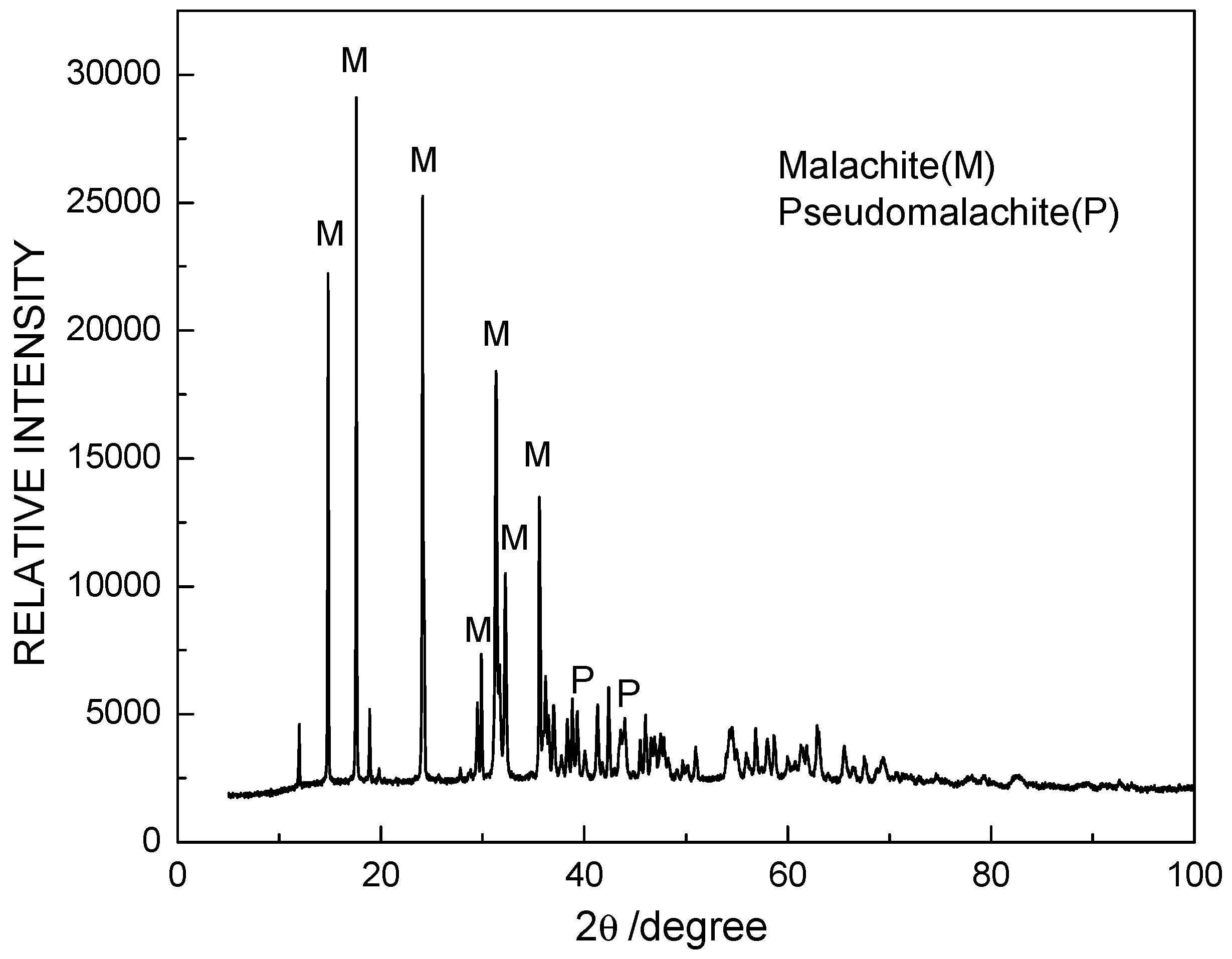
1. Introduction
With the depletion of easy-to-process sulfide copper ores, refractory copper oxides have been increasingly studied to process all over the world. Leaching-solvent extraction-electrowinning (L-SX-EW) and froth flotation are the two methods to beneficiate these ores, of which froth flotation is more economic. Particularly for mixed copper sulfide and oxide ores (e.g., Minto Mine in Yukon, Canada) that are not amenable to L-SX-EW, the development of complementary chemical agents to float the oxides becomes the primary option [
1]. However, oxide copper ores (e.g., malachite) respond poorly to traditional sulfide copper (e.g., thiol) collectors in flotation because of their more hydrophilic oxide surfaces [
2]. In practices, controlled potential sulfidization, prior to the addition of thiol collectors, has been applied to overcome this problem [
3,
4], but it is problematic in controlling accuracy, especially for the mixture of sulfide and oxide copper ores, because a slight excess of sulfidizing agents in the pulp depresses the flotation but an insufficient amount produces poor recoveries [
5]. Therefore, chelating reagents with superior collecting abilities and strong metal and mineral specificity to float copper oxides independently have been extensively explored. Chelating collector molecules contain a reactive functional group with ligand atoms such as S, N and O in positions capable of bonding the same metal atom through two or more different ligand atoms to form a heterocyclic ring in which the metal atom is one of the members. They are classified into S-S, S-N, N-N, N-O and O-O types based on their bidentate ligands [
6]. Although the study of chelating collectors dates back to 1940s and one type of S-S chelating collector, namely xanthate, has found great success in sulfide flotation [
7], in oxide flotations, most of the chelating reagents have only been synthesized and tested for their collecting power in laboratory without commercial applications.
