Geochemistry and Stable Isotopes of Travertine from Jordan Valley and Dead Sea Areas
Abstract
:1. Introduction
1.1. Location
1.2. Geological Setting
1.3. Hydrology and Hydrogeology
2. Materials and Methods
3. Results and Discussion
3.1. Mineralogy
3.1.1. MgCO3
3.1.2. Calcium Carbonate (CaCO3)
3.2. Geochemistry
3.2.1. CaO and MgO
3.2.2. Fe2O3 and MnO
3.2.3. Alkali Metals
3.2.4. Sr
3.3. Stable Isotopes
3.3.1. Background Information
3.3.2. Deir Alla Travertine
3.3.3. Suwayma Travertine
3.3.4. Az Zara Tufa
3.4. Travertine Dating
3.4.1. Background Information
3.4.2. Age of Travertine
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fouke, B.W.; Farmer, J.D.; Des Marais, D.J.; Pratt, L.; Sturchio, N.C.; Burns, P.C.; Discipulo, M.K. Depositional facies and aqueous-solid geochemistry of travertine-depositing hot springs (Angel Terrace, Mammoth Hot Springs, Yellowstone National Park, USA). J. Sediment. Res. 2000, 70, 565–585. [Google Scholar] [CrossRef]
- Jamtveit, B.; Hammer, Ø.; Andersson, C.; Dysthe, D.K.; Heldmann, J.; Vogel, M.L. Travertines from the Troll thermal springs, Svalbard. Norwegian. J. Geol. 2006, 86, 387–395. [Google Scholar]
- Fouke, B.W. Hot-spring systems geobiology: Abiotic and biotic influences on travertine formation at Mammoth hot springs, Yellowstone National Park, USA. Sedimentology 2011, 58, 170–219. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Capezzuoli, E.; Candin, A.; Pedley, M. Decoding tufa and travertine (fresh water carbonates) in the sedimentary record: The state of the art. Sedimentology 2014, 61, 1–21. [Google Scholar] [CrossRef]
- Rodrigo-Naharro, J.; Delgado, A.; Herrero, M.J.; Granados, A.; Pérez del Villar, L. Current travertines precipitation from CO2-rich groundwaters as an alert of CO2 leakages from a natural CO2 storage at Gañuelas-Mazarrón Tertiary Basin (Murcia, Spain). Inf. Técnicos Ciemat 2013, 1279, 1–53. [Google Scholar]
- Dreybrodt, W.; Buhmann, D.; Michaelis, J.; Usdowski, E. Geochemically controlled calcite precipitation by CO2 outgassing: Field measurements of precipitation rates in comparison to theoretical predictions. Chem. Geol. 1992, 97, 285–294. [Google Scholar] [CrossRef]
- Pentecost, A. Travertine, 1st ed.; Springer: London, UK, 2005; ISBN 978-1-4020-3606-4. [Google Scholar]
- Pentecost, A.; Vile, H.A. A review and reassessment of travertine classification. Géogr. Phys. Quat. 1994, 48, 305–314. [Google Scholar] [CrossRef]
- Alessandro, D.W.; Giammanco, S.; Bellomo, S.; Parello, F. Geochemistry and mineralogy of travertine deposits of the SW flank of Mt. Etna (Italy): Relationships with past volcanic and degassing activity. J. Volcanol. Geotherm. Res. 2007, 165, 64–70. [Google Scholar] [CrossRef]
- Ford, T.D.; Pedley, H.M. A review of tufa and travertine deposits of the world. Earth Sci. Rev. 1996, 41, 117–175. [Google Scholar] [CrossRef]
- Arenas, C.; Gutiérrez, F.; Osàcar, C.; Sancho, C. Sedimentology and geochemistry of fluvio-lacustrine tufa deposits controlled by evaporate solution subsidence in the central Ebro Depression, NE Spain. Sedimentology 2000, 47, 883–909. [Google Scholar] [CrossRef]
- Jasser, D. Investigation of Deir Alla Travertine; Natural Resources Authority: Amman, Jordan, 1984.
- Khoury, H.; Salameh, E.; Udluft, P. On the Zerqa Ma’in (thermal kallirrhoes) travertine/Dead Sea (hydrochemistry, geochemistry and isotopic composition). Neues Jahrb. Geol. Palaontol. Mh 1984, H8, 472–484. [Google Scholar]
- Obeidat, O. Geochemistry, Mineralogy, and Petrography of Travertine of Deir Alla and Zerqa Ma’in Hot Springs. Master’s Thesis, Yarmouk University, Irbid, Jordan, 1992. [Google Scholar]
- Ibrahim, K.M.; Moh’d, B.K.; Masri, A.I.; Al-Taj, M.M.; Musleh, S.M.; Alzughoul, K.A. Volcanotectonic evolution of central Jordan: Evidence from the Shihan volcano. J. Afr. Earth Sci. 2014, 100, 541–553. [Google Scholar] [CrossRef]
- Smit, J.; Brun, J.P.; Cloetingh, S.; Ben-Avraham, Z. The rift-like structure and asymmetry of the Dead Sea Fault. Earth Planet. Sci. Lett. 2010, 290, 74–82. [Google Scholar] [CrossRef]
- Ferry, M.; Meghraoui, M.; Abou Karaki, N.; Al-Taj, M.; Khalil, L. Episodic behavior of the Jordan Valley section of the Dead Sea fault inferred from a 14-kyr long integrated catalogue of large earthquakes. Bull. Seism. Soc. Am. 2011, 101, 39–87. [Google Scholar] [CrossRef]
- Al-Thawabteh, S.M. Sedimentology, Geochemistry, and Petrographic Study of Travertine Deposits along the Eastern Side of the Jordan Valley and Dead Sea Areas. Master’s Thesis, Hashemite University, Zarqa, Jordan, 2008. [Google Scholar]
- Salameh, E.; Bannayan, H. Water Resources of Jordan: Present Status and Future Potentials; Friedrich Ebert Shiftung: Amman, Jordan, 1993. [Google Scholar]
- Salameh, E.; Udluft, P. The Hydrodynamic Pattern of Central Jordan; Geol Jb, C38: Hannover, Germany, 1985. [Google Scholar]
- Water Authority of Jordan. Open Files of the Water Authority of Jordan; Water Authority of Jordan: Amman, Jordan, 1991.
