanomagnetite. Eur. J. Mineral. 1995, 7, 1361–1372. [Google Scholar] [CrossRef]
- Spenser, K.J.; Lindsley, D.H. A solution model for coexisting iron-titanium oxides. Am. Mineral. 1981, 66, 1189–1201. [Google Scholar]
- Andersen, D.J.; Lindsley, D.H. New (and final!) models for the Ti-magnetite/ilmenite geothermometer and oxygen barometer. In Spring Meeting Eos Transactions American Geophysical Union; American Geophysical Union: Washington, DC, USA, 1985; p. 416. [Google Scholar]
- Lehmann, J.; Roux, J. Experimental and theoretical study of (Fe2+, Mg)(Al, Fe3+)2O4 spinels: Activity-composition relationships, miscibility gaps, vacancy contents. Geochim. Cosmochim. Acta 1986, 50, 1765–1783. [Google Scholar] [CrossRef]
- Mattioli, G.S.; Wood, B.J. Magnetite activities across the MgAl2O4-Fe3O4 spinel join, with application to thermobarometric estimates of upper mantle oxygen fugacity. Contrib. Mineral. Petrol. 1988, 98, 148–162. [Google Scholar] [CrossRef]
- Bacon, C.R.; Hirschmann, M.M. Mg/Mn partitioning as a test for equilibrium between coexisting Fe-Ti oxides. Am. Mineral. 1988, 73, 57–61. [Google Scholar]
- Sack, R.O.; Ghiorso, M.S. An internally consistent model for the thermodynamic properties of Fe-Mg-titanomagnetite-aluminate spinels. Contrib. Mineral. Petrol. 1991, 106, 474–505. [Google Scholar] [CrossRef]
- Ghiorso, M.S.; Evans, B.W. Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen-barometer. Am. J. Sci. 2008, 308, 957–1039. [Google Scholar] [CrossRef]
- Lilova, K.I.; Pearce, C.I.; Gorski, C.; Rosso, K.M.; Navrotsky, A. Thermodynamics of the magnetite-ulvospinel (Fe3O4-Fe2TiO4) solid solution. Am. Mineral. 2012, 97, 1330–1338. [Google Scholar] [CrossRef]
- Harrison, R.J. Thermodynamics of the R-3 to R-3c phase transition in the ilmenite-hematite solid solution. Am. Mineral. 2000, 85, 1694–1705. [Google Scholar] [CrossRef]
- Harrison, R.J.; Redfern, S.A.T. Short- and long-range ordering in the ilmenite-hematite solid solution. Phys. Chem. Miner. 2001, 28, 399–412. [Google Scholar] [CrossRef]
- Lattard, D.; Sauerzapf, U.; Käsemann, M. New calibration data for the Fe–Ti oxide thermo-oxybarometers from experiments in the Fe–Ti–O system at 1 bar, 1000–1300 °C and a large range of oxygen fugacities. Contrib. Mineral. Petrol. 2005, 149, 735–754. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Yakovenchuk, V.N.; Pakhomovsky, Y.A. Kovdor; Laplandia Minerals: Apatity, Russia, 2002; ISBN 5900395413. [Google Scholar]
- Krasnova, N.I.; Petrov, T.G.; Balaganskaya, E.G.; García, D.; Moutte, J.; Zaitsev, A.N.; Wall, F. Introduction to phoscorites: Occurrence, composition, nomenclature and petrogenesis. In Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province; Zaitsev, A.N., Wall, F., Eds.; Mineralogical Society: London, UK, 2004; pp. 43–72. ISBN 0-903056-22-4. [Google Scholar]
- Rimskaya-Korsakova, O.M. On Question about Genesis of the Kovdor Iron-Ore Deposit. In Problems of Magmatism and Metamorphism; Leningrad State University Publishing: Leningrad, Russia, 1963; pp. 125–142. (In Russian) [Google Scholar]
- Kukharenko, A.A.; Orlova, M.P.; Bulakh, A.G.; Bagdasarov, E.A.; Rimskaya-Korsakova, O.M.; Nefedov, E.I.; Ilyinsky, G.A.; Sergeev, A.S.; Abakumova, N.B. Caledonian Complex of Ultrabasic, Alkaline Rocks and Carbonatites of Kola Peninsula and Northern Karelia (Geology, Petrology, Mineralogy and Geochemistry); Nedra: Moscow, Russia, 1965. (In Russian) [Google Scholar]
- Dolivo-Dobrovolsky, D.D. MINAL, Free Software. Available online: http://www.dimadd.ru (accessed on 8 July 2013).
- Lepage, L.D. ILMAT: An Excel worksheet for ilmenite–magnetite geothermometry and geobarometry. Comput. Geosci. 2003, 29, 673–678. [Google Scholar] [CrossRef]
- MELTS Fe-Ti Oxide Geothermometer. Available online: http://melts.ofm-research.org/CORBA_CTserver/OxideGeothrm/OxideGeothrm.php (accessed on 16 August 2016).