Hydroxamate, which is an O-O type chelating reagent, is the most intensively studied reagent in the development of novel collectors in oxide flotation. In the early stage, potassium octyl hydroxamate was found to chemically adsorb on the malachite surface, so as to be an effective collector for malachite flotation between pH 6 and 10 [
8]. While complete flotation of chrysocolla was obtained with potassium octyl hydroxamate as the collector at pH 6 at room temperature, and the flotation response was enhanced with increased temperature when low additions of hydroxamate were involved [
9]. Furthermore, hydroxamate was used for the removal of colored titaniferous impurities from kaolin clay in flotation industries [
10]. In recent years, advanced surface characterization techniques have been employed to study the interaction of hydroxamate on oxide minerals. For example, X-ray photoelectron spectroscopy (XPS) investigation of the copper oxide minerals cuprite and malachite, and the gangue mineral quartz, showed formation of a copper hydroxamate-like species on cuprite and malachite but no hydroxamate derived species was found on the quartz. Raman spectroscopy confirmed the existence of a copper n-octanohydroxamate layer on the surface of treated malachite [
11]. Density functional theory (DFT) computation indicated that the dianion of cyclohexyl hydroxamic acid (CHA) or benzoylhydroxamic acid (BHA) exhibited stronger chemical reactivity than their anions and neutral molecules, and that the replacement of the phenyl group by the cyclohexyl group in the BHA molecule significantly impacted the electron donating ability of hydroxamate collectors [
12]. Through studying the surface chemistry features of bastnaesite with respect to octyl hydroxamate adsorption, sum-frequency vibrational spectroscopy (SFVS) spectra indicate that a well-ordered monolayer was formed at a hydroxamate concentration of about 1 × 10
−4 mol/L [
13]. Meanwhile, some studies reported the chelating behavior of salicylaldoxime on oxide minerals, which is an O-N type chelating reagent. For example, salicylaldoxime was utilized for copper flotation from the synthetic mixtures malachite–quartz, of which a good copper recovery (92%) and a great copper percent (46%) demonstrated a good selectivity of the reagent [