- Salameh, E. Curative Water in Jordan; WRSC Bull, 7th Issue; University of Jordan: Amman, Jordan, 1988. [Google Scholar]
- Abu Ajamieh, M. The Geothermal Resources of Zarqa Ma’in and Zara; Natural Recourses Authority: Amman, Jordan, 1980.
- Vogel, J.C. Carbon-14 dating of groundwater. In Isotopes Hydrology; IAEA: Vienna, Austria, 1970. [Google Scholar]
- Thorpe, P.M.; Holyoak, D.T.; Preece, R.C.; Willing, M.J. Validity of corrected 14C dates from calcareous tufa. In Actes Colloq. I’AGF Form. Carbonatées Externs Travertines Paris; Springer: London, UK, 1981; pp. 151–156. [Google Scholar]
- Greensmith, J.T. Petrology of the Sedimentary Rocks, 6th ed.; George Allen and UNWIN: London, UK, 1978; ISBN 0 04 552011 9. [Google Scholar]
- Kawano, J.; Shimobayashi, N.; Miyake, A.; Kitamura, M. Precipitation diagram of calcium carbonate polymorphs: Its construction and significance. J. Phys. Condens. Matter 2009, 21, 425102–425107. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Renaut, R.W. Calcareous spring deposits in continental settings. In Carbonates in Continental Settings: Facies, Environments, and Processes; Alonso Zarza, A.M., Taner, L.H., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2010; Volume 61, pp. 177–224. [Google Scholar]
- Brian, J. Review of Calcium Carbonate Polymorph Precipitation in Spring Systems. Sediment. Geol. 2017, 353, 64–75. [Google Scholar] [CrossRef]
- Özkul, M.; Kele, S.; Gökgöz, A.; Shen, C.C.; Jones, B.; Baykara, M.O.; Fόrizs, I.; Nemeth, T.; Chang, Y.W.; Alcicek, M.C. Comparison of the Quaternary travertine sites in the Denizli Extensional Basin based on their depositional and geochemical data. Sediment. Geol. 2013, 294, 179–204. [Google Scholar] [CrossRef]
- Rao, C.P. Petrography, trace elements and oxygen and carbon isotopes of Grodon group carbonates (Ordovician), Florentine Valley, Tasmania, Australia. J. Sediment. Geol. 1990, 80, 221–231. [Google Scholar] [CrossRef]
