Effect of Intercalation Agents on Morphology of Exfoliated Kaolinite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
3.1. XRD Analysis
3.2. Morphology Characteristics of Kaolinite upon Intercalation and Exfoliation
3.3. FTIR Analysis of Exfoliated Kaolinite
3.4. Discussion on the Morphology Changes of Exfoliated Kaolinite
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frost, R.L.; Mako, E.; Kristof, J.; Kloprogge, J.T. Modification of kaolinite surfaces through mechanochemical treatment—A mid-IR and near-IR spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 2849–2859. [Google Scholar] [CrossRef]
- Martens, W.N.; Frost, R.L.; Kristof, J.; Horvath, E. Modification of kaolinite surfaces through intercalation with deuterated dimethylsulfoxide. J. Phys. Chem. B 2002, 106, 4162–4171. [Google Scholar] [CrossRef]
- Detellier, C.; Schoonheydt, R.A. From Platy Kaolinite to Nanorolls. Elements 2014, 10, 201–206. [Google Scholar] [CrossRef]
- Quiroz-Estrada, K.; Hernández-Espinosa, M.; Rojas, F.; Portillo, R.; Rubio, E.; López, L. N2 and CO2 adsorption by soils with high kaolinite content from San Juan Amecac, Puebla, México. Minerals 2016, 6, 73. [Google Scholar] [CrossRef]
