Selenium and Other Trace Element Mobility in Waste Products and Weathered Sediments at Parys Mountain Copper Mine, Anglesey, UK
Abstract
:1. Introduction
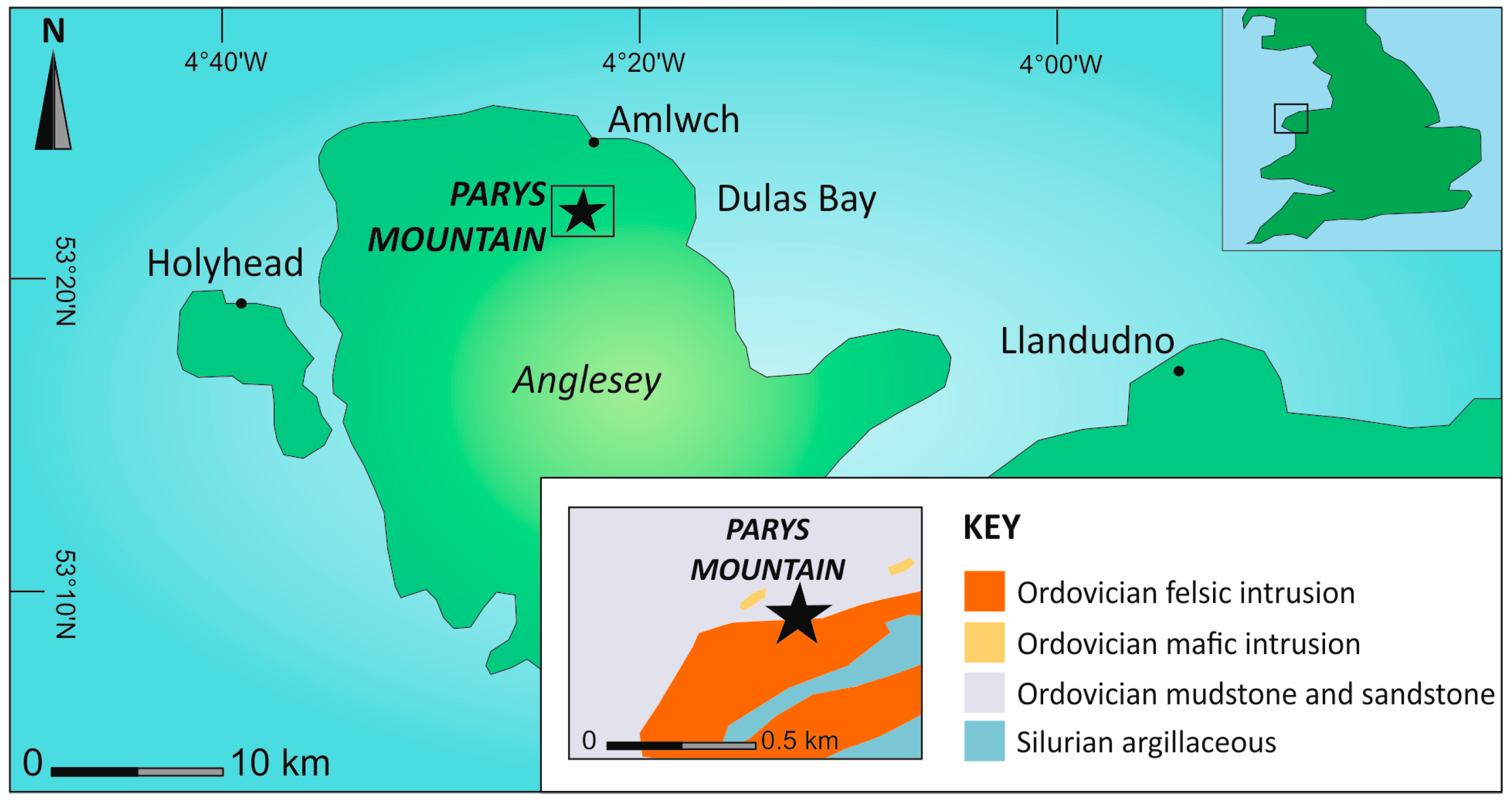
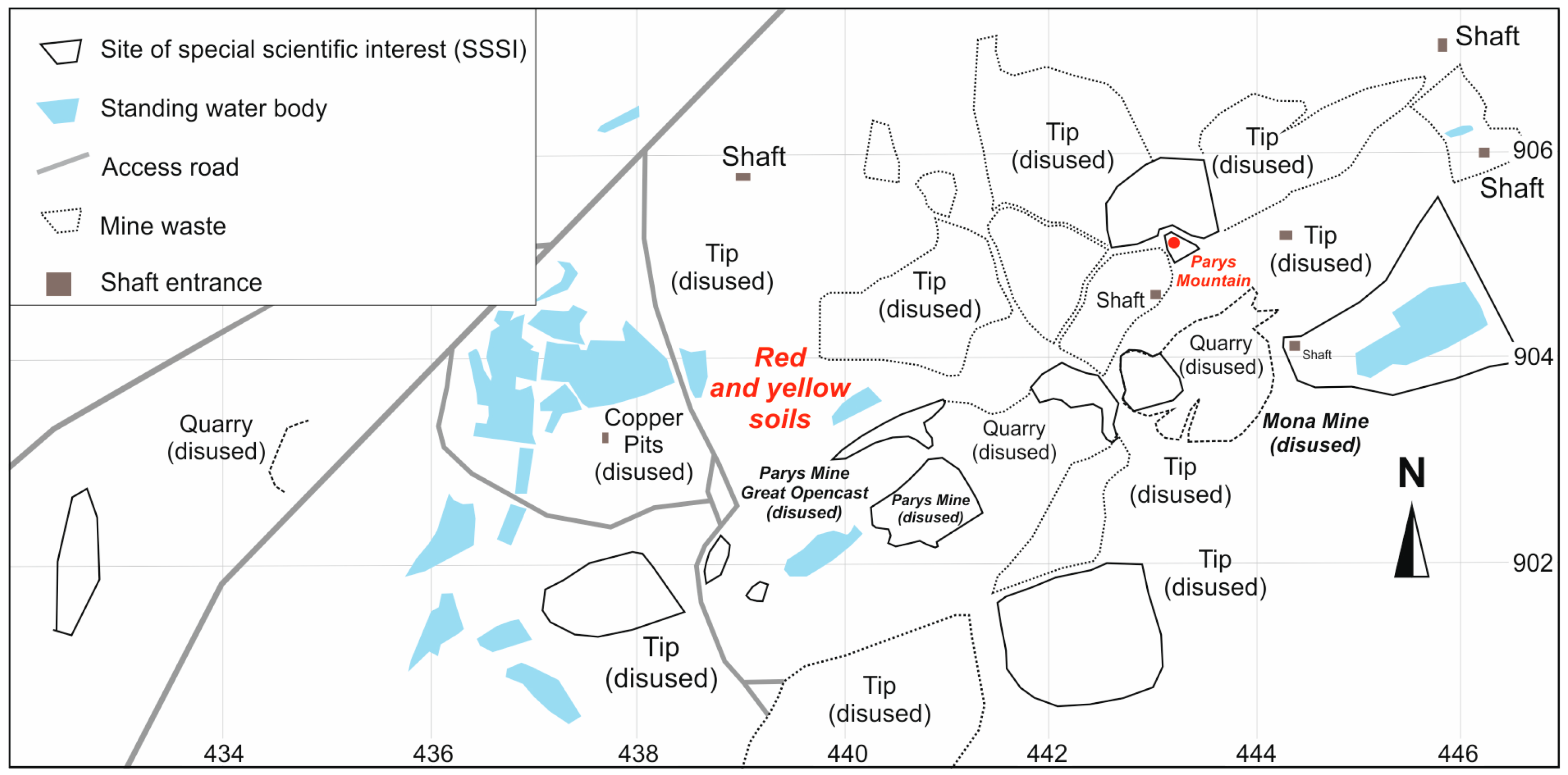
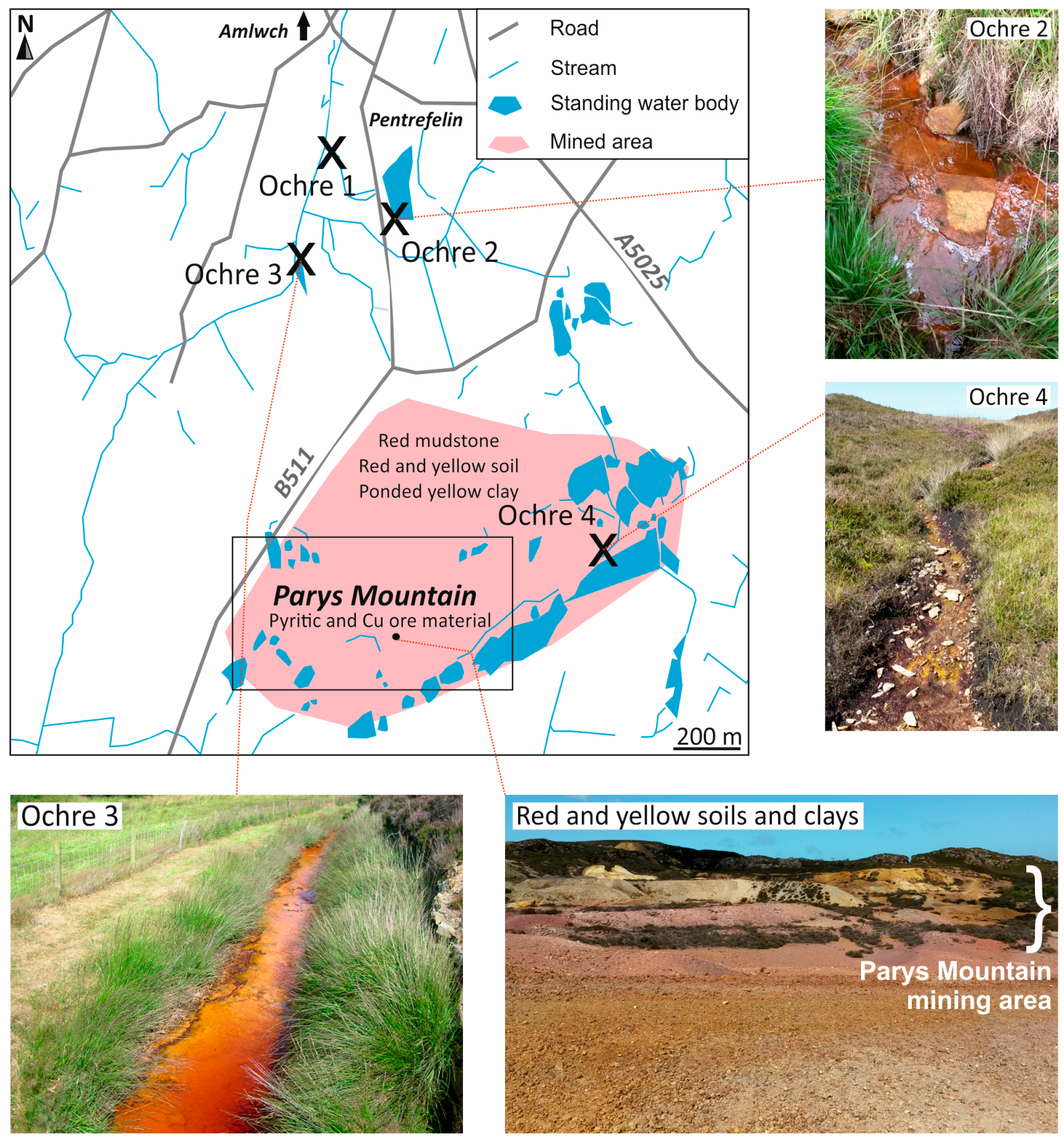
2. Study Area
3. Materials and Methods
3.1. Sampling
- Natural deposits: sulphidic bedrock (n = 4) and weathering soil (n = 8). Weathering soil formed from the natural weathering of bedrock deposits.
- Waste deposits: red smelt muds and soils (n = 7) and ochre sediments (n = 8). Red muds and soils formed from weathering of waste material from sintering, refining, and beneficiation activities, while ochre sediments formed from precipitation of Fe(III) (oxy)hydroxides from mine drainage systems.
- Underground pool water (n = 1) and surface stream water samples from mine drainage systems (n = 3) were also collected.
