Minor and Trace Elements in Natural Tetrahedrite-Tennantite: Effects on Element Partitioning among Base Metal Sulphides
Abstract
:1. Introduction
2. Background
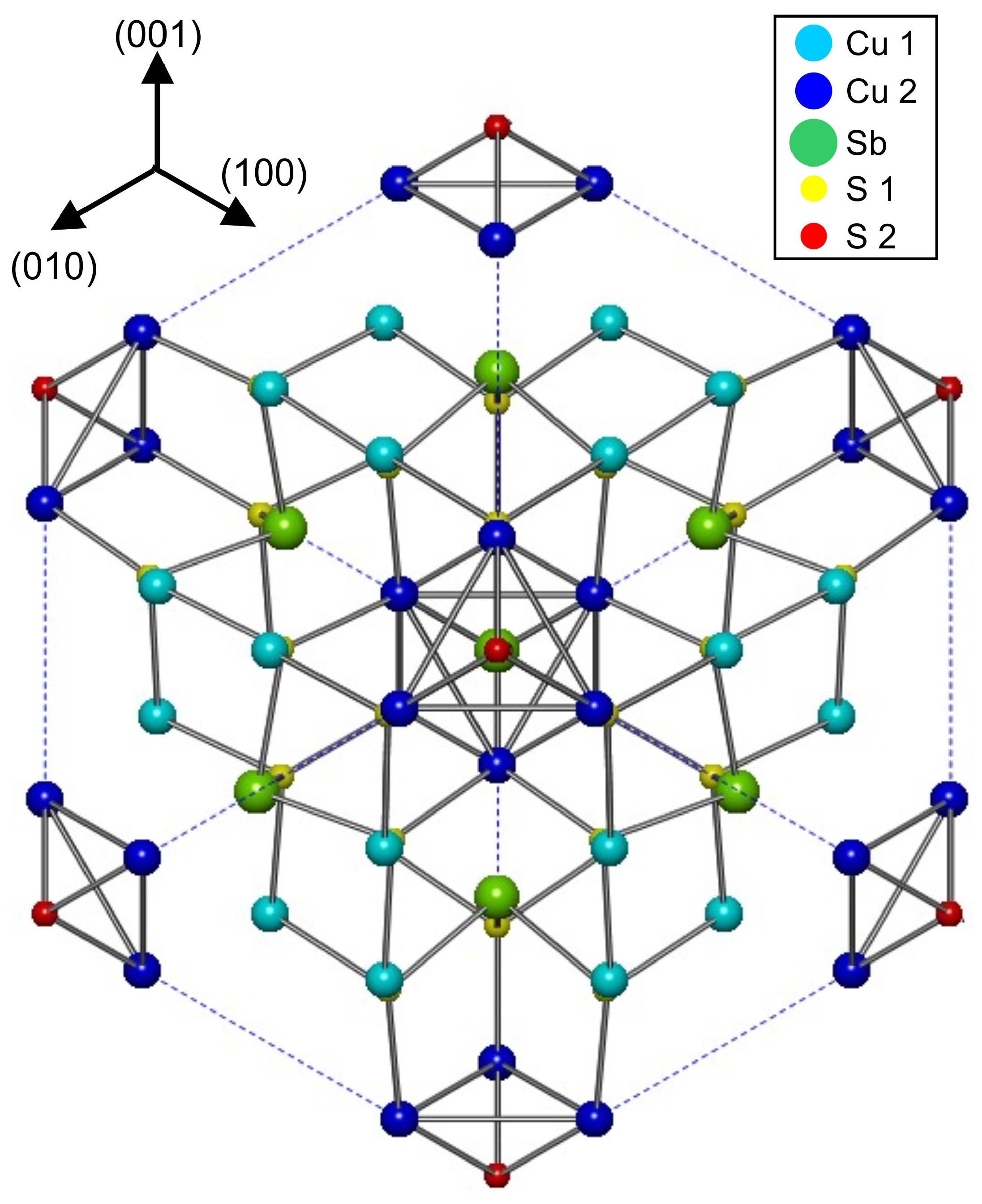
2.1. Crystal Structure
2.2. Documented Substitutions
3. Approach and Methodology
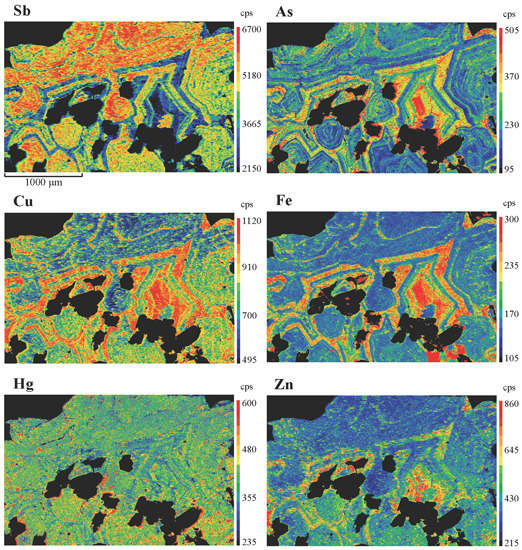
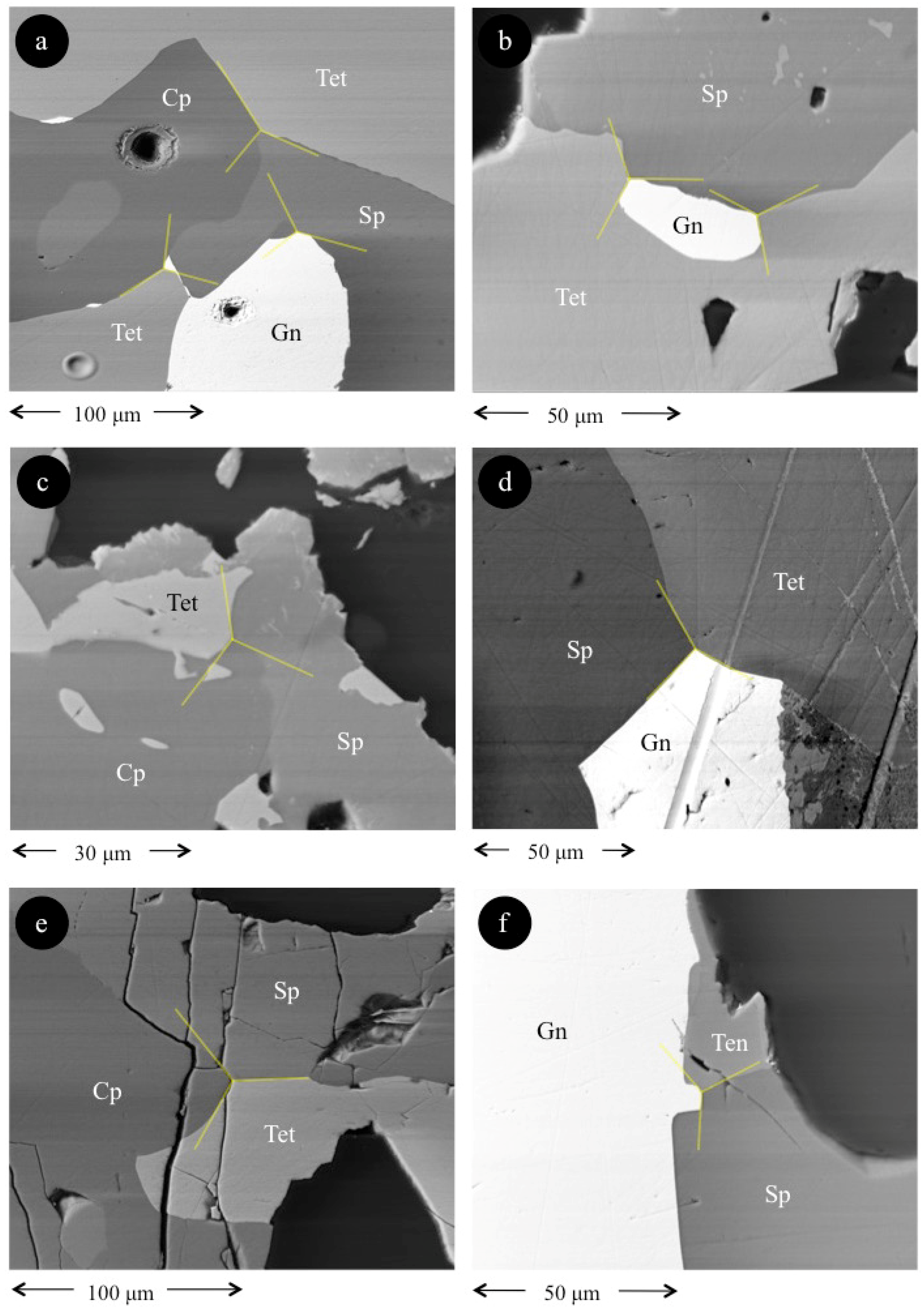
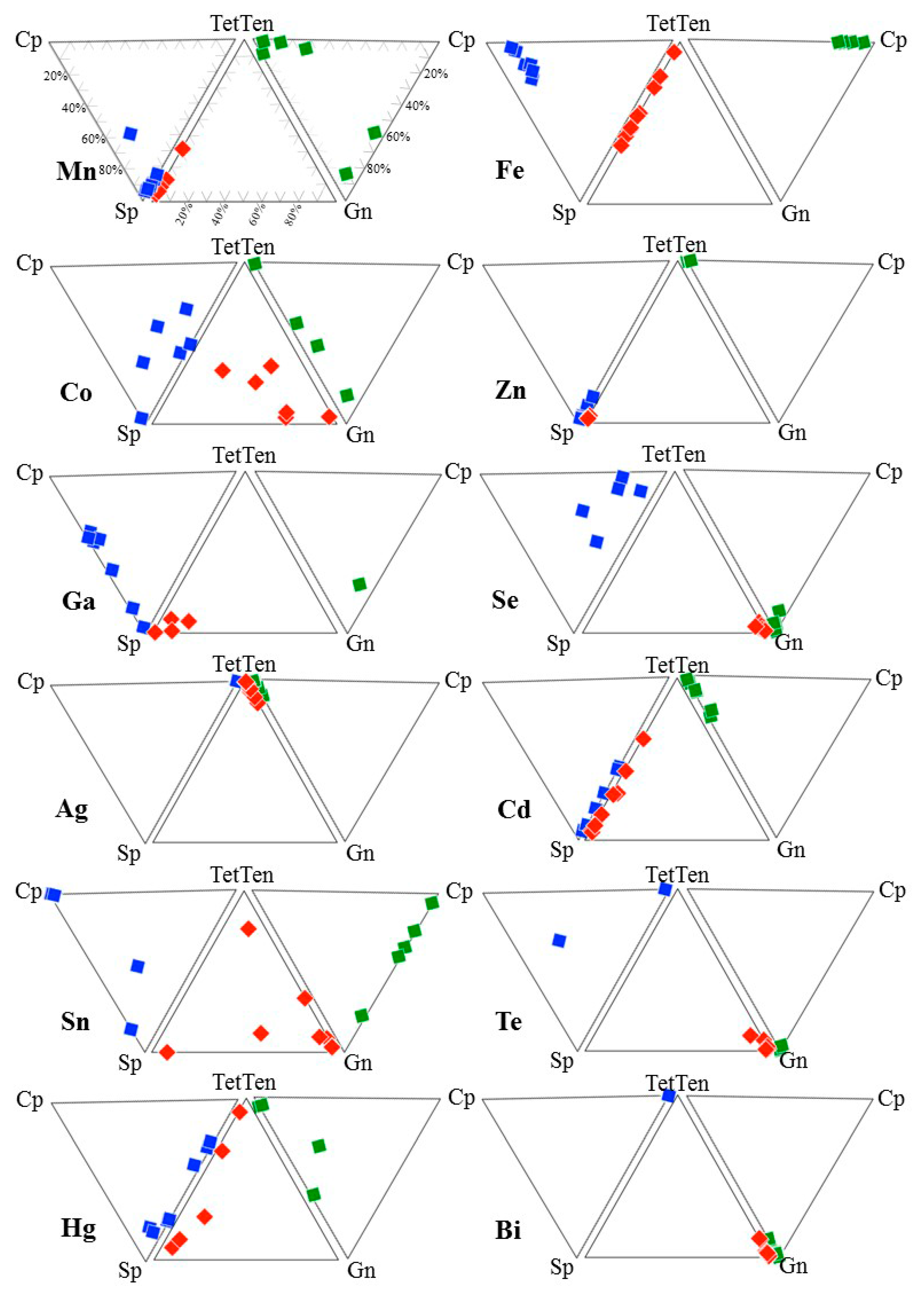
4. Results
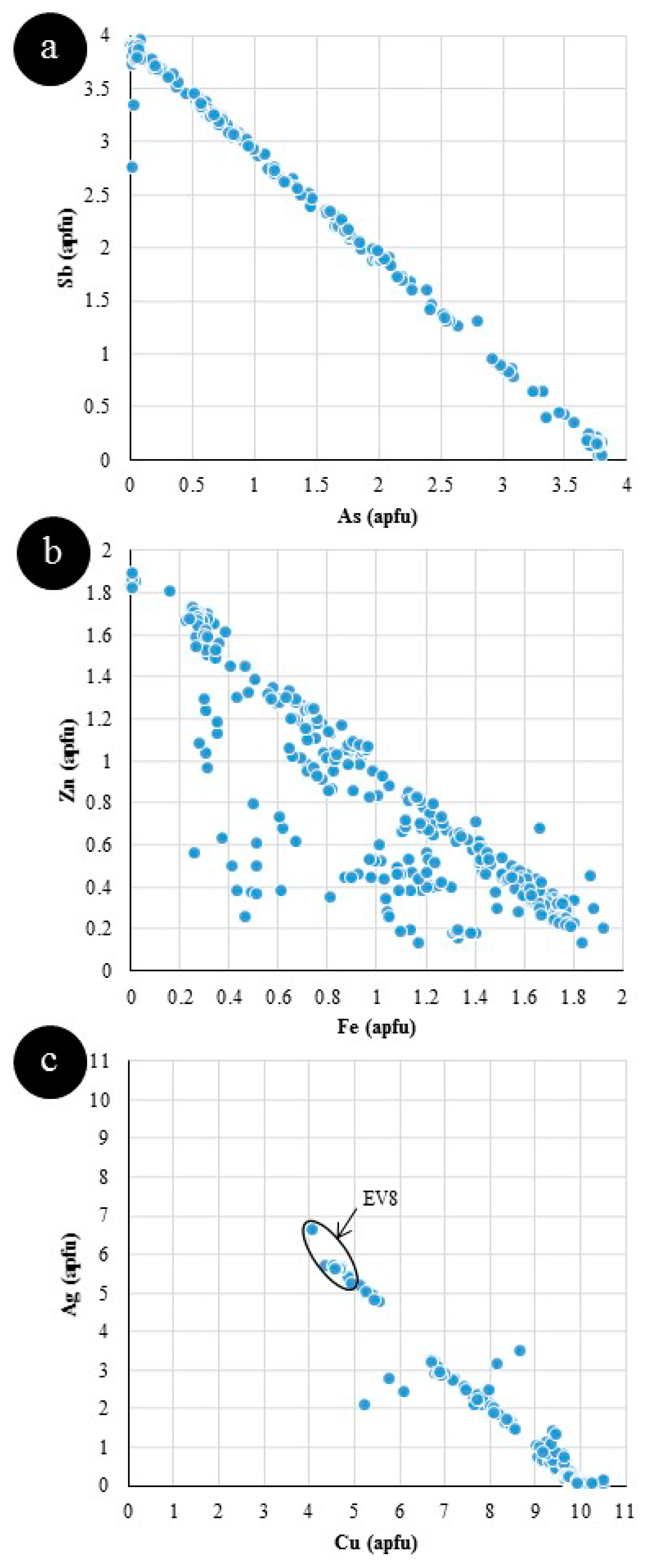
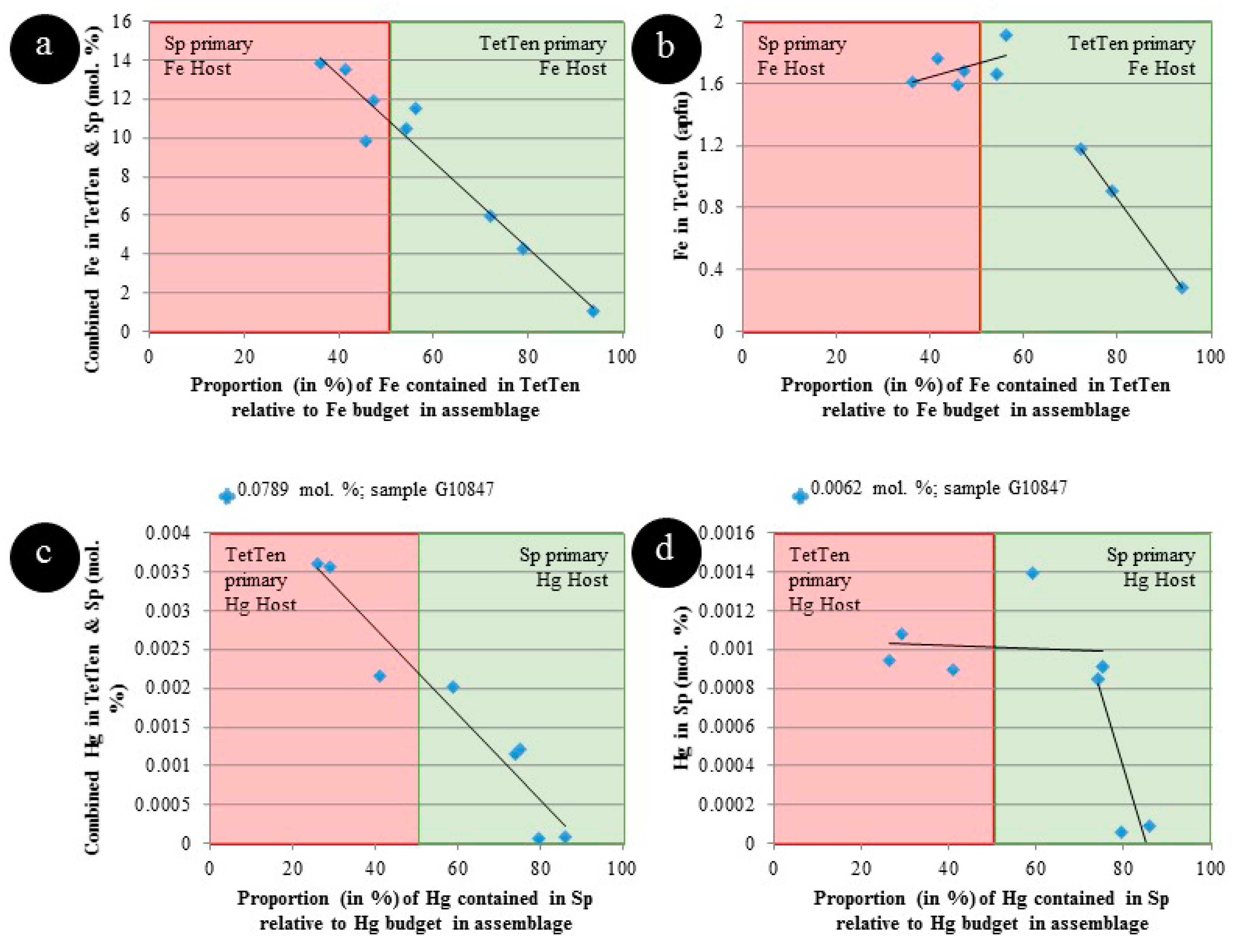
5. Discussion
5.1. Element Partitioning Between Tetrahedrite-Tennantite, Sphalerite, Galena, and Chalcopyrite
5.2. Controls on Fe and Hg Partitioning
6. Implications and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wuensch, B.J. The crystal structure of tetrahedrite, Cu12Sb4S13. Zeitschrift Krist. Cryst. Mater. 1964, 119, 437–453. [Google Scholar] [CrossRef]
- Wuensch, B.J.; Tackeuchi, Y.; Nowacki, W. Refinement of the crystal structure of binnite, Cu12As4S13. Zeitschrift Krist. Cryst. Mater. 1966, 123, 1–20. [Google Scholar] [CrossRef]
- Makovicky, E.; Skinner, B. Studies of the sulfosalts of copper. VII. Crystal structures of the exsolution products Cu12.3Sb4S13 and Cu13.8Sb4S13 of unsubstituted synthetic tetrahedrite. Can. Mineral. 1979, 17, 619–634. [Google Scholar]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.J.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, G.; Karup-Møller, S.; et al. Sulfosalt systematics: A review. Report of the sulfosalt sub-committee of the IMA commission on ore mineralogy. Eur. J. Mineral. 2008, 20, 7–46. [Google Scholar] [CrossRef]
- Repstock, A.; Voudouris, P.; Kolitsch, U. New occurrences of watanabeite, colusite, “arsenosulvanite” and “Cu-excess” tetrahedrite-tennantite at the Pefka high-sulfidation epithermal deposit, northeastern Greece. Neues Jahrb. Miner. Abh. J. Miner. Geochem. 2015, 192, 135–149. [Google Scholar] [CrossRef]
- Repstock, A.; Voudouris, P.; Zeug, M.; Melfos, V.; Zhai, M.; Li, H.; Kartal, T.; Matuszczak, J. Chemical composition and varieties of fahlore-group minerals from Oligocene mineralization in the Rhodope area, Southern Bulgaria and Northern Greece. Mineral. Petrol. 2016, 110, 103–123. [Google Scholar] [CrossRef]
- Makovicky, E.; Karanović, L.; Poleti, D.; Balić-Žunić, T.; Paar, W.H. Crystal structure of copper-rich unsubstituted tennantite, Cu12.5As4S13. Can. Mineral. 2005, 43, 679–688. [Google Scholar] [CrossRef]
- Sack, R.O.; Loucks, R.R. Thermodynamic properties of tetrahedrite-tennantites: Constraints on the interdependence of the Ag ↔ Cu, Fe ↔ Zn, Cu ↔ Fe, and As ↔ Sb exchange reactions. Am. Mineral. 1985, 70, 1270–1289. [Google Scholar]
- Johnson, N.E.; Craig, J.R.; Rimstidt, J.D. Compositional trends in tetrahedrite. Can. Mineral. 1986, 24, 385–397. [Google Scholar]
- Makovicky, E.; Karup-Möller, S. Exploratory studies on substitution of minor elements in synthetic tetrahedrite. Part I. Substitution by Fe, Zn, Co, Ni, Mn, Cr, V and Pb. Unit-cell parameter changes on substitution and the structural role of “Cu2+”. Neues Jahrb. Miner. Abh. 1994, 167, 89–123. [Google Scholar]
- Hackbarth, C.J.; Petersen, U. A fractional crystallization model for the deposition of argentian tetrahedrite. Econ. Geol. 1984, 79, 448–460. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Bortnikov, N.S. Chemical composition and mineral associations of sulphosalts in the precious metal deposits from different geological environment. Geol. Carpathica 1985, 36, 283–291. [Google Scholar]
- Staude, S.; Mordhorst, T.; Neumann, R.; Prebeck, W.; Markl, G. Compositional variation of the tennantite-tetrahedrite solid-solution series in the Schwarzwald ore district (SW Germany): The role of mineralization processes and fluid source. Mineral. Mag. 2010, 74, 309–339. [Google Scholar] [CrossRef]
- Apopei, A.I.; Damian, G.; Buzgar, N.; Buzatu, A. Mineralogy and geochemistry of Pb-Sb/As-sulfosalts from Coranda-Hondol ore deposit (Romania)—Conditions of telluride deposition. Ore Geol. Rev. 2016, 72, 857–873. [Google Scholar] [CrossRef]
- Peterson, R.C.; Miller, I. Crystal structure and cation distribution in freibergite and tetrahedrite. Mineral. Mag. 1986, 50, 717–721. [Google Scholar] [CrossRef]
- Pfitzner, A.; Evain, M.; Petricek, V. Cu12Sb4S13: A temperature-dependent structure investigation. Acta Cryst. 1997, B53, 337–345. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Zayakina, N.V.; Samusikov, V.P. Crystal structure features of minerals from the tetrahedrite-freibergite series. Mineral. Zhurnal 1993, 15, 9–17. (In Russian) [Google Scholar]
- Johan, Z.; Kvaček, M. La hakite, un nouveau minéral du groupe de la tétraédrite. Bull. Soc. Fr. Minéral. 1971, 94, 45–48. (In French) [Google Scholar]
- Johan, Z.; Picot, P.; Ruhlmann, F. Evolution paragénétique de la minéralisation uranifère de Chaméane (Puy-de-Dôme) France: Chaméanite, geffroyite et giraudite, trois séléniures nouveaux de Cu, Fe, Ag, and As. Tscher. Miner. Petrog. Mitt. 1982, 29, 151–167. (In French) [Google Scholar] [CrossRef]
- Kalbskopf, R. Synthese und Kristallstruktur von Cu12−xTe4S13, dem Tellur-Endglied der Fahlerze. Tscher. Miner. Petrog. Mitt. 1974, 21, 1–10. (In German) [Google Scholar] [CrossRef]
- Dmitrieva, M.T.; Bojik, G.B. The crystallochemical mechanism of formation of vacancies in goldfieldite structure. Zeitschrift Krist. 1988, 185, 601. [Google Scholar]
