2.1. Materials
The titanomagnetite concentrate used in this study was obtained from Panzhihua, Sichuan Province, China. The particle size distribution of the titanomagnetite concentrate is shown in
Table 1. Chemical analyses results are shown in
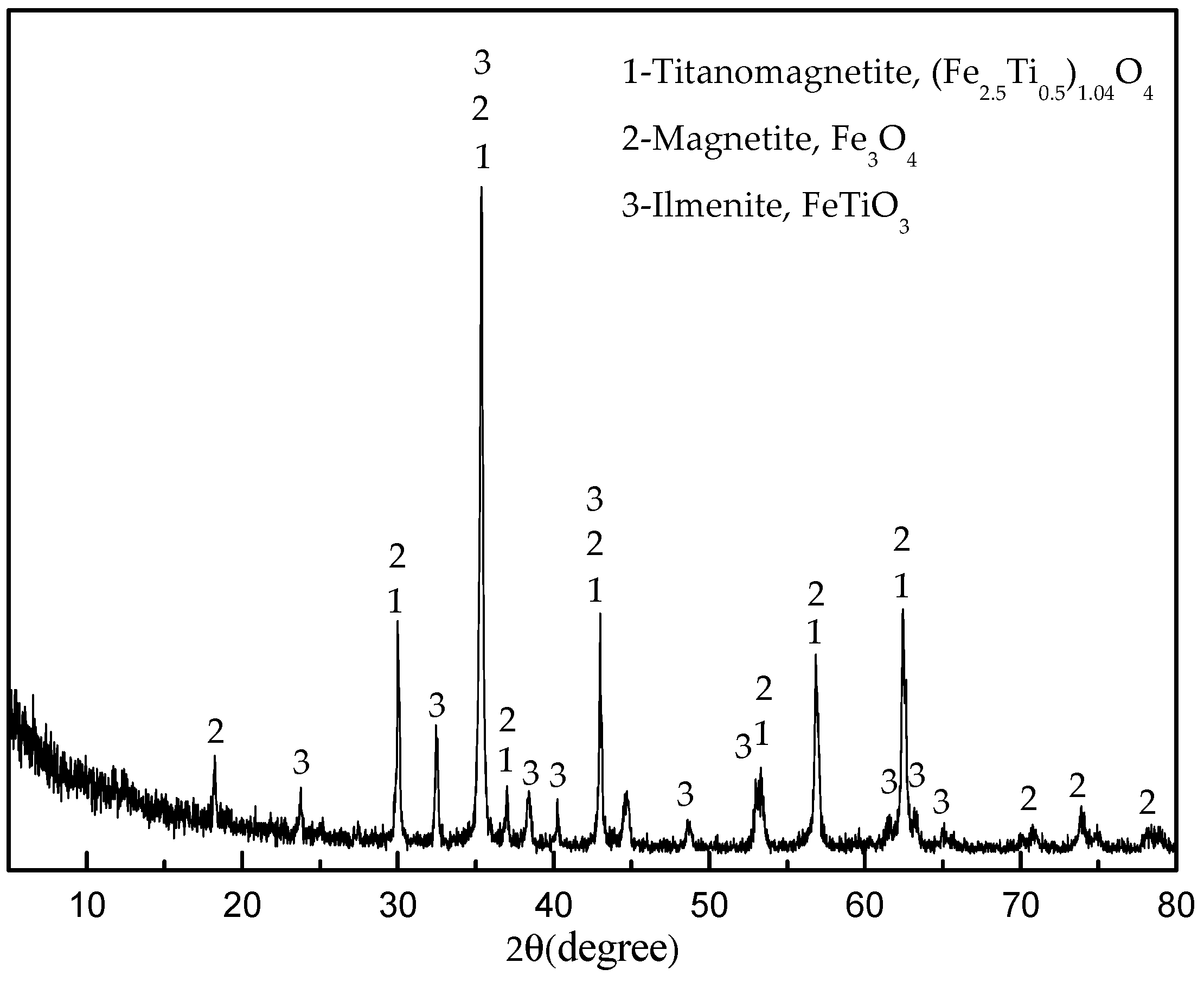
Table 2. X-ray diffraction (XRD, DX-2700, Hao Yuan Instrument Co., Ltd., Dandong, China) analyses revealed that the main crystalline phases of the sample were titanomagnetite ((Fe
2.5Ti
0.5)
1.04O
4), magnetite (Fe
3O
4) and ilmenite (FeTiO
3) (
Figure 1). The vanadium in the titanomagnetite is the trivalent vanadium (V
3+) forming a solid solution with the magnetite lattice [
2]. The reductant used in this work was anthracite coal. The proximate analysis of the coal was conducted according to China national standards GB/T212-2008 [
12], and the results are listed in
Table 3. The coal was ground to 100% passage through a 0.1 mm sieve.