- Kalashnikov, A.O.; Ivanyuk, G.Y.; Mikhailova, J.A.; Sokharev, V.A. Approach of automatic 3D geological mapping: The case of the Kovdor phoscorite-carbonatite complex, NW Russia. Sci. Rep. 2017, 7, 6893. [Google Scholar] [CrossRef] [PubMed]
- Zhitova, E.; Krivovichev, S.; Yakovenchuk, V.; Ivanyuk, G.; Pakhomovsky, Y.; Mikhailova, J. Crystal chemistry of natural layered double hydroxides. 4. Crystal structures and evolution of structural complexity of quintinite polytypes from the Kovdor alkaline massif, Kola peninsula, Russia. Mineral. Mag. 2017. [Google Scholar] [CrossRef]
- Randolph, A.D.; Larson, M.A. Theory of Particulate Processes; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Marsh, B.D. On the Interpretation of Crystal Size Distributions in Magmatic Systems. J. Petrol. 1998, 39, 553–599. [Google Scholar] [CrossRef]
- Clark, M.B.; Brantley, S.L.; Fisher, D.M. Power law vein thickness distribution and positive feedback in vein growth. Geology 1995, 23, 975–978. [Google Scholar] [CrossRef]
- Mercus, H.G. Particle Size Measurements Fundamentals, Practice, Quality; Springer: Berlin, Germany, 2009; ISBN 9781402090158. [Google Scholar]
- Gaydukova, V.S.; Sokolov, S.V.; Dubinchuk, V.T.; Sidorenko, G.A.; Il’yasov, A.M. About multistage decomposition of magnetite from iron ores of the Kovdor deposit. Mineral. Zhurnal 1984, 6, 64–70. (In Russian) [Google Scholar]
- Tan, W.; He, H.; Wang, C.Y.; Dong, H.; Liang, X.; Zhu, J. Magnetite exsolution in ilmenite from the Fe-Ti oxide gabbro in the Xinjie intrusion (SW China) and sources of unusually strong remnant magnetization. Am. Mineral. 2016, 101, 2759–2767. [Google Scholar] [CrossRef]
- Stormer, J., Jr. The effects of recalculation on estimates of temperature and oxygen fugacity from analyses of multi-component iron-titanium oxides. Am. Mineral. 1983, 68, 586–594. [Google Scholar]
- Frost, B.R. Introduction to oxygen fugacity and its petrologic importance. Rev. Mineral. Geochem. 1991, 25, 1–9. [Google Scholar]
- Anovitz, L.M.; Essene, E.J. Phase Equilibria in the System CaCO3-MgCO3-FeCO3. J. Petrol. 1987, 28, 389–415. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Bazai, A.V.; Sokharev, V.A.; Konopleva, N.G.; Mikhailova, J.A.; Goryainov, P.M.; Ivanyuk, G.Y. Scandium of the Kovdor baddeleyite–apatite–magnetite deposit (Murmansk Region, Russia): Mineralogy, spatial distribution, and potential resource. Ore Geol. Rev. 2016, 72, 532–537. [Google Scholar] [CrossRef]
- Yund, R.A.; McCallister, R.H. Kinetics and mechanisms of exsolution. Chem. Geol. 1970, 6, 5–30. [Google Scholar] [CrossRef]
- Putnis, A. An Introduction to Mineral Sciences; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Dieckmann, R.; Mason, T.O.; Hodge, J.D.; Schmalzried, H. Defects and Cation Diffusion in Magnetite (III.) Tracerdiffusion of Foreign Tracer Cations as a Function of Temperature and Oxygen Potential. Ber. Bunsengesellschaft Phys. Chem. 1978, 82, 778–783. [Google Scholar] [CrossRef]
- Dieckmann, R.; Schmalzried, H. Defects and Cation Diffusion in Magnetite (VI): Point Defect Relaxation and Correlation in Cation Tracer Diffusion. Ber. Bunsengesellschaft Phys. Chem. 1986, 90, 564–575. [Google Scholar] [CrossRef]