- Trudu, A.G.; Knittel, U. Crystallography, mineral chemistry and chemical nomenclature of goldfeldite, the tellurian member of the tetrahedrite solid-solution series. Can. Mineral. 1998, 36, 1115–1137. [Google Scholar]
- Spiridonov, E.M.; Sokolova, N.F.; Gapeyev, A.K.; Dashevskaya, D.M.; Yevstigneyeva, T.L.; Chvileva, T.N.; Demidov, V.G.; Balashov, Y.P.; Shul’ga, V.I. The new mineral argentotennantite. Dokl. Akad. Nauk SSSR 1986, 290, 206–210. (In Russian) [Google Scholar]
- Zhdanov, Y.Y.; Amuzinskii, V.A.; Andrianov, N.G. Discovery of a natural Ag-rich fahlore with the highest parameter of the unit cell. Dokl. Akad. Nauk SSSR 1992, 326, 337–340. (In Russian) [Google Scholar]
- Karup-Møller, S. Exploratory studies on element substitutions in synthetic tetrahedrite. Part V. Mercurian tetrahedrite. Neues Jahrb. Miner. Abh. 2003, 179, 73–83. [Google Scholar] [CrossRef]
- Karup-Møller, S.; Makovicky, E. Exploratory studies of element substitutions in synthetic tetrahedrite. Part II. Selenium and tellurium as anions in Zn-Fe tetrahedrites. Neues Jahrb. Miner. Abh. 1999, 9, 385–399. [Google Scholar]
- Karup-Møller, S.; Makovicky, E. Exploratory studies of the solubility of minor elements in tetrahedrite: VI. Zinc and the combined zinc-mercury and iron-mercury substitutions. Neues Jahrb. Miner. Mon. 2004, 11, 508–524. [Google Scholar] [CrossRef]
- Hansen, M.K.; Makovicky, E.; Karup-Møller, S. Exploratory studies on substitutions in tennantite-tetrahedrite solid solution. Part IV. Substitution of germanium and tin. Neues Jahrb. Miner. Abh. J. Miner. Geochem. 2003, 179, 43–71. [Google Scholar] [CrossRef]
- Klünder, M.H.; Karup-Møller, S.; Makovicky, E. Exploratory studies on substitutions in the tetrahedrite-tennantite solid solution series Part III. The solubility of bismuth in tetrahedrite-tennantite containing iron and zinc. Neues Jahrb. Miner. Mon. 2003, 2003, 153–175. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in co-crystallized sphalerite–galena–chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- Foit, F.F., Jr.; Hughes, M.J. Structural variations in mercurian tetrahedrite. Am. Mineral. 2004, 89, 159–163. [Google Scholar] [CrossRef]
- Johnson, M.L.; Burnham, C.W. Crystal structure refinement of an arsenic-bearing argentian tetrahedrite. Am. Mineral. 1985, 70, 165–170. [Google Scholar]
- Johnson, N.E.; Craig, J.R.; Rimstidt, J.D. Crystal chemistry of tetrahedrite. Am. Mineral. 1988, 73, 389–397. [Google Scholar]
- Pattrick, R.A.D.; Hall, A.J. Silver substitution into synthetic zinc, cadmium and iron tetrahedrites. Mineral. Mag. 1983, 47, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Charnock, J.M.; Garner, C.D.; Pattrick, R.A.D.; Vaughan, D.J. Co-ordination sites of metals in tetrahedrite minerals determined by EXAFS. J. Solid State Chem. 1989, 82, 279–289. [Google Scholar] [CrossRef]
- Charnock, J.M.; Garner, C.D.; Pattrick, R.A.D.; Vaughan, D.J. EXAFS and Mössbauer spectroscopic study of Fe-bearing tetrahedrites. Mineral. Mag. 1989, 53, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Pattrick, R.A.D.; van der Lann, G.; Vaughan, D.J.; Henderson, C.M.B. Oxidation state and electronic configuration determination of copper in tetrahedrite group minerals by L-edge X-ray absorption spectroscopy. Phys. Chem. Miner. 1993, 20, 395–401. [Google Scholar] [CrossRef]
- Oen, I.S.; Kieft, C. Bismuth-rich tennantite and tetrahedrite in the Mangualde pegmatite, Viseu district, Portugal. Neues Jahrb. Miner. Mon. 1976, 2, 94–96. [Google Scholar]
- Bortnikov, N.S.; Kudryavtsev, A.S.; Troneva, N.V. Bi-rich tetrahedrite from the Tary-Ekan deposit (East Karamazar, central Asia). Mineral. Zhurnal 1979, 198, 61–64. (In Russian) [Google Scholar]
- Kieft, K.; Eriksson, G. Regional zoning and metamorphic evolution of the Vindfall Pb-Zn ore, east central Sweden. GFF 1984, 106, 305–317. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Chvileva, T.N.; Borodaev, Y.S.; Vinogradova, R.A.; Kononov, O.V. The influence of bismuth on optical properties of gray copper. Dokl. Akad. Nauk SSSR 1986, 290, 1475–1478. (In Russian) [Google Scholar]
- Breskovska, V.V.; Tarkian, M. Compositional variation in Bi-bearing fahlores. Neues Jahrb. Miner. Mon. 1994, 5, 230–240. [Google Scholar]
- Gołębiowska, B.; Pieczka, A.; Parafiniuk, J. Substitution of Bi for Sb and As in minerals of the tetrahedrite series from Rędziny, Lower Silesia, southwestern Poland. Can. Mineral. 2012, 50, 267–279. [Google Scholar] [CrossRef]
- Nash, J.T. Geochemical studies in the Park City District; II, Sulfide mineralogy and minor-element chemistry, Mayflower Mine. Econ. Geol. 1975, 70, 1038–1049. [Google Scholar] [CrossRef]
- Basu, K.; Bortnykov, N.; Mookherjee, A.; Mozgova, N.; Tsepin, A.I. Rare minerals from Rajpura-Dariba, Rajasthan, India III: Plumbian tetrahedrite. Neues Jahrb. Miner. Abh. 1981, 141, 280–289. [Google Scholar]
- Mozgova, N.N.; Tsepin, A.I. Fahlore (Features of Chemical Composition and Properties); Nauka: Moscow, Russia, 1983; p. 279. (In Russian) [Google Scholar]
- Moh, G.H. Sulfosalts: Observations and mineral descriptions, experiments and applications. Neues Jahrb. Miner. Abh. 1984, 150, 25–64. [Google Scholar]
- Vavelidis, M.; Melfos, V. Two plumbian tetrahedrite-tennantite occurrences from Maronia area (Thrace) and Milos island (Aegean sea), Greece. Eur. J. Mineral. 1997, 9, 653–658. [Google Scholar]
