Study on the Dispersion Mechanism of Citric Acid on Chlorite in Hematite Reverse Flotation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Materials
2.1.2. Reagents
2.2. Analyzing Methods
2.2.1. Artificial Mixed Ore Flotation Experiment
2.2.2. Settlement Test of Artificial Mixed Ore
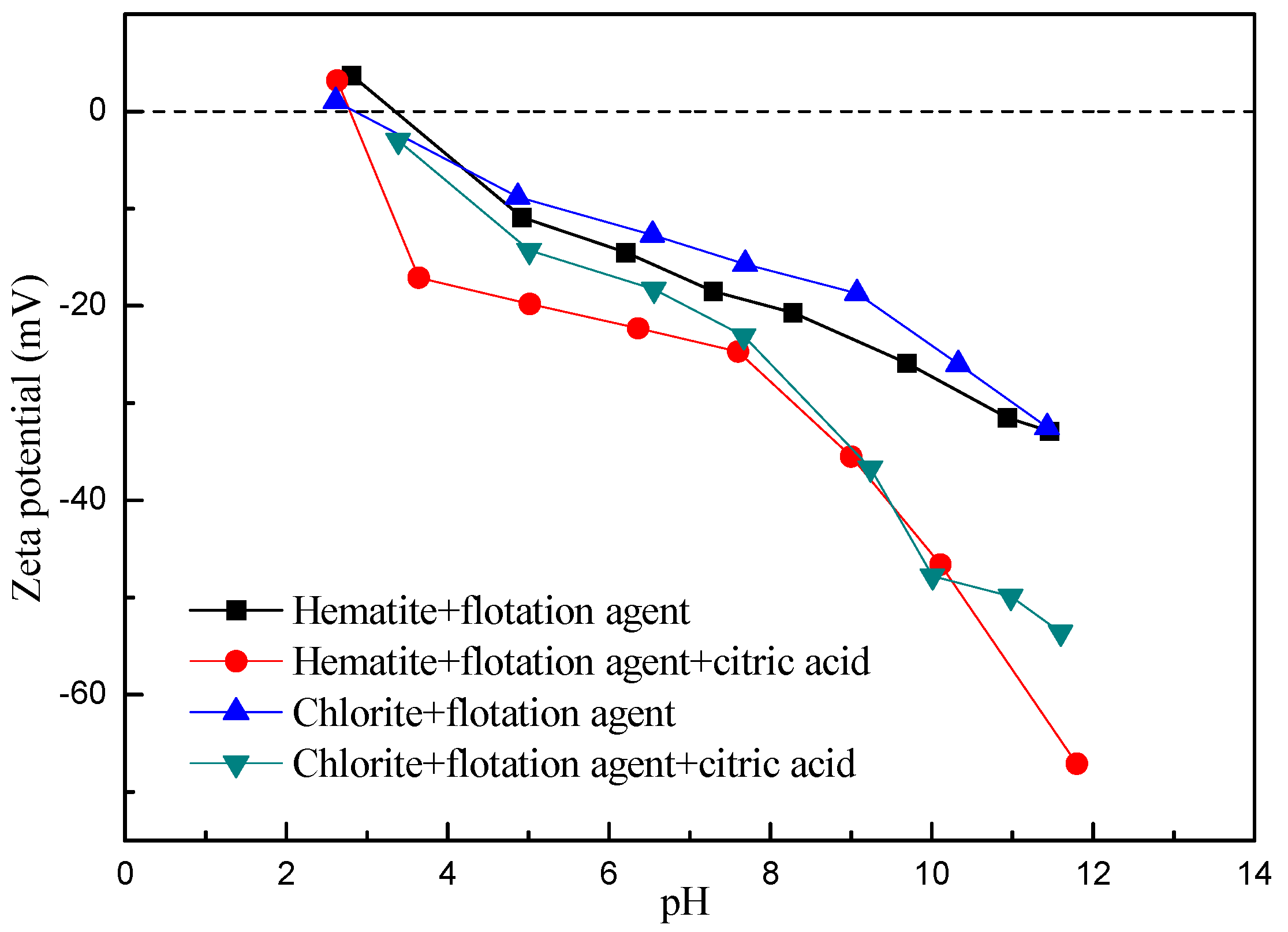
2.2.3. Contact Angle and Zeta Potential Measurements
2.2.4. Analysis of the Particle Size of Artificial Mixed Ore
2.2.5. Observation of Particle Aggregation and Dispersion
3. Results and Discussion
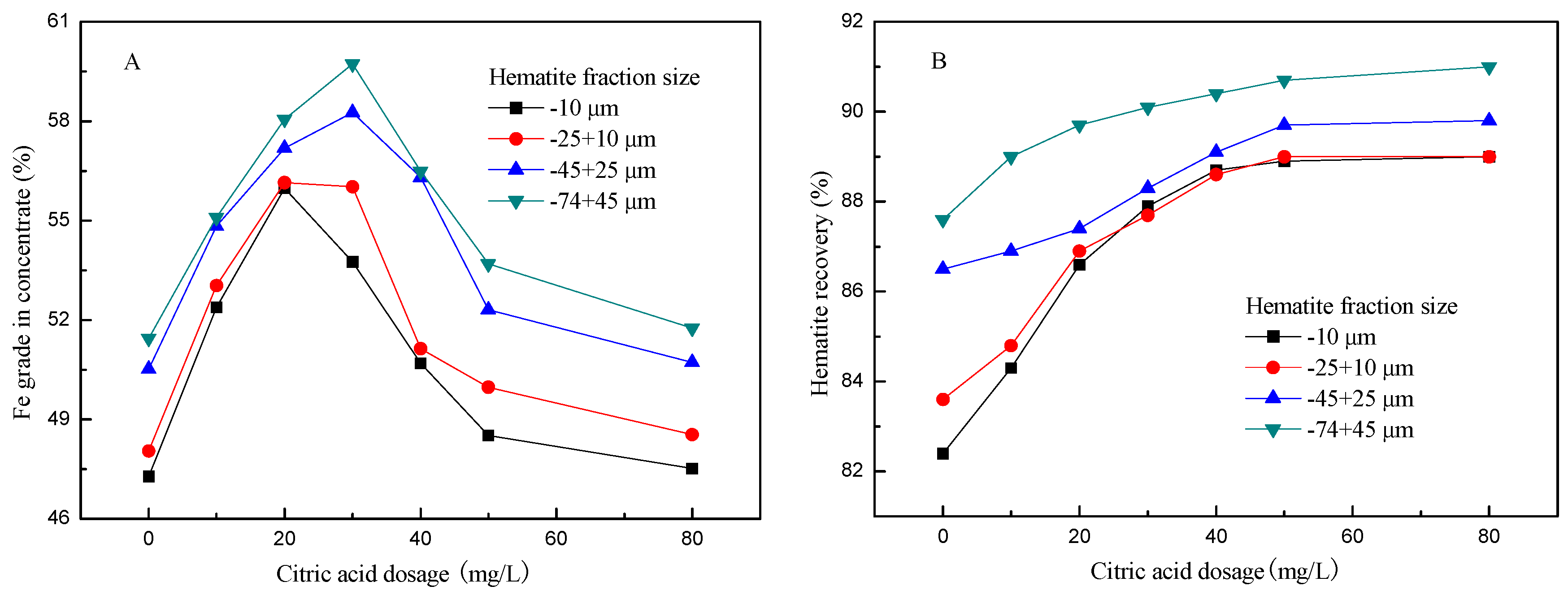
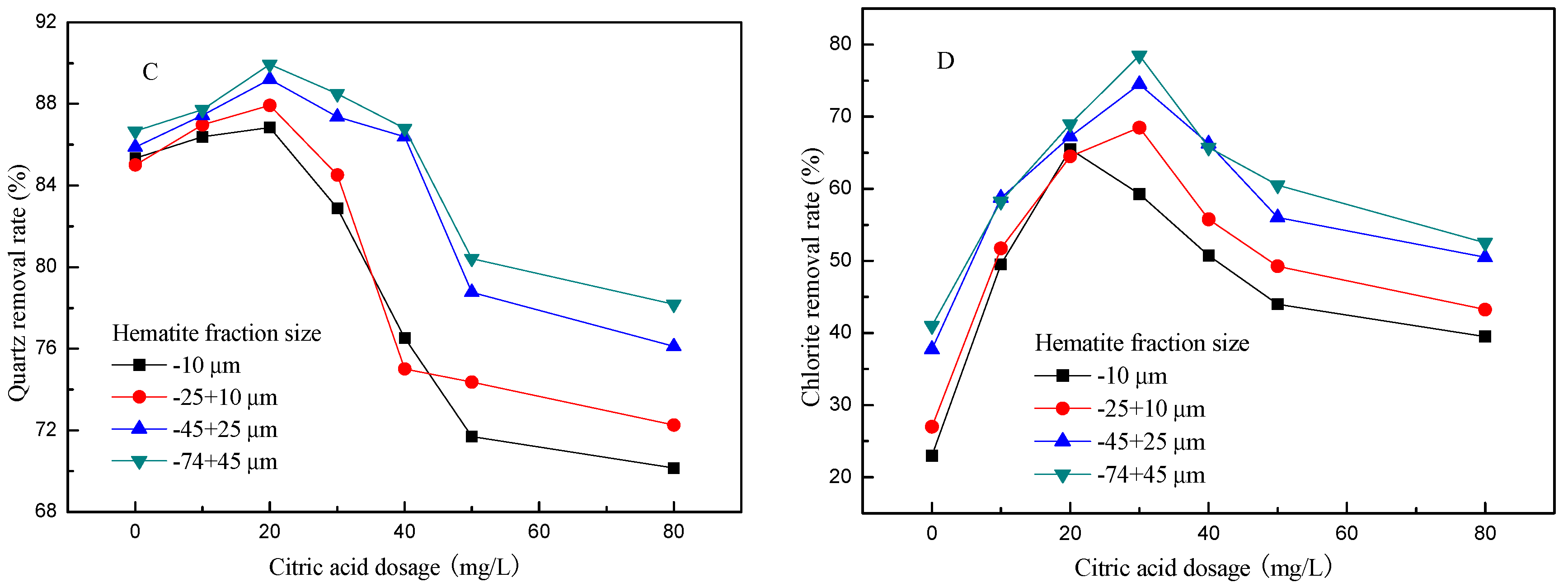
3.1. Effect of Citric Acid Dosage on Flotation of Artificial Mixed Ore
3.2. Citric Acid Dosage on the Settlement Effect of Artificial Mixed Ore
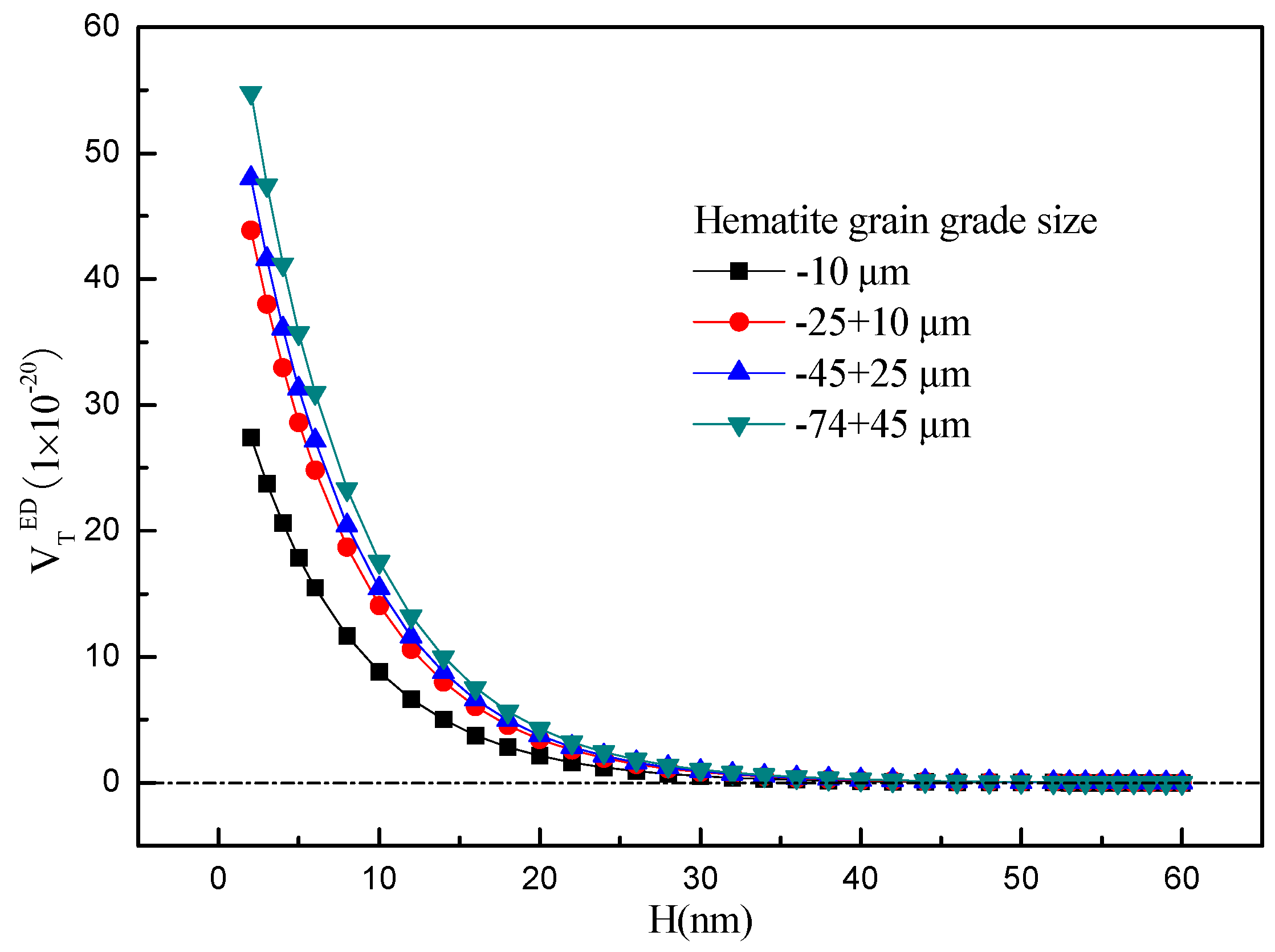
3.3. The Extended DLVO Theoretical Calculation
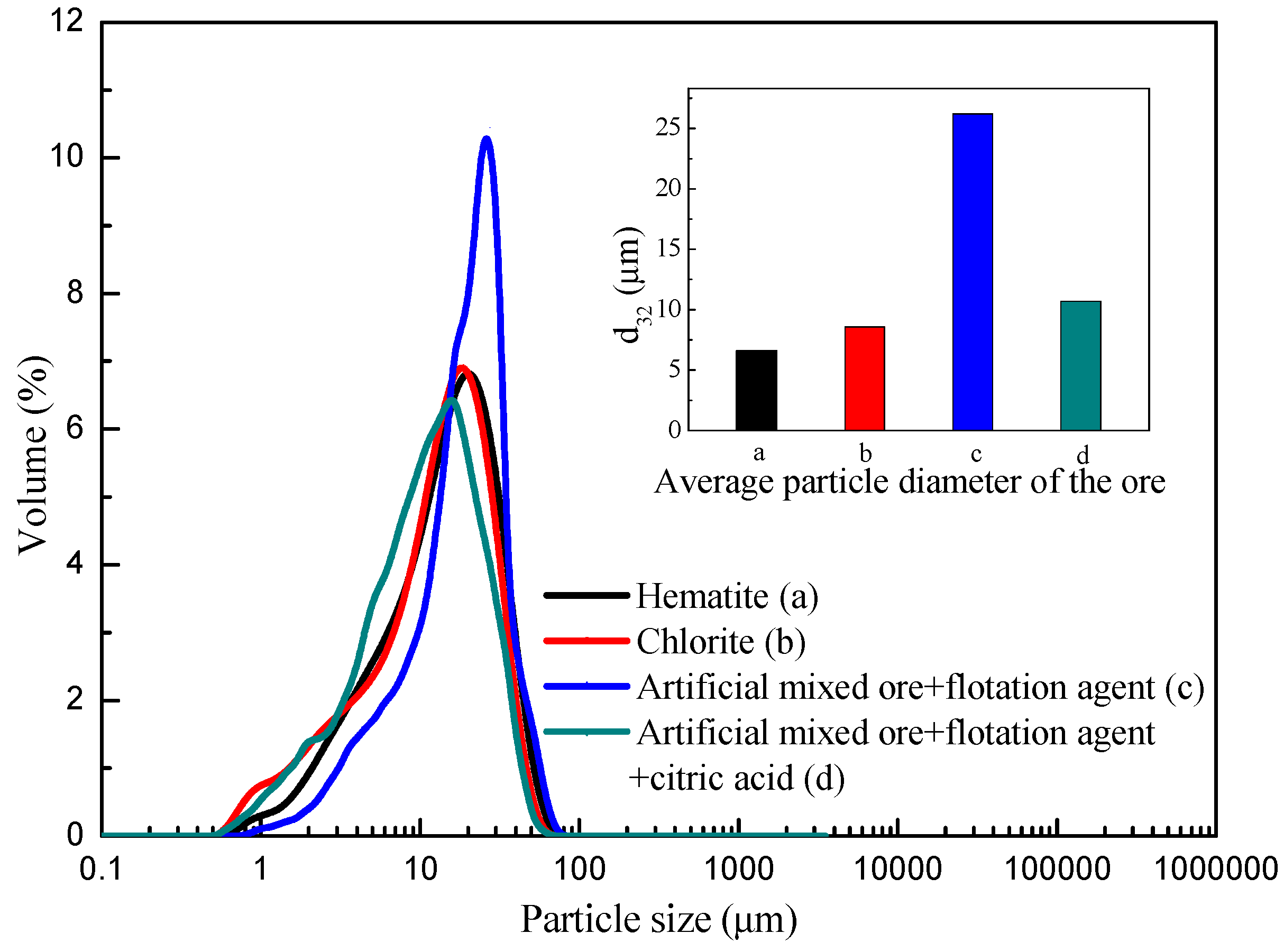
3.4. Particle Size Analysis of Artificial Mixed
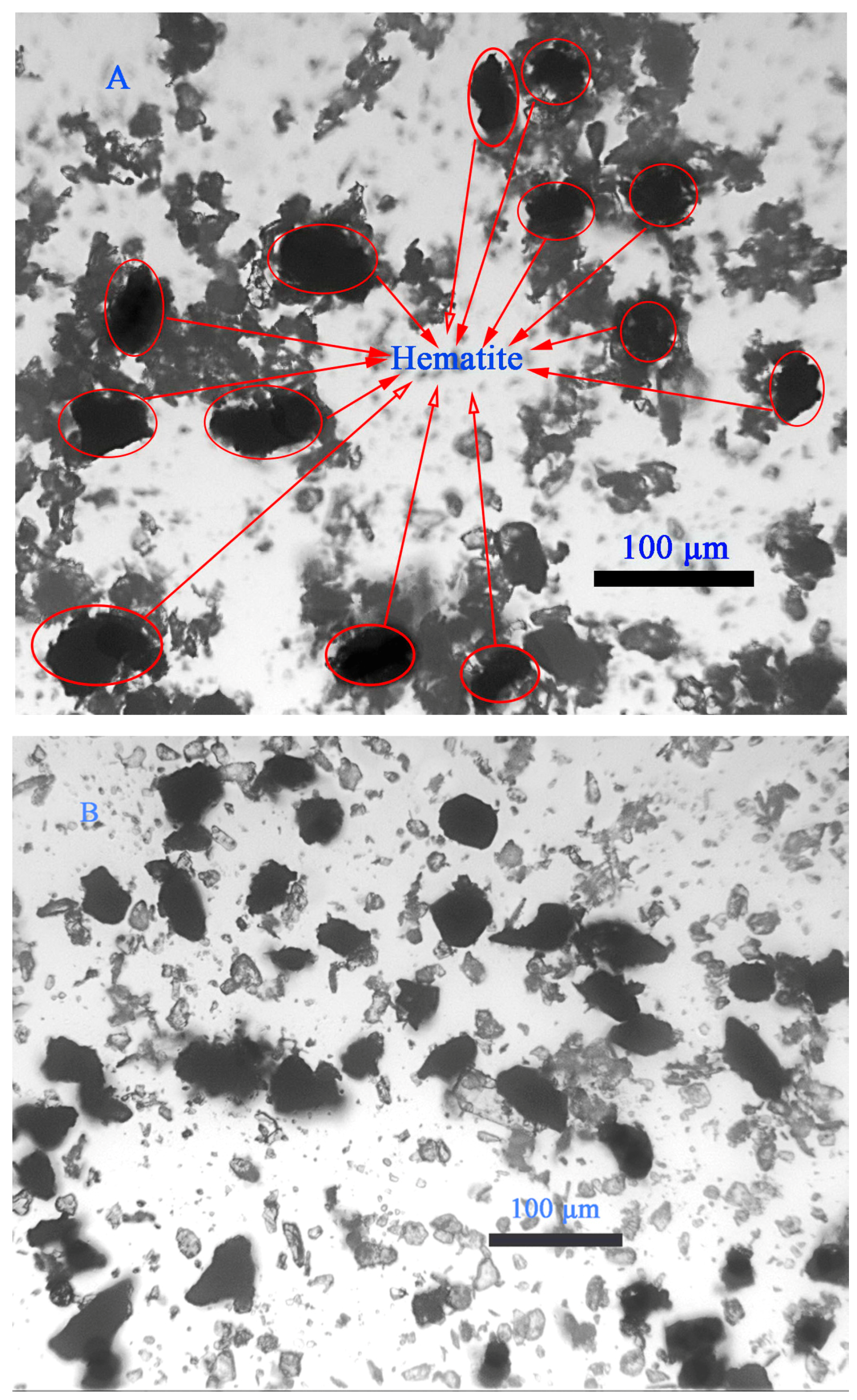
3.5. Optical Microscopy Image of Particles in the Pulp
4. Conclusions
