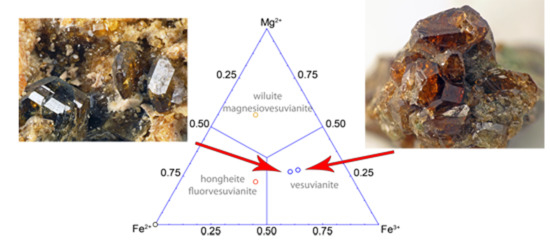
Vesuvianite from the Somma-Vesuvius Complex: New Data and Revised Formula
Abstract
:1. Introduction
| Т1B3+ + YMg2+ + 2O10,11O2−↔T1□ + YAl3+ + 2O10,11(OH)− and T2B3+ + 2O10,11O2−↔T2□ + O10,11(OH)− in wiluite [15] |
| Y1Mn3+↔Y1Fe3+ in manganvesuvianite [16] |
| Z1,2(SiO4)4−↔Z1,2(H4O4)4− [17] |
| O10OH− + O11OH−↔O10F− + O11F− in fluorvesuvianite [18] |
| O10OH−↔O10Cl− [19] |
| X3Bi3+ + Y2,3Mg2+↔X3Ca2+ + Y2,3Al3+ [20] |
| Y2Mg2+ + Y3Ti4+↔Y2Al3+ + Y3Al3+ [21] |
| X1,4Сa2+ + Y2,3Al3+↔X1,4Na+ + Y2,3Ti4+ in “natrovesuvianite” [22] |
| Y1Cu2+ + Y2,3Mn3+↔Y1Fe3+ + Y2,3Mg2+ in cyprine [23] |
| Y1Mg2+ + Y3Al3+↔Y1Fe3+ + Y3Mg2+ in magnesiovesuvianite [24] |
| Y1Fe3+↔Y1Al3+ in alumovesuvianite [25] |
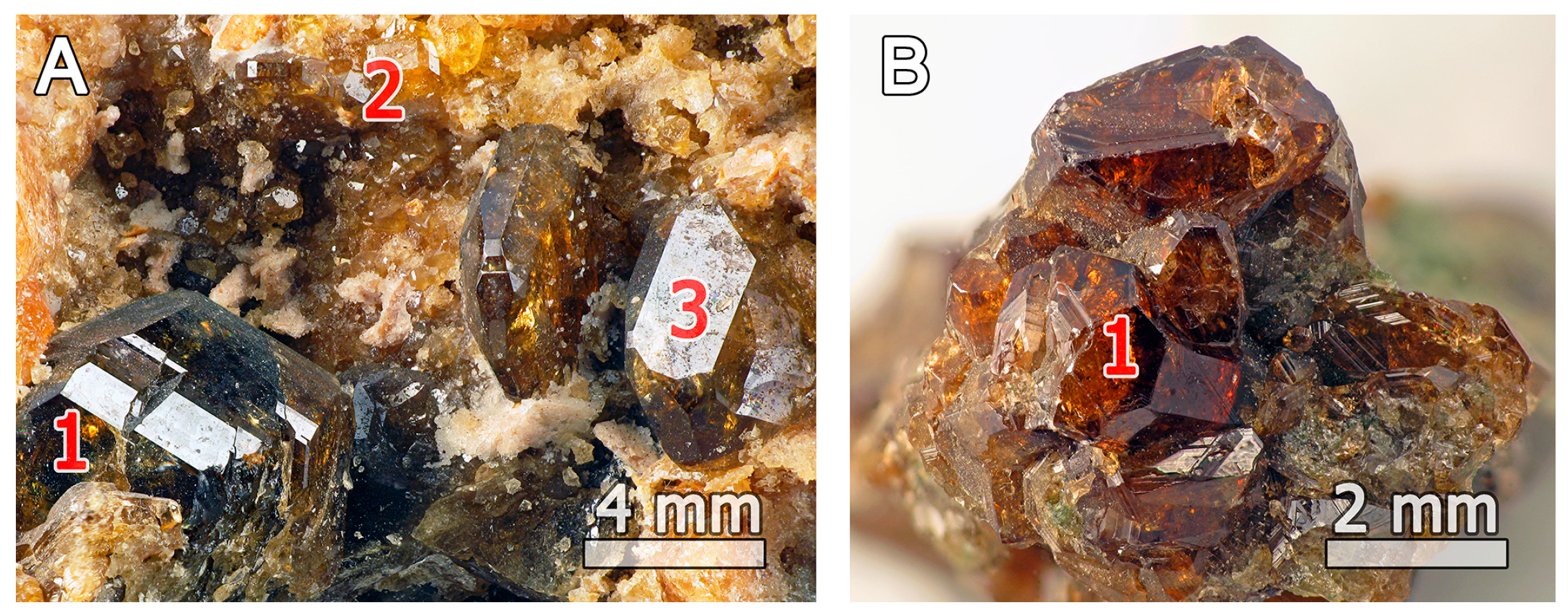
2. Occurrence
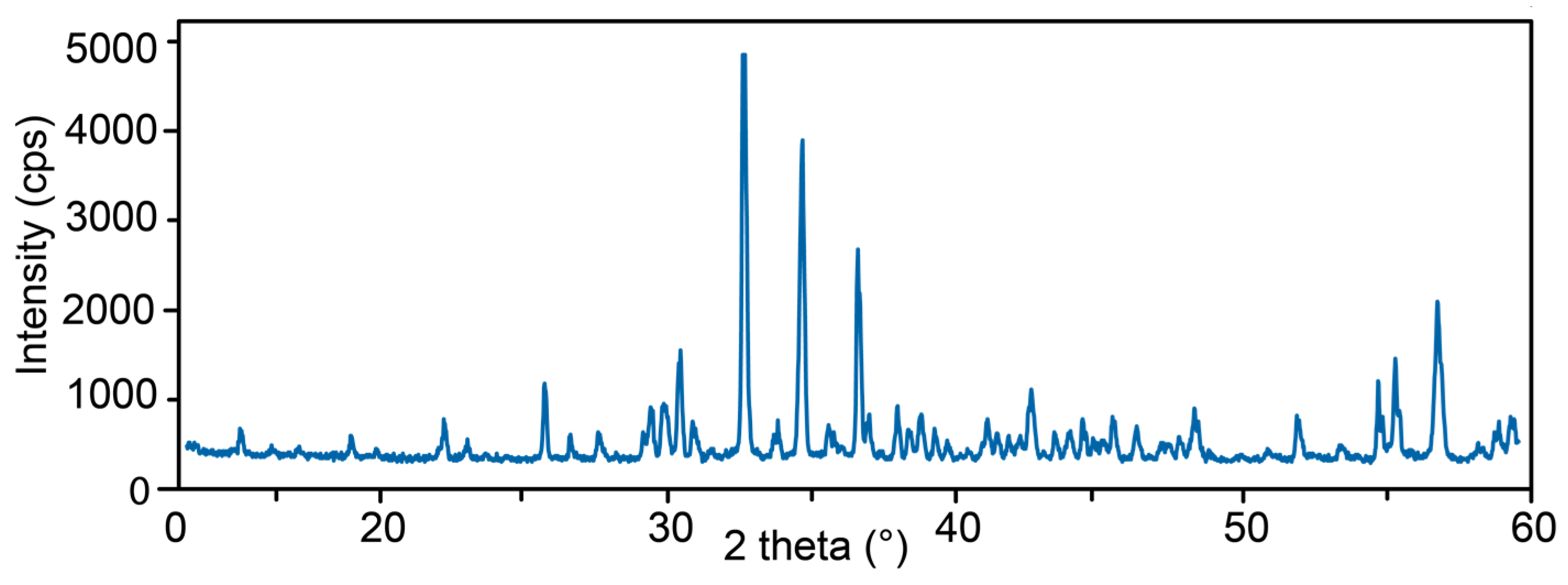
3. Materials and Methods
4. Results
4.1. Chemical Composition
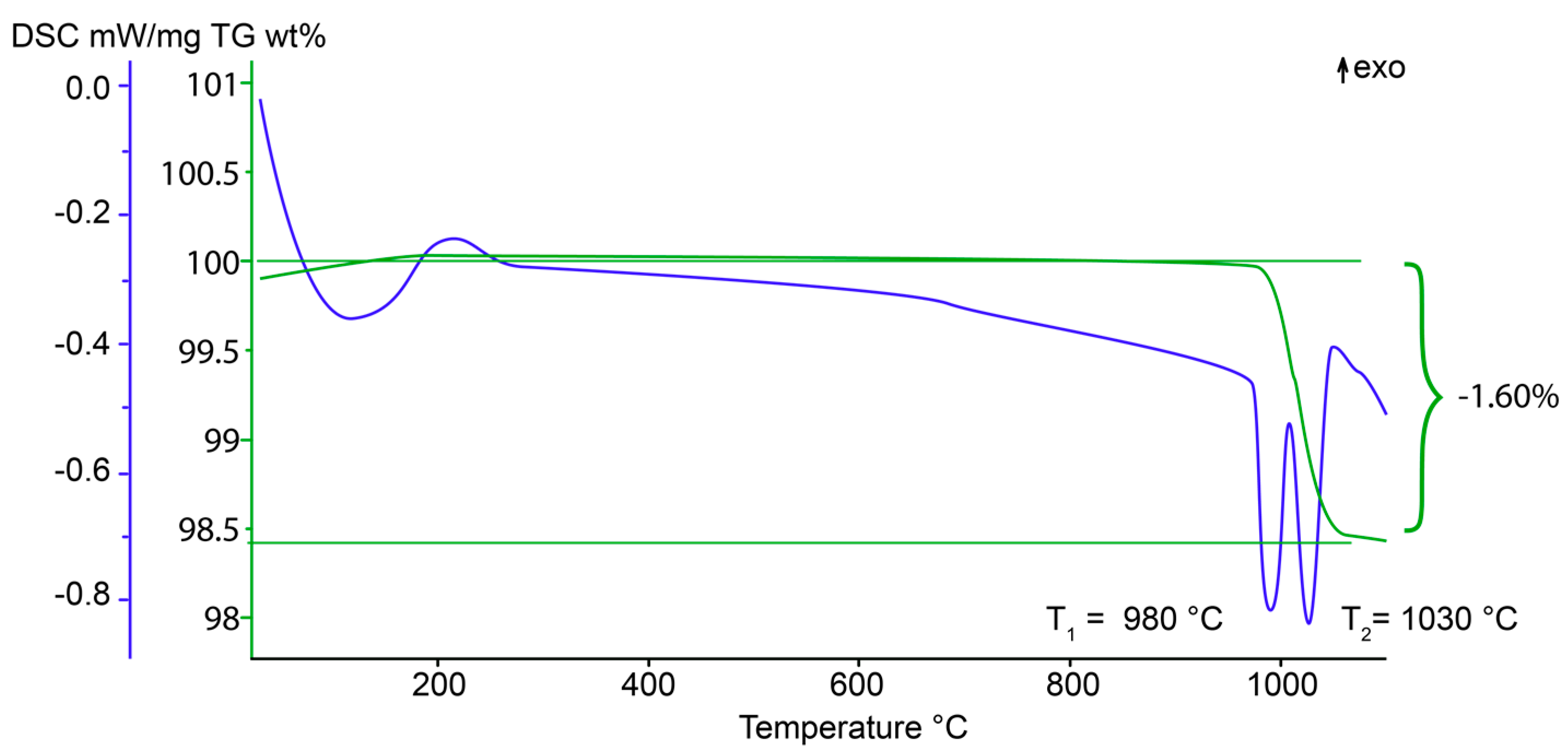
4.2. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA/DSC)
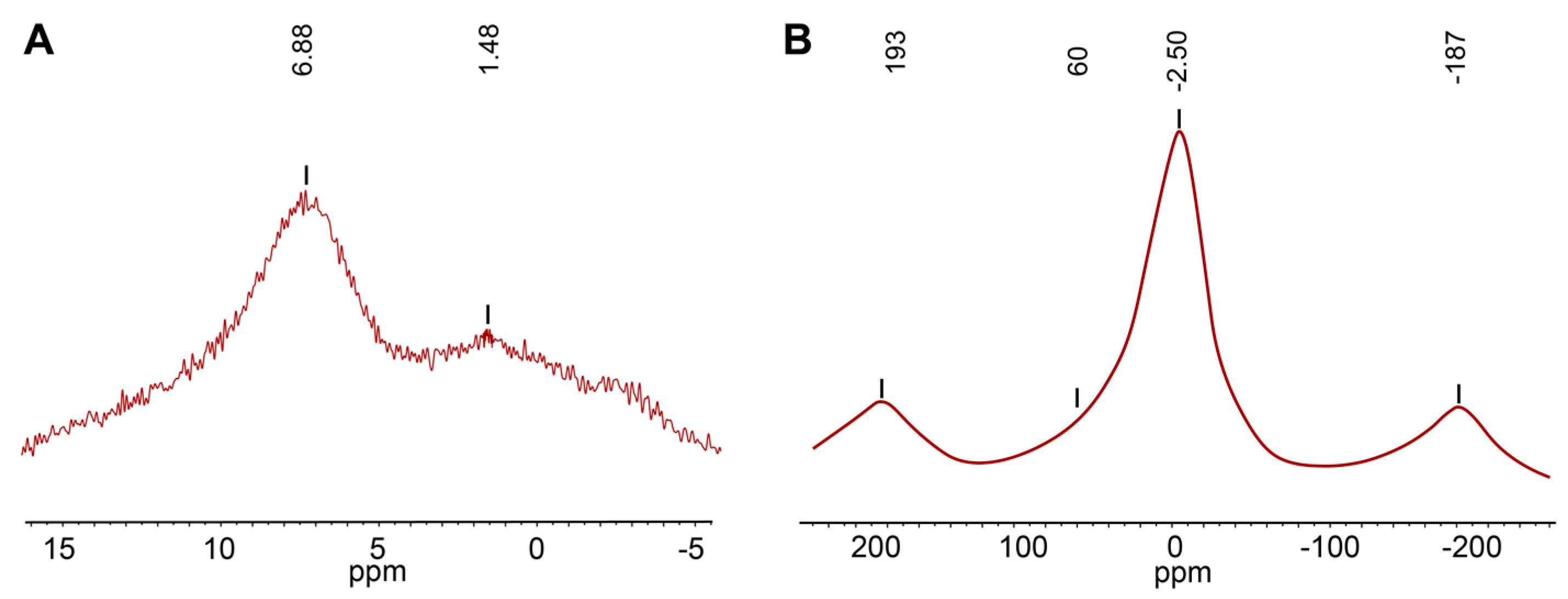
4.3. Solid State Magic-Angle Spinning Nuclear Magnetic Resonance (MAS NMR)
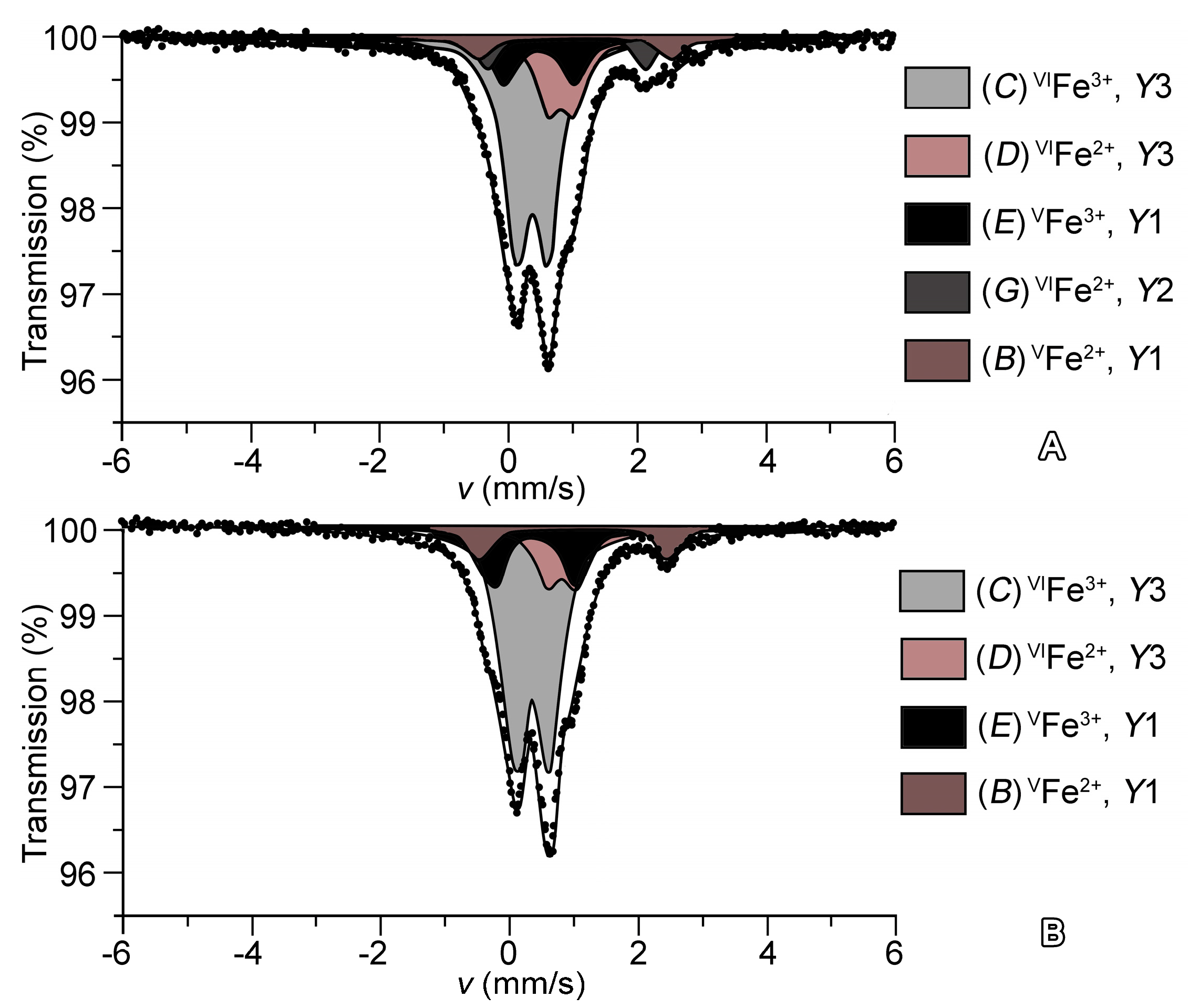
4.4. Mössbauer Measurenments
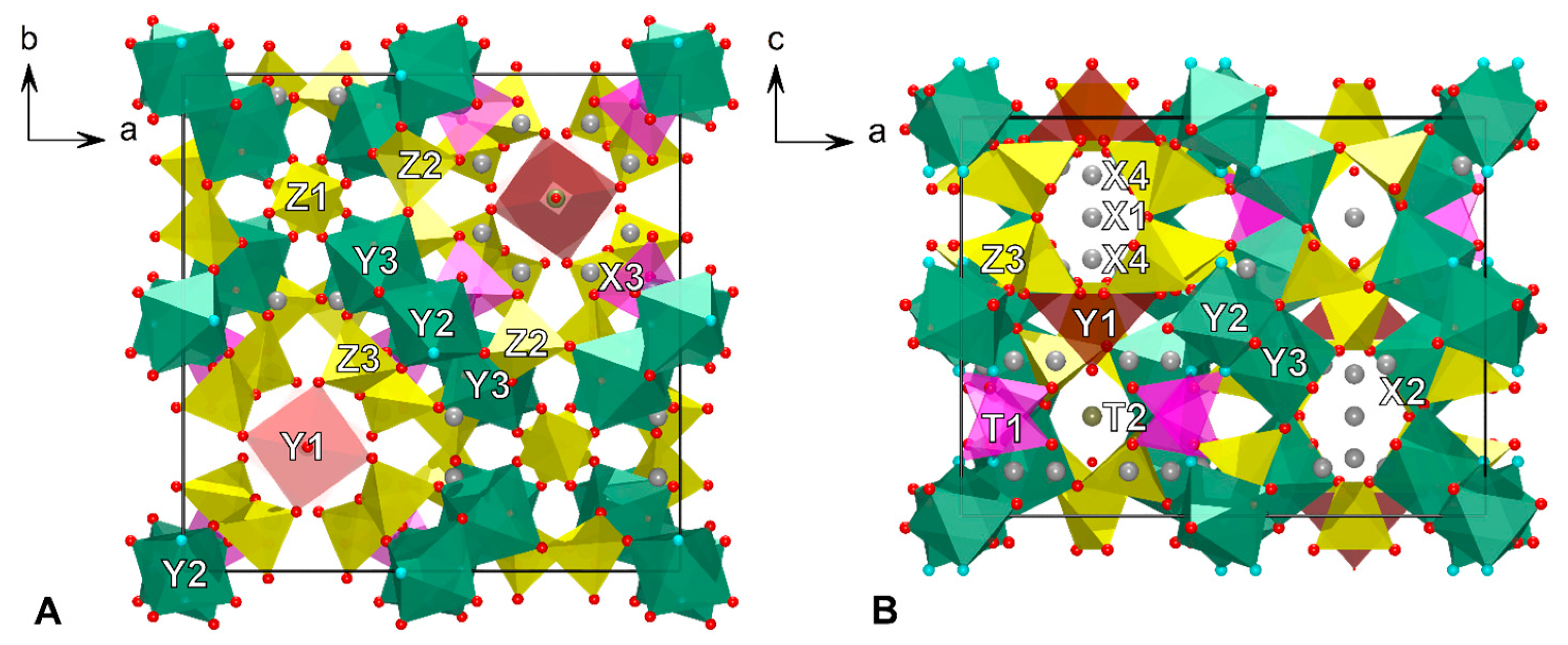
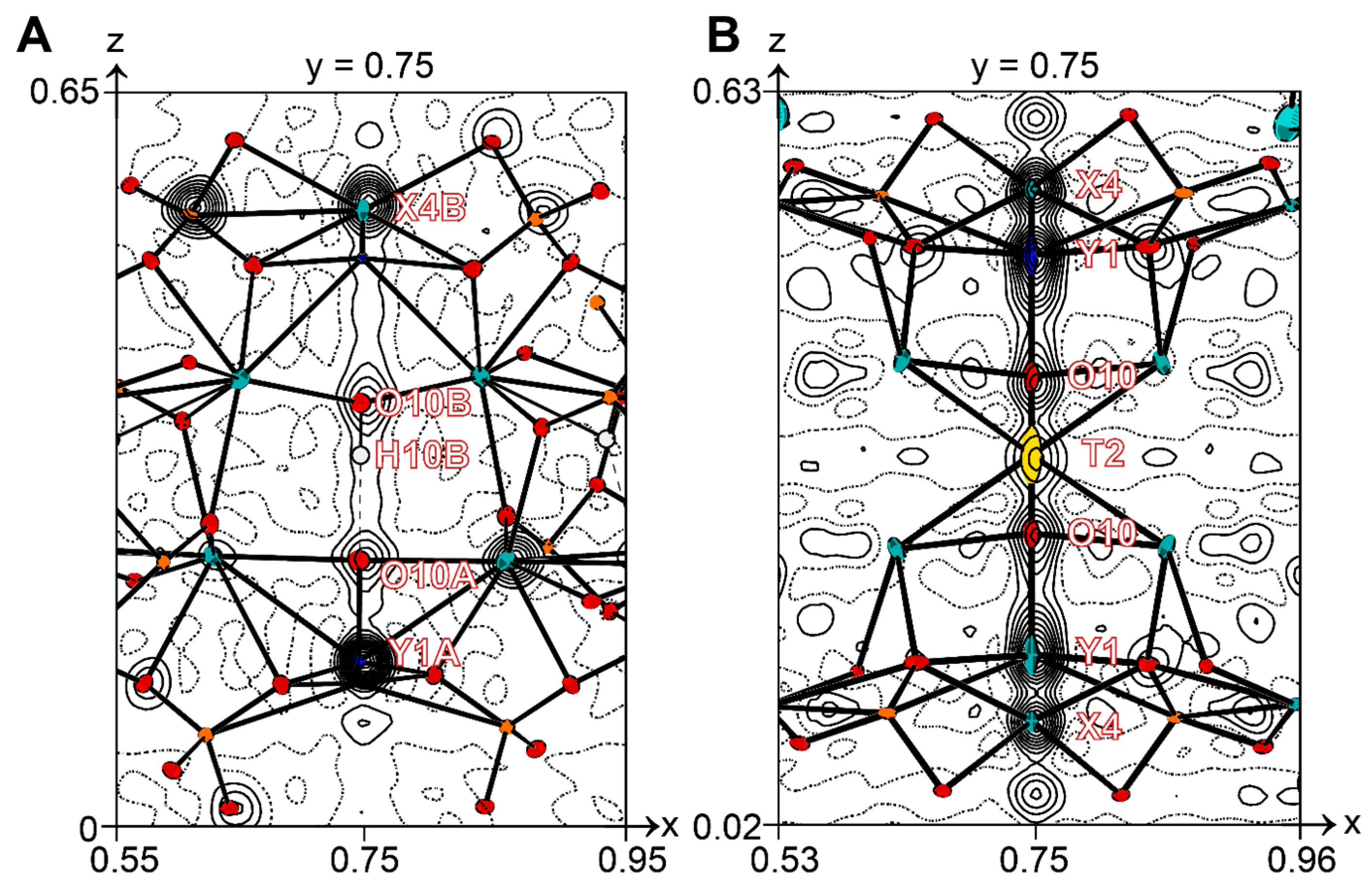
4.5. X-ray Crystallography
4.6. Infrared Spectroscopy
5. Discussion
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| dmeas Å | Imeas | dcalc Å | Icalc | hkl | dmeas Å | Imeas | dcalc Å | Icalc | hkl |
|---|---|---|---|---|---|---|---|---|---|
| 10.83 | 4 | 10.84 | 2 | 110 | 2.081 | 5 | 2.076 | 8 | 623 |
| 5.85 | 4 | 5.85 | 2 | 002 | 2.060 | 4 | 2.060 | 3 | 543 |
| 4.673 | 4 | 4.667 | 6 | 202 | 2.038 | 9 | 2.047 | 13 | 730 |
| 4.000 | 6 | 4.027 | 4 | 222 | 2.022 | 4 | 2.023 | 6 | 642 |
| 3.857 | 2 | 3.845 | 2 | 400 | 2.008 | 2 | 2.001 | 1 | 731 |
| 3.463 | 15 | 3.460 | 15 | 322 | 1.9941 | 8 | 1.9957 | 12 | 633 |
| 3.234 | 4 | 3.229 | 6 | 402 | 1.9602 | 6 | 1.6712 | 6 | 651 |
| 3.062 | 5 | 3.065 | 5 | 313 | 1.9281 | 2 | 1.9265 | 5 | 116 |
| 3.038 | 8 | 3.031 | 6 | 510 | 1.9171 | 2 | 1.9098 | 2 | 713 |
| 2.995 | 9 | 2.995 | 14 | 501 | 1.9029 | 4 | 1.9030 | 2 | 206 |
| 2.939 | 18 | 2.933 | 45 | 511 | 1.8823 | 7 | 1.8898 | 11 | 216 |
| 2.897 | 5 | 2.895 | 6 | 323 | 1.8647 | 2 | 1.8635 | 2 | 515 |
| 2.744 | 100 | 2.748 | 100 | 440 | 1.7960 | 1 | 1.7950 | 2 | 831 |
| 2.659 | 3 | 2.654 | 2 | 530 | 1.7614 | 9 | 1.7587 | 18 | 714 |
| 2.586 | 58 | 2.587 | 60 | 531 | 1.7158 | 2 | 1.7149 | 3 | 910 |
| 2.522 | 6 | 2.523 | 5 | 314 | 1.6768 | 17 | 1.6756 | 11 | 734 |
| 2.492 | 1 | 2.483 | 1 | 611 | 1.6603 | 22 | 1.6631 | 26 | 436 |
| 2.454 | 45 | 2.451 | 48 | 620 | 1.6215 | 30 | 1.6226 | 31 | 526 |
