Selenide Mineralization in the Příbram Uranium and Base-Metal District (Czech Republic)
Abstract
:1. Introduction
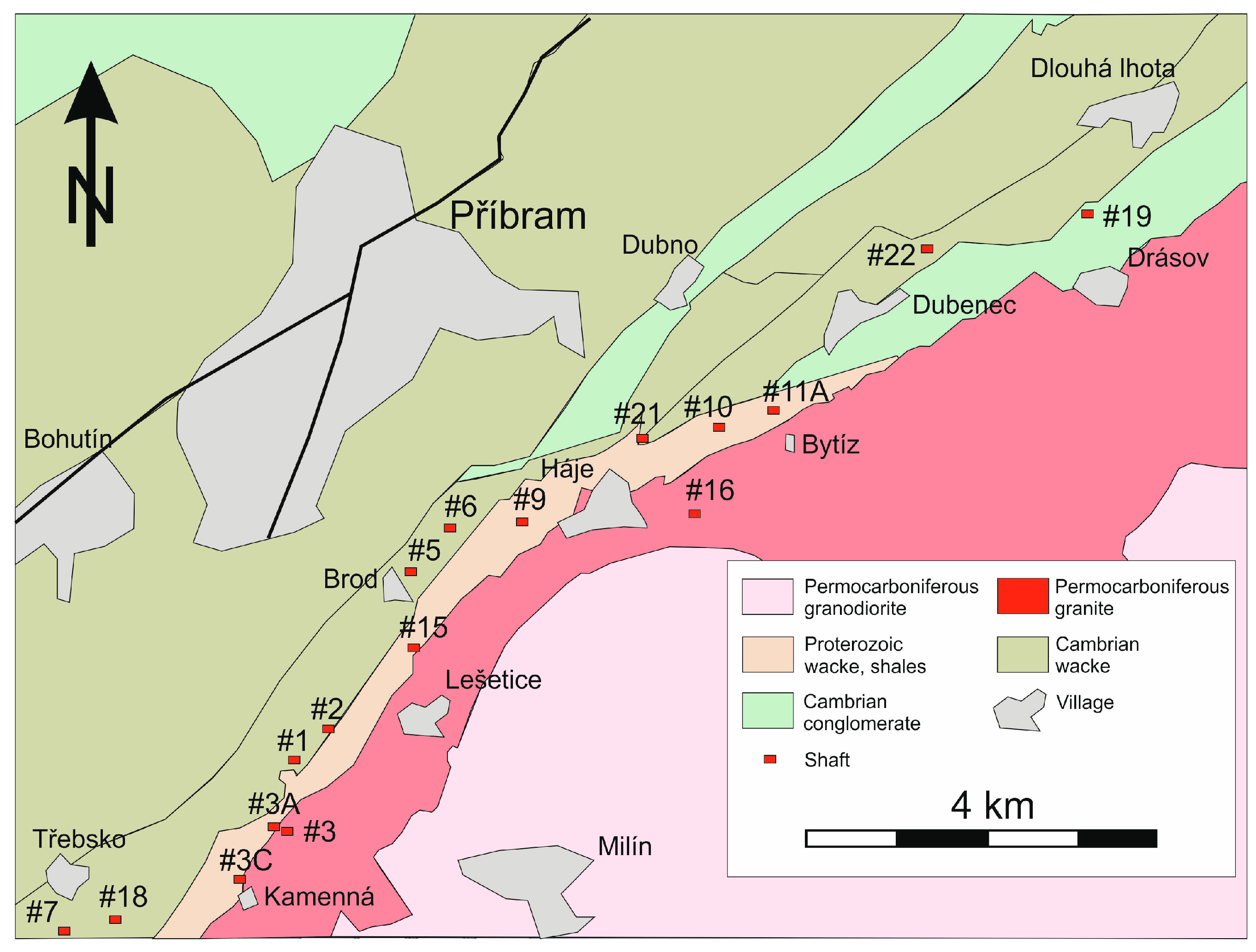
2. The Příbram Uranium and Base-Metal Ore District
2.1. History of Mining
2.2. Geological Situation
3. Methods of Research
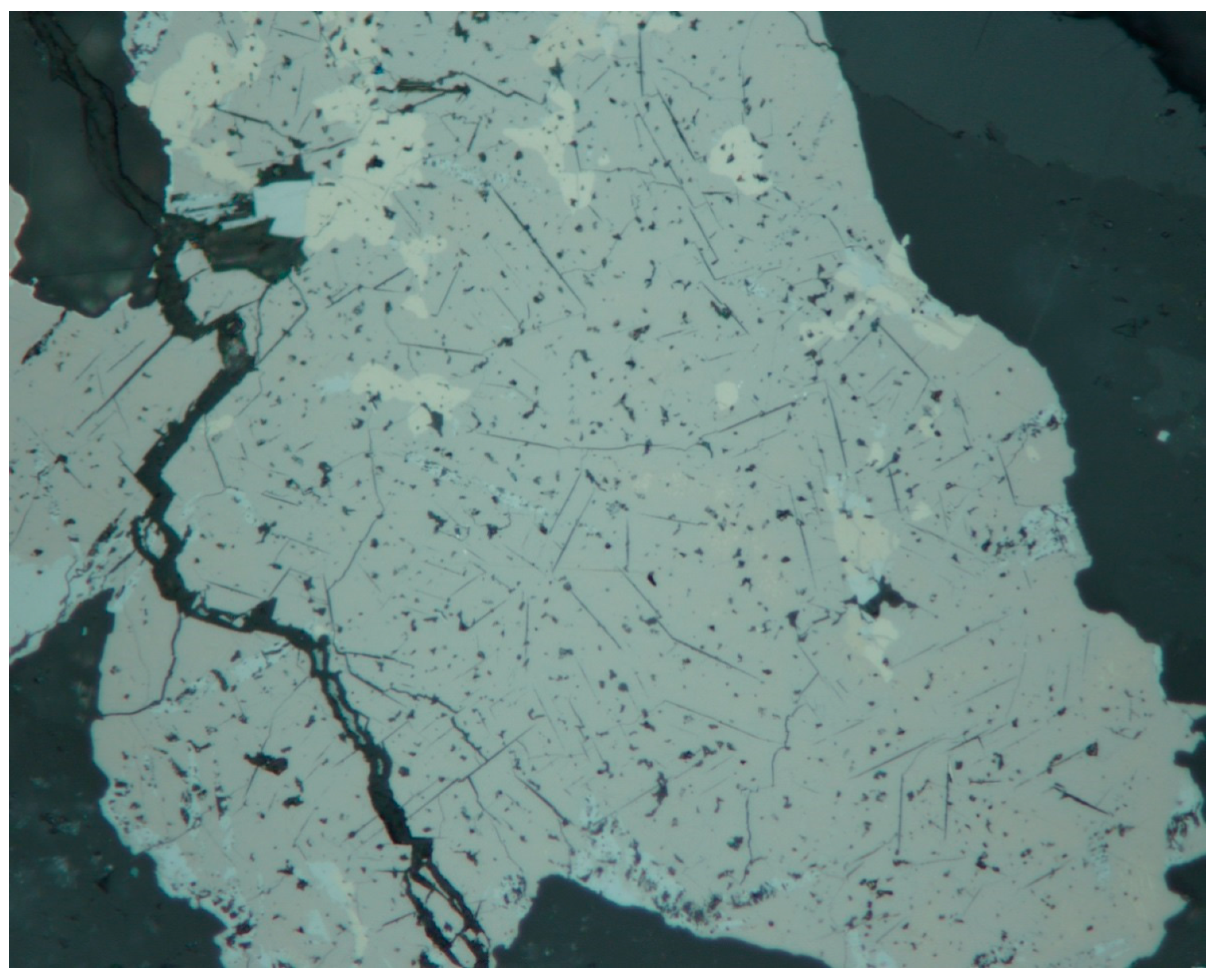
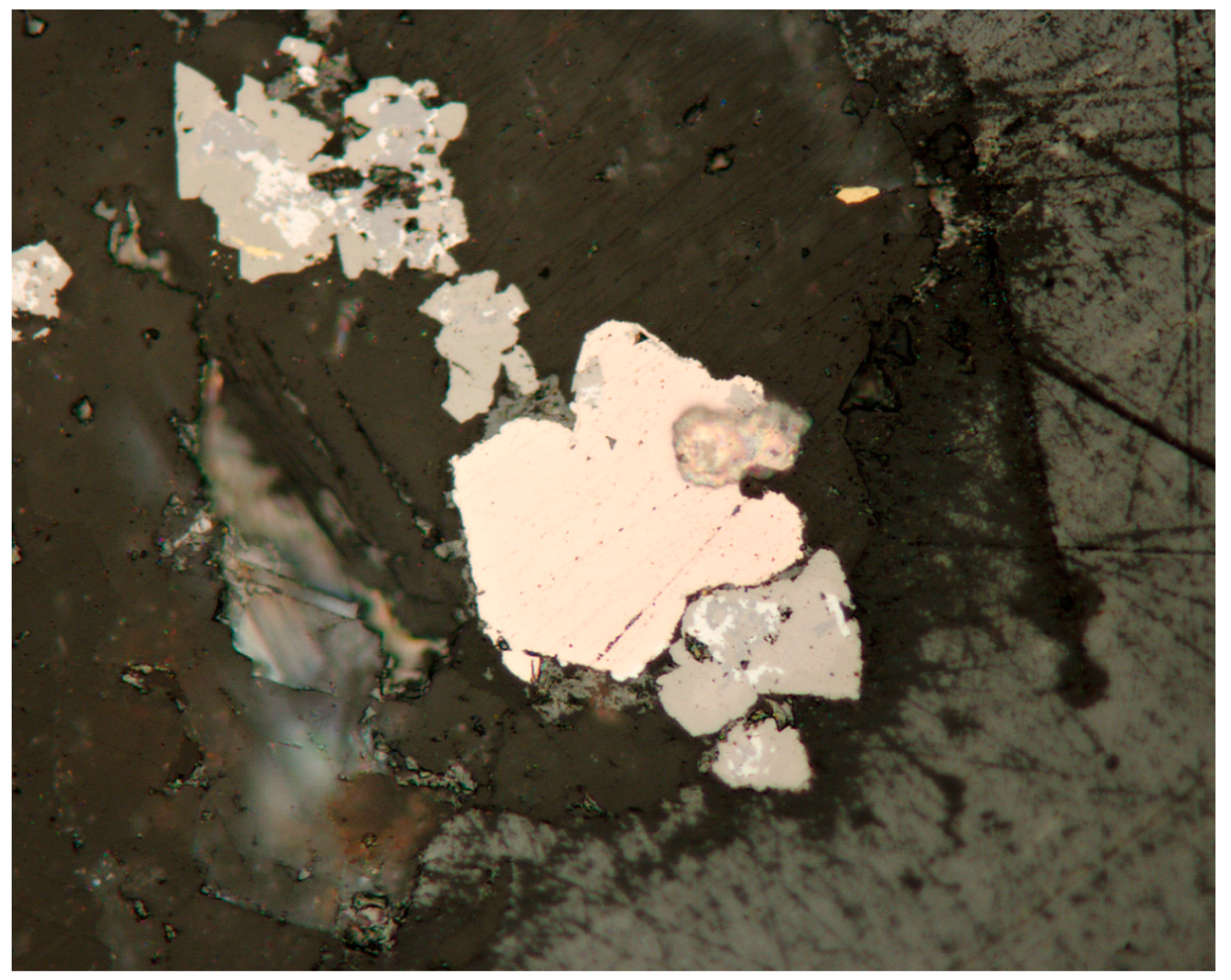
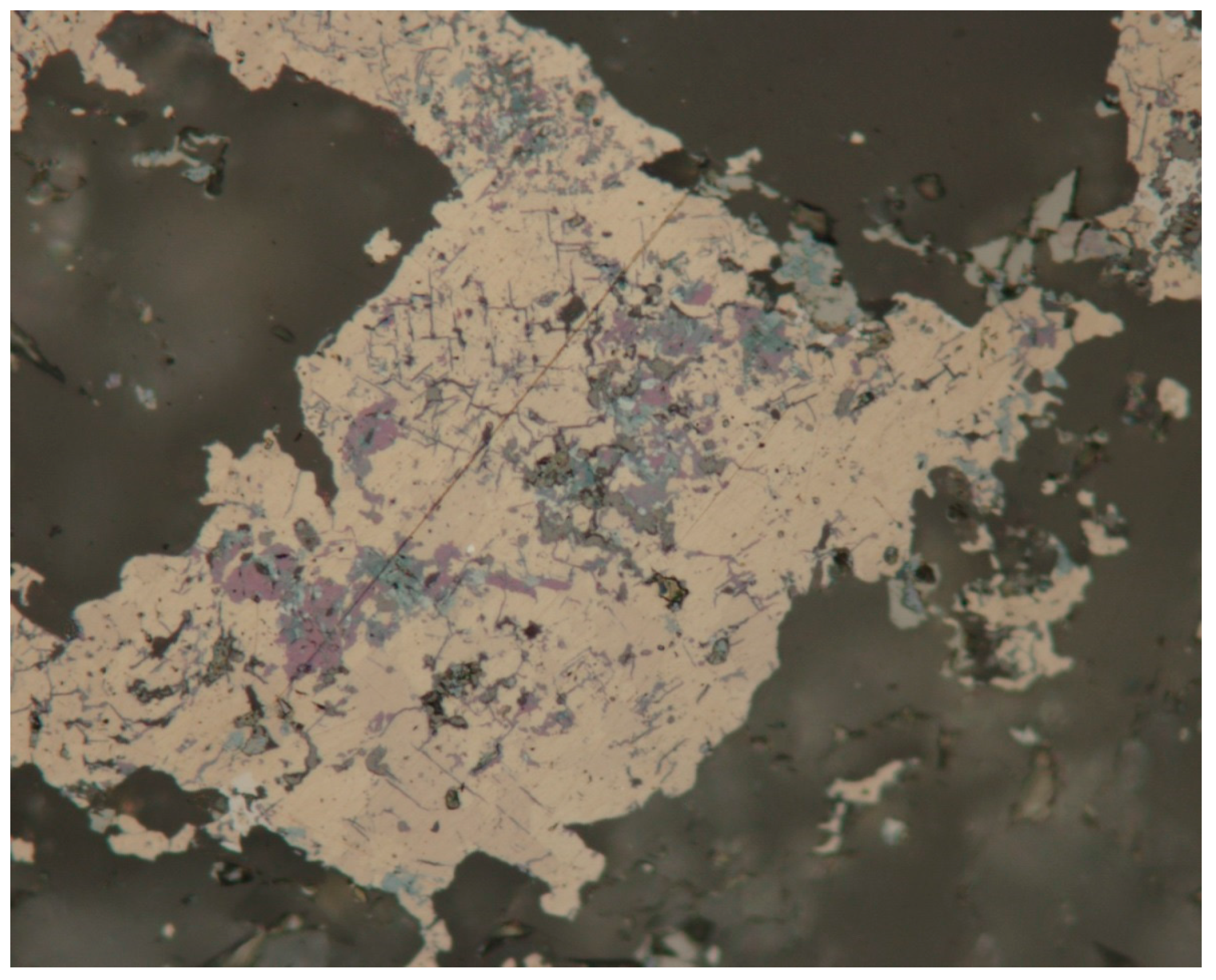
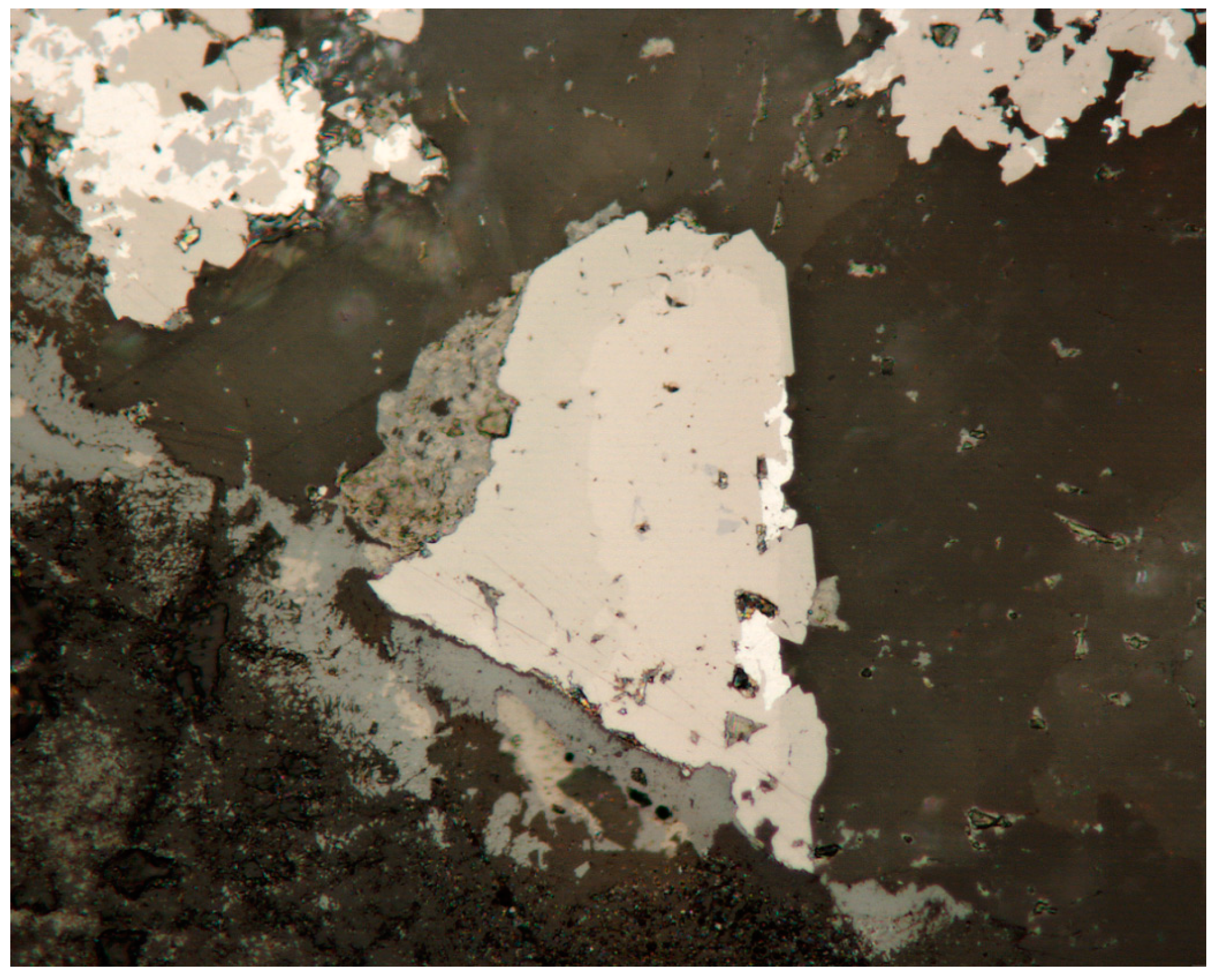
3.1. Reflected Light Microscopy
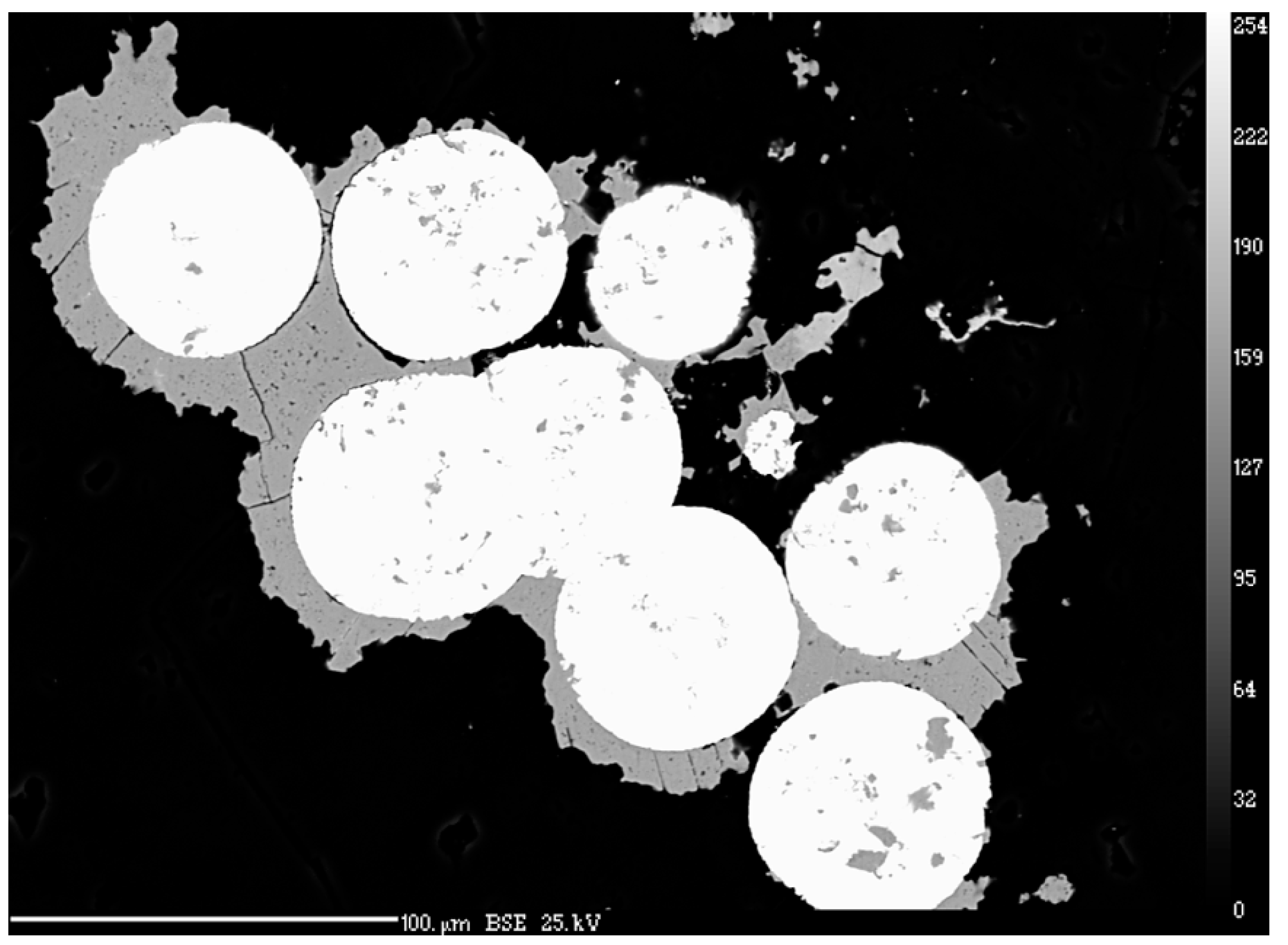
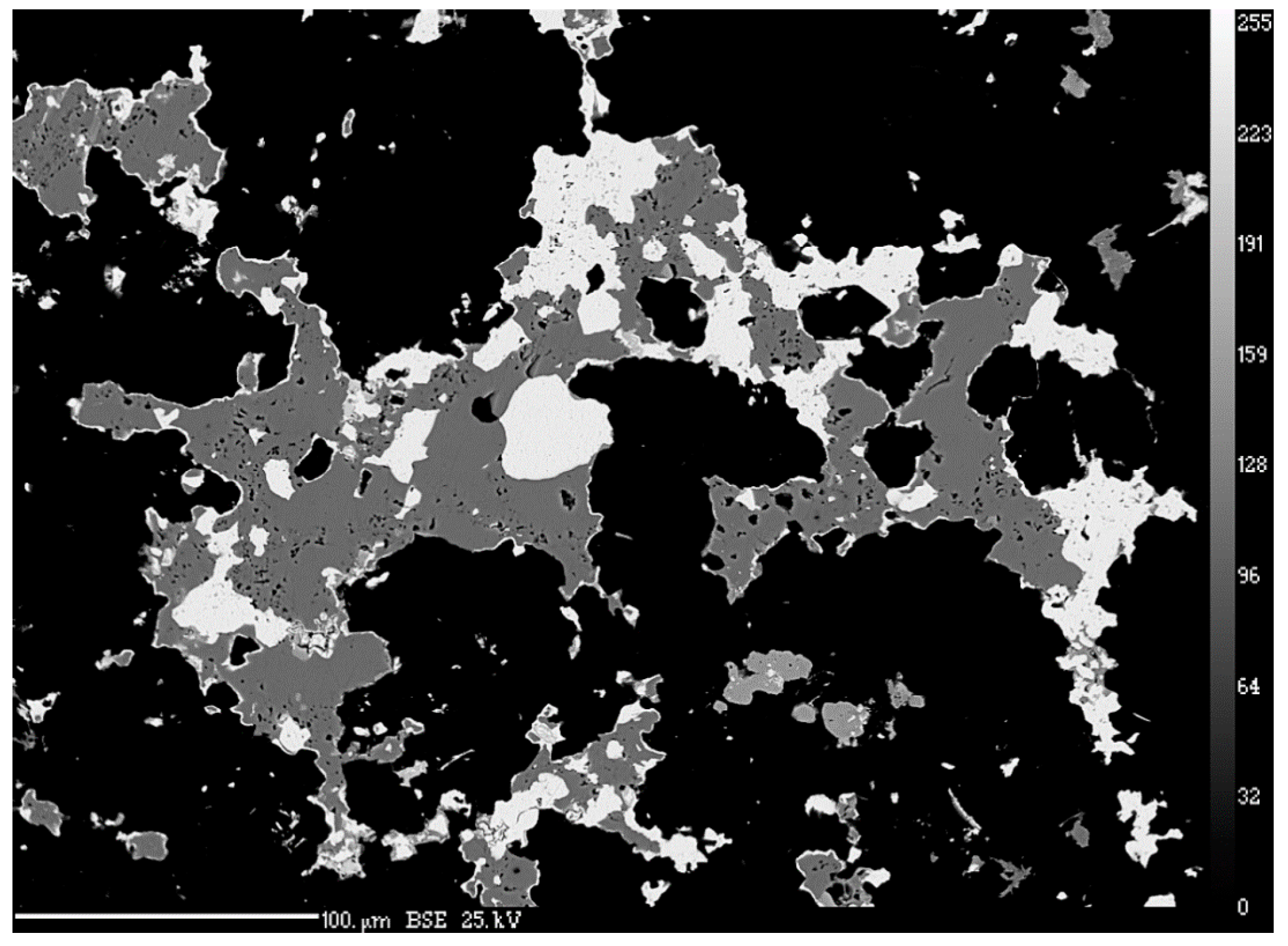
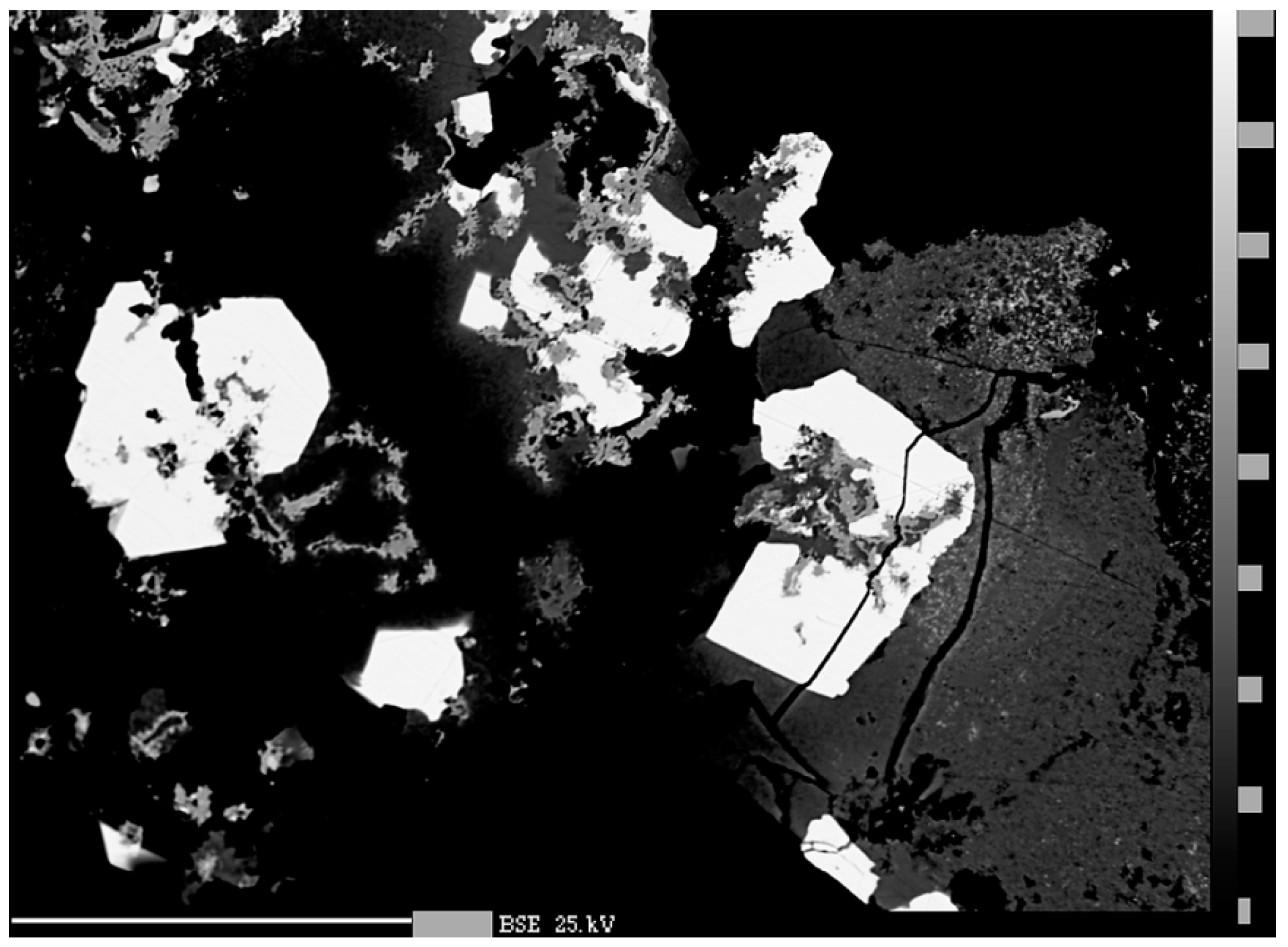
3.2. Quantitative Analysis of Chemical Composition
3.3. X-Ray Powder Diffraction Methods (PXRD)
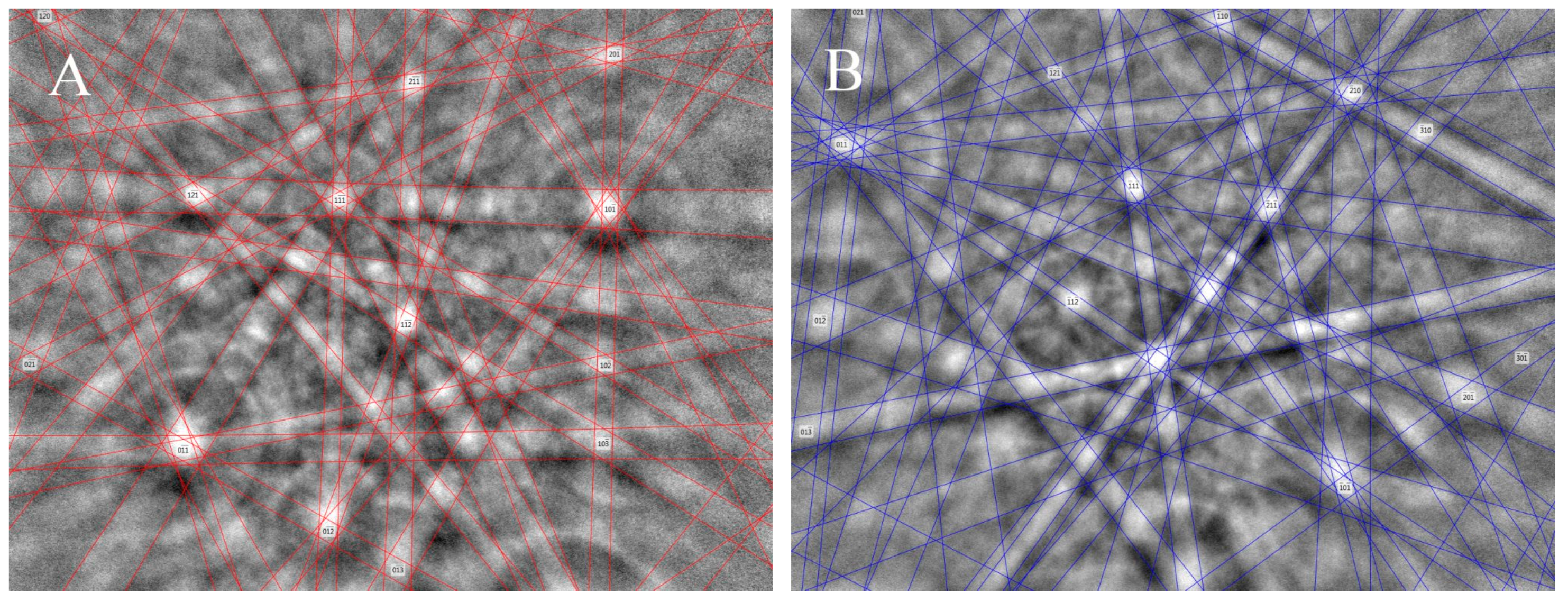
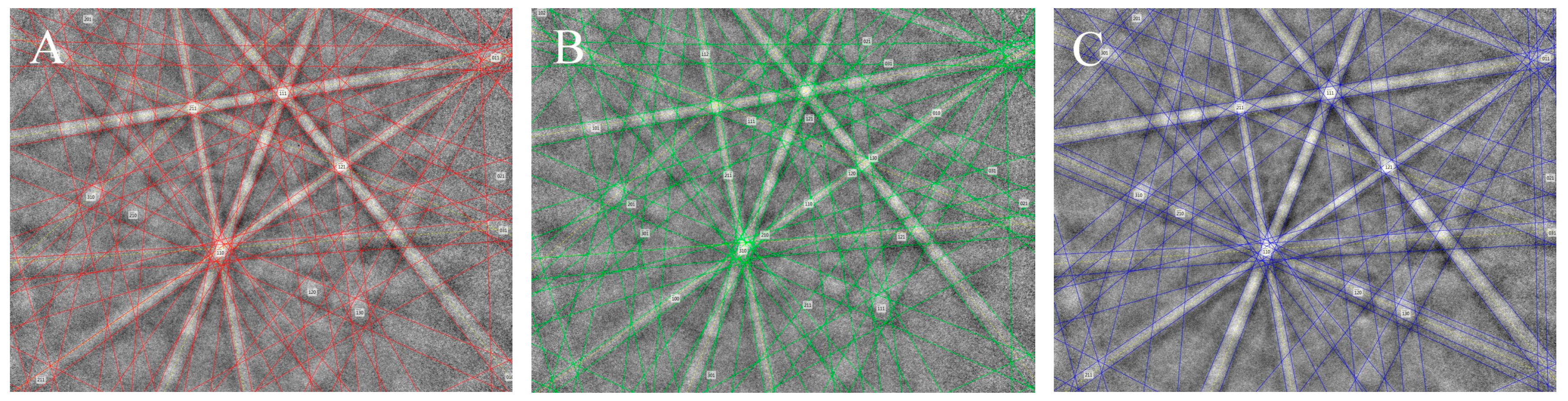
3.4. Single-Crystal X-ray Diffraction Methods (SXRD)
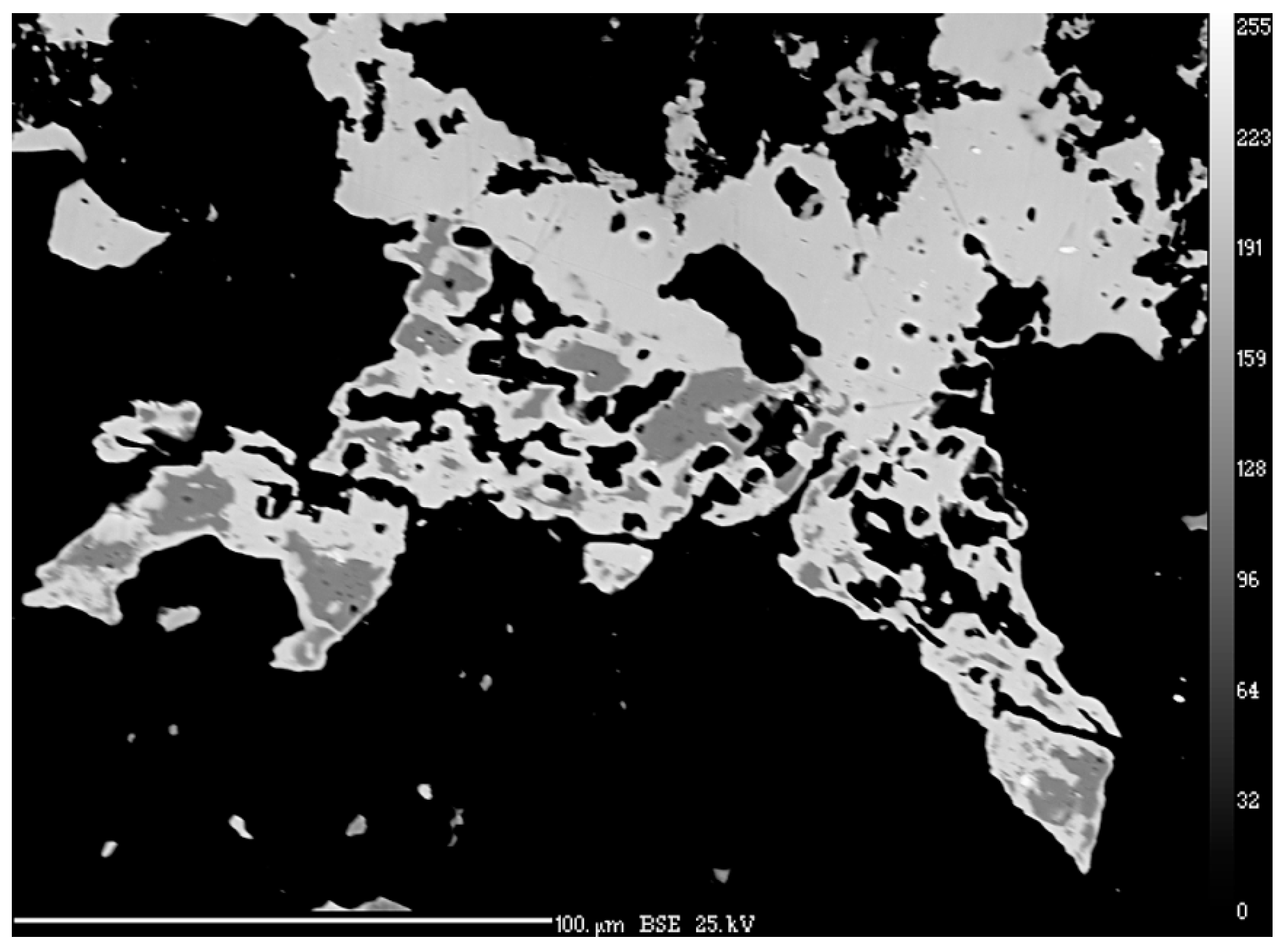
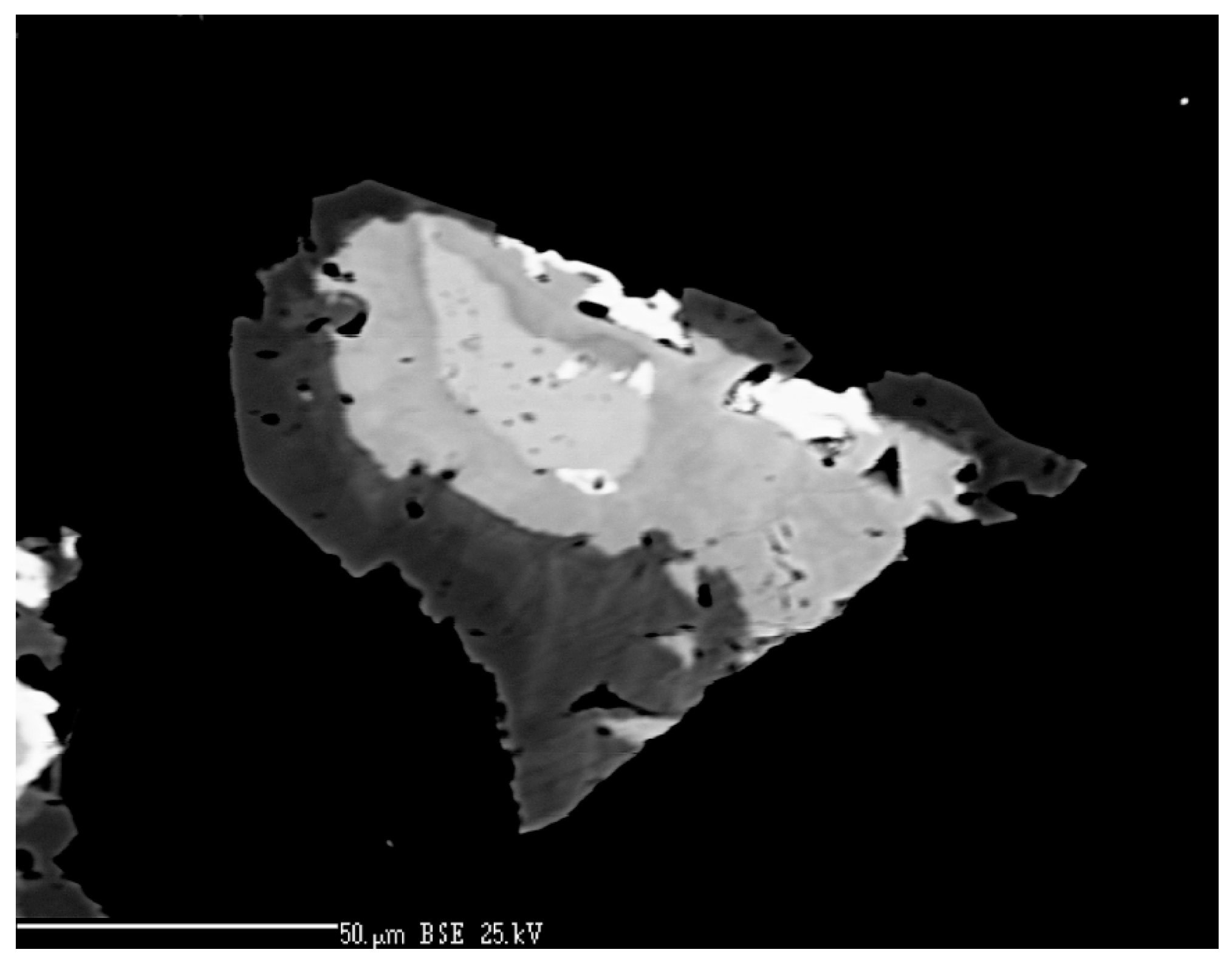
3.5. Electron Backscatter Diffraction (EBSD)
3.6. Raman Spectroscopy
4. Results
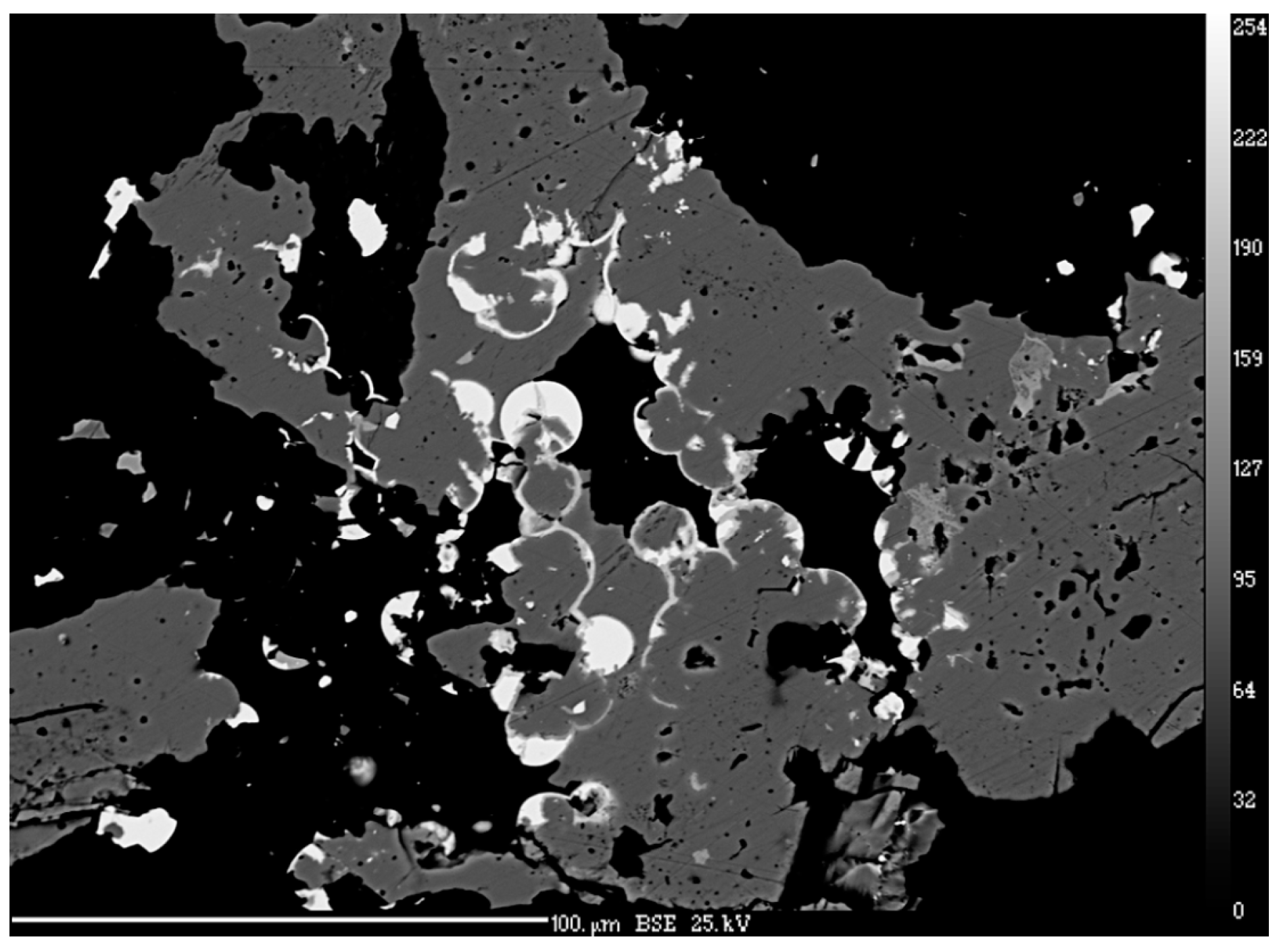
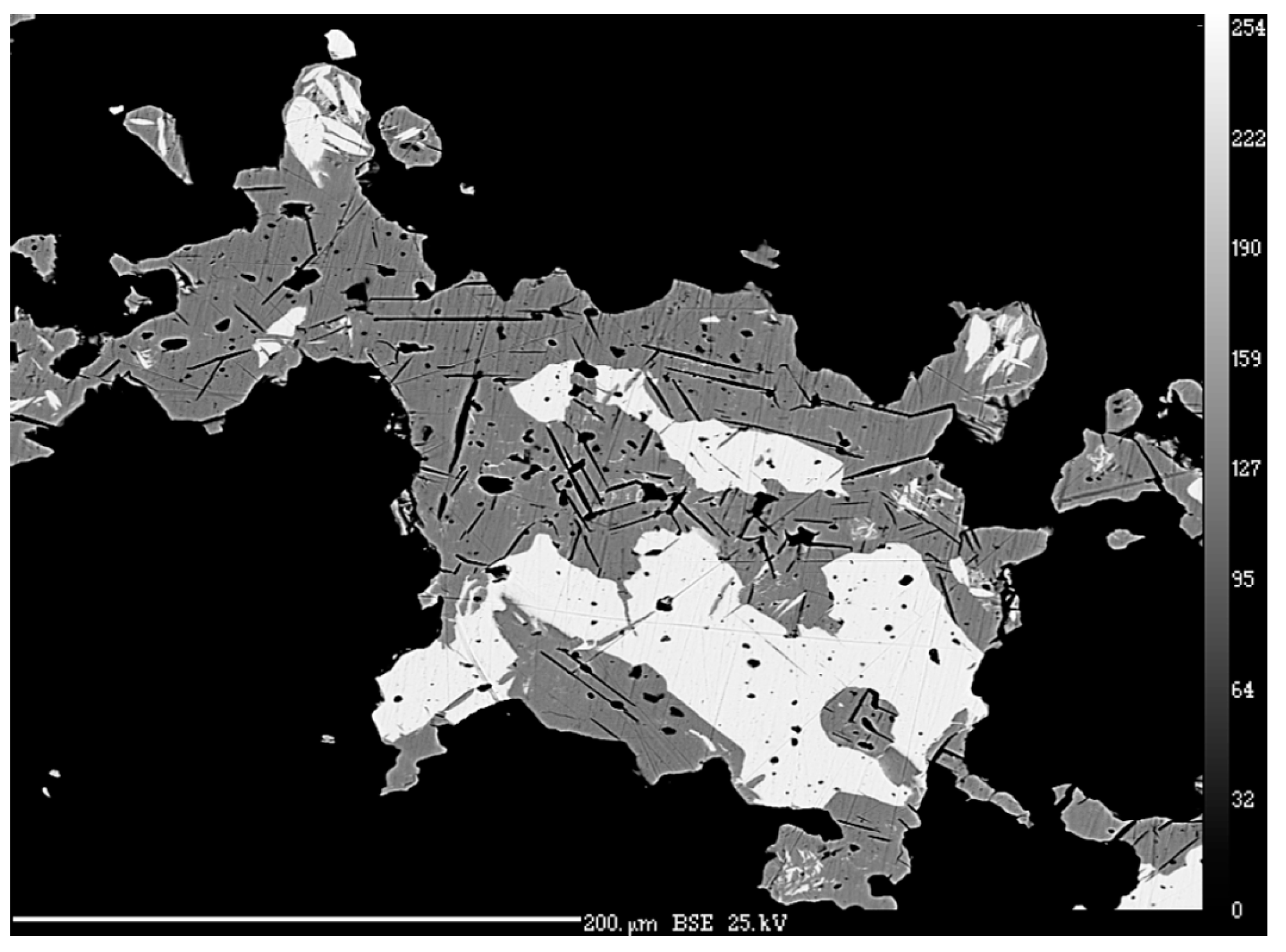
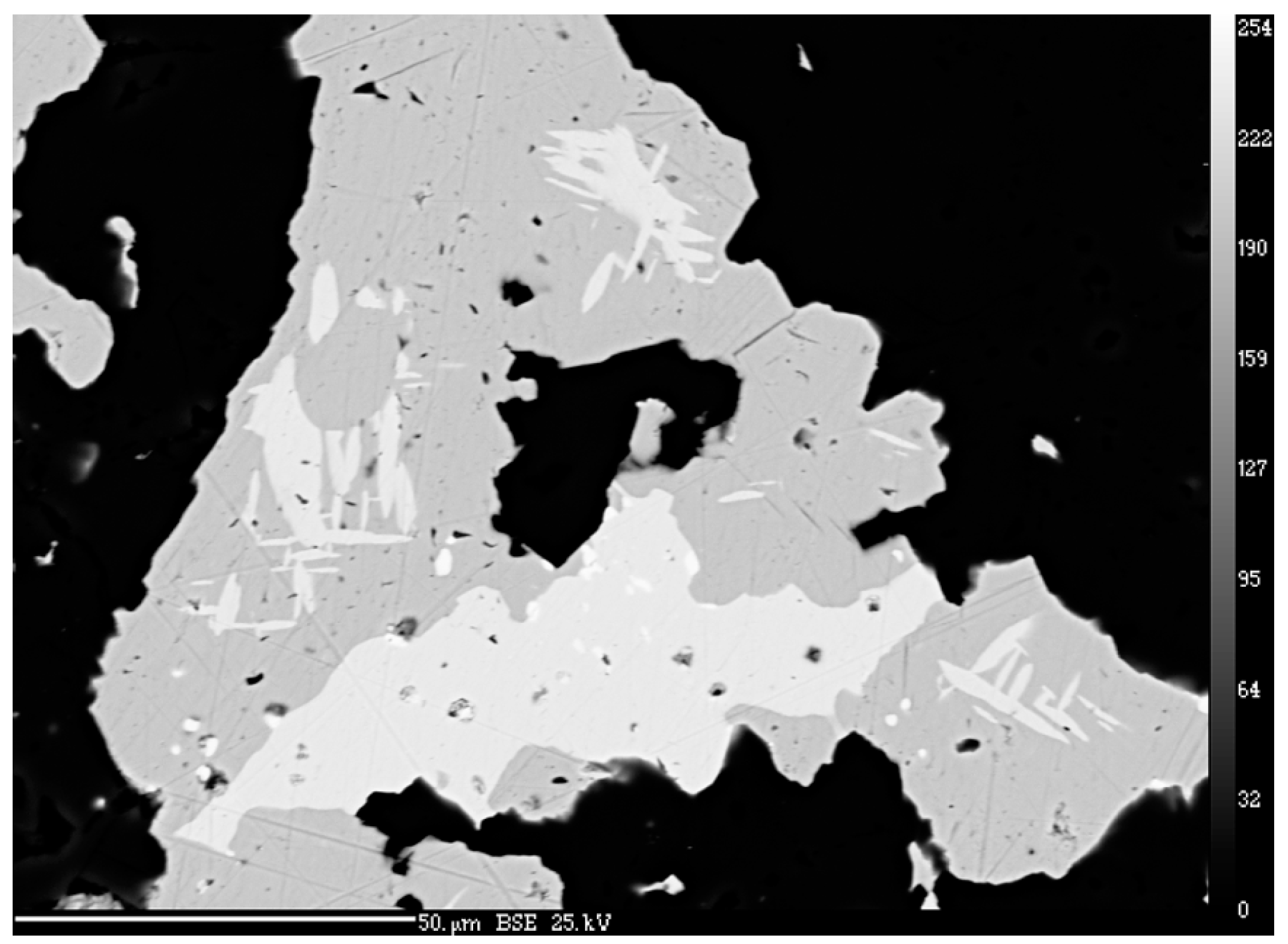
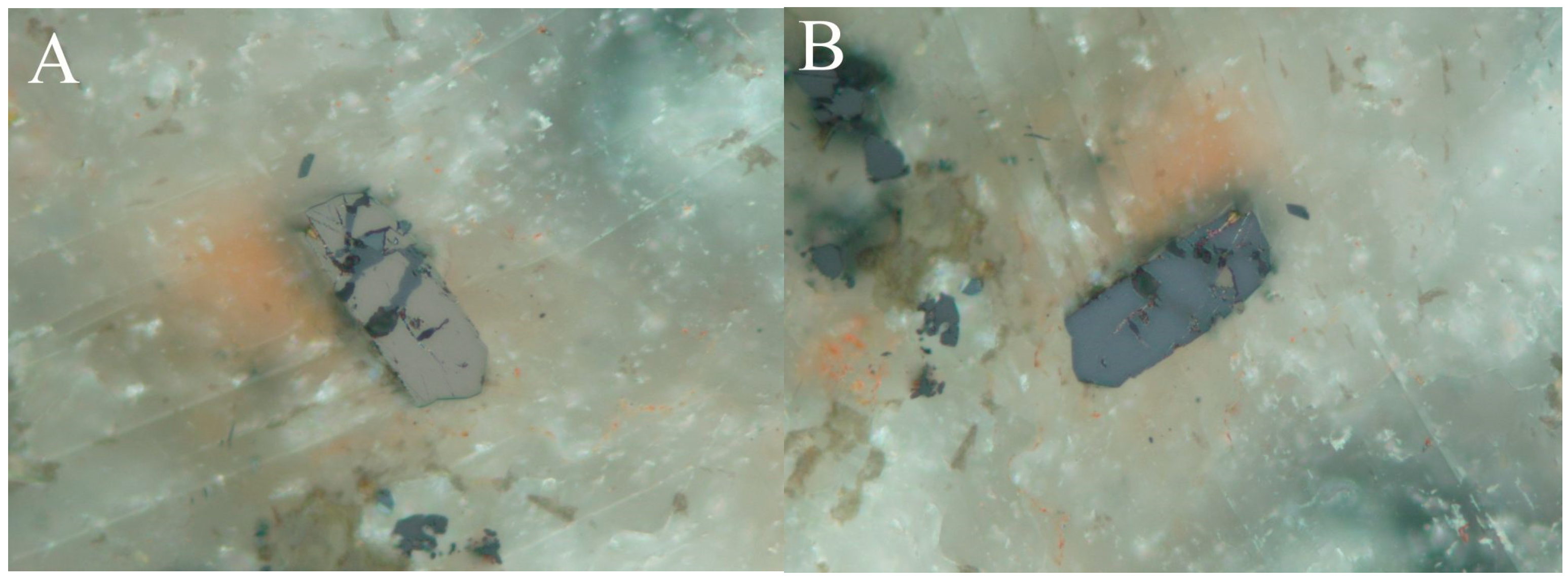
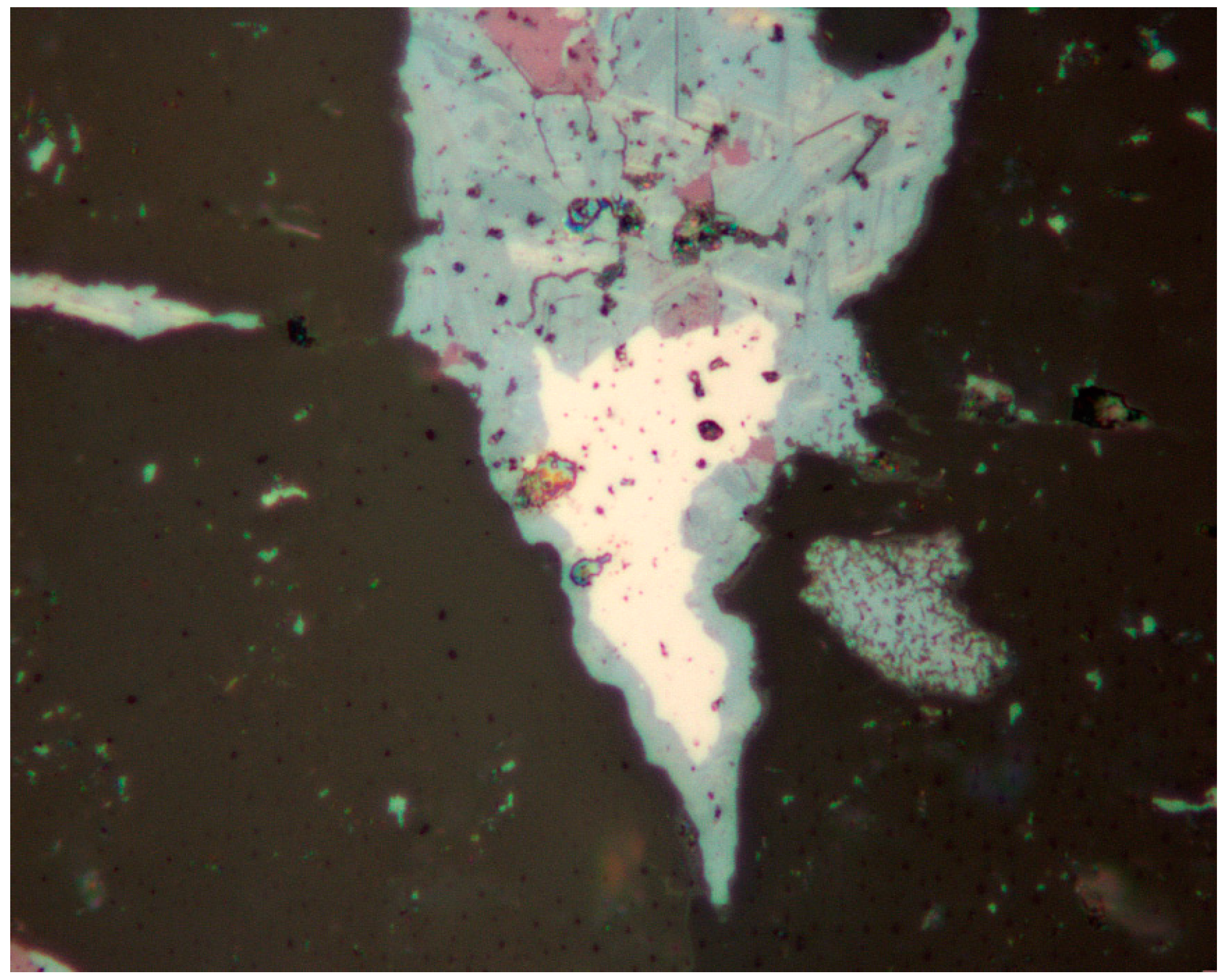
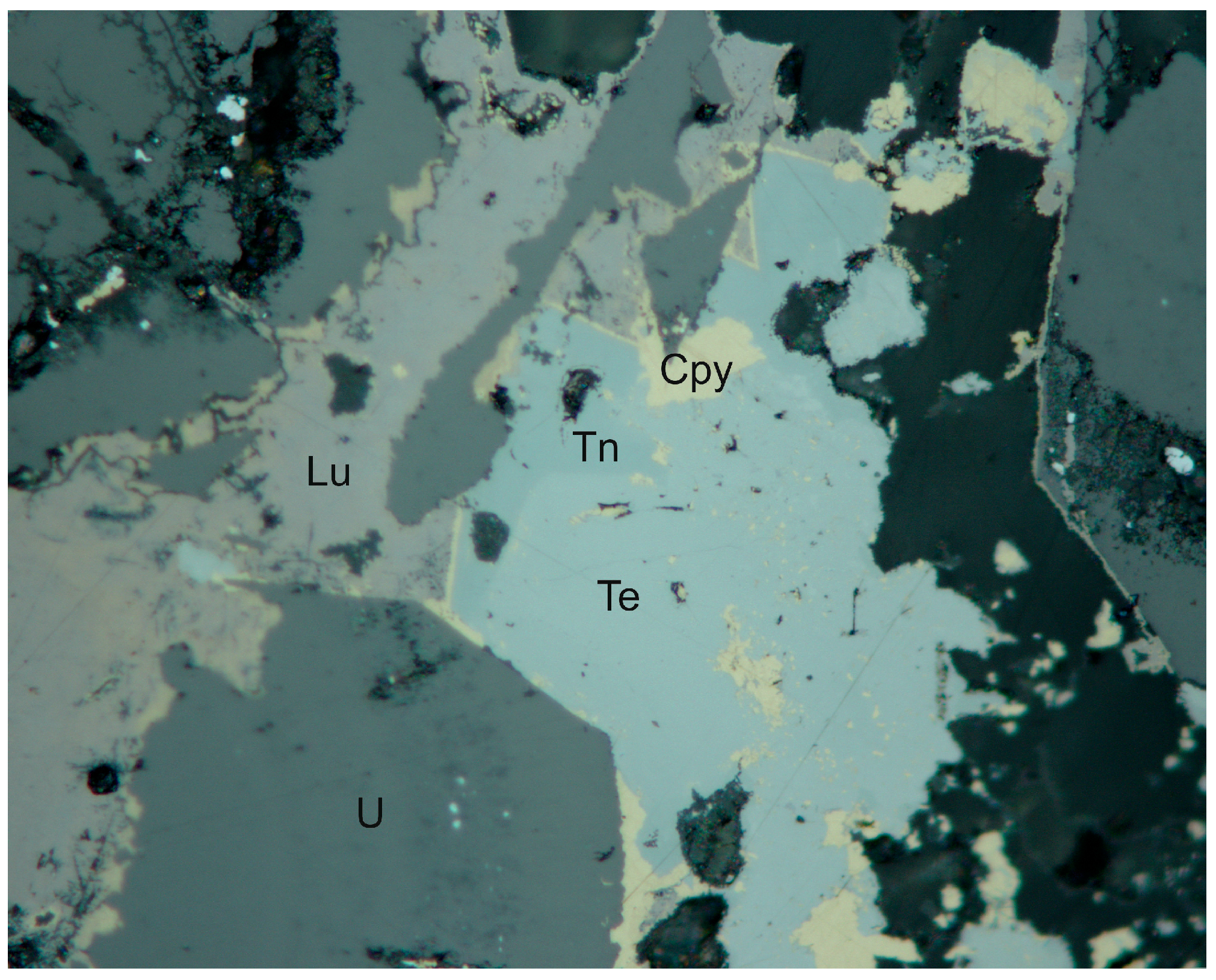
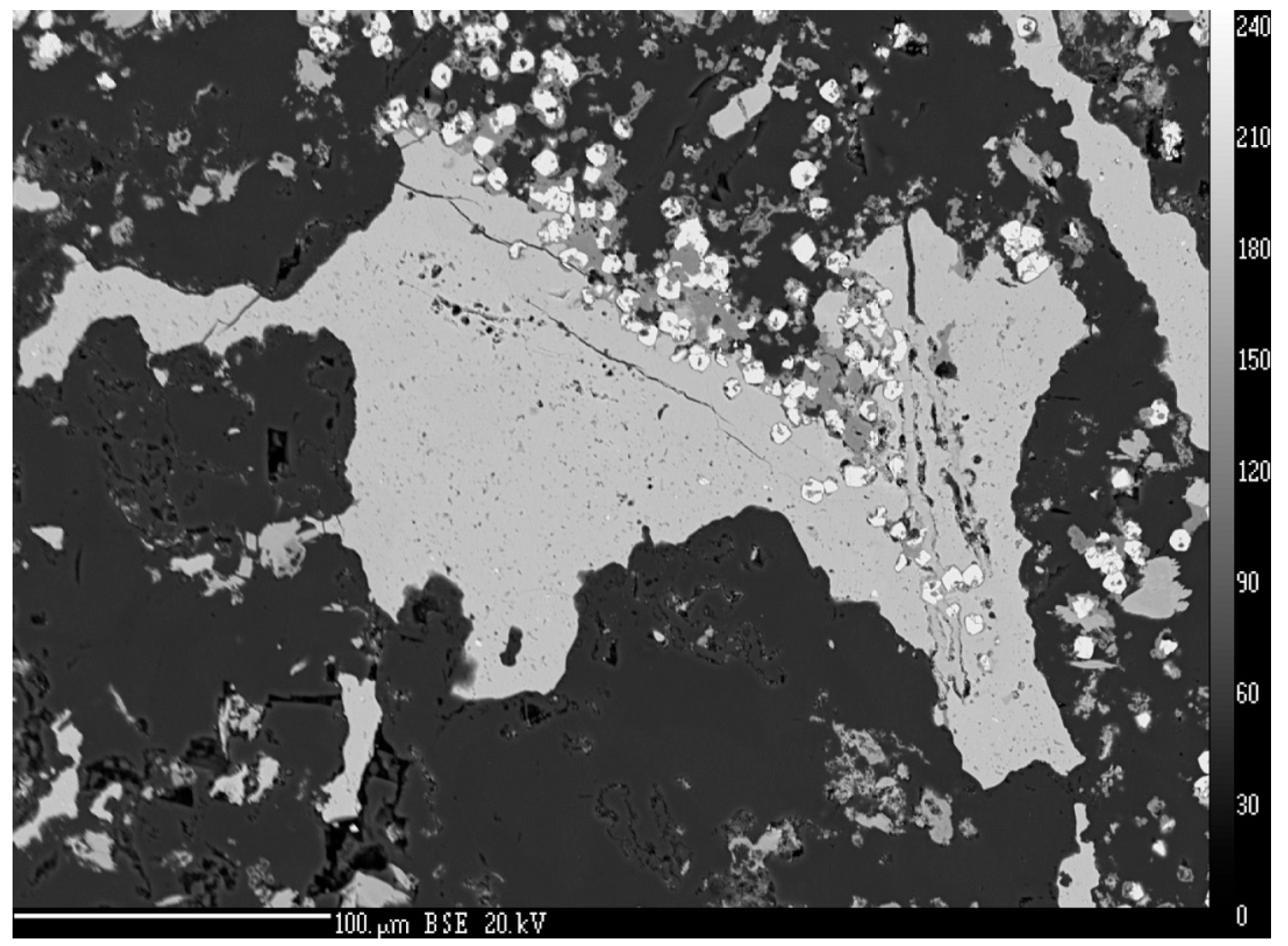
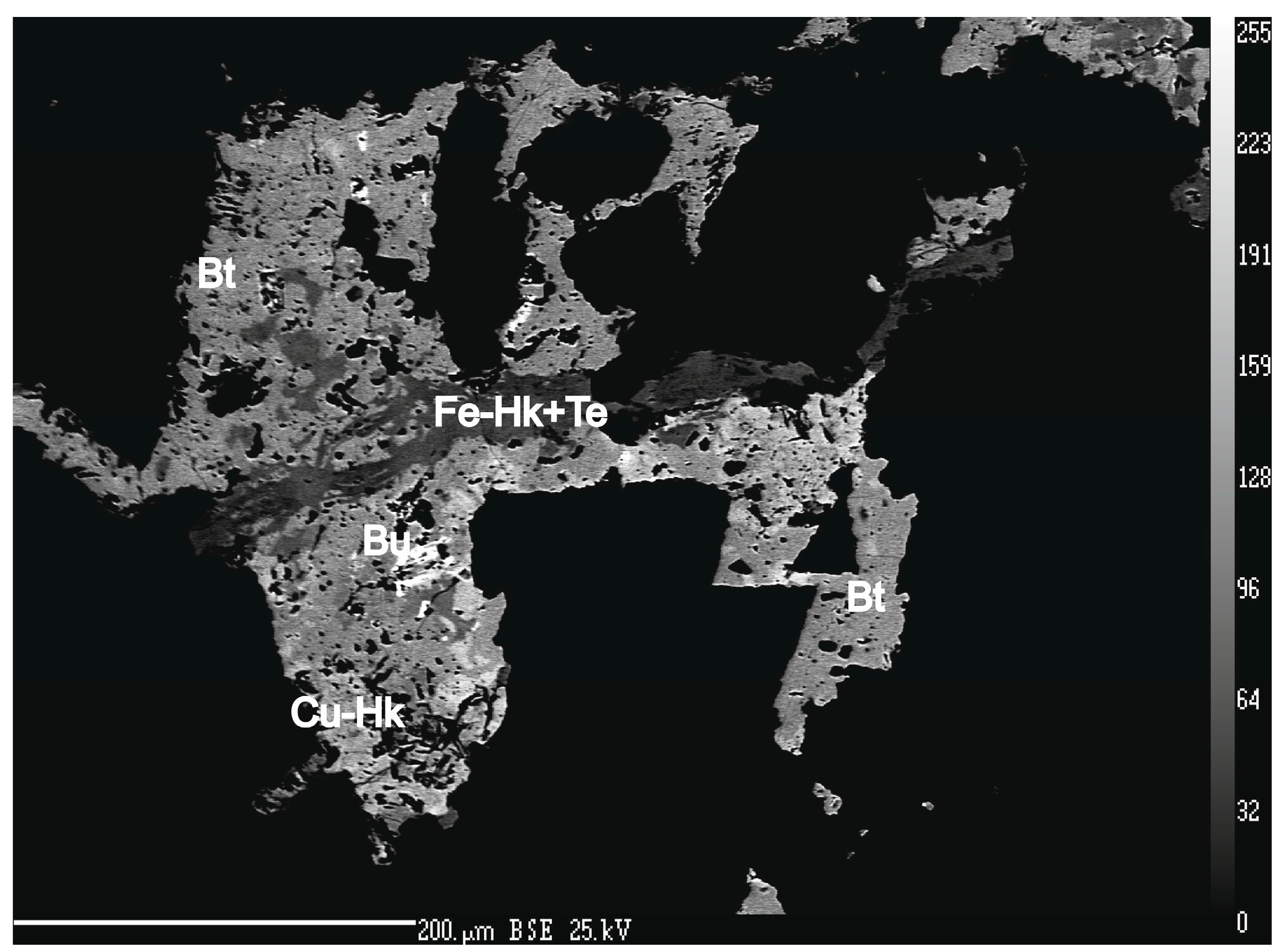
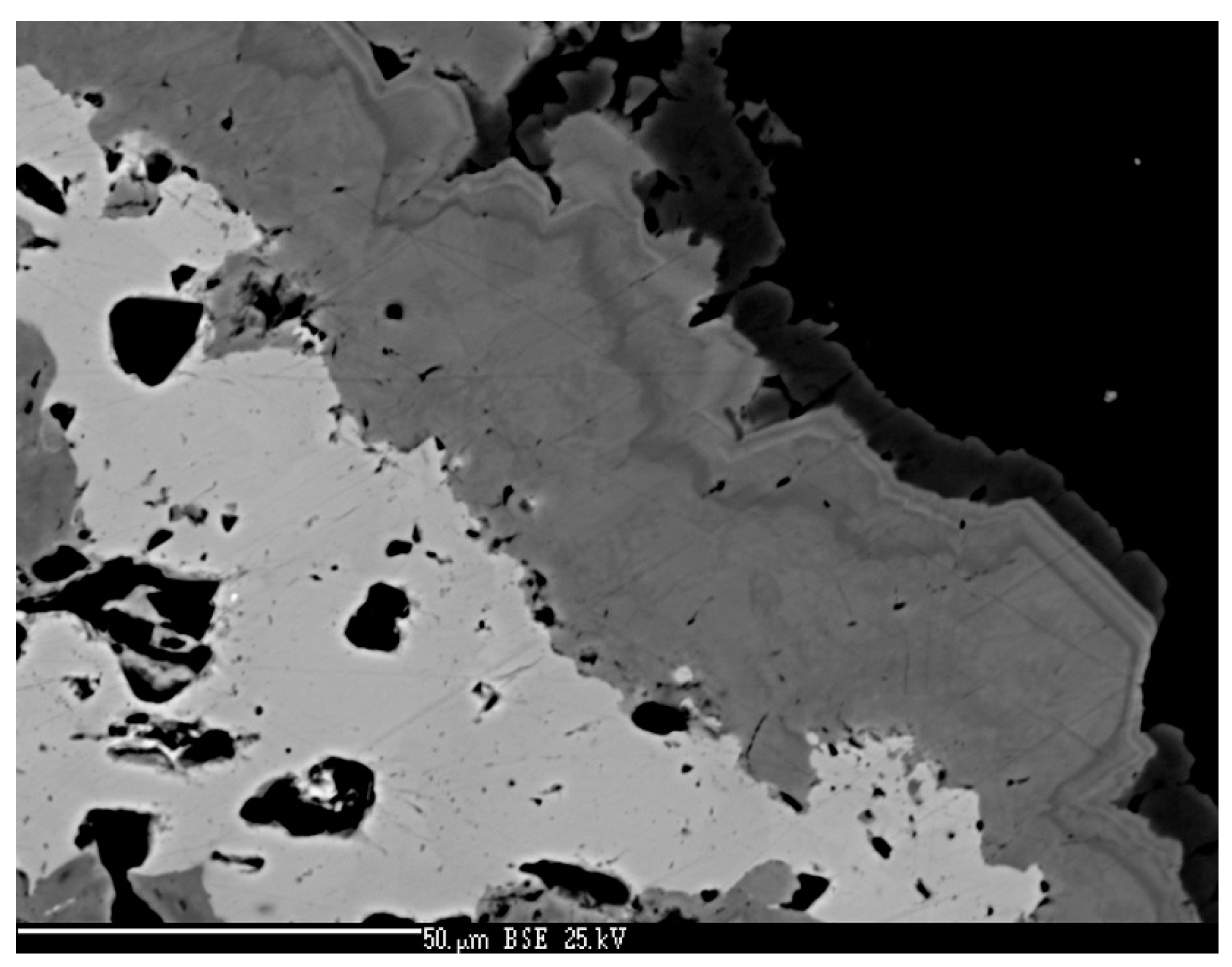
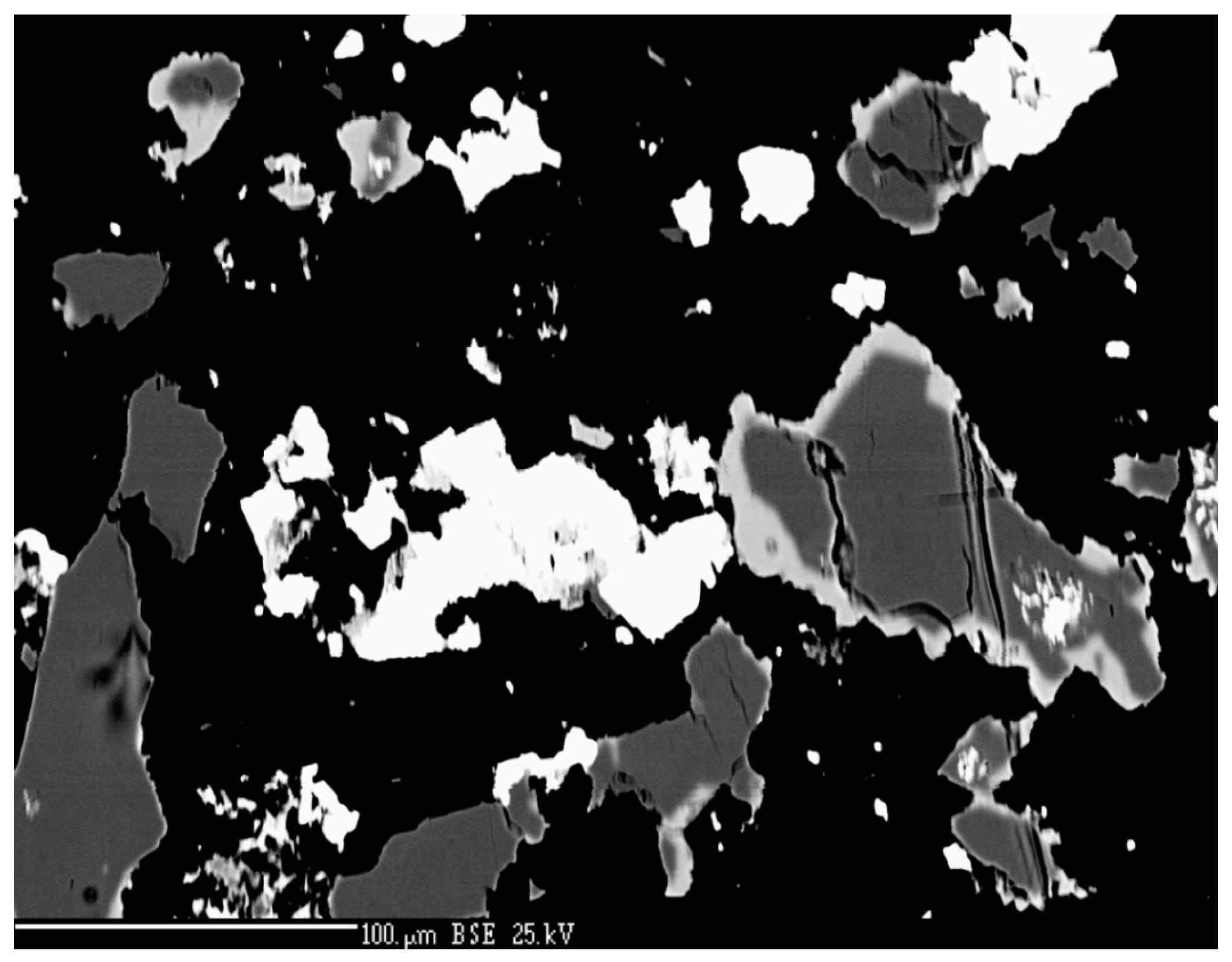
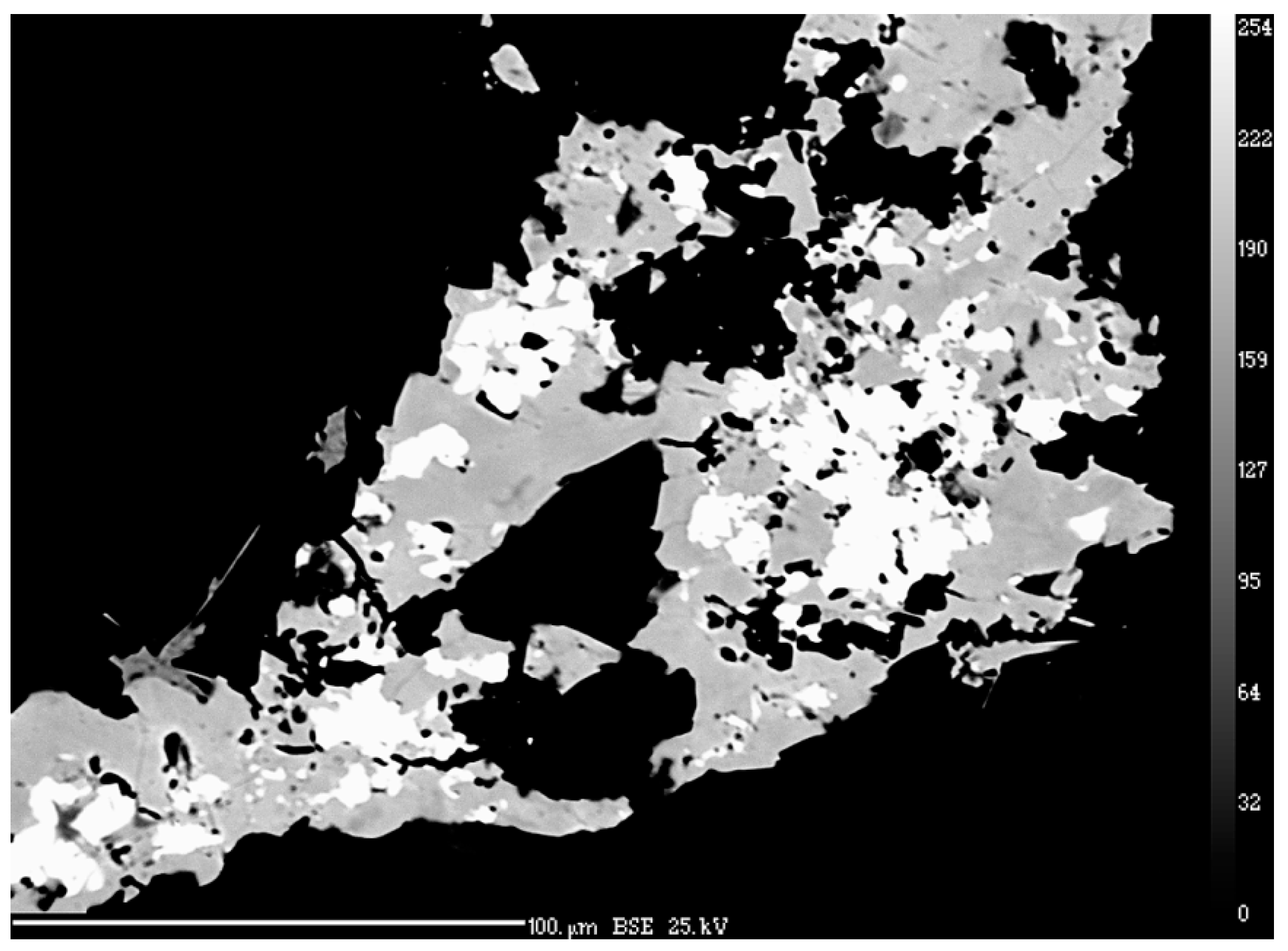
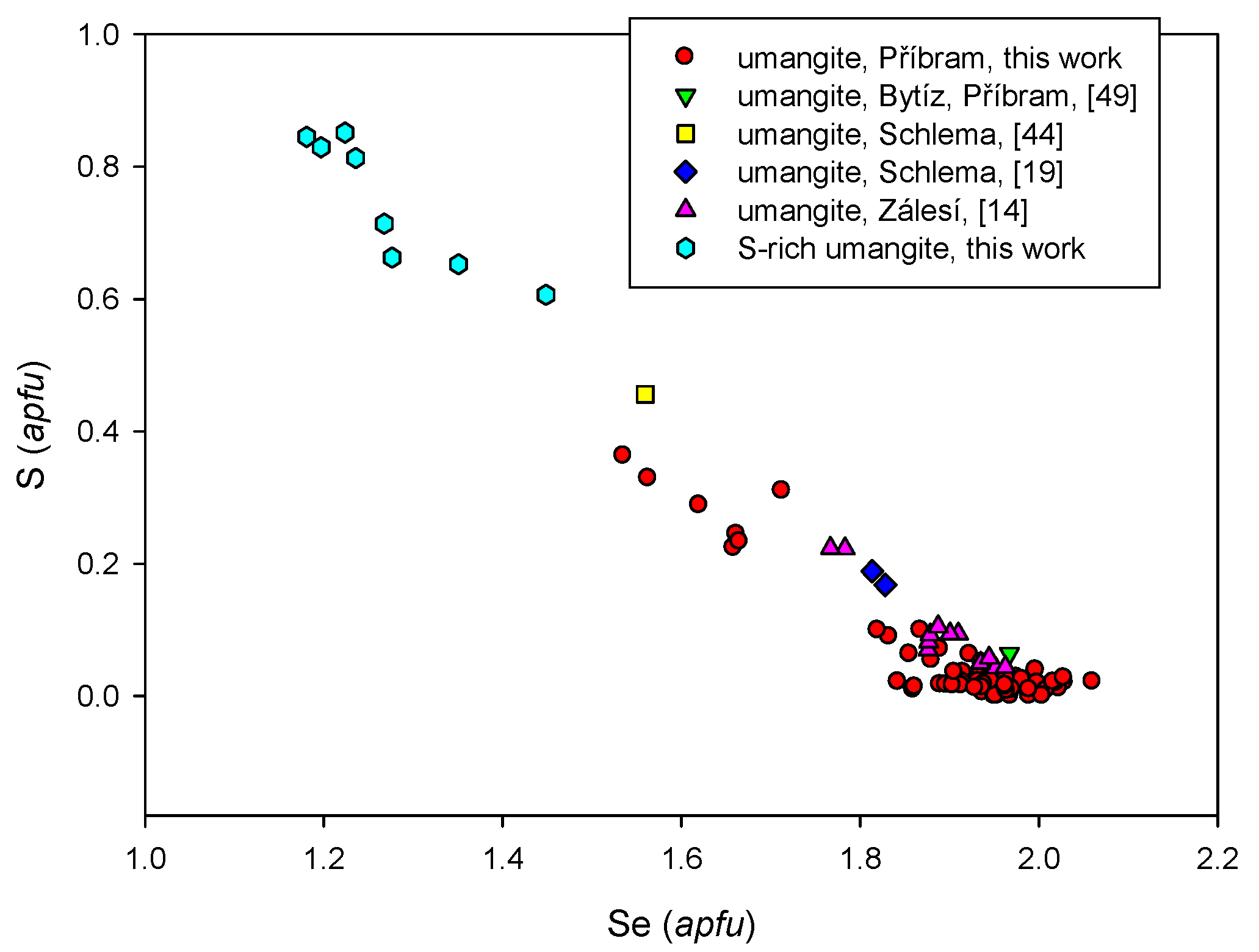
4.1. Characterization of Selenide Occurrences in Příbram
4.2. Characteristics of Individual Minerals of the Selenide Association
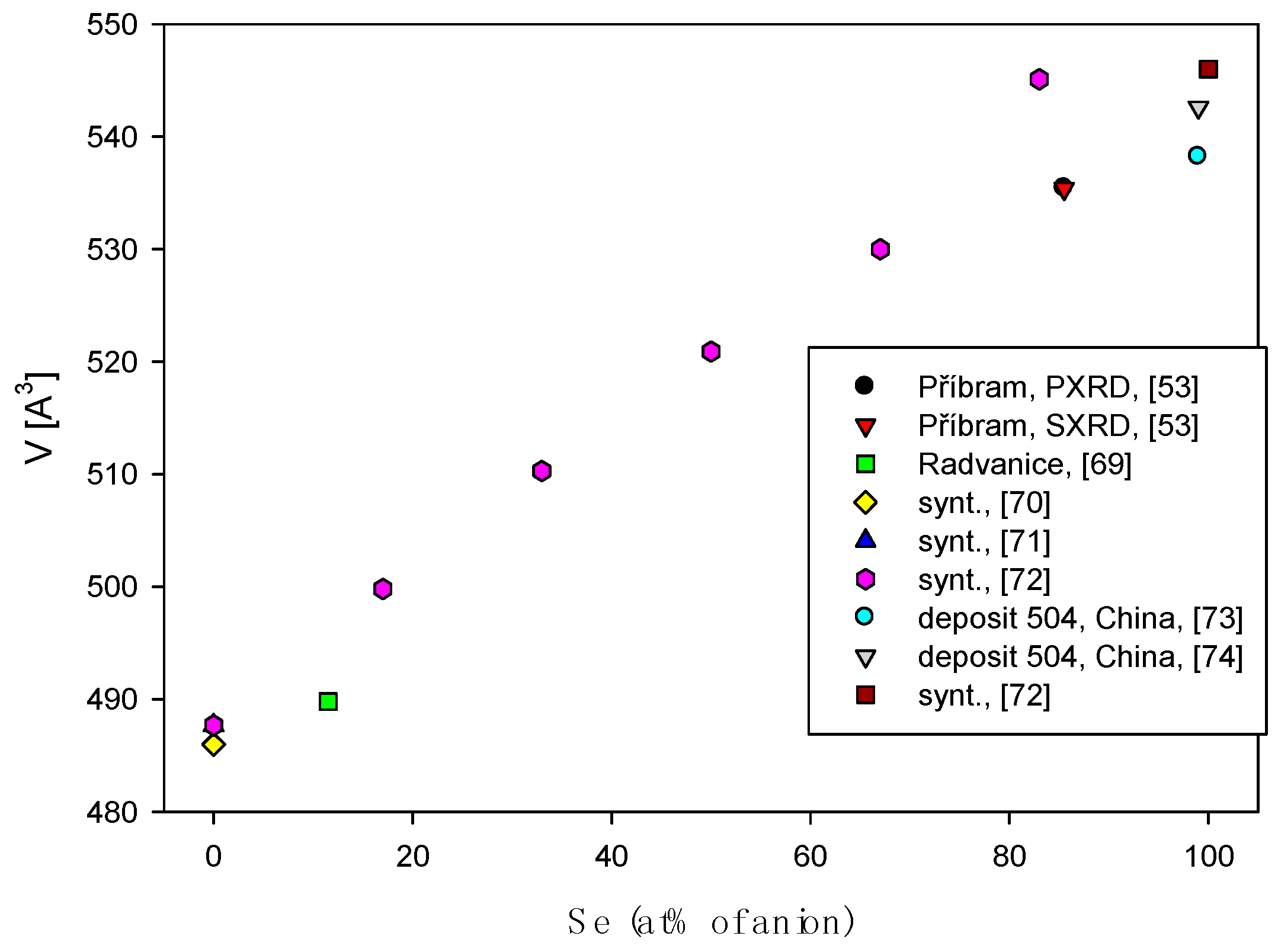
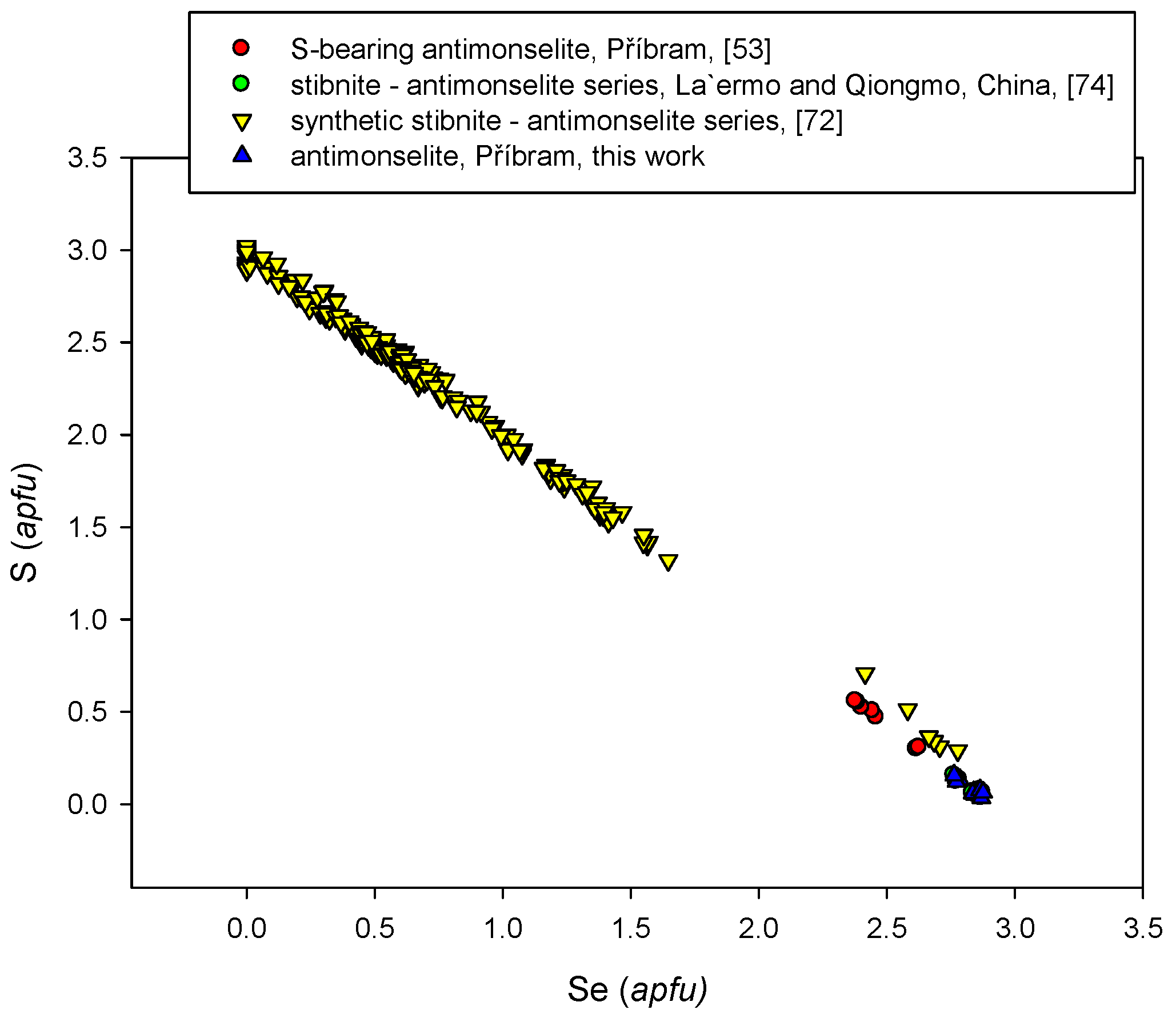
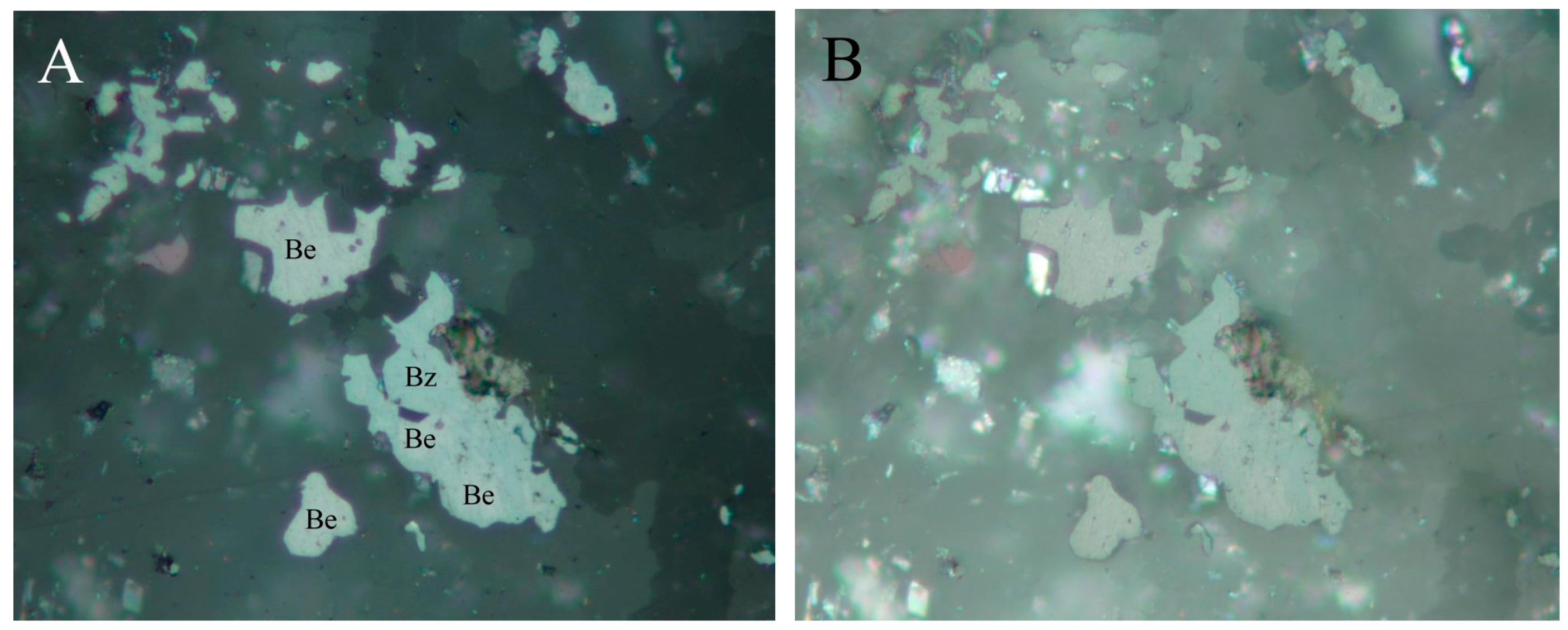
4.2.1. Antimonselite Sb2Se3
4.2.2. Athabascaite Cu5Se4
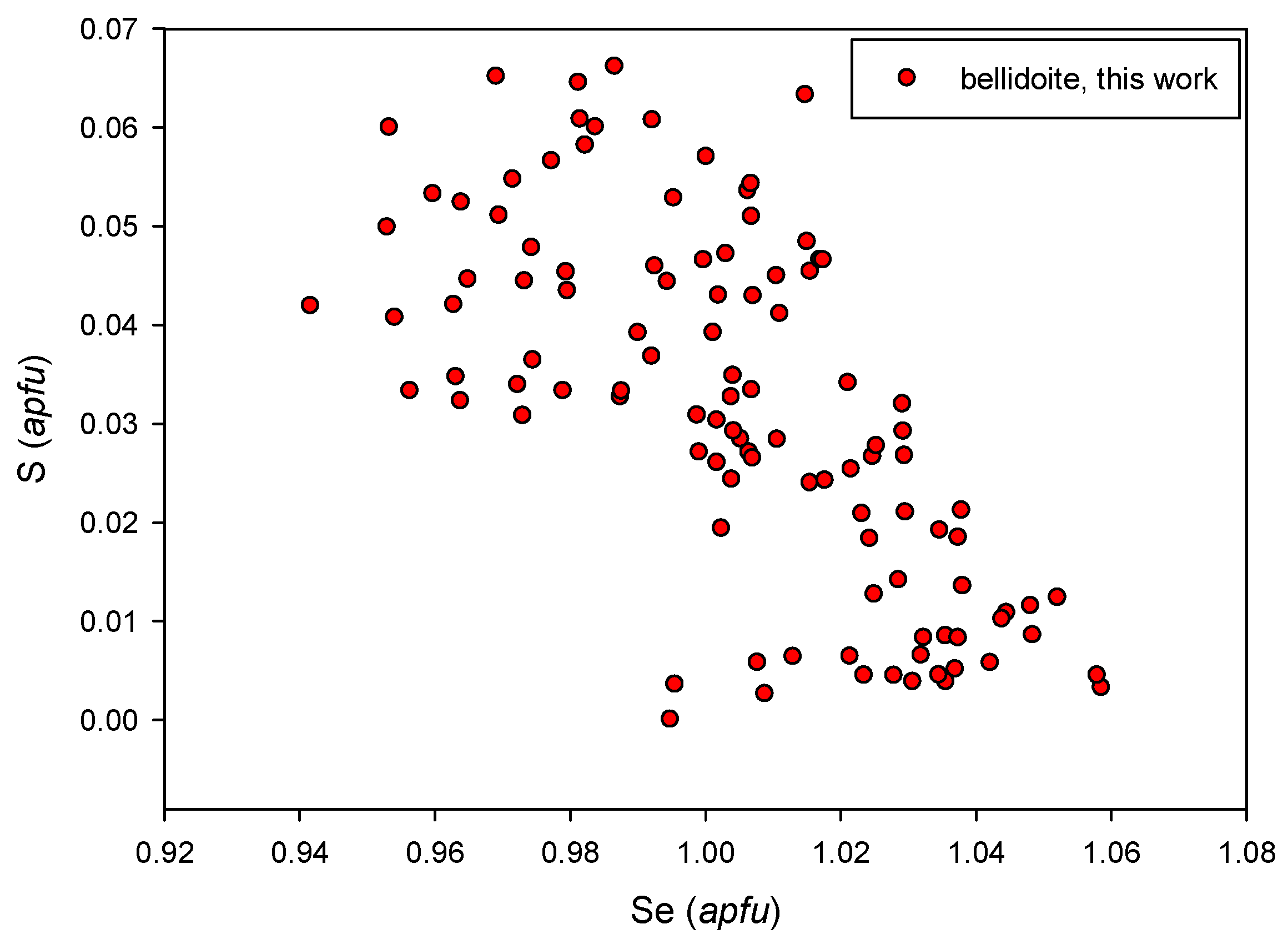
4.2.3. Bellidoite Cu2Se
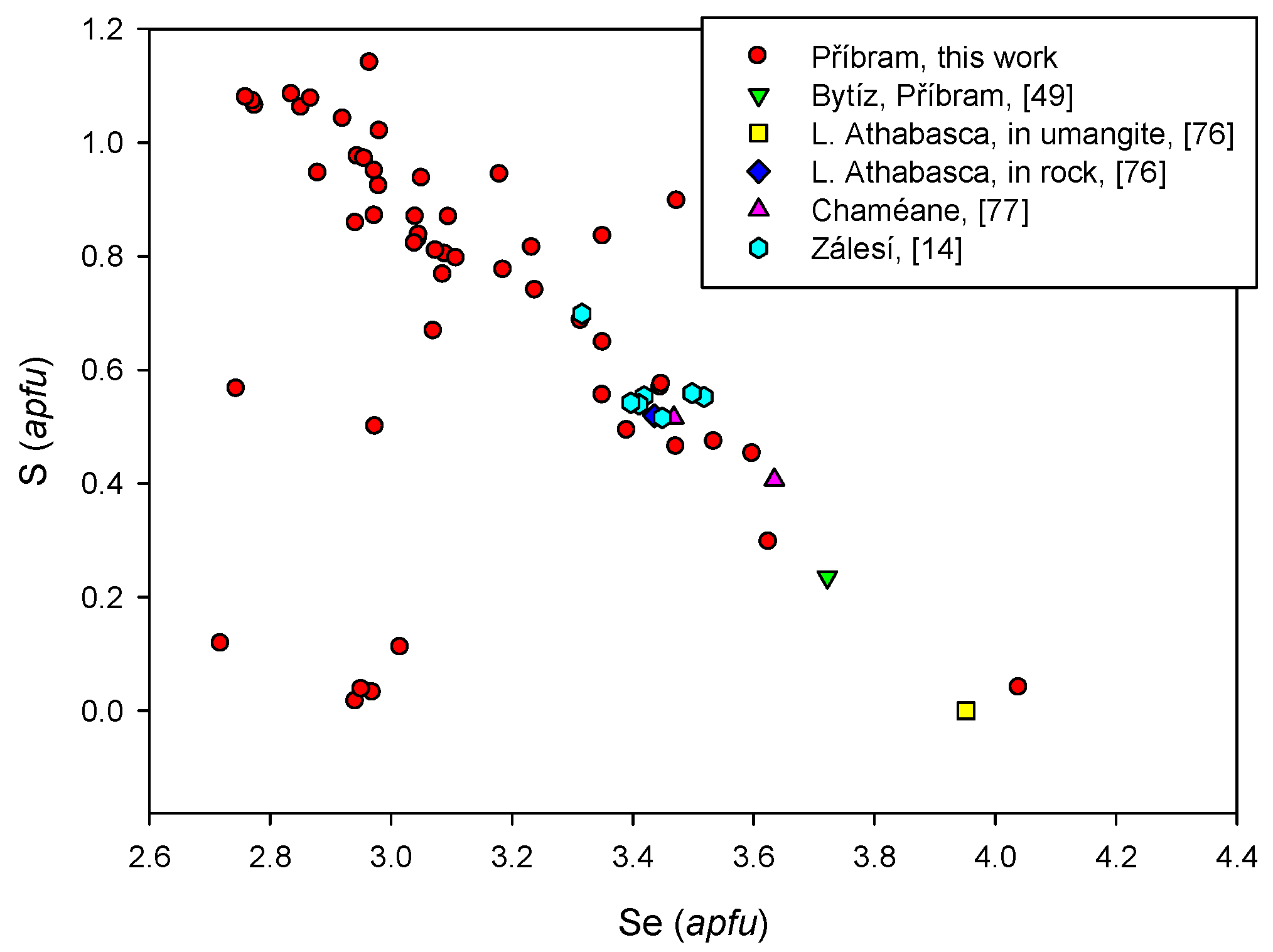
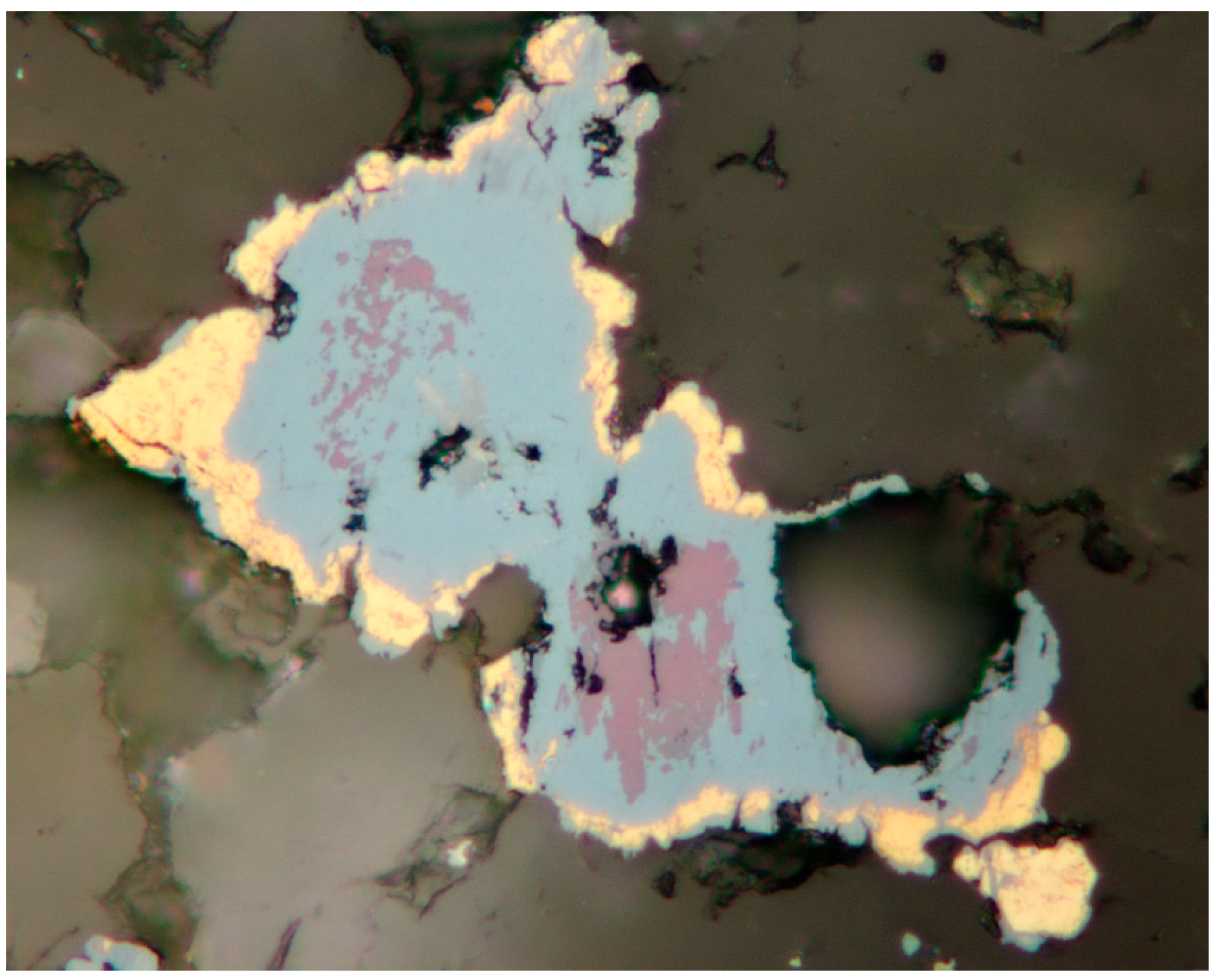
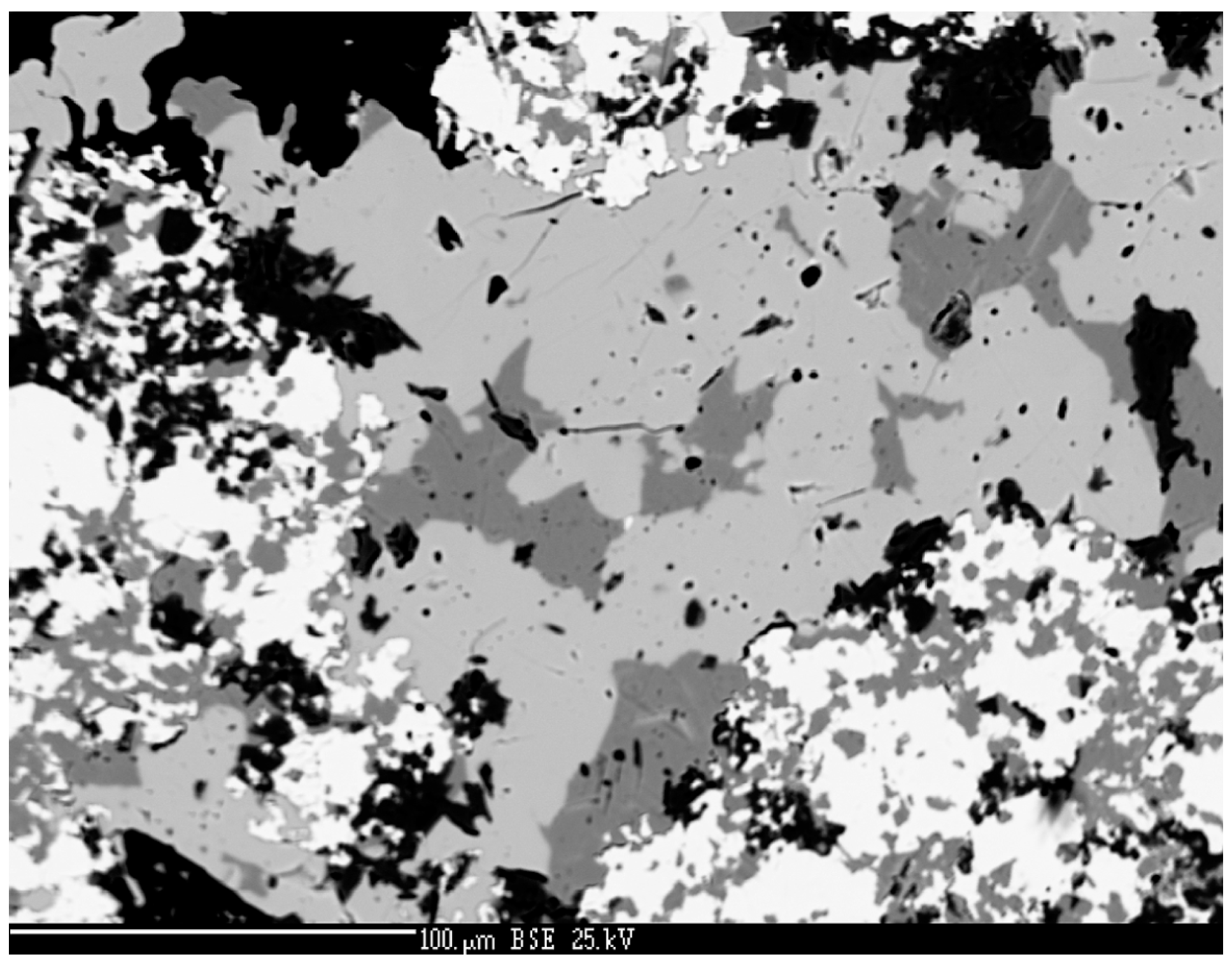
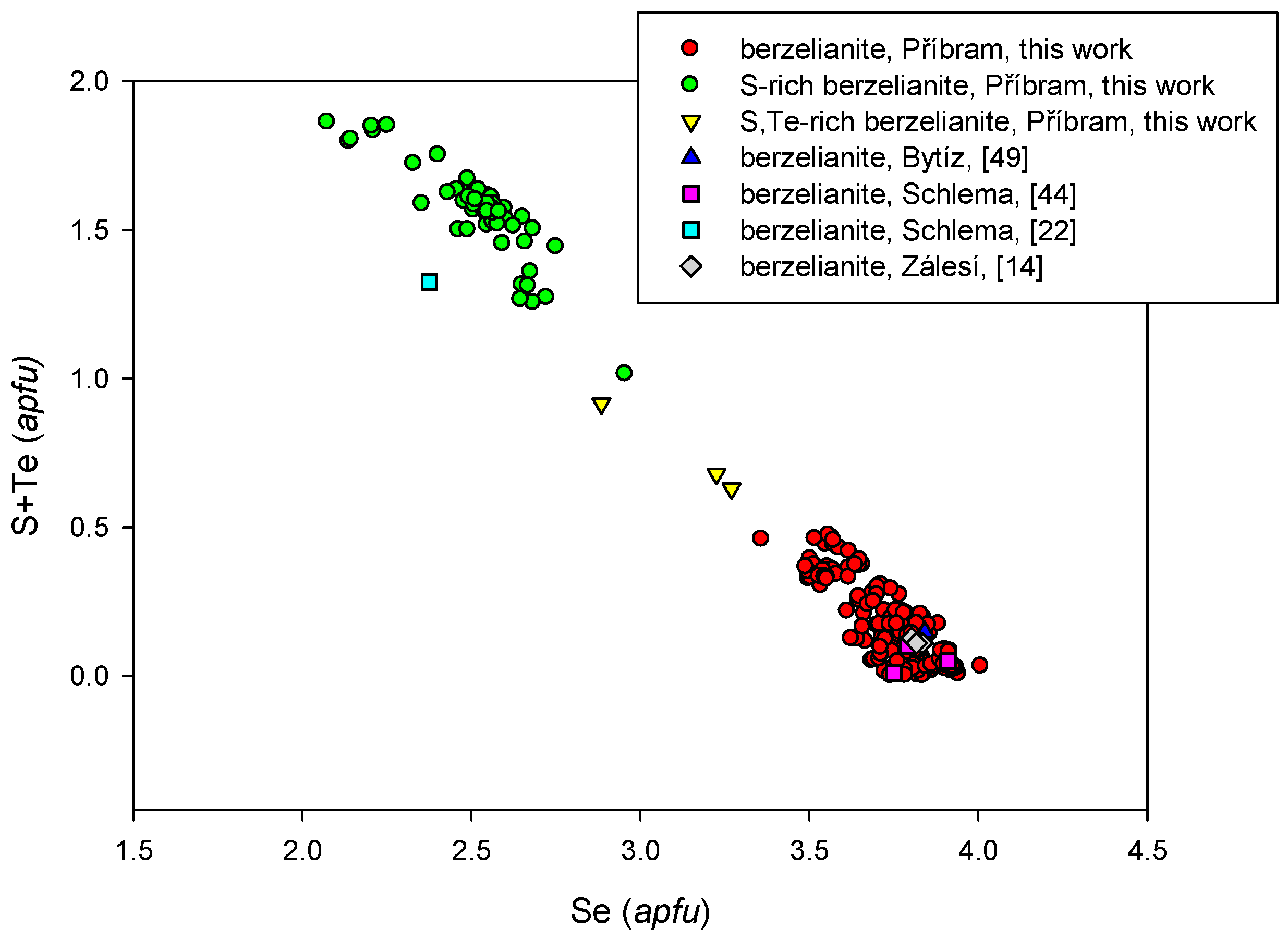
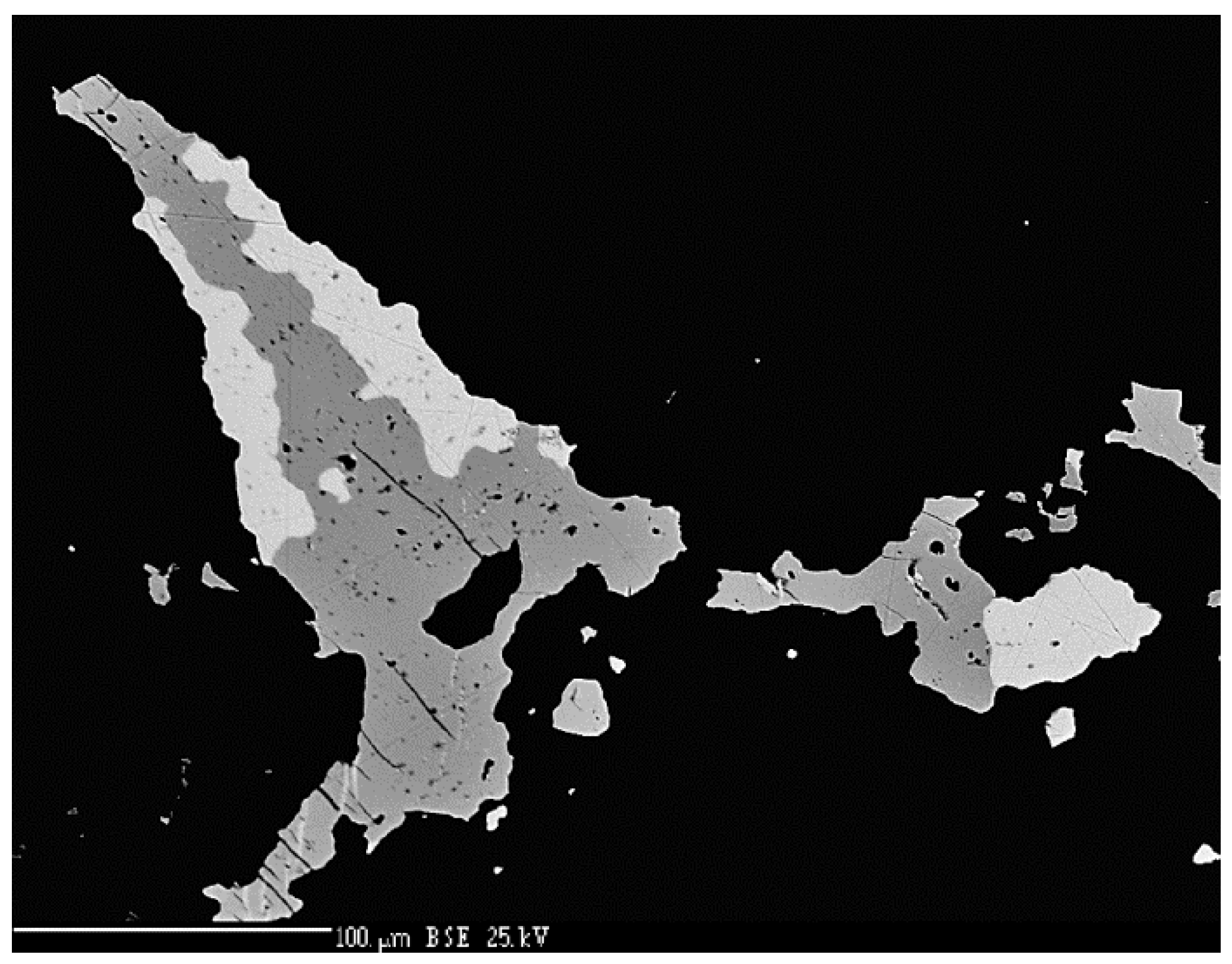
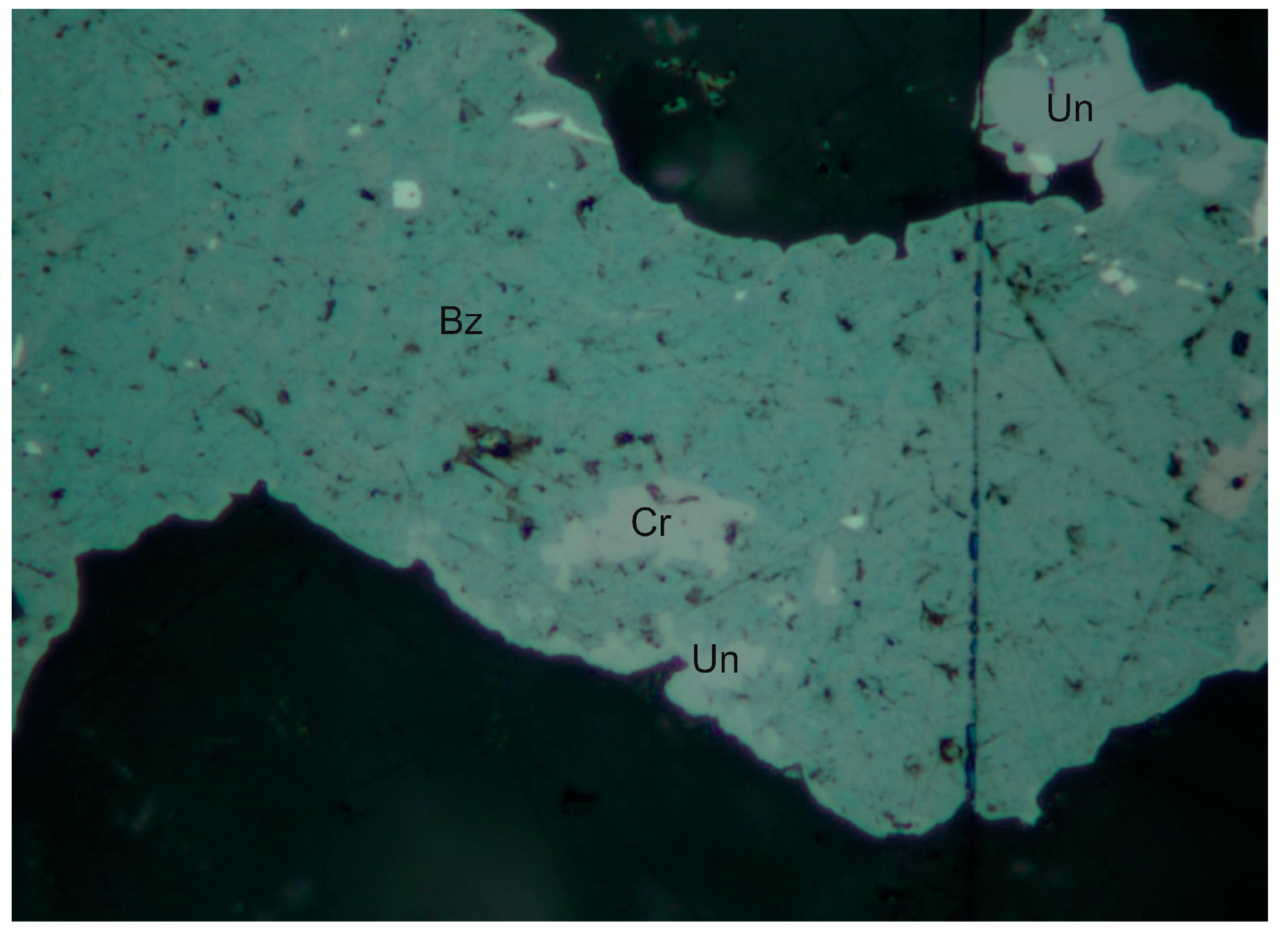
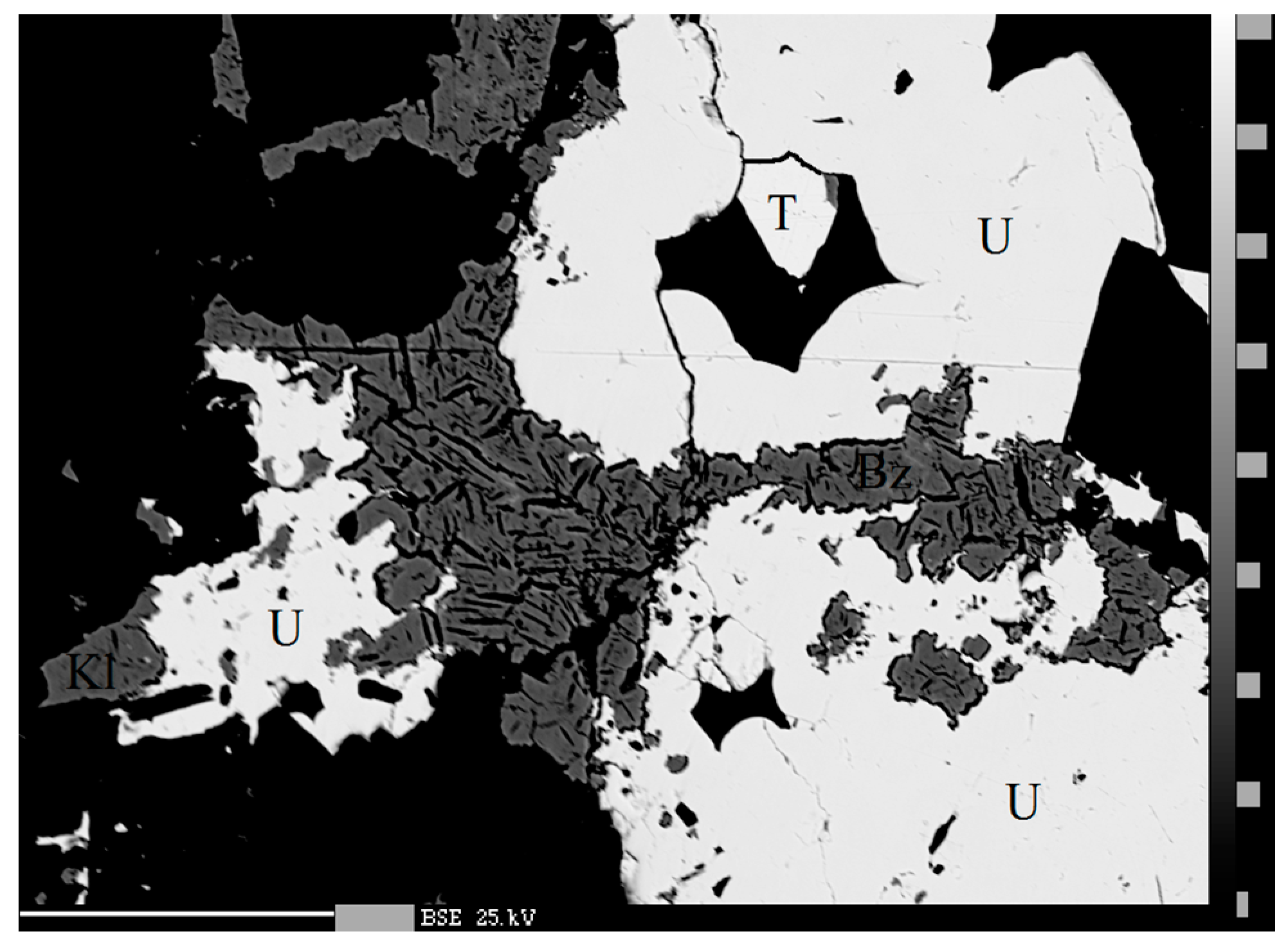
4.2.4. Berzelianite Cu2−xSe
4.2.5. Brodtkorbite, Cu2HgSe2
4.2.6. Bukovite, Tl2(Cu,Fe)4Se2
4.2.7. Bytízite, Cu3SbSe3
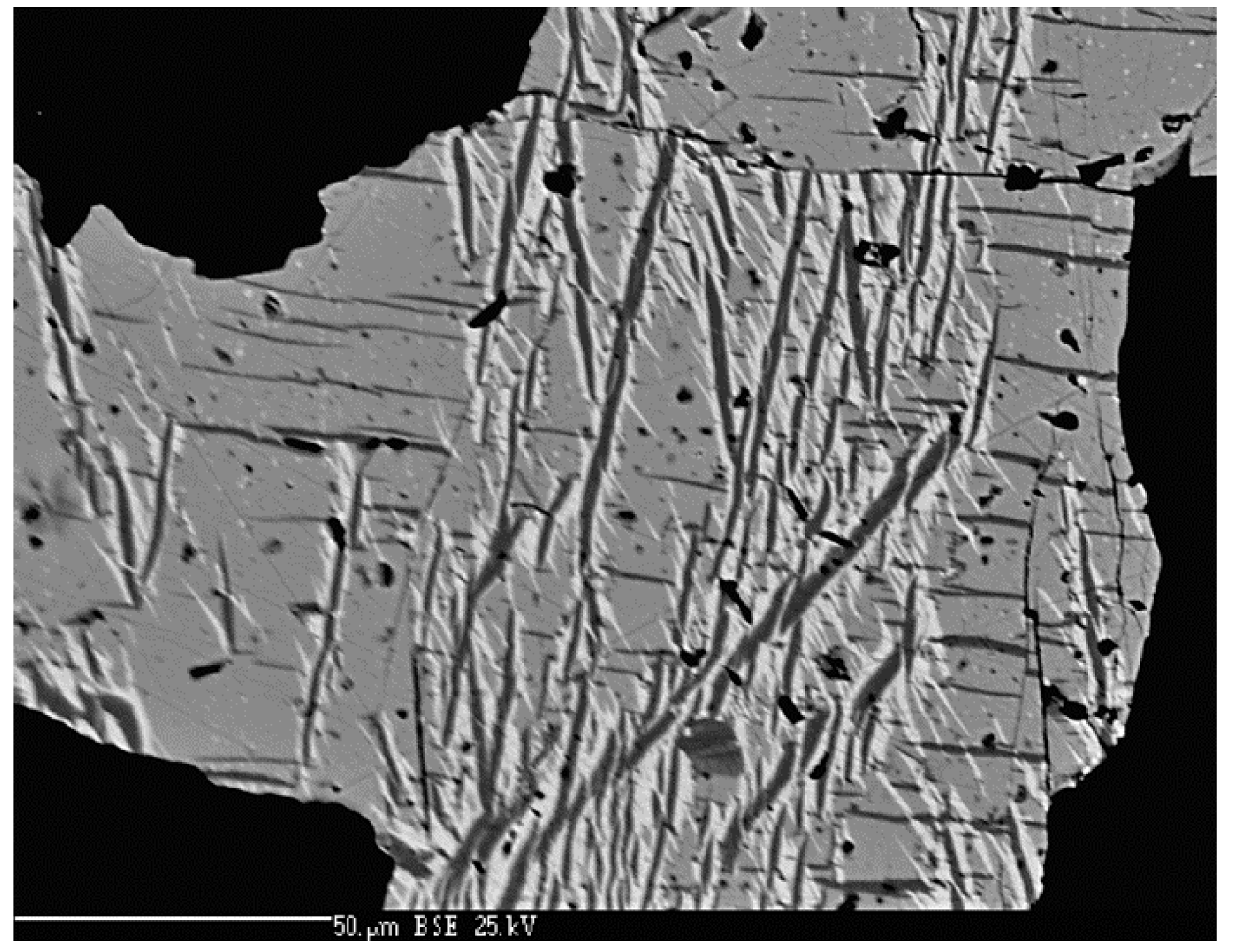
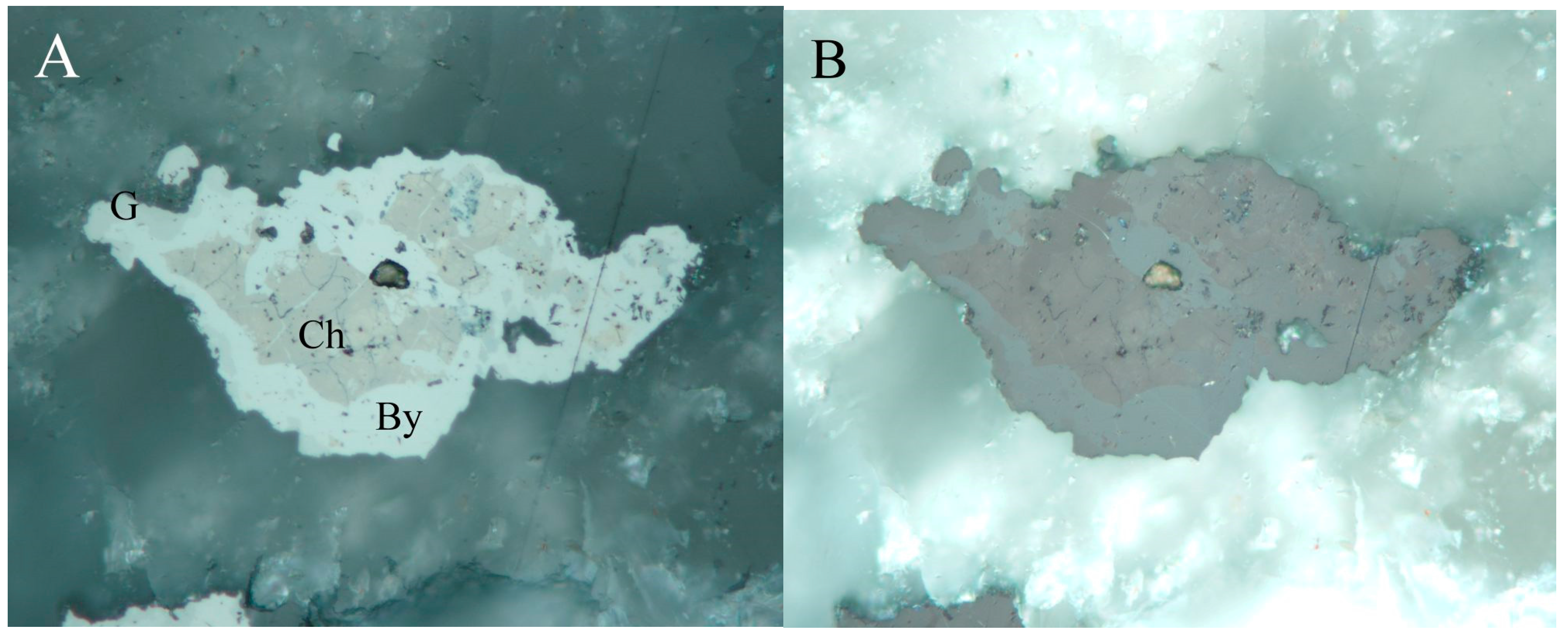
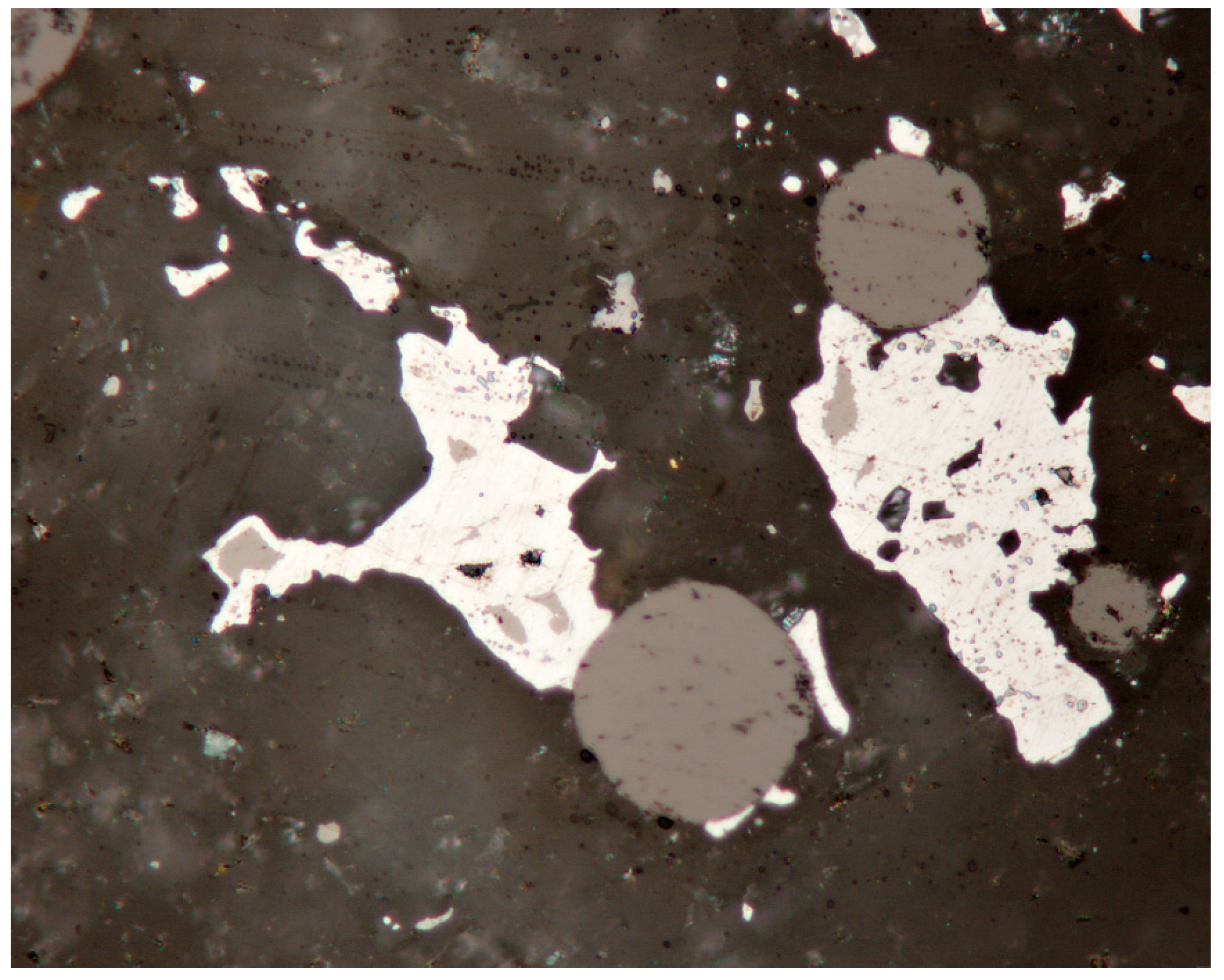
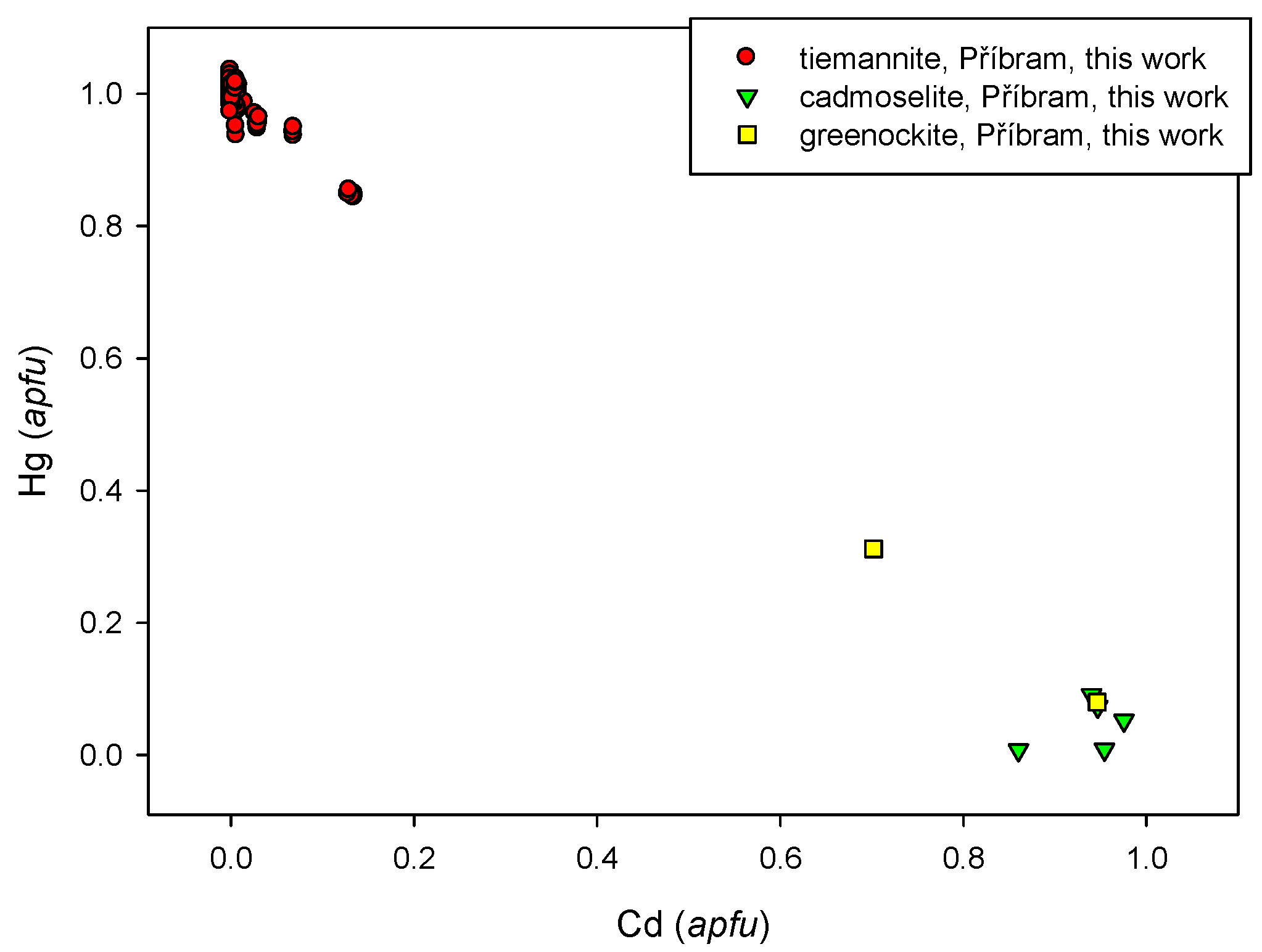
4.2.8. Cadmoselite, CdSe and Greenockite, CdS
4.2.9. Chaméanite, (Cu,Fe)4As(Se,S)4
4.2.10. Clausthalite, PbSe
4.2.11. Crookesite, Cu7(Tl,Ag)Se4
4.2.12. Dzharkenite-Pyrite Solid Solution, FeSe2-FeS2
4.2.13. Eskebornite-Chalcopyrite Solid Solution, CuFeSe2-CuFeS2
4.2.14. Eucairite, AgCuSe
4.2.15. Ferroselite, FeSe2
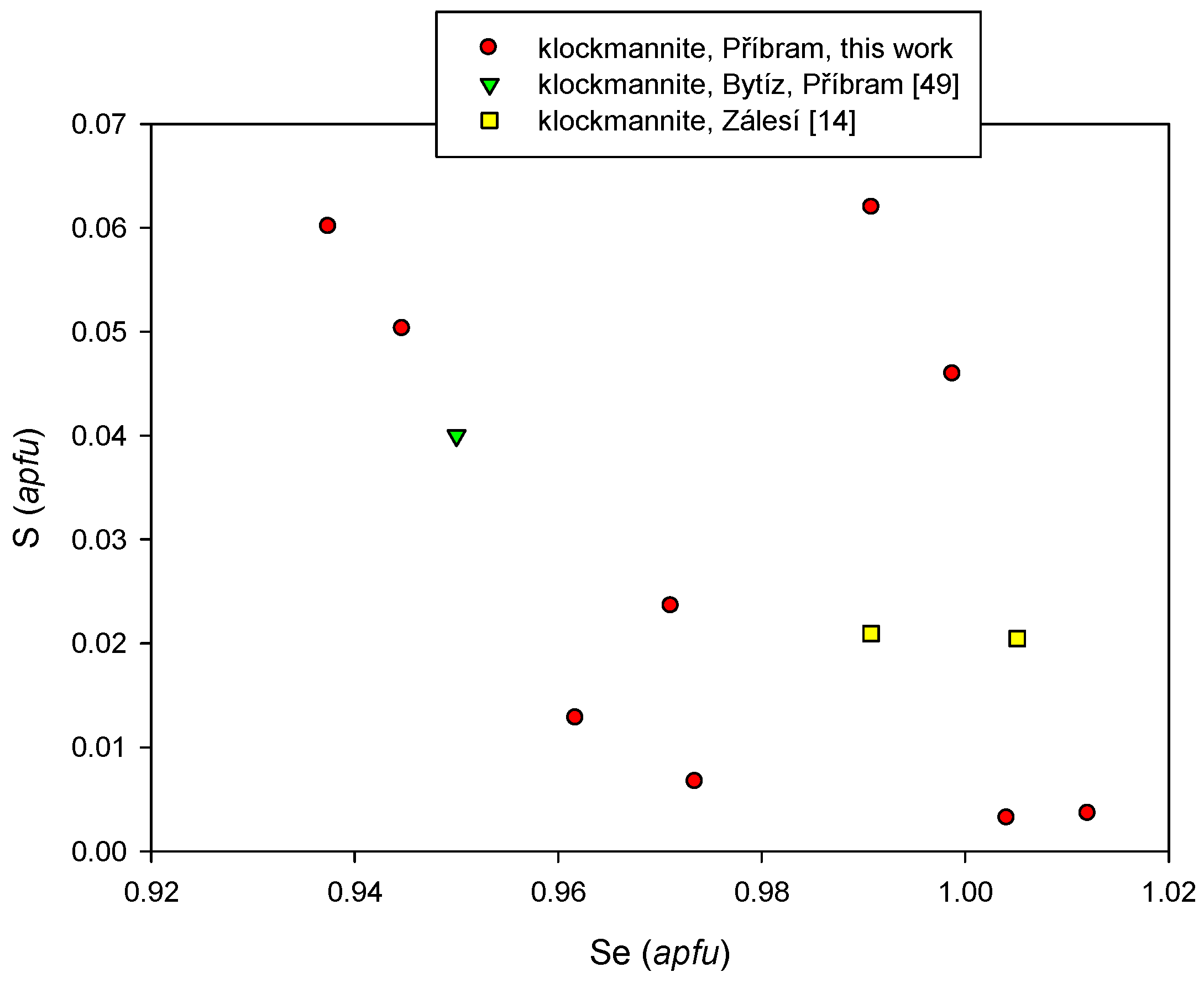
4.2.16. Klockmannite, CuSe
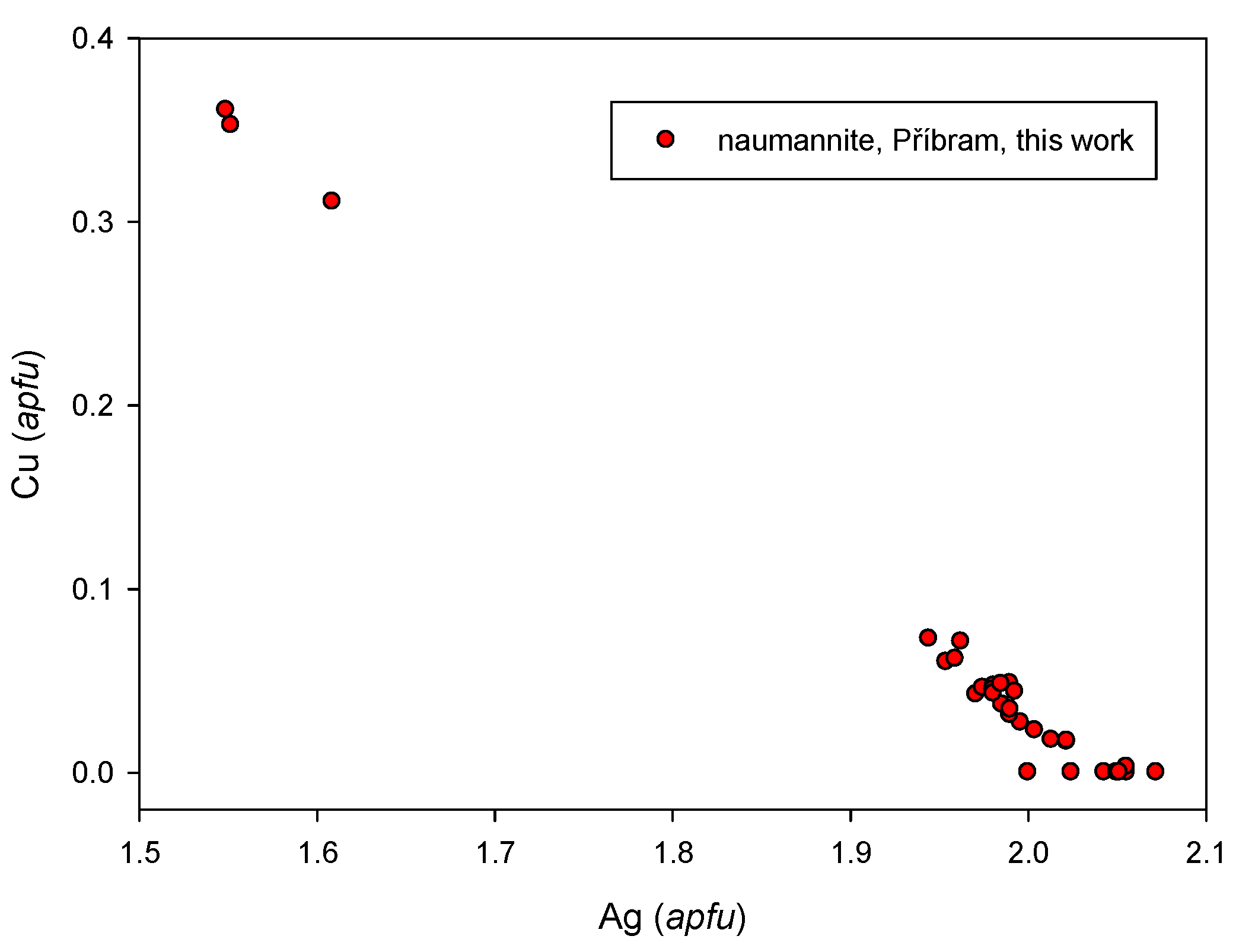
4.2.17. Naumannite, Ag2Se
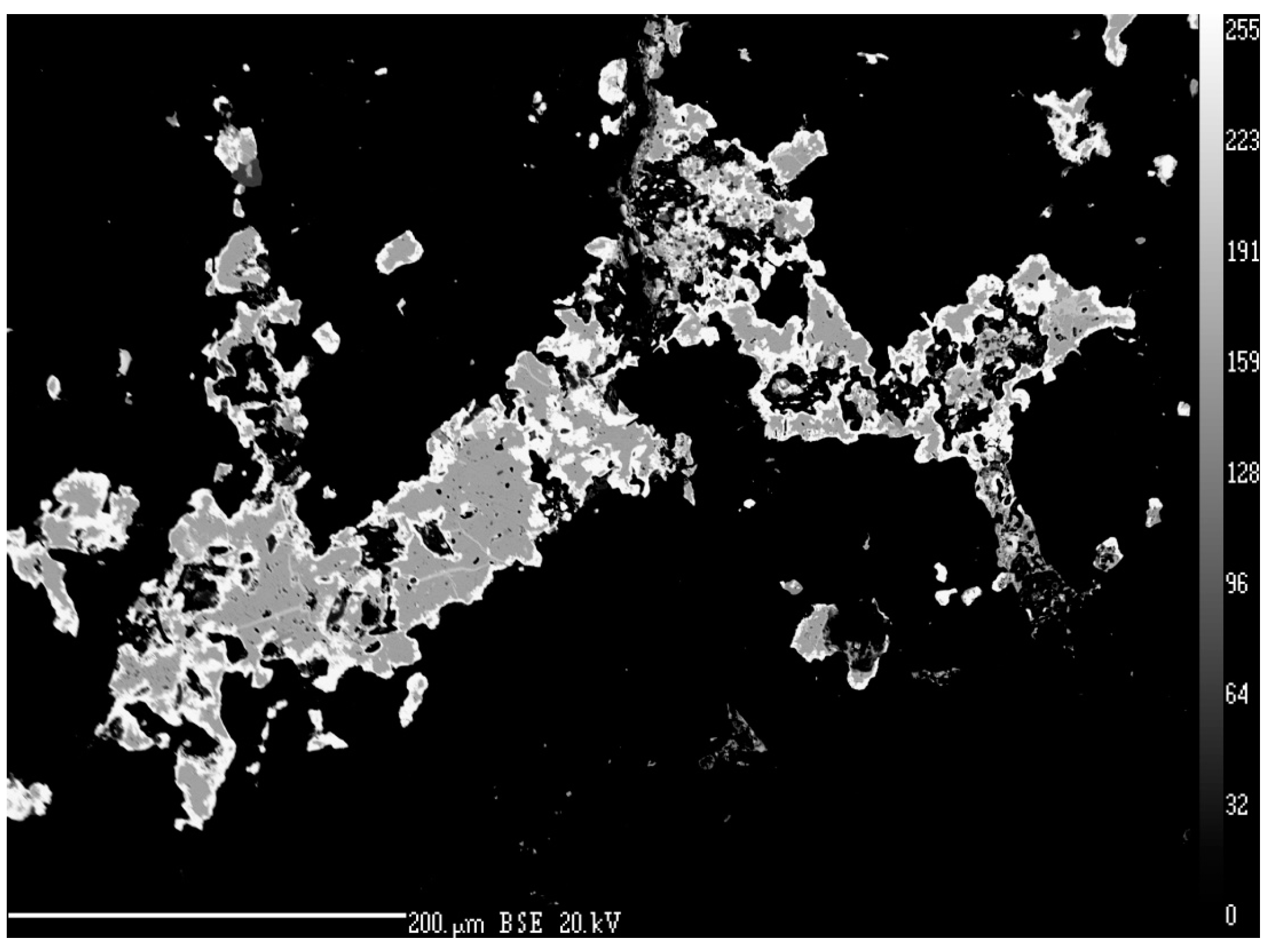
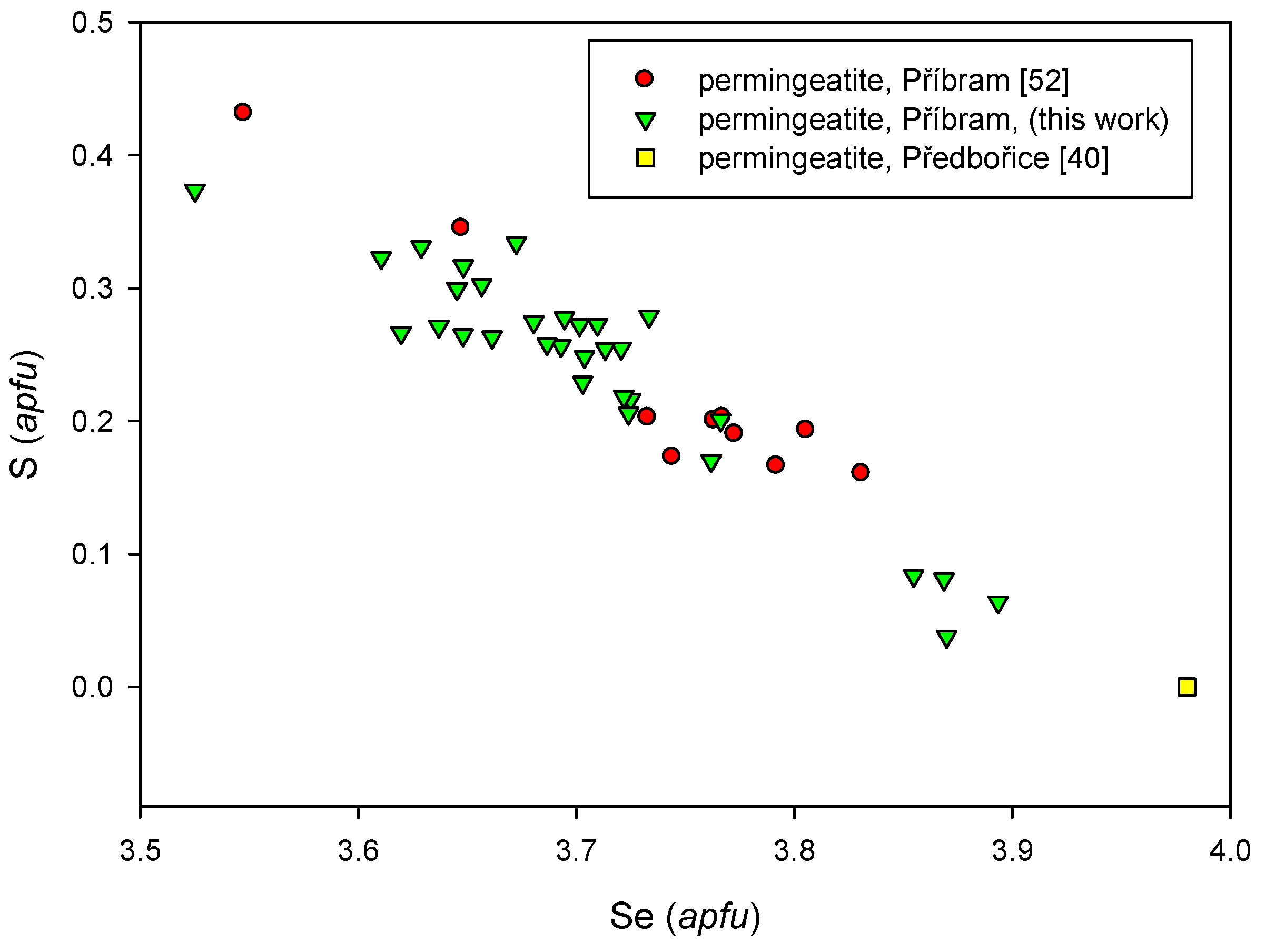
4.2.18. Permingeatite, Cu3SbSe4
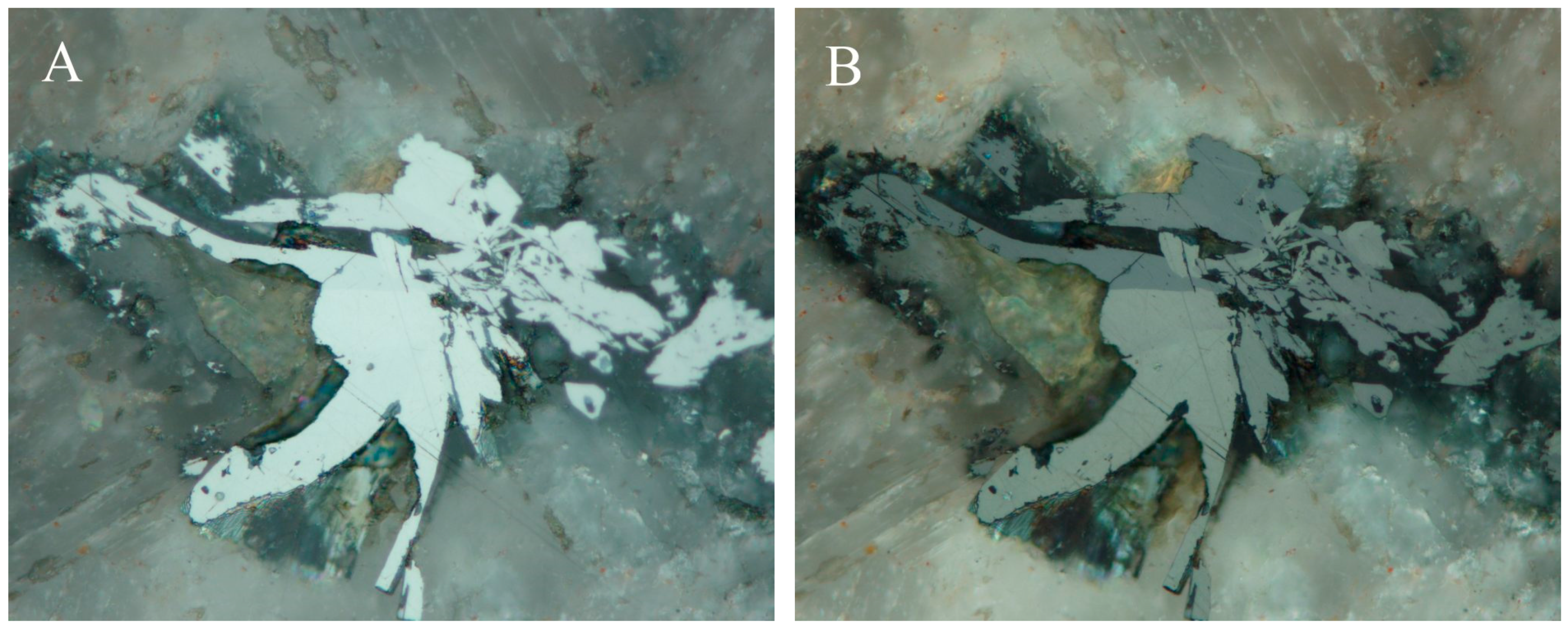
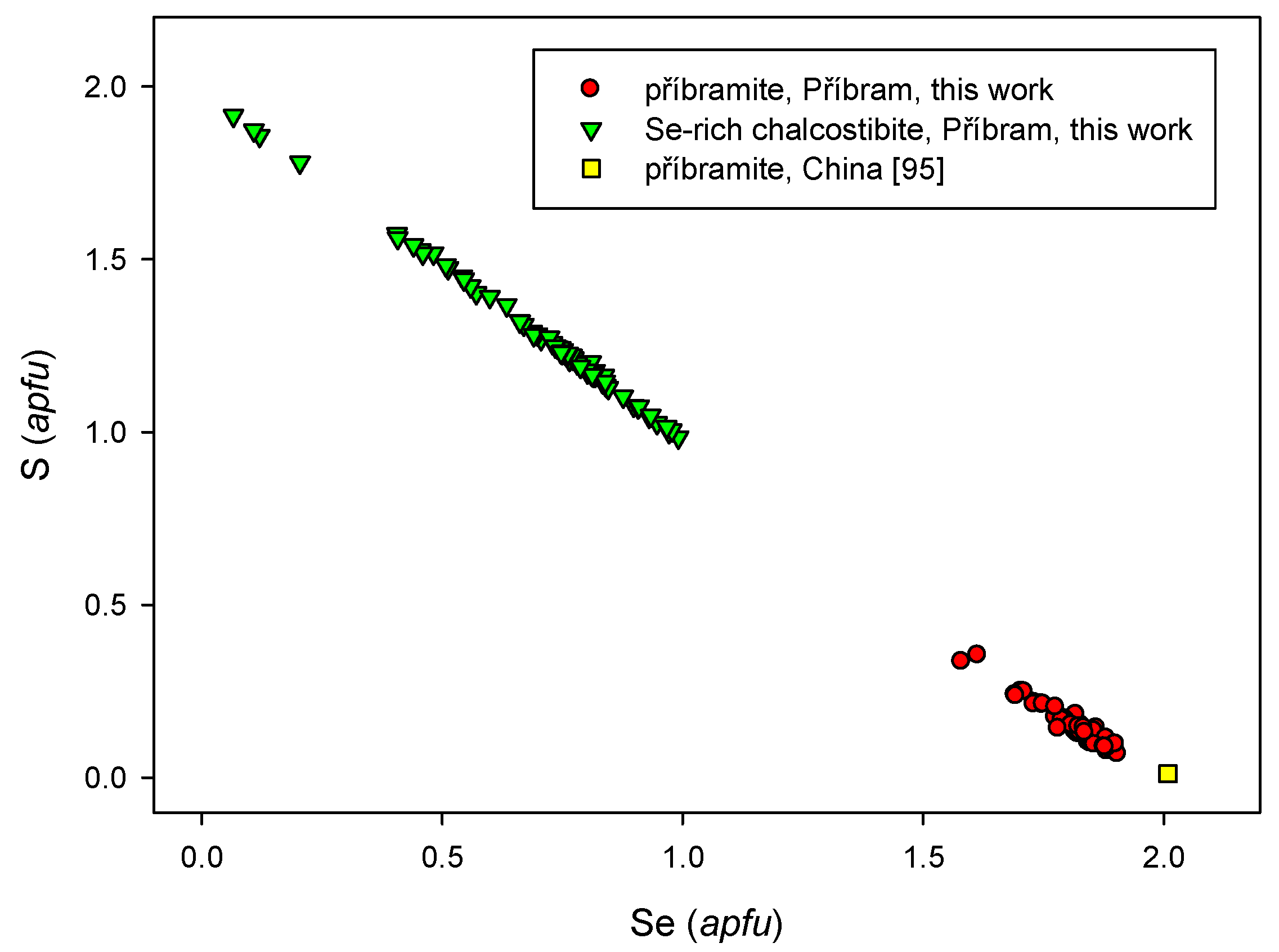
4.2.19. Příbramite-Chalcostibite Solid Solution CuSbSe2-CuSbS2
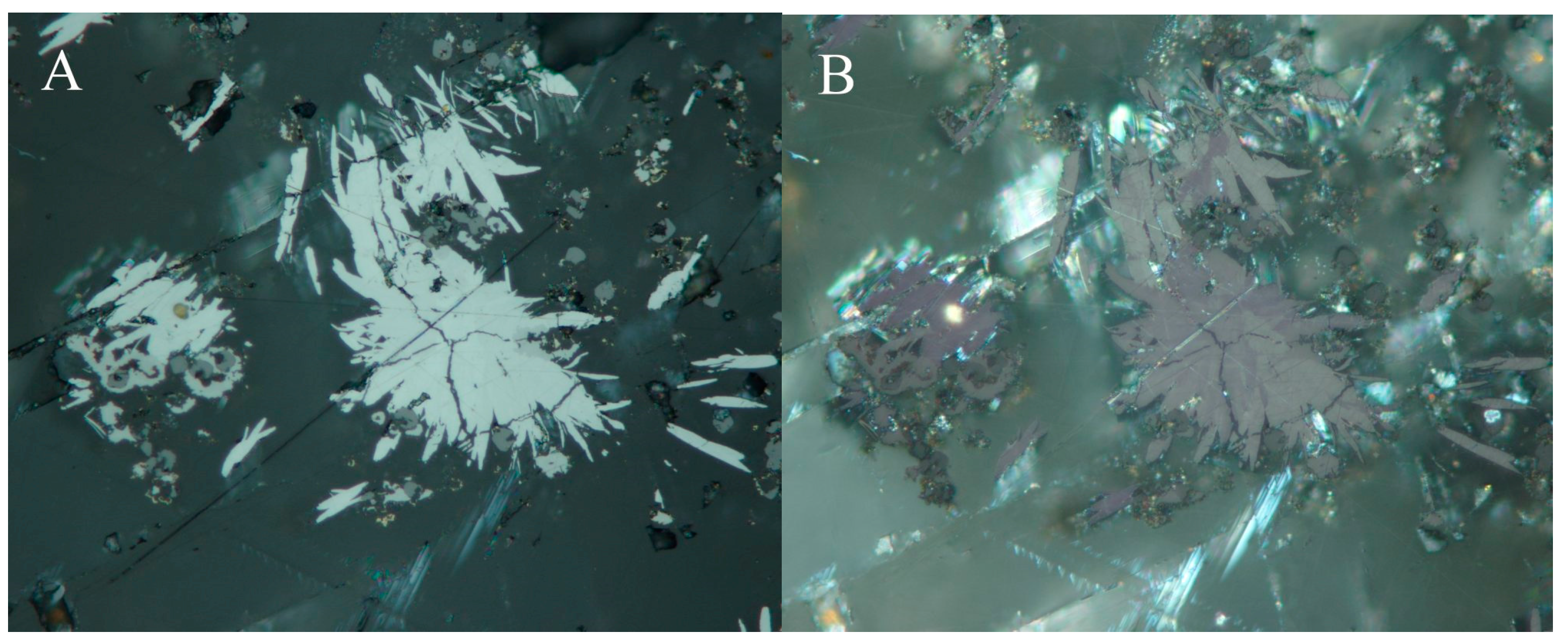
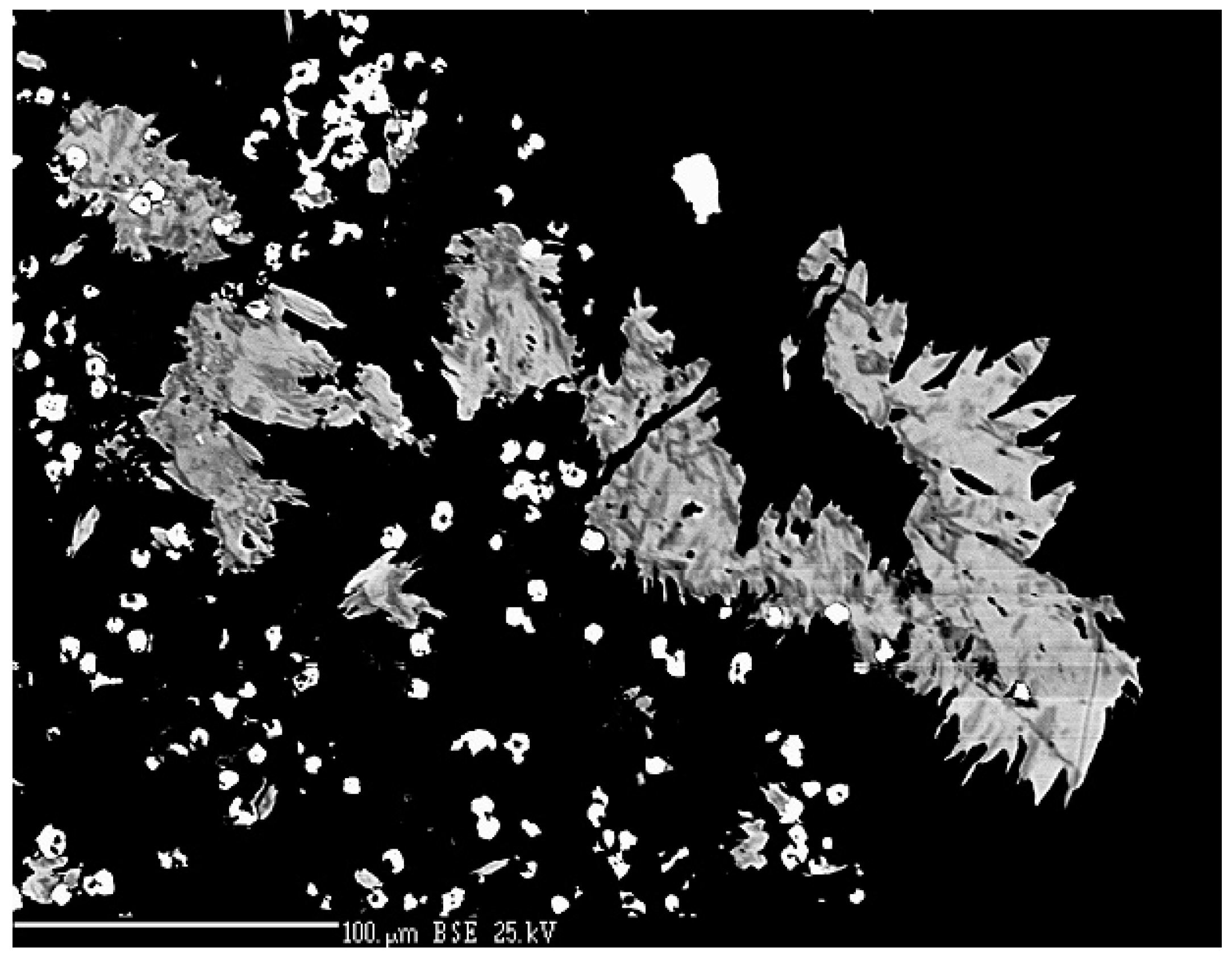
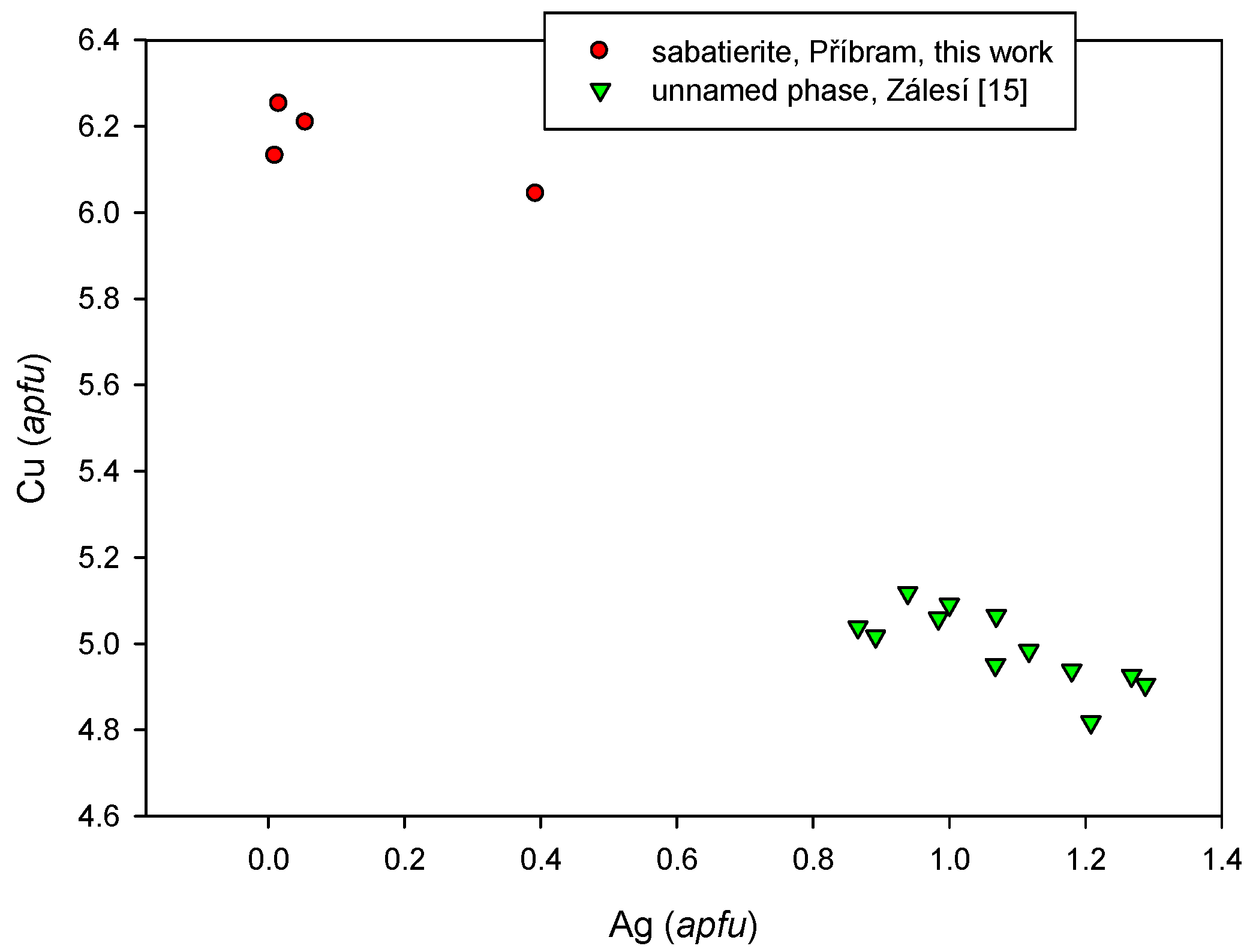
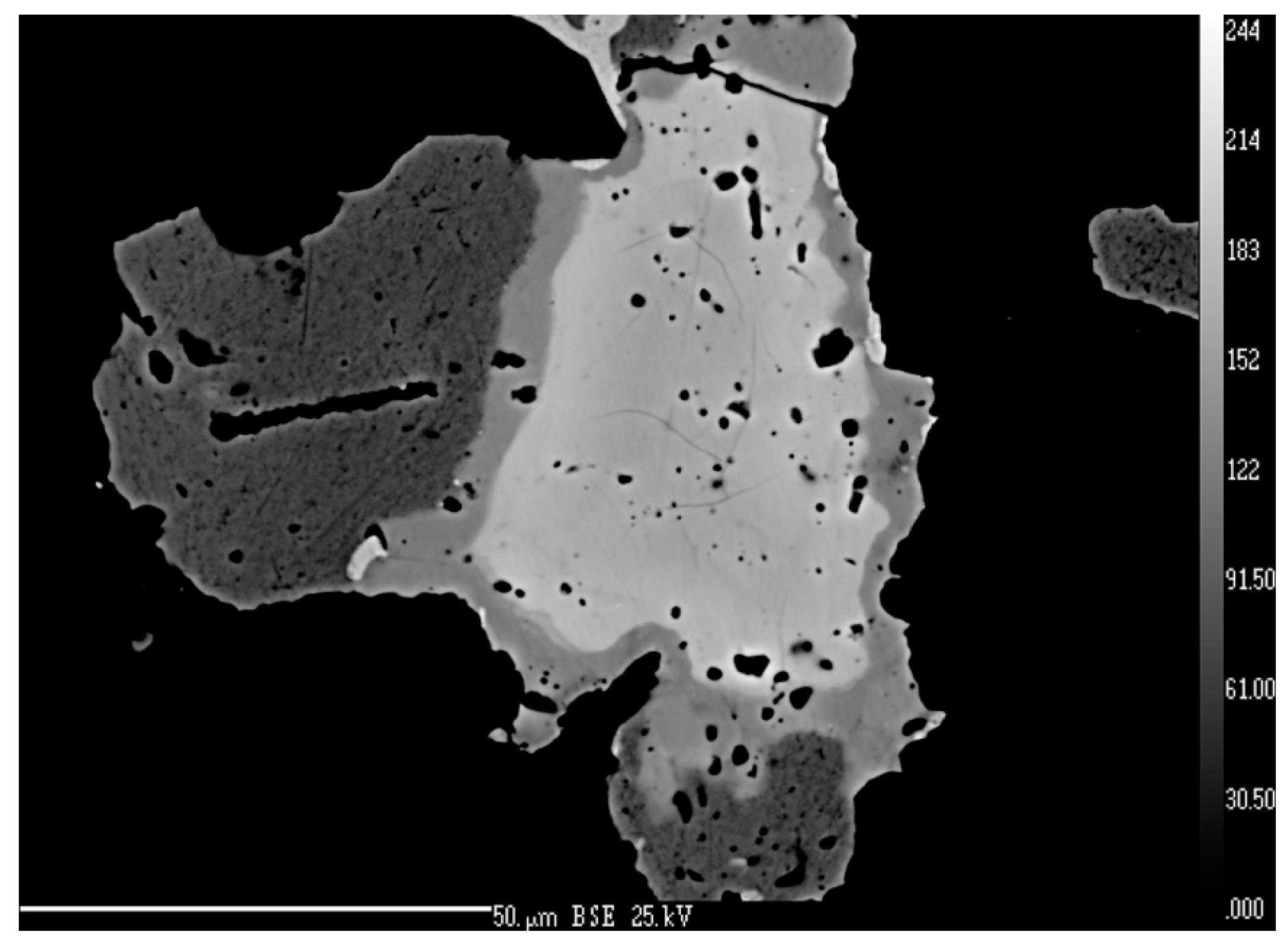
4.2.20. Sabatierite, Cu6TlSe4
4.2.21. Tetrahedrite Group
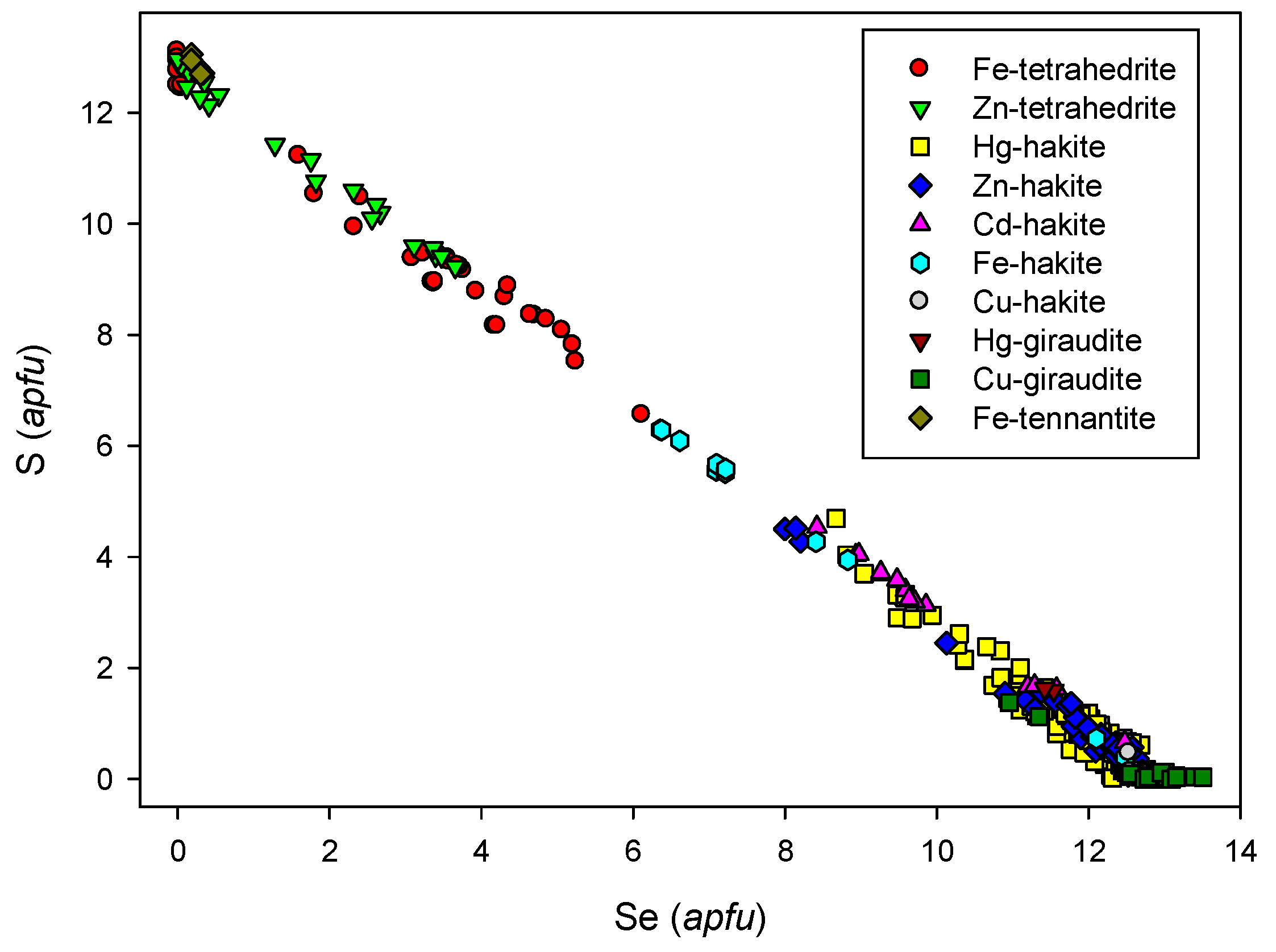
4.2.21.1. Giraudite-Tennantite Solid Solution, Cu6[Cu4(Fe,Zn)2]As4Se13-Cu6[Cu4(Fe,Zn)2]As4S13
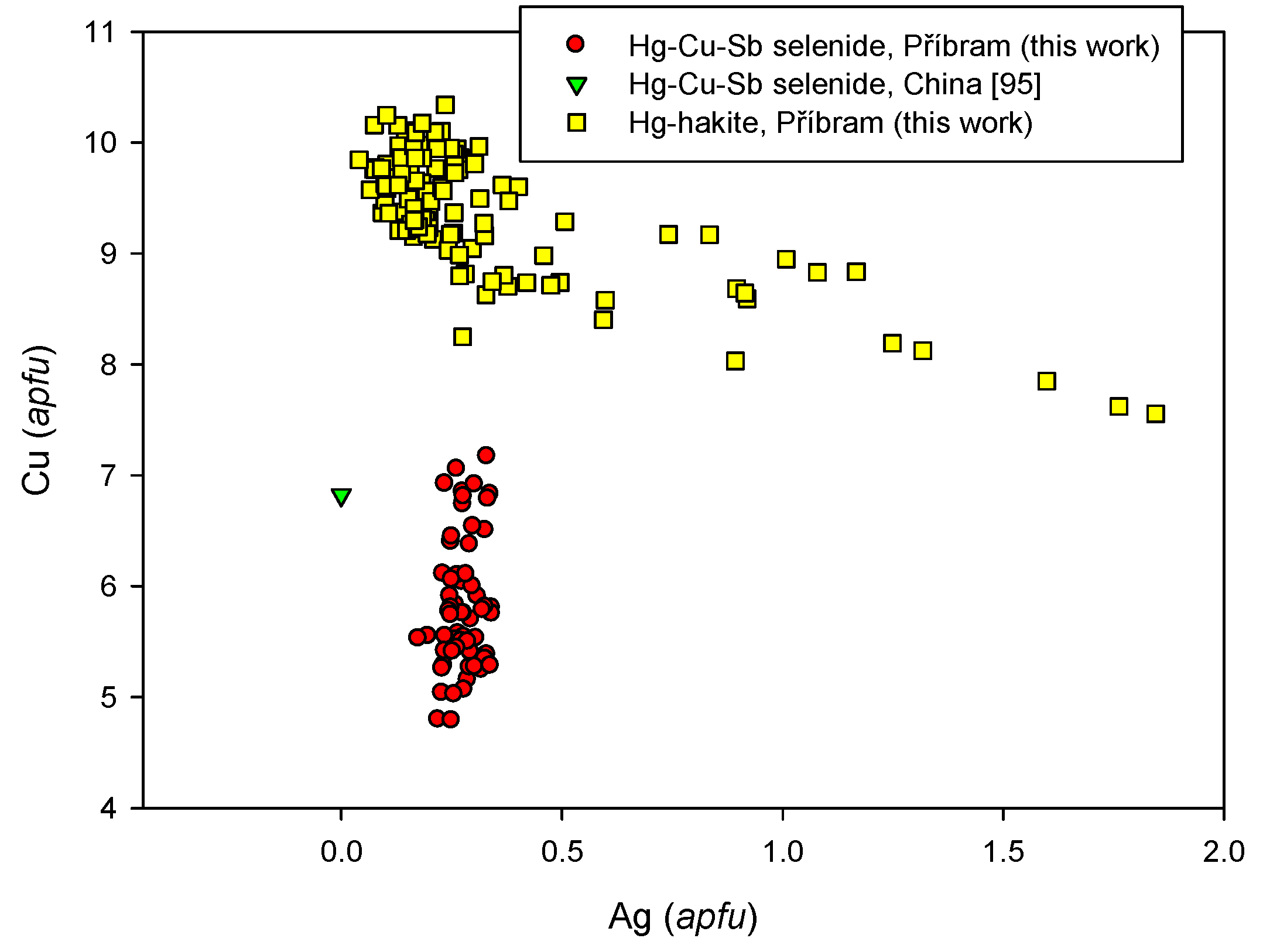
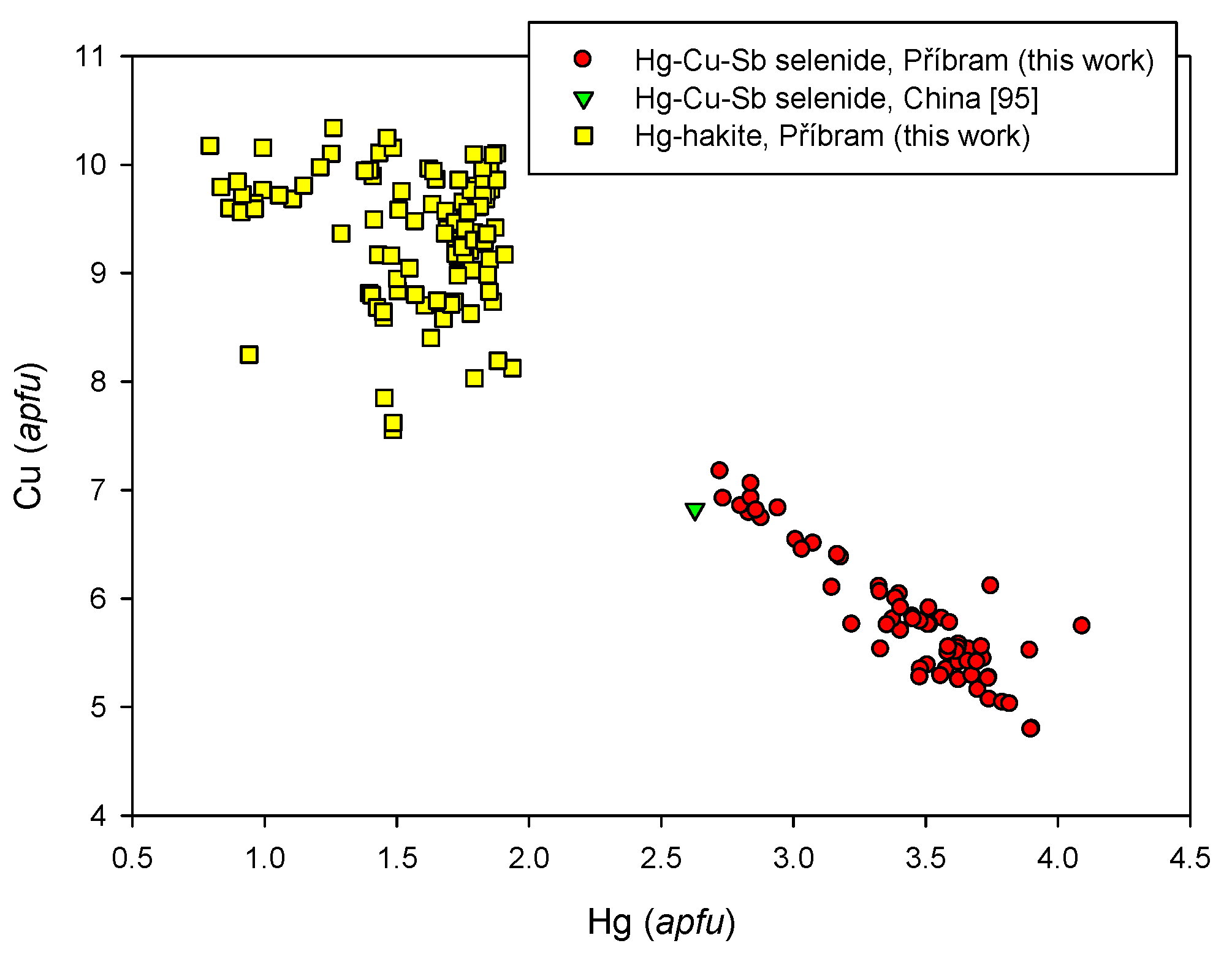
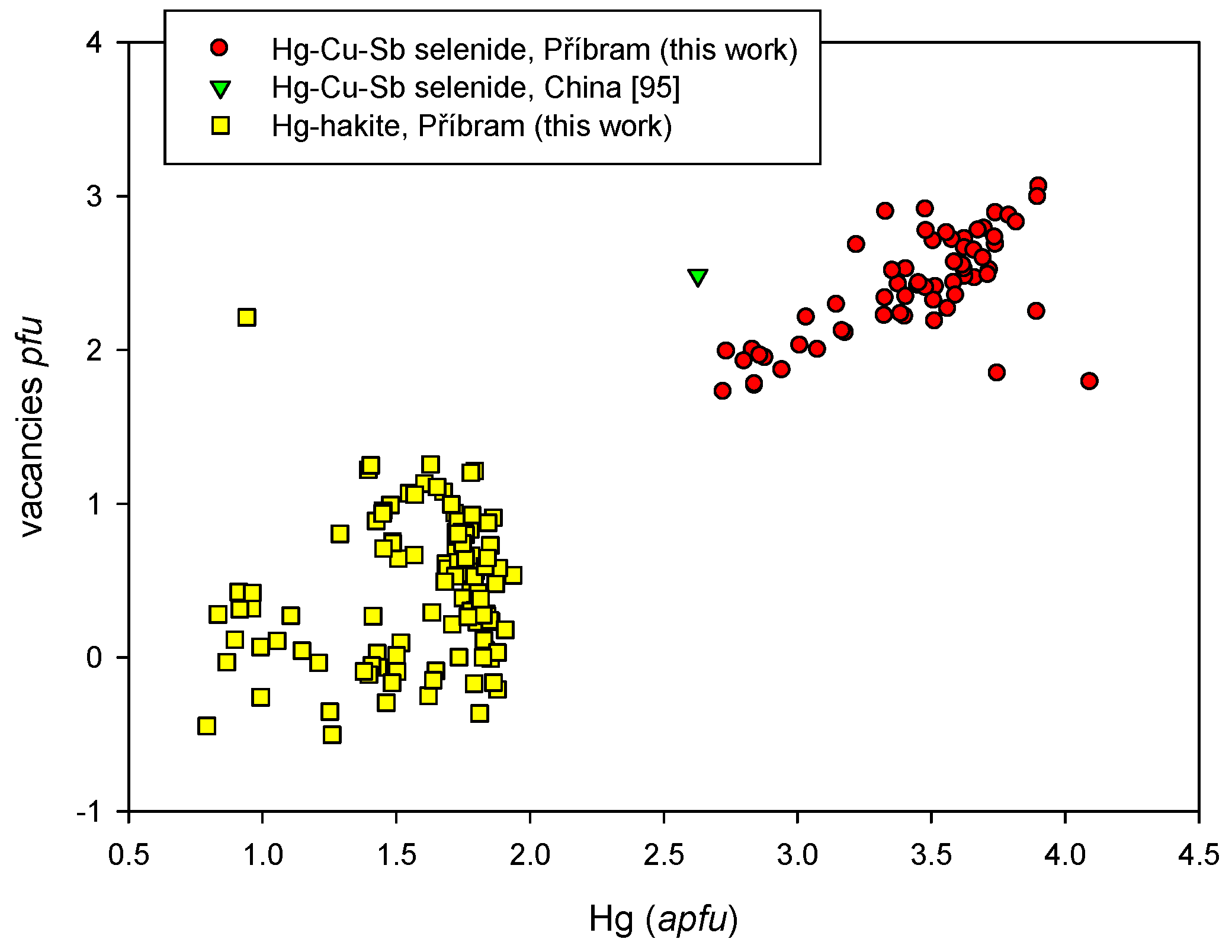
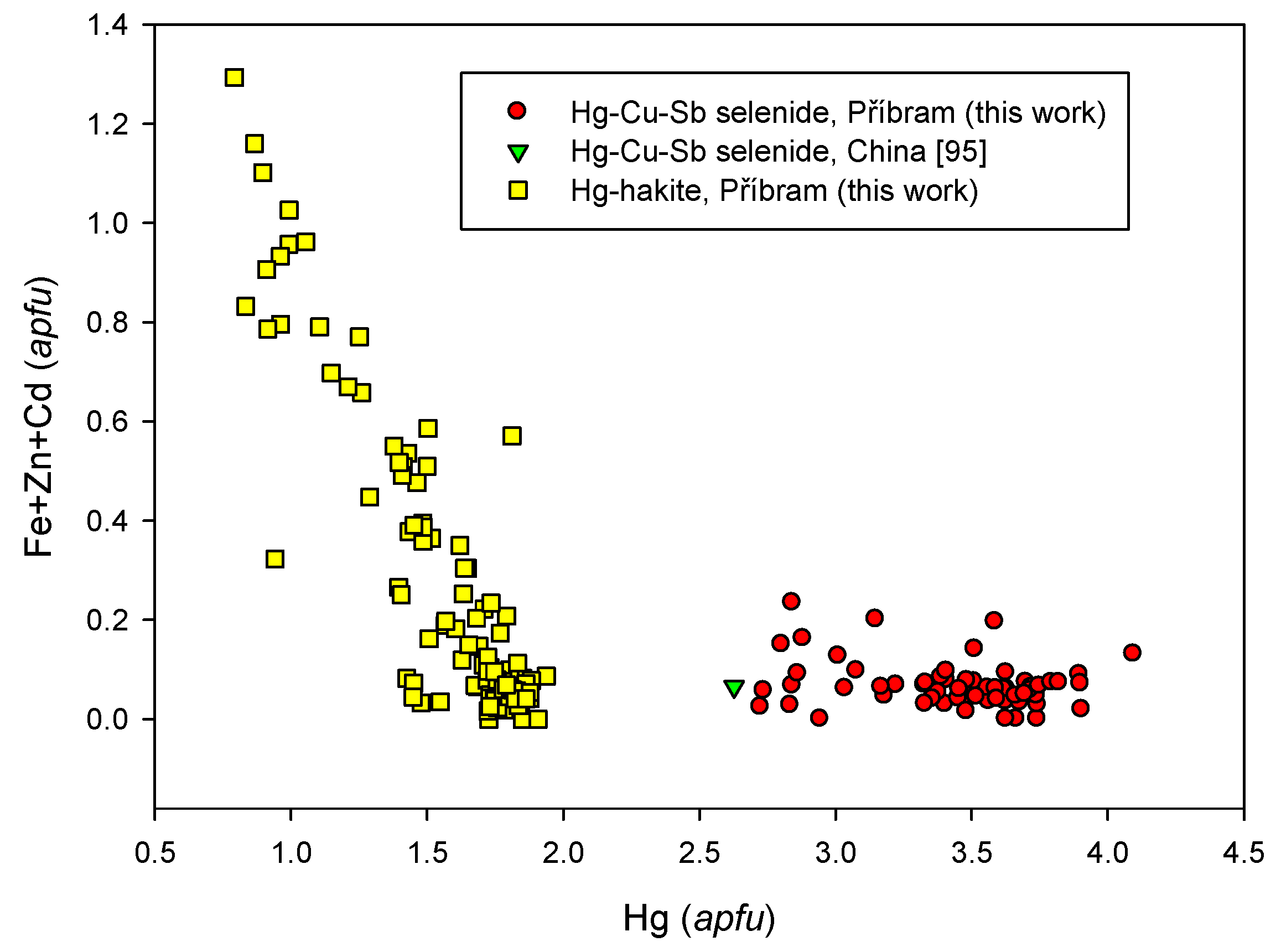
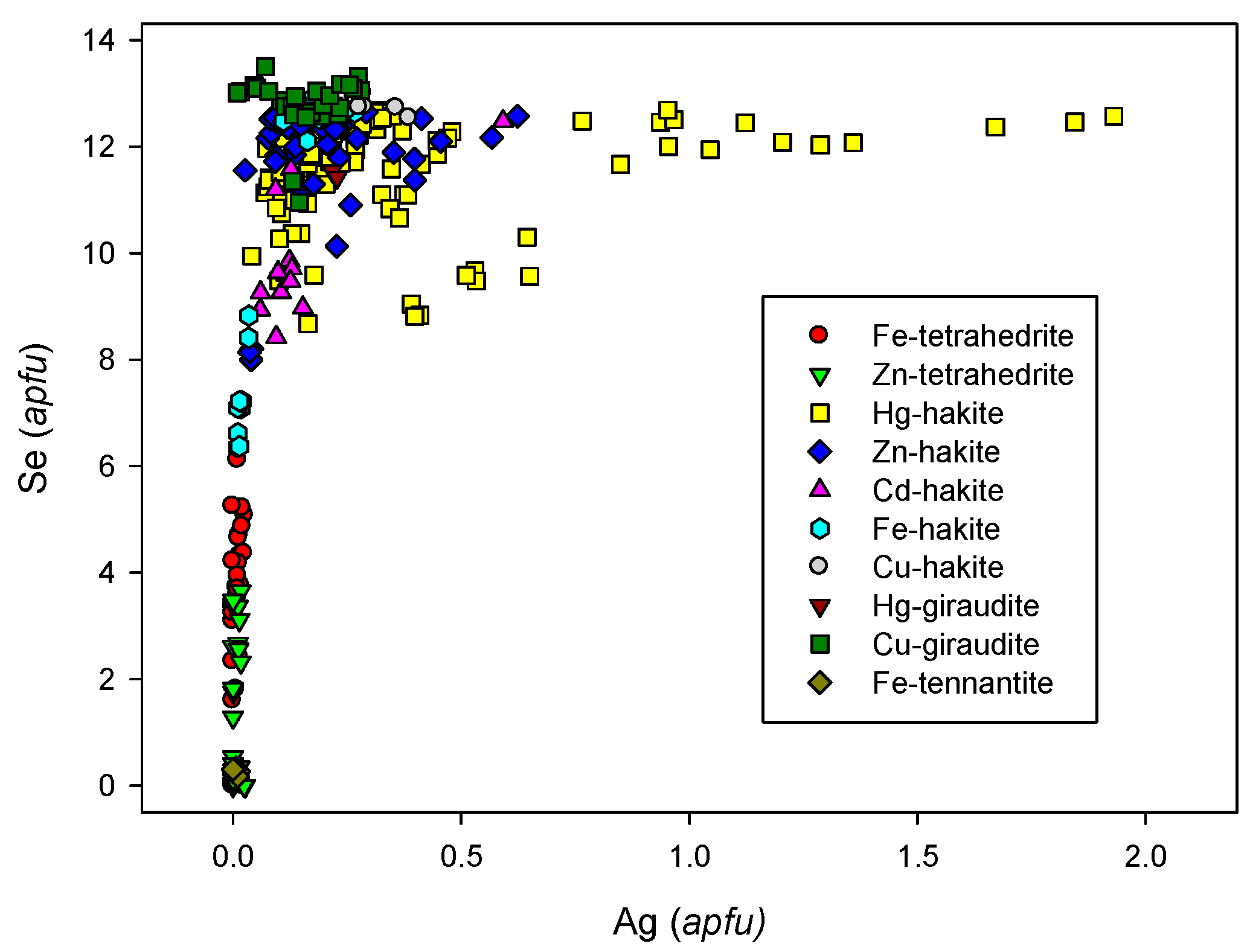
4.2.21.2. Hakite-Tetrahedrite Solid Solution, Cu6[Cu4(Hg,Zn)2]Sb4Se13-Cu6[Cu4(Fe,Zn)2]Sb4S13