- Bishop, A.C.; Criddle, A.J.; Clark, A.M. Plumbian tennantite from Sark, Channel Islands. Mineral. Mag. 1977, 41, 59–63. [Google Scholar] [CrossRef]
- Ixer, R.A.; Stanley, C.J. Silver mineralization at Sark’s Hope mine, Sark, Channel Islands. Mineral. Mag. 1983, 47, 539–545. [Google Scholar] [CrossRef]
- Förster, H.J.; Rhede, D.; Tischendorf, G. Continuous solid-solution between mercurian giraudite and hakite. Can. Mineral. 2002, 40, 1161–1170. [Google Scholar] [CrossRef]
- Jurković, I.B.; Garašić, V.; Jurković, I.M. Geochemical characteristics of mercurian tetrahedrite, barite and fluorite from the Duboki Vagan, Glumac and Dubrave-Dugi Dol barite deposits, south of Kreševo, Mid-Bosnian Schist Mts. Geol. Croat. 2011, 64, 49–59. [Google Scholar] [CrossRef]
- Basu, K.; Bortnykov, N.; Mookherjee, A.; Mozgova, N.; Sivtsov, A.V.; Tsepin, A.I.; Vrublevskaja, Z.V. Rare minerals from Rajpura-Dariba, Rajasthan, India V: The first recorded occurrence of a manganoan fahlore. Neues Jahrb. Miner. Abh. 1984, 149, 105–112. [Google Scholar]
- Voropayev, A.V.; Spiridonov, E.M.; Shchibrik, V.I. Cd-Tetrahedrite, first find in the USSR. Dokl. Acad. Sci. USSR 1988, 300, 1446–1468. [Google Scholar]
- Pattrick, R.A. Microprobe analyses of cadmium-rich tetrahedrites from Tyndrum, Perthshire, Scotland. Mineral. Mag. 1978, 42, 286–288. [Google Scholar] [CrossRef]
- Pattrick, R.A. Pb-Zn and minor U mineralization at Tyndrum, Scotland. Mineral. Mag. 1985, 49, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Huiwen, J.D.F.Z.Z.; Chunpei, Z. The first Discovery of Cd-Freibergite in China. Acta Mineral. Sin. 1988, 2, 005. [Google Scholar]
- Voudouris, P.C.; Spry, P.G.; Sakellaris, G.A.; Mavrogonatos, C. A cervelleite-like mineral and other Ag-Cu-Te-S minerals [Ag2CuTeS and (Ag, Cu)2TeS] in gold-bearing veins in metamorphic rocks of the Cycladic Blueschist Unit, Kallianou, Evia Island, Greece. Mineral. Petrol. 2011, 101, 169–183. [Google Scholar] [CrossRef]
- Dobbe, R.T. Manganoan-cadmian tetrahedrite from the Tunaberg Cu-Co deposit, Bergslagen, central Sweden. Mineral. Mag. 1992, 56, 113–115. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Rusinov, V.L. Goldfieldite: Chemical composition, parageneses and conditions of formation. Mineral. Zhurnal 1986, 8, 57–70. (In Russian) [Google Scholar]
- Kase, K. Tellurian tennantite from the Besshitype deposits in the Sambagawa metamorphic belt, Japan. Can. Mineral. 1986, 24, 399–404. [Google Scholar]
- Knittel, U. Composition and association of arsenian goldfieldite from the Marian gold deposit, Northern Luzon, Philippines. Mineral. Petrol. 1989, 40, 145–154. [Google Scholar] [CrossRef]
- Shimizu, M.; Stanley, C.J. Coupled Substitutions in Goldfieldite-Tetrahedrite Minerals from the Iriki Mine, Japan. Mineral. Mag. 1991, 55, 515–551. [Google Scholar] [CrossRef]
- Pohl, D.; Liessmann, W.; Okrugin, V.M. Rietveld analysis of selenium-bearing goldfieldites. Neues Jahrb. Miner. Mon. 1996, 1996, 1–8. [Google Scholar]
- Pinto, A.; Ferreira, A.; Bowles, J.F.W.; Gaspar, O.C. Mineralogical and textural characterization of the Neves-Corvo ores. Metallogenetic implications. In Geology and VMS Deposits of the Lberian Pyrite Belt; Ser. 27; Baniga, F.J.A.S., Carvalho, D., Eds.; SEG Neves Corvo Field Conference: Lisbon, Portugal, 1997; p. 90. [Google Scholar]
- Serranti, S.; Ferrini, V.; Masi, U.; Cabri, L.J. Trace-element distribution in cassiterite and sulfides from rubané and massive ores of the Corvo deposit, Portugal. Can. Mineral. 2002, 40, 815–835. [Google Scholar] [CrossRef]
- Figueiredo, M.O.; Silva, T.P.; de Oliveira, D.P.S.; Rosa, D.R.N. Searching for In-carrier minerals in polymetallic sulphide deposits: Digging deeper into the crystal chemistry of indium chalcogenides. In Digging Deeper, Proceedings of the 9th Biennial SGA Meeting, Ferrara, Italy, 29–31 August 2007; Andrew, C.J., Ed.; Irish Association Economic Geologists: Dublin, Ireland, 2007; pp. 1355–1358. [Google Scholar]
- McClenaghan, S.H.; Lentz, D.R.; Martin, J.; Diegor, W.G. Gold in the Brunswick No. 12 volcanogenic massive sulfide deposit, Bathurst Mining Camp, Canada: Evidence from bulk ore analysis and laser ablation ICP-MS data on sulfide phases. Miner. Depos. 2009, 44, 523–557. [Google Scholar] [CrossRef]
- Gaspar, O.C. Mineralogy and sulfide mineral chemistry of the Neves-Corvo ores, Portugal: Insight into their genesis. Can. Mineral. 2002, 40, 611–636. [Google Scholar] [CrossRef]
- Jurković, I.B.; Garašić, V.; Jurković, I.M. Cobalt, nickel, wolfram, cadmium, selenium, silver and gold-bearing mercurian tetrahedrite from the Saski Rad barite-siderite deposit in the Mid-Bosnian Schist Mts. Geol. Croat. 2011, 64, 223–237. [Google Scholar]
- Wohlgemuth-Ueberwasser, C.C.; Viljoen, F.; Petersen, S.; Vorster, C. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochim. Cosmochim. Acta 2015, 159, 16–41. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Crowe, B.B.P.; Ciobanu, C.L. Trace elements in hydrothermal chalcopyrite. Mineral. Mag. 2017, in press. [Google Scholar]
- Wilson, S.; Ridley, W.; Koenig, A. Development of sulphide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- United States Geological Survey. Microanalytical Reference Materials and Accessories. Available online: http://crustal.usgs.gov/geochemical_reference_standards/microanalytical_RM.html (accessed on 6 October 2016).