2.2. Methods
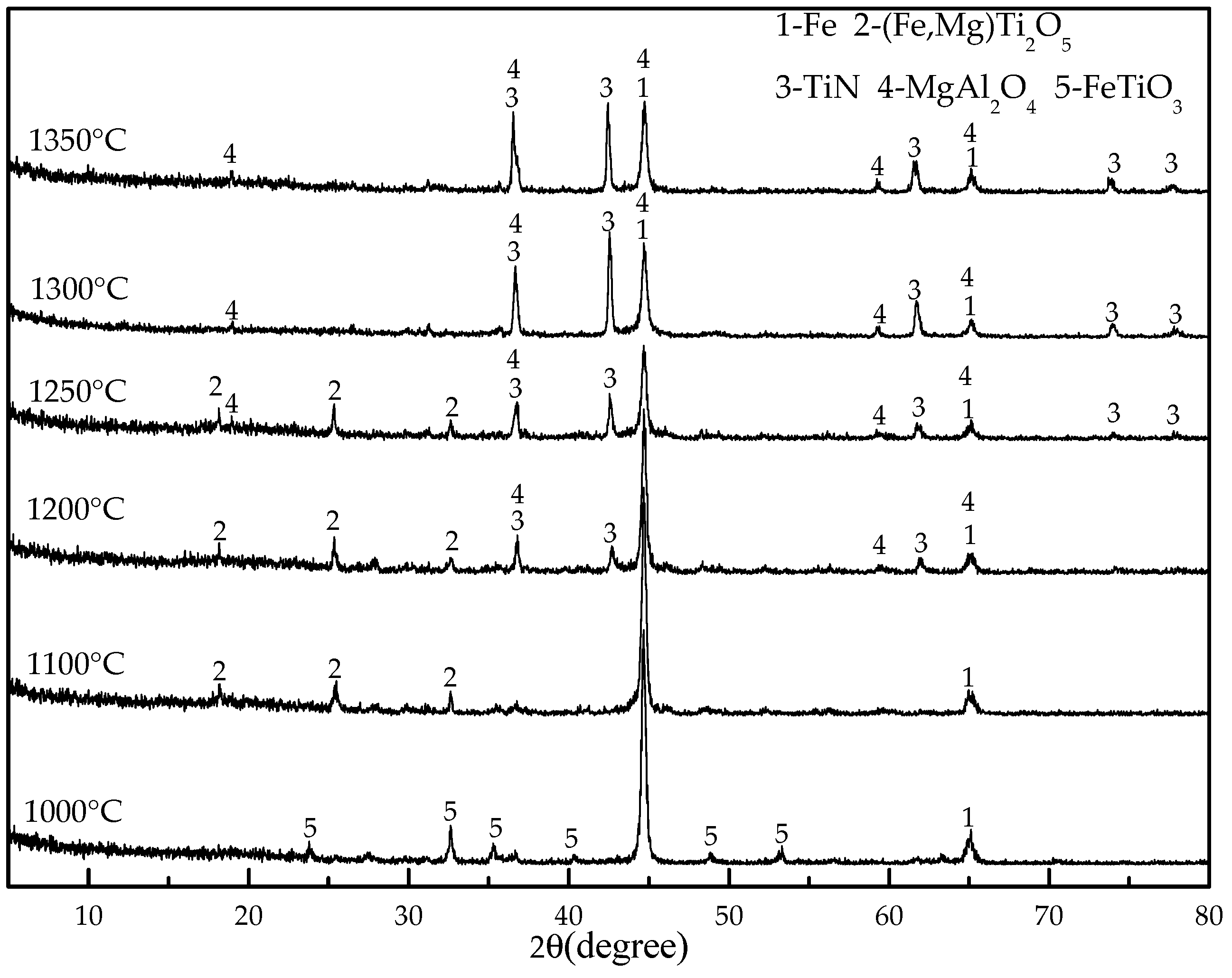
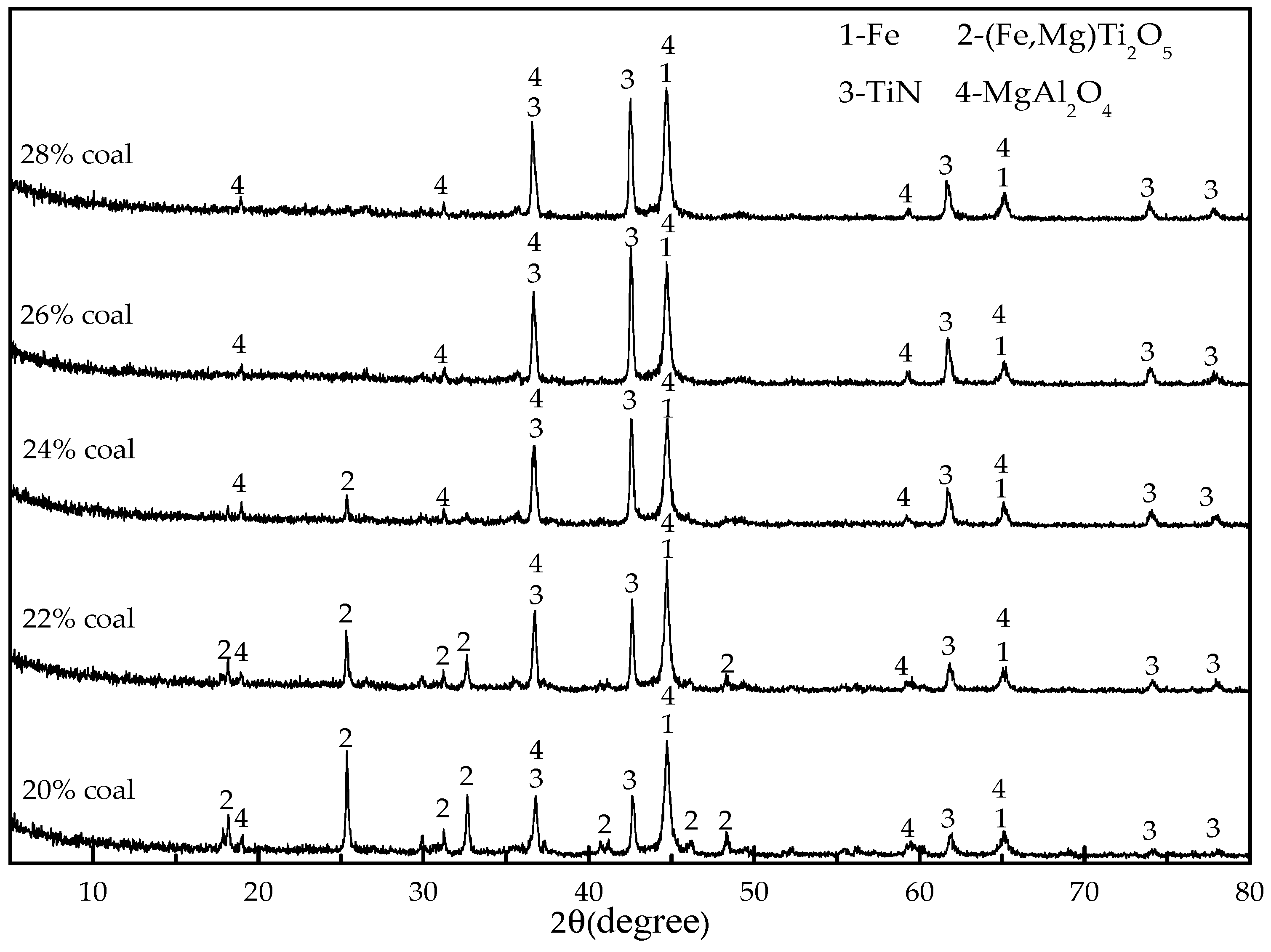
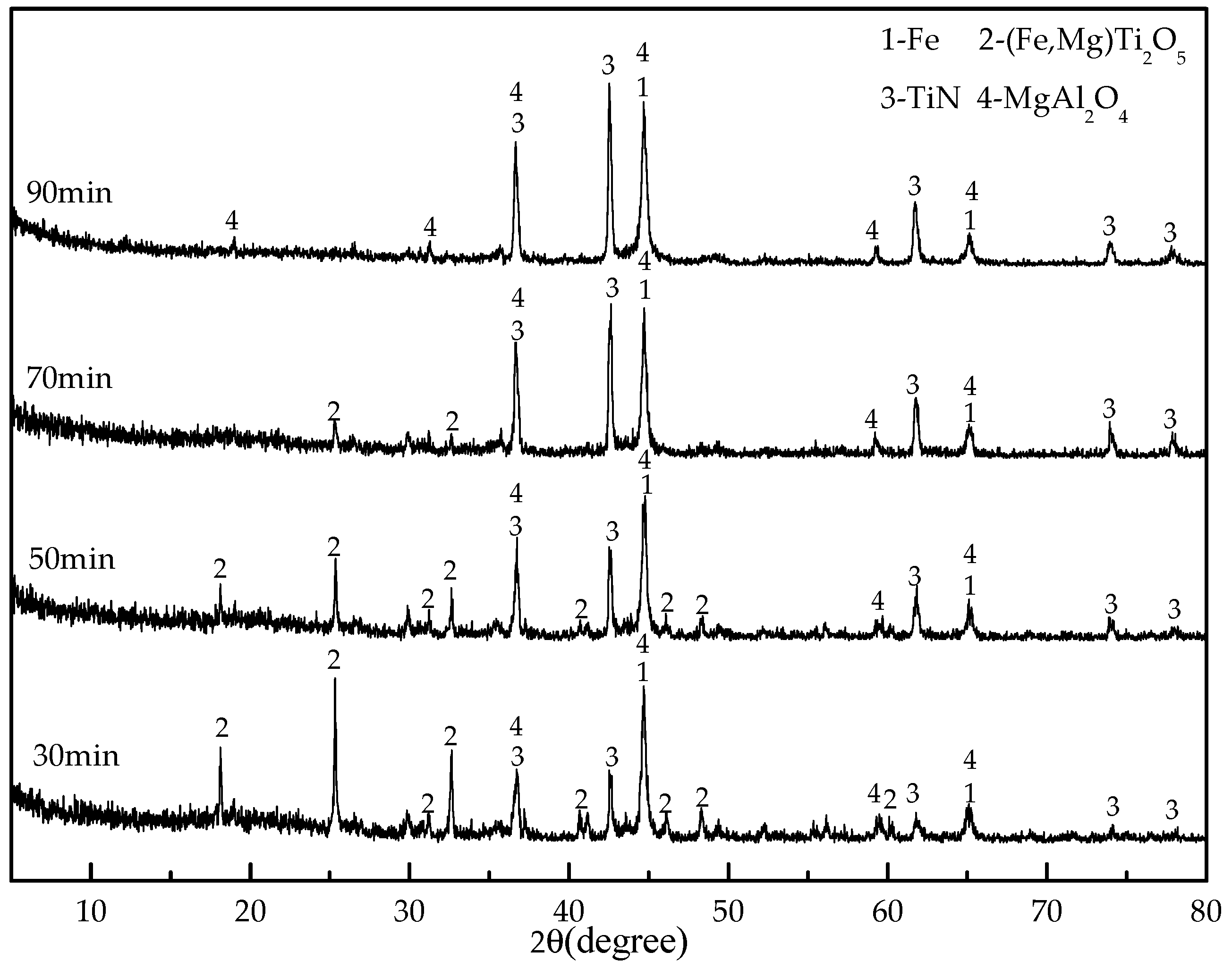
As a preparation for the reduction experiments, 20 g of titanomagentite were mixed with coal (20 wt %, 22 wt %, 24 wt %, 26 wt %, 28 wt %), cellulose binder (1 wt %) and water (about 25 wt %) to produce material that could be shaped by hands to make 6–8 mm diameter pellets. The dosages of coal, binder and water are expressed as percentages that refer to their mass ratios to the titanomagnetite concentrate. An analytical grade of sodium carboxymethylcellulose was employed as a binder. The green