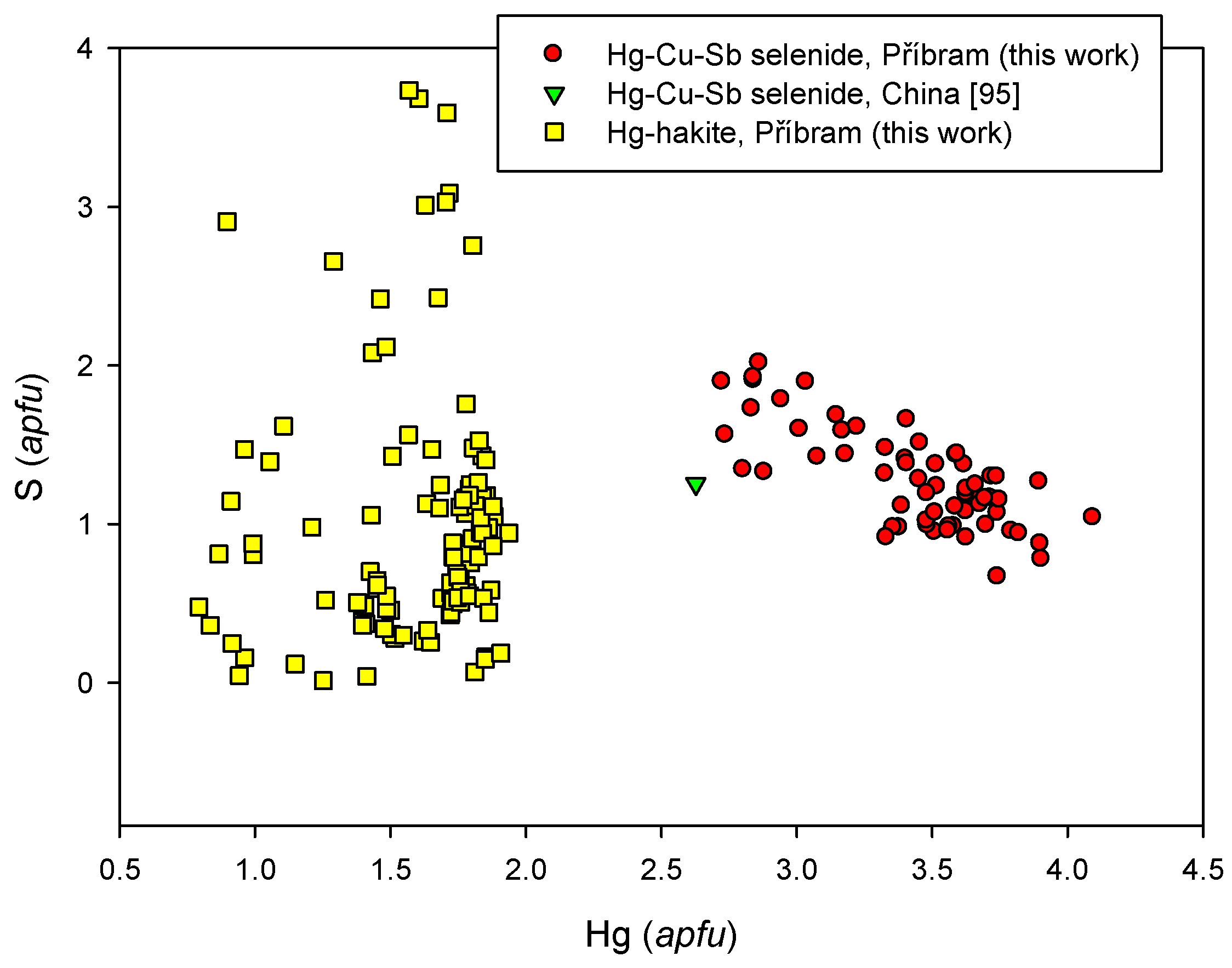
4.2.21.3. Unnamed Hg-Cu-Sb Selenide from the Tetrahedrite Group
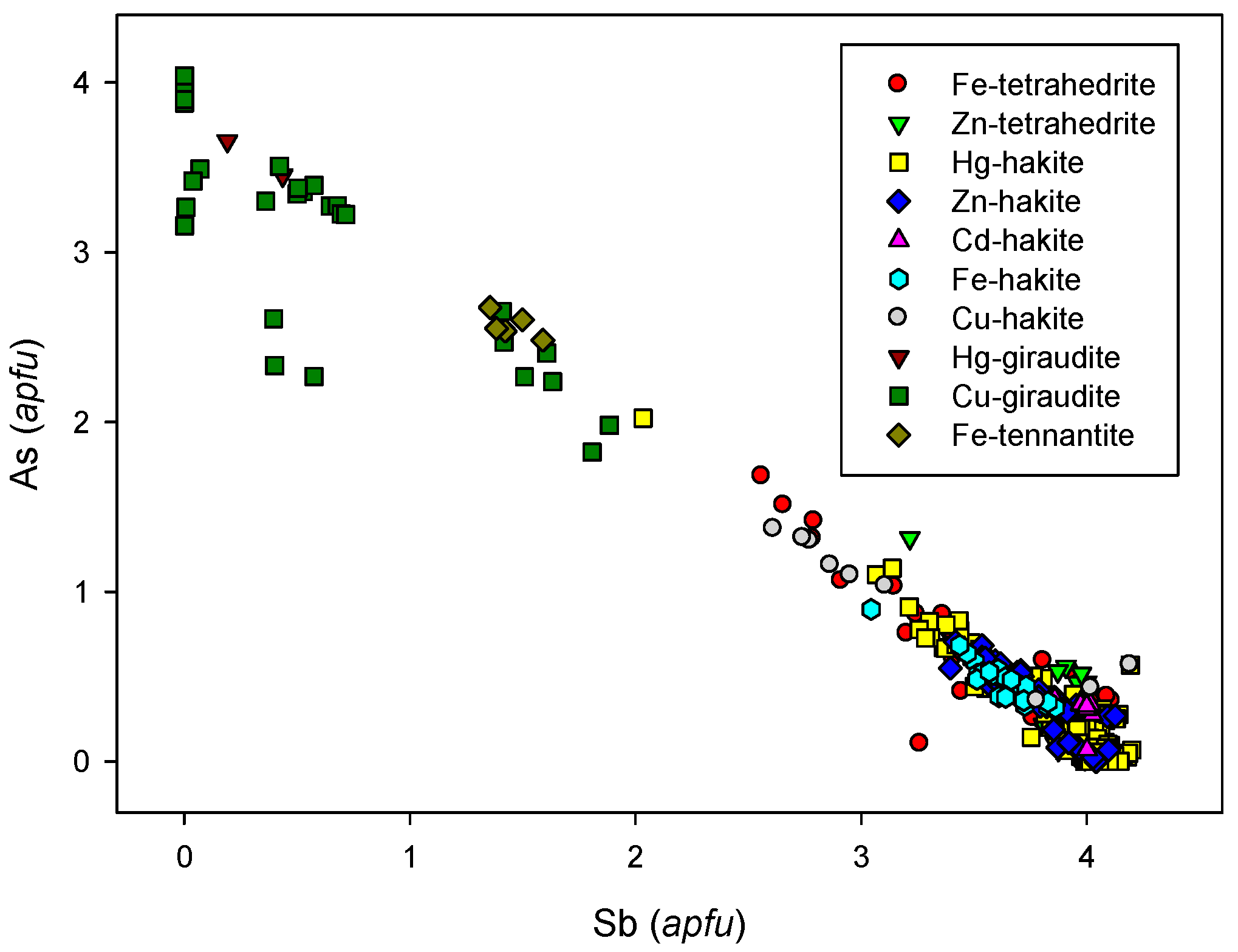
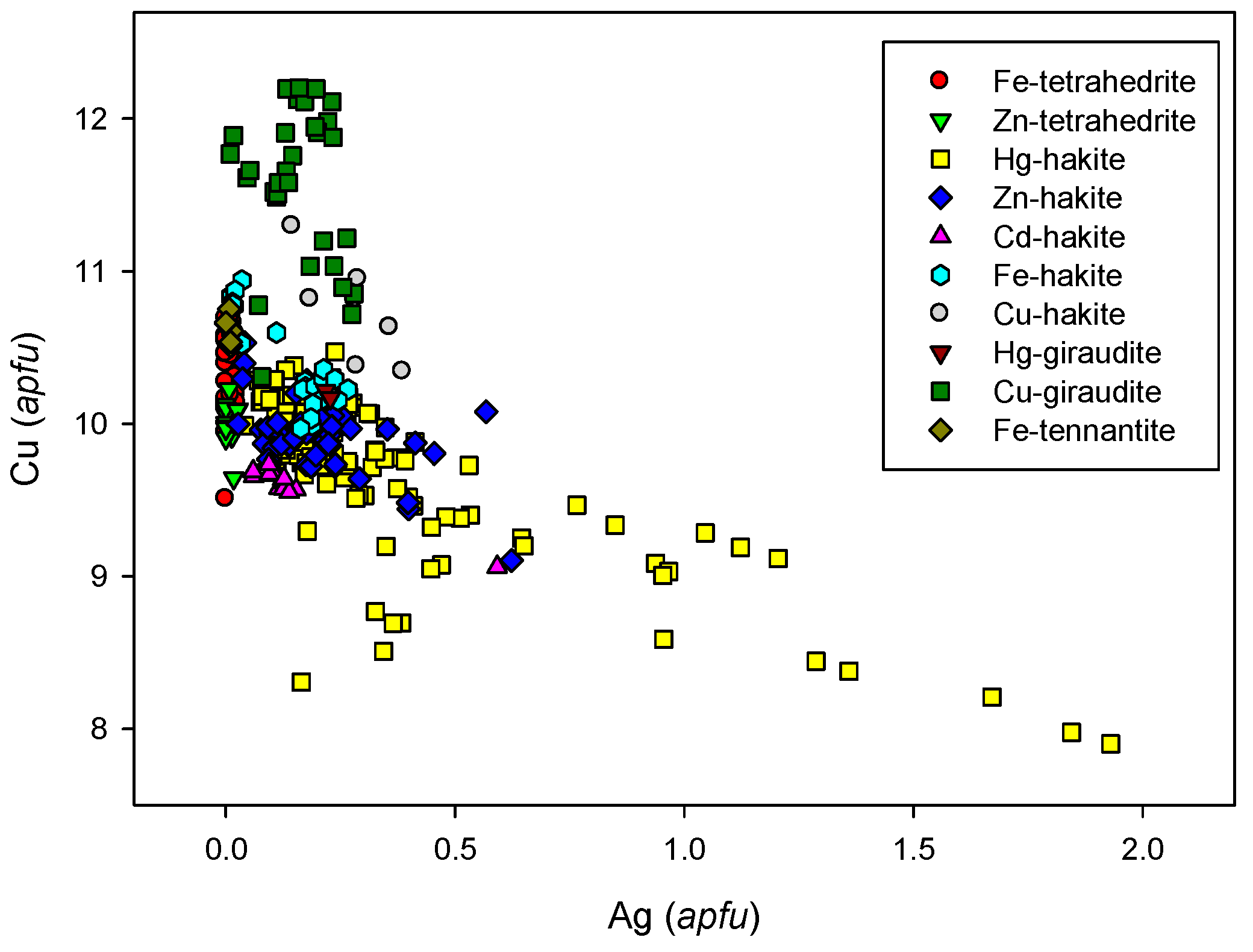
4.2.21.4 Trends in the Chemical Composition of Minerals of the Tetrahedrite Group at Příbram
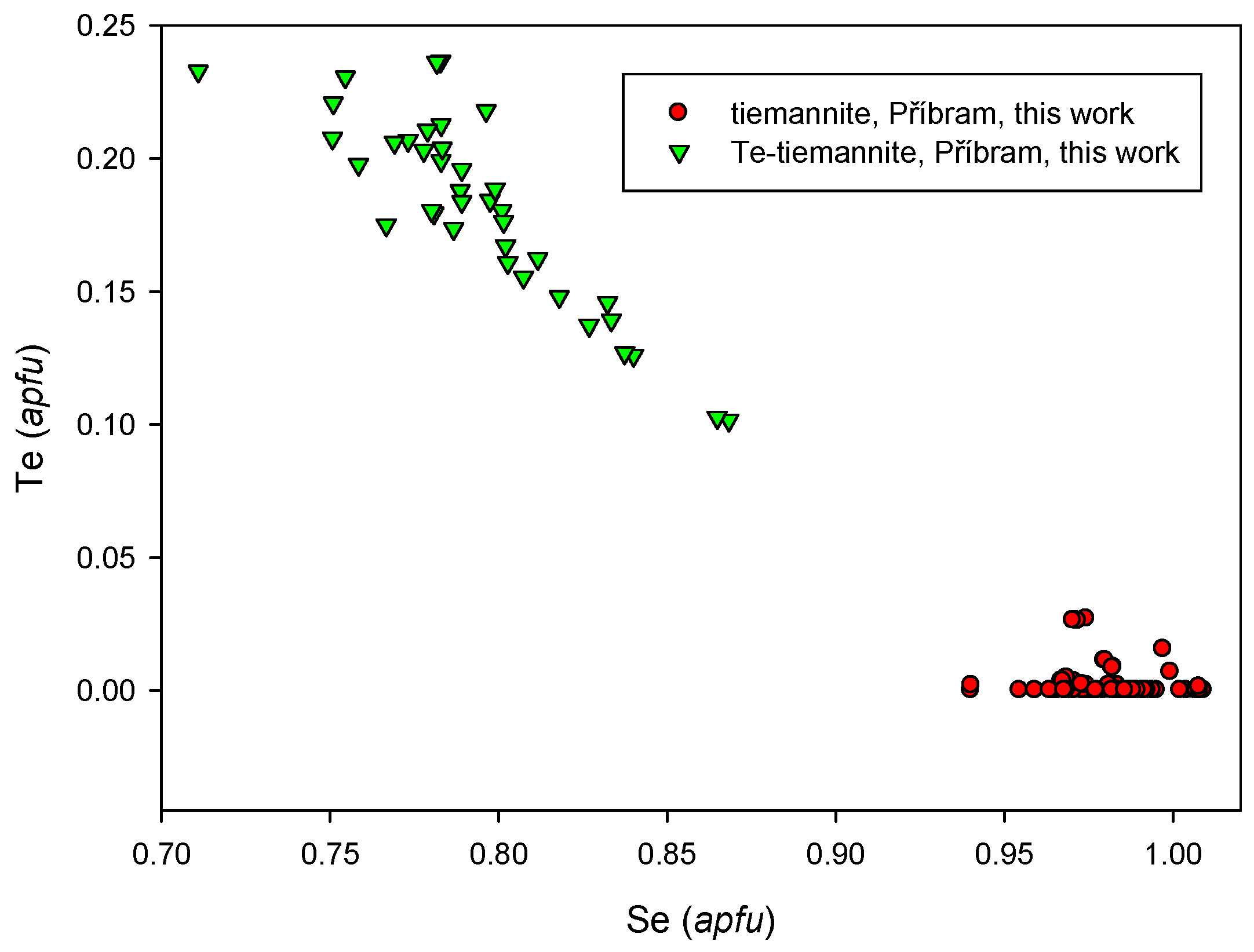
4.2.22. Tiemannite, HgSe
4.2.23. Umangite, Cu3Se2
4.2.24. Unnamed Cu-As Selenide, CuAsSe
5. Succession of Crystallization
6. Discussion of the Formation Conditions
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johan, Z. Preliminary report on the tiemannite-HgSe occurrence from Černý Důl in Krkonoše. Čas. Miner. Geol. 1960, 5, 65. (In Czech) [Google Scholar]
- Kvaček, M.; Plhal, J.; Matuška, J.; Kupka, F. The berzelianite Cu2−xSe finding in Morava. Čas. Miner. Geol. 1963, 8, 267. (In Czech) [Google Scholar]
- Kvaček, M. Selenides from the uranium deposits of western Moravia, Czechoslovakia—Part 1. Acta Univ. Carol. Geol. 1973, 1–2, 23–36. [Google Scholar]
- Kvaček, M. Selenides from the uranium deposits of western Moravia, Czechoslovakia—Part 2. Acta Univ. Carol. Geol. 1979, 1–2, 15–38. [Google Scholar]
- Blüml, A.; Tacl, A.; Rus, V. The occurrence of selenium minerals in southwest part of the selenides from the northwestern part of the Sedlčany-Krásná Hora metamorphosed island. Čas. Miner. Geol. 1964, 9, 73. (In Czech) [Google Scholar]
- Blüml, A.; Tacl, A.; Rus, V. Selenides from the northwestern part of the Sedlčany-Krásná Hora metamorphosed island. Čas. Miner. Geol. 1966, 11, 37–45. (In Czech) [Google Scholar]
- Johan, Z. Merenskyite, Pd(Te,Se)2, and the low-temperature selenide association from the Předbořice uranium deposit, Czechoslovakia. Neues Jahrb. Mineral. Monatsh. 1989, 4, 179–191. [Google Scholar]
- Čech, F.; Vavřín, I. Poubaite, PbBi2(Se,Te,S)4, a new Miner. Neues Jahrb. Mineral. Monatsh. 1978, 9–19. [Google Scholar]
- Čech, F.; Vavřín, I. Součekite, CuPbBi(S,Se)3, a new mineral of the bournonite group. Neues Jahrb. Mineral. Monatsh. 1979, 289–295. [Google Scholar]
- Novická, Z.; Kašpar, P. The new finding of selenides in exocontact of the Middle Bohemian Pluton. Geol. Průzk. 1976, 18, 250. [Google Scholar]
- Litochleb, J.; Šrein, V.; Novická, Z.; Šreinová, B. Selenides from the uranium deposit Ústaleč (nw. Bohemia). Bull. Mineral.-Petrol. Odd. Nár. Muz. 1999, 7, 98–108. (In Czech) [Google Scholar]
- Fojt, B.; Tenčík, I.; Panovský, K. Mineralogy and Geochemical Research of the Ore Deposits etc. Zálesí near Javorník Deposit; Lesní Čtvrť: Horní Hoštice, Bílá Voda, Czech Republic, Unpublished Report; 1971. (In Czech) [Google Scholar]
- Topa, D.; Makovicky, E.; Sejkora, J.; Dittrich, H. The crystal structure of watkinsonite, Cu2PbBi4Se8, from the Zálesí uranium deposit, Czech Republic. Can. Mineral. 2010, 48, 1109–1118. [Google Scholar] [CrossRef]
- Sejkora, J.; Plášil, J.; Litochleb, J.; Škácha, P.; Pavlíček, R. A selenide association with macroscopic umangite from the abandoned uranium deposit Zálesí, Rychlebské hory Mountains (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2012, 20, 187–196. (In Czech) [Google Scholar]
- Sejkora, J.; Macek, I.; Škácha, P.; Pauliš, P.; Plášil, J.; Toegel, V. An occurrence of Hg and Tl selenides association at the abandoned uranium deposit Zálesí, Rychlebské hory Mountains (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2014, 22, 333–345. (In Czech) [Google Scholar]
- Sejkora, J.; Škácha, P.; Kopecký, S.; Kopecký, S.; Pauliš, P.; Malíková, R.; Velebil, D. Se and Cu mineralization from Bílá Voda near Javorník (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2016, 24, 161–1177. (In Czech) [Google Scholar]