- Van Achterbergh, E.; Ryan, C.; Jackson, S.; Griffin, W. Data reduction software for LA-ICP-MS: Laser-Ablation-ICPMS in the earth sciences—Principles and applications. Mineral. Ass. Can. 2001, 29, 239–243. [Google Scholar]
- Danyushevsky, L.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.; McGoldrick, P.; Shelley, M. Routine quantitative multi-element analysis of sulphide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Patten, C.; Barnes, S.J.; Mathez, E.A.; Jenner, F.E. Partition coefficients of chalcophile elements between sulfide and silicate melts and the early crystallization history of sulfide liquid: LA-ICP-MS analysis of MORB sulfide droplets. Chem. Geol. 2013, 358, 170–188. [Google Scholar] [CrossRef]
- Cook, N.; Ciobanu, C.L.; George, L.; Zhu, Z.Y.; Wade, B.; Ehrig, K. Trace element analysis of minerals in magmatic-hydrothermal ores by laser ablation inductively-coupled plasma mass spectrometry: Approaches and opportunities. Minerals 2016, 6, 111. [Google Scholar] [CrossRef]
- George, L.; Cook, N.J.; Cristiana, L.; Wade, B.P. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef]
- Plotinskaya, O.Y.; Grabezhev, A.I.; Seltmann, R. Fahlores compositional zoning in a porphyry-epithermal system: Biksizak occurrence, South Urals, Russia as an example. Geol. Ore Depos. 2015, 57, 42–63. [Google Scholar] [CrossRef]
- Buzatu, A.; Damian, G.; Dill, H.G.; Buzgar, N.; Apopei, A.I. Mineralogy and geochemistry of sulfosalts from Baia Sprie ore deposit (Romania)—New bismuth minerals occurrence. Ore Geol. Rev. 2015, 65, 132–147. [Google Scholar] [CrossRef]
- Vokes, F.M. Geological studies on the Caledonian pyritic zinc-lead orebody at Bleikvassli, Norland, Norway. Nor. Geol. Unders. 1963, 222, 1–126. [Google Scholar]
- Vokes, F.M. On the possible modes of origin of the Caledonian sulfide ore deposit at Bleikvassli, Nordland, Norway. Econ. Geol. 1966, 61, 1130–1139. [Google Scholar] [CrossRef]
- Boulter, C.A.; Fotios, M.G.; Phillips, G.N. The Golden Mile, Kalgoorlie; a giant gold deposit localized in ductile shear zones by structurally induced infiltration of an auriferous metamorphic fluid. Econ. Geol. 1987, 82, 1661–1678. [Google Scholar] [CrossRef]

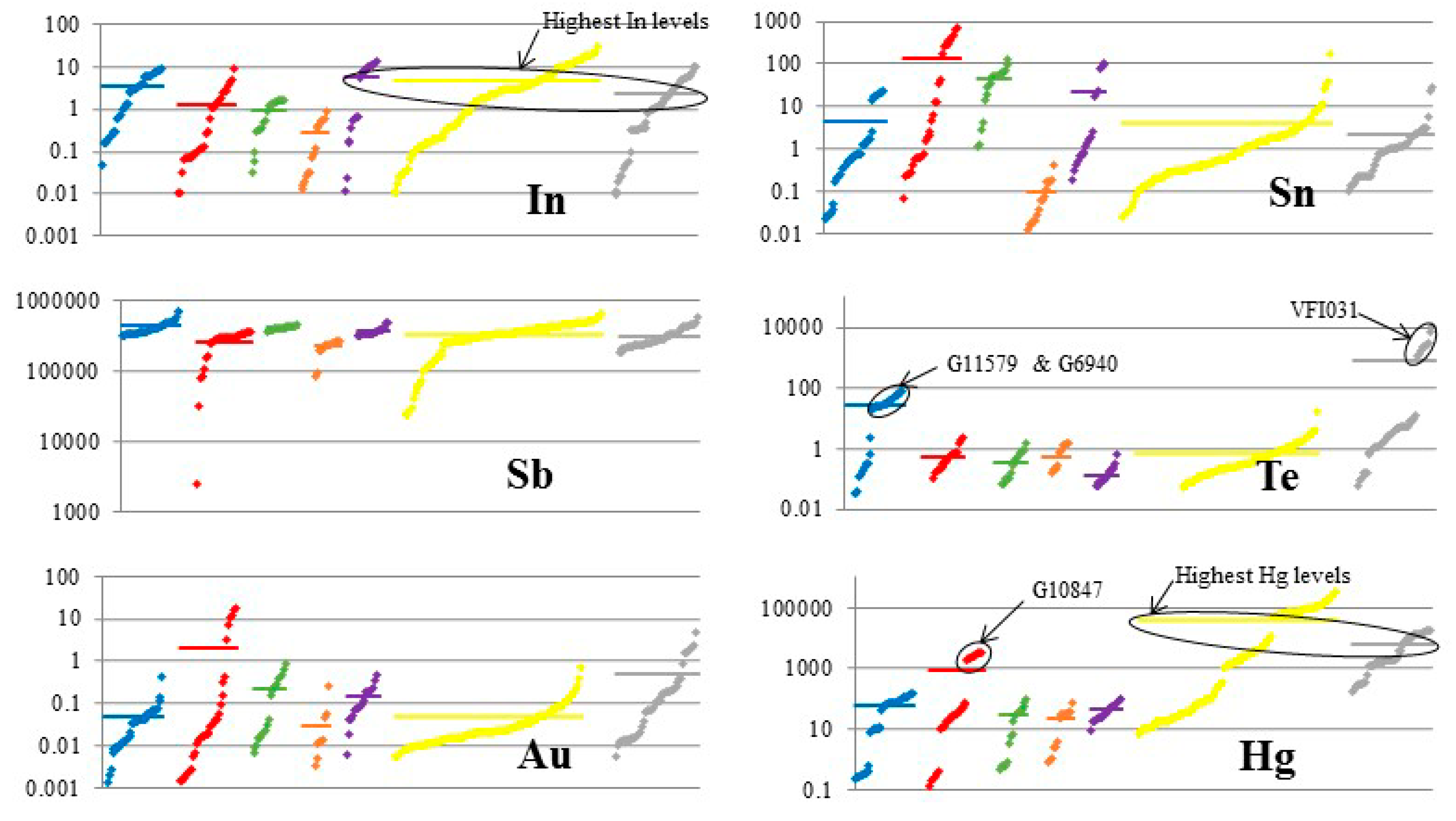
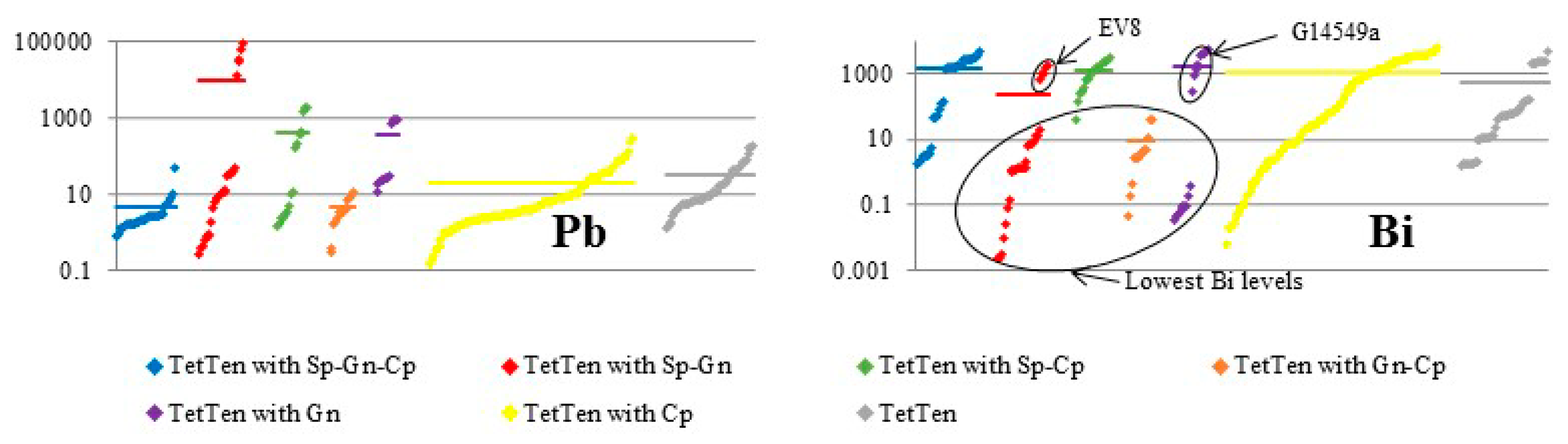
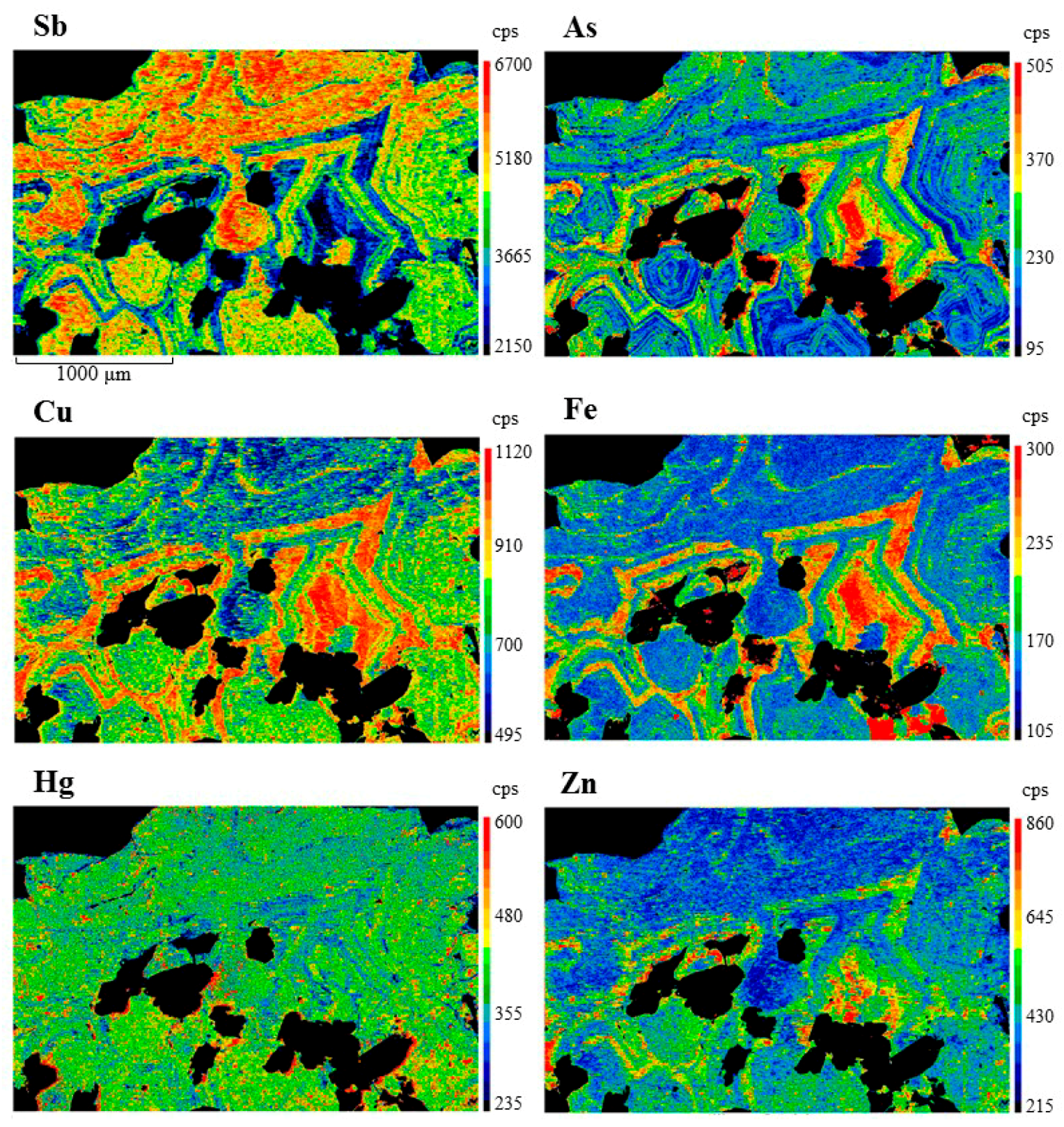
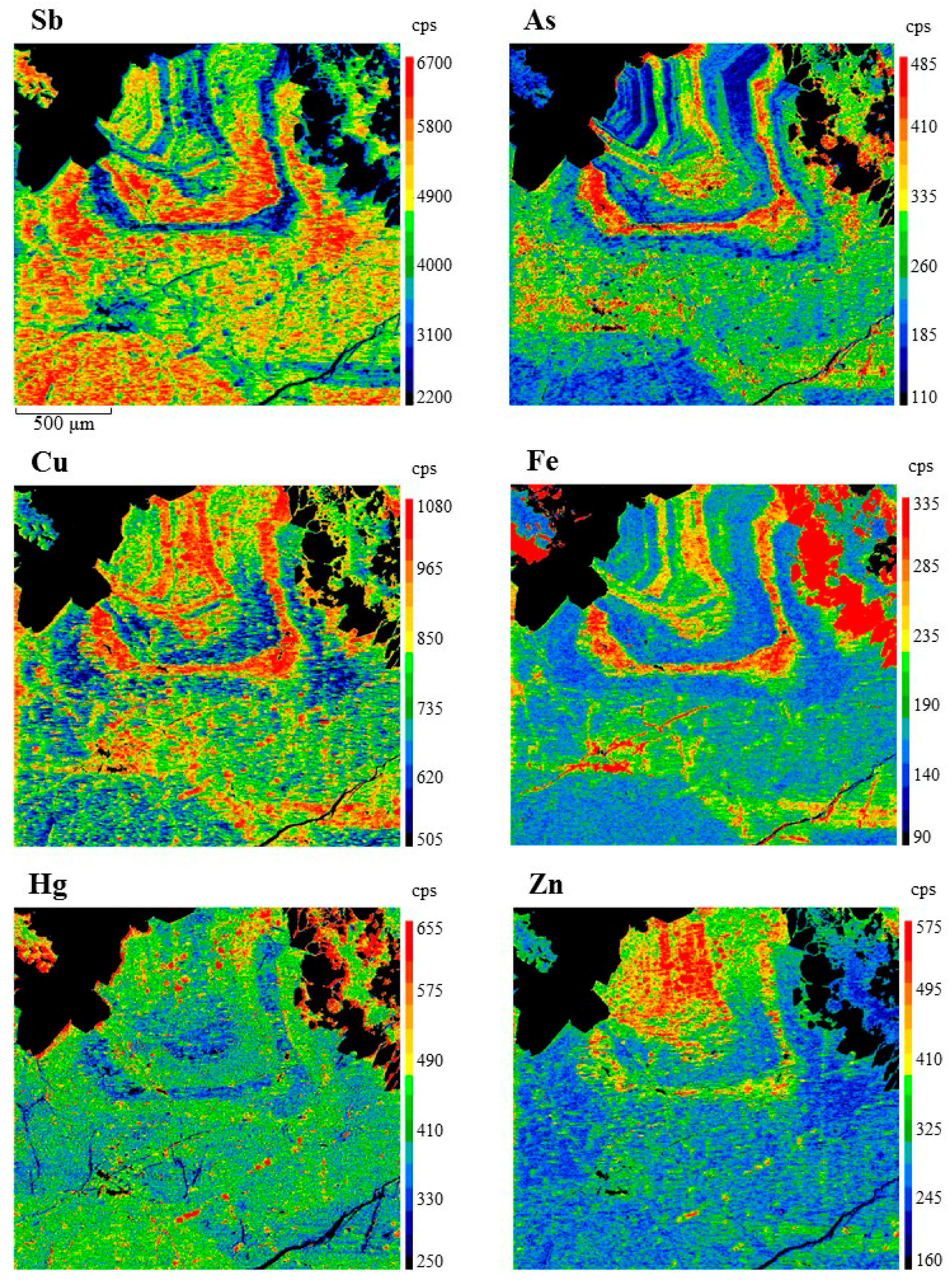
| Mineral | Ideal Formula | References |
|---|---|---|
| Tetrahedrite | Cu6[Cu4(Fe,Zn)2]Sb4S13 | [1,3,15,16] |
| Tennantite | Cu6[Cu4(Fe,Zn)2]As4S13 | [2,7] |
| Freibergite | Ag6[Cu4Fe2]Sb4S13−x | [15,17] |
| Hakite | Cu6[Cu4Hg2]Sb4Se13 | [18] |
| Giraudite | Cu6[Cu4(Fe,Zn)2]As4Se13 | [19] |
| Goldfieldite | Cu10Te4S13 | [20,21,22] |
| Argentotennantite | Ag6[Cu4(Fe,Zn)2]As4S13 | [23] |
| Argentotetrahedrite * | Ag10(Fe,Zn)2Sb4S13 | [24] |
| Sample | Tetrahedrite-Tennantite Composition | Assemblage | Locality | Ore Type |
|---|---|---|---|---|
| G16396 * | (Cu9,Ag1.2,Zn0.9,Fe0.9,Pb0.1) (Sb3.8,As0.1) S13 | Tet, Sp, Gn, Cp | Broken Hill, NSW, Australia | SEDEX (recrystallized) |
| G11579 * | (Cu9.6,Ag0.3,Zn1,Fe0.9) (Sb3.7,As0.2) S13.2 | Tet, Sp, Cp, Gn | Kalgoorlie, WA, Australia | Orogenic Au |
| G13289b * | (Cu10.1,Zn1.7,Fe0.3) (Sb3.2,As0.8) S12.9 | Tet, Gn, Cp, Sp | S. Wheal Exmouth, Devon, England | Low temperature replacement |
| G6940 * | (Cu9.5,Ag0.5,Fe1.2,Zn0.8) (Sb3.8,As0.2) S13 | Tet, Cp, Sp, Gn | Great Boulder Mine, WA, Australia | Orogenic Au |
| G13289a * | (Cu10.1,Zn1.7,Fe0.3) (Sb3.2,As0.7) S13 | Tet, Cp, Sp, Gn | S. Wheal Exmouth, Devon, England | Low temperature replacement |
| V446 | (Cu8.2,Ag1.9,Fe1.7,Zn0.4) Sb3.9 S13 | Sp, Gn, Cp, Tet | Bleikvassli, Norway | SEDEX (recrystallized) |
| V538 | (Cu7.8,Ag2.2,Fe1.7,Zn0.4) Sb3.9 S13.1 | Sp, Gn, Cp, Tet | Bleikvassli, Norway | SEDEX (recrystallized) |
| Hj13 | (Cu9.2,Ag0.6,Fe1.6,Zn0.4) Sb3.9 S13.2 | Gn, Cp, Sp, Tet | Herja, Romania | Epithermal |
| G6951 * | (Cu9.4,Ag0.5,Zn1.8,Cd0.1) (Sb3.5,As0.4) S13.2 | Tet, Sp, Gn, (Cp) | Yerranderie, NSW, Australia | Epithermal |
| Bv97-52 | (Cu9,Ag0.6,Fe1.6,Zn0.7,Pb0.2) (As2.8,Sb1.5) S13.2 | Ten, Sp, Gn | Bleikvassli, Norway | SEDEX (recrystallized) |
| G10847 * | (Cu10,Ag0.1,Zn1.7,Fe0.3) (Sb2.1,As1.8) S13 | Tet, Sp, Gn | Mt. Camel, Heathcote, Vic., Australia | Greenstone hosted |
| EV8 ** | (Ag5.6,Cu4.6,Fe1.9,Zn0.3) Sb3.9 S12.6 | Gn, Sp, Tet | Evelyn Mine, NT, Australia | VMS |
| Hj14 | (Cu6.8,Ag3.2,Fe1.8,Zn0.3) Sb3.8 S13.2 | Gn, Sp, Tet | Herja, Romania | Epithermal |
| G6948 * | (Cu9.6,Ag0.5,Fe1.4,Zn0.6) (Sb3.9,As0.1) S13 | Tet, Sp, Cp, (Gn) | Medcritting, Tas., Australia | Unknown |
| G14549b * | (Cu8.7,Ag1.8,Fe1.1,Zn0.7) (Sb3.8,As2.8) S12.8 | Tet, Sp, Cp | Consols Mine, Broken Hill, NSW, Australia | SEDEX (recrystallized) |
| Mo17A | (Cu7.2,Ag2.5,Fe1.6,Zn0.4,Pb0.4) (Sb3.7,As0.1) S13.1 | Cp, Gn, Tet | Mofjell, Norway | SEDEX (recrystallized) |
| ORV1 | (Cu10.1,Ag0.1,Zn1.3,Fe0.4) (Sb3.1,As0.8) S13.2 | Cp, Gn, Tet | Oravita, Romania | Skarn |
| G14549a * | (Cu8.7,Ag2.4,Fe1.1, Zn0.7) Sb3.7 S12.3 | Tet, Gn, (Sp) | Consols Mine, Broken Hill, NSW, Australia | SEDEX (recrystallized) |
| G873 * | (Cu9.5, Ag0.7, Zn1.2, Fe0.7) (Sb3,As0.9) S12.9 | Tet, Gn | Yerranderrie, NSW, Australia | Epithermal |
| G16152 * | (Cu10,Ag0.1,Zn1.1,Fe0.8,Co0.1) (Sb2.2,As1.8) S13 | Tet, Cp, (Gn) | Siegen, Westphalia, Germany | SEDEX? |
| G871 * | (Cu10.1,Hg0.9,Fe0.5,Zn0.5) (Sb3.2,As0.7) S13.1 | Tet, Cp | Pulganbar, Grafton, NSW, Australia | Vein hosted |
| G874 * | (Cu10.3,Zn1,Hg0.5,Fe0.4) (Sb3.3,As0.6) S12.8 | Tet, Cp | Pulganbar, Grafton, NSW, Australia | Vein hosted |
| G879 * | (Cu9.6,Ag0.4,Fe1.6,Zn0.4) (Sb3.8,As0.1) S13.2 | Tet, Cp | Ring Valley, Tas., Australia | Fissure fillings |
| G882 * | (Cu10.3,Fe1,Zn0.3,Hg0.2) (Sb2.5,As1.4) S13.1 | Tet, Cp | Pulganbar, Grafton, NSW, Australia | Vein hosted |
| G6946 * | (Cu10,Ag0.1,Zn1.3,Fe0.6) (Sb3.7,As0.2) S13.1 | Tet, Cp | Siegen, Westphalia, Germany | SEDEX? |