- Cheng, H.F.; Zhou, Y.; Feng, Y.P.; Geng, W.X.; Liu, Q.F.; Guo, W.; Jiang, L. Electro kinetic energy conversion in self-assembled 2D nanofluidic channels with janus nano building blocks. Adv. Mater. 2017, 29, 1700177–1700183. [Google Scholar] [CrossRef] [PubMed]
- Dedzo, G.K.; Detellier, C. Characterization and applications of kaolinite robustly grafted by an ionic liquid with naphthyl functionality. Materials 2017, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Bundy, W.M.; Ishley, J.N. Kaolin in paper filling and coating. Appl. Clay Sci. 1991, 5, 397–420. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Aras, A. The change of phase composition in kaolinite- and illite-rich clay-based ceramic bodies. Appl. Clay Sci. 2004, 24, 257–269. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Nkoumbou, C.; Njoya, A.; Njoya, D.; Grosbois, C.; Njopwouo, D.; Yvon, J.; Martin, F. Kaolin from Mayouom (Western Cameroon): Industrial suitability evaluation. Appl. Clay Sci. 2009, 43, 118–124. [Google Scholar] [CrossRef]
- Cheng, H.F.; Liu, Q.F.; Zhang, J.S.; Yang, J.; Frost, R.L. Delamination of kaolinite-potassium acetate intercalates by ball-milling. J. Colloid Interface Sci. 2010, 348, 355–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, Q.F.; Cheng, H.F.; Komarneni, S. High-yield production of mesoporous nanoscrolls from kaolinite by ultrasonic assisted exfoliation. Microporous Mesoporous Mater. 2017, 241, 66–71. [Google Scholar] [CrossRef]
- Komori, Y.; Sugahara, Y. A kaolinite-NMF-methanol intercalation compound as a versatile intermediate for further intercalation reaction of kaolinite. J. Mater. Res. 1998, 13, 930–934. [Google Scholar] [CrossRef]
- Olejnik, S.; Aylmore, L.; Posner, A.M.; Quirk, J.P. Infrared spectra of kaolin mineral-dimethyl sulfoxide complexes. J. Phys. Chem. 1968, 72, 241–249. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Composite of Coal-Series Kaolinite and Capric-lauricacid as form-stable phase-change material. Energy Technol. 2015, 3, 77–83. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Rintoul, L.; Kloprogge, J.T. Raman spectroscopy of urea and urea-intercalated kaolinites at 77 K. Spectrochim. Acta AMol. Biomol. Spectrosc. 2000, 56, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Kristof, J.; Paroz, G.N.; Kloprogge, J.T. Modification of the Kaolinite Hydroxyl Surfaces through Intercalation with Potassium Acetate under Pressure. J. Colloid Interface Sci. 1998, 208, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Li, K.; Liu, Q.F.; Zhang, S.; Li, X.G.; Frost, R.L. Insight into the thermal decomposition of kaolinite intercalated with potassium acetate: An evolved gas analysis. J. Therm. Anal. Calorim. 2014, 117, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Kloprogge, J.T.; Kristof, J.; Horvath, E. Deintercalation of hydrazine-intercalated low-defect kaolinite. Clays Clay Miner. 1999, 47, 732–741. [Google Scholar] [CrossRef]
- Komori, Y.; Sugahara, Y.; Kuroda, K. Intercalation of alkylamines and water into kaolinite with methanol kaolinite as an intermediate. Appl. Clay Sci. 1999, 15, 241–252. [Google Scholar] [CrossRef]
- Kuroda, Y.; Ito, K.; Itabshi, K.; Kuroda, K. One-Step Exfoliation of Kaolinites and Their Transformation into Nanoscrolls. Langmuir 2011, 27, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Cui, X.J.; Wang, S.; Wang, D.; Li, K.; Liu, Q.F.; Komarneni, S. Methoxy-grafted kaolinite preparation by intercalation of methanol: Mechanism of its structural variability. Appl. Clay Sci. 2017, 137, 241–248. [Google Scholar] [CrossRef]
- Matusik, J.; Scholtzová, E.; Tunega, D. Influence of synthesis conditions on the formation of a kaolinite-methanol complex and simulation of its vibrational spectra. Clays Clay Miner. 2012, 60, 227–239. [Google Scholar] [CrossRef]
- Gardolinski, J.E.F.C.; Lagaly, G. Grafted organic derivatives of kaolinite: II. Intercalation of primary n-alkylamines and delamination. Clay Miner. 2005, 40, 547–556. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q.F.; Cheng, H.F.; Zhang, S.; Zuo, X.C. Effect of reaction temperature on intercalation of octyltrimethyl ammonium chloride into kaolinite. J. Therm. Anal. Calorim. 2017, 128, 1555–1564. [Google Scholar] [CrossRef]