- Förster, H.J. Mineralogy of the Niederschlema-Alberoda U-Se-polymetallic deposit, Erzgebirge, Germany. II. Hessite, Ag2Te, and native Te (?), the first tellurium minerals. Neues Jahrb. Mineral. Abh. 2004, 180, 101–113. [Google Scholar] [CrossRef]
- Förster, H.J. Mineralogy of the Niederschlema-Alberoda U-Se-polymetallic deposit, Erzgebirge, Germany. IV. Continuous solid solutions between clausthalite and galena. Neues Jahrb. Mineral. Abh. 2005, 181, 2, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Förster, H.J.; Rhede, D. Mineralogy of the Niederschlema-Alberoda U-Se-polymetallic deposit, Erzgebirge, Germany. III. First indication of complete miscibility between tennantite and giraudite. Can. Mineral. 2004, 42, 1719–1732. [Google Scholar] [CrossRef]
- Förster, H.J.; Tischendorf, G. Se-rich tennantite and constraints on p-T-X conditions of selenide mineral formation in the Schlema-Alberoda uranium ore district (western Erzgebirge, Germany). Neues Jahrb. Mineral. Abh. 2001, 176, 109–126. [Google Scholar]
- Förster, H.J.; Rhede, D.; Tischendorf, G. Continuous solid-solution between mercurian giraudite and hakite. Can. Mineral. 2002, 40, 1161–1170. [Google Scholar] [CrossRef]
- Förster, H.J.; Rhede, D.; Tischendorf, G. Mineralogy of the Niederschlema-Alberoda U-Se-polymetallic deposit, Erzebirge, Germany. I. Jolliffeite, NiAsSe, the rare Se-dominant analogue of gersdorffite. Can. Mineral. 2004, 42, 841–849. [Google Scholar] [CrossRef]
- Förster, H.J.; Tischendorf, G.; Rhede, D. Mineralogy of the Niederschlema-Alberoda U-Se-polymetallic deposit, Erzgebirge, Germany. V. Watkinsonite, nevskite, bohdanowiczite and other bismuth minerals. Can. Mineral. 2005, 43, 899–908. [Google Scholar] [CrossRef]
- Sejkora, J.; Škácha, P.; Venclík, V.; Plášil, J. Vanadium-uranium mineralization in the Prachovice quarry (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2013, 21, 2, 113–130. (In Czech) [Google Scholar]
- Sejkora, J.; Škácha, P. The occurrence of selenides at the deposit Běstvina, Železné hory Mountains (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2015, 23, 255–260. (In Czech) [Google Scholar]
- Sejkora, J.; Škácha, P. Selenides from the fluorite deposit Moldava, Krušné hory Mountains (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2015, 23, 229–241. (In Czech) [Google Scholar]
- Malec, J. Morphology and composition of gold from the alluvial sediments In Czech Republic. Bull. Mineral.-Petrol. Odd. Nár. Muz. 2002, 10, 156–166. (In Czech) [Google Scholar]
- Pašava, J.; Sulovský, P.; Kovalová, M. Geochemistry and mineralogy of Proterozoic metal-rich black shales from the Bohemian Massif, Czech Republic, with a description of possible new molybdenum selenide and telluride phases. Can. Mineral. 1993, 31, 745–754. [Google Scholar]
- Scharm, B.; Scharmová, M. Accessory minerals in proterozoic silicit rocks in Kokšín near Mítov. Bull. Mineral.-Petrol. Odd. Nár. Muz. 1997, 4–5, 113–120. (In Czech) [Google Scholar]
- Scharmová, M. Mineralogical Evaluation of Wolframite Specimen from the Krupka-Barbora Adit; MS ČSUP: Stráž pod Ralskem, Česká Lípa, Czech Republic, 1989. (In Czech) [Google Scholar]
- Sejkora, J. Mineralogical Association of the Burning Dump of the Kateřina Mine in Radvanice Near Trutnov and Processes of Their Origin. Ph.D. Thesis, Faculty of Science, Masaryk University, Brno, Czech Rebublic, 2002. [Google Scholar]
- Johan, Z.; Kvaček, M. Bukovite, Cu3+xTl2FeSe4−x, new Miner. Bull. Soc. Franç. Minéral. Cristallogr. 1971, 94, 529–533. (In French) [Google Scholar]
- Johan, Z.; Kvaček, M.; Picot, P. Sabatierite, new selenide of copper and thallium. Bull. Minéral. 1978, 101, 557–560. (In French) [Google Scholar]
- Montreuil, L.A. Bellidoite: A new copper selenide. Econ. Geol. 1975, 70, 384–387. [Google Scholar] [CrossRef]