| G6949 * | (Cu9.7,Ag0.2,Fe1.7,Zn0.3) (Sb3.9,As0.1) S13.1 | Tet, Cp | Webb's Ag Mine, Emmaville, NSW, Australia | Veins and dissemination |
| G11701 * | (Cu5.2,Ag5.1,Fe1.6,Zn0.4) Sb4 S12.6 | Tet, Cp | Broken Hill, NSW, Australia | SEDEX (recrystallized) |
| G14246 * | (Cu9.8,Ag0.2,Fe1.5,Zn0.5) (Sb3.8,As0.1) S13.1 | Tet, Cp | Curtin Davis Mine, Dundas, Tas., Australia | Intrusion related? |
| G14867 * | (Cu10.5,Fe1.4,Zn0.2) (As3.1,Sb0.8) S13 | Ten, Cp | Oraparinna, SA, Australia | Diapir related |
| Mo16 | (Cu7.3,Ag2.6,Fe1.8,Zn0.2) (Sb3.8,As0.8) S13.2 | Cp, Tet, (Gn) | Mofjell, Norway | SEDEX (recrystallized) |
| G29851 * | (Cu10.2,Fe1.1, Zn0.5,Hg0.2) (As3.6,Sb0.3) S13 | Cp, Ten | Gortdrum Mine, Ireland | Carbonate hosted |
| ORV4 | (Cu10.1,Zn1.4,Fe0.4) (Sb2.7,As1.2) S13.2 | Cp, Tet | Oravita, Romania | Skarn |
| G12640 * | (Cu10.2,Zn1.1,Fe0.9) (As2.5,Sb1.4) S12.9 | Ten | Tinga, NSW, Australia | Unknown |
| G13301 * | (Cu10.2,Fe1.2,Zn0.4) (Sb3.3,As0.6) S13.3 | Tet | Allihies Mine, Castletown, Cork, Ireland | Unknown |
| G15977 * | (Cu10.1,Zn1.2,Fe0.7) (Sb3.2,As0.7) S13 | Tet | Mooloowatana HS, SA, Australia | Unknown |
| G16835 * | (Cu10.1,Ag0.1,Zn1,Fe0.7, Hg0.1) (As2,Sb1.9) S13.1 | Ten | Grosskogel Mine, Austria | MVT? |
| VFI031 ** | (Cu8.4,Ag1.7,Zn1.9) (Sb2.4,As1.4) S13.1 | Tet | Emperor Gold Mine, Fiji | Epithermal |
| Sample/Assemblage | S | Mn | Fe | Co | Cu | Zn | As | Se | Ag | Cd | Sn | Sb | Te | Hg | Pb | Bi | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G16396 * | Mean (10) | 24.4 | 0.016 | 3.07 | - | 33.3 | 3.59 | 0.237 | 0.021 | 7.25 | 0.051 | - | 27.3 | - | 0.069 | 0.994 | - | 100.1 |
| Tet, Sp, Gn, Cp | apfu | 13.0 | 0.005 | 0.942 | - | 8.97 | 0.942 | 0.054 | 0.005 | 1.16 | 0.008 | - | 3.84 | - | 0.006 | 0.083 | - | 29.0 |
| G11579 | Mean (10) | 25.9 | - | 3.08 | - | 37.3 | 4.19 | 0.903 | 0.018 | 2.27 | - | - | 27.7 | - | 0.079 | 0.076 | 0.110 | 101.6 |
| Tet, Sp, Cp, Gn | apfu | 13.2 | - | 0.902 | - | 9.59 | 1.05 | 0.197 | 0.004 | 0.344 | - | - | 3.72 | - | 0.006 | 0.006 | 0.009 | 29.0 |
| G13289b * | Mean (10) | 25.5 | - | 1.06 | - | 39.5 | 6.71 | 3.47 | - | 0.243 | - | - | 23.6 | - | 0.076 | 0.066 | - | 100.1 |
| Tet, Gn, Cp, Sp | apfu | 12.9 | - | 0.310 | - | 10.1 | 1.67 | 0.755 | - | 0.037 | - | - | 3.16 | - | 0.006 | 0.005 | - | 29.0 |
| G6940 | Mean (10) | 25.1 | 0.018 | 3.93 | 0.019 | 36.3 | 3.14 | 0.679 | 0.021 | 3.33 | - | - | 27.4 | - | 0.084 | 0.068 | 0.092 | 100.0 |
| Tet, Cp, Sp, Gn | apfu | 13.0 | 0.005 | 1.18 | 0.005 | 9.55 | 0.801 | 0.151 | 0.004 | 0.516 | - | - | 3.76 | - | 0.007 | 0.005 | 0.007 | 29.0 |
| G13289a * | Mean (10) | 25.7 | - | 0.976 | - | 39.4 | 6.77 | 3.20 | 0.031 | 0.212 | - | - | 24.1 | - | 0.073 | 0.079 | - | 100.4 |
| Tet, Cp, Sp, Gn | apfu | 13.0 | - | 0.284 | - | 10.1 | 1.68 | 0.693 | 0.006 | 0.032 | - | - | 3.21 | - | 0.006 | 0.006 | - | 29.0 |
| V446 | Mean (7) | 24.1 | 0.031 | 5.36 | 0.022 | 30.0 | 1.69 | - | - | 11.6 | - | - | 27.5 | 0.032 | 0.098 | 0.108 | - | 100.5 |
| Sp, Gn, Cp, Tet | apfu | 13.0 | 0.010 | 1.65 | 0.006 | 8.16 | 0.446 | - | - | 1.86 | - | - | 3.90 | 0.004 | 0.008 | 0.009 | - | 29.0 |
| V538 | Mean (10) | 24.2 | 0.020 | 5.42 | 0.023 | 28.7 | 1.33 | - | 0.033 | 13.5 | - | 0.029 | 27.3 | - | 0.100 | 0.086 | - | 100.6 |
| Sp, Gn, Cp, Tet | apfu | 13.1 | 0.006 | 1.68 | 0.007 | 7.82 | 0.352 | - | 0.007 | 2.16 | - | 0.004 | 3.88 | - | 0.009 | 0.007 | - | 29.0 |
| Hj13 | Mean (10) | 25.4 | 0.021 | 5.39 | 0.025 | 35.2 | 1.76 | - | 0.025 | 3.88 | - | 0.053 | 28.7 | - | 0.118 | 0.088 | - | 100.5 |
| Gn, Cp, Sp, Tet | apfu | 13.2 | 0.006 | 1.61 | 0.007 | 9.22 | 0.449 | - | 0.005 | 0.600 | - | 0.007 | 3.92 | - | 0.010 | 0.007 | - | 29.0 |
| G6951 * | Mean (10) | 25.6 | - | 0.043 | 0.018 | 36.4 | 7.30 | 1.86 | 0.019 | 3.45 | 0.674 | - | 26.0 | - | 0.082 | 0.078 | - | 101.4 |
| Tet, Sp, Gn, (Cp) | apfu | 13.2 | - | 0.013 | 0.005 | 9.44 | 1.84 | 0.408 | 0.004 | 0.526 | 0.099 | - | 3.51 | - | 0.007 | 0.006 | - | 29.0 |
| Bv97-52 | Mean (10) | 27.0 | 0.060 | 5.63 | 0.023 | 36.9 | 2.63 | 13.9 | 0.028 | 3.45 | 0.041 | 0.035 | 11.3 | - | 0.078 | 2.10 | - | 100.2 |
| Ten, Sp, Gn | apfu | 13.2 | 0.017 | 1.58 | 0.006 | 9.01 | 0.650 | 2.80 | 0.006 | 0.568 | 0.005 | 0.004 | 1.53 | - | 0.006 | 0.199 | - | 29.0 |
| G10847 | Mean (10) | 26.8 | - | 1.00 | - | 40.7 | 6.98 | 8.43 | - | 0.745 | - | - | 16.6 | - | 0.230 | 0.076 | - | 101.6 |
| Tet, Sp, Gn | apfu | 13.0 | - | 0.281 | - | 10.0 | 1.67 | 1.76 | - | 0.108 | - | - | 2.13 | - | 0.018 | 0.006 | - | 29.0 |
| EV8 | Mean (10) | 21.3 | - | 5.62 | - | 15.5 | 1.02 | - | 0.029 | 32.1 | - | 0.264 | 25.3 | - | 0.095 | 0.409 | 0.066 | 101.4 |
| Gn, Sp, Tet | apfu | 12.6 | - | 1.91 | - | 4.63 | 0.294 | - | 0.007 | 5.64 | - | 0.042 | 3.94 | - | 0.009 | 0.038 | 0.006 | 29.0 |
| Hj14 | Mean (6) | 24.0 | 0.018 | 5.56 | 0.022 | 24.4 | 0.937 | - | 0.018 | 19.3 | - | - | 26.5 | - | 0.082 | 0.102 | - | 100.9 |
| Gn, Sp, Tet | apfu | 13.2 | 0.006 | 1.76 | 0.007 | 6.77 | 0.253 | - | 0.004 | 3.15 | - | - | 3.85 | - | 0.007 | 0.009 | - | 29.0 |
| G6948 | Mean (10) | 24.9 | 0.047 | 4.60 | - | 36.3 | 2.33 | 0.234 | 0.027 | 3.39 | - | - | 28.1 | - | 0.086 | 0.071 | 0.091 | 100.0 |
| Tet, Sp, Cp, (Gn) | apfu | 13.0 | 0.014 | 1.38 | - | 9.55 | 0.597 | 0.052 | 0.006 | 0.526 | - | - | 3.86 | - | 0.007 | 0.006 | 0.007 | 29.0 |
| G14549b | Mean (10) | 24.0 | - | 3.73 | - | 32.5 | 2.66 | 0.134 | 0.017 | 11.1 | 0.147 | - | 27.3 | - | 0.094 | 0.255 | 0.129 | 101.7 |
| Tet, Sp, Cp | apfu | 12.8 | - | 1.14 | - | 8.72 | 0.696 | 0.031 | 0.004 | 1.84 | 0.022 | - | 3.82 | - | 0.008 | 0.023 | 0.011 | 29.0 |
| Mo17A | Mean (7) | 23.7 | - | 5.08 | - | 25.8 | 1.34 | 0.278 | 0.048 | 15.4 | 0.054 | - | 25.5 | - | 0.074 | 4.01 | - | 100.6 |
| Cp, Gn, Tet | apfu | 13.1 | - | 1.62 | - | 7.21 | 0.363 | 0.065 | 0.012 | 2.54 | 0.009 | - | 3.73 | - | 0.007 | 0.390 | - | 29.0 |