14]. Jain et al. [
15] performed DFT computations to study the interactions of salicylaldoxime (SALO) and its derivatives possessing appropriate alkyl group substitution in the main chain (CM-SALO) or side chain (CS-SALO) with copper, zinc and lead divalent ions. They found that the relative order of selectivity, as per the computed interaction energies, was Cu > Zn > Pb. In addition, the derivatives of hydroxamate and salicylaldoxime have been synthesized and applied in oxide flotations. Xu et al. [
16] prepared 2-ethyl-2-hexenoic hydroxamic acid (EHHA) for the adsorption and flotation of ilmenite. They found that EHHA exhibited superior flotation performance compared to isooctyl hydroximic acid (IOHA) and octyl hydroxamic acid (OHA), and floated out 84.03% ilmenite at pH 8.0 with 250 mg/L dosage. Liu et al. [
17] reported the adsorption of 3-hexyl-4-amino-1,2,4-triazole-5-thione (HATT) on the malachite surface via its anionic amino-triazole-thione group, thus inducing the malachite surface to be hydrophobic in flotation.
However, these studies were carried out on a case to case basis and the adsorption mechanism of the various chelating ligands is still elusive. Considering the important roles that chelating reagents play in developing oxide collectors, we attempt to first compare the adsorption of phenol O-O and N-O chelating collectors at the malachite/water interface and the corresponding flotation behavior of malachite. It aimed to find out the difference of chelating reactions on the malachite surface when their polar heads are different, so as to offer information for the design of novel collectors, and to provide a solid understanding of the commercial application of chelating collectors in the near further.
3. Results and Discussion
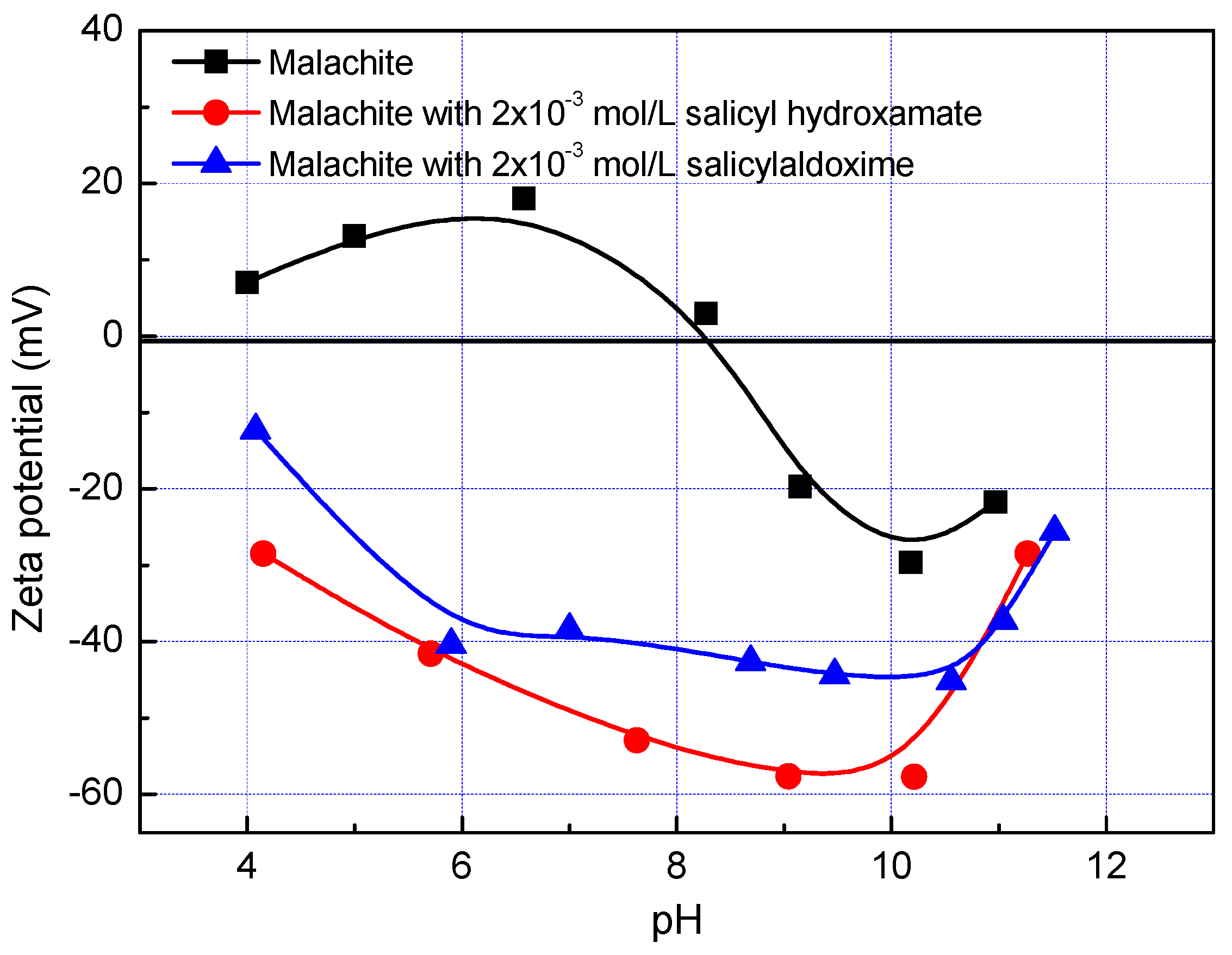
Figure 3 presents the effect of salicyl hydroxamate and salicylaldoxime on the zeta potential of malachite as a function of pH. As with most oxide minerals, the malachite has a positive surface charge at low pHs and a negative surface charge at high pHs. It shows the point of zero charge (PZC) of malachite at pH 8.2, which is in good accordance with the PZC (pH 7.9) reported by Lenormand et al. [