- Banas, M.; Atkin, D.; Bowles, J.F.W.; Simpson, P.R. Definitive data on bohdanowiczite, a new silver bismuth selenide. Mineral. Mag. 1979, 43, 131–133. [Google Scholar] [CrossRef]
- Johan, Z.; Picot, P.; Pierrot, R.; Kvaček, M. Kruťaite, CuSe2, new mineral of the pyrite group. Bull. Soc. Franç. Minéral. Cristallogr. 1972, 95, 475–481. (In French) [Google Scholar]
- Johan, Z.; Kvaček, M.; Picot, P. Petrovicite, Cu3HgPbBiSe5, new Miner. Bull. Soc. Franç. Minéral. Cristallogr. 1976, 99, 310–313. (In French) [Google Scholar]
- Johan, Z.; Picot, P.; Pierrot, R.; Kvaček, M. Fischesserite, Ag3AuSe2, the first selenide isotype of petzite. Bull. Soc. Franç. Minéral. Cristallogr. 1976, 94, 381–384. (In French) [Google Scholar]
- Johan, Z.; Kvaček, M. Hakite, new mineral of the tetrahedrite group. Bull. Soc. Franç. Minéral. Cristallogr. 1971, 94, 45–48. (In French) [Google Scholar]
- Johan, Z.; Picot, P.; Pierrot, R.; Kvaček, M. Permingeatite Cu3SbSe4, new mineral of the luzonite group. Bull. Soc. Franç. Minéral. Cristallogr. 1971, 94, 162–165. (In French) [Google Scholar]
- Paar, W.H.; Topa, D.; Makovicky, E.; Culetto, F.J. Milotaite, PdSbSe, a new palladium mineral species from Předbořice, Czech Republic. Can. Mineral. 2005, 43, 689–694. [Google Scholar] [CrossRef]
- Bindi, L.; Förster, H.J.; Grundmann, G.; Keutsch, F.N.; Stanley, C.J. Petříčekite, CuSe2, a new member of the marcasite group from the Předbořice Deposit, Central Bohemia Region, Czech Republic. Minerals 2016, 6, 33. [Google Scholar] [CrossRef]
- Dymkov, Y.M.; Loseva, T.I.; Zav’yalov, E.N.; Ryzhov, B.I.; Bochek, L.I. Mgriite, (Cu,Fe)3AsSe3, a new Miner. Zap. Vsesojuz. Mineral. Obshch. 1982, 111, 215–219. (In Russian) [Google Scholar]
- Förster, H.J.; Cooper, M.A.; Roberts, A.C.; Stanley, C.J.; Criddle, A.J.; Hawthorne, F.C.; Laflamme, J.H.G.; Tischendorf, G. Schlemaite, (Cu,□)6(Pb,Bi)Se4, a new mineral species from Niederschlema-Alberoda, Erzgebirge, Germany: Description and crystal structure. Can. Mineral. 2003, 41, 1433–1444. [Google Scholar] [CrossRef]
- Sejkora, J.; Makovicky, E.; Topa, D.; Putz, H.; Zagler, G.; Plášil, J. Litochlebite, Ag2PbBi4Se8, a new selenide mineral species from Zálesí, Czech Republic: Description and crystal structure. Can. Mineral. 2011, 49, 639–650. [Google Scholar] [CrossRef]
- Škácha, P.; Sejkora, J.; Plášil, J. Příbramite, CuSbSe2, the Se-analogue of chalcostibite, a new mineral from Příbram, Czech Republic. Eur. J. Mineral. 2017. [Google Scholar] [CrossRef]
- Škácha, P.; Sejkora, J.; Plášil, J. Bytízite, Cu3SbSe3, the Sb-Se analogue of witichenite, a new mineral from Příbram, Czech Republic. Mineral. Mag. 2017, in press. [Google Scholar]
- Růžička, J. Minerals of the Uranium Deposit Příbram; Vyd. Komitét Symp. Horn. Příbram ve Vědě a Technice: Pribram, Czech Republic, 1986; Volume 1–244. (In Czech) [Google Scholar]
- Litochleb, J.; Sejkora, J.; Šrein, V. Selenide minerals from the Bytíz deposit (Příbram uranium-base-metal ore district). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2004, 12, 113–123. (In Czech) [Google Scholar]
- Škácha, P.; Sejkora, J. The occurrence of arsenolamprite at the Příbram uranium-polymetallic district (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2007, 14–15, 131–133. (In Czech) [Google Scholar]
- Škácha, P.; Sejkora, J.; Litochleb, J.; Hofman, P. The occurrence of cuprostibite in the Příbram uranium-base metals ore district (the shaft 16, Příbram-Háje), Czech Republic. Bull. Mineral.-Petrol. Odd. Nár. Muz. 2009, 17, 73–78. (In Czech) [Google Scholar]
- Škácha, P.; Buixaderas, E.; Plášil, J.; Sejkora, J.; Goliáš, V.; Vlček, V. Permingeatite, Cu3SbSe4, from Příbram (Czech Republic): Description and Raman spectroscopy investigations of the luzonite-subgroup of minerals. Can Mineral. 2014, 52, 501–511. [Google Scholar] [CrossRef]