| 2.433 | 7 | 2.432 | 2 | 324 | 1.5853 | 2 | 1.5854 | 1 | 824 |
| 2.367 | 9 | 2.368 | 7 | 541 | 1.5712 | 5 | 1.5712 | 4 | 327 |
| 2.345 | 5 | 2.345 | 3 | 404 | 1.5580 | 7 | 1.5579 | 6 | 616 |
| 2.320 | 8 | 2.319 | 7 | 105 | 1.5397 | 2 | 1.5362 | 2 | 735 |
| 2.293 | 5 | 2.296 | 5 | 334 | 1.5318 | 1 | 1.5312 | 2 | 626 |
| 2.269 | 2 | 2.268 | 2 | 631 | 1.5235 | 4 | 1.5237 | 5 | 942 |
| 2.193 | 5 | 2.194 | 9 | 710 | 1.5089 | 2 | 1.5084 | 1 | 1021 |
| 2.177 | 5 | 2.169 | 5 | 701 | 1.5028 | 1 | 1.5029 | 1 | 1002 |
| 2.158 | 3 | 2.164 | 3 | 711 | 1.4979 | 8 | 1.4979 | 5 | 636 |
| 2.141 | 2 | 2.143 | 2 | 305 | 1.4753 | 1 | 1.4752 | 2 | 1022 |
| 2.121 | 11 | 2.122 | 14 | 315 |
References
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals: Orthosilicates, Vol 1A, 1st ed.; Geological Society: London, UK, 1982; pp. 701–719. [Google Scholar]
- Gnos, E.; Armbruster, T. Relationship among metamorphic grade, vesuvianite “rod polytypism”, and vesuvianite composition. Am. Mineral. 2006, 91, 862–870. [Google Scholar] [CrossRef]
- Olesch, M. Natürliche und synthetische Fe-haltige Vesuviane. Fortschr. Mineral. 1979, 57, 114–115. (In German) [Google Scholar]
- Kappeler, M.A. Prodromus Crystallographiae de Crystallis Improprie sic Dictis Commentarium; Heinrich, R.W., Ed.; HR Wyssing: Lucerna, Switzerland, 1723; pp. 1–43. [Google Scholar]
- Russo, M.; Punzo, I. I Minerali del Somma-Vesuvio, 1st ed.; AMI: Cremona, Italy, 2004; pp. 1–317. [Google Scholar]
- Werner, A.G. Klaproth’s Beiträge: Über Vesuvian; Klaproth, M.H., Ed.; Becker und Compagnie: Berlin, Germany, 1795; pp. 1–34. [Google Scholar]
- Clarke, F. The constitution of the silicates. Bull. U. S. Geol. Surv. 1895, 125, 109–110. [Google Scholar]
- Sjögren, H. Analyser pa tvenne vesuvian-varieter och vesuvianens kemiska constitution I Allmänhet. Geol. I Stockh. Forh. 1895, 17, 267–271. [Google Scholar] [CrossRef]
- Weibull, M. Studien über vesuvian. Z. Krystallogr. 1896, 35, 26–35. [Google Scholar] [CrossRef]
- Jannasch, P.; Weingarten, P. Uber die chemische Zusammensetzung und Konstitution des Vesuvians und des Wiluits. Z. Anorg. Allg. Chem. 1896, 11, 40–48. [Google Scholar] [CrossRef]
- Warren, B.E.; Modell, D.I. The structure of vesuvianite Ca10Al4(Mg,Fe)2Si9O34(OH)4. Z. Kristallogr. 1931, 78, 422–432. [Google Scholar] [CrossRef]
- Machatschki, F. Zur Formel des Vesuvian. Z. Kristallogr. 1932, 81, 148–152. [Google Scholar] [CrossRef]
- Coda, A.; Giusta, D.A.; Isetti, G.; Mazzi, F. On the structure of vesuvianite. Atti Accad. Sci. Torino 1970, 105, 1–22. [Google Scholar]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S.; Grice, J.D. Wiluite, Ca19(Al,Mg,Fe,Ti)13(B,Al,◻)5Si18O68(O,OH)10, a new mineral species isostructural with vesuvianite, from the Sakha Republic, Russian Federation. Can. Mineral. 1998, 36, 1301–1304. [Google Scholar] [CrossRef]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S. The chemistry of vesuvianite. Can. Mineral. 1992, 33, 19–48. [Google Scholar]