8]. With the addition of salicyl hydroxamate and salicylaldoxime, zeta potentials of malachite reverse from positive to negative at low pHs and become more negative at high pHs, indicating the chemical adsorption of these chelating reagents on its surface. However, this modification reduces at a pH higher than 10. At pH 11, the zeta potentials of malachite without and with addition of salicyl hydroxamate and salicylaldoxime are close, indicating a weak adsorption. It might be due to the fact that at a pH higher than 10, the predominant hydroxyl species weaken the interaction of chelating reagents on the malachite surface. It is interesting that the salicyl hydroxamate modifies the malachite surface more negatively than the salicylaldoxime does in the pH range of 5–10.
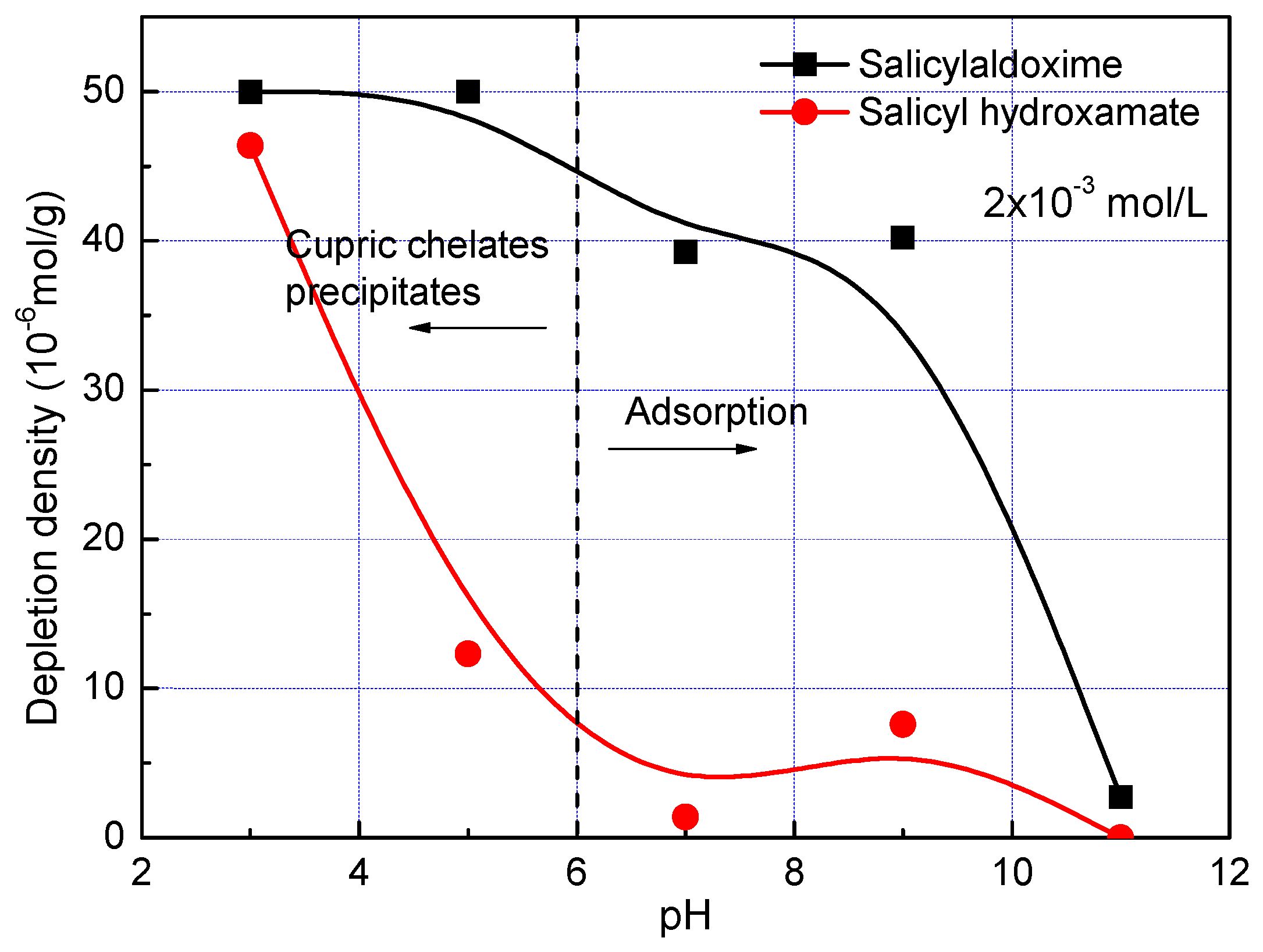
A batch of salicyl hydroxamate and salicylaldoxime depletion has been studied to explore their chemical adsorption on the malachite surface. At a pH lower than pH 6, cupric ions are highly dissolved from malachite, thus the precipitation of cupric salicyl hydroxamate or salicylaldoxime species are predominant reactions for the depletion of the chelating reagents [
8]. Then, at a pH higher than pH 6, chelating reactions (adsorption) on the malachite surface are mainly responsible for the salicyl hydroxamate or salicylaldoxime depletion.
Figure 4 gives their depletion densities in malachite slurry as a function of pH. The depletion of salicyl hydroxamate and salicylaldoxime at pH 3 are as high as 5 × 10
−5 mol per gram of malachite minerals, suggesting that both reagents are highly reactive with cupric ions in slurry or malachite surface. Increasing pH from 3 to 9, the depletion of salicylaldoxime decreases slightly but keeps a high amount, while the depletion of salicyl hydroxamate drops dramatically to the lower magnitude of 5 × 10
−6 mol/g. Then, the depletion decreases continually as the Ph is increased; and at pH 11, depletions of both salicyl hydroxamate and salicylaldoxime are around zero, which corresponds well with the zeta potential results in