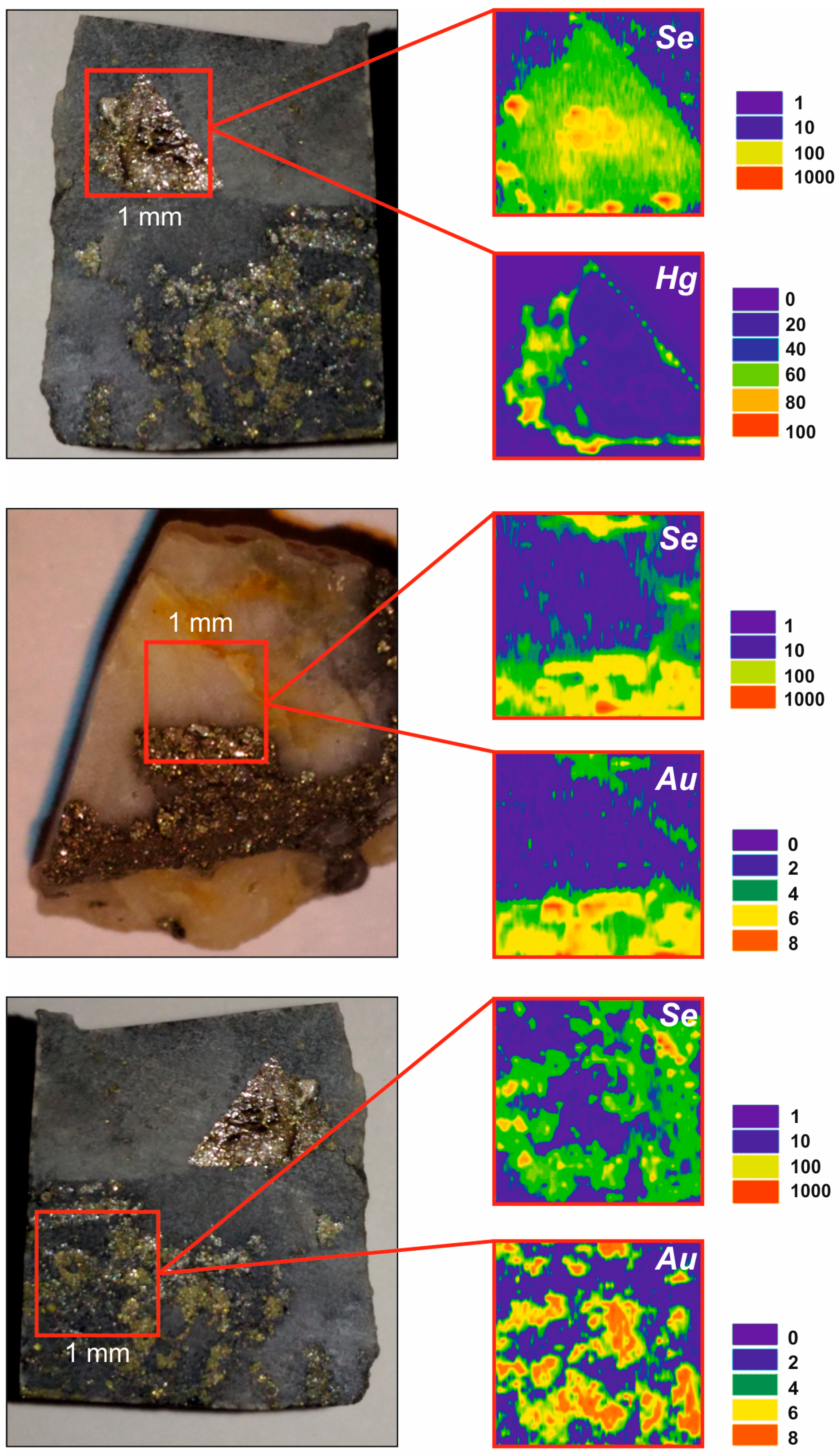
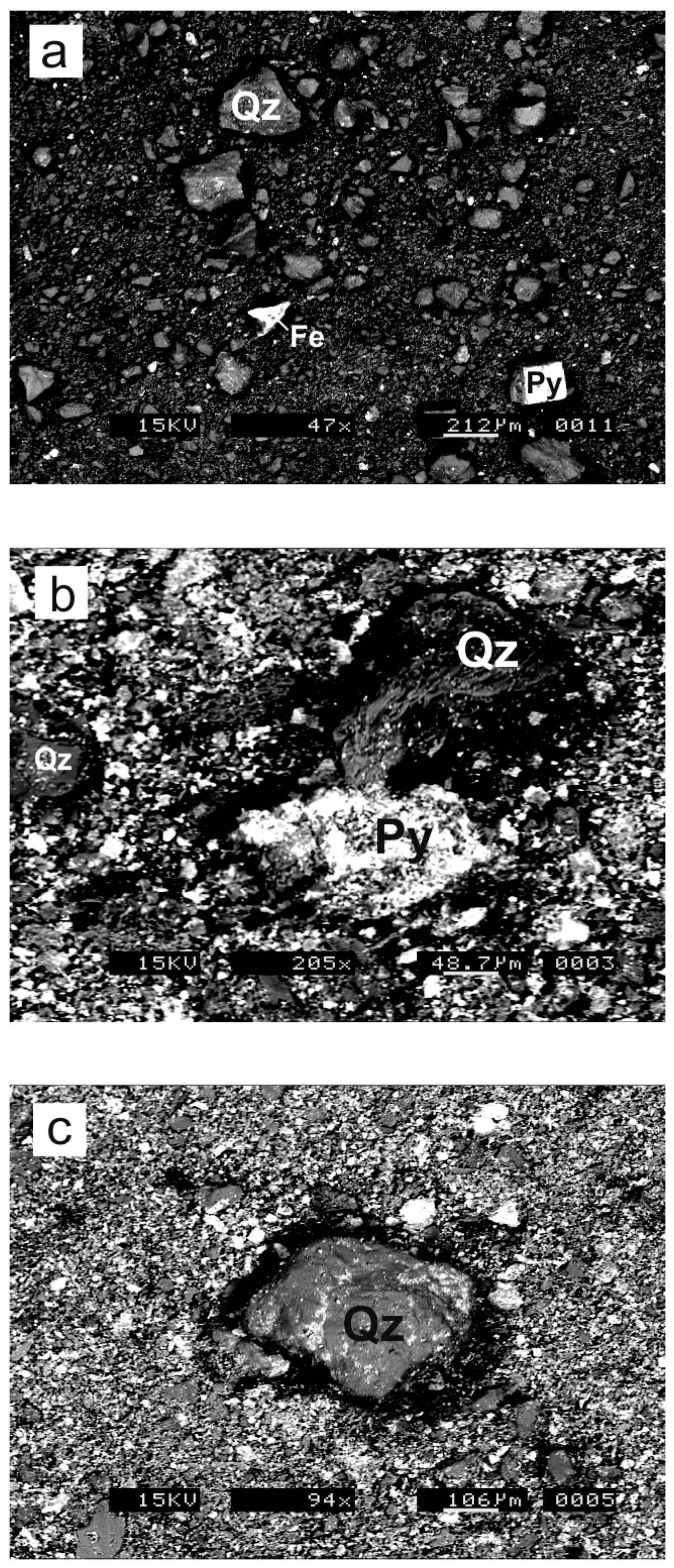
3.2. Scanning Electron Microscopy
3.3. Laser Ablation
3.4. Whole Rock and Sediment Geochemistry
3.5. Water Chemistry
4. Results
4.1. Sample Descriptions
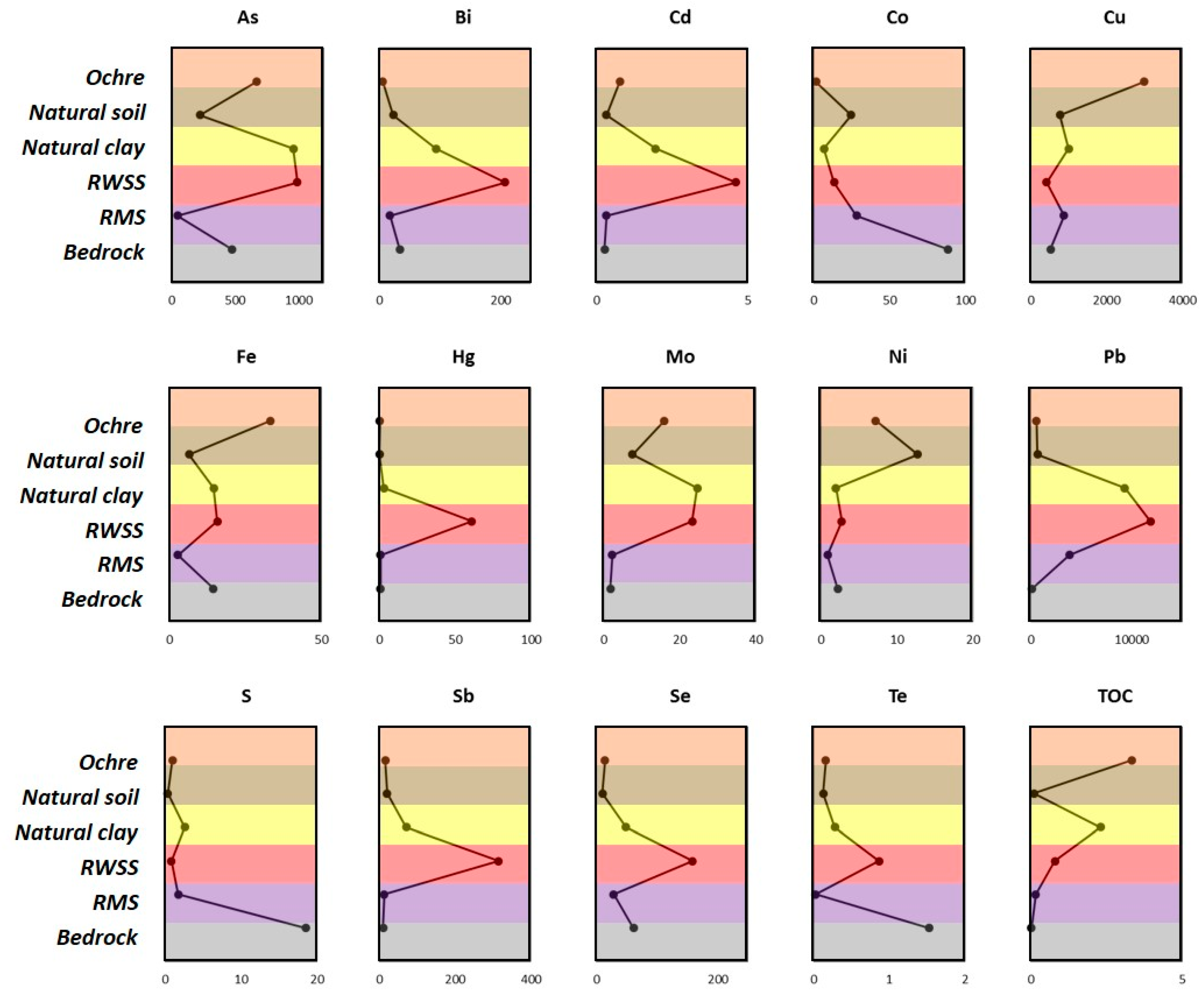
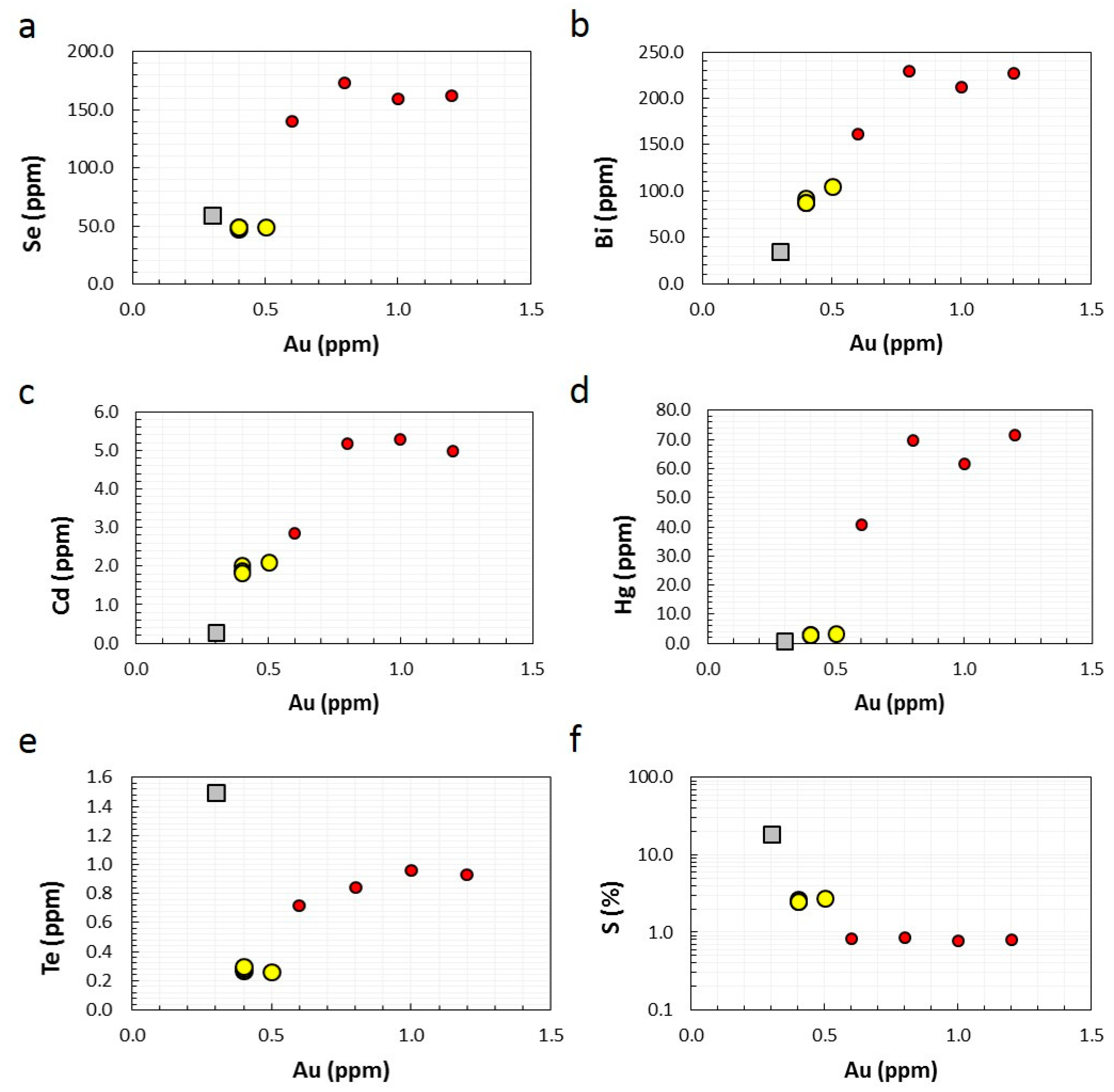
4.2. Whole Rock and Sediment Geochemistry (ICP-AES and ICP-MS)
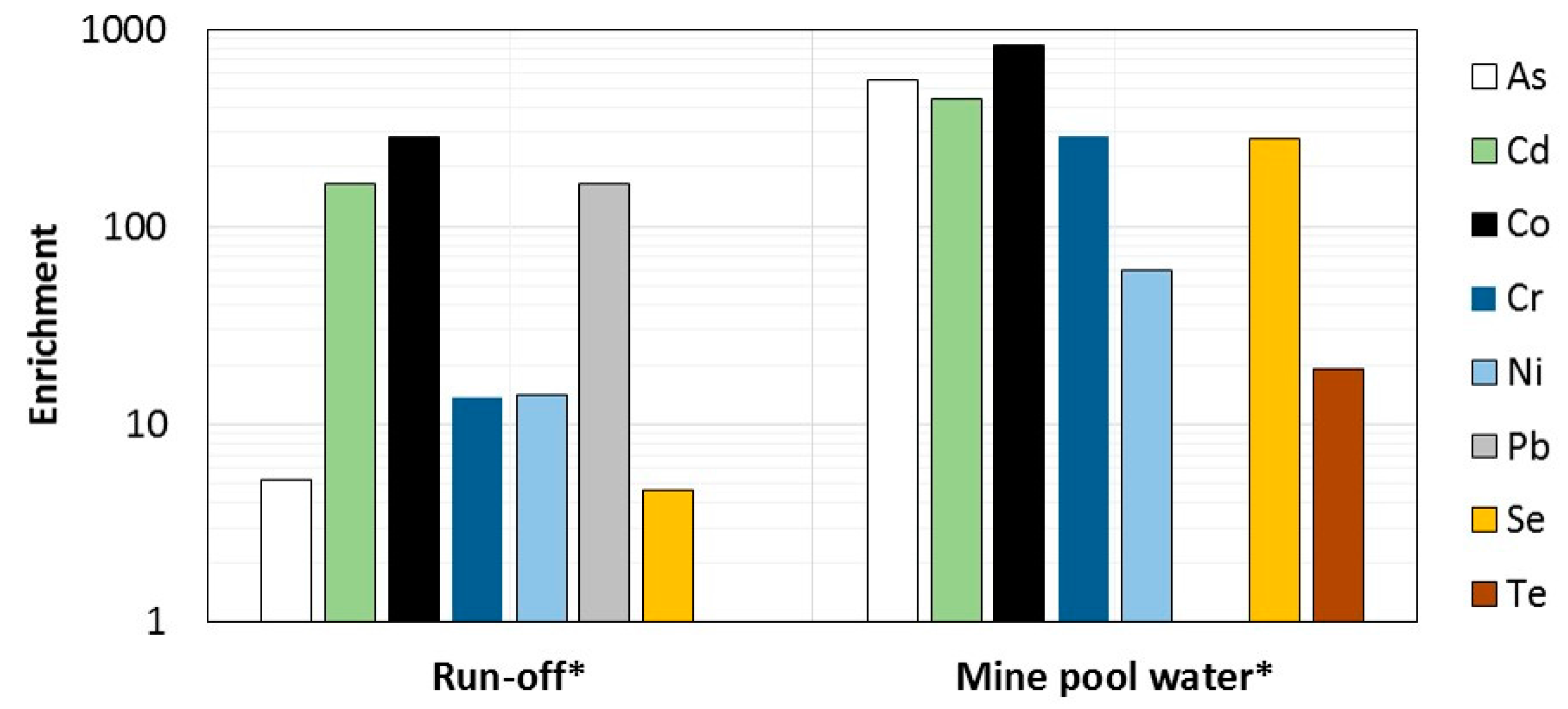
4.3. Water Chemistry
5. Discussion
5.1. Trace element Liberation and Fixation
5.2. Economic Considerations
5.3. Environmental Implications
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Šimonovičová, A.; Ferianc, P.; Vojtková, H.; Pangallo, D.; Hanajík, P.; Kraková, L.; Feketeová, Z.; Čerňanský, S.; Okenicová, L.; Žemberyová, M.; et al. Alkaline Technosol contaminated by former mining activity and its culturable autochthonous microbiota. Chemosphere 2017, 171, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G.; Paktunc, D.; Gould, W.D.; Johnson, D.B. The geochemistry of acid mine drainage. In Treatise on Geochemistry: Second Edition; Elsevier: Amsterdam, The Netherlands, 2013; Volume 11, pp. 131–190. ISBN 9780080983004. [Google Scholar]
- Fleming, G.A.; Walsh, T. Selenium occurrence in certain Irish soils and its toxic effects on animals. Proc. R. Ir. Acad. Sect. B 1957, 58, 151–166. [Google Scholar]
- Rogers, P.A.M.; Arora, S.P.; Fleming, G.A.; Crinion, R.A.P.; McLaughlin, J.G. Selenium toxicity in farm animals: Treatment and prevention. Ir. Vet. J. 1990, 43, 151–153. [Google Scholar]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453. [Google Scholar] [CrossRef]
- Carrillo-González, R.; Šimůnek, J.; Sauvé, S.; Adriano, D. Mechanisms and pathways of trace element mobility in soils. Adv. Agron. 2006, 91, 111–178. [Google Scholar]
- Alloway, B.J. Heavy Metals in Soils; Springer: Houten, The Netherlands, 2013; p. 368. ISBN 978-94-007-4469-1. [Google Scholar]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, T.; Koschinsky, A.; Bau, M. The ratio of tellurium and selenium in geological material as a possible paleo-redox proxy. Chem. Geol. 2014, 376, 44–51. [Google Scholar] [CrossRef]
- Parnell, J.; Brolly, C.; Spinks, S.; Bowden, S. Selenium enrichment in Carboniferous Shales, Britain and Ireland: Problem or opportunity for shale gas extraction? Appl. Geochem. 2016, 66, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Bullock, L.A.; Parnell, J. Selenium and molybdenum enrichment in uranium roll-front deposits of Wyoming and Colorado, USA. J. Geochem. Explor. 2017, 180, 101–112. [Google Scholar] [CrossRef]
- Department for Environment, Food & Rural Affairs (DEFRA). A Review of National Resource Strategies and Research; Department for Environment, Food and Rural Affairs: London, UK, 2012; p. 109.