- Caboi, R.; Cidu, R.; Fanfani, L.; Zuddas, P.; Zuddas, P.P. Geochemistry of Funtana Maore travertines (Central Sardinia, Italy). Miner. Petrogr. Acta 1991, 34, 77–93. [Google Scholar]
- Okumura, M.; Kitano, Y. Coprecipitation of alkali metal ions with calcium carbonate. Geochim. Cosmochim. Acta 1986, 50, 49–58. [Google Scholar] [CrossRef]
- Pentecost, A. The origin and development of the travertines and associated thermal waters at Matlock Bath, Derbyshire. Proc. Geol. Assoc. 1999, 110, 217–232. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W.; Rosen, M.R. High-temperature (>90 °C) calcite precipitation at Waikite Hot Springs, North Island, New Zealand. J. Geol. Soc. 1998, 153, 481–498. [Google Scholar] [CrossRef]
- Kinsman, D.J. Interpretation of Sr+2 concentration in carbonate minerals and rocks. J. Sediment. Pterol. 1989, 39, 488–508. [Google Scholar] [CrossRef]
- Veizer, J.; Demovic, R. Strontium as a tool in facies analysis. J. Sediment. Pterol. 1974, 44, 93–115. [Google Scholar] [CrossRef]
- Cipriani, N.; Ercoli, A.; Malesani, P.; Vannucci, S. I travertini di Rapolano Term (Siena). Mem. Soc. Geol. Ital. 1972, 11, 31–48. [Google Scholar]
- Cipriani, N.; Malesani, P.; Vannucci, S. I travertine dell’Italia centrale. Boll. Serv. Geol. Ital. 1977, 98, 85–115. [Google Scholar]
- Ichikuni, M. Partition of strontium between calcite and solution: Effect of substitution by manganese. Chem. Geol. 1973, 11, 315–319. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z. Wet-dry seasonal and spatial variations in the δ13C and δ18O values of the modern endogenic travertine at Baishuitai, Yunnan, SW China and their paleoclimatic and paleoenvironmental implications. Geochim. Cosmochim. Acta 2010, 74, 1018–1029. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Guidry, S.A. Bacterial shrubs, crystal shrubs, and ray-crystal crusts: Bacterially induced vs. a biotic mineral precipitation. Sediment. Geol. 1999, 128, 57–74. [Google Scholar] [CrossRef]