Figure 3. At pH 3 to 9, the higher precipitation and/or adsorption degree of salicylaldoxime on the malachite surface than that of salicyl hydroxamate might be attributed to the different stability constants of these chelating reagents with Cu
2+ complexes. The stability constants of Cu-salicylaldoxime and Cu-salicyl hydroxamate are 12 and 9.05 [
19,
20], respectively, indicating that it is easier for salicylaldoxime to react with Cu
2+ complexes in the form of cupric precipitates or adsorption on the malachite surface.
It is notable from previous discussions that salicyl hydroxamate has a lower adsorption density on the malachite surface (
Figure 4), but modifies the malachite surface to be more negative than salicylaldoxime (
Figure 3). This phenomenon might be due to the distinct chelating reactions of salicylaldoxime and salicyl hydroxamate on the malachite surface because of their different molecular structures. Based on the criteria that chelating reagents must possess at least two donor atoms carrying a long pair of electrons [
6], the donor atoms in salicylaldoxime are O (=N–OH, oxime), N (–N=, tertiary acyclic) and O (–OH, phenolic), while the donor atoms in salicyl hydroxamate are O, O (both in hydroxamate) and O (–OH, phenolic). As noted in
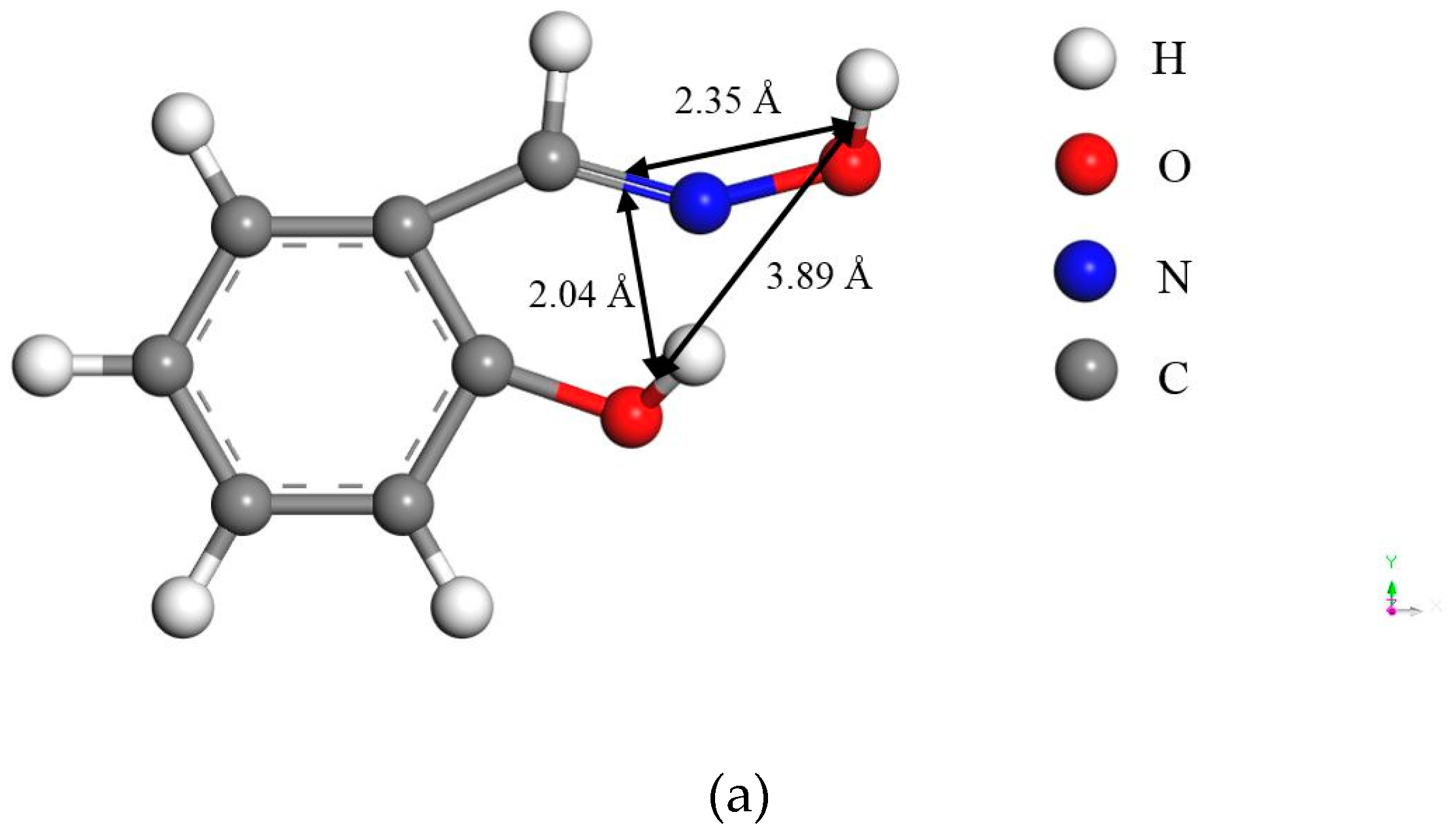
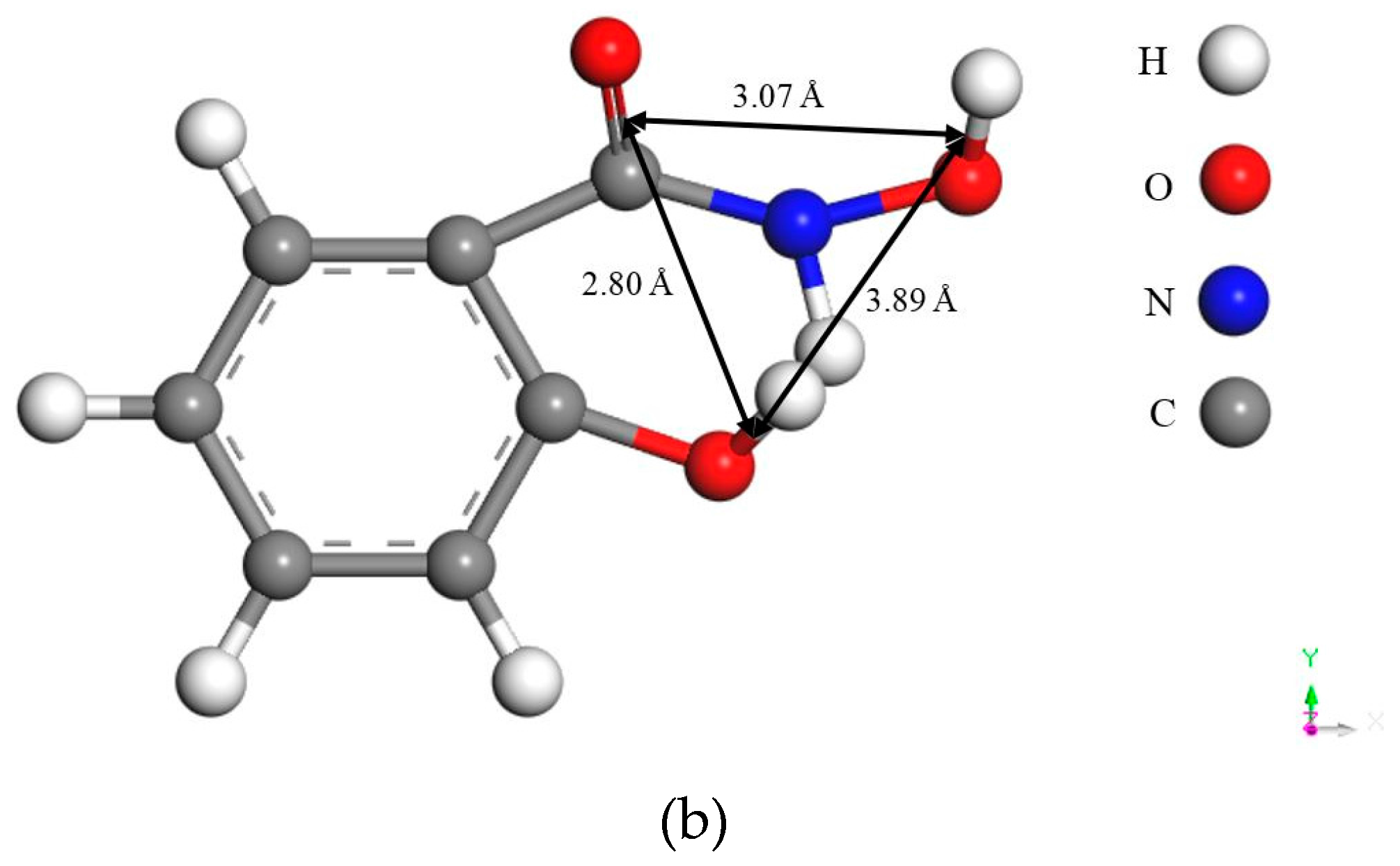
Figure 2, the bond distances between the ligands in the two reagents are different: the bond distances to the carbonyl O in salicyl hydroxamate are around 0.75 Å longer than those to the tertiary acyclic N in salicylaldoxime, while the third bond distance is of identical 3.89 Å. Based on the other criteria of the chelating reactions—that they must form a ring structure sterically including the metal atom [
6]—salicyl hydroxamate might form the ring structure with only two ligands because of the long bond distances, which leads to one O atom carrying a negative charge on the malachite surface after the adsorption. However, it is possible for salicylaldoxime to form the ring structure with three ligands because of the relatively short bond distances. Thus, compared with salicylaldoxime, salicyl hydroxamate modifies the malachite surface more negatively by a lower amount of adsorbed molecules.