- Van Orman, J.A.; Crispin, K.L. Diffusion in Oxides. Rev. Mineral. Geochem. 2010, 72, 757–825. [Google Scholar] [CrossRef]
- Aggarwal, S.; Dieckmann, R. Point defects and cation tracer diffusion in (TixFe1−x)3−δO4 1. Non-stoichiometry and point defects. Phys. Chem. Miner. 2002, 29, 695–706. [Google Scholar] [CrossRef]
- Eberl, D.D.; Kile, D.E.; Drits, V.A. On geological interpretations of crystal size distributions: Constant vs. proportionate growth. Am. Mineral. 2002, 87, 1235–1241. [Google Scholar] [CrossRef]
- Myerson, A. Handbook of Industrial Crystallization; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Garside, J.; Mersmann, A.; Nyult, J. Measurement of Crystal Growth and Nucleation Rates, 2nd ed.; Institute of Chemical Engineers: Rugby, UK, 2002. [Google Scholar]
- Kile, D.E.; Eberl, D.D. On the origin of size-dependent and size-independent crystal growth: Influence of advection and diffusion. Am. Mineral. 2003, 88, 1514–1521. [Google Scholar] [CrossRef]
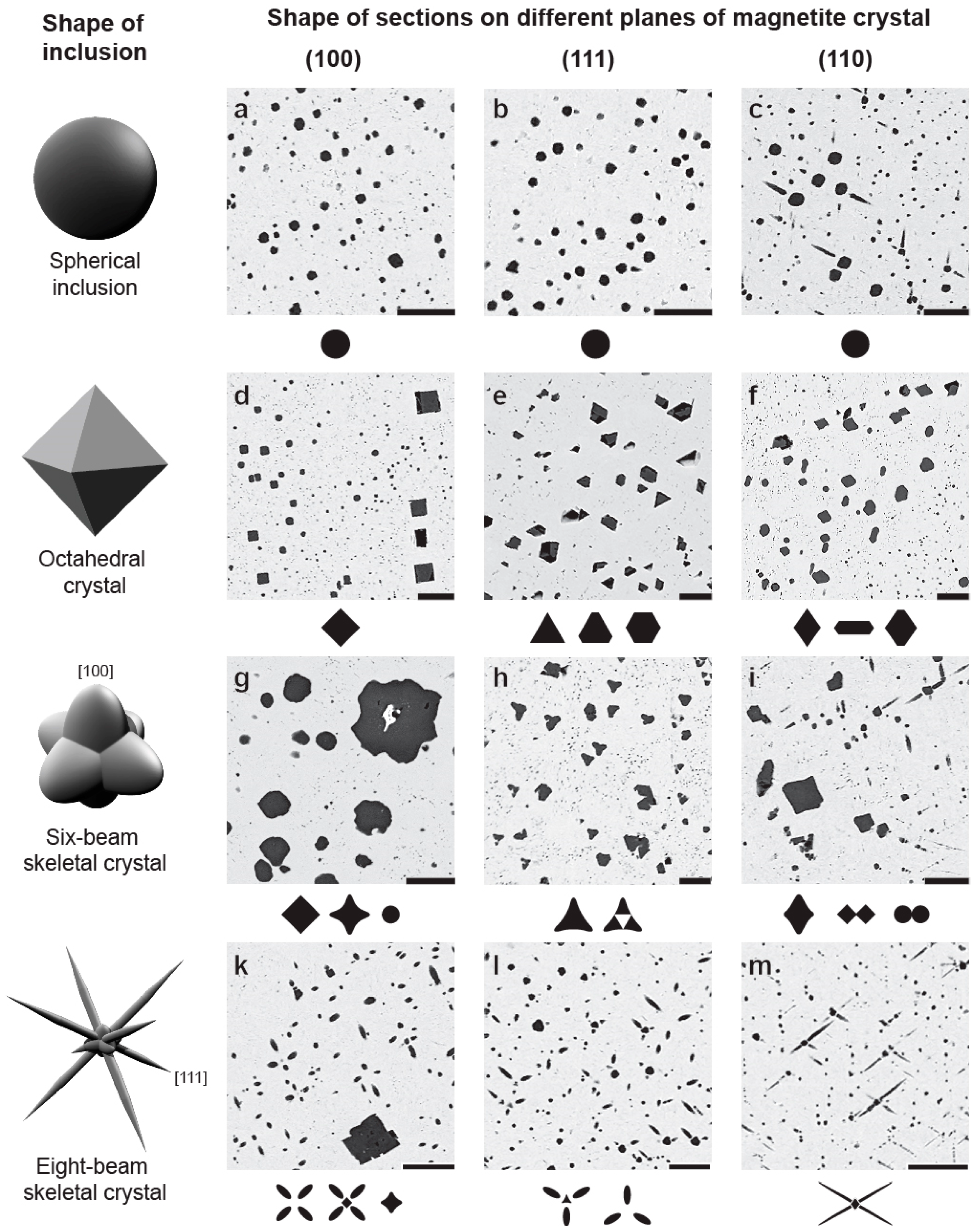
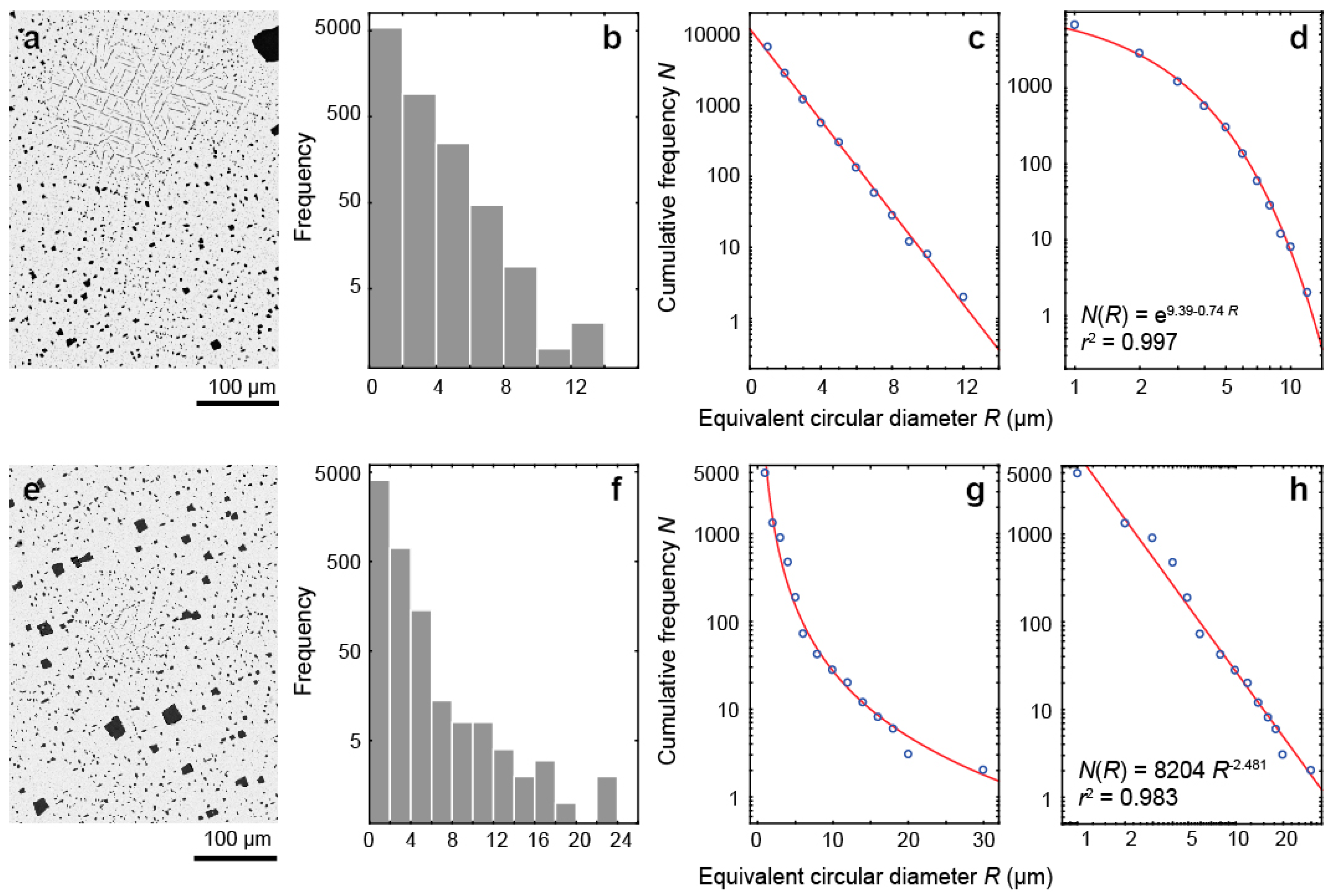

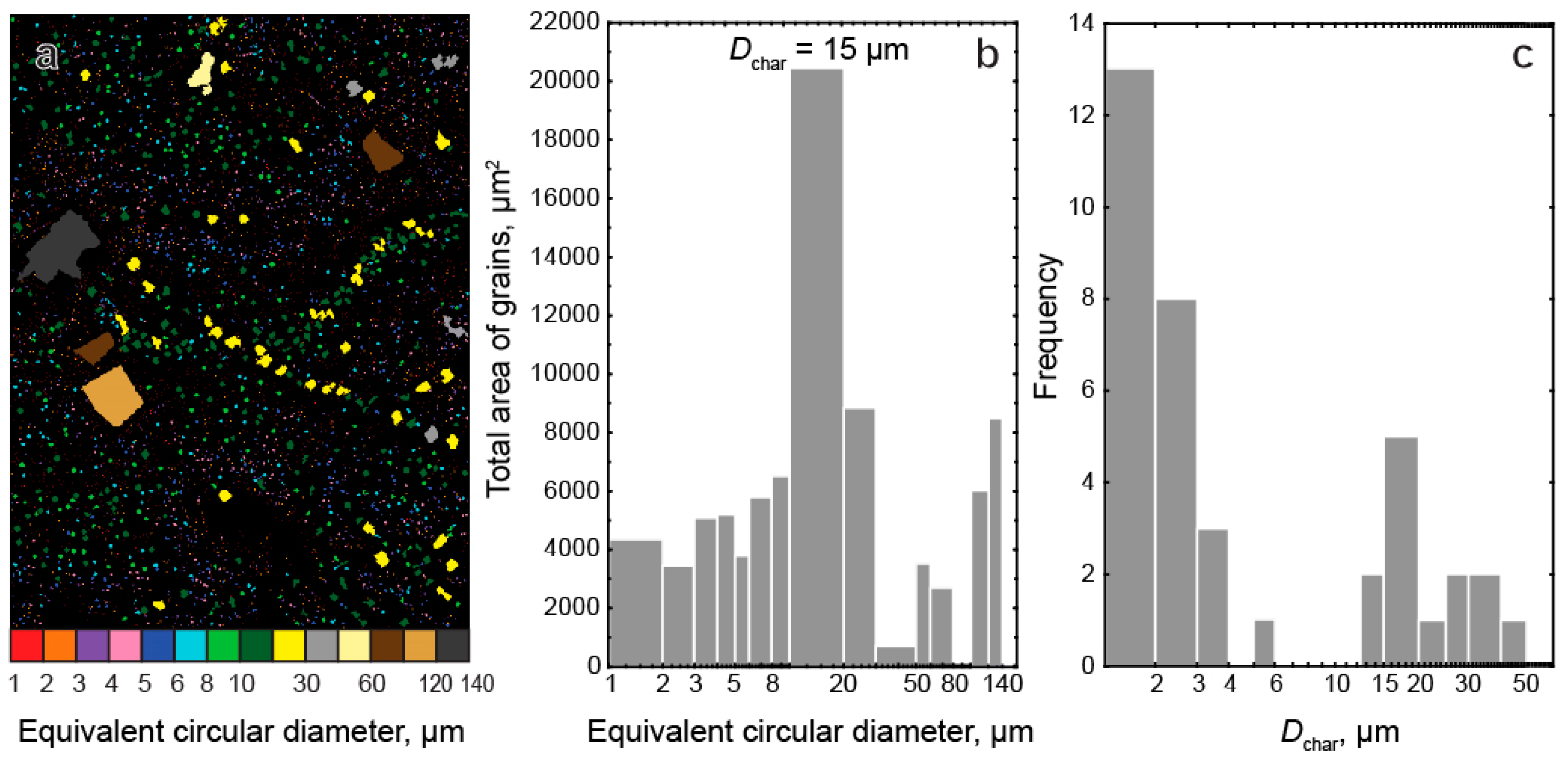
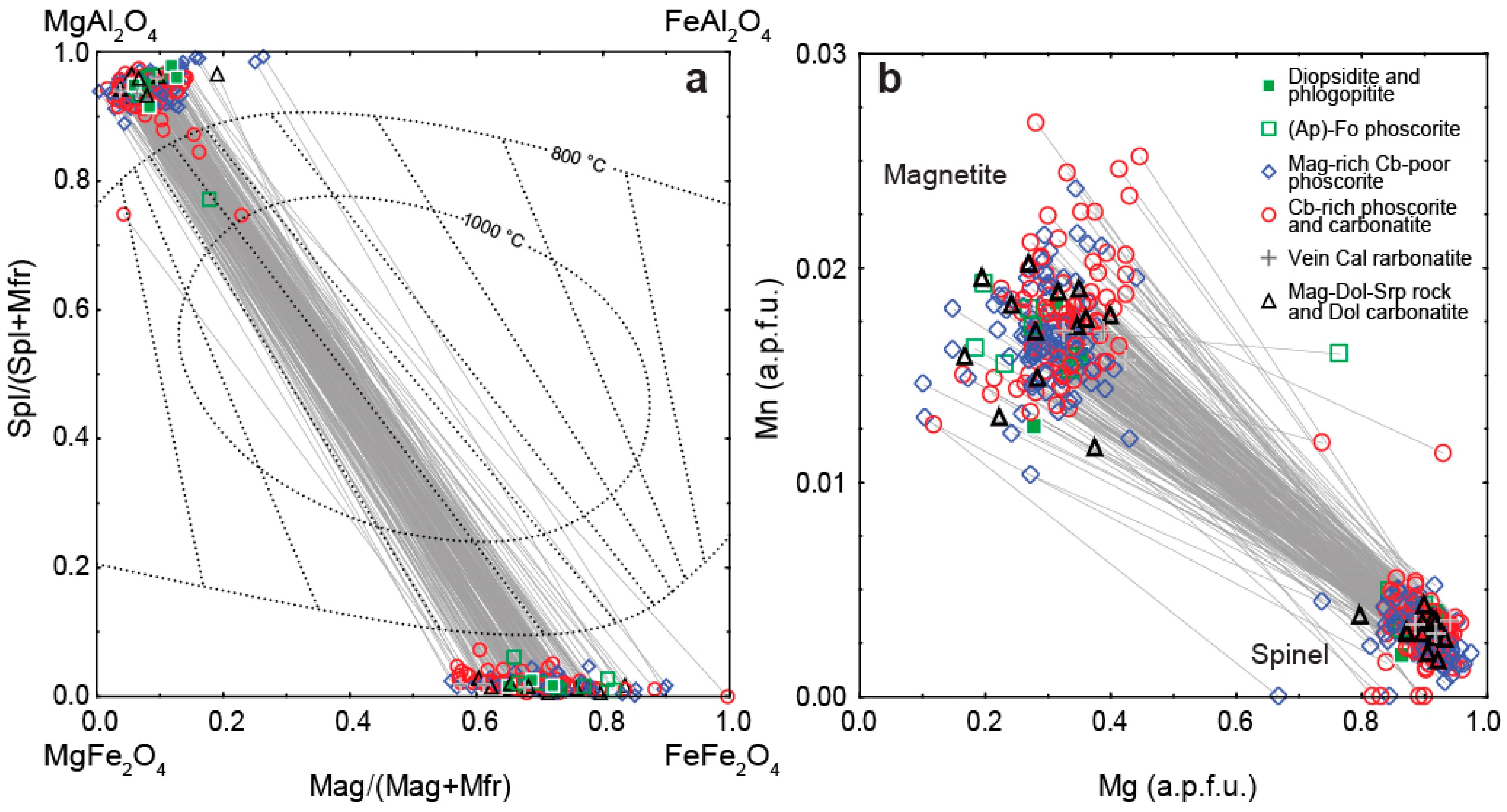

| Mineral | Sum of Oxides | Simplified Formula | Cation Positions | Reference | |
|---|---|---|---|---|---|
| Tetrahedral (8f) | Octahedral (16c) | ||||
| Normal spinellids | |||||
| Spinel | MgO·Al2O3 | MgAl2O4 | Mg2+ | Al3+ | [28] |
| Hercynite | FeO·Al2O3 | FeAl2O4 | Fe2+ | Al3+ | [29] |
| Galaxite | MnO·Al2O3 | MnAl2O4 | Mn2+ | Al3+ | [30] |
| Magnesiocoulsonite | MgO·V2O3 | MgV2O4 | Mg2+ | V3+ | [31] |
| Coulsonite | FeO·V2O3 | FeV2O4 | Fe2+ | V3+ | [32] |
| Inversed spinellids | |||||
| Magnetite | FeO·Fe2O3 | Fe3O4, Fe2+Fe3+2O4 | Fe3+ | Fe2+, Fe3+ | [33] |
| Magnesioferrite | MgO·Fe2O3 | MgFe2O4 | Fe3+ | Mg2+, Fe3+ | [34] |
| Qandilite | 2MgO·TiO2 | Mg2TiO4 | Mg2+ | Mg2+, Ti4+ | [35] |
| Ulvöspinel | 2FeO·TiO2 | Fe2TiO4 | Fe2+ | Fe2+, Ti4+ | [33] |
| Partially inversed spinellids | |||||
| Spinel | MgO·Al2O3 | MgAl2O4 | Mg2+, Al3+ | Al3+, Mg2+ | [36] |
| Magnesioferrite | MgO·Fe2O3 | MgFe2O4 | Fe3+, Mg2+ | Fe3+, Mg2+ | [34] |
| Jacobsite | MnO·Fe2O3 | MnFe2O4 | Fe3+, Mn2+ | Fe3+, Mn2+ | [37] |
| Titanomagnetite | 1.2FeO·0.8Fe2O3·0.2TiO2 | Fe2+1.2Fe3+1.6Ti0.2O4 | Fe3+, Fe2+ | Fe2+, Fe3+, Ti4+ | [33] |
| Rock | Description | Modal % | Minor Rock-Forming Minerals | Characteristic Accessories | Magnetite |
|---|---|---|---|---|---|
| Foidolite | Dark grey, medium- to fine-grained rock with massive or banded structure formed by alternating leucocratic nepheline-rich and melanocratic clinopyroxene- (Di–Aeg) and/or melilite-rich layers | Nph 10–50 Cpx 50–90 Phl 1–8 Me 0–10 | Cancrinite, magnetite, microcline, monticellite, natrolite, sodalite, titanite | Albite, ancylite-(Ce), andradite, baddeleyite, banalcite, barite, bornite, calcite, chalcopyrite, fluorapatite, galena, gonnardite, ilmenite, nordstrandite, nyerereite, pentlandite, perovskite, pyrite, pyrrhotite, pyrochlore, shortite, sphalerite, spinel, strontianite, thorianite | Irregularly shaped grains and skeletal crystals (0.2 ± 0.3 mm) |
