1. Introduction
The Fukushima Daiichi Nuclear Power Station (F1NPS) was damaged by the earthquake and subsequent tsunami that struck Japan on 11 March 2011, and cleanup has been conducted by the Tokyo Electric Power Company, Inc. (TEPCO, Tokyo, Japan). The radioactive contaminated water that accumulated inside the reactor, turbine, and other buildings is being processed, and the resulting freshwater is used as a coolant for the fuel in the damaged reactor. The composition of the radioactive contaminated water has varied since the accident [
1]. At the early stage, this contaminated water mixed with seawater originating from the tsunami, and also with the coolant used for the damaged reactor fuel. When TEPCO began the cleanup operations, the main aim was to remove Cesium (Cs) from the water. Currently, the radioactive contaminated water is treated by a “Treatment Facility” which has a “Cs Adsorption Apparatus” and a “Decontamination Facility” for removing radioactive Cs, together with a “Desalination Facility”, and a “Multi-nuclide Removal Facility”. The Multi-nuclide Removal Facility consists of “Pre-treatment Facilities” and an “Adsorption Tower”. This facility removes most of the radioactive material from the contaminated water, but does not extract
3H. In the Adsorption Tower of the Multi-nuclide Removal Facility, Strontium (Sr) is removed by hydrous sodium titanate (SrTreat
®) [
2], which is supplied as amorphous titanium oxide granules with the chemical formula Na
xTi
yO
z [
3].
Radioactive liquid waste has been generated during operation of nuclear power stations and reprocessing of spent nuclear fuels, in the production of nuclear materials for military applications, in mining and milling of Uranium (U), as well as during industrial and institutional application of radioisotopes. Several treatment options for these wastes are selected based on their consideration of chemical and biological characteristics of each waste, the costs associated with the treatment and disposal of the secondary liquid waste, and safety and risk assessment of physical protection and safeguards at a radioactive liquid waste management facility [
4,
5]. Titanate has been used in processes such as in-tank precipitation at the Savannah River Site in the United States of America, and for packing to a column at the Japan Atomic Energy Research Institute in Japan [
6,
7].
Defining the characteristics of spent adsorbents is important for effective disposal and deposition of the F1NPS waste. In particular, the amount of radioactive nuclides sorbed by adsorbents must be calculated accurately. In previous studies [
7,
8,
9], the Sr sorbed on SrTreat
® has only been quantified in the treatment of radioactive liquid waste generated by conventional nuclear facilities. In addition, those studies conducted sorption experiments with nitrate aqueous solutions and limited concentrations of metals in solution. However, the experimental conditions used in those studies are not suitable for quantifying the content of radioactive Sr in SrTreat
® used in the treatment of the radioactive contaminated water at F1NPS.
The composition of the radioactive contaminated water at F1NPS has varied as the coolant has changed from seawater to treated water. The process for treatment of radioactive contaminated water has changed over this period, and the adsorbents for the Adsorption Tower have been changed. As a result, the amount of sorbed elements on SrTreat
® varies according to the effects of competing soluble elements in the radioactive contaminated water. In addition, the ability of sorbing elements of SrTreat
® will change during use, and sorbed elements of SrTreat
® will volatilize and elute during storage after use. Thus, it is difficult to calculate a radioactive inventory of used SrTreat
® at processing from the reference data, because these matters cause uncertainties in the evaluation of the composition of the used SrTreat
®. However, the IAEA reports that knowing the radioactive inventory of waste is necessary for the planning of decontamination activities. These activities include the sizing and design of processing facilities for waste storage and disposal [
10]. Therefore, a method to characterize the radioactivity by analysis must be considered. However, the strong radioactivity of the used SrTreat
® precludes sampling and accurate radioactivity analysis.
Although the operating conditions of the Multi-nuclide Removal Facility were not sufficiently considered in the planning stages, the long-term exposure of SrTreat
® to the treated water can remove several elements from contaminated water at the upstream equipment. The pH of the treated water passing through the SrTreat
® in the Adsorption Tower is adjusted to approximately 12 at a “Carbonate Coprecipitation Treatment Facility”, which is part of the Pre-treatment Facilities [
11]. After use, the spent SrTreat
® is discharged and temporarily stored in high-integrity containers in the Adsorption Tower at the Multi-nuclide Removal Facility until further disposal and deposition. During use, it is expected that the reactive groups and crystallization of SrTreat
® change as a result of the exposure to treated water and radiation. During the storage period, the materials sorbed to SrTreat
® will elute into residual water, and the SrTreat
® will undergo radiolysis by the sorbing radioactive nuclides. In addition, the SrTreat
® will crystallize due to the heat generated by exothermic reactions on
90Sr.
Here, we aim to determine the Sr sorption ability of SrTreat®, which is important in calculating an initial radioactive inventory of used SrTreat®. This makes the present study relevant when considering variations in the Sr sorption ability of SrTreat®, and aids understanding of the causes of variation. The study used Sr sorption studies, acid–base titration, and X-ray photoelectron spectroscopy (XPS) of the as-received SrTreat®. Materials included two kinds of titanates, as well as SrTreat® following exposure to the simulated treated water (the TEPCO term for water treated and discharged from the Treatment Facility).
2. Samples
2.1. SrTreat®
The SrTreat
® is a commercial granular product made by Fortum, Finland. The granule size varies from 0.30 to 0.85 mm [
3]. To avoid hydrolysis reactions (1), Fortum adds 0.1 mol/L NaOH aqueous solution in the final process of manufacturing [
3].
From batchwise sorption experiments with metal nitrate solutions, it was found that sorption with the as-received SrTreat
® decreases in the order H
+ > Ca
2+ > Sr
2+ >> Mg
2+ > NH
4+ > K
+ > Li
+ [
12].
2.2. Sodium Titanate and Titanium Oxide
At the Multi-nuclide Removal Facility, the composition of SrTreat® was changed from NaxTiyOz to HxTiyOz by hydrolysis. To understand this change in more detail, customized sodium titanate and titanium oxide (Fuji Sangyo Co., Yokohama, Japan) were used. By comparing experimental and analytical Sr sorption behaviors on sodium titanate and titanium oxide, it becomes possible to understand how Na influences the adsorption affinity of Sr to SrTreat®.