Figure 5 schematically presents the chelating reactions of salicyl hydroxamate and salicylaldoxime on the malachite surface, in which CuOH
+ and HCO
3− are defined as the adsorption sites on the malachite surface, as analyzed elsewhere [
8].
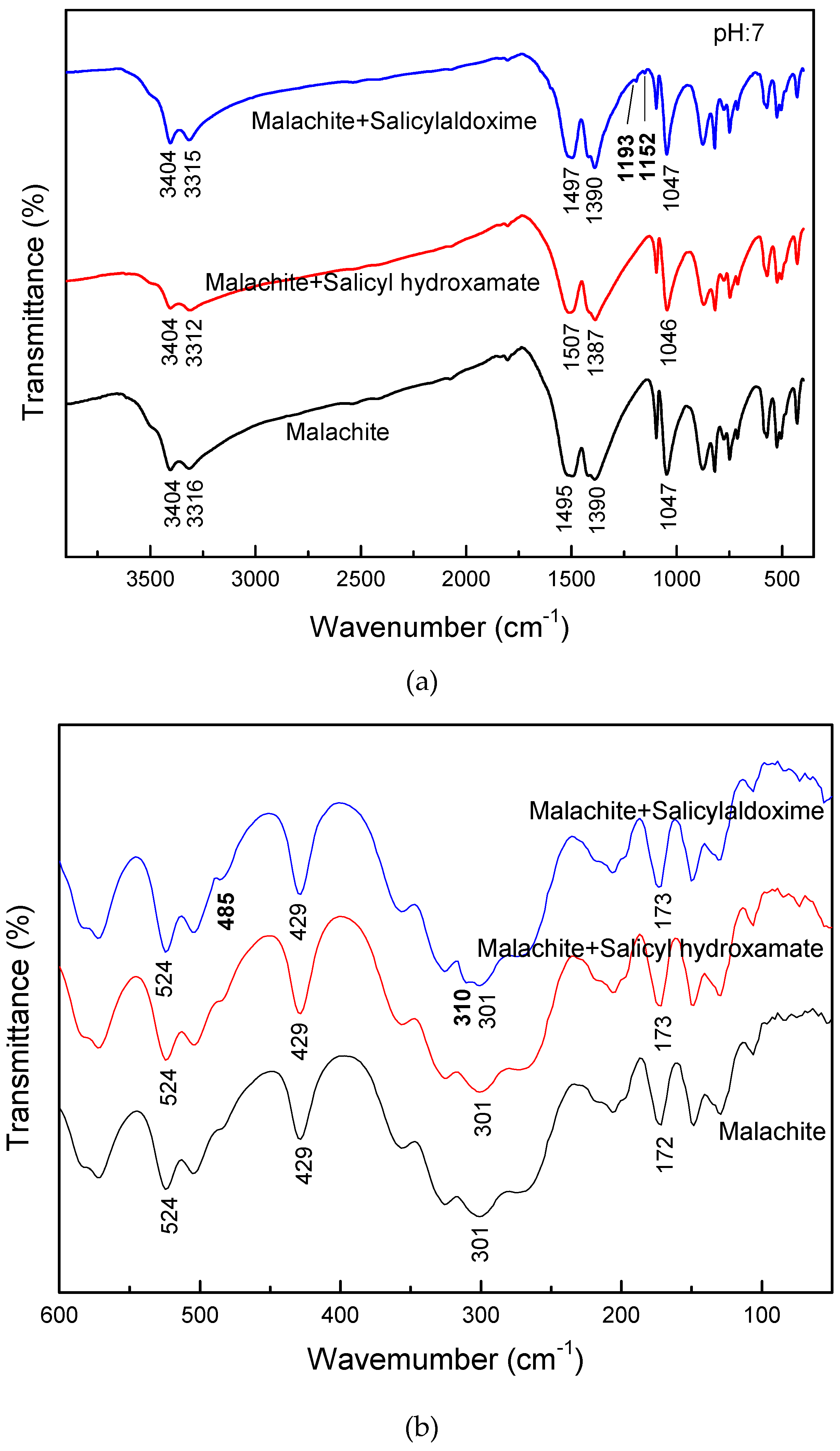
The proposed adsorption mechanism can be verified from the FTIR spectra of the malachite surface before and after salicylaldoxime or salicyl hydroxamate adsorption. As can be seen in
Figure 6, after salicylaldoxime treatment, the N-Cu and O-Cu stretch vibrations are found at 1193 and 1152 cm
−1 in the intermediate FTIR spectrum, and 310 and 485 cm
−1 in the far FTIR spectrum, respectively [
21], representing the chemical adsorption of salicylaldoxime on the malachite surface. In contrast, no new peak appears on the FTIR spectra of malachite after salicyl hydroxamate treatment, indicating that the adsorption density of salicyl hydroxamate on malachite is too low for FTIR to identify, which is in accordance with the adsorption behavior.