| Diopsidite and phlogopitite | Dark-green, medium- to coarse-grained massive rock composed mostly of short-prismatic diopside crystals, corroded by phlogopite lamellae. In phlogopitite, there are mica segregations (up to 1 cm in diameter) with diopside relics and newly-formed inclusions of hydroxylapatite and magnetite | Di 10–95 Phl 3–95 Fo 0–30 Nph 0–15 | Calcite, cancrinite, clinochlore, dolomite, edenite, gonnardite, hydroxylapatite, magnesioarfvedsonite, magnesiohastingsite, magnetite, natrolite, pargasite, pyrrhotite, richterite, serpentine, sodalite, vermiculite | Aegirine, albite, ancylite-(Ce), baddeleyite, barite, barytocalcite, calzirtite, cerussite, chalcopyrite, chamosite, chromite, cobaltpentlandite, freudenbergite, galena, grinalite, ilmenite, lueshite, mesolite, microcline, pentlandite, perovskite, pyrite, pyrochlore, pyrophanite, rutile, shortite, sphalerite, spinel, titanite, zircon, zirconolite | Irregularly shaped grains (1.0 ± 0.9 mm) in interstices of rock-forming silicates, and larger metacrysts (up to 5 mm) with poikilitic inclusions of surrounding minerals |
| (Ap)-Fo phoscorite | Greenish-gray fine-grained, massive, indistinctly banded or spotted rock consisting of rounded equant to short-prismatic grains of forsterite. Interstices between forsterite grains are filled with hydroxylapatite (±magnetite) that also forms monomineralic segregations and bands. Phlogopite replaces the forsterite. | Fo 10–90 Ap 0–80 Mag 0–8 Cal 0–5 Phl 1–10 | Calcite, diopside, dolomite, magnetite | Baddeleyite, brucite, chalcopyrite, cobaltpentlandite, geikielite, ilmenite, monazite-(Ce), pentlandite, pyrite, pyrochlore, pyrophanite, pyrrhotite, serpentine, sphalerite, spinel, strontianite, valleriite, zirconolite | Anhedral grains (0.2 ± 0.1 mm) in interstices of forsterite; rounded grains (0. 3 ± 0.1 mm) and larger metacrysts (up to 8 mm) in apatite-rich parts of the rock |
| Low-Cb Mag-rich phoscorite | Light greenish-grey to black, massive, spotted, spotty-banded rock consisting mainly of forsterite (altered grains, partially replaced by serpentine, clinochlore, phlogopite and valleriite) and hydroxylapatite (lens-like segregations of rounded or irregularly shaped grains) cemented by magnetite | Fo 0–70 Ap 0–70 Mag 10–95 Cal 0–8 | Calcite, chalcopyrite, dolomite, phlogopite, pyrrhotite, valleriite | Ancylite-(Ce), baddeleyite, bakhchisaraitsevite, barite, betafite, bobierrite, cobaltpentlandite, galena, geikielite, ilmenite, lueshite, magnesite, perovskite, pentlandite, pyrite, pyrochlore, pyrophanite, quintinite, sphalerite, spinel, thorianite, zirconolite | Irregularly shaped grains (3 ± 2 mm) and larger metacrysts (up to 2 cm) with inclusions of surrounding apatite-forsterite aggregates and separate grains of these minerals |