- Matusik, J.; Kłapyta, Z. Characterization of kaolinite intercalation compounds with benzylalkylammonium chlorides using XRD, TGA/DTA and CHNS elemental analysis. Appl. Clay Sci. 2013, 83–84, 433–440. [Google Scholar] [CrossRef]
- Matusik, J.; Kłapyta, Z.; Olejniczak, Z. NMR and IR study of kaolinite intercalation compounds with benzylalkylammonium chlorides. Appl. Clay Sci. 2013, 83–84, 426–432. [Google Scholar] [CrossRef]
- Sidheswaran, P.; Bhat, A.N.; Ganguli, P. Intercalation of salts of fatty acids into kaolinite. Clays Clay Miner. 1990, 38, 29–32. [Google Scholar] [CrossRef]
- Wang, S.; Zuo, X.C.; Cheng, H.F.; Yang, Y.J.; Liu, Q.F. Structural model and de-intercalation kinetics of kaolinite-methanol-sodium stearate intercalation compound. J. Braz. Chem. Soc. 2016, 27, 1311–1318. [Google Scholar] [CrossRef]
- Liu, Q.F.; Li, X.G.; Cheng, H.F. Insight into the self-adaptive deformation of kaolinite layers into nanoscrolls. Appl. Clay Sci. 2016, 124–125, 175–182. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.Y.; Annabi-Bergaya, F.; Yan, W.C.; Liu, D.; Liu, Z.W. From platy kaolinite to aluminosilicate nanoroll via one-step delamination of kaolinite: Effect of the temperature of intercalation. Appl. Clay Sci. 2013, 83–84, 68–76. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.F.; Cheng, H.F.; Zeng, F.G. Combined experimental and theoretical investigation of interactions between kaolinite inner surface and intercalated dimethyl sulfoxide. Appl. Clay Sci. 2015, 331, 234–240. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Cheng, H.; Zhang, S.; Frost, R.L. Mechanism of kaolinite sheets curling via the intercalation and delamination process. J. Colloid Interface Sci. 2015, 444, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G. Characterization of clays by organic compounds. Clay. Miner. 1981, 1–21. [Google Scholar] [CrossRef]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
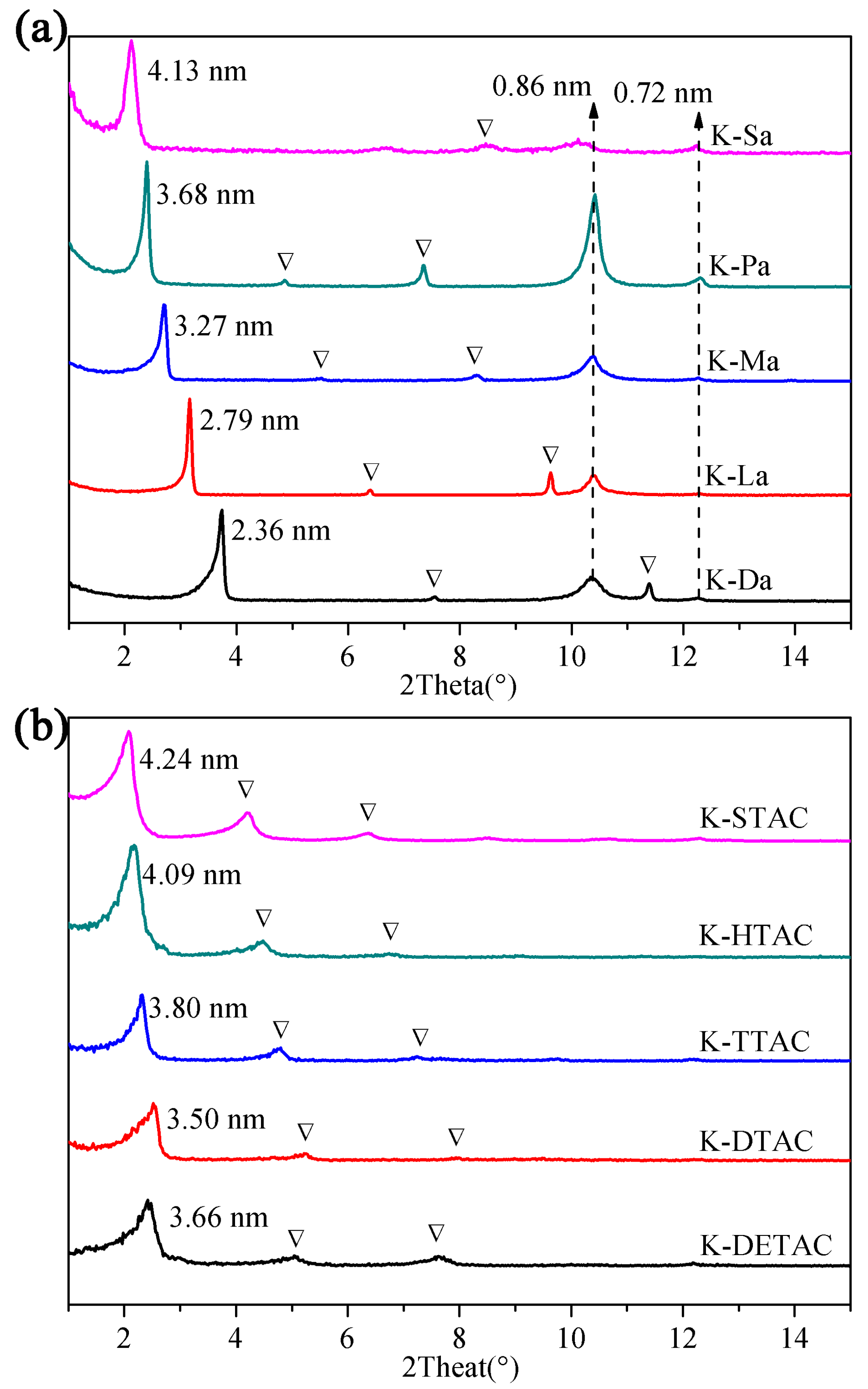
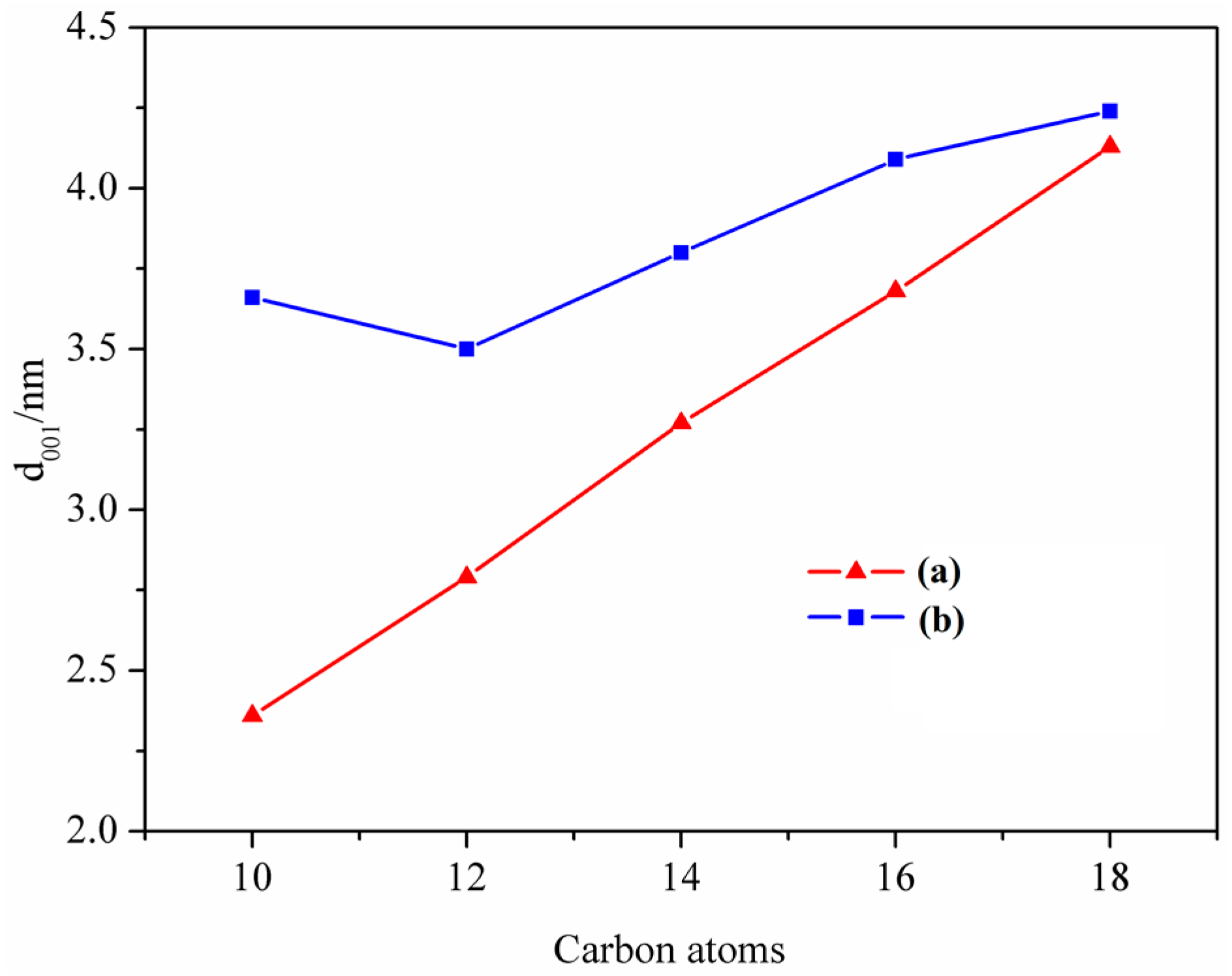

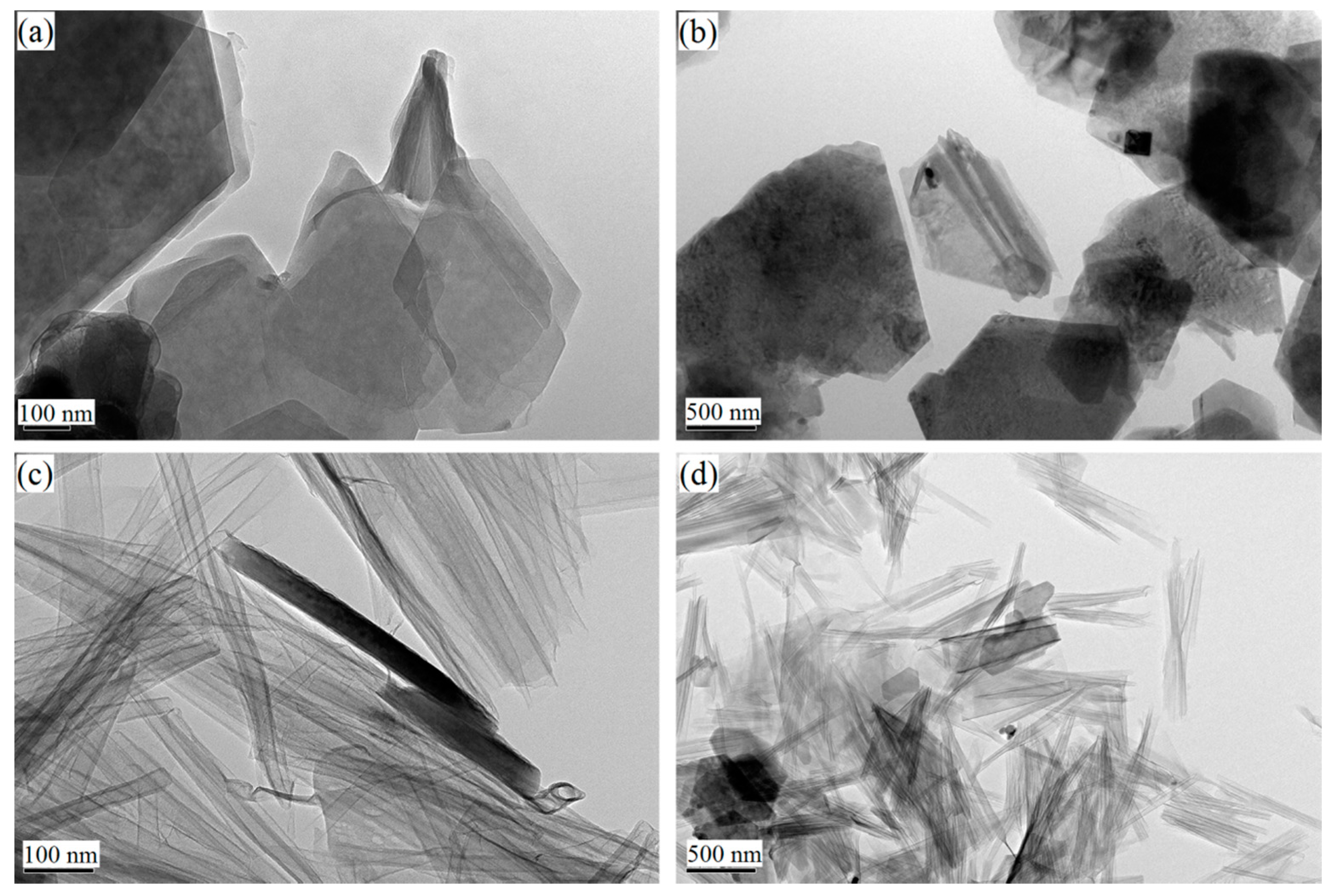
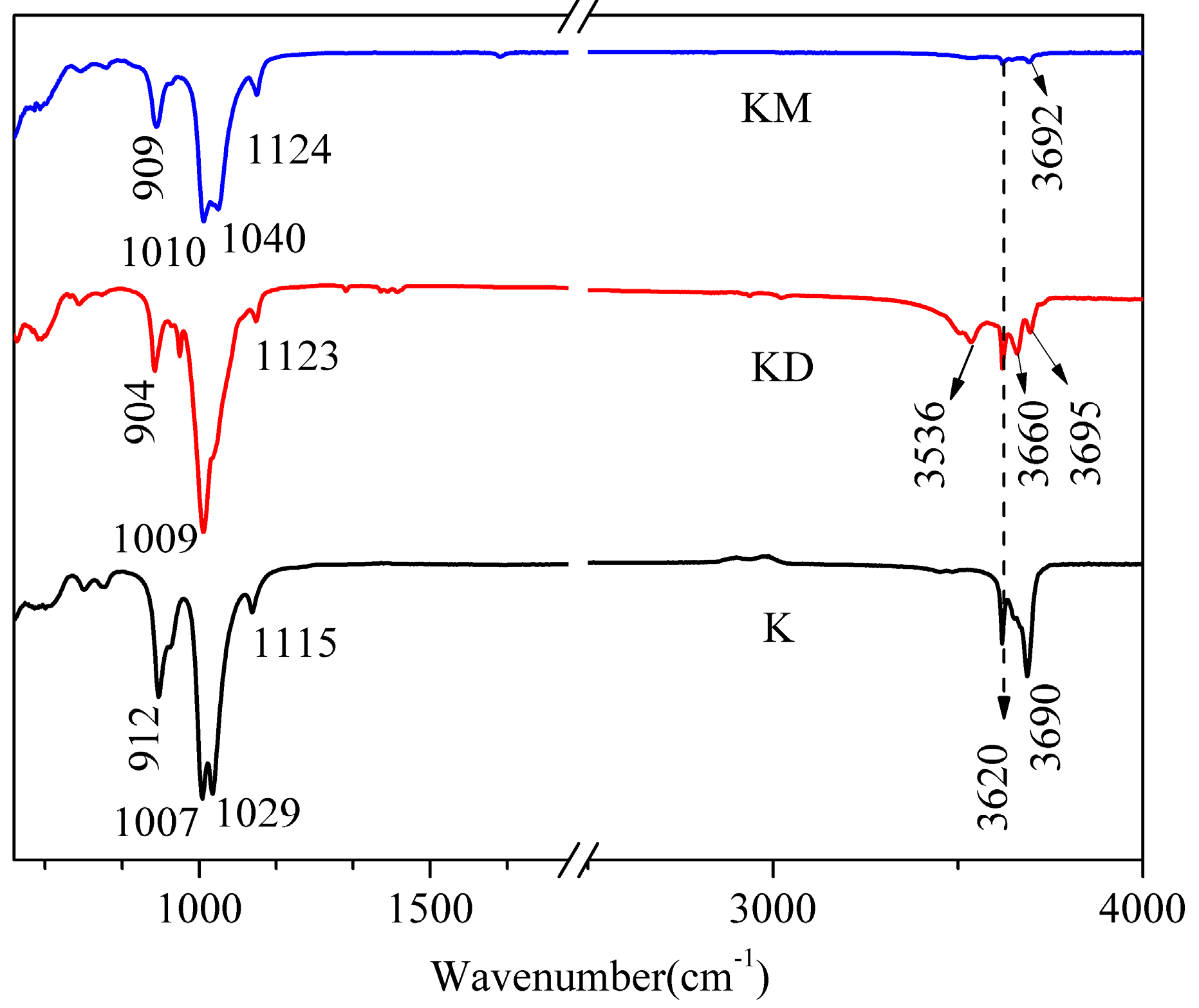
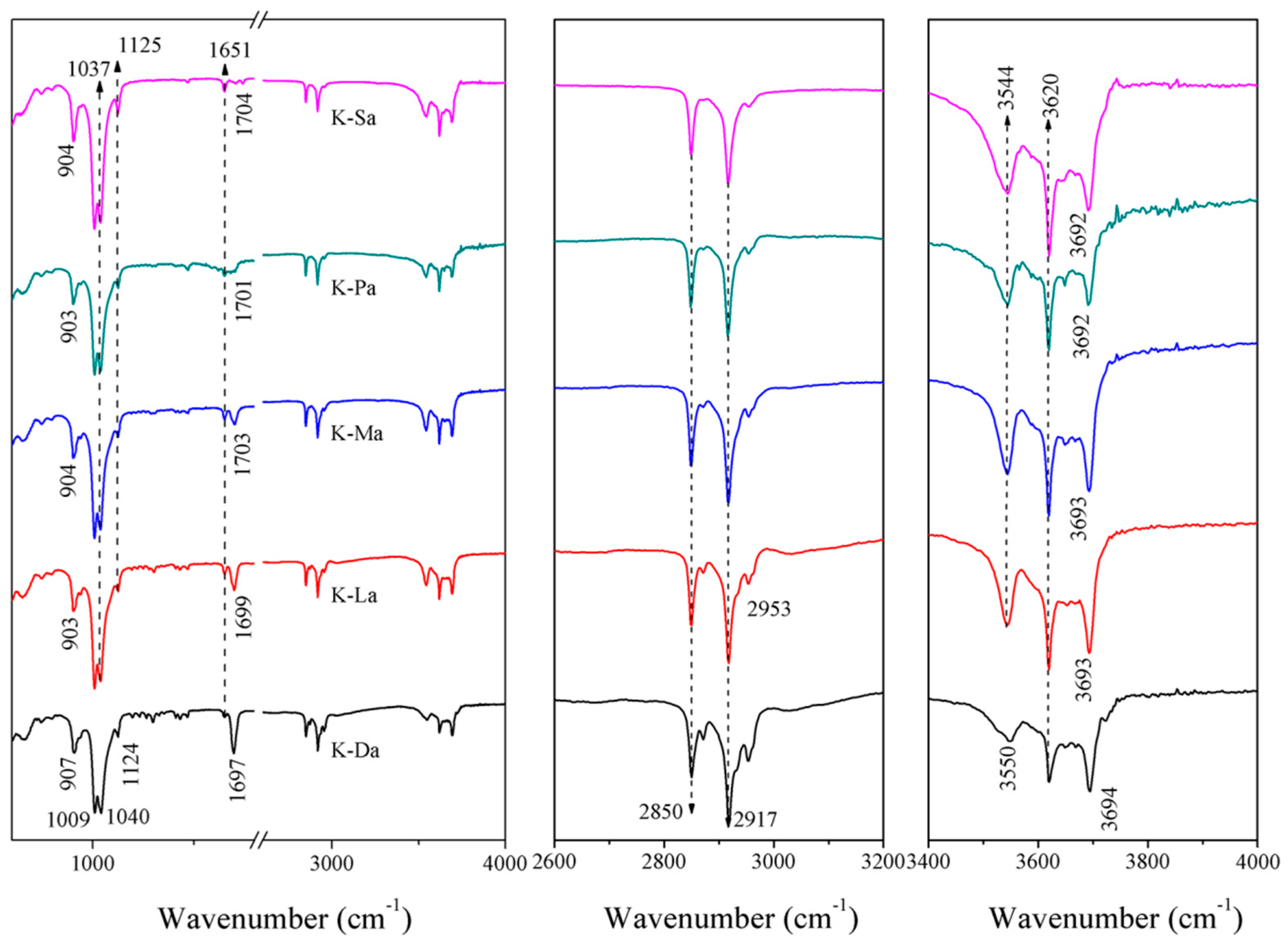
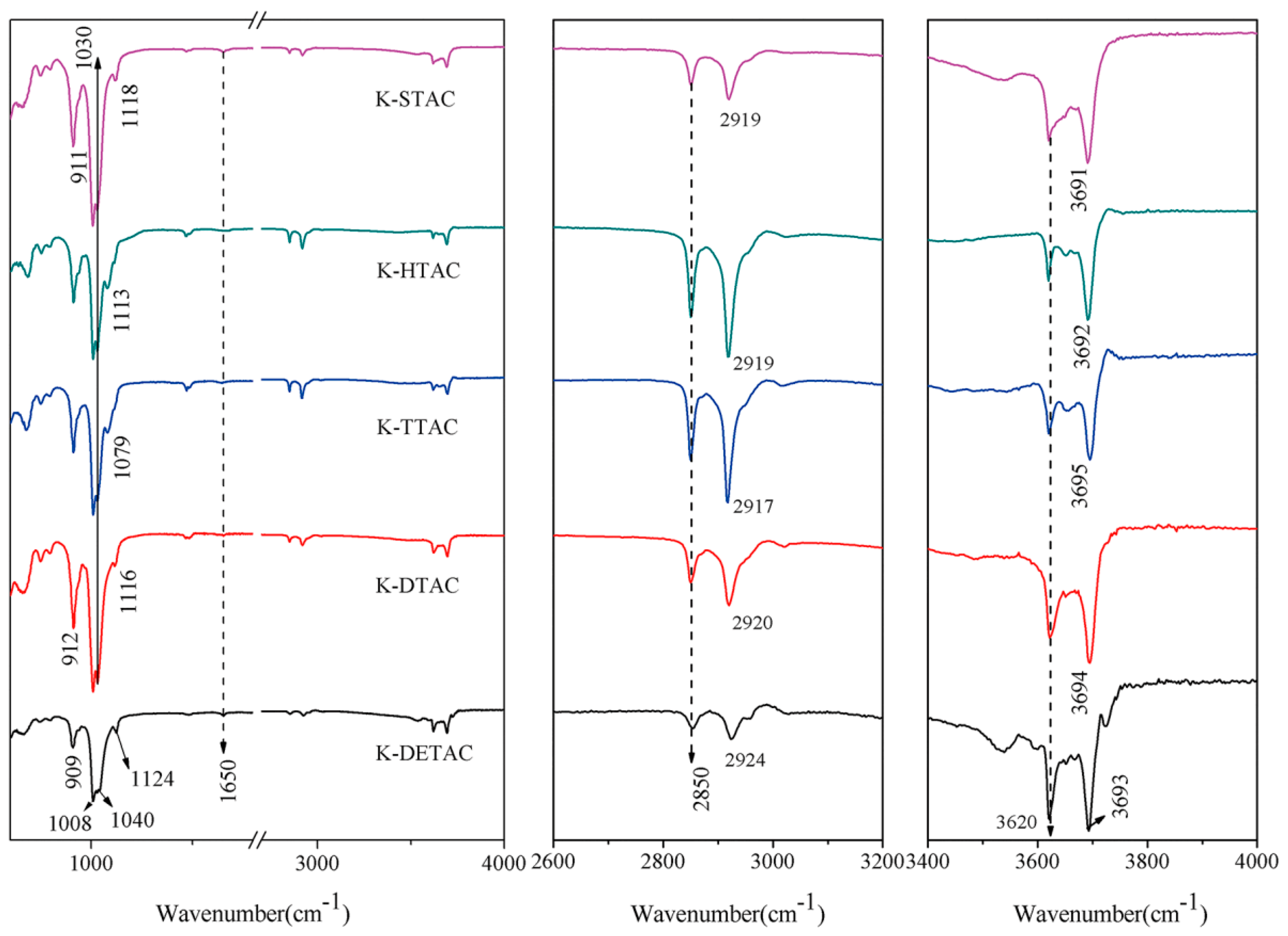
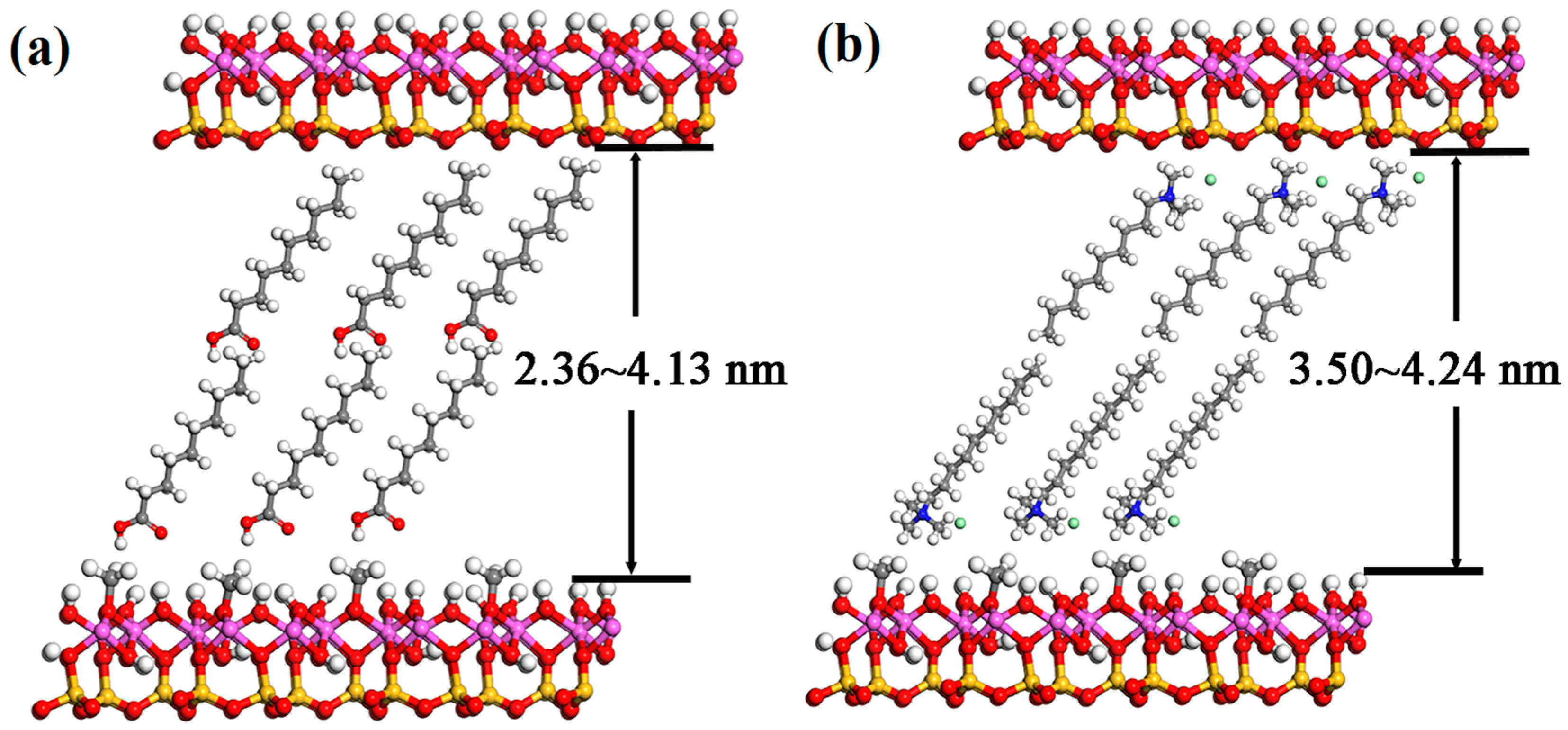