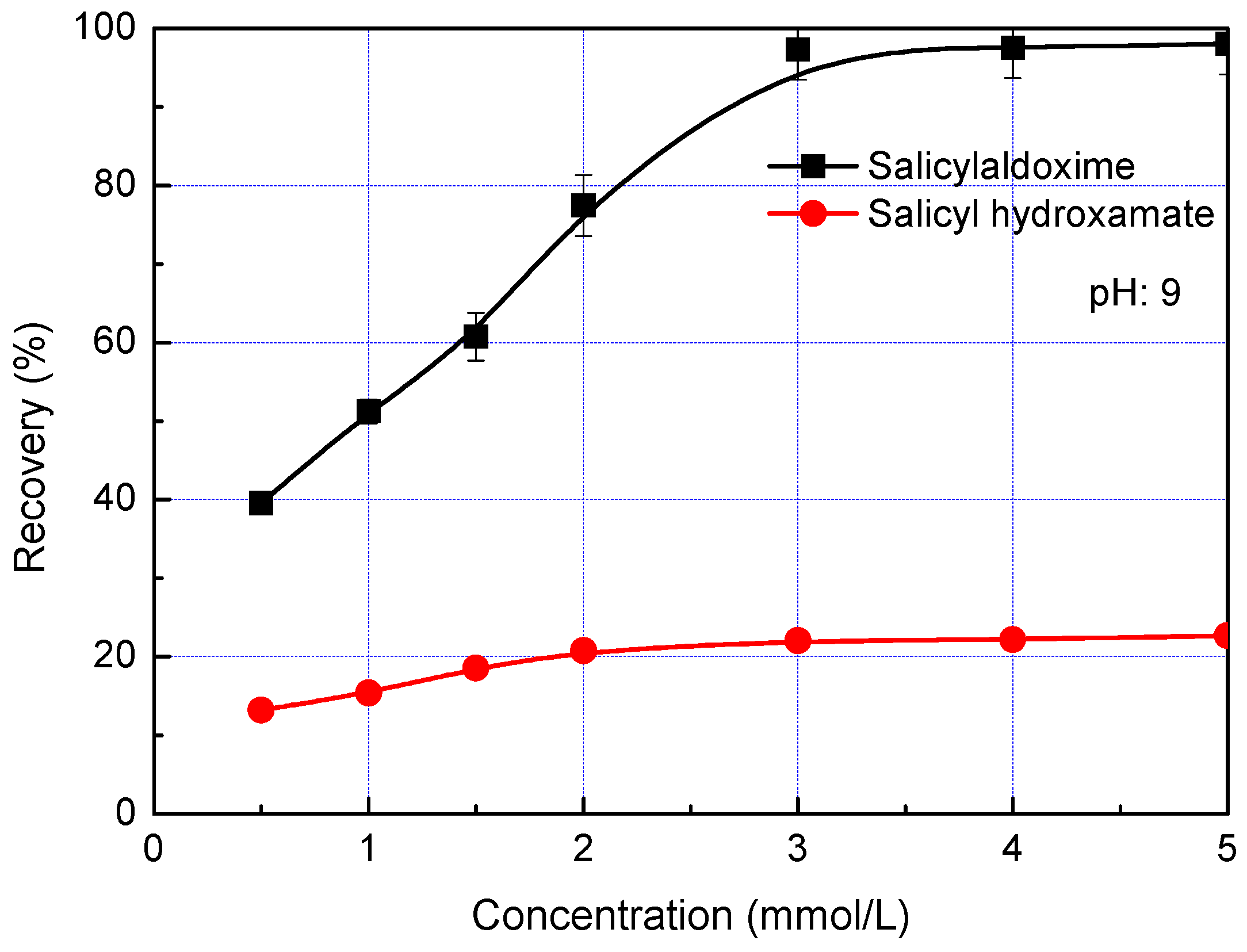
Figure 7 shows the flotation of malachite as a function of salicylaldoxime and salicyl hydroxamate concentrations at pH 9. By using salicylaldoxime as the collector, the malachite recovery increases to 97% as the salicylaldoxime is increased to 3 mmol/L. Then, the malachite recovery remains constant as the salicylaldoxime concentration is continually increased. In the case of salicyl hydroxamate collector, the malachite recovery increases slightly to 20% as the collector concentration is increased to 2 mmol/L, and then remains constant. It corresponds well with the adsorption phenomena that (i) both salicylaldoxime and salicyl hydroxamate are chemically adsorbed on the malachite surface; (ii) the adsorption density of salicylaldoxime is much higher than that of salicyl hydroxamate. Thus, compared with salicyl hydroxamate, salicylaldoxime possesses stronger collecting ability and the flotation of malachite reaches the maximum recovery at a higher collector concentration.