| Cb-rich phoscorite and phoscorite-related carbonatite | Spotted black-and-white rocks composed of forsterite (replaced by serpentine, dolomite, phlogopite, clinohumite and clinochlore), hydroxylapatite (anhedral grains between forsterite crystals, inclusions in calcite and monomineral segregations), magnetite (irregularly shaped metacrysts in apatite-rich parts and well-shaped octahedral crystals in calcite-rich parts) and calcite (monomineralic veinlets and nests between grains of forsterite, hydroxylapatite and magnetite). | Fo 0–70 Ap 0–60 Mag 8–80 Cal 10–82 Dol 1–10 Phl 0–15 Po 0–30 Cpp 0–10 | Clinochlore, clinohumite, richterite, tetraferriphlogopite, valleriite | Ancylite-(Ce), baddeleyite, bakhchisaraitsevite, barite, baritocalcite, bobierrite, bornite, burbankite, chalcocite, cobaltpentlandite, covellite, crandallite, cubanite, djerfisherite, galena, geikielite, ilmenite, juonniite, kovdorskite, mackinawite, magnesite, microlite, norsethite, northupite, nyerereite, perovskite, pyrite, pyrochlore, pyrophanite, quintinite, siderite, sphalerite, spinel, strontianite, thorianite, witherite, zircon, zirconolite | Rounded grains (4 ± 2 mm), larger metacrysts (up to 5 cm) with poikilitic inclusions of forsterite and hydroxylapatite, as well as well-shaped crystals with octahedral {111}, rhombic dodecahedral {110} and tetragonal trioctahedral {311} faces |
| Vein Cal carbonatite | White, light brown or light grey medium- to coarse-grained, massive to banded rock consisting of equant crystals of calcite and dolomite with interstitial hydroxylapatite, magnetite and phlogopite | Fo 0–5 Ap 0–15 Mag 0–10 Cal 70–95 Dol 5–15 | Forsterite, magnetite, phlogopite, pyrrhotite | Alstonite, ancylite-(Ce), baddeleyite, barite, calzirtite, chalcopyrite, cobaltpentlandite, djerfisherite, eitelite, galena, geikielite, ilmenite, microlite, monazite-(Ce), northupite, nyerereite, perovskite, pyrite, serpentine, sphalerite, spinel, strontianite, thorianite, zirconolite | Rounded grains (2 ± 1 mm) and well-shaped octahedral crystals (up to 3 cm in diameter) with minor rhombic dodecahedral faces |
| Vein Dol carbonatite | White, pale-pink or light-brown medium- to coarse-grained rock composed mainly of dolomite, with magnetite and pyrrhotite in selvages | Fo 0 Ap 0–6 Mag 0–40 Dol 50–95 Cal 5–10 Po 0–10 | Pyrite, richterite, phlogopite, serpentine, tetraferriphlogopite | Anatase, ancylite-(Ce), aragonite, baddeleyite, bakhchisaraitsevite, baricite, barite, barytocalcite, bobierrite, burbankite, catapleiite, chalcopyrite, collinsite, hydroxylapatite, ilmenite, juonniite, kovdorskite, labuntsovite-Mg, lueshite, magnesite, norsethite, rimkorolgite, serpentine, siderite, sphalerite, spinel, strontianite, zircon, zirconolite | Rounded grains (3 ± 2 mm) and well-shaped octahedral crystals (up to 4 cm) with minor rhombic dodecahedral faces |
| Mag-Dol-Srp rock | Dark green to black, fine-grained rock with massive, mottled or brecciated structure. Consists mainly of small grains of dolomite, phlogopite, serpentine (with phlogopite and forsterite relics), and poikilitic magnetite metacrysts of different size and shape | Fo 0–10 Ap 8–25 Mag 20–50 Dol 10–40 Srp 15–30 Phl 5–30 | Calcite, pyrrhotite, tetraferriphlogopite | Baddeleyite, baritocalcite, chalcopyrite, cobaltpentlandite, pentlandite, sphalerite, galena, strontianite, zircon, zirconolite | Irregularly shaped porous metacrysts (2 ± 1 mm) with numerous inclusions of phlogopite, apatite, calcite, dolomite and fragments of surrounding rock |