pellets were oven dried at 105 °C for 2 h. The reduction experiments were performed in a muffle furnace under air atmosphere with the following procedures: first, the pellets were placed in the clay–graphite crucible (70 mm in diameter and 75 mm in height) with a cap. Then, the crucibles were placed into the furnace and maintained for a certain time (30, 50, 70 and 90 min) after the temperature reached the designated temperature (1000, 1100, 1200, 1250, 1300 and 1350 °C). After heating, the crucibles were removed from the furnace and cooled to room temperature under air atmosphere.
After the reduced pellets cooling, they were crushed to −1 mm and then treated by two-stage grinding and magnetic separation. Grinding was done using a rod mill (RK/BM-1.0L, Wuhan Rock Crush & Grind Equipment Manufacture Co., Ltd, Wuhan, China) at 60 wt % solid density and with a speed of 192 rpm. The first stage grinding time was 10 min. A magnetic separator (XCGS-50, Nanchang Li Yuan Mining and Metallurgy Equipment Co., Ltd., Nanchang, China) with a magnetic field intensity of 1080 Oe was used to separate the slurry. The iron concentrates obtained from the first separation were reground for 20 min to 75.33 wt % −0.074 mm and then magnetically separated using the same conditions.
The resulting magnetic materials were labelled as DRI. The non-magnetic materials obtained from two-stage magnetic separation processes were mixed and labelled as rough TiN concentrates. As a result, DRI and rough TiN concentrates were obtained. The Fe, TiO2 and V2O5 contents of the DRI were chemically analyzed, and the composition of the rough TiN concentrates was analyzed by X-ray fluorescence analyser (Axios max, PANalytical, Almelo, The Netherlands). This XRF analyzer is a wavelength dispersive spectrometer equipped with Omnian standardless analysis software. The samples were measured as pressed powders.