Sodium titanate was synthesized by adding NaOH aqueous solution (0.8 mol/L) to dilute Ti{OCH(CH3)2}4 (tetraisopropyl orthotitanate) in C6H5CH3 (toluene) (2 mol-Ti/L) in equal volume. All reagents were purchased Wako Pure Chemical Industries, Ltd. (Osaka, Japan). After 4 h mixing, white precipitate was collected by filtration with filter paper No.5A (Advantec Co., Ltd., Tokyo, Japan). Then, white precipitate was washed with distilled water, dried at 105 °C, and crushed to 0.30–0.85 mm. Titanium oxide was synthesized by a similar procedure with synthesized sodium titanate. However, this process used diluted water in the place of NaOH aqueous solution.
2.3. Solid Phase of Titanates
The X-ray diffractometry (XRD) patterns of the three titanates were obtained to verify that the as-received SrTreat® was amorphous, and that the ordered titanates were not appreciably crystalized. The samples were well-ground in an agate mortar to a particle size below 10 µm, and then placed in a reflection-free sample holder. The crystallographic structure of the samples was identified in the 2θ = 2°–70° range by a Rigaku XRD instrument (RINT-2100, Rigaku Co., Ltd., Akishima-shi, Japan) with Cu-Kα radiation.
The XRD patterns of the samples are shown in
Figure 1. The XRD pattern of the as-received SrTreat
® featured a broad diffraction peak at 9°, and small peaks at 18°–19°, 29°, and 34°. The peaks at 9° and 29° agreed with those reported in the patent [
3], but the other peaks are not reported there. The unidentified small peak at 19° appeared at this position in the XRD pattern of sodium titanate. The continuous diffraction peak at 45°–49° appeared in the XRD patterns of all samples. This peak would appear to be associated with the 48.02° peak on the anatase spectrum.
The absence of noticeable peaks in the spectra of all titanates in the present study confirmed the approximately amorphous state of the fine crystal structure, and sodium titanate was confirmed as similar to SrTreat® in crystallinity.
2.4. Chemical Compositions of Samples
The chemical compositions and impurity amounts in the titanates investigated in the present study were analyzed by energy dispersive X-ray (EDX) spectroscopy operated at 15 kV on a Swift ED 3000 OXFORD instrument (Abingdon, UK). For the EDX, a granule of sample was affixed on a specimen support (Nisshin EM Co., Ltd., Tokyo, Japan) by aluminum-based carbon tape (Nisshin EM Co., Ltd., Tokyo, Japan), and three granules of each of the samples were analyzed.
The ratios of the elements in the samples measured by EDX are listed in
Table 1. Only Ti and Na were detected in the samples. Titanium oxide contained a small amount of Na, and the sample was considered to be pure titanium oxide. The chemical compositions of the titanates in this study were estimated to be Na
xH
(2−x)TiO
3, assuming that TiO
3 combines with one of the two of Na or H.
Table 2 shows the estimated chemical compositions. This assumption is suggested by the ideal chemical composition of perovskite (CaTiO
3).
3. Experimental
Solutions for all experiments were prepared by dissolving the components in ion-exchanged water. All reagents were special grade (Japanese Industrial Standards), and were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan.
3.1. Changes to SrTreat® after the Simulated Treated Water Exposure
To understand the initial reaction of the SrTreat
® in the Adsorption Tower at the Multi-nuclide Removal Facility, a solution was passed through a column filled with SrTreat
®. The composition of the solution matched that of the treated water flowing to the Adsorption Tower during the initial period of the SrTreat
® usage in the Multi-nuclide Removal Facility. When the Multi-nuclide Removal Facility commenced operation in March of 2013, the radioactive contaminated water was still mixed with the seawater washed ashore by the tsunami. This water also contained coolant from the damaged reactors. The equipment upstream of the Adsorption Tower did not remove Na
+, K
+, and Cl
− ions from the radioactive contaminated water, and a large part of the content of the treated water flowing to the Adsorption Tower contained these ions. To match this, the simulated treated water was prepared by dissolving NaCl and KCl in ion-exchanged water, and the concentrations of Na
+, K
+, and Cl
− in the simulated treated water were 0.16, 0.0034, and 0.15 mol/L respectively. These values are in accordance with typical seawater ratios [
13]. The pH of the simulated treated water was adjusted to 12 using NaOH. This value replicates the pH of the Pre-treatment Facility water.
Table 3 details the particulars of the simulated treated water flowing through the SrTreat
® column.
Ion chromatography was used to analyze the Na+, K+, and Cl− concentrations in the simulated treated water, as well as and their fractions of the effluent through the column. Concentrations of Ti in the fractions of effluent through the column were analyzed by Inductively Coupled Plasma–Optical Emission Spectrometry (ICP–OES) SPS5000 (Seiko Instruments Inc., Chiba, Japan), and the pH was measured by an HM-30R meter (DKK-TOA Co., Tokyo, Japan) with a glass electrode.
3.2. Sorption Experiments
Batchwise sorption experiments were carried out with Sr solution in order to estimate the amount of Sr sorbed at various pHs with the as-received SrTreat®, sodium titanate, titanium oxide, and SrTreat® after the simulated treated water exposure.
The experimental conditions were determined by batchwise Sr sorption experiments for shaking for 5–360 min at room temperature for two basic titanates. The experiments were then conducted at various pH levels. Batchwise experiments were conducted on the as-received SrTreat® by shaking with 20 mL of 50 mmol/L SrCl2 solution and 0.89 g of titanate. Experiments on sodium titanate were conducted with 10 mL of 20 mmol/L and 0.10 g of titanate. The solid and liquid phases were separated by filtering through a polytetrafluoroethylene membrane with a pore size of 0.45 µm. Concentrations of Sr and Na in the liquid phases were measured by Atomic Absorption Spectroscopy (AAS) iCE3300 (Thermo Fisher Scientific Inc., Waltham, MA, USA).
The stock solutions contained 0.1–10 mmol/L SrCl
2 and 0.1 mol/L NaCl. Fifty milliliters of a stock solution was added to 0.050 g of a sample weighed in a polypropylene bottle. After adding HCl or NaOH solution to maintain the pH, the bottle was shaken at 25 °C for 5 h. The solid and liquid phases were separated by filtering through a polytetrafluoroethylene membrane with a pore size of 0.45 µm. The pH and concentrations of Sr in the liquid phases were measured by a pH meter with a glass electrode, and by ICP–OES. When the pH of the liquid exceeded 9, the experiments were conducted in the absence of CO
2, because the Sr
2+ in the stock solution precipitates as SrCO
3 above a pH of 10 [
14]. Before adding the stock and pH-adjusted solutions to the bottle with a sample, both the stock and pH-adjusted solutions were purged with N
2 gas for 30 min. The addition of the solutions and pH measurements were then conducted in a glove bag under an N
2 atmosphere.