- (1)
- In the reverse flotation process of hematite, chlorite is easily adsorbed on the surface of hematite, resulting in a lower chlorite removal rate and a poor concentrate index.
- (2)
- Extended DLVO theory studies indicate that citric acid by changing the contact angle and zeta potential of minerals, thus the interactions between hematite and chlorite is from attractive forces to repulsive forces, so that the particles are scattered between each other, and ultimately significantly improve the removal rate of chlorite in the flotation.
- (3)
- Artificial mixed ore settlement test and laser particle size analysis results showed that, with the addition of citric acid, the settlement rate of chlorite was decreased significantly, and the apparent particle size of the pulp was decreased obviously, the results further confirmed the dispersion effect of citric acid in the hematite flotation system.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, X.M.; Yin, W.Z.; Sun, C.Y.; Wang, N.L.; Ma, Y.Q.; Wang, Y.F. Improved flotation performance of hematite fines using citric acid as a dispersant. Int. J. Miner. Metall. Mater. 2016, 23, 1119–1125. [Google Scholar] [CrossRef]
- Seaman, D.R.; Lauten, R.A.; Kluck, G.; Stoitis, N. Usage of anionic dispersants to reduce the impact of clay particles in flotation of copper and gold at the Telfer mine. In Proceedings of the 11th AusIMM Mill Operators’ Conference, Hobart, Australia, 29–31 October 2012; p. 207. [Google Scholar]
- Wang, Y.; Lauten, R.A.; Peng, Y. The effect of biopolymer dispersants on copper flotation in the presence of kaolinite. Miner. Eng. 2016, 96, 123–129. [Google Scholar] [CrossRef]
- Zhang, G.F. Theory and Technology of Flotation on Bauxite Desilicated; Central South University: Changsha, China, 2001; pp. 95–99. (In Chinese) [Google Scholar]
- Wang, Y.H.; Chen, X.H.; Hu, Y.M. Effects of Sodium Carbonate on the Dispersion of Fine Alumin-Silicate Minerals. J. China Univ. Min. Technol. 2007, 36, 292. [Google Scholar]