- Armbruster, T.; Gnos, E.; Dixon, R.; Gutzmer, J.; Hejny, C.; Döbelin, N.; Medenbach, O. Manganvesuvianite and tweddillite, two new Mn3+-silicate minerals from the Kalahari manganese fields, South Africa. Mineral. Mag. 2002, 66, 137–150. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Sitarz, M.; Stadnicka, K. Si-deficient, OH-substituted, boron-bearing vesuvianite from the Wiluy River, Yakutia, Russia. Can. Mineral. 2003, 41, 833–842. [Google Scholar] [CrossRef]
- Britvin, S.N.; Antonov, A.A; Krivovichev, S.V.; Armbruster, T.; Burns, P.C.; Chukanov, N.V. Fluorvesuvianite, Ca19(Al,Mg,Fe 2+)13[SiO4]10[Si2O7]4O(F,OH)9, a new mineral species from Pitkäranta, Karelia, Russia: Description and crystal structure. Can. Mineral. 2003, 41, 1371–1380. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Dzierżanowski, P. Chlorine in vesuvianites. Miner. Pol. 2005, 36, 51–61. [Google Scholar]
- Hålenius, U.; Bosi, F.; Gatedal, K. Crystal structure and chemistry of skarn-associated bismuthian vesuvianite. Am. Mineral. 2013, 98, 566–573. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Chukanov, N.V.; Rusakov, V.S.; Panikorovskii, T.L.; Gainov, R.R.; Vagizov, F.G.; Rastsvetaeva, R.K.; Lyssenko, K.A.; Belakovskiy, D.I. Towards a revisitation of vesuvianite-group nomenclature: The crystal structure of Ti-rich vesuvianite from Alchuri, Shigar valley, Pakistan. Acta Crystallogr. B 2016, 72, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Panikorovskii, T.L.; Krivovichev, S.V.; Yakovenchuk, V.N.; Shilovkikh, V.V.; Mazur, A.S. Crystal chemistry of Na-bearing vesuvianite from fenitized gabbroid of the Western Keivy (Kola peninsula, Russia). Zap. Ross. Mineral. Obsh. 2016, 145, 83–95. (In Russian) [Google Scholar]
- Panikorovskii, T.L.; Shilovskikh, V.V.; Avdontseva, E.Y.; Zolotarev, A.A.; Pekov, I.V.; Britvin, S.N.; Hålenius, U.; Krivovichev, S.V. Cyprine, Ca19Cu2+(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group mineral from the Wessels mine, South Africa. Eur. J. Mineral. 2017, 29, 295–307. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Shilovskikh, V.V.; Avdontseva, E.Y.; Zolotarev, A.A.; Karpenko, V.Y.; Mazur, A.S.; Yakovenchuk, V.N.; Krivovichev, S.V.; Pekov, I.V. Magnesiovesuvianite, Ca19Mg(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group mineral. J. Geosci. 2017, 1, 25–36. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Chukanov, N.V.; Aksenov, S.M.; Mazur, A.S.; Avdontseva, E.Y.; Shilovskikh, V.V.; Krivovichev, S.V. Alumovesuvianite, Ca19Al(Al,Mg)12Si18O69(OH)9, a new vesuvianite-group member from the Jeffrey mine, Asbestos, Estrie Region, Québec, Canada. Mineral. Petrol. 2017, 111, 833–842. [Google Scholar] [CrossRef]