The recoveries of Fe, Ti, and V in the DRI were calculated according to the following formula:
2.3. Characterisation
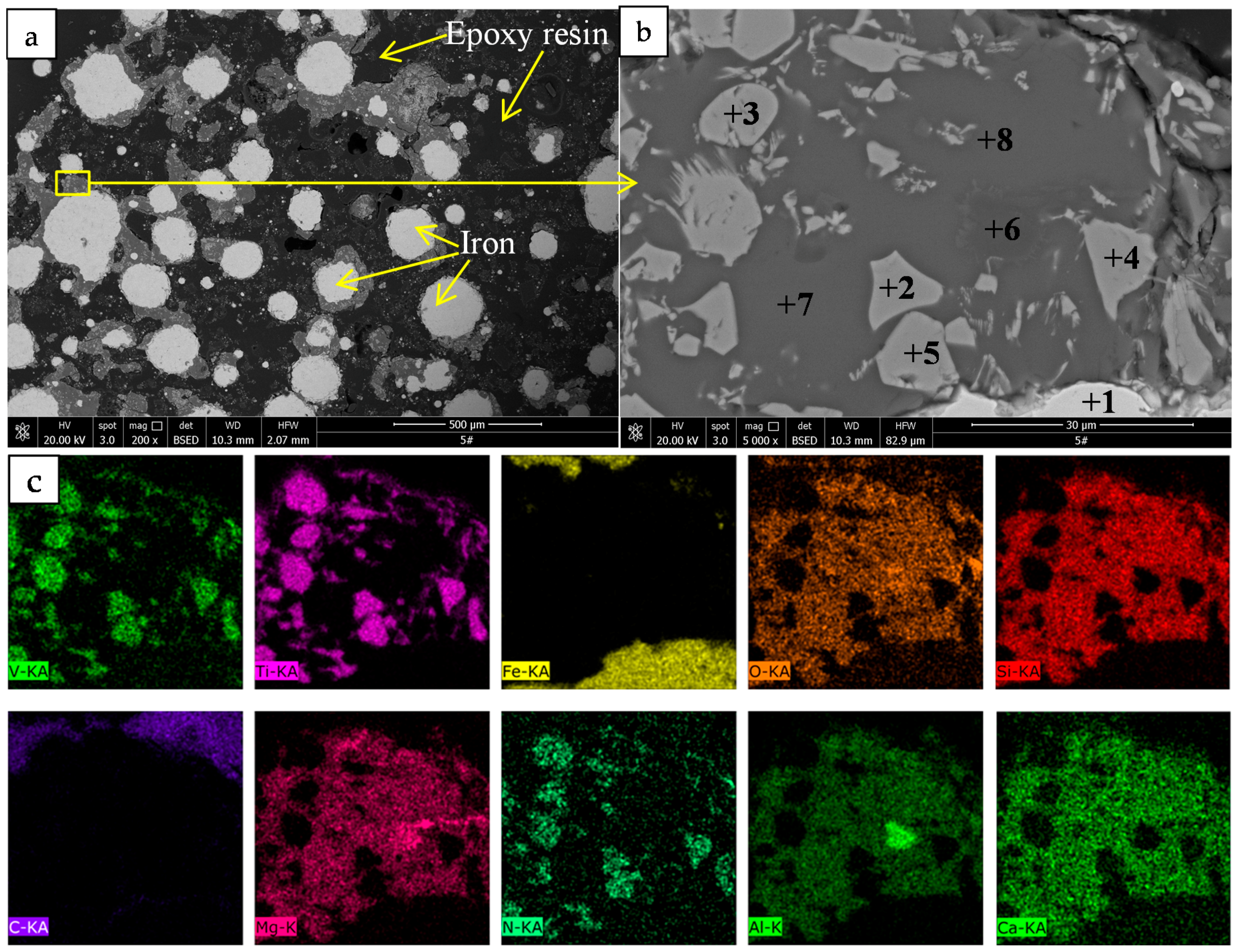
XRD with Cu-Kα radiation and secondary monochromator in the 2θ range from 5° to 80° was used to identify the phases present in the titanomagnetite concentrate and the reduced pellets. SEM and EDS analysis (MLA650F, FEI, Hillsboro, OR, USA) were performed on the samples mounted in epoxy resin, polished, and sputter coated with gold. The electron optical system of SEM consists of the Schottky field emission gun; an acceleration voltage of 0~30 kV; electron images were obtained at an acceleration voltage of 20.0 kV and a working distance of 10.3 mm. A silicon drift detector was employed for the EDS analysis. The EDS results were obtained at an acceleration voltage of 20.0 kV and pulse of 10–15 kcps.