3.3. Acid–Base Titration
To evaluate the amount of cations and anions that reacted with the as-received SrTreat®, sodium titanate, titanium oxide, and SrTreat® after the simulated treated water exposure, an acid–base titration curve was created for each sample: a suspension with 0.075 g of a sample in 75 mL of 0.1 mol/L NaCl solution, and a solution of titrants HCl and NaOH. The suspension was placed in a glass vessel with a stirring bar supported over its base by a fish-clip® to avoid disturbing the structure of the granules, and purged with moist N2 gas to remove any CO2. Titrants were added to the suspension by a fixed-volume micropipette (Eppendorf Reference 2, Eppendorf AG, Hamburg, Germany). The pH of the suspension was measured by the pH meter using a glass electrode. The pH was assumed to be equilibrated when the pH varied by less than ±0.1 over a 30-min interval.
3.4. XPS Analysis
Analysis by X-ray photoelectron spectrometer (XPS) was conducted to understand details of the mechanism of Sr sorption with the titanate investigated in the present study. This was performed by analyzing the binding condition of the elements composing the surface of the samples. The analysis was carried out on a JPS-9200, (JEOL Ltd. Tokyo, Japan). The XPS spectra were recorded with Mg-Kα radiation (hv = 1253.6 eV), with the pressure of the analyzer chamber maintained at around 5 × 10−8 Pa, and the shift of the binding energy was corrected by the C1s peak of 285.0 eV as an internal standard.
4. Results and Discussion
4.1. SrTreat® Reaction by Exposure to the Simulated Treated Water
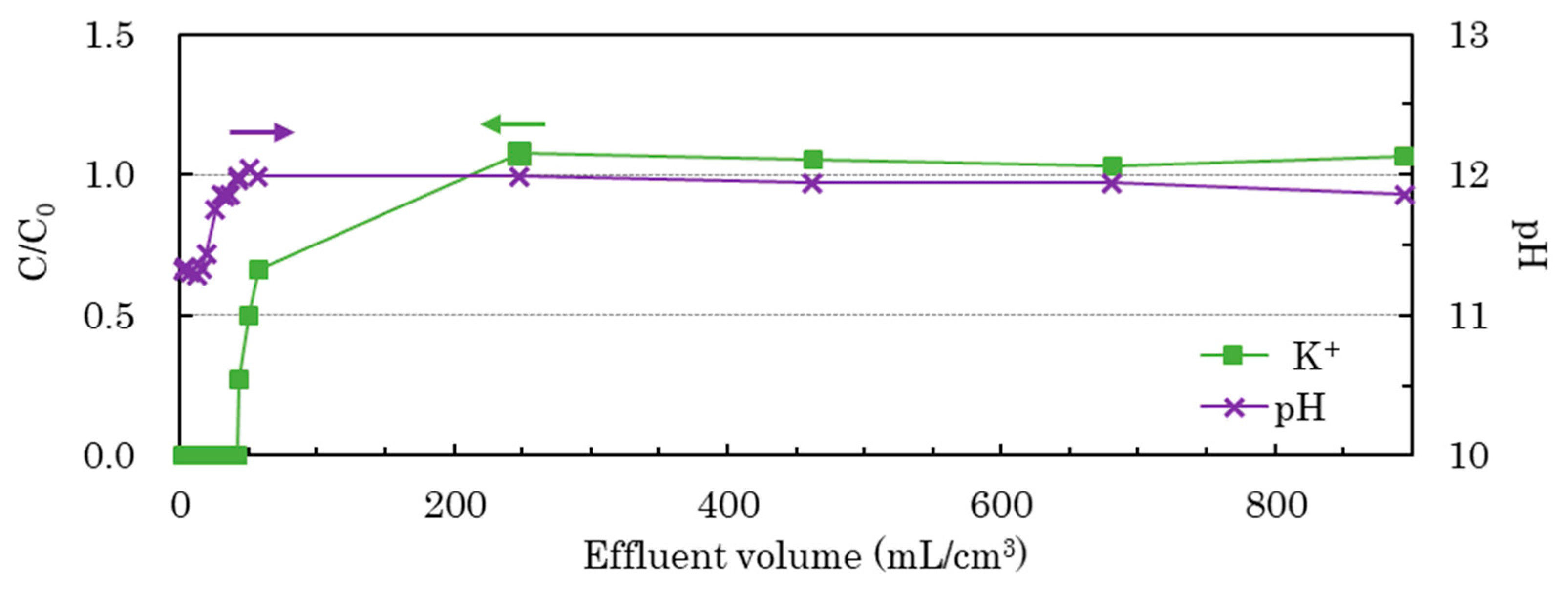
The Na
+ and Cl
− concentrations in the fractions of effluent through the column were not different from those in the simulated treated water, and the K
+ concentration and pH in the fractions varied as shown in
Figure 2. Along the left vertical axis of
Figure 2, the K
+ concentration of the fraction of effluent through the column (
C, mol/L) is normalized with that in the simulated treated water (
C0, mol/L). The horizontal axis is calculated as the volume of the sum of effluent from the column (mL) divided by the volume of the column filled with SrTreat
® (cm
3).
The C/C0 of K+ in the elute was not calculated at the start, because the concentrations of K+ in the fractions were below the determination limit. After the initial period, the C/C0 increased and saturated at 1.0. This implies that K+ was sorbed to saturation on the SrTreat®. Initially, the pH was lower in the fractions of effluent than in the simulated treated water, because H+ ions were released from SrTreat®. The concentration of Ti in the fractions of effluent was below 0.58 µmol/L, and the total Ti discharge from the column was calculated as 7.5 mg. This is negligible compared to the weight of SrTreat® filled in the column (34 g).
After the simulated treated water exposure, the ratios of Ti, Na, and K on the SrTreat® measured by EDX were 69, 27, and 1.6 mol %, respectively. The chemical composition of SrTreat® after the simulated treated water exposure was estimated as Na0.39K0.023H1.6TiO3, assuming the chemical composition of NaxKyH(2−x−y)TiO3. This is the likely chemical composition of the SrTreat® in the Adsorption Tower at the Multi-nuclide Removal Facility after 4 days of use.