- Guo, L.; Andrews, J.; Riding, R.; Dennis, P.; Dresser, Q. Possible microbial effects on stable carbon isotopes in hot-spring travertines. J. Sediment. Res. 1996, 66, 468–473. [Google Scholar] [CrossRef]
- Turi, B. Stable isotope geochemistry of travertines. In Handbook of Environmental Isotope Geochemistry; Fritz, B.P., Fontes, L.J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; ISBN 9781483289830. [Google Scholar]
- Fritz, P. Der Isotopengehalt der Mineralwasser von Stuttgart und Umgebung und ihrer ittel pleistozaenen Travertin-Ablagerungen. Jber. Mitt. Oberrhein. Geol. Ver. 1968, 50, 53–58. [Google Scholar]
- Srdoč, D.; Chafetz, H.; Utech, N. Radiocarbon dating of travertine deposits, Arbuckle Mountains, Oklahoma. Radiocarbon 1989, 31, 819–828. [Google Scholar] [CrossRef]
- Nishikawaa, O.; Furuhashia, K.; Masuyamaa, M.; Ogatab, T.; Shiraishia, T.; Shenc, C. Radiocarbon dating of residual organic matter in travertine formed along the Yumoto Fault in Oga Peninsula, northeast Japan: Implications for long-term hot spring activity under the influence of earthquakes. Sediment. Geol. 2012, 243, 181–190. [Google Scholar] [CrossRef]
- Lovea, A.J.; Shanda, P.; Karlstromd, K.; Crosseyd, L.; Rousseau-Gueutina, P.; Priestleya, S.; Wholinge, D.; Fultonf, S.; Keppela, M. Geochemistry and travertine dating provide new insights into the hydrogeology of the Great Artesian Basin, South Australia. Procedia Earth Planet. Sci. 2013, 7, 521–524. [Google Scholar] [CrossRef]
- Lebatard, A.; Alcicek, M.C.; Rochette, P.; Khatib, S.; Vialet, A.; Boulbes, N.; Bourles, D.L.; Demory, F.; Guipert, G.; Mayda, S.; et al. Dating the Homo erectus bearing travertine from Kocabas, (Denizli, Turkey) at least 1.1 Ma. Earth Planet. Sci. Lett. 2014, 390, 8–18. [Google Scholar] [CrossRef]