- Škácha, P.; Plášil, J.; Sejkora, J.; Goliáš, V. Suphur-rich antimonselite, Sb2(Se,S)3 in the Se bearing mineral association from the uranium and base metal ore district Příbram, Czech Republic. J. Geosci. 2015, 60, 23–29. [Google Scholar] [CrossRef]
- Škácha, P.; Sejkora, J.; Palatinus, L.; Makovicky, E.; Plášil, J.; Macek, I.; Goliáš, V. Hakite from Příbram, Czech Republic: Compositional variability, crystal structure and the role in the Se-mineralization. Mineral. Mag. 2016, 80, 1115–1128. [Google Scholar] [CrossRef]
- Sejkora, J.; Škácha, P.; Laufek, F.; Plášil, J. Brodtkorbite from Příbram, Czech Republic: Crystal structure and description. Eur. J. Mineral. 2017. [Google Scholar] [CrossRef]
- Škácha, P. Role of the Selenium in the Late Hydrothermal Phase of the Příbram Uranium Region. Ph.D. Thesis, Faculty of Science, Charles University, Prague, Czech Republic, 2015; pp. 1–235. [Google Scholar]
- Ettler, V.; Sejkora, J.; Drahota, P.; Litochleb, J.; Pauliš, P.; Zeman, J.; Novák, M.; Pašava, J. Příbram and Kutná Hora mining districts—From historical mining to recent environmental impact. IMA 2010, Budapest, Acta Mineral.-Petrol. Field Guide Ser. 2010, 7, 1–23. [Google Scholar]
- Litochleb, J.; Černý, P.; Litochlebová, E.; Sejkora, J.; Šreinová, B. The deposits and occurrences of mineral raw materials in the Střední Brdy Mts. and the Brdy piedmont area (Central Bohemia). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2003, 12, 57–86. (In Czech) [Google Scholar]
- Komínek, J. Geology of the Wide Surroundings and of the Deposit, Part I and II. Final Report on the Uranium District Příbram; MS DIAMO: Příbram, Czech Republic, 1995; p. 418. [Google Scholar]
- Anderson, E.B. Isotopic-Geochronological Investigation of the Uranium Mineralization of Czechoslovakia; MS DIAMO: Příbram, Czech Republic, 1987. [Google Scholar]
- Škácha, P.; Goliáš, V.; Sejkora, J.; Plášil, J.; Strnad, L.; Škoda, R.; Ježek, J. Hydrothermal uranium-base metal mineralization of the Jánská vein, Březové Hory, Příbram, Czech Republic: Lead isotopes and chemical dating of uraninite. J. Geosci. 2009, 54, 1–13. [Google Scholar] [CrossRef]
- Žák, K.; Dobeš, P. Stable isotopes and fluid inclusions in hydrothermal deposits: The Příbram ore region. Rozpr. ČSAV, řada mat. a přír. věd, 1991, 1–109. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. “PAP” (φ ρZ) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Ondruš, P. ZDS—Software for Analysis of X-ray Powder Diffraction Patterns; Version 6.01; User’s Guide; ZDS System: Prague, Czech Republic, 1993; pp. 1–120. [Google Scholar]
- Yvon, K.; Jeitschko, W.; Parthé, E. Lazy Pulverix, a computer program for calculation X-ray and neutron diffraction powder patterns. J. Appl. Crystallogr. 1977, 10, 73–74. [Google Scholar] [CrossRef]
- Burnham, C.W. Lattice Constant Refinement; Carnegie Institute Washington Yearbook: Washington, DC, USA, 1962; Volume 61, pp. 132–135. [Google Scholar]
- Sejkora, J.; Černý, P.; Černý, P. The occurrence of rare selenate, munakataite, at mine dump of the Lill mine, Příbram (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2010, 18, 87–90. (In Czech) [Google Scholar]
- Rigaku. CrysAlis CCD and CrysAlis RED; Oxford Diffraction Ltd.: Yarnton, Oxfordshire, UK, 2016. [Google Scholar]
- Sejkora, J.; Litochleb, J. Se-rich stibnite from the burning coal mine dump of the Kateřina mine, Radvanice near Trutnov, Czech Republic. Bull. Mineral.-Petrol. Odd. Nár. Muz. 2005, 13, 204–207. (In Czech) [Google Scholar]
- Lundegaard, L.F.; Miletich, R.; Balic-Zunic, T.; Makovicky, E. Equation of state and crystal structure of Sb2S3 between 0 and 10 GPa. Phys. Chem. Miner. 2003, 30, 463–468. [Google Scholar]