- Dyrek, K.; Platonov, A.N.; Sojka, Z.; Żabinski, W. Optical absorption and EPR study of Cu2+ ions in vesuvianite (“cyprine”) from Sauland, Telemark, Norway. Eur. J. Mineral. 1992, 4, 1285–1289. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Krivovichev, S.V.; Zolotarev, A.A.; Antonov, A.A. Crystal chemistry of low-symmetry (P4nc) vesuvianite from the Kharmankul’ Cordon (South Urals, Russia). Zap. Ross. Mineral. Obsh. 2016, 145, 94–104. (In Russian) [Google Scholar]
- Panikorovskii, T.L.; Krivovichev, S.V; Galuskin, E.V.; Shilovskikh, V.V.; Mazur, A.S.; Bazai, A.V. Si-deficient, OH-substituted, boron-bearing vesuvianite from Sakha-Yakutia, Russia: A combined single-crystal, 1H MAS-NMR and IR spectroscopic study. Eur. J. Mineral. 2016, 28, 931–941. [Google Scholar] [CrossRef]
- Panikorovskii, T.L.; Mazur, A.S.; Bazai, A.V.; Shilovskikh, V.V.; Galuskin, E.V.; Chukanov, N.V.; Rusakov, V.S.; Zhukov, Y.M.; Avdontseva, E.Y.; Aksenov, S.M.; et al. X-ray diffraction and spectroscopic study of wiluite: Implications for the vesuvianite-group nomenclature. Phys. Chem. Mineral. 2017, 44, 577–593. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Mazzi, F. The crystal structure of a vesuvianite with P4/n symmetry. Tsch. Mineral. Petrol. 1983, 31, 277–288. [Google Scholar] [CrossRef]
- Xu, J.; Li, G.; Fan, G.; Ge, X.; Zhu, X.; Shen, G. Hongheite, IMA 2017-027. CNMNC Newsletter No. 39, October 2017, page 1283. Mineral. Mag. 2017, 81, 1279–1286. [Google Scholar]
- Veblen, D.R.; Wiechmann, M.J. Domain structure of low-symmetry vesuvianite from Crestmore, California. Am. Mineral. 1991, 76, 397–404. [Google Scholar]
- Allen, F.M.; Burnham, C.W. A comprehensive structure-model for vesuvianite: Symmetry variation and crystal growth. Can. Mineral. 1992, 30, 1–18. [Google Scholar]
- Groat, L.A.; Hawthorne, F.C.; Ercit, T.S.; Putnis, A. The symmetry of vesuvianite. Can. Mineral. 1993, 31, 617–635. [Google Scholar]
- Tanaka, T.; Akizuki, M.; Hudoh, Y. Optical properties and crystal structure of triclinic growth sectors in vesuvianite. Mineral. Mag. 2002, 66, 261–274. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Leavens, P.B.; Rossman, G.R.; Yap, G.P.A.; Rose, T. Vesuvianite from Pajsberg, Sweden, and the role of Be in the vesuvianite structure. Can. Mineral. 2016, 54, 1525–1537. [Google Scholar] [CrossRef]