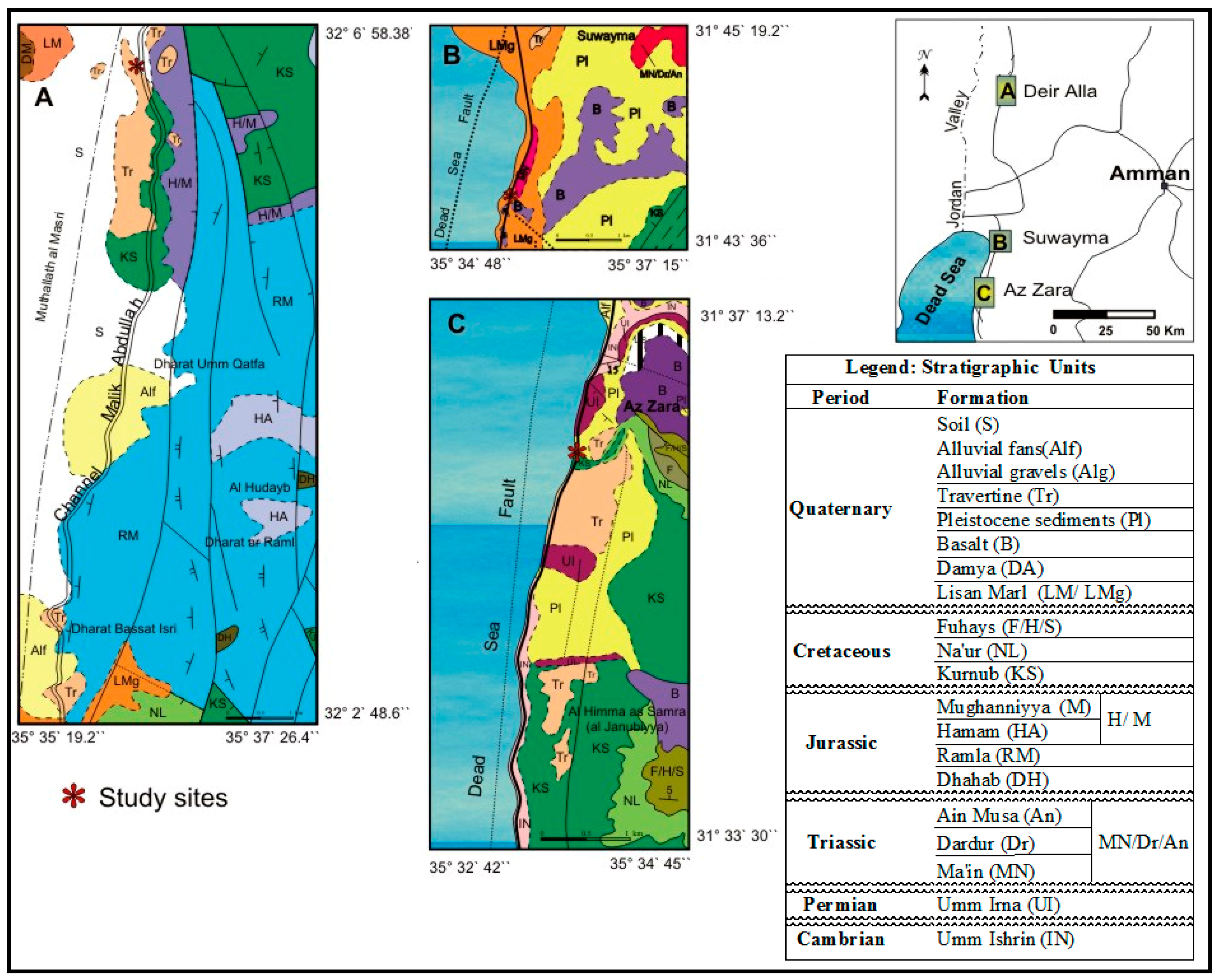

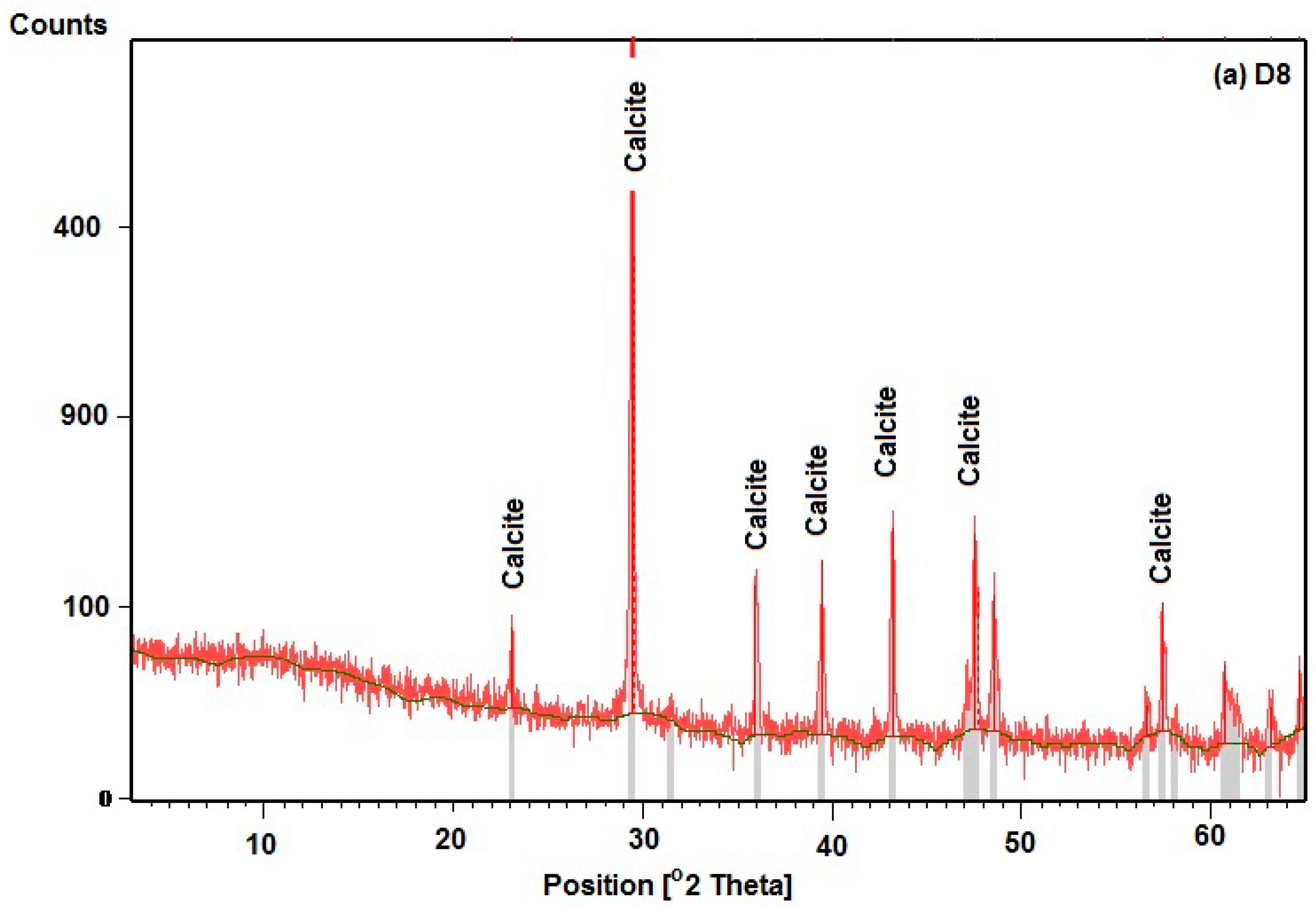

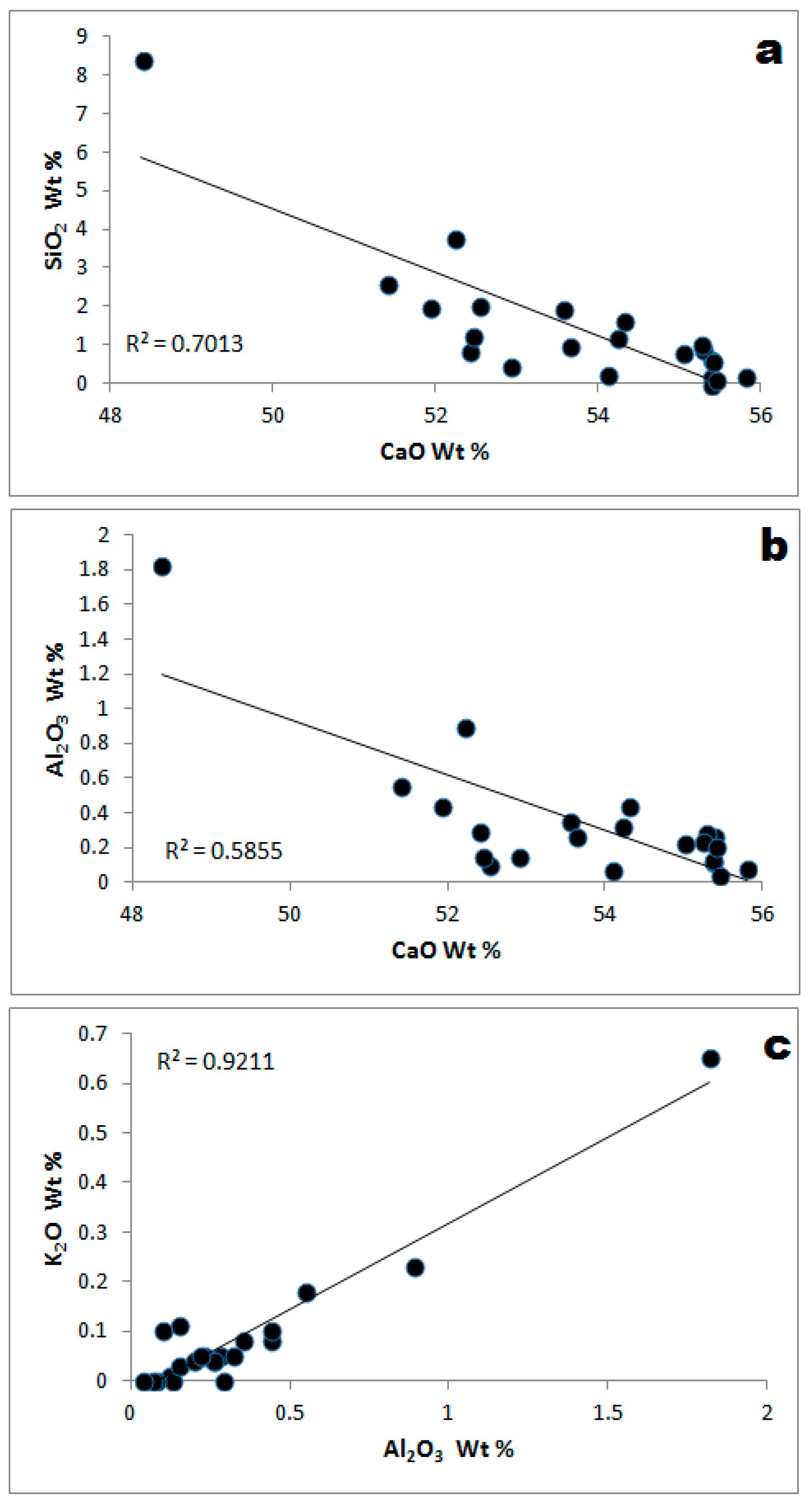
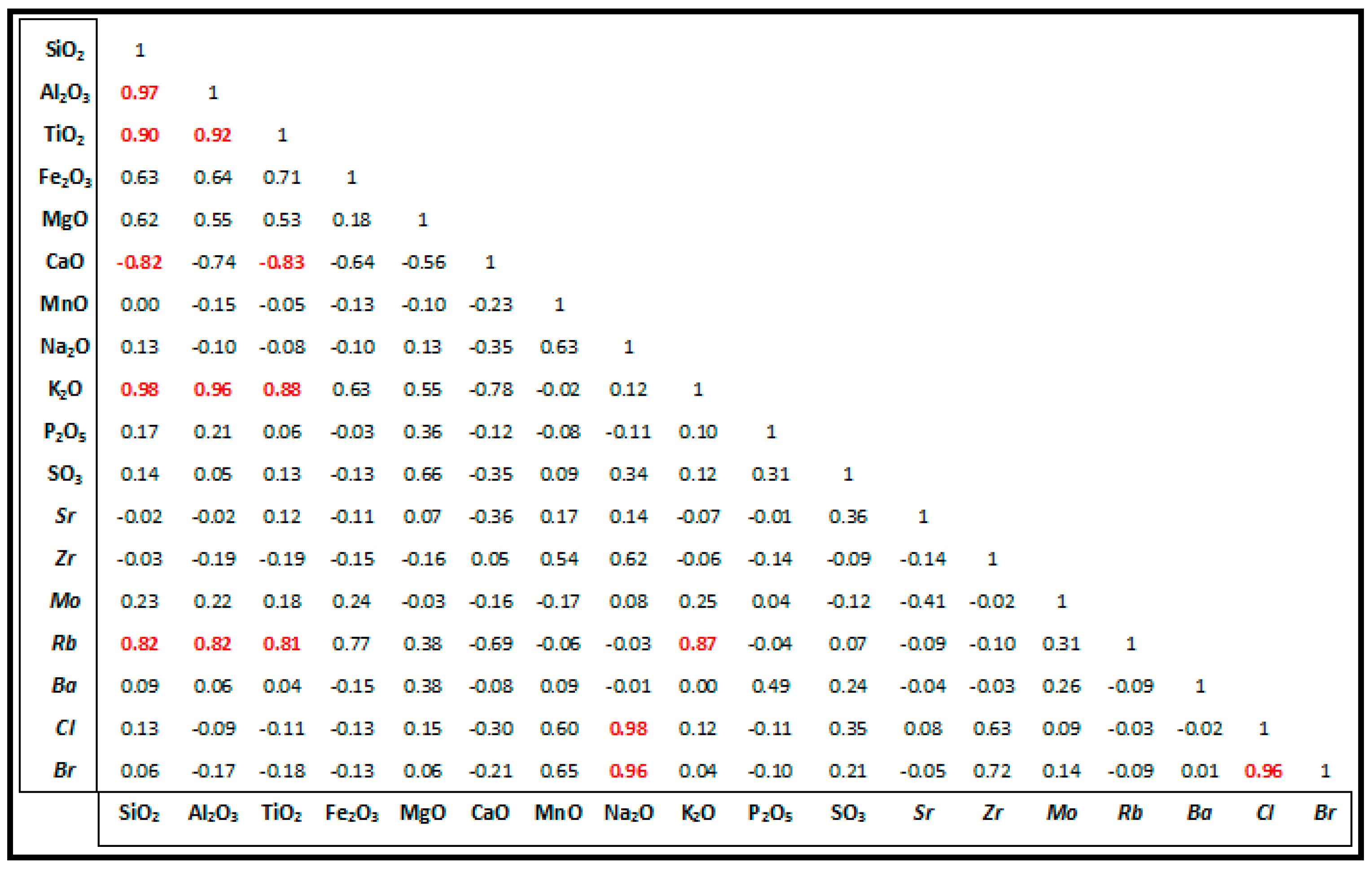
| Locality | Lithofacies | Description |
|---|---|---|
| Deir Alla & Suwayma Travertine | Crystalline crust | Dense, crudely fibrous, elongated calcite crystals (ray crystals) |
| Shrub | Small bush-like growths, on horizontal-semi-horizontal surfaces | |
| Rafts | Thin, delicate and brittle crystalline layers | |
| Lithoclast | Penecontemporaneous, angular to subangular travertine fragments in different size, formed by erosion of the upper slope and collapse of waterfalls and terrace cliffs | |
| Coated gas bubble | Gas bubbles are coated by rapid precipitation of calcium carbonate | |
| Reed | Rich in molds of reed and coarse grass | |
| Pebbly | Rounded pebbles of limestone, chert and basalt | |
| Palaeosol | Exposure of travertine surface to rainwater, subaerial desiccation, and biological activities associated with soil formation | |
| Az Zara Tufa | Macrophyte encrustation | Calcified stems, twigs and leaves that represent rushes, reeds and unidentifiable bushes. |
| Bryophyte build-ups | Laminated deposits that commonly form asymmetrical mounds or small domes, up to 1.75 m thick or stacked sequences up to 2 m high. | |
| Biomicrites | Tabular layers, tens of centimeters thick, mostly at the top of the tufa succession. They consist of massive biomicrites or, in some places, biosparites. | |
| Marls | Massive lenticular beds that are associated with macrophyte palisades. They contain plant debris. |
| No. | D3 | D8 | D11 | D18 | D23b | D26a | D26b | D29 | ||
| Clay | 0.8 | 0.7 | 0.4 | 1.5 | 0.4 | 0.7 | 1.3 | 0.2 | ||