- Moss, R.; Tzimas, E.; Kara, H.; Willis, P.; Koorosh, J. Critical Metals in Strategic Energy Technologies—Assessing Rare Metals as Supply-Chain Bottlenecks in Low-Carbon Energy Technologies; Publications Office of the European Union: Luxembourg, 2011; p. 164. ISBN 9789279206993. [Google Scholar]
- Parnell, J.; Bellis, D.; Feldmann, J.; Bata, T. Selenium and tellurium enrichment in palaeo-oil reservoirs. J. Geochem. Explor. 2015, 148, 169–173. [Google Scholar] [CrossRef]
- Brown, R.D. Selenium and Tellurium. In US Geological Survey Minerals Yearbook; United States Geological Survey: Reston, VA, USA, 2000; pp. 1–8. [Google Scholar]
- Plant, J.A.; Bone, J.; Voulvoulis, N.; Kinniburgh, D.G.; Smedley, P.L.; Fordyce, F.M.; Klinck, B. Arsenic and Selenium. In Treatise on Geochemistry: Second Edition; Elsevier: Amsterdam, The Netherlands, 2013; Volume 11, pp. 13–57. ISBN 9780080983004. [Google Scholar]
- Swallow, M. Parys Mountain a mine in prospect. Min. Mag. 1990, 163, 334–336. [Google Scholar]
- O’Brien, W. Bronze Age Copper Mining in Britain and Ireland; Shire Publications Ltd.: Buckinghamshire, UK, 1996; p. 64. [Google Scholar]
- Summers, N. Parys Underground Grosuplup/Grwp Tanddaearol Parys. Available online: http://www.parysmountain.co.uk/ (accessed on 31 August 2017).
- Anglesey Mining plc. Parys Mountain Mineral Resource Estimate at $80 per tonne GMPV* Cut-Off. Available online: http://angleseymining.co.uk/projects/parysresources.html (accessed on 31 August 2017).
- Barrett, T.J.; MacLean, W.H.; Tennant, S.C. Volcanic sequence and alteration at the Parys Mountain volcanic-hosted massive sulfide deposit, Wales, United Kingdom: Applications of immobile element lithogeochemistry. Econ. Geol. 2001, 96, 1279–1305. [Google Scholar] [CrossRef]
- Barrett, T.J.; Tennant, S.C.; Daliran, F. Volcanic sequence and position of massive sulfides and quartz-rock at Parys Mountain, Wales. In Proceedings of the SEG 2009 Conference, Houston, TX, USA, 25–30 October 2009; Conference Draft Abstract. Society of Exploration Geophysicists: Townsville, Australia, 2009. [Google Scholar]
- Younger, P.L.; Jenkins, D.A.; Rees, S.B.; Robinson, J.; Jarvis, A.P.; Ralph, J.; Johnston, D.N.; Coulton, R.H. Mine waters in Wales: Pollution, risk management and remediation. In Urban Geology in Wales; Nichol, D., Bassett, M.G., Deisler, V.K., Eds.; National Museums and Galleries of Wales Geological Series Number 23; National Museums and Galleries of Wales: Cardiff, UK, 2004; pp. 138–154. [Google Scholar]
- Younger, P.; Potter, H.A.B. Parys in springtime: Hazard management and steps towards remediation of the UK’s most polluted acidic mine discharge. In Proceedings of the 9th International Conference on Acid Rock Drainage (ICARD), Ottawa, ON, Canada, 20–26 May 2012; p. 12. [Google Scholar]
- Parrish, R.R. The age of the volcanic rocks at the Parys Mountain VMS deposit, Wales. In Geology and Mineralization of the Parys Mountain Polymetallic Deposit; Barrett, T.J., Tennant, S.C., MacLean, W.H., Eds.; Unpublished Report 1992, v. 1 (text) and v. 2 (appendix); Anglesey Mining plc: Bangor, UK, 1992. [Google Scholar]
- Walton, K.C.; Johnson, D.B. Microbiological and chemical characteristics of an acidic stream draining a disused copper mine. Environ. Pollut. 1992, 76, 169–175. [Google Scholar] [CrossRef]
- Fuge, R.; Pearce, F.M.; Pearce, N.J.G.; Perkins, W.T. Acid mine drainage in wales and influence of ochre precipitation on water chemistry. In Environmental Geochemistry of Sulfide Oxidation; American Chemical Society: Washington, DC, USA, 1994; pp. 261–274. ISBN 0-8412-2772-1. [Google Scholar]
- Parkman, R.H.; Curtis, C.D.; Vaughan, D.J.; Charnock, J.M. Metal fixation and mobilisation in the sediments of the Afon Goch estuary—Dulas Bay, Anglesey. Appl. Geochem. 1996, 11, 203–210. [Google Scholar] [CrossRef]
- Hodson, M.E.; Valsami-Jones, E.; Cotter-Howells, J.D. Bonemeal additions as a remediation treatment for metal contaminated soil. Environ. Sci. Technol. 2000, 34, 3501–3507. [Google Scholar] [CrossRef]
- Whiteley, J.D.; Pearce, N.J.G. Metal distribution during diagenesis in the contaminated sediments of Dulas Bay, Anglesey, N. Wales, UK. Appl. Geochem. 2003, 18, 901–913. [Google Scholar] [CrossRef]
- Wilson, B.; Pyatt, F.B. Heavy metal dispersion, persistance, and bioccumulation around an ancient copper mine situated in Anglesey, UK. Ecotoxicol. Environ. Saf. 2007, 66, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.P.G.; Bradshaw, A.D. Heavy-metal tolerance in populations of Agrostis tenuis Sibth and other grasses. New Phytol. 1965, 64, 131–143. [Google Scholar] [CrossRef]
- Gartside, D.W.; McNeilly, T. Genetic studies in heavy metal tolerant plants. Heredity (Edinb.) 1974, 33, 303–308. [Google Scholar] [CrossRef]
- Humphreys, M.O.; Nicholls, M.K. Relationships between tolerance to heavy metals in Agrostis capillaris L. {A. tenuis Sibth.). New Phytol. 1984, 98, 177–190. [Google Scholar]
- Ye, Z.H.; Baker, A.J.M.; Wong, M.H.; Willis, A.J. Copper tolerance, uptake and accumulation by Phragmites australis. Chemosphere 2003, 50, 795–800. [Google Scholar] [CrossRef]
- Alloway, B.J.; Davies, B.E. Trace element content of soils affected by base metal mining in Wales. Geoderma 1971, 5, 197–208. [Google Scholar] [CrossRef]
- White, R.A. The Behaviour of the Rare Earth Elements in Ochreous Mine Drainage. Unpubl. Ph.D. Thesis, University of Wales, Aberystwyth, UK, 2000. [Google Scholar]