- Nickel, E.H.; Grice, J.D. The IMA Commission on New Minerals and Mineral Names: Procedures and guidelines on mineral nomenclature, 1998. Can. Mineral. 1998, 36, 913–926. [Google Scholar] [CrossRef]
- Balassone, G.; Talla, D.; Beran, A.; Mormone, A.; Altomare, A.; Moliterni, A.; Mondillo, N.; Saviano, M.; Petti, C. Vesuvianite from Somma-Vesuvius volcano (Southern Italy): Chemical, X-ray diffraction and single-crystal polarized FTIR investigations. Period. Mineral. 2011, 80, 369–384. [Google Scholar] [CrossRef]
- Balassone, G.; Bellatreccia, F.; Mormone, A.; Biagioni, C.; Pasero, M.; Petti, C.; Mondillo, N.; Fameli, G. Sodalite-group minerals from the Somma-Vesuvius volcanic complex, Italy: A case study of K-feldspar-rich xenoliths. Mineral. Mag. 2012, 76, 191–212. [Google Scholar] [CrossRef]
- Balassone, G.; Kahlenberg, V.; Altomare, A.; Mormone, A.; Rizzi, R.; Saviano, M.; Mondillo, M. Nephelines from the Somma-Vesuvius volcanic complex (Southern Italy): Crystal chemical, structural and genetic investigations. Mineral. Petrol. 2014, 108, 71–90. [Google Scholar] [CrossRef]
- Balassone, G.; Bellatreccia, F.; Ottolini, L.; Mormone, A.; Petti, C.; Ghiara, M.R.; Altomare, A.; Saviano, M.; Rizzim, R.; D’oraziom, L. Sodalite-group minerals from Somma-Vesuvius volcano (Naples, Italy): A combined EPMA, SIMS and FTIR crystal chemical study. Can. Mineral. 2016, 54, 583–604. [Google Scholar] [CrossRef]
- Gilg, A.H.; Lima, A.; Somma, R.; Belkin, H.E.; De Vivo, B.; Ayuso, R.A. Isotope geochemistry and fluid inclusion study of skarns from Vesuvius. Mineral. Petrol. 2001, 73, 145–176. [Google Scholar] [CrossRef]
- Hermes, O.D.; Cornell, W.C. Petrochemical significance of xenolithic nodules associated with potash-rich lavas of Somma-Vesuvius volcano. In NSF Final Technical Report; University Rhode Island: Kingston, RI, USA, 1978; p. 58. [Google Scholar]
- Joron, J.L.; Metrich, N.; Rosi, M.; Santacroce, R.; Sbrana, A. Chemistry and petrography. CNR Quad. Ric. Sci. 1987, 114, 105–174. [Google Scholar]
- Gruppo Mineralogico Geologico Napoletano. Available online: http://www.gmgn.it/index.html (accessed on 1 November 2017).
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Elmi, C.; Brigattim, M.F.; Pasqualim, L.; Montecchi, M.; Laurora, A.; Malferrari, D.; Nannarone, S. High-temperature vesuvianite: Crystal chemistry and surface considerations. Phys. Chem. Miner. 2011, 38, 459–468. [Google Scholar] [CrossRef]
- Żabiński, W.; Wactawska, Z.; Paluszkiewicz, C. Thermal decomposition of vesuvianite. J. Therm. Anal. 1996, 46, 1437–1447. [Google Scholar] [CrossRef]
- Foldvari, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011; pp. 1–180. [Google Scholar]