4.2. Sr Sorption
Figure 3 shows the time dependency of the distribution ratio on the as-received SrTreat
® and sodium titanate. A distribution ratio (
Kd) was calculated by Equation (2);
where
Ctot and
Caq are the concentrations of Sr in the stock solution (mmol/L) and the liquid phase of the mixture (mmol/L), V is the total volume (mL), and m is mass of titanate (g).
Kd of two titanates increased depending on shaking time, and became constant at 240 min.
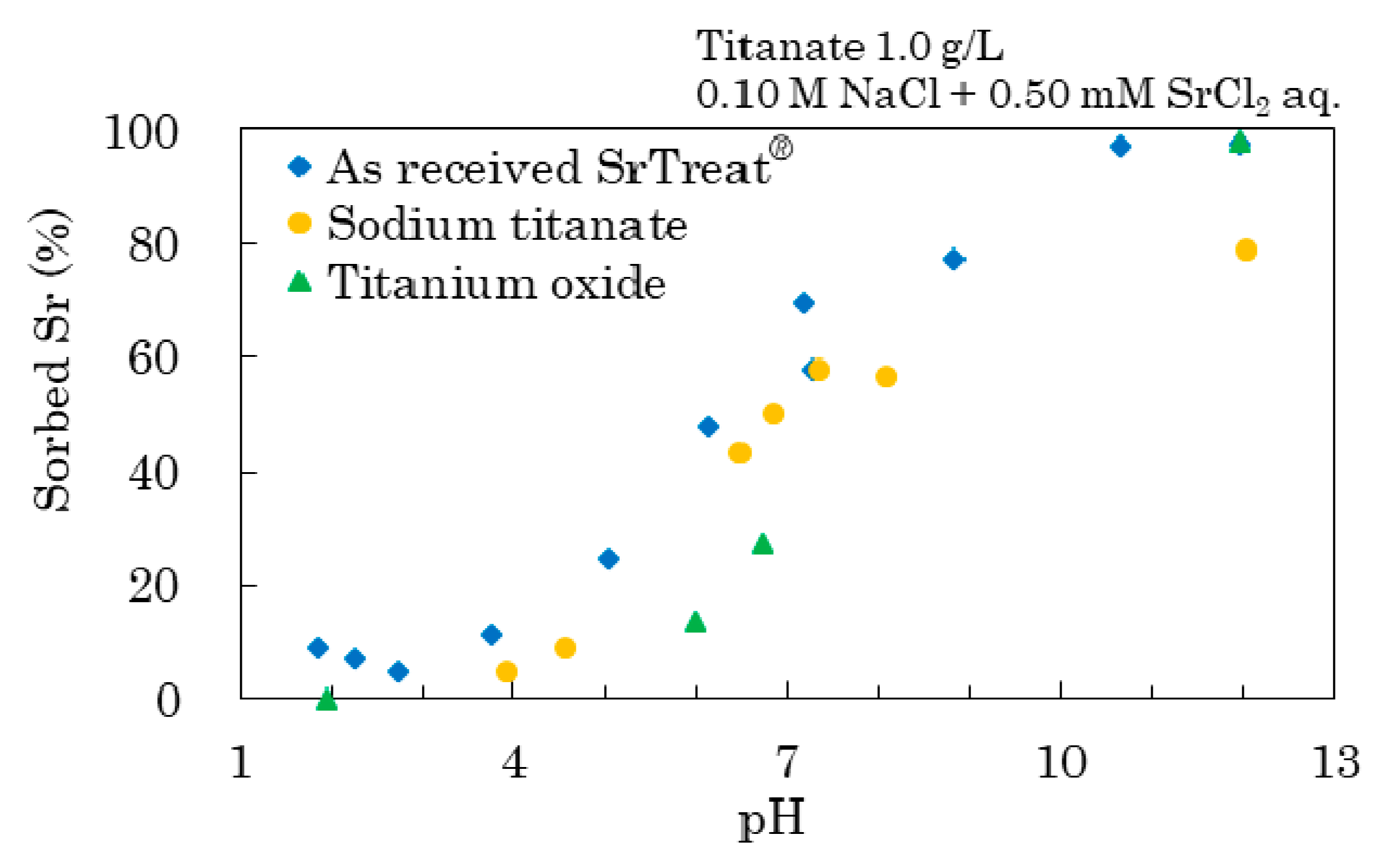
Figure 4 shows the percentage of sorbed Sr with the titanates calculated as:
The as-received SrTreat® and sodium titanate were not sorbed on Sr in the 2–4 pH range. The percentage of sorbed Sr increased with increasing pH of the as-received SrTreat®. Sodium titanate sorbed Sr less than the as-received SrTreat® at each pH, and largely agreed with the percentage of sorbed Sr, except above pH 8 where it was considerably lower. The percentage of sorbed Sr on titanium oxide was lowest below pH 7, and exceeded that on sodium titanate at pH 12.