| Dol | 0.5 | 0.7 | 0.5 | 0.7 | 0.4 | 0.9 | 0.5 | 0.4 | ||
| Cc | 98.4 | 94.3 | 97.2 | 93.0 | 94.3 | 93.7 | 98.5 | 98.8 | ||
| Qz | nd | nd | nd | 0.1 | 0.1 | 0.1 | nd | nd | ||
| Mgs | 0.4 | 0.4 | 0.3 | 0.5 | 0.3 | 0.8 | 0.4 | 0.3 | ||
| Others | 2.4 | 4.3 | 1.8 | 4.7 | 4.8 | 4.4 | 1.8 | 0.7 | ||
| No. | S1d | S10 | S19 | S27 | S32 | S34 | S38 | S39 | S45 | S47b |
| Clay | 9.2 | 2.2 | 4.5 | 1.4 | 1.2 | 1.8 | 1.8 | 1.3 | 2.2 | 2.8 |
| Dol | 2.1 | 2.3 | 2.7 | 1.3 | 3.0 | 3.4 | 2.0 | 2.2 | 2.5 | 2.9 |
| Cc | 72.0 | 90.7 | 88.9 | 94.4 | 92.3 | 90.7 | 93.8 | 93.1 | 90.7 | 87.8 |
| Qz | 3.7 | 0.5 | 1.5 | 0.2 | 0.4 | 1.1 | 0.4 | 0.3 | 0.9 | 1.2 |
| Mgs | 1.9 | 1.59 | 1.99 | 0.9 | 1.9 | 2.2 | 1.3 | 1.4 | 1.7 | 1.9 |
| Others | 12.9 | 4.0 | 4.4 | 2.7 | 3 | 3.0 | 2.3 | 2.7 | 3.5 | 5.3 |
| No. | Z1 | Z1* | Z3 | Z4 | ||||||
| Clay | 0.8 | 0.5 | 1.0 | 1.1 | ||||||
| Dol | 2.5 | 1.8 | 1.5 | 2.2 | ||||||
| Cc | 91.8 | 88.7 | 93.5 | 94.2 | ||||||
| Qz | 0.8 | 1.8 | 0.1 | 0.2 | ||||||
| Mgs | 1.8 | 1.1 | 1.0 | 1.4 | ||||||
| Others | 3.9 | 7.1 | 3.7 | 2.1 | ||||||
| Chemical Constituents | D3 | D8 | D11 | D18 | D23b | D26a | D26b | D 29 | S1d | S10 | S19 |
| wt % | |||||||||||
| CaO | 55.38 | 55.38 | 55.81 | 52.42 | 54.1 | 52.92 | 55.39 | 55.45 | 48.38 | 54.31 | 52.22 |
| SiO2 | 0.22 | nd | 0.22 | 0.85 | 0.24 | 0.44 | 0.82 | 0.09 | 8.39 | 1.85 | 3.77 |
| Al2O3 | 0.12 | 0.13 | 0.08 | 0.29 | 0.07 | 0.15 | 0.28 | 0.04 | 1.82 | 0.44 | 0.89 |
| TiO2 | nd | nd | nd | 0.05 | nd | 0.05 | nd | nd | 0.28 | nd | 0.13 |
| Fe2O3 | 0.28 | 1.3 | 0.8 | 2.13 | 3.81 | 0.24 | 0.4 | 0.55 | 4.72 | 0.47 | 1.83 |
| MgO | 0.17 | 0.21 | 0.18 | 0.28 | 0.14 | 0.27 | 0.19 | 0.12 | 0.9 | 0.74 | 0.91 |
| MnO | nd | nd | nd | 0.03 | 0.03 | 1.93 | nd | 0.01 | 0.08 | 0.08 | 0.08 |
| Na2O | nd | nd | nd | 0.04 | 0.04 | 0.13 | nd | 0.04 | 0.13 | 0.02 | 0.03 |