| Fatty Acid | Molecular Chain Length (Å) | d001 (Å) | △d (Å) | Quaternary Ammonium Salt | Molecular Chain Length (Å) | d001 (Å) | △d (Å) |
|---|---|---|---|---|---|---|---|
| Decanoic acid | 12.42 | 23.6 | - | DETAC | 14.60 | 36.6 | - |
| Lauric acid | 14.97 | 27.9 | 4.3 | DTAC | 17.15 | 35.0 | −1.6 |
| Myristic acid | 17.52 | 32.7 | 4.8 | TTAC | 19.70 | 38.0 | 3.0 |
| Palmitic acid | 20.07 | 36.8 | 4.1 | HTAC | 22.25 | 40.9 | 2.9 |
| Stearic acid | 22.62 | 41.3 | 4.5 | STAC | 24.80 | 42.4 | 1.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Wang, D.; Zhang, S.; Liu, Q.; Yang, H. Effect of Intercalation Agents on Morphology of Exfoliated Kaolinite. Minerals 2017, 7, 249. https://doi.org/10.3390/min7120249
Zuo X, Wang D, Zhang S, Liu Q, Yang H. Effect of Intercalation Agents on Morphology of Exfoliated Kaolinite. Minerals. 2017; 7(12):249. https://doi.org/10.3390/min7120249
Chicago/Turabian StyleZuo, Xiaochao, Ding Wang, Shilong Zhang, Qinfu Liu, and Huaming Yang. 2017. "Effect of Intercalation Agents on Morphology of Exfoliated Kaolinite" Minerals 7, no. 12: 249. https://doi.org/10.3390/min7120249