| ORV1 | Mean (10) | 26.2 | - | 1.47 | - | 39.8 | 5.47 | 3.65 | 0.018 | 0.531 | - | - | 23.5 | - | 0.088 | 0.087 | 0.065 | 100.8 |
| Cp, Gn, Tet | apfu | 13.2 | - | 0.427 | - | 10.1 | 1.34 | 0.783 | 0.004 | 0.079 | - | - | 3.11 | - | 0.007 | 0.007 | 0.005 | 29.0 |
| G14549a | Mean (10) | 22.4 | - | 3.55 | - | 31.7 | 2.58 | 0.195 | 0.023 | 13.5 | 0.047 | - | 25.8 | - | 0.083 | 0.254 | 0.181 | 100.2 |
| Tet, Gn, (Sp) | apfu | 12.3 | - | 1.12 | - | 8.74 | 0.694 | 0.045 | 0.005 | 2.36 | 0.008 | - | 3.73 | - | 0.007 | 0.021 | 0.016 | 29.0 |
| G873 | Mean (10) | 24.8 | - | 2.48 | - | 36.3 | 4.76 | 3.96 | - | 4.59 | 0.034 | - | 22.2 | - | 0.077 | 0.113 | - | 99.3 |
| Tet, Gn | apfu | 12.9 | - | 0.740 | - | 9.52 | 1.21 | 0.881 | - | 0.710 | 0.005 | - | 3.04 | - | 0.006 | 0.009 | - | 29.0 |
| G16152 | Mean (10) | 26.6 | - | 2.82 | 0.362 | 40.7 | 4.38 | 8.48 | - | 0.449 | - | - | 16.8 | - | 0.185 | 0.066 | - | 100.9 |
| Tet, Cp, (Gn) | apfu | 13.0 | - | 0.792 | 0.096 | 10.0 | 1.05 | 1.77 | - | 0.065 | - | - | 2.17 | - | 0.014 | 0.005 | - | 29.0 |
| G871 | Mean (10) | 24.2 | - | 1.50 | 0.068 | 37.1 | 1.70 | 3.17 | - | 0.051 | - | - | 22.6 | - | 10.6 | 0.076 | - | 101.1 |
| Tet, Cp | apfu | 13.1 | - | 0.464 | 0.020 | 10.1 | 0.449 | 0.726 | - | 0.008 | - | - | 3.23 | - | 0.922 | 0.006 | - | 29.0 |
| G874 | Mean (10) | 24.1 | - | 1.32 | 0.079 | 38.3 | 3.81 | 2.72 | - | 0.050 | - | - | 23.7 | - | 5.79 | 0.056 | - | 99.8 |
| Tet, Cp | apfu | 12.8 | - | 0.404 | 0.023 | 10.3 | 1.00 | 0.621 | - | 0.008 | - | - | 3.33 | - | 0.494 | 0.005 | - | 29.0 |
| G879 | Mean (10) | 25.6 | - | 5.43 | - | 37.0 | 1.48 | 0.291 | 0.025 | 2.59 | - | - | 28.5 | - | 0.077 | 0.064 | 0.073 | 101.0 |
| Tet, Cp | apfu | 13.2 | - | 1.60 | - | 9.58 | 0.371 | 0.064 | 0.005 | 0.395 | - | - | 3.85 | - | 0.006 | 0.005 | 0.006 | 29.0 |
| G882 | Mean (10) | 26.4 | - | 3.54 | 0.087 | 41.2 | 1.34 | 6.78 | - | - | - | - | 19.0 | - | 3.10 | 0.070 | - | 101.6 |
| Tet, Cp | apfu | 13.1 | - | 1.01 | 0.024 | 10.3 | 0.327 | 1.44 | - | - | - | - | 2.50 | - | 0.247 | 0.005 | - | 29.0 |
| G6946 | Mean (10) | 25.7 | - | 2.05 | - | 39.0 | 5.14 | 1.04 | 0.023 | 0.600 | - | - | 27.5 | - | 0.158 | 0.059 | - | 101.3 |
| Tet, Cp | apfu | 13.1 | - | 0.600 | - | 10.0 | 1.28 | 0.226 | 0.005 | 0.091 | - | - | 3.69 | - | 0.013 | 0.005 | - | 29.0 |
| G6949 | Mean (10) | 25.8 | 0.015 | 5.72 | - | 37.9 | 1.30 | 0.331 | 0.020 | 1.23 | - | - | 28.9 | - | 0.081 | 0.057 | 0.136 | 101.4 |
| Tet, Cp | apfu | 13.1 | 0.004 | 1.67 | - | 9.75 | 0.326 | 0.072 | 0.004 | 0.186 | - | - | 3.87 | - | 0.007 | 0.005 | 0.011 | 29.0 |
| G11701 | Mean (10) | 21.5 | - | 4.72 | 0.026 | 17.6 | 1.48 | - | 0.025 | 29.2 | - | - | 26.1 | - | 0.081 | 0.055 | - | 100.6 |
| Tet, Cp | apfu | 12.6 | - | 1.59 | 0.008 | 5.21 | 0.425 | - | 0.006 | 5.10 | - | - | 4.03 | - | 0.008 | 0.005 | - | 29.0 |
| G14246 | Mean (10) | 25.6 | - | 5.10 | - | 37.6 | 1.86 | 0.298 | 0.029 | 1.41 | - | - | 28.3 | - | 0.082 | 0.073 | 0.173 | 100.6 |
| Tet, Cp | apfu | 13.1 | - | 1.50 | - | 9.76 | 0.470 | 0.065 | 0.006 | 0.215 | - | - | 3.83 | - | 0.007 | 0.006 | 0.014 | 29.0 |
| G14867 | Mean (10) | 28.0 | - | 5.06 | 0.023 | 44.7 | 0.765 | 15.5 | 0.020 | 0.049 | - | - | 6.40 | - | 0.392 | 0.073 | 0.135 | 101.0 |
| Ten, Cp | apfu | 13.0 | - | 1.35 | 0.006 | 10.5 | 0.175 | 3.10 | 0.004 | 0.007 | - | - | 0.786 | - | 0.029 | 0.005 | 0.010 | 29.0 |
| Mo16 | Mean (7) | 24.3 | - | 5.62 | 0.024 | 26.5 | 0.933 | 3.60 | 0.024 | 15.9 | 0.054 | - | 26.7 | - | 0.089 | 0.088 | - | 100.6 |
| Cp, Tet, (Gn) | apfu | 13.2 | - | 1.76 | 0.007 | 7.25 | 0.247 | 0.802 | 0.005 | 2.58 | 0.008 | - | 3.83 | - | 0.008 | 0.007 | - | 29.0 |
| G29851 | Mean (10) | 27.9 | - | 4.10 | 0.021 | 43.2 | 2.01 | 18.0 | - | 0.093 | - | 0.026 | 2.72 | - | 3.28 | 0.084 | 0.087 | 101.4 |
| Cp, Ten | apfu | 13.0 | - | 1.10 | 0.005 | 10.2 | 0.461 | 3.60 | - | 0.013 | - | 0.003 | 0.337 | - | 0.246 | 0.006 | 0.006 | 29.0 |
| ORV4 | Mean (10) | 26.6 | - | 1.48 | - | 40.3 | 5.89 | 5.68 | - | 0.156 | 0.048 | - | 20.6 | - | 0.070 | 0.070 | - | 100.9 |
| Cp, Tet | apfu | 13.2 | - | 0.419 | - | 10.1 | 1.43 | 1.20 | - | 0.023 | 0.007 | - | 2.70 | - | 0.006 | 0.005 | - | 29.0 |
| G12640 | Mean (10) | 27.1 | 0.017 | 3.46 | - | 42.3 | 4.53 | 12.1 | - | 0.212 | - | - | 11.2 | - | 0.122 | 0.072 | - | 101.1 |
| Ten | apfu | 12.9 | 0.005 | 0.947 | - | 10.2 | 1.06 | 2.46 | - | 0.030 | - | - | 1.41 | - | 0.009 | 0.005 | - | 29.0 |
| G13301 | Mean (10) | 26.7 | - | 4.24 | 0.070 | 40.8 | 1.59 | 3.01 | 0.020 | - | 0.069 | - | 25.0 | - | 0.149 | 0.074 | - | 101.7 |
| Tet | apfu | 13.3 | - | 1.21 | 0.019 | 10.2 | 0.387 | 0.639 | 0.004 | - | 0.010 | - | 3.27 | - | 0.012 | 0.006 | - | 29.0 |
| G15977 | Mean (10) | 26.0 | - | 2.53 | - | 39.8 | 5.07 | 3.45 | - | 0.102 | 0.243 | - | 24.2 | - | 0.117 | 0.083 | - | 101.3 |
| Tet | apfu | 13.0 | - | 0.726 | - | 10.1 | 1.24 | 0.739 | - | 0.015 | 0.035 | - | 3.19 | - | 0.009 | 0.006 | - | 29.0 |
| G16835 | Mean (10) | 27.1 | - | 2.68 | 0.027 | 41.5 | 4.00 | 9.61 | - | 0.398 | - | - | 14.9 | - | 0.904 | 0.093 | 0.093 | 101.1 |
| Ten | apfu | 13.1 | - | 0.746 | 0.007 | 10.1 | 0.952 | 1.99 | - | 0.057 | - | - | 1.90 | - | 0.070 | 0.007 | 0.007 | 29.0 |
| VFI031 | Mean (5) | 25.5 | 0.107 | 0.039 | - | 32.2 | 7.47 | 6.52 | 0.036 | 10.8 | - | - | 17.9 | 0.159 | 0.469 | 0.083 | - | 101.2 |
| Tet | apfu | 13.1 | 0.032 | 0.011 | - | 8.37 | 1.89 | 1.43 | 0.008 | 1.65 | - | - | 2.43 | 0.020 | 0.039 | 0.007 | - | 29.0 |
| Sample/Assemblage | Mn | Fe | Co | Ni | Zn | Ga | As | Se | Mo | Ag | Cd | In | Sn | Te | W | Au | Hg | Tl | Pb | Bi | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G16396 * | Mean (10) | 1.4 | ME | 2.7 | 0.54 | ME | 0.09 | 4620 | - | 0.12 | ME | 4968 | 0.57 | 28 | 1.4 | 0.04 | 0.74 | 93 | 0.09 | 61 | 445 |