- Phillips, B.L.; Allen, F.M.; Kirkpatrick, R.J. High-resolution solid-state 27Al NMR spectroscopy of Mg-rich vesuvianite. Am. Mineral. 1987, 72, 1190–1194. [Google Scholar]
- Olejniczak, Z.; Żabiński, W. 27Al NMR study of white vesuvianite from Piz Lunghin, Switzerland. Miner. Pol. 1996, 27, 41–45. [Google Scholar]
- Yesinowski, J.P.; Eckert, H.; Rossman, G.R. Characterization of hydrous species in minerals by high-speed 1H MAS-NMR. J. Am. Chem. Soc. 1988, 110, 1367–1375. [Google Scholar] [CrossRef]
- Lager, G.A.; Xie, Q.; Ross, F.K.; Rossman, G.R.; Armbruster, T.; Rotella, F.J.; Schultz, A.J. Hydrogen-atom position in P4/nnc vesuvianite. Can. Mineral. 1999, 37, 763–768. [Google Scholar]
- Ohkawa, M.; Yoshiasa, A.; Takeno, S. Crystal chemistry of vesuvianite: Site preferences of square-pyramidal coordinated sites. Am. Mineral. 1992, 77, 945–953. [Google Scholar]
- Ohkawa, M.; Armbruster, T.; Galuskin, E. Structural investigation of low symmetry vesuvianite collected from Tojyo, Hiroshima, Japan: Implications for hydrogarnet-like substitution. J. Mineral. Petrol. Sci. 2009, 104, 69–76. [Google Scholar] [CrossRef]
- Pavese, A.; Prencipe, M.; Tribaudino, M.; Aagaard, S.S. X-ray and neutron single-crystal study of P4/n vesuvianite. Can. Mineral. 1998, 36, 1029–1037. [Google Scholar]
- Armbruster, T.; Gnos, E. “Rod” polytypism in vesuvianite: Crystal structure of a low-temperature P4nc with pronounced octahedral cation ordering. Schweiz. Mineral. Petrogr. 2000, 80, 109–116. [Google Scholar]
- Galuskin, E.V. Vesuvianite-Group Minerals from Achtarandite Rocks (Wiluy River, Yakutia), 1st ed.; University of Silesia: Katowice, Poland, 2005; pp. 123–137. (In Polish) [Google Scholar]
- Kraczka, J.; Żabiński, W. Mössbauer study of iron in some vesuvianites. Mineral. Pol. 2003, 34, 37–44. [Google Scholar]
- Rusakov, V.S.; Kovalchuk, R.V.; Borovikova, E.Y.; Kurazhkovskaya, V.S. State of iron atoms in high vesuvianites according to Mössbauer spectroscopy data. Zap. Ross. Mineral. Obsh. 2006, 135, 91–100. (In Russian) [Google Scholar]
- Manning, P.G.; Tricker, M.J. Optical absorption and Mössbauer spectral studies of iron and titanium site-populations in vesuvianites. Can. Mineral. 1975, 13, 259–265. [Google Scholar]
- Groat, L.A.; Evans, R.J.; Cempírek, J.; McCammon, C.; Houzar, S. Fe-rich and As-bearing vesuvianite and wiluite from Kozlov, Czech Republic. Am. Mineral. 2013, 98, 1330–1337. [Google Scholar] [CrossRef]
- Bruker AXS. Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS GmbH: Karlsruhe, Germany, 2009. [Google Scholar]
- Groat, L.A.; Evans, R.J. Crystal chemistry of Bi- and Mn-bearing vesuvianite from Langban, Sweden. Am. Mineral. 2012, 97, 1627–1634. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Leavens, P.B.; Rheingold, A.L.; Nelen, J.A. Crystal structure of a REE-bearing vesuvianite from San Benito County, California. Am. Mineral. 1987, 72, 625–628. [Google Scholar]