| Element | Limit of Accuracy, wt % | Standards for Wavelength Dispersive X-ray Spectroscopy (WDS) Analyses |
|---|---|---|
| Mg | 0.1 | Pyrope |
| Al | 0.05 | Pyrope |
| Si | 0.05 | Diopside |
| Ca | 0.03 | Diopside |
| Sc | 0.02 | Thortveitite |
| Ti | 0.02 | Lorenzenite |
| V | 0.1 | Metallic vanadium |
| Cr | 0.02 | Chromite |
| Mn | 0.01 | Synthetic MnCO3 |
| Fe | 0.01 | Hematite |
| Co | 0.01 | Metallic cobalt |
| Ni | 0.01 | Metallic nickel |
| Zn | 0.01 | Synthetic ZnO |
| Nb | 0.05 | Metallic niobium |
| Ta | 0.05 | Metallic tantalum |
| Rock Group | Foidolite | Diopsidite and Phlogopitite | (Ap)-Fo Phoscorite | Low-Cb Mag-rich Phoscorite | Cb-rich Phoscorite and PhoscoriteRelated Carbonatite | Vein Cal Carbonatite | Vein Dol Carbonatite and Mag-Dol-Srp Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 971/609.8 | 900/592.3 | 946/540.4 | 917/32.8 | 1007/33.0 | 989/92.2 | 977/129.5 | |||||||
| Rock | Ijolite | Diopsidite | AF-phoscorite | MF-phoscorite | Cal carbonatite | Cal carbonatite | Mag-Dol-Srp rock | |||||||
| Mineral | Mag | Spl | Mag | Spl | Mag | Spl | Mag | Spl | Mag | Spl | Mag | Spl | Mag | Spl |
| MgO | 5.74 | 24.53 | 6.57 | 24.93 | 4.23 | 23.50 | 5.65 | 25.15 | 6.43 | 25.17 | 8.14 | 25.55 | 7.12 | 25.35 |
| Al2O3 | 0.80 | 66.86 | 0.69 | 64.94 | 0.67 | 64.72 | 0.71 | 66.21 | 0.74 | 64.49 | 0.88 | 64.46 | 0.69 | 67.02 |
| CaO | - | - | - | - | - | - | - | - | 0.03 | - | - | - | - | - |
| TiO2 | 0.75 | - | 1.04 | - | 1.20 | - | 0.77 | - | 0.91 | - | 0.69 | 0.09 | 0.59 | - |
| V2O3 | 0.04 | - | 0.07 | - | 0.17 | - | 0.08 | - | 0.06 | - | 0.05 | - | 0.03 | - |
| MnO | 0.53 | 0.10 | 0.52 | 0.09 | 0.50 | 0.13 | 0.55 | 0.25 | 0.56 | 0.10 | 0.53 | 0.17 | 0.39 | 0.08 |
| FeO | 84.54 | 7.05 | 85.07 | 7.76 | 86.79 | 7.40 | 85.68 | 6.94 | 84.77 | 8.23 | 85.47 | 7.57 | 86.32 | 6.00 |
| ZnO | - | 1.56 | - | 0.96 | - | 1.55 | - | 1.31 | - | 1.26 | - | 1.13 | - | 1.07 |
| Total | 92.40 | 100.10 | 93.96 | 98.68 | 93.56 | 97.30 | 93.44 | 99.86 | 93.50 | 99.25 | 95.76 | 98.97 | 95.14 | 99.52 |
| Cation Content on the Basis of 4 O and 3 Me a.p.f.u. (atoms per formula unit) | ||||||||||||||
| Fe2+ | 0.69 | 0.07 | 0.66 | 0.06 | 0.78 | 0.08 | 0.70 | 0.05 | 0.66 | 0.05 | 0.58 | 0.04 | 0.63 | 0.05 |
| Mg | 0.32 | 0.90 | 0.35 | 0.92 | 0.23 | 0.89 | 0.31 | 0.92 | 0.35 | 0.93 | 0.42 | 0.94 | 0.38 | 0.92 |
| Mn | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | 0.01 | 0.02 | - | 0.02 | - | 0.01 | - |
| Zn | - | 0.03 | - | 0.02 | - | 0.03 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 |
| Fe3+ | 1.92 | 0.07 | 1.91 | 0.10 | 1.91 | 0.07 | 1.92 | 0.09 | 1.92 | 0.12 | 1.92 | 0.12 | 1.93 | 0.07 |
| Al | 0.03 | 1.93 | 0.03 | 1.90 | 0.03 | 1.93 | 0.03 | 1.91 | 0.03 | 1.88 | 0.04 | 1.88 | 0.03 | 1.94 |
| Ti | 0.02 | - | 0.03 | - | 0.03 | - | 0.02 | - | 0.02 | - | 0.02 | - | 0.02 | - |