- Kyono, A.; Kimata, M.; Matsuhisa, M.; Miyashita, Y.; Okamoto, K. Low-temperature crystal structures of stibnite implying orbital overlap of Sb 5s2 inert pair electrons. Phys. Chem. Miner. 2002, 29, 254–260. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Li, J.; Xie, H.; Wang, J.; Deng, J.; Feng, C.; Qi, F.; Zhang, N. Experimental synthesis of the stibnite-antimonselite solid solution series. Int. Geol. Rev. 2008, 50, 163–176. [Google Scholar] [CrossRef]
- Min, M.; Zhai, J.; Wang, X.; Shen, B.; Wen, G.; Fan, T. Refinement of the crystal structure for a new mineral—Antimonselite. Chin. Sci. Bull. 2008, 43, 413–416. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Li, D.; Wang, G. Antimonselite, a new Miner. Acta Miner Sin. 1993, 13, 7–11. (In Chinese) [Google Scholar]
- Liu, J.; Liu, J.; Liu, C.; Lu, W.; Liu, S.; Su, W. Mineralogy of the Stibnite-Antimonselite Series. Int. Geol. Rev. 1999, 41, 1042–1050. [Google Scholar]
- Harris, D.C.; Cabri, L.J.; Kaiman, S. Athabascaite: A new copper selenide mineral from Martin Lake, Saskatchewan. Can. Mineral. 1970, 10, 207–215. [Google Scholar]
- Johan, Z.; Picot, P.; Ruhlmann, F. Paragenetic evolution of uranium mineralization from Chaméane (Puy-de-Dôme), France: Chaméanite, geffroyite and giraudite, three new selenides of Cu, Fe, Ag and As. Tscherm. Min. Petr. Mitt. 1982, 29, 151–167. (In French) [Google Scholar] [CrossRef]
- Grundmann, G.; Lehrberger, G.; Schnorrer-Köhler, G. The El Dragón mine, Potosí, Bolivia. Mineral. Rec. 1990, 21, 133–146. [Google Scholar]
- Paar, W.H.; Topa, D.; Roberts, A.C.; Criddle, A.J.; Amann, G.; Sureda, R.J. The new mineral species brodtkorbite, Cu2HgSe2, and the associated selenide assemblage from Tuminico, Sierra de Cacho, La Rioja, Argentina. Can. Mineral. 2002, 40, 225–237. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Bi, C. Geology of gold-bearing skarn deposits in the middle and lower Yangtze River Valley and adjacent regions. Ore Geol. Rev. 1999, 14, 227–249. [Google Scholar] [CrossRef]
- Heyding, R.D.; Murray, R.M. The crystal structures of Cu1.8Se, Cu3Se2, α-and γ CuSe, CuSe2, and CuSe2II. Can. J. Chem. 1976, 54, 841–848. [Google Scholar] [CrossRef]
- Harris, D.C.; Cabri, L.J.; Murray, E.J. An occurrence of a sulphur-bearing berzelianite. Can. Mineral. 1970, 10, 737–740. [Google Scholar]
- Makovicky, E.; Johan, Z.; Karup-Møller, S. New data on bukovite, thalcusite, chalcothallite and rohaite. Neues Jahrb. Mineral. Abh. 1980, 138, 122–146. [Google Scholar]
- Bur´yanova, E.Z.; Kovalev, G.A.; Komkov, A.I. The new mineral cadmoselite. Zap. Vsesojuz. Miner. Obshch. 1957, 86, 626–628. [Google Scholar]
- Vavřín, I. New findings of selenides in Bohemian Massif. Bull. Mineral.-Petrol. Odd. Nár. Muz. 1994, 2, 82–89. (In Czech) [Google Scholar]
- Scharmová, M. New findings of selenides and tellurides on the uranium deposits Zadní Chodov and Vítkov II (western Bohemia). Bull. Mineral.-Petrol. Odd. Nár. Muz. 1998, 6, 212–216. (In Czech) [Google Scholar]
- Yashunsky, Y.V.; Ryabeva, E.G.; Abramov, M.V.; Rasulova, S.D. Dzharkenite FeSe2—New Miner. Zap. Vses. Miner. Obsh. 1995, 124, 1, 85–90. (In Russian) [Google Scholar]
- Yakovleva, V.A.; Belogub, E.V.; Novoselov, K.A. Supergene iron sulpho-selenides from the Zapadno-Ozernoe copper-zinc massive sulphide deposit, South Urals, Russia: A new solid-solution series between pyrite FeS2 and dzharkenite FeSe2. Mineral. Mag. 2003, 67, 2, 355–361. [Google Scholar] [CrossRef]
- Jolyon, R. Available online:. Available online: https://www.mindat.org/min-6907.html (accessed on 9 February 2017).
- Kvaček, M.; Šuráň, J.; Ambrož, F. Eskebornite—New mineral for ČSSR. Čas. Miner. Geol. 1965, 10, 441. (In Czech) [Google Scholar]
- Kvaček, M. Contribution to geochemistry of selenide mineralization in Českomoravská vrchovina-ČSSR. Sbor. 1. Geoch. Konf. Ostrav. 1965, 393–402. (In Czech) [Google Scholar]