- Galuskin, E.V.; Galuskina, I.O.; Stadnicka, K.; Armbruster, T.; Kozanecki, M. The crystal structure of Si-deficient, OH-substituted, boron-bearing vesuvianite from the Wiluy River, Sakha-Yakutia, Russia. Can. Mineral. 2007, 45, 239–248. [Google Scholar] [CrossRef]
- Groat, L.A.; Hawthorne, F.C.; Rossman, G.R.; Scott, T.E. The infrared spectroscopy of vesuvianite in the OH region. Can. Mineral. 1995, 33, 609–626. [Google Scholar]
- Chukanov, N.V.; Panikorovskii, T.L.; Chervonnyi, A.D. On the relationships between crystal-chemical characteristics of vesuvianite-group minerals and their IR spectra. Zap. Ross. Mineral. Obsh. 2017. in print (In Russian) [Google Scholar]
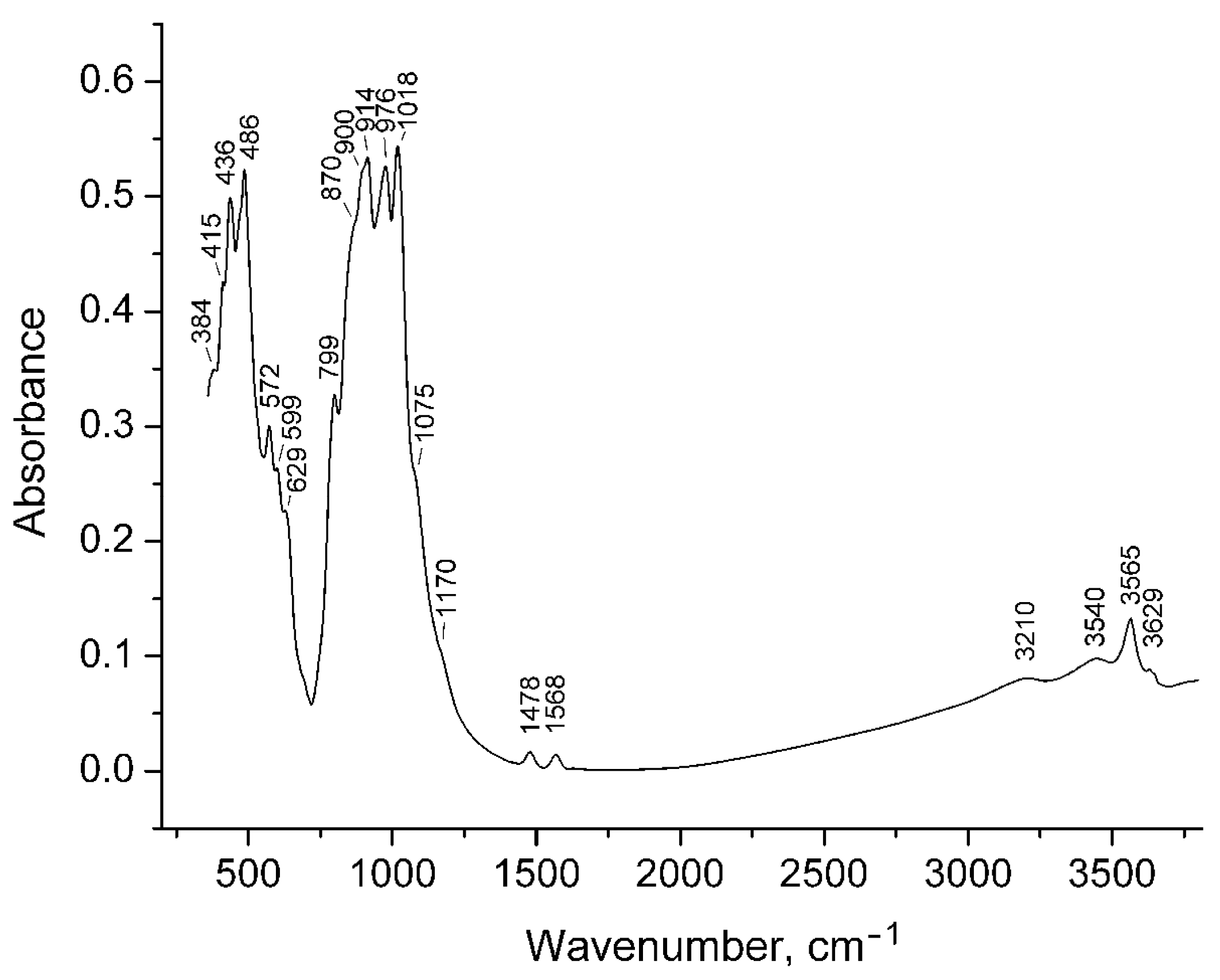
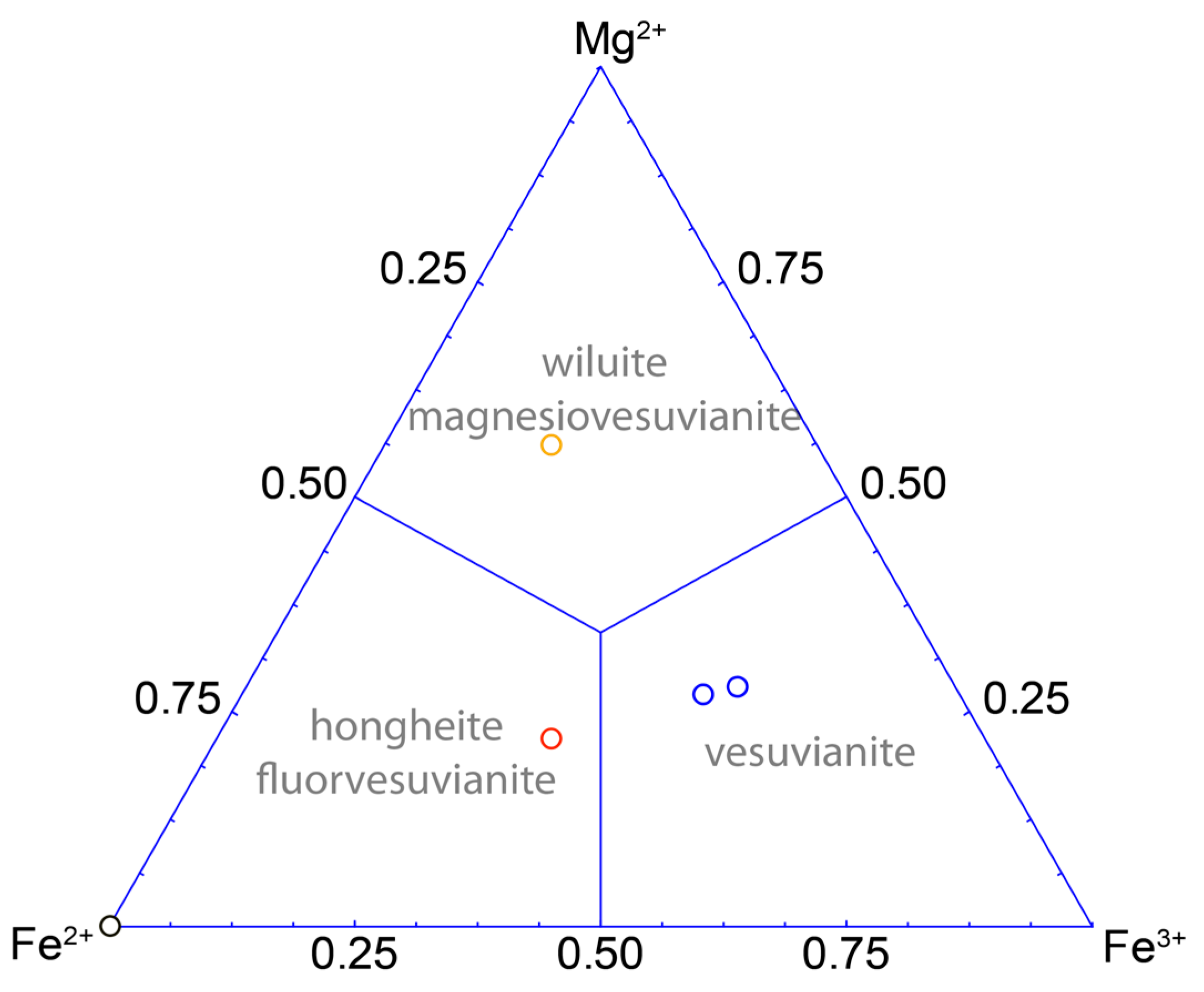
| Mineral/Formula | X1, X2, X3 | X4 | Y1 | Y2 | Y3 | T1 | T2 | O10 | O11 | O12 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vesuvianite s.s. | Ca | Ca | Fe3+ | Al | Al | □ | □ | OH | OH | □ | [30] |
| Fluorvesuvianite | Ca | Ca | Fe2+ | Al | Al | □ | □ | F | F | □ | [18] |
| Manganvesuvianite | Ca | Ca | Mn3+ | Al | Al | □ | □ | OH | OH | □ | [16] |
| Cyprine | Ca | Ca | Cu | Al | Al | □ | □ | OH | OH | □ | [23] |
| Magnesiovesuvianite | Ca | Ca | Mg | Al | Al | □ | □ | OH | OH | □ | [24] |
| Alumovesuvianite | Ca | Ca | Al | Al | Al | □ | □ | OH | OH | □ | [25] |
| Wiluite | Ca | Ca | Mg | Al | Al | B | B | O | O | O | [14,29] |
| Hongheite | Ca | Ca | Fe2+ | Al | Fe3+ | □ | B | O | O | □ | [31] |
| Sample | 27844 | 51062 |
|---|---|---|
| Temperature/K | 293(2) | 293(3) |
| Crystal system | Tetragonal | Tetragonal |
| Space group | P4/nnc | P4/nnc |
| a = b (Å) | 15.5720(3) | 15.5459(3) |
| c (Å) | 11.8158(5) | 11.7988(4) |
| Volume (Å3) | 2865.16(16) | 2851.48(16) |
| Z | 2 | 2 |
| ρcalc (g/cm−3) | 3.395 | 3.405 |
| μ (mm−1) | 2.839 | 2.837 |
| F(000) | 2941.0 | 2901.0 |
| Crystal size (mm3) | 0.21 × 0.15 × 0.14 | 0.22 × 0.19 × 0.17 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2θ range for data collection (°) | 5.232–54.982 | 6.802–54.974 |
| Index ranges | −20 ≤ h ≤ 19 | −13 ≤ h ≤ 20 |
| −15 ≤ k ≤ 20 | −14 ≤ k ≤ 15 | |
| −15 ≤ l ≤ 14 | −15 ≤ l ≤ 7 | |
| Reflections collected | 14,428 | 5687 |
| Independent reflections | 1653 [Rint = 0.0440 Rsigma = 0.0178] | 1622 [Rint = 0.0274 Rsigma = 0.0267] |
| Data/restraints/parameters | 1653/0/162 | 1622/0/162 |
| Goodness of fit on F2 | 1.184 | 1.114 |
| Final R indices [I > 2σ (I)] | R1 = 0.027 | R1 = 0.030 |
| wR2 = 0.072 | wR2 = 0.078 | |
| Final R indices [all data] | R1 = 0.028 | R1 = 0.035 |