| Total | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| End Members, Mole % | ||||||||||||||
| Mag | 64.78 | 0.27 | 61.20 | 0.31 | 72.09 | 0.31 | 65.59 | 0.24 | 61.64 | 0.30 | 54.62 | 0.22 | 59.82 | 0.19 |
| Mfr | 29.75 | 3.23 | 32.89 | 4.78 | 21.40 | 3.33 | 29.03 | 4.19 | 32.58 | 5.80 | 40.18 | 5.74 | 35.98 | 3.21 |
| Jcb | 1.64 | 0.20 | 1.57 | 0.20 | 1.53 | 0.28 | 1.66 | 0.52 | 1.69 | 0.22 | 1.54 | 0.35 | 1.15 | 0.17 |
| Hc | 1.18 | 7.39 | 0.94 | 5.70 | 1.11 | 8.25 | 1.04 | 5.18 | 1.02 | 4.60 | 1.03 | 3.51 | 0.89 | 5.40 |
| Spl | 0.54 | 88.91 | 0.51 | 89.01 | 0.33 | 87.82 | 0.46 | 89.87 | 0.54 | 89.08 | 0.76 | 90.00 | 0.54 | 91.03 |
| Usp | 1.40 | - | 1.81 | - | 2.54 | - | 1.45 | - | 1.60 | - | 1.04 | 0.01 | 0.98 | - |
| Qnd | 0.64 | - | 0.97 | - | 0.75 | - | 0.64 | - | 0.85 | - | 0.76 | 0.17 | 0.59 | - |
| Cul | 0.04 | - | 0.07 | - | 0.19 | - | 0.08 | - | 0.05 | - | 0.04 | - | 0.03 | - |
| Mcul | 0.02 | - | 0.04 | - | 0.06 | - | 0.04 | - | 0.03 | - | 0.03 | - | 0.02 | - |
| Total | 99.99 | 100.00 | 100.00 | 100.00 | 100.0 | 99.99 | 99.99 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Rock Group | Foidolite | Diopsidite and Phlogopitite | (Ap)-Fo Phoscorite | Low-Cb Mag-Rich Phoscorite | Cb-Rich Phoscorite and Phoscorite-Related Carbonatite | Vein Cal Carbonatite | Vein Dol Carbonatite and Mag-Dol-Srp Rock | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 931/405.9 | 963/92.2 | 937/147.4 | 976/96.9 | 970/124.0 | 967/66.5 | 987/198.0 | |||||||
| Rock | Ijolite | Diopsidite | F-phoscorite | MA-phoscorite | Cal carbonatite | Cal carbonatite | Mag-Dol-Srp rock | |||||||
| Mineral | Mag | Ilm | Mag | Ilm | Mag | Ilm | Mag | Ilm | Mag | Ilm | Mag | Ilm | Mag | Ilm |
| MgO | 0.21 | 1.73 | 0.37 | 2.12 | 2.04 | 8.66 | 1.86 | 10.87 | 0.84 | 10.55 | 0.93 | 10.77 | 1.60 | 10.57 |
| Al2O3 | - | - | 0.16 | 0.06 | 0.36 | - | - | - | - | - | - | - | - | - |
| SiO2 | 0.20 | 0.18 | 0.20 | 0.20 | - | - | - | 0.13 | - | - | - | - | - | - |
| CaO | 0.32 | 0.07 | 0.39 | 0.07 | - | - | - | 0.22 | 0.08 | 0.04 | 0.03 | - | 0.06 | 0.05 |
| TiO2 | 0.32 | 50.25 | 0.85 | 51.32 | 1.33 | 54.61 | 0.98 | 55.20 | 0.98 | 55.99 | 0.82 | 51.00 | 0.89 | 55.25 |
| V2O3 | 0.38 | - | 0.07 | - | 0.16 | - | 0.20 | - | 0.22 | 0.38 | 0.12 | - | 0.20 | - |
| Cr2O3 | 0.11 | - | 0.06 | - | - | - | - | - | - | - | - | - | - | - |
| MnO | 0.09 | 3.80 | 0.19 | 4.37 | 0.72 | 6.84 | 0.25 | 3.06 | 0.15 | 2.76 | 0.12 | 3.38 | 0.29 | 3.95 |
| FeO | 92.52 | 41.82 | 92.25 | 39.26 | 89.22 | 28.37 | 90.27 | 28.01 | 92.63 | 29.75 | 93.49 | 34.04 | 90.08 | 28.06 |
| ZnO | - | - | - | - | - | - | - | - | - | - | 0.06 | - | - | - |
| Nb2O5 | - | 0.38 | - | 0.20 | - | - | - | 0.45 | - | - | - | 0.79 | - | 0.14 |
| Total | 94.15 | 98.23 | 94.54 | 97.60 | 93.83 | 98.48 | 93.56 | 97.94 | 94.90 | 99.47 | 95.57 | 99.98 | 93.12 | 98.02 |
| Cation Content on the Basis of 4 O and 3 Me a.p.f.u., and 3 O and 2 Me a.p.f.u., Respectively for the Spinel-group and Ilmenite-group Minerals. | ||||||||||||||
| Fe2+ | 0.99 | 0.82 | 0.99 | 0.82 | 0.90 | 0.54 | 0.91 | 0.55 | 0.97 | 0.56 | 0.97 | 0.47 | 0.92 | 0.54 |