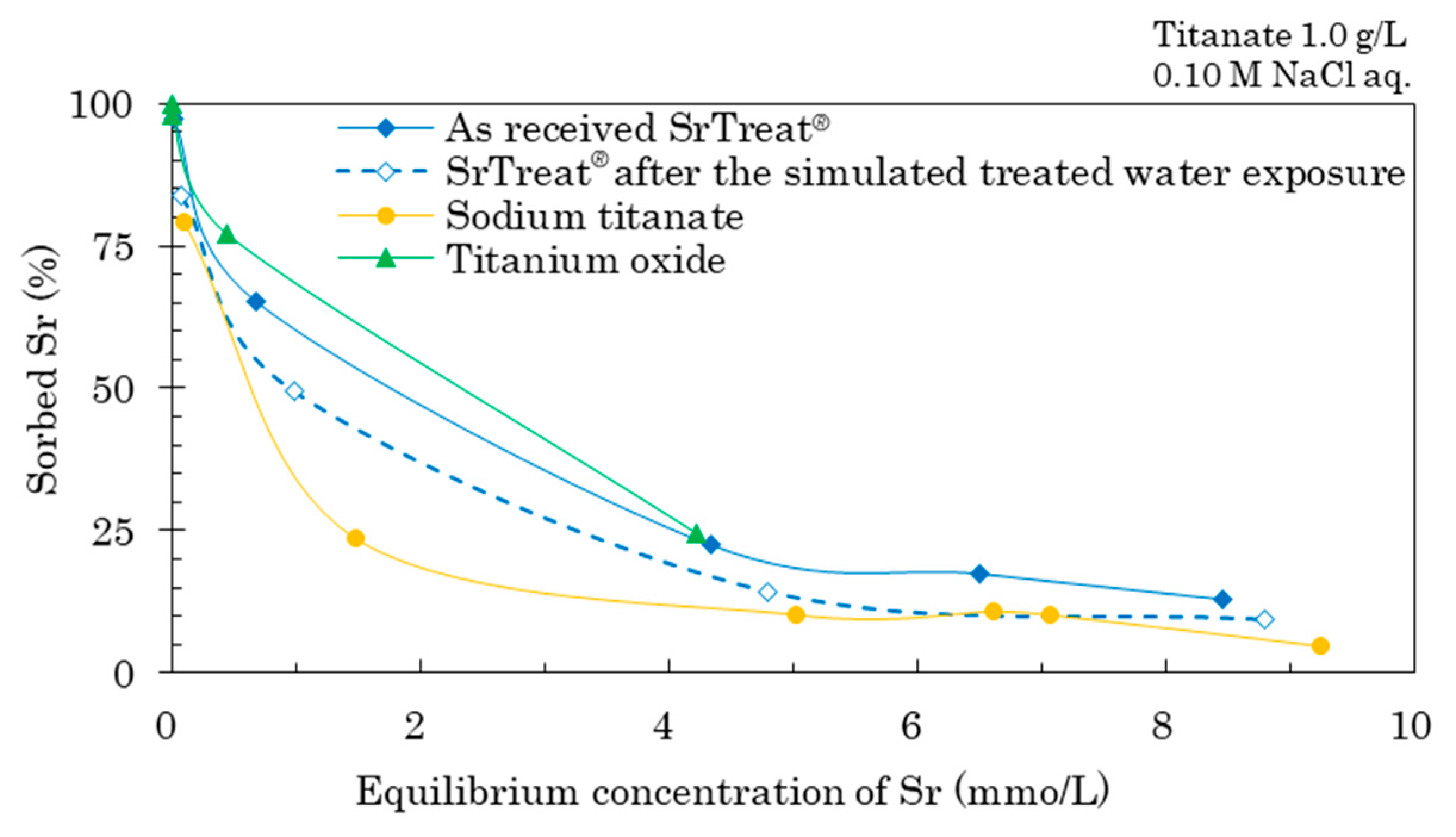
Figure 5 compares the percentage of sorbed Sr on the as-received SrTreat
®, the SrTreat
® after the simulated treated water exposure, sodium titanate, and titanium oxide. The amount of Sr sorbed on the SrTreat
® decreased after the simulated treated water exposure, when the equilibrium Sr concentration was 0.01–5 Sr mmol/L. The EDX results suggest that after the simulated treated water exposure, some of the Na at the SrTreat
® surface was exchanged for H and K, and this exchange may have decreased the amount of Sr sorbed on SrTreat
®. The percentage of sorbed Sr was higher on titanium oxide than on sodium titanate at all equilibrium Sr concentrations. For the order of sorption ability of ions on the as-received SrTreat
® [
12], the few H
+ ions available at pH 12 may be a cause of the sorption of the other cation onto titanate under highly alkaline conditions. Titanium oxide captures these cations into its structure more easily than at a lower pH. Titanium oxide sorbed Sr when the equilibrium pH of the mixture was 12, whereas sodium titanate sorbed Sr after releasing Na. As Sr sorption on titanium oxide is a single-step process, the percentage of sorbed Sr at pH 12 was higher on titanium oxide than on sodium titanate.
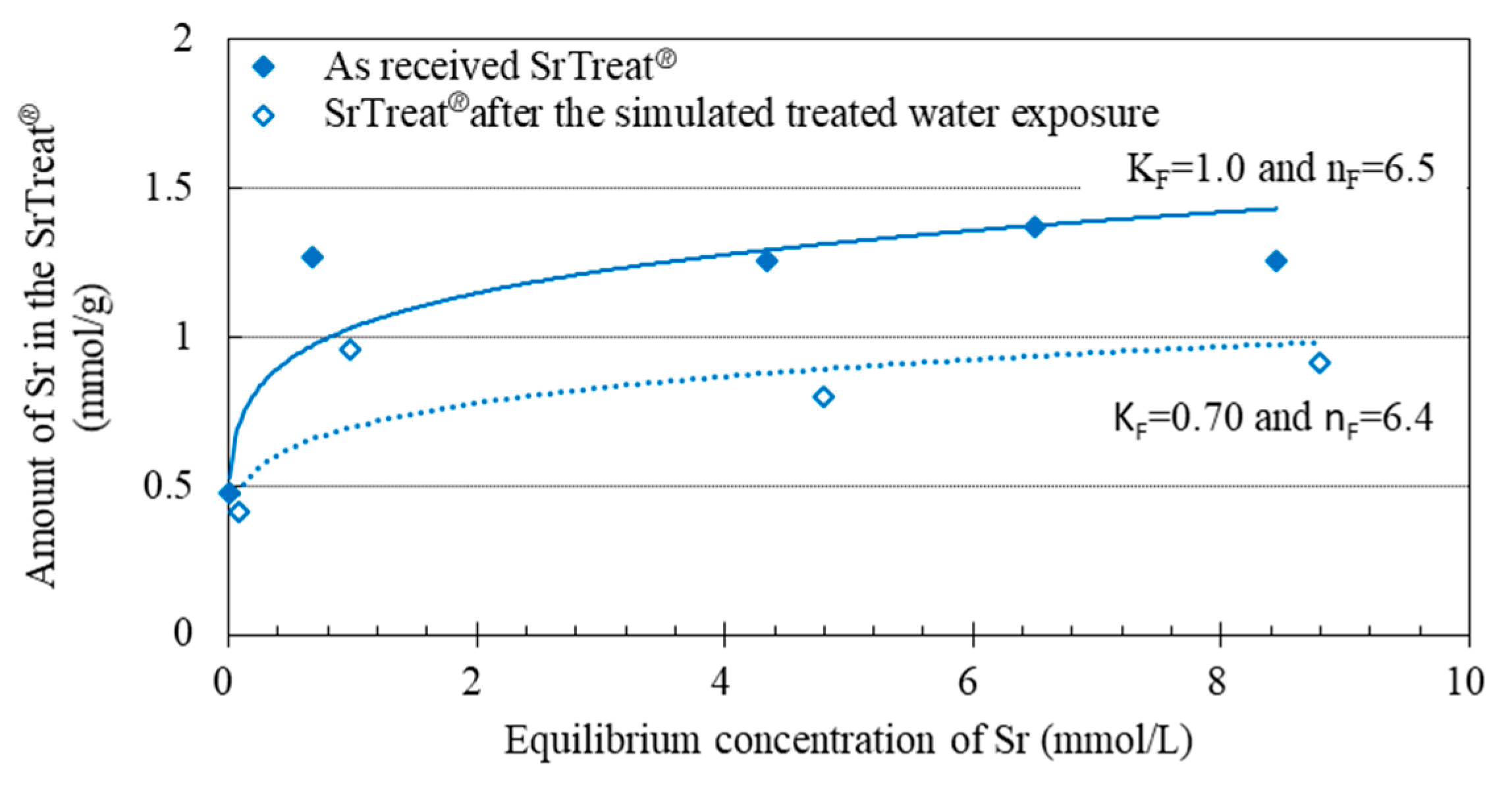
The amount of Sr in the SrTreat
® (
C′) was calculated by Equation (4).