- Zijlstra, J.J.P.; Dessì, R.; Peretti, R.; Zucca, A. Treatment of percolate from metal sulfide mine tailings with a permeable reactive barrier of transformed red mud. Water Environ. Res. 2010, 82, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Jayasankar, K.; Ray, P.K.; Chaubey, A.K.; Padhi, A.; Satapathy, B.K.; Mukherjee, P.S. Production of pig iron from red mud waste fines using thermal plasma technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Ma, Y.; Si, C.; Lin, C. Comparison of copper scavenging capacity between two different red mud types. Materials (Basel) 2012, 5, 1708–1721. [Google Scholar] [CrossRef] [Green Version]
- Lockwood, C.L.; Stewart, D.I.; Mortimer, R.J.G.; Mayes, W.M.; Jarvis, A.P.; Gruiz, K.; Burke, I.T. Leaching of copper and nickel in soil-water systems contaminated by bauxite residue (red mud) from Ajka, Hungary: The importance of soil organic matter. Environ. Sci. Pollut. Res. 2015, 22, 10800–10810. [Google Scholar] [CrossRef] [PubMed]
- United States Geological Survey (USGS). Characterization of Mine Waste at the Elizabeth Copper Mine; Open-File Report 990-564; USGS: Orange County, VT, USA, 1999.
- Liao, M.; Xie, X.M. Effect of heavy metals on substrate utilization pattern, biomass, and activity of microbial communities in a reclaimed mining wasteland of red soil area. Ecotoxicol. Environ. Saf. 2007, 66, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Koljonen, T.; Gustavsson, N.; Noras, P.; Tanskanen, H. Geochemical Atlas of Finland: Preliminary aspects. J. Geochem. Explor. 1989, 32, 231–242. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 4, pp. 1–51. ISBN 9780080983004. [Google Scholar]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskiene, V.; Halamic, J.; et al. Geochemical Atlas of Europe. Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005; p. 525. [Google Scholar]
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Boyle, R.W. The Geochemistry of Gold and Its Deposits; Geological Survey of Canada Bulletins 280; Geological Survey of Canada: Ottawa, ON, Canada, 1979; p. 584.
- Malisa, E.P. The behaviour of selenium in geological processes. Environ. Geochem. Health 2001, 23, 137–158. [Google Scholar] [CrossRef]
- Kovalenker, V.A. Ore-forming systems of epithermal gold–silver deposits: Concepts, reality, and models. In Problems of Ore Geology, Petrology, and Geochemistry; IGEM RAN: Moscow, Russia, 2004; pp. 160–183. (In Russian) [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Spry, P.G. Preface-Special issue: Telluride and selenide minerals in gold deposits—How and why? Mineral. Petrol. 2006, 87, 163–169. [Google Scholar] [CrossRef]
- Budyak, A.E.; Bryukhanova, N.N. Selenium, bismuth, and mercury in black shale-hosted gold deposits of different genetic types. Geochem. Int. 2012, 50, 791–797. [Google Scholar] [CrossRef]
- Parnell, J.; Spinks, S.; Bellis, D. Low-temperature concentration of tellurium and gold in continental red bed successions. Terra Nova 2016, 28, 221–227. [Google Scholar] [CrossRef]
- Ivanov, V.V. Ekologicheskaya Geokhimia Elementov; Ecology 1-6: Moscow, Russia, 1996. (In Russian) [Google Scholar]
- Savage, K.S.; Tingle, T.N.; O’Day, P.A.; Waychunas, G.A.; Bird, D.K. Arsenic speciation in pyrite and secondary weathering phases, Mother Lode Gold District, Tuolumne County, California. Appl. Geochem. 2000, 15, 1219–1244. [Google Scholar] [CrossRef]
- Moses, C.O.; Kirk Nordstrom, D.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Paktunc, D.; Kingston, D.; Pratt, A.; McMullen, J. Distribution of gold in pyrite and in products of its transformation resulting from roasting of refractory gold ore. Can. Mineral. 2006, 44, 213–227. [Google Scholar] [CrossRef]
- Paktunc, D. Speciation of Arsenic in Pyrite by Micro-X-ray Absorption Fine-Structure Spectroscopy (XAFS); Argonne National Laboratory (ANL): Argonne, IL, USA, 2008; pp. 8–10.
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Younger, P.L. Possible Environmental Impact of the Closure of Two Collieries in County Durham. Water Environ. J. 1993, 7, 521–531. [Google Scholar] [CrossRef]
- Younger, P.L.; Sherwood, J.M. The cost of decommissioning a coalfield: Potential environmental problems in County Durham. Miner. Plan. 1993, 57, 26–29. [Google Scholar]
- Banks, D.; Younger, P.L.; Arnesen, R.T.; Iversen, E.R.; Banks, S.B. Mine-water chemistry: The good, the bad and the ugly. Environ. Geol. 1997, 32, 157–174. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur isotope geochemistry of sulfide minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Parks, G.A. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Blowes, D.W.; Condon, P.D.; Ptacek, C.J. Managing pore-water quality in mine tailings by inducing microbial sulfate reduction. Environ. Sci. Technol. 2009, 43, 7086–7091. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.B.J.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Martínez, M.; Giménez, J.; De Pablo, J.; Rovira, M.; Duro, L. Sorption of selenium(IV) and selenium(VI) onto magnetite. Appl. Surf. Sci. 2006, 252, 3767–3773. [Google Scholar] [CrossRef]
- Jordan, N.; Lomenech, C.; Marmier, N.; Giffaut, E.; Ehrhardt, J.J. Sorption of selenium(IV) onto magnetite in the presence of silicic acid. J. Colloid Interface Sci. 2009, 329, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Missana, T.; Alonso, U.; Scheinost, A.C.; Granizo, N.; García-Gutiérrez, M. Selenite retention by nanocrystalline magnetite: Role of adsorption, reduction and dissolution/co-precipitation processes. Geochim. Cosmochim. Acta 2009, 73, 6205–6217. [Google Scholar] [CrossRef]
- Kearney, J. Anglesey Mining plc Annual Report; Anglesey Mining plc: Amlwch, UK, 2003; p. 27. [Google Scholar]
- Avala Resources Ltd. Avala Resources Intersects 83.5 Meters of 1.28 g/t Gold from a Depth of 2.5 Meters at Kraku Pestar in Serbia. Available online: https://www.yahoo.com/news/Avala-Resources-Intersects-iw-3806121069.html (accessed on 31 August 2017).