| Tet, Sp, Gn, Cp | St. Dev. | 1.0 | ME | 1.1 | 0.78 | ME | 0.12 | 943 | - | 0.15 | ME | 751 | 0.16 | 39 | 1.1 | 0.06 | 0.53 | 20 | 0.16 | 40 | 768 |
| G11579 | Mean (10) | 32 | ME | 22 | 0.87 | ME | 0.06 | 28,446 | 53 | 0.09 | ME | 2692 | 7.0 | 0.54 | 50 | - | 0.03 | 93 | 0.05 | 3.1 | 3007 |
| Tet, Sp, Cp, Gn | St. Dev. | 2.1 | ME | 4.5 | 1.0 | ME | 0.03 | 6725 | 23 | 0.08 | ME | 324 | 1.1 | 0.37 | 25 | - | 0.02 | 31 | 0.05 | 2.2 | 595 |
| G13289b * | Mean (10) | 0.77 | ME | 44 | 0.14 | ME | 0.03 | ME | 11 | 0.03 | 2038 | 2187 | 0.25 | 0.80 | 0.07 | 0.01 | 0.02 | 9.3 | 0.15 | 270 | 8.2 |
| Tet, Gn, Cp, Sp | St. Dev. | 0.54 | ME | 7.8 | 0.08 | ME | 0.02 | ME | 8.6 | 0.04 | 538 | 274 | 0.34 | 0.49 | 0.16 | 0.01 | 0.04 | 3.2 | 0.22 | 257 | 8.5 |
| G6940 | Mean (10) | 43 | ME | 0.55 | 0.33 | ME | 0.05 | 12,626 | 73 | 0.06 | ME | 1860 | 3.4 | 1.1 | 38 | 0.02 | 0.02 | 86 | 0.02 | 1.8 | 1539 |
| Tet, Cp, Sp, Gn | St. Dev. | 7.9 | ME | 0.14 | 0.19 | ME | 0.04 | 5590 | 20 | 0.05 | ME | 231 | 0.92 | 0.71 | 21 | 0.03 | 0.02 | 28 | 0.02 | 0.68 | 142 |
| G13289a * | Mean (10) | 0.83 | 10,116 | 38 | 0.25 | ME | 0.03 | ME | 21 | 0.04 | 1866 | 2052 | 0.17 | 0.47 | 0.09 | 0.01 | 0.01 | 13 | 0.05 | 108 | 11 |
| Tet, Cp, Sp, Gn | St. Dev. | 0.65 | 1653 | 11 | 0.19 | ME | 0.03 | ME | 29 | 0.05 | 615 | 262 | 0.40 | 0.35 | 0.18 | 0.01 | 0.00 | 2.9 | 0.05 | 130 | 16 |
| V446 | Mean (4) | 204 | ME | 0.06 | 0.13 | ME | 0.19 | 38 | 12 | 0.02 | ME | 170 | 1.2 | 16 | 0.06 | - | 0.01 | 10 | 0.16 | 1.9 | 111 |
| Sp, Gn, Cp, Tet | St. Dev. | 12 | ME | 0.05 | 0.25 | ME | 0.05 | 11 | 6.1 | 0.03 | ME | 24 | 0.21 | 1.7 | 0.09 | - | 0.02 | 1.5 | 0.21 | - | 22 |
| V538 | Mean (3) | 122 | ME | 0.02 | 0.00 | ME | 0.11 | 92 | 6.0 | 0.00 | ME | 158 | 0.64 | 22 | 0.13 | - | 0.00 | 10 | 0.21 | 21 | 45 |
| Sp, Gn, Cp, Tet | St. Dev. | 6.9 | ME | 0.03 | 0.01 | ME | 0.10 | 44 | 2.6 | 0.00 | ME | 12 | 0.08 | 0.42 | 0.17 | - | 0.01 | 1.3 | 0.31 | 23 | 4.2 |
| Hj13 | Mean (9) | 109 | ME | 0.24 | - | ME | 0.03 | 117 | 8.9 | 0.08 | ME | 110 | 0.21 | 0.15 | 0.42 | 0.00 | 0.11 | 0.33 | 0.48 | 3.0 | 3.1 |
| Gn, Cp, Sp, Tet | St. Dev. | 19 | ME | 0.38 | - | ME | 0.02 | 24 | 4.7 | 0.07 | ME | 18 | 0.08 | 0.23 | 0.73 | 0.01 | 0.13 | 0.10 | 0.24 | 1.4 | 1.0 |
| G6951 * | Mean (10) | 1.5 | 369 | 27 | - | ME | 0.04 | ME | - | 0.06 | ME | 29,893 | 0.10 | 0.31 | 0.29 | - | 0.06 | 29 | 0.20 | 85 | 0.03 |
| Tet, Sp, Gn, (Cp) | St. Dev. | 0.75 | 138 | 6.0 | - | ME | 0.02 | ME | - | 0.07 | ME | 7569 | 0.02 | 0.18 | 0.35 | - | 0.04 | 14 | 0.12 | 36 | 0.04 |
| Bv97-52 | Mean (8) | 389 | ME | 0.01 | 0.15 | ME | 0.32 | ME | 3.8 | 0.05 | ME | 682 | 1.9 | 46 | - | - | 0.00 | 46 | 0.00 | ME | 9.1 |
| Ten, Sp, Gn | St. Dev. | 332 | ME | 0.01 | 0.37 | ME | 0.51 | ME | 1.9 | 0.05 | ME | 351 | 1.6 | 128 | - | - | 0.00 | 18 | 0.00 | ME | 5.9 |
| G10847 | Mean (10) | 18 | ME | 0.03 | 0.35 | ME | 0.34 | ME | 11 | 0.07 | 5164 | 1325 | 0.09 | 0.92 | 0.40 | - | 0.06 | 2668 | 0.11 | 13 | 1.3 |
| Tet, Sp, Gn | St. Dev. | 4.7 | ME | 0.02 | 0.24 | ME | 0.13 | ME | 7.2 | 0.06 | 4579 | 169 | 0.02 | 0.78 | 0.41 | - | 0.09 | 493 | 0.22 | 9.3 | 0.22 |
| EV8 | Mean (10) | 7.6 | ME | 0.20 | 0.31 | ME | 0.21 | 121 | 19 | - | ME | 870 | 2.6 | 404 | 0.82 | 0.02 | 0.08 | 19 | 3.7 | 46,078 | 1174 |
| Gn, Sp, Tet | St. Dev. | 3.0 | ME | 0.07 | 0.41 | ME | 0.11 | 142 | 16 | - | ME | 418 | 2.5 | 185 | 0.82 | 0.05 | 0.13 | 8.5 | 2.1 | 29,774 | 456 |
| Hj14 | Mean (6) | 80 | ME | 0.01 | 0.02 | 10,882 | 0.01 | 310 | 2.7 | 0.08 | ME | 161 | 0.05 | 18 | 0.09 | - | 11 | 0.26 | 0.24 | 39 | 0.03 |
| Gn, Sp, Tet | St. Dev. | 22 | ME | 0.03 | 0.04 | 587 | 0.01 | 135 | 2.6 | 0.12 | ME | 22 | 0.05 | 16 | 0.15 | - | 5.2 | 0.09 | 0.58 | 10 | 0.06 |
| G6948 | Mean (10) | 517 | ME | 0.11 | 0.36 | ME | 0.06 | 8222 | 135 | 0.06 | ME | 1033 | 1.4 | 65 | 0.54 | - | 0.02 | 2.4 | 0.02 | 4.6 | 1933 |
| Tet, Sp, Cp, (Gn) | St. Dev. | 71 | ME | 0.10 | 0.21 | ME | 0.06 | 5983 | 68 | 0.06 | ME | 89 | 0.17 | 28 | 0.47 | - | 0.01 | 2.6 | 0.01 | 3.9 | 621 |
| G14549b | Mean (10) | 2.3 | ME | 1.2 | 0.56 | ME | 0.12 | 1921 | 11 | 0.10 | ME | 4731 | 0.33 | 19 | 0.14 | - | 0.39 | 47 | 0.83 | 871 | 475 |
| Tet, Sp, Cp | St. Dev. | 1.2 | ME | 1.0 | 0.61 | ME | 0.09 | 805 | 9.0 | 0.13 | ME | 1272 | 0.24 | 19 | 0.18 | - | 0.22 | 23 | 0.58 | 775 | 386 |
| Mo17A | Mean (6) | 0.50 | ME | 0.16 | 0.03 | ME | 0.01 | 20,439 | 5.8 | 0.03 | ME | 1751 | 0.02 | 0.06 | 0.83 | - | 0.06 | 1.9 | 0.12 | ME | 3.2 |
| Cp, Gn, Tet | St. Dev. | 0.12 | ME | 0.20 | 0.07 | ME | 0.01 | 29,371 | 2.1 | 0.04 | ME | 373 | 0.01 | 0.06 | 0.71 | - | 0.10 | 1.1 | 0.15 | ME | 0.82 |
| ORV1 | Mean (10) | 0.18 | ME | 23 | 2.9 | ME | 0.04 | ME | 21 | 0.12 | 4023 | 979 | 0.40 | 0.11 | 0.34 | - | 0.01 | 36 | 0.00 | 4.0 | 12 |
| Cp, Gn, Tet | St. Dev. | 0.13 | ME | 12 | 2.3 | ME | 0.05 | ME | 16 | 0.14 | 880 | 393 | 0.26 | 0.12 | 0.46 | - | 0.02 | 13 | 0.01 | 3.1 | 16 |
| G14549a | Mean (10) | 1.4 | ME | 0.74 | 0.84 | ME | 0.13 | 3988 | 13 | 0.03 | ME | 3104 | 0.82 | 42 | 0.07 | 0.01 | 0.20 | 25 | 0.30 | 849 | 2794 |
| Tet, Gn, (Sp) | St. Dev. | 0.61 | ME | 0.36 | 1.2 | ME | 0.07 | 1181 | 8.7 | 0.03 | ME | 929 | 1.5 | 41 | 0.10 | 0.01 | 0.13 | 11 | 0.16 | 106 | 1842 |
| G873 | Mean (10) | 1.6 | ME | 1.4 | 0.35 | ME | 0.09 | ME | 13 | 0.33 | ME | 4310 | 10 | 0.70 | 0.19 | 0.03 | 0.08 | 62 | 0.08 | 23 | 0.11 |
| Tet, Gn | St. Dev. | 1.1 | ME | 2.0 | 0.25 | ME | 0.05 | ME | 11 | 0.89 | ME | 1422 | 1.9 | 0.37 | 0.20 | 0.03 | 0.07 | 25 | 0.09 | 5.7 | 0.10 |
| G16152 | Mean (10) | 0.47 | ME | 2512 | 12 | ME | 0.05 | ME | 13 | 1.9 | 4452 | 683 | 3.0 | 28 | - | 0.01 | 0.02 | 3456 | 0.02 | 16 | 29 |
| Tet, Cp, (Gn) | St. Dev. | 0.32 | ME | 880 | 7.4 | ME | 0.03 | ME | 12 | 2.2 | 634 | 70 | 1.4 | 51 | - | 0.02 | 0.02 | 921 | 0.02 | 20 | 30 |
| G871 | Mean (10) | 0.46 | ME | 382 | 0.52 | ME | 0.04 | ME | 17 | 0.12 | 372 | 139 | 0.09 | 0.35 | 0.20 | 0.02 | 0.02 | ME | 0.02 | 1.8 | 0.25 |