Amount of Sr in the SrTreat
® before and after the simulated water exposure is shown in
Figure 6. The isotherm was expressed by the following equation;
where q
e is the amount of Sr in the titanate, K
F and 1/n
F are Freundlich’s constants of sorption capacity and sorption intensity.
The data of batchwise Sr sorption experiments on SrTreat followed the Freundlich isotherm, and KF decreased after the simulated treated water exposure. Batchwise Sr sorption experiments for sodium titanate were given the same pretreatment as for SrTreat®. Experiments were then conducted, and the decrease of sorbed Sr arose on Sodium titanate after the simulated treated water exposure. SrTreat® is supplied to the Multi-nuclide Removal Facility for treatment of the radioactive contaminated water at F1NPS. Thus only the Sr sorption data about SrTreat® was discussed in this paper.
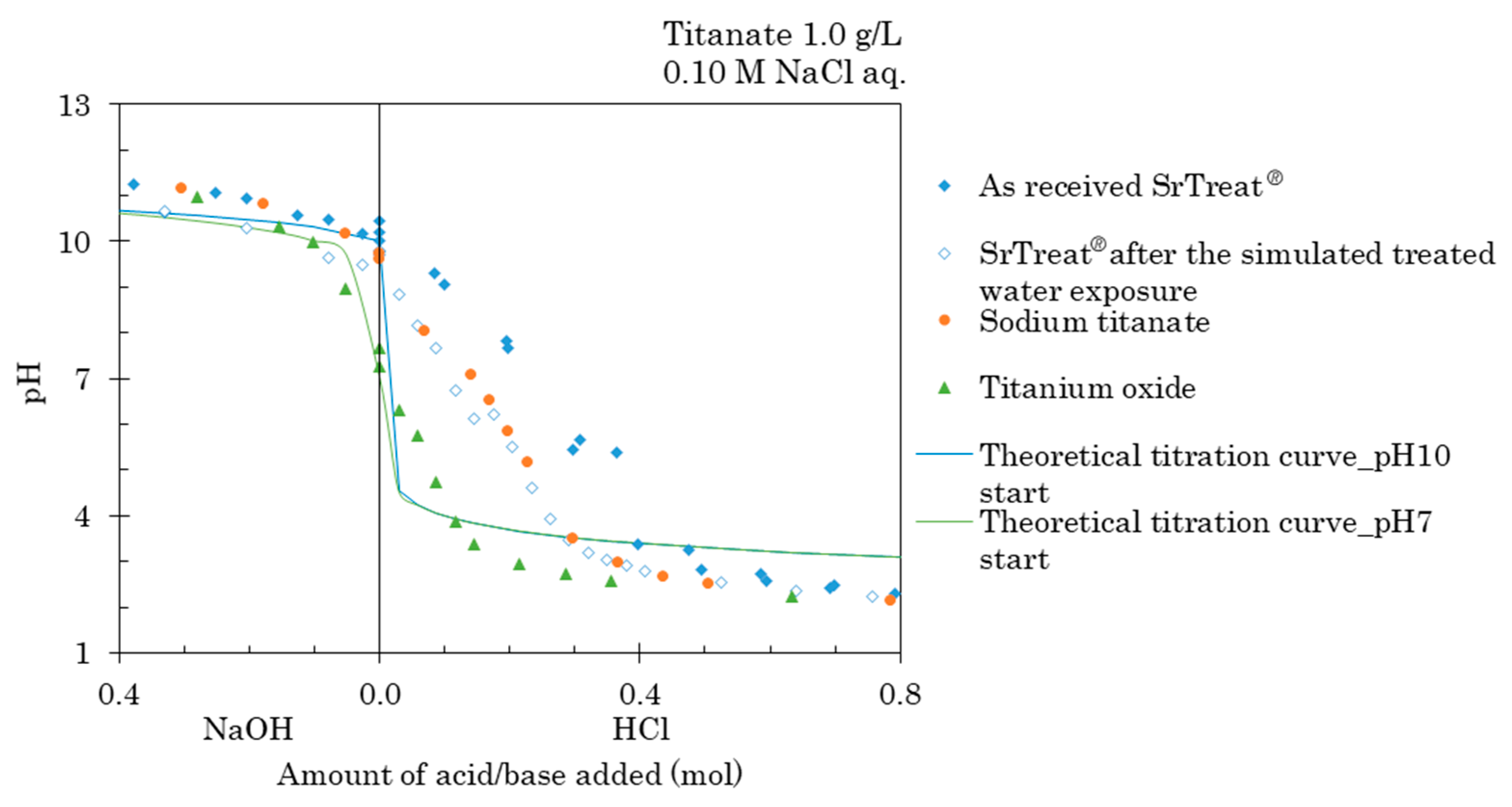
4.3. Acid–Base Titration
The measured and theoretical titration curves are shown in
Figure 7. The buffer capacity for H
+ was larger in the as-received SrTreat
® than in the SrTreat
® after the simulated treated water exposure. This demonstrated that the amount of reaction with cations on SrTreat
® decreases after the simulated treated water exposure. The buffer capacity for H
+ was significantly higher in sodium titanate than in titanium oxide. The results confirmed that the Na on the titanate group increased the buffer capacity for H
+. The buffer capacity for OH
− was small in all samples, and overall the titanates in the present study reacted little with anions.