- Liu, D.; Peng, Y. Exploring the different dispersion effect of anionic polymeric dispersant on clay minerals in the flotation using fresh and saline water. Int. Miner. Process. Congr. 2014, 2–12. [Google Scholar]
- Gan, W.; Liu, Q. Coagulation of bitumen with kaolinite in aqueous solutions containing Ca2+, Mg2+ and Fe3+: Effect of citric acid. J. Colloid Interface Sci. 2008, 324, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. An overview of the beneficiation of iron ores via reverse cationic flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Luo, X.M.; Yin, W.Z.; Yao, J.; Sun, C.Y.; Cao, Y.; Ma, Y.Q.; Hou, Y. Flotation separation of magnetic separation concentrate of refractory hematite containing carbonate with enhanced dispersion. Chin. J. Nonferrous Met. 2013, 23, 238–245. [Google Scholar]
- Liu, Q.; Zhang, Y. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Tomasik, P.; Schilling, C.H.; Jankowiak, R.; Kim, J.C. The role of organic dispersants in aqueous alumina suspensions. J. Eur. Ceram. Soc. 2003, 23, 913–919. [Google Scholar] [CrossRef]
- Yao, J.; Li, D.; Yin, W.Z.; Han, H.L. Dispersion Mechanism of Citric Acid in Flotation System of Hematite Containing Carbonate. J. Northeast. Univ. 2017, 38, 720–724. [Google Scholar]
- Zhang, Y.; Matijević, E. Stability of monodispersed hematite sols in the presence of citric acid. Colloid Polym. Sci. 1984, 262, 723–726. [Google Scholar] [CrossRef]
- Luo, X.M.; Wang, Y.F.; Wen, S.M.; Ma, M.Z.; Sun, C.Y.; Yin, W.Z.; Ma, Y.Q. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Ng, W.S.; Sonsie, R.; Forbes, E.; Franks, G.V. Flocculation/flotation of hematite fines with anionic temperature-responsive polymer acting as a selective flocculant and collector. Miner. Eng. 2015, 77, 64–71. [Google Scholar] [CrossRef]
- Zhang, M. Regulation and Control Mechanism Study on the Heterogeneous Dispersion of Serpentine and Pyrite. Ph.D. Thesis, Central South University, Changsha, China, 2010. [Google Scholar]
- Qiu, G.Z.; Hu, Y.H.; Wang, D.Z. Interactions between Particles and Flotation of Fine Particles; Central South University of Technology Press: Changsha, China, 1993; pp. 129–131. [Google Scholar]