- Johan, Z. Crystal symmetry of eskebornite, CuFeSe2. Neues Jahrb. Mineral. Monatsh. 1988, 337–343. [Google Scholar]
- Frueh, A.J.; Czamanske, G.K.; Knight, C. The crystallography of eucairite, CuAgSe, Zeit. Kristallogr. 1957, 108, 389–396. [Google Scholar] [CrossRef]
- Pfitzner, A. Crystal structure of tricopper tetraselenoantimonate (V), Cu3SbSe4. Z. Kristallogr. 1994, 209, 685. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Wang, G.; Zhang, Q. A study of two unnamed selenide minerals. Acta Miner. Sinica 1995, 15, 418–421. (In Chinese) [Google Scholar]
- Berger, R.A. Crookesite and sabatierite in a new light. A crystallographer's comment. Zeit. Kristallogr. 1987, 181, 241–249. [Google Scholar] [CrossRef]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.J.; Cook, N.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, S.; Karup-Møller, S.; et al. Sulphosalts systematics: A review. Report of the sulphosalt sub-committee of the IMA Commission on Ore Mineralogy. Eur. J. Miner. 2008, 20, 7–46. [Google Scholar] [CrossRef]
- Makovicky, E. Crystal structures of sulfides and other chalcogenides. Rev. Miner. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Hatert, F.; Burke, E.A.J. The IMA-CNMNC dominant-constituent rule revisited and extended. Can. Miner. 2008, 46, 717–728. [Google Scholar] [CrossRef]
- Velebil, D.; Macek, I.; Soumar, J. A contribution to the knowledge of the chemistry of tetrahedrites from the Czech localities: Příbram, Obecnice, Zvěstov, Mníšek pod Brdy, Ratibořské Hory, Stará Vožice, Jáchymov, Kutná Hora and Stříbrná Skalice. Bull. Mineral.-Petrol. Odd. Nár. Muz. 2016, 24, 132–143. (In Czech) [Google Scholar]
- Kharbish, S.; Libowitzky, E.; Beran, A. The effect of As-Sb substitution in the Raman spectra of tetrahedrite-tennantite and pyrargyrite-proustite solid solution. Eur. J. Miner. 2007, 19, 567–574. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Nováček, R. Mercury rich tetrahedrites from Slovak. Zpr. Geol. Úst. 1942, 18, 107–110. (In Czech) [Google Scholar]
- Karanović, L.; Cvetković, L.; Poleti, D.; Balić-Žunić, T.; Makovicky, E. Structural and optical properties of schwazite from Dragodol (Serbia). Neues Jahrb. Mineral. Monatsh. 2003, 11, 503–520. [Google Scholar] [CrossRef]
- Arlt, T.; Diamond, L.W. Composition of tetrahedrite-tennantite and “schwazite” in the Schwaz silver mines, North Tyrol, Austria. Miner. Mag. 1998, 62, 801–820. [Google Scholar] [CrossRef]
- Foit, F.F.; Ulbricht, M.E. Compositional variation in mercurian tetrahedrite-tennantite from the epithermal deposits of the Steens and Pueblo Mountains, Harney County, Oregon. Can. Mineral. 2001, 39, 819–830. [Google Scholar] [CrossRef]
- Karup-Møller, S.; Makovicky, E. Exploratory studies of the solubility of minor elements in tetrahedrite. Part II. Selenium and tellurium as anions in Zn-Fe tetrahedrites. Neues Jahrb. Mineral. Monatsh. 1999, 7, 385–399. [Google Scholar]
- Karup-Møller, S.; Makovicky, E. Exploratory studies of element substitutions in synthetic tetrahedrite. Part V. Mercurian tetrahedrite. Neues Jahrb. Mineral. Abh. 2003, 179, 73–83. [Google Scholar] [CrossRef]
- Biagioni, C.; Moëlo, Y.; Orlandi, P. Lead-antimony sulfosalts from Tuscany (Italy). XV. (Tl-Ag)-bearing rouxelite from Monte Arsiccio mine: Occurrence and crystal-chemistry. Miner. Mag. 2014, 78, 651–661. [Google Scholar] [CrossRef]
- Bindi, L.; Keutsch, F.N.; Francis, C.A.; Menchetti, S. Fettelite, [Ag6As2S7][Ag10HgAs2S8] from Chañarcillo, Chile: Crystal structure, pseudosymmetry, twinning, and revised chemical formula. Am. Mineral. 2009, 94, 609–615. [Google Scholar] [CrossRef]
- Johnson, N.E.; Craig, J.R.; Rimstidt, J.D. Compositional trends in tetrahedrite. Can. Mineral. 1986, 24, 385–397. [Google Scholar]