In suspensions of the as-received SrTreat
®, the SrTreat
® after the simulated treated water exposure, and sodium titanate, the initial pH was approximately 10. Hydrogen ions react with anatase PK5585 (Bayer AG, Leverkusen, Germany) as shown in Equations (6)–(8). Ludwig and Schindler, who described these reactions, also confirm the dominance of the postulated surface species in 0.1 mol/L KNO
3 aqueous solution, (TiOH)(TiOH
2)
+ at pH below 4.5 in solution, (TiOH)
2 at pH 4.5–8.0, (TiOH)(TiO)
− at pH 8.0–11.0, and
at pH over 11.0 [
15].
From the EDX results, the as-received SrTreat
® and sodium titanate differ from anatase only by the presence of Na or H. From the XRD results, the crystal structure of these two samples was suggested to be associated with anatase. Based on Ludwig and Schindler, the following reactions of the as-received SrTreat
® and sodium titanate in the acid–base titration were:
where X denotes H and Na, Na is for the composition of titanate. From the suspension of the as-received SrTreat
® and sodium titanate with an approximate pH of 10, the dominant surface species was initially (TiOX)(TiO)
−. During the base titration, the dominant surface species shifted to
as the reaction proceeded to the right-hand side of Equation (11). In the acid titration, increasing amounts of H
+ were added to the (TiOX)(TiO)
−, and the dominant surface species shifted from (TiOX)(TiOH) to (TiOX)(TiOH
2)
+ at pH below 4.5. The amount of reacting H
+ with the as-received SrTreat
® was larger than in sodium titanate considering the differences in the slope of the titration curves. This suggested a preferential reaction to the right side of Equation (9), and to the left side of Equation (10) on the as-received SrTreat
® compared to sodium titanate.
From the XRD and the EDX results, the titanium oxide in this study had a crystal structure and similar chemical composition compared with the anatase in Ludwig and Schindler. These suggest that the pH-dependence of the surface species and reactions would follow Equations (6)–(8). As the pH of the initial titanium oxide suspension was approximately 7, the dominant surface species may be considered to be (TiOH)2. During the base titration, the dominant surface species shifted to by way of (TiOH)(TiO)−. During the acid titration, it further shifted to (TiOH)(TiOH2)+ along Equation (6).
4.4. Relation between the Titanate Surface Structure and Sr Sorption Ability
Despite their similar chemical compositions, the as-received SrTreat
® and sodium titanate exhibited different Sr sorption abilities and different buffer capacities for H
+, and these differences were observed in SrTreat
® both before and after the simulated treated water exposure. To understand these differences, the different samples were analyzed by XPS.
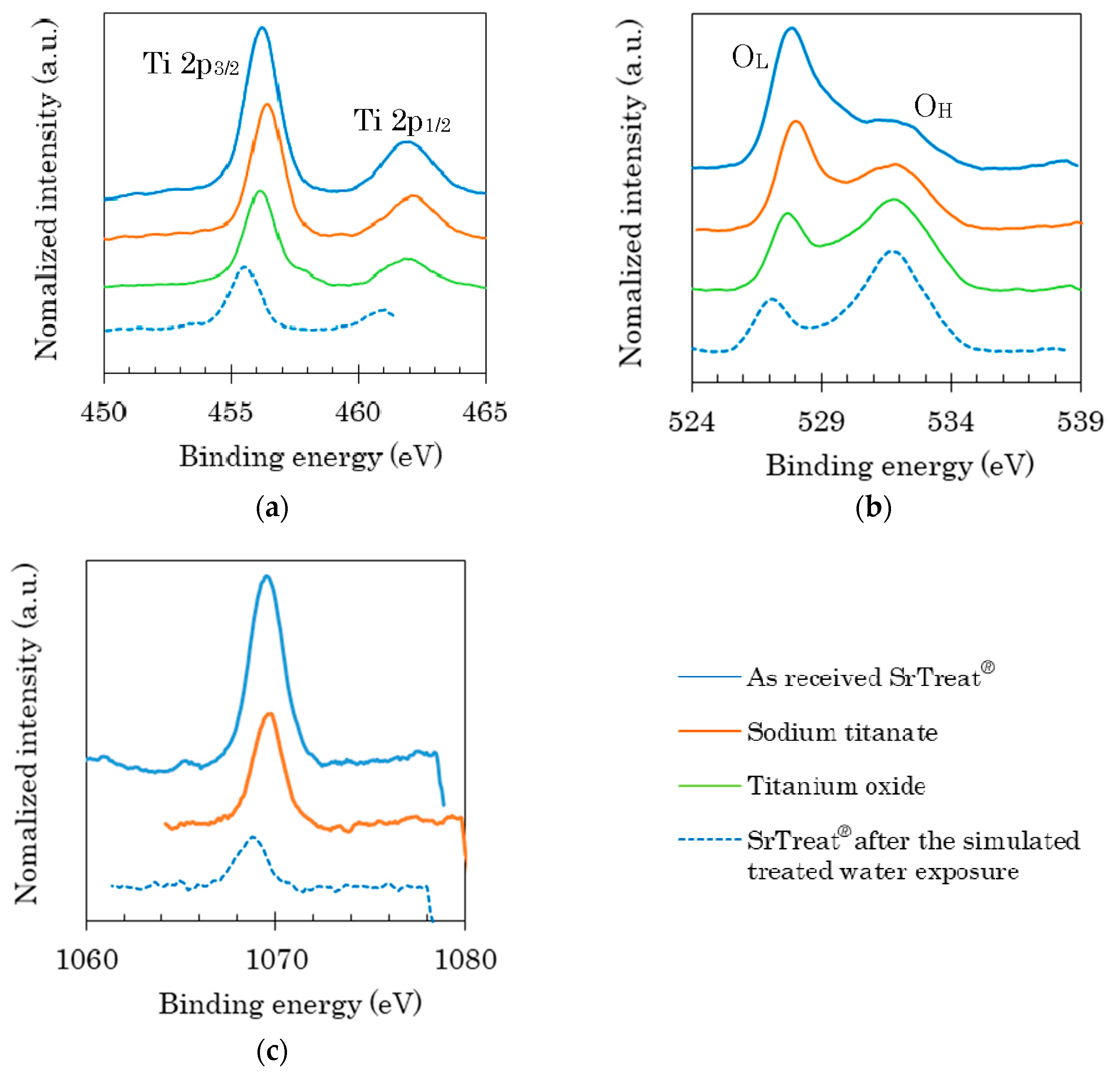
Figure 8 shows the XPS spectra of the four samples at bond energies corresponding to Ti2p, O1s, and Na1s in panels (a–c), respectively. The intensity ratios of the peaks are listed in