| Tet, Cp | St. Dev. | 0.45 | ME | 220 | 0.42 | ME | 0.03 | ME | 9.5 | 0.17 | 48 | 71 | 0.13 | 0.32 | 0.29 | 0.03 | 0.02 | ME | 0.01 | 1.4 | 0.21 |
| G874 | Mean (10) | 0.46 | ME | 557 | 0.20 | ME | 0.03 | ME | 14 | 0.11 | 206 | 263 | 1.8 | 0.47 | 0.17 | 0.02 | 0.01 | ME | 0.01 | 5.9 | 0.47 |
| Tet, Cp | St. Dev. | 0.41 | ME | 397 | 0.12 | ME | 0.02 | ME | 15 | 0.07 | 40 | 157 | 1.2 | 0.35 | 0.16 | 0.02 | 0.01 | ME | 0.01 | 3.5 | 0.54 |
| G879 | Mean (10) | 7.1 | ME | 3.6 | 1.9 | ME | 0.14 | 8555 | - | - | ME | 380 | 15 | 1.4 | 0.29 | 0.04 | 0.09 | 20 | 0.11 | 18 | 1229 |
| Tet, Cp | St. Dev. | 3.7 | ME | 2.6 | 2.9 | ME | 0.16 | 9952 | - | - | ME | 57 | 4.5 | 1.5 | 0.35 | 0.04 | 0.12 | 10 | 0.12 | 28 | 549 |
| G882 | Mean (10) | 0.70 | ME | 613 | 0.56 | ME | 0.13 | ME | 56 | 0.36 | 303 | 328 | 2.3 | 0.68 | 0.69 | 0.04 | 0.05 | ME | 0.03 | 37 | 4.8 |
| Tet, Cp | St. Dev. | 0.62 | ME | 475 | 0.30 | ME | 0.10 | ME | 69 | 0.36 | 90 | 283 | 2.2 | 0.56 | 0.79 | 0.04 | 0.04 | ME | 0.04 | 40 | 3.3 |
| G6946 | Mean (10) | 1.4 | ME | 53 | 28 | ME | 0.14 | ME | 63 | 0.08 | 6202 | 1268 | 1.4 | 2.3 | 0.35 | 0.01 | 0.03 | 1917 | 0.01 | 7.3 | 98 |
| Tet, Cp | St. Dev. | 1.2 | ME | 40 | 9.1 | ME | 0.09 | ME | 28 | 0.05 | 420 | 163 | 0.61 | 2.2 | 0.36 | 0.02 | 0.02 | 710 | 0.01 | 8.2 | 147 |
| G6949 | Mean (10) | 11 | ME | 0.32 | 2.6 | ME | 0.09 | 8922 | 16 | 0.09 | ME | 283 | 15 | 3.0 | 0.12 | - | 0.10 | 13 | 0.65 | 19 | 2821 |
| Tet, Cp | St. Dev. | 7.9 | ME | 0.31 | 2.8 | ME | 0.07 | 2833 | 11 | 0.12 | ME | 47 | 5.9 | 2.5 | 0.13 | - | 0.08 | 4.5 | 0.39 | 15 | 1101 |
| G11701 | Mean (10) | 1.1 | ME | 78 | 0.55 | ME | 0.05 | 134 | 13 | 0.07 | ME | 1124 | 0.14 | 5.1 | 0.12 | 0.02 | 0.04 | 192 | 0.02 | 6.1 | 23 |
| Tet, Cp | St. Dev. | 0.64 | ME | 21 | 0.56 | ME | 0.05 | 36 | 14 | 0.07 | ME | 902 | 0.05 | 7.5 | 0.22 | 0.02 | 0.06 | 91 | 0.04 | 3.0 | 23 |
| G14246 | Mean (10) | 18 | ME | 3.0 | 0.23 | ME | 0.06 | 5264 | - | 0.06 | ME | 365 | 7.3 | 1.5 | 0.22 | 0.01 | 0.02 | 58 | 0.08 | 3.2 | 2269 |
| Tet, Cp | St. Dev. | 13 | ME | 1.4 | 0.13 | ME | 0.05 | 3493 | - | 0.06 | ME | 63 | 4.5 | 1.0 | 0.26 | 0.01 | 0.01 | 29 | 0.08 | 3.2 | 1035 |
| G14867 | Mean (10) | 0.36 | ME | 146 | 0.54 | 16,696 | 0.07 | ME | 376 | 0.15 | 70 | 183 | 3.0 | 0.68 | 0.34 | - | 0.02 | 5310 | 0.14 | 3.0 | 3768 |
| Ten, Cp | St. Dev. | 0.15 | ME | 3.6 | 0.49 | 903 | 0.05 | ME | 293 | 0.12 | 20 | 19 | 0.35 | 0.44 | 0.53 | - | 0.01 | 3594 | 0.18 | 0.91 | 1038 |
| Mo16 | Mean (3) | 15 | ME | 7.0 | 1.7 | 8795 | 0.06 | ME | 11 | 0.01 | ME | 1213 | 0.03 | 0.10 | 2.0 | - | 0.02 | 31 | 0.03 | 2.2 | 2.7 |
| Cp, Tet, (Gn) | St. Dev. | 6.6 | ME | 12 | 2.9 | 6021 | 0.09 | ME | 3.4 | 0.02 | ME | 599 | 0.01 | 0.04 | 1.2 | - | 0.02 | 10 | 0.06 | 1.0 | 1.0 |
| G29851 | Mean (10) | 0.88 | ME | 78 | 1.1 | ME | 0.10 | ME | 33 | 1.0 | 909 | 535 | 0.31 | 0.91 | 1.7 | 0.07 | 0.11 | ME | 0.12 | 108 | 1361 |
| Cp, Ten | St. Dev. | 0.79 | ME | 28 | 0.75 | ME | 0.10 | ME | 22 | 1.0 | 382 | 97 | 0.12 | 0.77 | 1.3 | 0.07 | 0.22 | ME | 0.21 | 89 | 1471 |
| ORV4 | Mean (10) | 29 | ME | 3.3 | 1.9 | ME | 0.02 | ME | 12 | 0.19 | 1409 | 1269 | 4.5 | 0.07 | 3.0 | - | - | 37 | 0.01 | 2.9 | 140 |
| Cp, Tet | St. Dev. | 29 | ME | 2.7 | 1.6 | ME | 0.04 | ME | 4.5 | 0.31 | 274 | 395 | 2.3 | 0.06 | 4.9 | - | - | 21 | 0.02 | 2.1 | 136 |
| G12640 | Mean (10) | 79 | ME | 3.1 | 0.62 | ME | 0.08 | ME | 107 | 0.15 | 1301 | 658 | 0.34 | 2.6 | 7.1 | 0.03 | 0.03 | 1654 | 0.14 | 7.3 | 54 |
| Ten | St. Dev. | 13 | ME | 0.49 | 0.47 | ME | 0.06 | ME | 22 | 0.18 | 185 | 39 | 0.05 | 1.2 | 2.7 | 0.03 | 0.03 | 329 | 0.22 | 2.3 | 12 |
| G13301 | Mean (10) | 1.0 | ME | 633 | 31 | ME | 0.27 | ME | 127 | - | 158 | 7005 | 2.7 | 0.66 | 3.4 | 0.03 | 0.02 | 1747 | 0.02 | 11 | 100 |
| Tet | St. Dev. | 2.2 | ME | 18 | 26 | ME | 0.20 | ME | 43 | - | 27 | 991 | 0.79 | 0.60 | 2.7 | 0.03 | 0.02 | 322 | 0.02 | 6.1 | 49 |
| G15977 | Mean (10) | 9.2 | ME | 19 | 0.28 | ME | 0.11 | ME | 32 | 0.06 | 630 | 1488 | 0.04 | 0.77 | 1.0 | - | 0.18 | 321 | 0.01 | 3.9 | 10.9 |
| Tet | St. Dev. | 2.0 | ME | 2.7 | 0.16 | ME | 0.11 | ME | 15 | 0.08 | 181 | 114 | 0.03 | 0.42 | 0.73 | - | 0.05 | 129 | 0.02 | 2.4 | 1.0 |
| G16835 | Mean (10) | 2.0 | ME | 85 | 0.88 | ME | 0.11 | ME | 12 | 0.14 | 3942 | 331 | 1.1 | 0.70 | 1.1 | 0.05 | 0.18 | 14,761 | 0.06 | 61 | 2385 |
| Ten | St. Dev. | 2.8 | ME | 8.4 | 0.76 | ME | 0.10 | ME | 11 | 0.13 | 314 | 45 | 0.19 | 0.65 | 1.3 | 0.12 | 0.13 | 2281 | 0.08 | 24 | 770 |
| VFI031 | Mean (10) | 1019 | 153 | - | 0.16 | ME | 1.2 | ME | 624 | 0.17 | ME | 1095 | 6.9 | 6.1 | 3648 | 0.01 | 2.2 | 8965 | 0.22 | 67 | 1.7 |
| Tet | St. Dev. | 236 | 57 | - | 0.10 | ME | 0.42 | ME | 213 | 0.34 | ME | 129 | 2.1 | 11 | 3185 | 0.01 | 1.1 | 3439 | 0.13 | 76 | 0.11 |
| Trace Element | Mn | Fe | Cu | Zn |
| Preferred Host | Sp 1 | TetTen >Sp 3 | TetTen >Sp 3 | TetTen 1 |
| Trace Element | Ga | As | Se | Ag |
| Preferred Host | Sp 2 | TetTen >Gn 1 | Gn 1 | TetTen >Gn 1 |
| Trace Element | Cd | In | Sn | Sb |
| Preferred Host | Sp >TetTen 3 | Sp > Cp 2 | ? * | TetTen >Gn 1 |
| Trace Element | Te | Hg | Tl | Bi |
| Preferred Host | Gn 1 | Sp >TetTen 3 | Gn 1 | Gn >TetTen 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, L.L.; Cook, N.J.; Ciobanu, C.L. Minor and Trace Elements in Natural Tetrahedrite-Tennantite: Effects on Element Partitioning among Base Metal Sulphides. Minerals 2017, 7, 17. https://doi.org/10.3390/min7020017
George LL, Cook NJ, Ciobanu CL. Minor and Trace Elements in Natural Tetrahedrite-Tennantite: Effects on Element Partitioning among Base Metal Sulphides. Minerals. 2017; 7(2):17. https://doi.org/10.3390/min7020017
Chicago/Turabian StyleGeorge, Luke L., Nigel J. Cook, and Cristiana L. Ciobanu. 2017. "Minor and Trace Elements in Natural Tetrahedrite-Tennantite: Effects on Element Partitioning among Base Metal Sulphides" Minerals 7, no. 2: 17. https://doi.org/10.3390/min7020017