| K2O | 0.01 | nd | nd | nd | nd | 0.03 | 0.04 | nd | 0.85 | 0.08 | 0.23 |
| P2O5 | dl | 0.01 | dl | 0.02 | 0.01 | 0.01 | 0.01 | dl | 0.01 | 0.19 | 0.08 |
| SO3 | 0.02 | 0.02 | 0.02 | nd | 0.01 | 0.07 | 0.02 | 0.01 | 0.03 | 0.11 | 0.08 |
| mg/kg | |||||||||||
| Sr | 842 | 423 | 539 | 4189 | 833 | 3919 | 1200 | 788 | 511 | 1051 | 898 |
| Zr | 31 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Mo | 307 | 357 | 349 | 377 | 285 | 287 | 378 | 379 | 389 | 350 | 321 |
| Rb | nd | nd | nd | nd | nd | nd | nd | nd | 87 | nd | nd |
| Ba | nd | 447 | nd | 378 | nd | 2825 | nd | nd | 1035 | 8339 | 758 |
| Cl | nd | nd | nd | nd | nd | 88 | nd | 40 | 188 | 31 | 12 |
| Br | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Chemical Constituents | S27 | S32 | S34 | S38 | S39 | S45 | S47b | Z1 | Z1* | Z3 | Z4 |
| wt % | |||||||||||
| CaO | 55.29 | 55.28 | 53.57 | 54.23 | 53.84 | 51.93 | 51.41 | 52.54 | 52.45 | 55.41 | 55.03 |
| SiO2 | 0.89 | 1.04 | 1.95 | 1.21 | 1 | 2.01 | 2.81 | 2.03 | 1.24 | 0.81 | 0.82 |
| Al2O3 | 0.28 | 0.23 | 0.35 | 0.32 | 0.28 | 0.44 | 0.55 | 0.1 | 0.15 | 0.2 | 0.22 |
| TiO2 | nd | nd | 0.08 | 0.05 | 0.09 | 0.08 | 0.11 | nd | nd | nd | nd |
| Fe2O3 | 0.83 | 0.47 | 0.87 | 0.91 | 1.81 | 0.53 | 3.43 | 0.88 | 0.18 | 0.17 | 0.47 |
| MgO | 0.42 | 0.9 | 1.03 | 0.82 | 0.89 | 0.79 | 0.92 | 0.54 | 0.74 | 0.47 | 0.85 |
| MnO | 0.03 | 0.04 | nd | nd | 0.04 | 0.12 | 0.15 | 2.1 | 0.03 | nd | nd |
| Na2O | nd | nd | 0.02 | 0.02 | 0.05 | 0.28 | 0.04 | 1.17 | 0.73 | nd | 0.02 |
| K2O | 0.05 | 0.05 | 0.08 | 0.05 | 0.04 | 0.1 | 0.18 | 0.1 | 0.11 | 0.04 | 0.05 |
| P2O5 | dl | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 |
| SO3 | 0.02 | 0.04 | 0.09 | 0.05 | 0.12 | 0.14 | 0.14 | 0.07 | 0.18 | 0.07 | 0.1 |
| mg/kg | |||||||||||
| Sr | 588 | 555 | 1008 | 802 | 980 | 1811 | 7987 | 2213 | 897 | 585 | 837 |
| Zr | nd | nd | nd | nd | nd | nd | nd | 33 | nd | nd | nd |