Table 4. The peaks at 456 eV and 462 eV in
Figure 8a can be attributed to Ti2p
3/2 and Ti2p
1/2 [
16]. The peaks at 527.5 eV and 531.5 eV on
Figure 8b apply to lattice oxygen and O–H bonds [
17], and the peaks of lattice oxygen is termed O
L, and that of the O–H bond O
H. Here, the lattice oxygen refers to a bond between oxygen and a metal.
The peak positions for Ti2p differed by 0.2 eV in sodium titanate and titanium oxide, both Ti2p peaks shifting toward higher energies when the Ti content of the material is lower [
18], indicating a decrease in Ti
3+ and an increase in Ti
4+ [
16,
19]. Replacement with Na in the titanium oxide was confirmed to increase the Ti
4+ proportion. The O
L/O
H ratio was higher in sodium titanate than in titanium oxide, because the replacement of Na increased the peak intensity of O
L.
The intensity ratio of Ti2p
3/2/Ti2p
1/2 was higher in the as-received SrTreat
® than in sodium titanate, and the binding energies of both Ti peaks were lower in the as-received SrTreat
® than in sodium titanate. Therefore, the energy levels of the 2p orbital of Ti
4+ on the two kinds of sodium titanates were different, showing a slightly higher ratio of Ti
3+ on the as-received SrTreat
® than on sodium titanate. The ratio of O
L/O
H on the as-received SrTreat
® was higher than in sodium titanate. In addition, the peak intensity of Na1s on SrTreat
® was larger than in the sodium titanate, as shown in
Figure 8c. A high availability of Na causes a high value of the ratio of O
L/O
H on the as-received SrTreat
®, as the ratio of O
L/O
H of sodium titanate was higher than that of titanium oxide. There were some differences in the surface composition between the as-received SrTreat
® and sodium titanate. These differences determine the Sr sorption abilities and the buffer capacity for H
+ of the two kinds of titanate.
The positions of the Ti peaks shifted to lower energies, and the Ti2p3/2/Ti2p1/2 ratio also decreased on the SrTreat® after the simulated treated water exposure. Exposing the simulated treated water to the SrTreat® increased the Ti3+ ratio at the surface of SrTreat®, and changed the energy level of the 2p orbital in the Ti4+ state of the SrTreat®. After the simulated treated water exposure, the OL peak position in the SrTreat® spectrum shifted to a lower energy, and the OL/OH ratio and peak Na1s intensity decreased. The decreased OL/OH ratio after the simulated treated water exposure may be ascribed to a reduced number of Na ions in the titanate groups in SrTreat®. This result cannot be attributed to fewer Ti–O bonds (the basic bond of SrTreat®) because the Ti eluted from the SrTreat® column was not evaluated from the concentrations of Ti in the fractions of effluent through the column. The exposure to the simulated treated water clearly changed the chemical bond conditions at the surface of SrTreat®. These changes were likely related to the decreased percentage of sorbed Sr, and the buffer capacity for H+ on the SrTreat® after the simulated treated water exposure.
From the XPS analyses of the SrTreat® surfaces before and after the simulated treated water exposure, it may be assumed that the surface structure of SrTreat® in the Multi-nuclide Removal Facility changed from the situation at the start of its use. Furthermore, the Sr sorption experiments imply that the amount of Sr sorbed on the used SrTreat® was smaller than that estimated from the ability of Sr sorption of the as-received SrTreat®. In conclusion, when the Sr content in the used SrTreat® is calculated from the data of the as-received SrTreat®, the results will lead to an overestimation of the amount of radioactive Sr in the used SrTreat®. When the radiation and the radioactive inventory of the used SrTreat® rise above accuracy estimated values, the need for radiation shielding at the treatment facility will be excessive for the actual radioactivity on the used SrTreat®. In addition, the treatment and end-point for used SrTreat® will be inaccurate and exceed the radioactive inventory of the used SrTreat®. The excessively large values will demand an increase in the disposal and deposition costs of the used SrTreat®.
5. Conclusions
The TEPCO decontaminated radioactive contaminated water generated at Fukushima Daiichi Nuclear Power Station (F1NPS) generated after the major earthquakes and tsunami that struck the region in March of 2011 has been treated by use of SrTreat® to recover Sr at the Adsorption Tower of the Multi-nuclide Removal Facility. It is important to distinguish the changing chemical composition of SrTreat® during its use in order to evaluate the amount of radioactive nuclides sorbed in the used SrTreat®. To understand the changes in the Sr sorption ability of SrTreat® after the simulated treated water exposure, as well as the cause of this change, we carried out Sr sorption experiments and an XPS analysis. Additionally, we established the acid–base titration curves of the as-received SrTreat®, sodium titanate, titanium oxide, and SrTreat® after exposure to the simulated treated water that was injected to the Adsorption Tower filled with SrTreat®. After simulated treated water exposure for 99 h, the chemical composition of SrTreat® changed from Na0.64H1.5TiO3 to Na0.39K0.023H1.6TiO3. The chemical bond conditions at the surface of SrTreat® also changed. In addition, a decrease in the percentage of sorbed Sr on SrTreat® was observed. The buffer capacity for H+ of the SrTreat® also declined after the simulated treated water exposure. The surface structure of the SrTreat® in the Multi-nuclide Removal Facility changes from the start of its use, and the amount of Sr sorbed on the used SrTreat® is lower than that estimated from the percentage of sorbed Sr on the as-received SrTreat®. From these results, the amount of radioactive nuclides in the used SrTreat® can be calculated from the sorption data of the as-received SrTreat®. However, the nuclide content in the used SrTreat® will be overestimated, resulting in unnecessary and increased costs of disposal and deposition of the used SrTreat®.