- Brodin, B.V. Silver-bearing hakite. Int. Geol. Rev. 1981, 23, 71–73. [Google Scholar] [CrossRef]
- Scharmová, M.; Scharm, B. The selenium minerals on the uranium deposit Zadní Chodov. Bull. Mineral.-Petrol. Odd. Nár. Muz. 1995, 3, 43–47. (In Czech) [Google Scholar]
- Kopecký, S.; Pauliš, P.; Škoda, R. New occurrence of selenides from the Černý Důl uranium deposit in the Giant Mountains (Czech Republic). Bull. Mineral.-Petrol. Odd. Nár. Muz. 2010, 18, 43–49. (In Czech) [Google Scholar]
- Wallis, W. The Paragenetic and Mineralogy Research of Selenides in Harz. Unpublished Thesis. 1994; pp. 1–195. [Google Scholar]
- Chakrabarti, D.J.; Laughlin, D.E. The Cu-Se (copper-selenium) system. Bull. Alloy Phase Diag. 1981, 2, 305–315. [Google Scholar] [CrossRef]
- Whitfield, H.J. The crystal structure of hcc-CuAsSe. J. Solid State Chem. 1981, 39, 209–214. [Google Scholar] [CrossRef]
- Bindi, L.; Catelani, T.; Chelazi, L.; Bonazzi, P. Reinvestigation of the crystal structure of lautite, CuAsS. Acta Crystallogr. Sect. E 2008, 64, i22. [Google Scholar] [CrossRef] [PubMed]
- Litochleb, J.; Šrein, V. Silver minerals of the Příbram uranium deposit. Bull. Mineral.-Petrol. Odd. Nár. Muz. 1994, 2, 76–81. (In Czech) [Google Scholar]
- Litochleb, J.; Šrein, V.; Knížek, F.; Sejkora, J. Mineralogy characteristic of bournonite occurrences of the Bytíz base-metal (uranium deposit Příbram). Bull. Mineral.-Petrol. Odd. Nár. Muz. 1995, 3, 219–221. (In Czech) [Google Scholar]
- Litochleb, J.; Sejkora, J.; Šrein, V. Sulphoantimonides of lead from the base-metal vein H32A from the uranium deposit Příbram (ore knob Háje). Bull. Mineral.-Petrol. Odd. Nár. Muz. 1997, 4–5, 163–169. (In Czech) [Google Scholar]
- Dymkov, J.M. Selenides of uraninite-carbonate veins. In Paragenezis Mineralov Uranonosnych Žil; Izd. Nedra: Moscow, Russia, 1985; pp. 153–162. (In Russian) [Google Scholar]
- Kvaček, M. Mineralogical-geochemical characteristic of selenide mineralization in the uranium deposit in Bohemian Massif. In Sbor. Celoštát. Miner. Semin. Mineralógia Uránových a s Nimi Súvisiacích Nerastných Surovin; Čingov: Spišská Nová Ves, Slovakia, 1987; pp. 89–95. (In Czech) [Google Scholar]
- Simon, G.; Essene, E.J. Phase relations among selenides, sulfides, tellurides, and oxides. I. Thermodynamic properties and calculated equilibria. Econ. Geol. 1996, 91, 1183–1208. [Google Scholar] [CrossRef]
- Simon, G.; Kessler, S.E.; Essene, E.J. Phase relations among selenides, sulfides, tellurides, and oxides. II. Application to selenide-bearing ore deposits. Econ. Geol. 1997, 92, 468–484. [Google Scholar] [CrossRef]
- Škácha, P.; Sejkora, J.; Knížek, F.; Slepička, V.; Litochleb, J.; Jebavá, I. Occurrences of unique monometallic Ag mineralization at the H14F3 vein between the 7th and 9th level of the shaft No. 21 Háje, the Příbram uranium-base metal ore district, Czech Republic. Bull. Mineral.-Petrol. Odd. Nár. Muz. 2012, 20, 230–254. (In Czech) [Google Scholar]
- Schønwandt, H.K. Interpretation of ore microstructures from a selenous Cu-mineralization in South Greenland. Neues Jahrb. Mineral. Abh. 1983, 146, 302–332. [Google Scholar]














© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škácha, P.; Sejkora, J.; Plášil, J. Selenide Mineralization in the Příbram Uranium and Base-Metal District (Czech Republic). Minerals 2017, 7, 91. https://doi.org/10.3390/min7060091
Škácha P, Sejkora J, Plášil J. Selenide Mineralization in the Příbram Uranium and Base-Metal District (Czech Republic). Minerals. 2017; 7(6):91. https://doi.org/10.3390/min7060091
Chicago/Turabian StyleŠkácha, Pavel, Jiří Sejkora, and Jakub Plášil. 2017. "Selenide Mineralization in the Příbram Uranium and Base-Metal District (Czech Republic)" Minerals 7, no. 6: 91. https://doi.org/10.3390/min7060091