- Hoek, E.M.; Agarwal, G.K. Extended DLVO interactions between spherical particles and rough surfaces. J. Colloid Interface Sci. 2006, 298, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Churaev, N.V. The current state of the theory of long-range surface forces. Colloid Surf. 1989, 41, 223–237. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, G.; Miller, J.D. Hydrodynamic interactions between particles in aggregation and flotation. Int. J. Miner. Process. 2003, 70, 157–170. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Giese, R.F.; Costanzo, P.M. DLVO and non-DLVO interactions in hectorite. Clays Clay Miner. 1990, 38, 151–159. [Google Scholar] [CrossRef]
- Liu, A.; Fan, M.Q.; Fan, P.P. Interaction mechanism of miscible DDA—Kerosene and fine quartz and its effect on the reverse flotation of magnetic separation concentrate. Miner. Eng. 2014, 65, 41–50. [Google Scholar] [CrossRef]


| Measuring Medium | Without Citric Acid | Citric Acid Added | ||||
|---|---|---|---|---|---|---|
| Hematite | Chlorite | Quartz | Hematite | Chlorite | Quartz | |
| Water | 36.3° | 114.6° | 128.5° | 32.2° | 123.4° | 133.2° |
| Glycerol | 61.8° | 136.2° | 136.4° | 45.1° | 111.8° | 127.5° |
| Liquid | (mJ/m2) | (mJ/m2) | (mJ/m2) | (mJ/m2) |
|---|---|---|---|---|
| Water | 72.8 | 25.5 | 21.8 | 25.5 |
| Glycerol | 64 | 3.92 | 34 | 57.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, W.; Fu, Y.; Yao, J.; Yang, B.; Cao, S.; Sun, Q. Study on the Dispersion Mechanism of Citric Acid on Chlorite in Hematite Reverse Flotation System. Minerals 2017, 7, 221. https://doi.org/10.3390/min7110221
Yin W, Fu Y, Yao J, Yang B, Cao S, Sun Q. Study on the Dispersion Mechanism of Citric Acid on Chlorite in Hematite Reverse Flotation System. Minerals. 2017; 7(11):221. https://doi.org/10.3390/min7110221
Chicago/Turabian StyleYin, Wanzhong, Yafeng Fu, Jin Yao, Bin Yang, Shaohang Cao, and Qianyu Sun. 2017. "Study on the Dispersion Mechanism of Citric Acid on Chlorite in Hematite Reverse Flotation System" Minerals 7, no. 11: 221. https://doi.org/10.3390/min7110221