| Mo | 343 | 234 | 385 | 410 | 328 | 373 | 228 | 378 | 358 | 308 | 331 |
| Rb | nd | nd | nd | nd | nd | 48 | nd | nd | nd | nd | nd |
| Ba | 547 | 535 | 8341 | 10,958 | 1019 | 1159 | 1812 | nd | 2955 | 822 | 1305 |
| Cl | nd | nd | 24 | nd | 39 | 23 | 297 | 877 | 1545 | 45 | 338 |
| Br | nd | nd | nd | nd | nd | nd | nd | 54 | 134 | nd | nd |
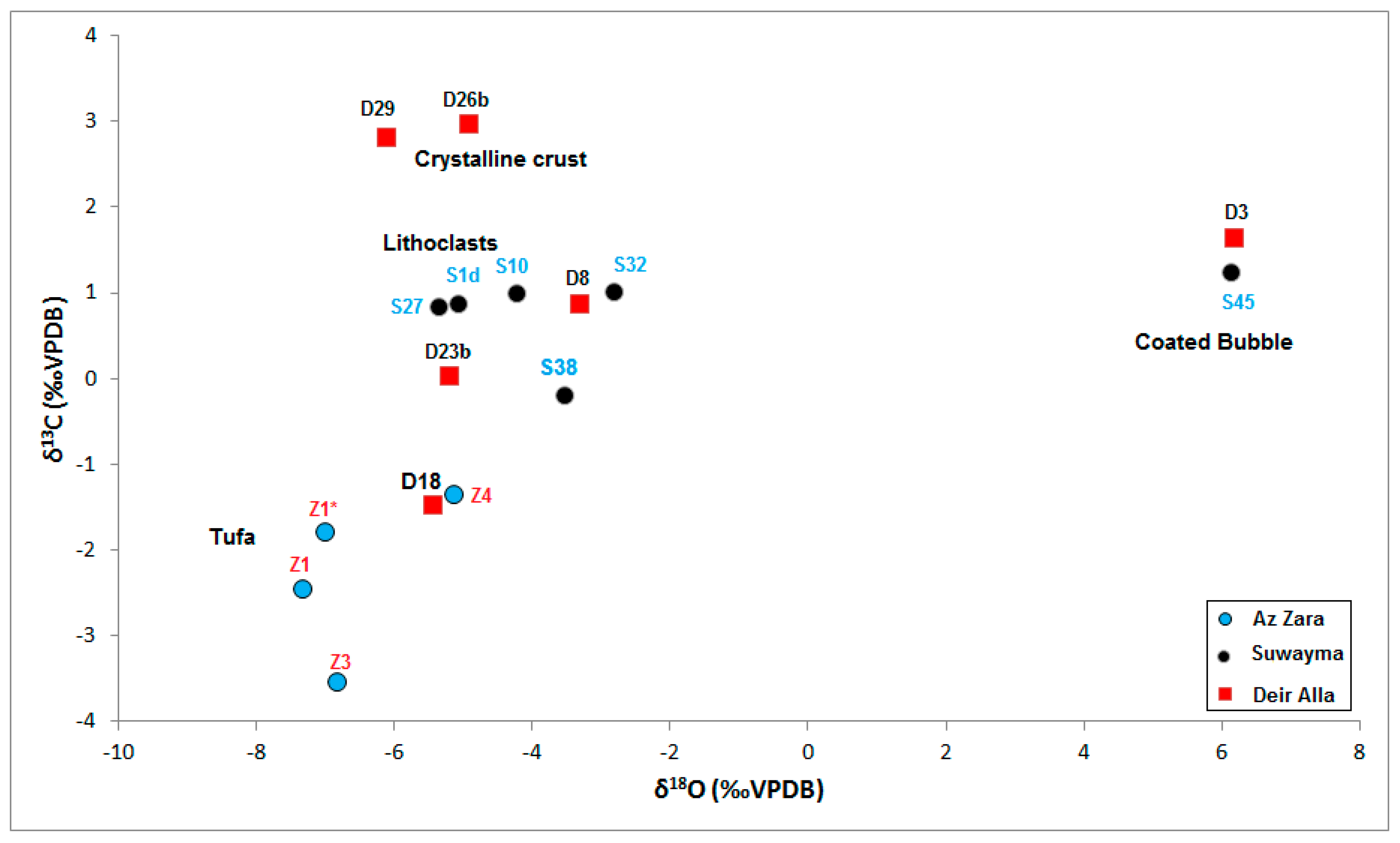
| Sample | Facies | δ18O (‰VPDB) | δ13C (‰VPDB) |
|---|---|---|---|
| D3 | coated bubble | 8.17 | 1.88 |
| D8 | paper-raft + reed | −3.32 | 0.89 |
| D18 | Shrub | −5.48 | −1.45 |
| D23b | paper-raft + reed | −5.22 | 0.05 |
| D26b | crystalline crust + palaeosol | −4.93 | 2.99 |
| D29 | crystalline crust | −8.13 | 2.83 |
| S1d | lithoclast + pebbly | −5.1 | 0.89 |
| S10 | Lithoclast | −4.25 | 1.01 |
| S27 | Lithoclast | −5.38 | 0.85 |
| S32 | Lithoclast | −2.84 | 1.03 |
| S38 | Reed | −3.58 | −0.18 |
| S45 | coated bubble | 8.12 | 1.28 |
| Z1 | macrophyte tufa | −7.34 | −2.45 |
| Z1* | macrophyte tufa | −7.02 | −1.78 |
| Z3 | coated bubble travertine | −8.83 | −3.52 |
| Z4 | lithoclast + paper-raft travertine | −5.14 | −1.34 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, K.M.; Makhlouf, I.M.; El Naqah, A.R.; Al-Thawabteh, S.M. Geochemistry and Stable Isotopes of Travertine from Jordan Valley and Dead Sea Areas. Minerals 2017, 7, 82. https://doi.org/10.3390/min7050082
Ibrahim KM, Makhlouf IM, El Naqah AR, Al-Thawabteh SM. Geochemistry and Stable Isotopes of Travertine from Jordan Valley and Dead Sea Areas. Minerals. 2017; 7(5):82. https://doi.org/10.3390/min7050082
Chicago/Turabian StyleIbrahim, Khalil M., Issa M. Makhlouf, Ali R. El Naqah, and Sana’ M. Al-Thawabteh. 2017. "Geochemistry and Stable Isotopes of Travertine from Jordan Valley and Dead Sea Areas" Minerals 7, no. 5: 82. https://doi.org/10.3390/min7050082