| wR2 = 0.072 | wR2 = 0.080 | |
| Largest diff. peak/hole (e Å−3) | 0.56/−1.05 | 0.53/−1.08 |
| Component | wt % | Component | apfu | ||
|---|---|---|---|---|---|
| 27844 | 51062 | 27844 | 51062 | ||
| SiO2 | 36.41 | 35.89 | Si | 17.81 | 17.99 |
| Al2O3 | 15.82 | 15.93 | Al | 9.12 | 9.41 |
| TiO2 | bd | 0.41 | Ti | bd | 0.16 |
| Fe2O3 1 | 3.48 | 2.97 | Fe3+ | 1.28 | 1.12 |
| FeO 1 | 0.98 | 1.29 | Fe2+ | 0.40 | 0.54 |
| MnO | 0.27 | 0.38 | Mn | 0.11 | 0.16 |
| MgO | 2.51 | 3.21 | Mg | 1.83 | 2.40 |
| CaO | 36.19 | 35.19 | Ca | 18.97 | 18.90 |
| Ce2O3 | bd | 0.33 | Ce | bd | 0.06 |
| Nd2O3 | bd | 0.22 | Nd | bd | 0.04 |
| Na2O | 0.03 | bd | Na | 0.03 | bd |
| B2O3 2 | 0.83 | 0.79 | B | 0.70 | 0.68 |
| Cl | 0.06 | 0.05 | Cl | 0.05 | 0.04 |
| F | 1.29 | 1.22 | F | 2.00 | 1.93 |
| H2O 3 | 1.60 | 1.76 | OH | 5.22 | 5.88 |
| –O=F,Cl | −0.56 | −0.52 | O | 73.04 | 74.75 |
| Total | 98.90 | 98.57 | |||
| Designation of the Quadrupole Doublet | Isomer Shift (mm/s) | Quadrupole Splitting (mm/s) | Line Width (mm/s) | Relative Area (%) | Assignment 1 |
|---|---|---|---|---|---|
| Sample | 27844 | ||||
| C | 0.377 ± 0.002 | 0.492 ± 0.004 | 0.460 ± 0.004 | 54.4 ± 0.6 | VIFe3+ in Y3 |
| E | 0.481 ± 0.006 | 1.076 ± 0.011 | 12.8 ± 0.7 | VFe3+ in Y1 | |
| D | 0.821 ± 0.003 | 0.396 ± 0.004 | 17.9 ± 0.5 | VIFe2+ in Y3 | |
| B | 1.039 ± 0.012 | 2.920 ± 0.031 | 6.1 ± 0.5 | VFe2+ in Y1 | |
| G | 0.921 ± 0.009 | 2.400 ± 0.022 | 8.8 ± 0.5 | VIFe2+ in Y2 | |
| Sample | 51062 | ||||
| C | 0.377 ± 0.007 | 0.528 ± 0.014 | 0.432 ± 0.004 | 59.9 ± 0.4 | VIFe3+ in Y3 |
| E | 0.405 ± 0.004 | 1.312 ± 0.010 | 16.3 ± 0.5 | VFe3+ in Y1 | |
| D | 0.842 ± 0.030 | 0.438 ± 0.060 | 14.8 ± 0.4 | VIFe2+ in Y3 | |
| B | 1.030 ± 0.007 | 2.920 ± 0.014 | 9.0 ± 0.3 | VFe2+ in Y1 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panikorovskii, T.L.; Chukanov, N.V.; Rusakov, V.S.; Shilovskikh, V.V.; Mazur, A.S.; Balassone, G.; Ivanyuk, G.Y.; Krivovichev, S.V. Vesuvianite from the Somma-Vesuvius Complex: New Data and Revised Formula. Minerals 2017, 7, 248. https://doi.org/10.3390/min7120248
Panikorovskii TL, Chukanov NV, Rusakov VS, Shilovskikh VV, Mazur AS, Balassone G, Ivanyuk GY, Krivovichev SV. Vesuvianite from the Somma-Vesuvius Complex: New Data and Revised Formula. Minerals. 2017; 7(12):248. https://doi.org/10.3390/min7120248
Chicago/Turabian StylePanikorovskii, Taras L., Nikita V. Chukanov, Vyacheslav S. Rusakov, Vladimir V. Shilovskikh, Anton S. Mazur, Giuseppina Balassone, Gregory Yu. Ivanyuk, and Sergey V. Krivovichev. 2017. "Vesuvianite from the Somma-Vesuvius Complex: New Data and Revised Formula" Minerals 7, no. 12: 248. https://doi.org/10.3390/min7120248