- Alchemy Resources Ltd. June 2012 Quarterly Report. Available online: http://www.asx.com.au/asxpdf/20120720/pdf/427hl8hl7zjfjy.pdf (accessed on 31 August 2017).
- Minnett, M.; Sandeman, H.; Wilton, D.; Section, M.D. Geochemistry of the Host Rocks and Timing of Gold-Electrum Mineralization at the Viking Property, Newfoundland; Newfoundland and Labrador Department of Natural Resources: St. John’s, NL, Canada, 2012; pp. 61–84.
- Joseph Ifeanyi, N.; Okengwu, K.; Adesoji, A. predicting the concentration characteristics of itakpe iron ore for cut-off grade estimation. J. Appl. Sci. Environ. Manag. 2013, 17, 315–319. [Google Scholar] [CrossRef]
- Arne, D.; Mackie, R.; Pennimpede, C.; Jones, S. The Kiyuk Lake Deposit—A New Style of Gold Mineralization in the Western Churchill Province of Southern Nunavut, Canada; Society of Economic Geologists Annual Convention poster presentation: Whistler, BC, Canada, 2013. [Google Scholar]
- Nevada Copper. Reserves and Resources. Available online: http://www.nevadacopper.com/s/Resources.asp (accessed on 31 August 2017).
- Red Metal Ltd. Maronan Deposit—Summary of Inferred Resource Estimates. Available online: http://www.redmetal.com.au/images/stories/pdf/A_1510_RDM_ASX_Maronan_JORC_Inferred_Resource_October2015.pdf (accessed on 31 August 2017).
- Bhappu, R.B. Economic Recovery of Selenium by Flotation from Sandstone Ores of New Mexico. Available online: https://geoinfo.nmt.edu/publications/monographs/circulars/58/ (accessed on 31 August 2017).
- Saunders, J.A.; Hofstra, A.H.; Goldfarb, R.J.; Reed, M.H. Geochemistry of Hydrothermal Gold Deposits. In Treatise on Geochemistry: Second Edition; Elsevier: Amsterdam, The Netherlands, 2013; Volume 13, pp. 383–424. ISBN 9780080983004. [Google Scholar]
- Hedin, R.S. Long-term minimization of mine water treatment costs through passive treatment and production of a saleable iron oxide sludge. In Mining Meets Water–Conflicts and Solutions, Proceedings of the International Mine Water Association Annual Conference, Freiberg, Germany, 11–15 July 2016; IMWA: Wendelstein, Germany, 2016; pp. 1267–1273. [Google Scholar]
- Dahlkamp, F.J. Uranium Deposits of the World: USA and Latin America; Springer Science & Business Media: Berlin, Germany, 2010; p. 520. ISBN 9783540785552. [Google Scholar]
- Abzalov, M.Z. Sandstone-hosted uranium deposits amenable for exploitation by In Situ leaching technologies. Appl. Earth Sci. 2012, 121, 55–64. [Google Scholar] [CrossRef]
- Legendre, G.R.; Runnels, D.D. Removal of dissolved molybdenum from wastewater by precipitates of ferric iron. Environ. Sci. Technol. 1975, 9, 744–748. [Google Scholar] [CrossRef]
- Harris, R.E. Industrial Minerals and Construction Materials of Wyoming; Reprint No. 50; The Geological Survey of Wyoming: Laramie, WY, USA, 1992; pp. 91–102.
- Ramirez, P.; Rogers, B.P. Selenium in a Wyoming grassland community receiving wastewater from an in situ uranium mine. Arch. Environ. Contam. Toxicol. 2002, 42, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ewers, W. Standards, guidelines and legislative regulatory concerning metals and their compounds. In Metals and Their Compounds in the Environment; Merian, E., Ed.; Wiley-VCH: Weinheim, Germany, 1991; pp. 707–711. [Google Scholar]
- Environmental Protection Agency (EPA). Contaminants and Remedial Options at Selected Metal-Contaminated Sites; US-EPA. 540. R-95/512; EPA: Washington, DC, USA, 1995; p. 268.
- Iversen, E.R.; Johannessen, M. Water Pollution from Abandoned Mines; Norsk Institutt for Vannforskning (NIVA) Report O-82068; NIVA: Oslo, Norway, 1984; p. 62. (In Norwegian) [Google Scholar]
- Schartau, A.K.L. Extent and effects of environmental toxins in fresh water. Vann 1992, 27, 41–48. (In Norwegian) [Google Scholar]
- Marchand, L.; Sabaris, C.Q.; Desjardins, D.; Oustrière, N.; Pesme, E.; Butin, D.; Wicart, G.; Mench, M. Plant responses to a phytomanaged urban technosol contaminated by trace elements and polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2016, 23, 3120–3135. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A.; Zornoza, R. Creation of Technosols to Decrease Metal Availability in Pyritic Tailings with Addition of Biochar and Marble Waste; Geophysical Research Abstracts 19; EGU2017-3276; 2017 EGU General Assembly; EGU: Vienna, Austria, 2017. [Google Scholar]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullock, L.A.; Parnell, J.; Perez, M.; Feldmann, J.; Armstrong, J.G. Selenium and Other Trace Element Mobility in Waste Products and Weathered Sediments at Parys Mountain Copper Mine, Anglesey, UK. Minerals 2017, 7, 229. https://doi.org/10.3390/min7110229
Bullock LA, Parnell J, Perez M, Feldmann J, Armstrong JG. Selenium and Other Trace Element Mobility in Waste Products and Weathered Sediments at Parys Mountain Copper Mine, Anglesey, UK. Minerals. 2017; 7(11):229. https://doi.org/10.3390/min7110229
Chicago/Turabian StyleBullock, Liam A., John Parnell, Magali Perez, Joerg Feldmann, and Joseph G. Armstrong. 2017. "Selenium and Other Trace Element Mobility in Waste Products and Weathered Sediments at Parys Mountain Copper Mine, Anglesey, UK" Minerals 7, no. 11: 229. https://doi.org/10.3390/min7110229