| Mn | - | 0.08 | 0.01 | 0.09 | 0.02 | 0.14 | 0.01 | 0.06 | - | 0.05 | - | 0.07 | 0.01 | 0.08 |
| Mg | 0.01 | 0.07 | 0.02 | 0.08 | 0.11 | 0.31 | 0.10 | 0.38 | 0.05 | 0.37 | 0.05 | 0.37 | 0.09 | 0.37 |
| Ca | 0.01 | - | 0.02 | - | - | - | - | 0.01 | - | - | - | - | - | - |
| Al | - | - | 0.01 | - | 0.02 | - | - | - | - | - | - | - | - | - |
| Fe3+ | 1.96 | 0.06 | 1.92 | 0.02 | 1.91 | 0.03 | 1.94 | 0.01 | 1.94 | 0.02 | 1.96 | 0.19 | 1.94 | 0.02 |
| V3+ | 0.01 | - | - | - | - | - | 0.01 | - | 0.01 | 0.01 | - | - | 0.01 | - |
| Ti | 0.01 | 0.97 | 0.02 | 0.99 | 0.04 | 0.98 | 0.03 | 0.99 | 0.03 | 0.99 | 0.02 | 0.89 | 0.03 | 0.99 |
| Si | 0.01 | - | 0.01 | - | - | - | - | - | - | - | - | - | - | - |
| Nb | - | - | - | - | - | - | - | - | - | - | - | 0.01 | - | - |
| Total | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 |
| End Members, Mole % | ||||||||||||||
| Mag | 96.11 | 93.70 | 82.47 | 86.23 | 91.98 | 92.20 | 87.58 | |||||||
| Mfr | 1.17 | 1.97 | 10.45 | 9.88 | 4.45 | 4.94 | 8.63 | |||||||
| Jcb | 0.30 | 0.61 | 2.28 | 0.81 | 0.48 | 0.39 | 0.92 | |||||||
| Hc | - | 0.34 | 0.71 | - | - | - | - | |||||||
| Spl | - | 0.01 | 0.09 | - | - | - | - | |||||||
| Usp | 0.90 | 2.38 | 3.33 | 2.49 | 2.63 | 2.17 | 2.32 | |||||||
| Qnd | 0.01 | 0.05 | 0.42 | 0.28 | 0.13 | 0.12 | 0.23 | |||||||
| Cul | 0.56 | 0.10 | 0.22 | 0.27 | 0.31 | 0.17 | 0.28 | |||||||
| Mcul | 0.01 | - | 0.03 | 0.03 | 0.02 | 0.01 | 0.03 | |||||||
| Ilm | 82.00 | 81.32 | 53.56 | 54.48 | 56.19 | 46.64 | 53.43 | |||||||
| Ppn | 8.13 | 9.38 | 13.88 | 6.13 | 5.47 | 6.62 | 7.94 | |||||||
| Gkl | 6.50 | 8.02 | 30.92 | 38.35 | 36.83 | 37.13 | 37.38 | |||||||
| Hem | 3.18 | 0.99 | 1.63 | 0.48 | 1.00 | 9.61 | 1.11 | |||||||
| Total | 99.06 | 99.81 | 99.16 | 99.71 | 100.00 | 99.99 | 99.99 | 99.44 | 100.00 | 99.49 | 100.00 | 100.00 | 99.99 | 99.86 |
| Temperature (°C) and Oxygen Fugacity | ||||||||||||||
| T1 | 504 | 452 | 504 | 455 | 455 | 651 | 470 | |||||||
| T2 | 287 | 265 | 289 | 232 | 240 | 411 | 250 | |||||||
| ΔNNO1 | +0.15 | −4.09 | −2.73 | −4.47 | −4.18 | +2.55 | −3.29 | |||||||
| ΔNNO2 | +0.66 | −2.80 | −3.85 | −5.15 | −4.66 | +0.96 | −4.13 | |||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.V.; Goryainov, P.M.; Mikhailova, J.A.; Yakovenchuk, V.N.; Konopleva, N.G. Subsolidus Evolution of the Magnetite-Spinel-UlvöSpinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia. Minerals 2017, 7, 215. https://doi.org/10.3390/min7110215
Ivanyuk GY, Kalashnikov AO, Pakhomovsky YA, Bazai AV, Goryainov PM, Mikhailova JA, Yakovenchuk VN, Konopleva NG. Subsolidus Evolution of the Magnetite-Spinel-UlvöSpinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia. Minerals. 2017; 7(11):215. https://doi.org/10.3390/min7110215
Chicago/Turabian StyleIvanyuk, Gregory Yu., Andrey O. Kalashnikov, Yakov A. Pakhomovsky, Ayya V. Bazai, Pavel M. Goryainov, Julia A. Mikhailova, Victor N. Yakovenchuk, and Nataly G. Konopleva. 2017. "Subsolidus Evolution of the Magnetite-Spinel-UlvöSpinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia" Minerals 7, no. 11: 215. https://doi.org/10.3390/min7110215