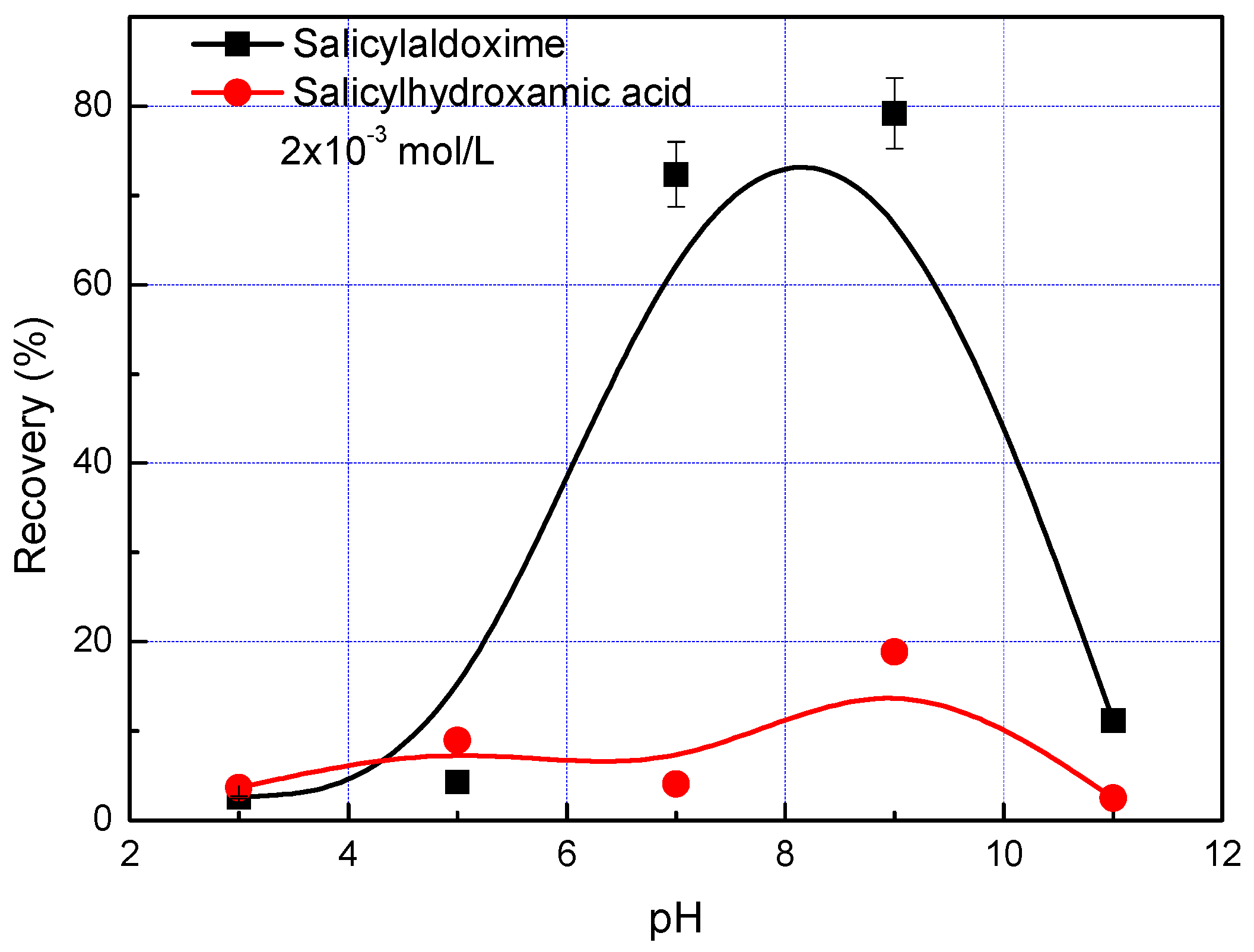
The flotation of malachite using salicylaldoxime and salicylhydroxamic collectors as a function of pH is given in
Figure 8. The malachite recovery is less than 20% when using a salicyl hydroxamate collector in the pH range 3–11, in which the maximum recovery of 19% is obtained at pH 9. In contrast, the malachite recovery is around 80% at pH 7–9 with salicylaldoxime as the collector. These results are in good agreement with the precipitation and adsorption of salicylaldoxime and salicyl hydroxamate in aqueous malachite slurries. At a pH lower than 6, the precipitation of cupric salicylaldoxime and salicyl hydroxamate species are predominant reactions of the depletion of collectors, leading to a low adsorption amount and poor flotation performance. At pH 7–9, the recoveries of malachite reach the maximum with both collectors because of the chelating (adsorption) reactions, and because salicylaldoxime possesses a stronger collecting ability than salicyl hydroxamate due to its higher adsorption density. Then, at a pH higher than pH 9 (e.g., pH 11), due to the competition between chelating collectors and hydroxyls on the malachite surface, a low adsorption density takes place, leading to a low malachite recovery. Thus, the proper pH range for malachite flotation with chelating collectors is pH 7–9, which is consistent with malachite flotation by using an octyl hydroxamate collector [
8].
In addition, octyl hydroxamate has been reported as an effective collector for oxide (malachite) flotations [
22,
23], but in our results, salicyl hydroxamate shows a weak collecting ability on malachite. This might be due to the fact that (i) the longer alkyl chain in octyl hydroxamate can render the oxide surfaces hydrophobic more effectively than the benzene ring in salicyl hydroxamate; (ii) the leaving O
− after salicyl hydroxamate adsorption not only modifies the malachite surface more negatively, but also renders it hydrophilic. These results might provide clues for designing a novel collector of oxide flotations in both the carbon chains and the polar heads.